Abstract
In last few years, a rapid advancement in nanotechnology have provided many applications in the health sector and have great potential to solve the day-to-day problems faced during the treatment of various chronic diseases. One of the most important applications is the use of nanocarriers (NCs) for efficient and targeted drug delivery. Now a days, NCs such as organic, inorganic and hybrid NCs are of great interest owing to their wide application as drug delivery systems (DDSs). These NCs have a great deal of potential to minimize the drawbacks of present pharmacotherapeutics by improving therapeutic efficacy and biomolecular interactions, thereby lowering the side effects of various drugs. These systems often exhibit excellent stability, effective drug encapsulation and drug preservation against degradation. NCs are considered an alternative to drug diffusion into cells and can also be used as a diagnostic tool for various diseases. This chapter provides an overview of the functionalization of NCs by different classes of DDSs for diagnosing and treating human diseases.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
11.1 Introduction
Nanotechnology entails functional systems at the molecular level. Such systems have distinctive electrical, optical, physicochemical and biological characteristics that make them interesting candidates for applications in fields such as materials science and biomedicine. In biomedicine, drug delivery systems (DDSs) entail the administration of therapeutic or pharmaceutical components to a precise area of the body with better efficacy and safety (Hong et al., 2020). Delivering therapeutic components to the specific targeted site or cells is a noteworthy requirement for curing several ailments. A conventional DDS is sometimes characterized by a lack of selectivity, poor biodistribution and limited efficacy. Therefore, the development of drug delivery techniques could strategically use nanotechnology to expand the drug market. Nanoparticles (NPs) are colloidal nanocarriers (NCs) of synthetic or semisynthetic polymers with a size of 1–100 nm, which are helpful in addressing concerns about the delivery of both modern and conventional drugs (Sur et al., 2019). NPs have been shown to possess better flexibility in accessing deep molecular targeting tissues and in regulating drug release (Karuppusamy & Venkatesan, 2017). When formulated properly, nanodrug particles can have greater adherence to biological surfaces, better saturation solubility, quick dissolution and resistance to settling, all of which contribute to a faster beginning of therapeutic action and higher bioavailability. In addition, the nanostructure’s surface contains the vast bulk of its molecules (Bamrungsap et al., 2012). Most of the molecules within a nanostructure are found on the particle’s surface, maximizing the delivery and loading tendency of cargoes such as various therapeutic drugs, polynucleotides, enzymes, proteins and genes to specific tissues or cells. Different types of nanostructures, including NPs, nanocomposites, nanotubes and nanofibres, effectively aid in the screening and treatment of a wide range of disorders (Baskar et al., 2017a, b; Chamundeeswari et al., 2013; Verma et al., 2012, 2013). Moreover, NCs with optimal biological and physicochemical properties could be applicable for delivering presently available bioactive chemicals, as cells can absorb them more readily than larger molecules (Saman & Iqbal, 2012; Zahin et al., 2020; Wilczewska et al., 2012). This chapter elaborates the different classes of colloidal NCs, which play a significant role in DDSs and are applied as a suitable factor for biological applications.
11.2 Nanocarriers as Drug Delivery Systems
NCs are the colloidal particle system of NPs that are frequently employed to carry therapeutic agents or any other compounds to a targeted site (Qian et al., 2012). As the size of microcapillaries in a body is 200 nm, NCs should be of a size less than 200 nm for their therapeutic applications in the body (Singh & Lillard, 2009). NCs are inactive and typically regarded as a safe medium and thus offer good biocompatibility, fewer side effects and many other physicochemical features (Kingsley et al., 2006), depending on their composition, shape and surface (Sun et al., 2014). As a result, they have a broad spectrum of drug delivery. Several types of NCs have been reported to exhibit remarkable site-specific drug delivery (Mishra et al., 2010), including applications such as enhanced pharmacokinetics and biodistribution, enhanced solubility and stability, toxicity reduction and sustainability.
11.2.1 Types of NCs and Their Classification
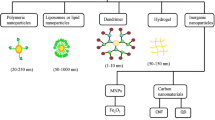
NCs possess a high surface-to-volume ratio and are categorized as inorganic, organic and hybrid NCs, which are further distributed into various classes (Fig. 11.1).
11.3 Inorganic NCs
Inorganic NCs have been classified into various types, including gold NPs (AuNPs), ceramic and superparamagnetic NCs, quantum dots (QDs), mesoporous silica, carbon nanotubes (CNTs), etc. (Fig. 11.2). These NCs have enormous applications in therapeutics and pharmacology, including biosensing, diagnostics, bioimaging, cell labelling, biocatalysis and gene delivery and targeting (Santos et al., 2014). Inorganic NCs also exhibit various other clinical applications, namely the treatment of tumours (Shi et al., 2020), chronic myelogenous leukaemia (Ghosn et al., 2019), inflammatory disease (Prosperi et al., 2017) and many others. In addition, the modification of the size and arrangement of inorganic NCs can lead to remarkable plasmonic and optical properties (Goldenberg et al., 2020; Gellini & Feis, 2021). Moreover, its composition with heavy metals could raise significant drawbacks which can lead to chronic health diseases (Ma et al., 2015).
11.3.1 Gold NCs
AuNPs are inorganic NCs in which the inner core contains the gold atom and its surface has negative groups. A monolayer of surface ligands can easily functionalize the surface for active targeting. AuNPs have surface biofunctionalization with biomolecules, including proteins, carboxylic acid, enzymes and so on (Mohammed & Al-Gawhari, 2020). These NPs display low toxicity and high surface area and thus show greater drug-loading tendency. Due to the uniform dispersity of AuNPs, they can reach the active targeted site and thus provide new delivery strategies. AuNPs have shown tremendous biomedical applications in optics, chemotherapy, photoacoustic imaging, gene delivery, photothermal therapy, etc. Furthermore, due to their optical properties, biomolecules such as proteins, enzymes, peptides, carbohydrates, genes and fluorophores can be attached to AuNPs, thus making possible the effective delivery of AuNPs within the cell (Khandelia et al., 2013). Developments in the pharmacokinetics, pharmacology and biodistribution of AuNPs are also imperative for enhancing their applications in medicinal drugs (Wang et al., 2004; Qian et al., 2008; García, 2011; Lu et al., 2010; Huang et al., 2006).
11.3.2 Ceramic NCs
Ceramic NCs are made of inorganic materials having pore-like properties, such as titania, silica and alumina (Medina et al., 2007; Nutter & Ratts, 1973). Silica has been proven to have better features owing to its biocompatibility, easy synthesis and surface modification (Bottini et al., 2007; Ohulchanskyy et al., 2007). Well-understood silane chemistry also makes it easier for drugs to cross-link with silica particles (Slowing et al., 2007). Furthermore, NCs of mesoporous silica are porous structures with a two-dimensional network of several mesopores, which resembles a honeycomb. Recent studies have shown that these NCs show exclusive biocompatibility in pharmacological applications as compared to amorphous silica materials of low biocompatibility (Descalzo et al., 2006; Trewyn et al., 2007). To deliver drug molecules at levels that are pharmacologically efficacious after the vehicle has been localized in the cytoplasm, it is preferable to have effectual control over their release. To accomplish this, it is advantageous to be able to selectively functionalize the internal nanochannel surface of mesoporous silica and their exterior particle surfaces (Angelos et al., 2007). To attain tissue specificity, the mesoporous silica surface can be modified with cell-specific moieties, such as organic compounds, peptides, antibodies and aptamers. Furthermore, versatile DDSs can be created using optical and magnetic contrast agents (Slowing et al., 2008).
11.3.3 Carbon-Based NCs
Carbon-based NCs have a tube-like assembly of carbon atoms. CNTs are considered carbon-based nanocarriers, which act as an excellent source of delivering drugs, due to their unique biological and physical–chemical features. CNTs belong to the family of fullerenes, which are made by wrapping graphene sheets into a tube-like shape (Bianco, 2004). CNTs are suitable for numerous applications due to their high surface area with ultralight weight, nano-sized needle structure, high aspect ratio and thermal, mechanical, electrical and distinctive chemical properties (Ng et al., 2016; O’Regan & Gratzer, 1991). Moreover, their surface modification, structural flexibility and stability make them effective agents for destroying cancer cells. According to that theory, anti-cancer medications like paclitaxel are frequently encapsulated in or linked to functionalized carbon nanotubes (Liu et al., 2008; Thiruvengadam et al., 2021).
11.3.4 Quantum Dots
Elements such as Te, Se, Zn, As, P and so on are included in QD formulation and are considered energy carriers (Corrocher et al., 1975). The emission of light in the ultraviolet (UV) region is dependent on the quantum dot’s size; for example, small-size QDs (~2 nm) lead to the emission of blue fluorescence, whereas large-size QDs (~5 nm) emit red fluorescence. Their optical quality sets them apart from other organic dyes, and thus, they can be utilized for cell imaging. For instance, the in vivo targeting of rat tumour vasculature uses a quantum dot–peptide conjugate (Åkerman et al., 2002). In addition, QDs are known for their effectiveness as delivery and reporting systems (Christian et al., 2003; Derfus et al., 2007). In the charge transfer process, these colloidal nanocrystals are used as an energy transfer quencher (Medintz et al., 2009), chemiluminescence resonance energy transfer acceptors (Freeman et al., 2011) and quantum dot–fluorescence resonance energy transfer system (Geißler et al., 2010).
11.3.5 Magnetic NCs
Magnetic NCs have shown an extensive range of applications for the diagnosis and treatment of diseases that pose risks to human life, such as cancer and neurological and cardiovascular conditions (Stergar et al., 2019; Abulibdeh et al., 2019; Almessiere et al., 2018a). These NCs work by magnetic absorption of specific tissues. They consist of supermagnetic and magnetic susceptibility and super-saturation properties (Üzek et al., 2019; Almessiere et al., 2018b; Advanced C, 2022). In contrast to metal oxide NPs, metal NPs are often more magnetic. They are used in biosensing. Among superparamagnetic and paramagnetic NPs, the former are more susceptible to magnetic fields than the latter. These NCs show good biocompatibility and offer good ease of surface modification and are considered for use in biomedical and industrial applications (Kianfar, 2021).
11.3.6 Mesoporous NCs
Mesoporous NCs have a porous honeycomb-like structure, which makes it possible to incorporate more drug molecules into them. These NCs have been applied in the biomedical industry due to their accessibility and simplicity. Both hydrophilic and aquaphobic drugs can bind to a ligand for targeted drug administration and can be encapsulated by mesoporous NCs (Li et al., 2017). Mesoporous silica possesses thermochemical properties and shows good biocompatibility, a large porous volume, a high surface area and drug-loading capacity (Wang et al., 2015). Some of the anti-cancer drugs such as camptothecin and methotrexate are proficiently distributed by using mesoporous silica.
11.4 Organic NCs
Organic NCs possess good drug-loading capability, biocompatibility and less toxicity. The first-generation NCs were basic excipients called polymeric NCs (PNCs) and liposomes that were used for drug delivery. Moreover, liposomes and micelles can amass at the specific spot due to their improved permeability and retention impact (Peng et al., 2020). Figure 11.3 shows the various classes of organic drug NCs.
11.4.1 Solid Lipid NCs (SLNCs)
Solid lipid NCs (SLNCs) are submicron spherical colloidal carriers with a typical size of nearly 40–1000 nm. SLNCs are composed of solid biodegradable lipids and biocompatible material (Liu et al., 2010). These are non-toxic alternative lipophilic colloidal drug carriers (Yadav et al., 2013). SLNCs are formed by dispersing melted solid lipids in water, followed by their stabilization with the addition of emulsifiers through the process of high-pressure homogenization or microemulsification (Yadav et al., 2013; Malam et al., 2009). Mono-, di- or triglycerides; steroids; free fatty alcohol or acids; and wax are some of the solid lipids used for the production of SLNCs, as shown in Fig. 11.4 (Torchilin, 2011; Rouco et al., 2020; Mäder & Mehnert, 2004).
SLNCs can be classified into two types: solid lipid NPs (SLNPs) and nanostructured lipid carriers (NLCs) (Naseri et al., 2015; Schwarz et al., 1994). Solid lipids are the major components of SLNPs, whereas NLCs contain solid and liquid lipids (Müller et al., 2002). SLNPs can carry both micro- and macro-molecules (protein and peptides) (Mu & Holm, 2018), using appropriate excipients and adopting suitable method of formulation or preparation, whereas NLCs are designed to improve the shortcomings of SLNCs (Das & Chaudhury, 2011; Kim et al., 2005). The drug loading and release profile are both significantly impacted by the change in the lipid composition of SLNCs (Das et al., 2012; Balguri et al., 2016). Molecular drugs can be integrated into the matrix, shell or core of the solid lipid depending on the manufacturing conditions and conformation. Due to their versatility, SLNCs can overcome the limitations of conventional chemotherapy (Hallan et al., 2016). SLNCs, when loaded with curcumin, have also been investigated for breast cancer treatment (Wang et al., 2018). Furthermore, ionic and hydrophilic anti-cancer drugs can now be added to lipophilic drugs using SLNCs. These can also be utilized in parenteral and oral drug delivery (Chamundeeswari et al., 2019). SLNCs have provided value-added advantages as drug carriers in the field of pharmaceutics (Yaghmur & Mu, 2021).
11.4.2 Liposomes
Liposomes are the colloidal spherical structure made up of self-assembled phospholipids or amphiphilic lipid molecules (Guimarães et al., 2021; Sebaaly et al., 2016). Liposomal NCs possess a size of 50–100 nm. The liposomal membrane is composed of lamellas, that is, unilamellar or multilamellar lipid bilayers, forming a spherical vesicle (Nisini et al., 2018; Laouini et al., 2012). The lipid bilayers serve as the vehicles for hydrophilic and lipophilic drug delivery at the specific site. However, in systemic circulation, these molecules possess limited half-life. Therefore, polymeric molecules like polyethylene glycol (PEG) can be used to coat liposomes to create PEGylated liposomes or stealth liposomes. The stealth liposomes can evade the reticulate endothelial system owing to their long stability in blood, which results in producing sustained drug release (Torchilin, 2000). Liposomes ultimately enhance the biodistribution and pharmacokinetics of incorporated drug molecules (Wang et al., 2012). Moreover, because of their structural versatility, biocompatibility and non-immunogenic nature, they are well sought as a good drug delivery agent. The amphiphilic nature of phospholipids in solution is similar to that of natural cell membranes, and this results in an effective interaction of liposomes with mammalian cell membranes to promote cellular absorption (Laouini et al., 2012). Liposomes are capable of carrying large drug payloads and have a wide range of physicochemical properties (Sercombe et al., 2015). Liposomes have enhanced biomedical and therapeutic properties that enable the biodistribution of drugs to the target site in vivo (Hua & Wu, 2013; Ding et al., 2006).
11.4.3 Polymeric Micelles (PMs)
Polymeric micelles (PMs) are the multifunctional NPs (10–100 nm) formed by the spontaneous association of di- or tri-block polymeric components (copolymers) or synthetic amphiphilic surfactants in an aqueous milieu to form micelle core–shell structures. A micelle’s hydrophobic inner core is enclosed by a shell of hydrophilic polymers such as polyethylene glycol. The hydrophobic inner core contains amphiphilic and poorly water-soluble drugs, whereas the hydrophilic shell stabilizes the core. However, the hydrophilic shell of PMs allows solubility in aqueous media and modulates in vivo pharmacokinetics (Begines et al., 2020; Majumder et al., 2020). Until now, various drug components can be incorporated in PMs via covalent/chemical attachment (Wu et al., 2012) or physical attachment (Din et al., 2017; Batrakova et al., 1996; Nakanishi et al., 2001). PMs can be prepared by oil-in-water emulsion, dialysis, cosolvent evaporation, freeze-drying and solvent evaporation methods (Rapoport, 2007). PMs have been thought of as suitable NCs for the controlled release of biomedical drug delivery (Kaur et al., 2022). Anti-cancer medications are aquaphobic, and PMs can entrap these aquaphobic drug components within their core, which ultimately increases their water solubility. Drugs can be loaded into micellar systems with efficiency and ease via physical entrapment. Several anticancer medications, such as doxorubicin and paclitaxel, have been physically trapped for ultrasonic delivery in PMs (Rapoport et al., 1999a, b, 2000). In addition, PM-based nucleic acid carriers have been studied as nucleic acid therapeutics permit for therapeutic modulation of gene expression (Jarak et al., 2021; Toscanini et al., 2021; Howard et al., 2006).
11.4.4 Dendrimers
Dendrimers are multivalent globular nanoscale macromolecules with an initiator core in the centre, forming a star- or tree-like shape (Pawar et al., 2020), with a size of 1–10 nm. They have active terminal groups and provide a high range of surface functionality. Dendrimers are made up of nucleotides, amino acids and sugar molecules. The core cavities encapsulate the drug molecules within them via chemical interaction, hydrophobic bonds and hydrogen (H) bonds or are attached to the active terminal groups by covalent bonds. This class of NCs is used to encapsulate drugs like rifampicin, which are further used for the treatment of tuberculosis due to their structural applications (Mignani et al., 2018). Numerous anti-cancer medications, including dox and cisplatin, coupled with dendrimers create improved anti-cancer activity (Lai et al., 2007). Dendrimers play a significant role in DDS which include Oral drug delivery, transdermal drug delivery, ocular drug delivery, targeted gene delivery, and anticancer drug delivery (Mathur et al., 2015).
11.4.5 Polymeric Nanocarriers
Polymeric NCs (PNCs) are made from biodegradable synthetic polymers, semi-synthetic polymers or natural polymers. Figures 11.5 and 11.6 show the different classes and chemical structures of synthetic PNCs, respectively (Avramović et al., 2020; Wang et al., 2009). The solid colloidal PNCs can act as a reservoir type for nanocapsules that diffuse or encapsulate the molecular drugs in the matrix of polymers, that is, nanospheres (Prabhu et al., 2015). PNCs undergo a polymerization reaction involving several monomer units (Calzoni et al.; Zhu & Liao, 2015). When compared to other NCs, PNCs provide better stability, good drug payload tendency, adequate half-life in systemic circulation and prolonged drug release. To target cancerous cells, an anti-cancer medicine like dox is encapsulated inside PNCs. Physicochemical changes in the polymeric source can improve the regulated release of the medication. Multifunctional PNCs can also be made, which allow for the inclusion of various medications within them (Zhu & Liao, 2015; Nelemans & Gurevich, 2020). PNCs allow the active and passive modes of drug delivery, provide high concentration of drug delivery and preserve constancy of volatile pharmaceutical agents (López-Dávila et al., 2012).
11.5 Hybrid NCs
The combination of more than two organic and inorganic NCs, either organic–organic or inorganic–inorganic NCs, are termed as hybrid NCs. These NCs overcome all the disadvantages of the individual ones by incorporating two or more NPs together (Qian et al., 2012). This can be seen in the case of liposomes. Being unstable, liposomes are easily removed from the bloodstream, which makes them less effective. On the other hand, using hybrid liposomes can solve such problems. Further, these are classified into lipid polymer hybrid and ceramic polymer hybrid (Peer et al., 2007). During the selection of NPs to form hybrid NCs, various parameters should be followed such as drug type to be conjugated, site of action, physiological barrier while delivering the drugs and stability and solubility of the NCs. These hybrid NCs offer larger bioavailability of therapeutic substances with fewer adverse effects. Some of the most important examples of hybrid NCs are mesoporous silica NP–lipid bilayer hybrid NC system, which is useful for intracellular delivery of zoledronic acid with a high retention rate in breast cancer and prevention of the premature release of drug (Desai et al., 2017).
11.6 Safety Concerns and Future Perspectives
With advancements in the field of nanotechnology, a new field has emerged which is nanotoxicology. As the name suggests, nanotoxicology is concerned with the toxic effect of nanomaterials on biological systems. Some of the studies have shown that nanomaterials lead to the formation of free radicals that can further damage our brain cells and can also cause unnecessary penetration through the epidermis. This can lead to many other toxic effects on the biological system (Shvedova et al., 2011; Niemeier et al., 2006; Oberdörster et al., n.d.; Lovrić et al., 2005). This is a serious obstacle confronted by pharmaceutical companies during NP formulation and encapsulation. Nanomaterials have great potential as nanomedicines because of their many advantages. Thus, a well-defined database is required to be followed by the experts, which should contain information about the nano DDS, storage, handling protocols and most importantly its toxicology towards biological systems (Kirchner et al., 2005; Choi et al., 2007; Chang et al., 2007). Moreover, a detailed investigation over the issues related to their shape, size, production and surface characterization should be performed to enhance their bioavailability and long-term advantages.
11.7 Conclusion
Pharmaceutical nanocarriers including micelles, nano emulsions, liposomes, PMs, NPs, etc., exhibit a wide range of beneficial properties, which includes longevity in blood, enabling their desired concentration in pathophysiological areas with the compromised vascular system; precise affected site targeting (due to various targeting ligands linked to the NCs surface), and many more. NCs have shown high efficacy and biocompatibility compared to traditional DDSs. Potential for creating extremely effective and precise systems for medications, diagnostics and genetic agents using pharmaceutical nanocarriers is practically limitless. Thus, the combination of nanotechnology and medicinal drugs has produced an offspring that is expected to lead to significant advancements in the treatment of several ailments. It is crucial to comprehend how the biodistribution of NCs affects the body’s intricate biological network and mass transport throughout the compartmental boundaries. In addition, the development of a toxicological catalogue to facilitate safety and hazard assessments is essential for the healthy progress of this area. A well-defined database can be of great importance to formulate more nanodrugs. In a nutshell, NCs are the future of DDS owing to their specificity and effectiveness in treating a wide range of diseases, as well as their numerous therapeutic uses.
References
Abulibdeh, N., Kumar, K. V., Karthika, C., Jarin, T., Gopi, A., & Bouzidi, A. (2019). Exploring magnetic fluid sensor using dual circular core elliptical cladding photonic crystal fiber. Results in Physics, 13(February), 4–5.
Advanced C. Pe Off r ic s e o Phone: Education formation Foreign Languages. Research Areas Published journal articles indexed by a n d. 2022;2022.
Åkerman, M. E., Chan, W. C. W., Laakkonen, P., Bhatia, S. N., & Ruoslahti, E. (2002). Nanocrystal targeting in vivo, 99(20), 12617–12621.
Almessiere, M. A., Slimani, Y., & Baykal, A. (2018a). Author’s accepted manuscript. Ceramics International [Internet]. https://doi.org/10.1016/j.ceramint.2018.09.272
Almessiere, M. A., Slimani, Y., & Baykal, A. (2018b). Structural, morphological and magnetic properties of hard/soft SrFe12-xVxO19/(Ni0.5Mn0.5Fe2O4)y nanocomposites: Effect of vanadium substitution. Journal of Alloys and Compounds, 767, 966–975.
Angelos, S., Johansson, E., Stoddart, J. F., & Zink, J. I. (2007). Mesostructured silica supports for functional materials and molecular machines. Advanced Functional Materials, 17(14), 2261–2271.
Avramović, N., Mandić, B., Savić-Radojević, A., & Simić, T. (2020). Polymeric nanocarriers of drug delivery systems in cancer therapy. Pharmaceutics, 12(4), 1–17.
Balguri, S. P., Adelli, G. R., & Majumdar, S. (2016). Topical ophthalmic lipid nanoparticle formulations (SLN, NLC) of indomethacin for delivery to the posterior segment ocular tissues. European Journal of Pharmaceutics and Biopharmaceutics [Internet], 109, 224–235. https://doi.org/10.1016/j.ejpb.2016.10.015
Bamrungsap, S., Zhao, Z., Chen, T., Wang, L., Li, C., Fu, T., et al. (2012). Nanotechnology in therapeutics: A focus on nanoparticles as a drug delivery system. Review. Carbohydrate Polymers [Internet], 1(1), 71–88. Available from: https://doi.org/10.1016/j.nano.2010.07.004%5Cn., http://linkinghub.elsevier.com/retrieve/pii/S1818087616300502%5Cn, https://doi.org/10.1016/j.carbpol.2016.06.026%5Cn, http://www.cancerjournal.net/article.asp?issn=0973-1482%5Cnyear=2014%5Cnvolume=10%5Cnissue=
Baskar, G., Lalitha, K., Garrick, B. G., & Chamundeeswari, M. (2017a). Conjugation, labeling and characterization of asparaginase bound silver nanoparticles for anticancer applications. Indian Journal of Experimental Biology, 55(7), 421–426.
Baskar, G., Bikku George, G., & Chamundeeswari, M. (2017b). Synthesis and characterization of asparaginase bound silver nanocomposite against ovarian cancer cell line A2780 and lung cancer cell line A549. Journal of Inorganic and Organometallic Polymers and Materials [Internet], 27(1), 87–94. https://doi.org/10.1007/s10904-016-0448-x
Batrakova, E. V., Dorodnych, T. Y., Klinskii, E. Y., Kliushnenkova, E. N., Shemchukova, O. B., Goncharova, O. N., et al. (1996). Anthracycline antibiotics non-covalently incorporated into the block copolymer micelles: In vivo evaluation of anti-cancer activity. British Journal of Cancer, 74(10), 1545–1552.
Begines, B., Ortiz, T., Pérez-Aranda, M., Martínez, G., Merinero, M., Argüelles-Arias, F., et al. (2020). Polymeric nanoparticles for drug delivery: Recent developments and future prospects. Nanomaterials, 10(7), 1–41.
Bianco, A. (2004). Carbon nanotubes for the delivery of therapeutic molecules. Expert Opinion on Drug Delivery, 1(1), 57–65.
Bottini, M., D’Annibale, F., Magrini, A., Cerignoli, F., Arimura, Y., Dawson, M. I., et al. (2007). Quantum dot-doped silica nanoparticles as probes for targeting of T-lymphocytes. International Journal of Nanomedicine [Internet], 2(2), 227–233. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17722550%0A, http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=PMC2673975
Calzoni, E., Cesaretti, A., Polchi, A., Michele, A. D., Tancini, B., & Emiliani, C. Biocompatible polymer nanoparticles for drug delivery applications in cancer and neurodegenerative disorder therapies. Journal of Functional Biomaterials, 10, 1–15.
Chamundeeswari, M., Sastry, T. P., Lakhsmi, B. S., Senthil, V., & Agostinelli, E. (2013). Iron nanoparticles from animal blood for cellular imaging and targeted delivery for cancer treatment. Biochimica et Biophysica Acta (BBA) - General Subjects, 1830(4), 3005–3010.
Chamundeeswari, M., Jeslin, J., & Verma, M. L. (2019). Nanocarriers for drug delivery applications. Environmental Chemistry Letters [Internet], 17(2), 849–865. https://doi.org/10.1007/s10311-018-00841-1
Chang, J. S., Chang, K. L. B., Hwang, D. F., & Kong, Z. L. (2007). In vitro cytotoxicitiy of silica nanoparticles at high concentrations strongly depends on the metabolic activity type of the cell line. Environmental Science and Technology, 41(6), 2064–2068.
Choi, A. O., Ju, S. J., Desbarats, J., Lovrić, J., & Maysinger, D. (2007). Quantum dot-induced cell death involves Fas upregulation and lipid peroxidation in human neuroblastoma cells. Journal of Nanobiotechnology, 5, 1–13.
Christian, S., Pilch, J., Akerman, M. E., Porkka, K., Laakkonen, P., & Ruoslahti, E. (2003). Nucleolin expressed at the cell surface is a marker of endothelial cells in angiogenic blood vessels, 163(4), 871–878.
Corrocher, R., Tedesco, F., Rabusin, P., & De Sandre, G. (1975). Effect of human erythrocyte stromata on complement activation. British Journal of Haematology, 29, 235–241.
Das, S., & Chaudhury, A. (2011). Recent advances in lipid nanoparticle formulations with solid matrix for oral drug delivery. AAPS PharmSciTech, 12(1), 62–76.
Das, S., Ng, W. K., & Tan, R. B. H. (2012). Are nanostructured lipid carriers (NLCs) better than solid lipid nanoparticles (SLNs): Development, characterizations and comparative evaluations of clotrimazole-loaded SLNs and NLCs? European Journal of Pharmaceutical Sciences [Internet], 47(1), 139–151. https://doi.org/10.1016/j.ejps.2012.05.010
Derfus, A. M., Chen, A. A., Min, D., Ruoslahti, E., & Bhatia, S. N. (2007). Targeted quantum dot conjugates for siRNA delivery, 18, 1391–1396.
Desai, D., Zhang, J., Sandholm, J., Lehtimäki, J., Grönroos, T., Tuomela, J., et al. (2017). Lipid bilayer-gated mesoporous silica nanocarriers for tumor-targeted delivery of zoledronic acid in vivo. Molecular Pharmaceutics, 14(9), 3218–3227.
Descalzo, A. B., Martínez-Máñez, R., Sancenón, F., Hoffmann, K., & Rurack, K. (2006). The supramolecular chemistry of organic-inorganic hybrid materials. Angewandte Chemie - International Edition, 45(36), 5924–5948.
Din, F. U., Aman, W., Ullah, I., Qureshi, O. S., Mustapha, O., et al. (2017). Effective use of nanocarriers as drug delivery systems for the treatment of selected tumors. International Journal of Nanomedicine, 12, 7291–7309.
Ding, B. S., Dziubla, T., Shuvaev, V. V., Muro, S., & Muzykantov, V. R. (2006). Advanced drug delivery systems that target the vascular endothelium. Molecular Interventions [Internet]. [cited 2022 Aug 8], 6(2), 98–112. Available from: https://pubmed.ncbi.nlm.nih.gov/16565472/
Freeman, R., Liu, X., & Willner, I. (2011). Chemiluminescent and Chemiluminescence Resonance Energy Transfer (CRET) detection of DNA, metal ions, and aptamer À substrate complexes using hemin / G-quadruplexes and CdSe / ZnS quantum dots. Journal of the American Chemical Society, 133, 11597–11604.
García, M. A. (2011). Surface plasmons in metallic nanoparticles: Fundamentals and applications. Journal of Physics D: Applied Physics, 44(8), 283001.
Geißler, D., Charbonni, L. J., Ziessel, R. F., Butlin, N. G., Löhmannsröben, H., & Hildebrandt, N. (2010). Quantum dot biosensors for ultrasensitive multiplexed diagnostics. Angewandte Chemie International Edition., 49, 1396–1401.
Gellini, C., & Feis, A. (2021). Optothermal properties of plasmonic inorganic nanoparticles for photoacoustic applications. Photoacoustics, 23, 100281.
Ghosn, Y., Kamareddine, M. H., Tawk, A., Elia, C., El Mahmoud, A., Terro, K., et al. (2019). Inorganic nanoparticles as drug delivery systems and their potential role in the treatment of chronic myelogenous leukaemia. Technology in Cancer Research & Treatment, 18, 1–12.
Goldenberg, L. M., Köhler, M., Kahle, O., & Dreyer, C. (2020). Impact of inorganic nanoparticles on optical properties of low refractive index waveguiding polymers. Optical Materials Express, 10(11), 2987.
Guimarães, D., Cavaco-Paulo, A., & Nogueira, E. (2021). Design of liposomes as drug delivery system for therapeutic applications. International Journal of Pharmaceutics, 601(March).
Hallan, S. S., Kaur, P., Kaur, V., Mishra, N., & Vaidya, B. (2016). Lipid polymer hybrid as emerging tool in nanocarriers for oral drug delivery. Artificial Cells, Nanomedicine and Biotechnology, 44(1), 334–349.
Hong, S., Choi, D. W., Kim, H. N., Park, C. G., Lee, W., & Park, H. H. (2020). Protein-based nanoparticles as drug delivery systems. Pharmaceutics, 12(7), 1–28.
Howard, B., Gao, Z., Lee, S. W., Seo, M. H., & Rapoport, N. (2006). Ultrasound-enhanced chemotherapy of drug-resistant breast cancer tumors by micellar-encapsulated paclitaxel. American Journal of Drug Delivery, 4(2), 97–104.
Hua, S., & Wu, S. Y. (2013). The use of lipid-based nanocarriers for targeted pain therapies, 4(November), 1–7.
Huang, X., Li, L., Qian, H., Dong, C., & Ren, J. (2006). A resonance energy transfer between chemiluminescent donors and luminescent quantum-dots as acceptors (CRET). Angewandte Chemie - International Edition, 45(31), 5140–5143.
Jarak, I., Pereira-Silva, M., Santos, A. C., Veiga, F., Cabral, H., & Figueiras, A. (2021). Multifunctional polymeric micelle-based nucleic acid delivery: Current advances and future perspectives. Applied Materials Today, 25, 101217.
Karuppusamy, C., & Venkatesan, P. (2017). Role of nanoparticles in drug delivery system: A comprehensive review. Journal of Pharmaceutical Sciences and Research, 9(3), 318–325.
Kaur, J., Gulati, M., Jha, N. K., Disouza, J., Patravale, V., Dua, K., et al. (2022). Recent advances in developing polymeric micelles for treating cancer: Breakthroughs and bottlenecks in their clinical translation. Drug Discovery Today, 27(5), 1495–1512.
Khandelia, R., Jaiswal, A., Ghosh, S. S., & Chattopadhyay, A. (2013). Gold nanoparticle-protein agglomerates as versatile nanocarriers for drug delivery. Small, 9(20), 3494–3505.
Kianfar, E. (2021). Magnetic nanoparticles in targeted drug delivery: A review. Journal of Superconductivity and Novel Magnetism [Internet], 34(7), 1709–1735. https://doi.org/10.1007/s10948-021-05932-9
Kim, B. D., Na, K., & Choi, H. K. (2005). Preparation and characterization of solid lipid nanoparticles (SLN) made of cacao butter and curdlan. European Journal of Pharmaceutical Sciences, 24(2–3), 199–205.
Kingsley, J. D., Dou, H., Morehead, J., Rabinow, B., Gendelman, H. E., & Destache, C. J. (2006). Nanotechnology: A focus on nanoparticles as a drug delivery system. Journal of Neuroimmune Pharmacology, 1(3), 340–350.
Kirchner, C., Liedl, T., Kudera, S., Pellegrino, T., Javier, A. M., Gaub, H. E., et al. (2005). Cytotoxicity of colloidal CdSe and CdSe/ZnS nanoparticles. Nano Letters, 5(2), 331–338.
Lai, P. S., Lou, P. J., Peng, C. L., Pai, C. L., Yen, W. N., Huang, M. Y., et al. (2007). Doxorubicin delivery by polyamidoamine dendrimer conjugation and photochemical internalization for cancer therapy. Journal of Controlled Release, 122(1), 39–46.
Laouini, A., Jaafar-Maalej, C., Limayem-Blouza, I., Sfar, S., Charcosset, C., & Fessi, H. (2012). Preparation, characterization and applications of liposomes: State of the art. Journal of Colloid Science and Biotechnology, 1(2), 147–168.
Li, Y., Li, N., Pan, W., Yu, Z., Yang, L., & Tang, B. (2017). Hollow mesoporous silica nanoparticles with tunable structures for controlled drug delivery. ACS Applied Materials & Interfaces, 9, 2123.
Liu, Z., Chen, K., Davis, C., Sherlock, S., Cao, Q., Chen, X., et al. (2008). Drug delivery with carbon nanotubes for in vivo cancer treatment. Cancer Research, 68(16), 6652–6660.
Liu, D., Liu, C., Zou, W., & Zhang, N. (2010). Enhanced gastrointestinal absorption of N3-O-toluylfluorouracil by cationic solid lipid nanoparticles. Journal of Nanoparticle Research, 12(3), 975–984.
López-Dávila, V., Seifalian, A. M., & Loizidou, M. (2012). Organic nanocarriers for cancer drug delivery. Current Opinion in Pharmacology, 12(Figure 1), 414–419.
Lovrić, J., Bazzi, H. S., Cuie, Y., Fortin, G. R. A., Winnik, F. M., & Maysinger, D. (2005). Differences in subcellular distribution and toxicity of green and red emitting CdTe quantum dots. Journal of Molecular Medicine, 83(5), 377–385.
Lu, W., Huang, Q., Ku, G., Wen, X., Zhou, M., Guzatov, D., et al. (2010). Photoacoustic imaging of living mouse brain vasculature using hollow gold nanospheres. Biomaterials, 31(9), 2617–2626.
Ma, P., Xiao, H., Li, C., Dai, Y., Cheng, Z., Hou, Z., et al. (2015). Inorganic nanocarriers for platinum drug delivery. Materials Today [Internet], 18(10), 554–564. https://doi.org/10.1016/j.mattod.2015.05.017
Mäder, K., & Mehnert, W. (2004). Solid lipid nanoparticles - Concepts, procedures, and physicochemical aspects. In Lipospheres in drug targets and delivery: Approaches, methods, and applications, 1–22 p.
Majumder, N., Das, N. G., & Das, S. K. (2020). Polymeric micelles for anticancer drug delivery. Therapeutic Delivery, 11(10), 613–635.
Malam, Y., Loizidou, M., & Seifalian, A. M. (2009). Liposomes and nanoparticles: Nanosized vehicles for drug delivery in cancer. Trends in Pharmacological Sciences, 30(11), 592–599.
Mathur, V., Satrawala, Y., & Rajput, M. S. (2015). Dendrimers: A review Dendrimers (January 2010).
Medina, C., Santos-Martinez, M. J., Radomski, A., Corrigan, O. I., & Radomski, M. W. (2007). Nanoparticles: Pharmacological and toxicological significance. British Journal of Pharmacology, 150(5), 552–558.
Medintz, I. L., Farrell, D., Susumu, K., Trammell, S. A., Deschamps, J. R., Brunel, F. M., et al. (2009). Multiplex charge-transfer interactions between quantum dots and peptide-bridged ruthenium complexes, 81(12), 4831–4839.
Mignani, S., Tripathi, R. P., Chen, L., Caminade, A. M., Shi, X., & Majoral, J. P. (2018). New ways to treat tuberculosis using dendrimers as nanocarriers. Pharmaceutics, 10(3).
Mishra, B., Patel, B. B., & Tiwari, S. (2010). Colloidal nanocarriers: a review on formulation technology, types and applications toward targeted drug delivery. Nanomedicine: Nanotechnology, Biology and Medicine, 6(1), 9–24.
Mohammed, I. A., & Al-Gawhari, F. J. (2020). Gold nanoparticle: Synthesis, functionalization, enhancement, drug delivery and therapy: A review. Systematic Reviews in Pharmacy, 11(6), 888–910.
Mu, H., & Holm, R. (2018). Solid lipid nanocarriers in drug delivery: Characterization and design. Expert Opinion on Drug Delivery [Internet], 15(8), 771–785. https://doi.org/10.1080/17425247.2018.1504018
Müller, R. H., Radtke, M., & Wissing, S. A. (2002). Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) in cosmetic and dermatological preparations. Advanced Drug Delivery Reviews, 54(SUPPL), 131–155.
Nakanishi, T., Fukushima, S., Okamoto, K., Suzuki, M., Matsumura, Y., Yokoyama, M., et al. (2001). Development of the polymer micelle carrier system for doxorubicin. Journal of Controlled Release, 74(1–3), 295–302.
Naseri, N., Valizadeh, H., & Zakeri-Milani, P. (2015). Solid lipid nanoparticles and nanostructured lipid carriers: Structure, preparation and application. Advanced Pharmaceutical Bulletin [Internet], 5(3), 305–313. Available from: http://apb.tbzmed.ac.ir
Nelemans, L. C., & Gurevich, L. (2020). Drug delivery with polymeric nanocarriers — Cellular uptake mechanisms. Materials, 13(Figure 1), 366.
Ng, C. M., Loh, H. S., Muthoosamy, K., Sridewi, N., & Manickam, S. (2016). Conjugation of insulin onto the sidewalls of single-walled carbon nanotubes through functionalization and diimide-activated amidation. International Journal of Nanomedicine, 11, 1607–1614.
Niemeier, U., Granier, C., Kornblueh, L., Walters, S., & Brasseur, G. P. (2006). Global impact of road traffic on atmospheric chemical composition and on ozone climate forcing. Journal of Geophysical Research Atmospheres, 111(9), 4326–4335.
Nisini, R., Poerio, N., Mariotti, S., De Santis, F., & Fraziano, M. (2018). The multirole of liposomes in therapy and prevention of infectious diseases. Frontiers in Immunology, 9(FEB).
Nutter, D. O., & Ratts, T. (1973). Direct actions of prostaglandins E1, A1, and F2α on myocardial contraction. Prostaglandins, 3(3), 323–336.
O’Regan, B., & Gratzer, M. (1991). © 19 9 1 Nature Publishing Group 그라첼꺼. Nature, 354, 737–740.
Oberdörster, G., Oberdörster, E., & Oberdörster, J. Nanotoxicology: An emerging discipline evolving from studies of ultrafine particles supplemental web sections. Environmental Medicine.
Ohulchanskyy, T. Y., Roy, I., Goswami, L. N., Chen, Y., Bergey, E. J., Pandey, R. K., et al. (2007). Organically modified silica nanoparticles with covalently incorporated photosensitizer for photodynamic therapy of cancer. Nano Letters, 7(9), 2835–2842.
Pawar, R. S., Dimri, M., Maithani, A., & Luv, K. (2020). Asian Journal of Pharmaceutical Research and Development. The application of Marine Natural Products (MNPS) in anti-Covid-19 therapeutics, 8(6), 77–80.
Peer, D., Karp, J. M., Hong, S., Farokhzad, O. C., Margalit, R., & Langer, R. (2007). 84 Nat nanotech 2007 R Langer Nanocarriers as an emerging platform for cancer therapy.pdf. Nature Nanotechnology, 2, 751–760.
Peng, Y., Bariwal, J., Kumar, V., Tan, C., & Mahato, R. I. (2020). Organic nanocarriers for delivery and targeting of therapeutic agents for cancer treatment. Advanced Therapeutics, 3(2), 1–45.
Prabhu, R. H., Patravale, V. B., & Joshi, M. D. (2015). Polymeric nanoparticles for targeted treatment in oncology: Current insights. International Journal of Nanomedicine, 10, 1001–1018.
Prosperi, D., Colombo, M., Zanoni, I., & Granucci, F. (2017 Dec 1). Drug nanocarriers to treat autoimmunity and chronic inflammatory diseases. Seminars in Immunology, 34, 61–67.
Qian, X., Peng, X. H., Ansari, D. O., Yin-Goen, Q., Chen, G. Z., Shin, D. M., et al. (2008). In vivo tumor targeting and spectroscopic detection with surface-enhanced Raman nanoparticle tags. Nature Biotechnology, 26(1), 83–90.
Qian, W. Y., Sun, D. M., Zhu, R. R., Du, X. L., Liu, H., & Wang, S. L. (2012). pH-sensitive strontium carbonate nanoparticles as new anticancer vehicles for controlled etoposide release. International Journal of Nanomedicine, 7, 5781–5792.
Rapoport, N. (2007). Physical stimuli-responsive polymeric micelles for anti-cancer drug delivery. Progress in Polymer Science, 32(8–9), 962–990.
Rapoport, N. Y., Herron, J. N., Pitt, W. G., & Pitina, L. (1999a). Micellar delivery of doxorubicin and its paramagnetic analog, ruboxyl, to HL-60 cells: effect of micelle structure and ultrasound on the intracellular drug uptake. Journal of Controlled Release, 58(2), 153–162.
Rapoport, N., Smirnov, A. I., Pitt, W. G., & Timoshin, A. A. (1999b). Bioreduction of tempone and spin-labeled gentamicin by gram-negative bacteria: Kinetics and effect of ultrasound. Archives of Biochemistry and Biophysics, 362(2), 233–241.
Rapoport, N., Marin, A. P., & Timoshin, A. A. (2000). Effect of a polymeric surfactant on electron transport in HL-60 cells. Archives of Biochemistry and Biophysics, 384(1), 100–108.
Rouco, H., Diaz-Rodriguez, P., Remuñán-López, C., & Landin, M. (2020). Recent advances in solid lipid nanoparticles formulation and clinical applications. Nanomaterials for Clinical Applications, 1, 213–247.
Saman RA, Iqbal M. Nanotechnology-based drug delivery systems: Past, present and future. 2012;175–185.
Santos, H. A., Bimbo, L. M., Peltonen, L., & Hirvonen, J. (2014). Inorganic nanoparticles in targeted drug delivery and imaging. [cited 2022 Aug 22], 571–613. Available from: https://pureportal.strath.ac.uk/en/publications/inorganic-nanoparticles-in-targeted-drug-delivery-and-imaging
Schwarz, C., Mehnert, W., Lucks, J. S., & Müller, R. H. (1994). Solid lipid nanoparticles (SLN) for controlled drug delivery. I. Production, characterization and sterilization. Journal of Controlled Release, 30(1), 83–96.
Sebaaly, C., Greige-Gerges, H., Stainmesse, S., Fessi, H., & Charcosset, C. (2016). Effect of composition, hydrogenation of phospholipids and lyophilization on the characteristics of eugenol-loaded liposomes prepared by ethanol injection method. Food Bioscience, 15, 1–10.
Sercombe, L., Veerati, T., Moheimani, F., Wu, S. Y., Sood, A. K., & Hua, S. (2015). Advances and challenges of liposome assisted drug delivery. Frontiers in Pharmacology, 6(DEC), 1–13.
Shi, Z., Zhou, Y., Fan, T., Lin, Y., Zhang, H., & Mei, L. (2020). Inorganic nano-carriers based smart drug delivery systems for tumor therapy. Smart Materials in Medicine, 1, 32–47.
Shvedova, A., Castranova, V., Kisin, E., Murray, A., Gandelsman, V., & Baron, P. (2011). Current issues exposure to carbon nanotube material: Assessment of nanotube cytotoxicity using human keratinocyte cells. Journal of Toxocology and Environmental Health, Part A, 66(June 2012), 1909–1926.
Singh, R., & Lillard, J. W. (2009). Nanoparticle-based targeted drug delivery. Experimental and Molecular Pathology, 86(3), 215–223.
Slowing, I. I., Trewyn, B. G., & Lin, V. S. Y. (2007). Mesoporous silica nanoparticles for intracellular delivery of membrane-impermeable proteins. Journal of the American Chemical Society, 129(28), 8845–8849.
Slowing, I. I., Vivero-Escoto, J. L., Wu, C. W., & Lin, V. S. Y. (2008). Mesoporous silica nanoparticles as controlled release drug delivery and gene transfection carriers. Advanced Drug Delivery Reviews, 60(11), 1278–1288.
Stergar, J., Ban, I., & Maver, U. (2019). The potential biomedical application of NiCu magnetic nanoparticles. Magnetochemistry, 5(4).
Sun, T., Zhang, Y. S., Pang, B., Hyun, D. C., Yang, M., & Xia, Y. (2014). Engineered nanoparticles for drug delivery in cancer therapy. Angewandte Chemie - International Edition., 53(46), 12320–12364.
Sur, S., Rathore, A., Dave, V., Reddy, K. R., Chouhan, R. S., & Sadhu, V. (2019). Recent developments in functionalized polymer nanoparticles for efficient drug delivery system. Nano-Structures and Nano-Objects [Internet], 20, 100397. https://doi.org/10.1016/j.nanoso.2019.100397
Thiruvengadam, M., Rajakumar, G., Swetha, V., Ansari, M. A., Alghamdi, S., Almehmadi, M., et al. (2021). Recent insights and multifactorial applications of carbon nanotubes. Micromachines, 12(12).
Torchilin, V. P. (2000). Drug targeting. European Journal of Pharmaceutical Science, 11(SUPPL. 2), S81–S91.
Torchilin, V. (2011). Tumor delivery of macromolecular drugs based on the EPR effect. Advanced Drug Delivery Reviews, 63(3), 131–135.
Toscanini, M. A., Limeres, M. J., Garrido, A. V., Cagel, M., Bernabeu, E., Moretton, M. A., et al. (2021). Polymeric micelles and nanomedicines: Shaping the future of next generation therapeutic strategies for infectious diseases. Journal of Drug Delivery Science and Technology, 66, 102927.
Trewyn, B. G., Slowing, I. I., Giri, S., Chen, H. T., & Lin, V. S. Y. (2007). Synthesis and functionalization of a mesoporous silica nanoparticle based on the sol-gel process and applications in controlled release. Accounts of Chemical Research, 40(9), 846–853.
Üzek, R., Sari, E., & Merkoçi, A. (2019). Optical-based (Bio) sensing systems using magnetic nanoparticles. Magnetochemistry, 5(4), 1–25.
Verma, M. L., Barrow, C. J., & Puri, M. (2012). Nanobiotechnology as a novel paradigm for enzyme immobilisation and stabilisation with potential applications in biodiesel production. Applied Microbiology and Biotechnology [Internet]. [cited 2022 Aug 4], 97(1), 23–39. Available from: https://springerlink.bibliotecabuap.elogim.com/article/10.1007/s00253-012-4535-9
Verma, M. L., Chaudhary, R., Tsuzuki, T., Barrow, C. J., & Puri, M. (2013). Immobilization of β-glucosidase on a magnetic nanoparticle improves thermostability: Application in cellobiose hydrolysis. Bioresource Technology, 135, 2–6.
Wang, Y., Xie, X., Wang, X., Ku, G., Gill, K. L., O’Neal, D. P., et al. (2004). Photoacoustic tomography of a nanoshell contrast agent in the in vivo rat brain. Nano Letters [Internet]. [cited 2022 Aug 22], 4(9), 1689–1692. Available from: https://pubs.acs.org/doi/abs/10.1021/nl049126a
Wang, X., Wang, Y., Chen, Z. G., & Shin, D. M. (2009). Advances of cancer therapy by nanotechnology. Cancer Research and Treatment, 41(1), 1.
Wang, A. Z., Langer, R., & Farokhzad, O. C. (2012). Nanoparticle delivery of cancer drugs. Annual Review of Medicine, 63, 185–198.
Wang, Y., Zhao, Q., Han, N., Bai, L., Li, J., Liu, J., et al. (2015). Mesoporous silica nanoparticles in drug delivery and biomedical applications. Nanomedicine: Nanotechnology, Biology, and Medicine [Internet], 11(2), 313–327. https://doi.org/10.1016/j.nano.2014.09.014
Wang, W., Chen, T., Xu, H., Ren, B., Cheng, X., Qi, R., et al. (2018). Curcumin-loaded solid lipid nanoparticles enhanced anticancer efficiency in breast cancer. Molecules, 23(7), 1–13.
Wilczewska, A. Z., Niemirowicz, K., Markiewicz, K. H., & Car, H. (2012). Nanoparticles as drug delivery systems. Pharmacological Reports [Internet]., 64(5), 1020–1037. https://doi.org/10.1016/S1734-1140(12)70901-5
Wu, Z., Zeng, X., Zhang, Y., Feliu, N., Lundberg, P., Fadeel, B., et al. (2012). Linear-dendritic polymeric amphiphiles as carriers of doxorubicin-In vitro evaluation of biocompatibility and drug delivery. Journal of Polymer Science, Part A: Polymer Chemistry, 50(2), 217–226.
Yadav, N., Khatak, S., & Singh Sara, U. V. (2013). Solid lipid nanoparticles- A review. International Journal of Applied Pharmaceutics, 5(2), 8–18.
Yaghmur, A., & Mu, H. (2021). Recent advances in drug delivery applications of cubosomes, hexosomes, and solid lipid nanoparticles. Acta Pharmaceutica Sinica B, 11(4), 871–885.
Zahin, N., Anwar, R., Tewari, D., Kabir, M. T., Sajid, A., Mathew, B., et al. (2020). Nanoparticles and its biomedical applications in health and diseases: special focus on drug delivery. Environmental Science and Pollution Research, 27(16), 19151–19168.
Zhu, Y., & Liao, L. (2015). Applications of nanoparticles for anticancer drug deliver: A review, 15(7), 4753–4773.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Thakur, S., Mutreja, V., Sharma, A. (2023). Nanoparticles Function as Delivery Systems for Immune Potentiation. In: Pal, K. (eds) Nanovaccinology. Springer, Cham. https://doi.org/10.1007/978-3-031-35395-6_11
Download citation
DOI: https://doi.org/10.1007/978-3-031-35395-6_11
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-35394-9
Online ISBN: 978-3-031-35395-6
eBook Packages: MedicineMedicine (R0)