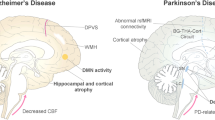
Abstract
Neurodegeneration is a progressive and irreversible process of structural and/or functional loss in neuronal cells, which affects vulnerable cell subsets in certain anatomic brain regions leading to various neurodegenerative diseases. Although the exact etiology of neurodegenerative diseases largely remains unknown, aging is the primary risk factor and some of the main mechanisms underlying neurodegenerative diseases include protein misfolding, protein aggregation, autophagy, lysosomal dysfunction, oxidative injury, mitochondrial dysfunction, and neuroinflammation. Structural MRI, diffusion MRI, arterial spin labeling, MR spectroscopy, FDG, SV2A, beta-amyloid, and tau PET are among common imaging modalities used for neurodegeneration evaluation. Moreover, dopaminergic and cholinergic imaging techniques are used to investigate neurotransmitter systems in neurodegenerative diseases. Emerging imaging techniques of neurodegeneration comprise ultra-field MRI, functional MRI, quantitative susceptibility imaging, and TSPO PET.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Neurodegeneration
- Aging
- Protein misfolding
- Protein aggregation
- Autophagy
- Lysosomal dysfunction
- Oxidative injury
- Mitochondrial dysfunction
- Neuroinflammation
- Neurovascular unit
- Multimodality imaging
-
1.
Neurodegeneration is defined as the process of structural and/or functional loss in neuronal cells.
-
2.
The primary mechanisms underlying neurodegeneration include protein misfolding, protein aggregation, autophagy, lysosomal dysfunction, oxidative injury, mitochondrial dysfunction, and neuroinflammation.
-
3.
The neurovascular unit comprises the multi-dimensional relationship between brain cells and their microvasculature.
-
4.
Among the main imaging modalities of neurodegeneration are structural MRI, diffusion MRI, arterial spin labeling, MR spectroscopy, FDG, SV2A, beta-amyloid, and tau PET.
-
5.
The neurotransmitter systems in neurodegenerative diseases can be investigated by dopaminergic and cholinergic imaging techniques.
-
6.
Emerging imaging techniques of neurodegeneration include ultra-field MRI, functional MRI, quantitative susceptibility imaging, and TSPO PET.
Pathophysiology of Neurodegeneration
Neurodegeneration is defined as the process of structural and/or functional loss in neuronal cells. Neurodegenerative diseases such as Alzheimer’s disease (AD) and Parkinson’s disease (PD) comprise a progressive, irreversible, and often slow process affecting specific vulnerable subsets of cells in certain anatomic regions of the brain, which determine the clinical presentation and disease course [1]. Neurodegenerative diseases stand out with their high prevalence and cost as well as the challenge in discovery of mechanism-targeted effective treatments [2].
The concept of neurovascular unit includes the multi-dimensional relationship between brain cells and their microvasculature as well as the organized reaction of brain cells and vessels to injury [3]. Neurons, microglia, astrocytes, basal membrane, pericytes, and endothelial cells are the main components of the neurovascular unit [4]. The developmental, structural, and functional interactions of brain cells and blood vessels in the neurovascular unit contribute to the maintenance and regulation of cerebral blood flow, blood–brain barrier function, and brain homeostasis [5, 6]. Therefore, the changes in the neurovascular unit function may trigger neurodegeneration by the decrease in cerebral blood flow leading to hypoxia, the decrease in production of trophic factors resulting in increased cell vulnerability, irregularities in the blood–brain barrier causing dysfunction in homeostasis, and the decrease in the clearance of metabolites leading to accumulation of proteins such as beta-amyloid and tau [5].
The main risk factor for neurodegenerative diseases is aging. Neuronal loss and alterations in neurotransmitters happen both during aging and also with neurodegenerative diseases that lead to cognitive and motor dysfunction in older individuals [2]. The precise etiology of neurodegenerative diseases mostly remains unknown, but the main underlying mechanisms are usually shared among distinct neurodegenerative diseases [7] and are likely to be influenced or triggered by numerous metabolic, genetic, or environmental factors [2].
Some of the main mechanisms underlying neurodegenerative diseases that result in progressive neuronal cell dysfunction and ultimately cell death through common neuronal pathways are: (1) Protein misfolding, defective degradation, extra and intracellular aggregation of misfolded proteins, (2) autophagy and lysosomal dysfunction, (3) reactive oxidative species and free radical formation, mitochondrial deficits, excitotoxicity, and dysregulation of intracellular calcium, and (4) neuroinflammation [2, 7,8,9].
Protein Misfolding and Aggregation
Protein aggregation is one of the basic underlying mechanisms in neurodegenerative diseases. Based on their flexibility, proteins shift between a variety of conformational substrates. Newly synthesized proteins convert to functional molecules after folding. Abnormal interactions between highly soluble proteins lead to protein misfolding through alterations in protein conformation. The insoluble, improperly folded or misfolded proteins self-accumulate as a result of structural change of the normal, functional proteins [9, 10]. Defective intra and extracellular protein aggregation and accumulation leads to mitochondrial dysfunction, oxidative stress, and reactive oxygen species, defects in ubiquitin-proteasome system and abnormal alternative mRNA exon splicing [11]. AD and PD are main examples of neurodegenerative diseases with protein misfolding and aggregation.
Autophagy and Lysosomal Dysfunction
As a catabolic process, autophagy is the degradation of protein aggregates, excess or damaged organelles and cytosolic contents in lysosomes [12]. In case of abnormalities in autophagy and lysosomal dysfunction, the cell contents are not degraded properly and they start to accumulate [13]. CAG-polyglutamine repeat diseases such as Huntington disease is an example of aberrant degradation of autophagy pathway [14].
Oxidative Injury and Mitochondrial Dysfunction
Oxidative stress is a major contributor in the development of neurodegeneration. The formation of reactive oxidative species may be due to mechanisms such as metal-associated Fenton reactions, electrons that escape from respiratory chain reactions with oxygen and lipid peroxidation [15]. Once the free radical formation exceeds antioxidant mechanisms, oxidative injury takes place. Consequently, oxidative injury leads to mitochondrial deficits, excitotoxity and dysregulation of intracellular calcium resulting in neuronal cell dysfunction [9].
Damage to mitochondrial DNA and oxidative stress lead to mitochondrial impairment, which particularly increase with aging. Mitochondrial dysfunction is followed by the promotion of cell death as the cells become more vulnerable to degeneration and neurotoxic insults once the cell energy metabolism and ion homeostasis get compromised [9, 16].
Neuroinflammation
Chronic inflammatory reactions play an important role in the pathogenesis of neurodegenerative diseases. With aging, inflammatory pathways lead to neurodegeneration by either becoming hyperactivated (too much function) or inadequate to manage aging associated stress (too little function) [9, 17]. Microglia activation is a key component of neuroinflammatory reactions as it has both neuroprotective and neurotoxic features and is seen as a double-edged sword in neurodegeneration, especially in AD [18]. Microglia is a key factor in beta-amyloid clearance, but continued beta-amyloid production leads to reduction in the ability of microglial beta-amyloid clearance and increase in beta-amyloid deposition.
Multimodality Imaging of Neurodegeneration
Pathology is the current gold standard for definite diagnosis of neurodegenerative diseases [8]. However, multimodality imaging biomarkers of neurodegeneration have recently provided a more objective and in vivo understanding of the pathological changes. By enlightening the pathophysiology and detecting subtle structural and molecular changes, imaging biomarkers offer a complementary window of opportunity to help diagnose neurodegenerative diseases early and accurately, even in preclinical stages, to delay or avoid hospitalization, and to initiate early symptomatic management. It is also used for evaluating disease severity and disease course. Moreover, imaging biomarkers of neurodegeneration help develop disease-modifying treatments [19]. Some of these practical imaging biomarkers are already included in diagnostic criteria of neurodegenerative diseases for more accurate diagnosis.
Primary clinical symptoms, anatomical distribution of regions affected by neurodegeneration, or the main cellular/molecular abnormality may be used in classification of neurodegenerative diseases [8]. Often, the underlying pathology is complex with contribution from multiple etiologies such as AD, dementia with Lewy bodies (DLB), limbic pre-dominant age-related TDP-43 encephalopathy (LATE), and cerebrovascular disease [20]. This etiological heterogeneity tends to increase with age.
Structural MRI
Structural MRI is the main and most widely used imaging technique in neurodegenerative diseases. Structural MRI is also used to exclude other possible etiologies of cognitive dysfunction such as mass lesions and intracranial hemorrhage [21]. It is a common biomarker of progression in neurodegenerative diseases and have been used as an outcome measure in disease-modifying intervention trials.
In assessment of neurodegenerative diseases, structural MRI primarily targets atrophy and is able to detect even subtle morphological changes by utilizing volumetry and regional morphometry metrics, but also qualitative measures such as visual ratings [22]. Although it can assess changes in brain volume globally, it can also evaluate region-specific volume loss in brain and by identifying these atrophy patterns specific to the disease process, it can help differentiate neurodegenerative diseases from each other.
The anatomic changes in hippocampus and entorhinal cortex of the limbic system and precuneus, which is part of medial parietal lobe are essential in interpreting early neurodegenerative processes [23, 24]. Significant and disproportionate structural atrophy in medial and lateral temporal lobes and medial parietal cortex is a biomarker of neurodegeneration used for AD as a diagnostic criterion [25]. The lack of medial temporal lobe atrophy or minimal atrophy in medial temporal lobe is more consistent with DLB [26]. However, as AD and DLB may coexist quite commonly [26], medial temporal lobe atrophy is not an exclusion criterion for DLB.
The frontal or anterior temporal lobe atrophy with relatively preserved hippocampal and medial temporal lobe volume is suggestive for the behavioral variant of frontotemporal dementia [27], whereas putamen, pons, middle cerebellar peduncle, or cerebellum atrophy on MRI is suggestive of multiple system atrophy (MSA) [28]. Relative midbrain atrophy compared to pons is a characteristic imaging finding for progressive supranuclear palsy (PSP) [29]. Lacunar and cortical infarcts and moderate to severe white matter hyperintensities seen on the FLAIR MRI may suggest cognitive impairment due to vascular disease.
Diffusion MRI
Diffusion MRI evaluates the random and thermally induced displacement of water molecules as they diffuse within the tissue [30] and provides information about the microstructural integrity and complexity of the white matter. This data can be used to make inferences on membrane permeability, myelination, and axonal density. Among diffusion MRI techniques, diffusion tensor imaging (DTI) is the most commonly used technique to study neurodegenerative diseases, but other new techniques such as neurite orientation dispersion and density (NODDI) and free-water imaging (FWI) have also been developed, to improve the specificity of DTI for axonal integrity and free-water in the tissue [31].
Diffusion MRI facilitates interpreting pathophysiological and microstructural alterations underlying neurodegenerative diseases such as AD, PD, and amyotrophic lateral sclerosis (ALS). For example, in AD, in addition to gray matter injury, white matter injury and related changes also occur and can be measured through the DTI metrics [32]. In AD, DTI can particularly detect the microstructural changes in white matter tracts that link regions affected early in the disease course such as parahippocampus and fornix [33, 34]. These microstructural changes also associate with abnormal beta-amyloid and tau deposits in cognitively unimpaired individuals [35].
Because diffusion MRI is a quantitative imaging tool of cell pathophysiology, tissue microstructure, and structural connectivity, it is also a good candidate for detecting and monitoring early pathological changes and can be used as a biomarker in clinical trials [36, 37].
Arterial Spin Labeling (ASL) MRI
Arterial spin labeling (ASL) is an emerging MRI technique that measures cerebral blood flow quantitively and provides information about perfusion changes in neurodegenerative diseases. Classically, the metabolic changes in the brain are identified by FDG PET. However, especially in patients who are already undergoing an MRI scan, ASL MRI can be a good alternative for FDG PET, because hypoperfusion patterns on ASL MRI generally overlap with hypermetabolism patterns on FDG PET [38, 39]. For example, in DLB, ASL MRI can help detect the cingulate island sign [39]. Yet, in patients with mild cognitive impairment (MCI) and AD, apart from the typical hypoperfused regions, ASL also detects regions of hyperperfusion that reflects the functional response to neurodegeneration [40]. In addition to being more accessible, faster and cheaper in acquisition compared to FDG PET, ASL MRI can be used for evaluation of vascular factors that play a role in neurodegeneration as well [40].
MR Spectroscopy
Although conventional MRI is adequately utilized for morphological changes in neurodegenerative diseases, it does not particularly provide information on molecular changes. Conversely, MR spectroscopy can illustrate alterations in cell type, cell density, metabolite levels/biochemical composition using the proton [1H] of water, and it helps enlighten underlying disease mechanisms. Hence, conventional MRI and MR spectroscopy complement each other at every stage of the disease course including diagnosis, follow-up, and therapy response [41].
MR spectroscopy is utilized in biomarker research in many diseases including brain tumors, epilepsy, multiple sclerosis, traumatic brain injury, and stroke. In neurodegenerative diseases including AD, PD, and ALS, a decrease in total N-acetlyaspartate (tNAA) in the regions that reflect the characteristic pattern of neurodegenerative process of each disease is typically detected by MR spectroscopy [42, 43]. As a prognostic biomarker, the decrease in tNAA associates with clinical metrics and pathological severity [44, 45].
Elevation in myoinositol is closely associated with microglial activation seen in neurodegeneration [46]. It precedes reduction of total NAA, neuronal loss, and cognitive impairment in dementia [47, 48]. Besides the decrease in the total NAA and elevation in myoinositol levels [49, 50], reduction in glutamate [50, 51] and elevation in total choline levels [42] are other changes in neurochemical profiles commonly seen in neurodegeneration that are detected by MR spectroscopy. Moreover, functional response to treatment in neurodegenerative diseases can be observed with MR spectroscopy monitoring [41]. Temporary increase in tNAA level [52] and decreased myoinositol/total creatinine level [53] were observed following donepezil treatment in AD.
Fluorodeoxyglucose (FDG) PET
Fluorodeoxyglucose (FDG) is the most common radionucleotide ligand used in clinical practice. FDG is taken up by the cells through regular glucose transporters and its uptake is higher by cells which are metabolically more active. The brain uses glucose as its main energy source and most of the glucose utilization occurs with synaptic activity. The detection of hypometabolism by FDG PET may be used for evaluation of neurodegenerative diseases as neuronal injury and synaptic inactivity leads to metabolic dysfunction [21].
FDG PET helps in identification of regional glucose metabolism patterns, which may be useful in differential diagnosis of dementias. On FDG PET, the parietotemporal hypometabolism including precuneus and posterior cingulate cortex is considered the neurodegeneration biomarker of AD [25]. Conversely, the FDG uptake is decreased in the occipital lobe in DLB along with the cingulate island sign, which is supportive of the DLB diagnosis [26]. The cingulate island sign is observed when the metabolism of midcingulate and posterior cingulate cortex is relatively preserved, while there is hypometabolism in the cuneus and precuneus [54, 55].
In line with structural MRI findings, hypometabolism in the frontal or anterior temporal lobe is characteristic of the FDG PET in behavioral variant of FTD [27]. In PSP, midbrain hypometabolism relative to pons is observed in FDG PET [29]. Putamen hypometabolism in MSA-Cerebellar (MSA-C) and decreased metabolism in putamen, brainstem, and cerebellum may be seen in MSA-Parkinsonian (MSA-P) [28].
Synaptic Vesicle Glycoprotein 2A (SV2A) PET
Synapses are one of the main components of neurotransmission, linking neurons to each other via neurotransmitters. Loss or dysfunction in synapses is associated with motor, sensory, and cognitive impairment and is a crucial mechanism in neurodegeneration. Particularly, synaptic loss is a key feature and one of the earliest hallmarks of AD. It precedes beta-amyloid and tau accumulation in the preclinical stage of AD [56] and is correlated with cognitive impairment and disease severity in AD [57]. Similarly, synaptic dysfunction and loss is a characteristic component of PD and DLB pathogenesis. Apart from the loss of dopaminergic neurons in the nigrostriatal system, synaptic loss is found outside the nigrostriatal system as well in the nondopaminergic neurons of the cortex in neurodegenerative diseases including PD [58, 59].
Synaptic vesicle glycoprotein 2A (SV2A) is a widely expressed component of the synaptic vesicle in neuronal cells. In the central nervous system, it is commonly found as a presynaptic protein in GABAergic and glutamatergic neurons [60]. SV2A PET is the first noninvasive and in vivo method to directly evaluate synaptic density [61] and evaluation of synaptic density by SV2A PET is important at every stage of the disease course from diagnosis to prognosis in neurodegenerative diseases. So far, as a candidate biomarker of synaptic density, SV2A PET seems to have its highest potential in AD and PD. However, the use of SV2A PET is also promising in other diseases with synaptic abnormalities such as Huntington’s disease, epilepsy, stroke, multiple sclerosis, depression, and autism spectrum disorders [59].
Amyloid PET
One of the main pathological hallmarks of AD is postmortem beta-amyloid plaques. As a molecular imaging technique, amyloid PET offers an in vivo antemortem histopathological picture of the central nervous system by demonstrating a characteristic distribution of amyloid-affected areas of the brain, in line with the pathological distribution [62].
Amyloid PET tracers can reliably quantify cortical beta-amyloid deposition with high sensitivity and specificity by crossing the blood–brain barrier and binding to beta-amyloid plaques. Among amyloid tracers, C11-Pittsburgh compound-B (PiB) tracer is widely used and PiB was the first published human amyloid PET tracer [63]. However, newer tracers such as F18-Florbetapir, F18-Florbetaben, and F18-Flutemetamol have been developed, which have longer radioactive half-lives with commercial availability.
Using amyloid PET in addition to non-PET biomarkers improves diagnostic precision in neurodegenerative diseases [64]. Increased cortical C11-PiB uptake is observed on PET in patients with AD compared to controls, showing the deposition of beta-amyloid plaques in the cerebral cortex [63]. Amyloid PET is also found positive in about 10–44% of cognitively unimpaired individuals aged 50–90 years, but the clinical relevance is not known [65]. Moreover, amyloid PET alone is not sufficient in determining the clinical transition from prodromal stages and disease staging in AD [66]. However, the use of amyloid PET has been suggested in identification of individuals with MCI with clinical uncertainty, individuals with dementia suggestive of AD, but with a mixed or atypical presentation, and individuals who present with early-onset progressive cognitive decline [67].
Tau PET
Tau pathology plays an important role in the development of various neurodegenerative diseases such as AD. Tau function depends on phosphorylation; however, its physiology is modified if tau becomes hyperphosphorylated and hyperphosphorylation of tau leads to increase in intracellular aggregation of tau [68].
Tau PET enables quantification of tau deposition in the brain by using tau PET tracers that target tau deposits in vivo in the brain [69]. As tau PET visualizes and reflects the regional patterns of tau throughout the brain in different pathologies, it is a promising tool for diagnosis of neurodegenerative diseases [70]. Tau PET also correlates with cognitive impairment and neurodegeneration and therefore provides information on prognosis [70, 71].
Imaging of Neurotransmitter Systems
Dopaminergic Imaging (DatSCAN)
For evaluation of parkinsonian syndromes, dopamine transporter (DAT) can be measured with single-photon emission computed tomography (SPECT). DAT SPECT serves as the standard in vivo molecular imaging biomarker of presynaptic dopaminergic nigrostriatal neurons. It is a supportive diagnostic tool used in differentiation of PD and atypical parkinsonisms (such as PSP, MSA, corticobasal disease) from vascular or drug-induced parkinsonisms, avoiding misdiagnosis and unnecessary dopaminergic treatment [72, 73]. Although DAT imaging (DatSCAN) cannot differentiate PD from atypical parkinsonisms, it improves the accuracy of diagnosis and shortens the time to diagnosis in PD [72, 74]. A normal DatSCAN is one of the Movement Disorders Society PD exclusion criteria [75] because DatSCAN is considered as a very reliable biomarker of degenerative parkinsonism.
DAT decline appears to be non-linear in PD [76] and DAT imaging is helpful in both diagnosis and early stages of PD, but also in monitoring treatment response. As downregulation of DAT happens early in the disease course [77], DATSCaN can detect changes in DAT density before symptoms become evident showing early synaptic dysfunction. Once the dopaminergic neuronal terminal loss surpasses 50% at symptom onset later in the disease course, DATSCaN shows the decline in striatal DAT uptake reflecting the neurodegeneration of presynaptic dopaminergic nerve terminals [73].
F18-DOPA PET is another functional imaging technique of dopamine deficiency that is especially useful in differential diagnosis of patients with early age at onset, atypical presentations, or mild symptoms of parkinsonian syndrome [78]. F18 DOPA PET evaluates the integrity of presynaptic dopaminergic activity and dopamine terminal loss by quantifying dopamine precursor uptake [79, 80] and is a reliable in vivo diagnostic tool for PD with high sensitivity and specificity [78].
Cholinergic Imaging
The cholinergic system is crucial in cognitive function and is involved in the processing of numerous circuits associated with cognition [81]. Consequently, the dysfunction in the cholinergic system is closely related to mechanisms involved in neurodegenerative processes underlying cognitive impairment and dementia [82]. Thus, targeting the cholinergic (both pre- and post-synaptic) system using molecular imaging of PET or SPECT provides an opportunity to investigate the multiple elements of dementia pathophysiology.
PET studies using ligands targeting acetylcholine esterase (AChE) show decreased AChE activity in AD, which is associated with attention and working memory [83] and based on the age of onset, the binding patterns appear to vary [84]. Furthermore, cholinergic imaging can be used for evaluation of treatment response. In patients with AD treated with donepezil and rivastigmine, cholinergic PET studies detected inhibition of AChE activity [85]. Molecular imaging of the cholinergic system is also used in parkinsonian dementias and depict significantly decreased cortical AChE activity in PD dementia and DLB [86].
Emerging Imaging Techniques
Ultra-High Field MRI
3 T and 1.5 T MRIs are widely used in clinic and research for investigation of neurodegenerative diseases. However, higher field 7T MRI has several benefits over lower field MRIs, given its increased sensitivity in early detection of neurodegenerative changes. With increase in magnetic field strength and associated higher signal-to-noise ratio, high field MRI provides significant improvement in image quality along with higher spatial resolution and reduced acquisition time [87]. Furthermore, iron depositions leading to neurotoxicity can be detected early on with high field imaging, because the sensitivity of image contrast increases to iron levels in the tissue. Similarly, image quality is increased, and background suppression is improved in high field MR angiography with longer T1 values of blood and tissue [87].
In comparison to MR spectroscopy at lower magnetic fields, higher field MR spectroscopy enables a more accurate in vivo quantification of brain metabolites as a result of better resolution, greater dispersion of chemical shifts, and increased signal-to-noise ratio [88]. With improved sensitivity [89], higher field MR spectroscopy can reliably quantify a greater range of metabolites, even from small structures of the brain, due to higher signal-to-noise ratio and associated higher anatomical consistency [88, 90]. The improvement in MR spectroscopy performance (including quantification and dispersion) becomes more relevant as the alterations in metabolite concentrations are often small and therefore may be harder to detect. With reliable quantification of metabolites, higher field MR spectroscopy helps clinical decisions regarding patient management including but not limited to early diagnosis, evaluation of treatment response, and longitudinal changes in metabolite levels in AD [88].
Functional Connectivity
Functional MRI specifically during the resting state is a noninvasive technique for evaluation of the strength and spatial topology of interactions between brain networks [91,92,93]. By utilizing the blood-oxygenation-level-dependent (BOLD) signals, functional MRI provides information on specific brain networks through quantification of temporal association of functional activation in different brain areas [92].
Functional MRI is helpful in investigating mechanisms underlying neurodegenerative diseases [93], because the connectivity of distinct large-scale distributed brain networks is impacted early on in neurodegenerative diseases [94]. Functional MRI studies reveal individual patterns of atrophy within functional networks [94] and detect alterations in the default mode network connectivity in neurodegenerative diseases [91].
Quantitative Susceptibility Mapping (QSM)
Iron is critical in metabolic pathways, but also is a key player in neurotransmitter and myelin synthesis [95]. Excess iron deposition plays an important role in pathology of neurodegenerative diseases such as AD and PD [96] as iron triggers oxidative injury and cell death [97]. However, iron also interacts with proteins such as beta-amyloid and tau leading to their aggregation and escalation in subsequent cell death.
Although it cannot quantify iron content directly, a recently developed MRI technique, QSM can offer a reliable evaluation of tissue magnetic susceptibility and changes in brain iron content [98, 99]. Quantitative Susceptibility Mapping (QSM) can provide a comprehensive investigation in vivo of the brain iron profile and related pathophysiology underlying neurodegenerative diseases [100]. Most importantly, it can help define patterns of iron distribution in the brain, which are disease-specific and reflect brain regions associated with pathology of each disease [100]. For example, QSM is sensitive to the increased magnetic susceptibility due to higher iron content in the substantia nigra in DLB [101].
Translocator Protein (TSPO) PET
Chronic neuroinflammation is a common hallmark of many neurodegenerative diseases and is critical in pathogenesis and progression of neurodegeneration [102]. Hence, the evaluation of neuroinflammation is important in identification of underlying mechanisms and the disease spectrum [103].
The translocator protein 18 kDa (TSPO), which is a mitochondrial membrane protein, is upregulated in neuroinflammation and TSPO PET is an emerging imaging technique for evaluating neuroinflammation. In numerous diseases of the central nervous system including AD, PD, and multiple sclerosis, TSPO PET detects the fluctuations in TSPO expression [104,105,106], and it provides information on microglia activity, microglia phenotypes and temporal changes in microglia and astrocyte function during neuroinflammation [102]. Therefore, TSPO PET seems to be a promising molecular imaging biomarker in vivo in tracking neuroinflammation, treatment response in clinical trials, and disease progression.
Clinical Trials and Future Perspectives
Molecular imaging may provide much needed information on enrichment of clinical trials with individuals who may respond to disease-modifying treatments targeting a specific pathological process. A good example is the use of beta-amyloid PET for enrollment of participants to beta-amyloid modifying treatments as well as determining treatment efficacy. Overall, imaging biomarkers are becoming central to patient selection, assessment of target engagement, and treatment efficacy in disease-modifying clinical trials [107]. Furthermore, imaging biomarkers may be critical in determining multiple etiologies contributing to cognitive impairment for individualized approaches to patient care and potentially identifying new targets for drug development in neurodegenerative diseases.
References
Przedborski S, Vila M, Jackson-Lewis V. Neurodegeneration: what is it and where are we? J Clin Invest. 2003;111:3–10. https://doi.org/10.1172/JCI17522.
Relja M. Pathophysiology and classification of neurodegenerative diseases. EJIFCC. 2004;15:97–9.
Iadecola C. The pathobiology of vascular dementia. Neuron. 2013;80:844–66. https://doi.org/10.1016/j.neuron.2013.10.008.
Bell AH, Miller SL, Castillo-Melendez M, et al. The neurovascular unit: effects of brain insults during the perinatal period. Front Neurosci. 2019;13:1452. https://doi.org/10.3389/fnins.2019.01452.
Iadecola C. The neurovascular unit coming of age: a journey through neurovascular coupling in health and disease. Neuron. 2017;96:17–42. https://doi.org/10.1016/j.neuron.2017.07.030.
Schaeffer S, Iadecola C. Revisiting the neurovascular unit. Nat Neurosci. 2021;24:1198–209. https://doi.org/10.1038/s41593-021-00904-7.
Fan J, Dawson TM, Dawson VL. Cell death mechanisms of neurodegeneration. Adv Neurobiol. 2017;15:403–25. https://doi.org/10.1007/978-3-319-57193-5_16.
Dugger BN, Dickson DW. Pathology of neurodegenerative diseases. Cold Spring Harb Perspect Biol. 2017;9:a028035. https://doi.org/10.1101/cshperspect.a028035.
Jellinger KA. Basic mechanisms of neurodegeneration: a critical update. J Cell Mol Med. 2010;14:457–87. https://doi.org/10.1111/j.1582-4934.2010.01010.x.
Hartl FU, Hayer-Hartl M. Converging concepts of protein folding in vitro and in vivo. Nat Struct Mol Biol. 2009;16:574–81. https://doi.org/10.1038/nsmb.1591.
Tan SH, Karri V, Tay NWR, et al. Emerging pathways to neurodegeneration: dissecting the critical molecular mechanisms in Alzheimer’s disease, Parkinson’s disease. Biomed Pharmacother. 2019;111:765–77. https://doi.org/10.1016/j.biopha.2018.12.101.
Gan L, Cookson MR, Petrucelli L, et al. Converging pathways in neurodegeneration, from genetics to mechanisms. Nat Neurosci. 2018;21:1300–9. https://doi.org/10.1038/s41593-018-0237-7.
Menzies FM, Fleming A, Caricasole A, et al. Autophagy and neurodegeneration: pathogenic mechanisms and therapeutic opportunities. Neuron. 2017;93:1015–34. https://doi.org/10.1016/j.neuron.2017.01.022.
Ochaba J, Lukacsovich T, Csikos G, et al. Potential function for the Huntingtin protein as a scaffold for selective autophagy. Proc Natl Acad Sci U S A. 2014;111:16889–94. https://doi.org/10.1073/pnas.1420103111.
Halliwell B. Oxidative stress and neurodegeneration: where are we now? J Neurochem. 2006;97:1634–58. https://doi.org/10.1111/j.1471-4159.2006.03907.x.
Yang JL, Weissman L, Bohr VA, et al. Mitochondrial DNA damage and repair in neurodegenerative disorders. DNA Repair (Amst). 2008;7:1110–20. https://doi.org/10.1016/j.dnarep.2008.03.012.
Lucin KM, Wyss-Coray T. Immune activation in brain aging and neurodegeneration: too much or too little? Neuron. 2009;64:110–22. https://doi.org/10.1016/j.neuron.2009.08.039.
Hickman S, Izzy S, Sen P, et al. Microglia in neurodegeneration. Nat Neurosci. 2018;21:1359–69. https://doi.org/10.1038/s41593-018-0242-x.
Shimizu S, Hirose D, Hatanaka H, et al. Role of neuroimaging as a biomarker for neurodegenerative diseases. Front Neurol. 2018;9:265. https://doi.org/10.3389/fneur.2018.00265.
Schneider JA, Arvanitakis Z, Bang W, et al. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology. 2007;69:2197–204. https://doi.org/10.1212/01.wnl.0000271090.28148.24.
Patel KP, Wymer DT, Bhatia VK, et al. Multimodality imaging of dementia: clinical importance and role of integrated anatomic and molecular imaging. Radiographics. 2020;40:200–22. https://doi.org/10.1148/rg.2020190070.
Koikkalainen J, Rhodius-Meester H, Tolonen A, et al. Differential diagnosis of neurodegenerative diseases using structural MRI data. Neuroimage Clin. 2016;11:435–49. https://doi.org/10.1016/j.nicl.2016.02.019.
Karas G, Scheltens P, Rombouts S, et al. Precuneus atrophy in early-onset Alzheimer’s disease: a morphometric structural MRI study. Neuroradiology. 2007;49:967–76. https://doi.org/10.1007/s00234-007-0269-2.
Morris JC, Csernansky J, Price JL. MRI measures of entorhinal cortex versus hippocampus in preclinical AD. Neurology. 2002;59:1474; author reply 1474–5. https://doi.org/10.1212/wnl.59.9.1474.
Jack CR Jr, Albert MS, Knopman DS, et al. Introduction to the recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:257–62. https://doi.org/10.1016/j.jalz.2011.03.004.
McKeith IG, Boeve BF, Dickson DW, et al. Diagnosis and management of dementia with Lewy bodies: fourth consensus report of the DLB Consortium. Neurology. 2017;89:88–100. https://doi.org/10.1212/WNL.0000000000004058.
Rascovsky K, Hodges JR, Knopman D, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011;134:2456–77. https://doi.org/10.1093/brain/awr179.
Gilman S, Wenning GK, Low PA, et al. Second consensus statement on the diagnosis of multiple system atrophy. Neurology. 2008;71:670–6. https://doi.org/10.1212/01.wnl.0000324625.00404.15.
Hoglinger GU, Respondek G, Stamelou M, et al. Clinical diagnosis of progressive supranuclear palsy: the movement disorder society criteria. Mov Disord. 2017;32:853–64. https://doi.org/10.1002/mds.26987.
Assaf Y, Pasternak O. Diffusion tensor imaging (DTI)-based white matter mapping in brain research: a review. J Mol Neurosci. 2008;34:51–61. https://doi.org/10.1007/s12031-007-0029-0.
Kamagata K, Andica C, Kato A, et al. Diffusion magnetic resonance imaging-based biomarkers for neurodegenerative diseases. Int J Mol Sci. 2021;22:5216. https://doi.org/10.3390/ijms22105216.
Brun A, Englund E. A white matter disorder in dementia of the Alzheimer type: a pathoanatomical study. Ann Neurol. 1986;19:253–62. https://doi.org/10.1002/ana.410190306.
Kantarci K, Murray ME, Schwarz CG, et al. White-matter integrity on DTI and the pathologic staging of Alzheimer’s disease. Neurobiol Aging. 2017;56:172–9. https://doi.org/10.1016/j.neurobiolaging.2017.04.024.
Acosta-Cabronero J, Nestor PJ. Diffusion tensor imaging in Alzheimer’s disease: insights into the limbic-diencephalic network and methodological considerations. Front Aging Neurosci. 2014;6:266. https://doi.org/10.3389/fnagi.2014.00266.
Jacobs HIL, Hedden T, Schultz AP, et al. Structural tract alterations predict downstream tau accumulation in amyloid-positive older individuals. Nat Neurosci. 2018;21:424–31. https://doi.org/10.1038/s41593-018-0070-z.
Kantarci K, Schwarz CG, Reid RI, et al. White matter integrity determined with diffusion tensor imaging in older adults without dementia: influence of amyloid load and neurodegeneration. JAMA Neurol. 2014;71:1547–54. https://doi.org/10.1001/jamaneurol.2014.1482.
Goveas J, O’Dwyer L, Mascalchi M, et al. Diffusion-MRI in neurodegenerative disorders. Magn Reson Imaging. 2015;33:853–76. https://doi.org/10.1016/j.mri.2015.04.006.
Young PNE, Estarellas M, Coomans E, et al. Imaging biomarkers in neurodegeneration: current and future practices. Alzheimers Res Ther. 2020;12:49. https://doi.org/10.1186/s13195-020-00612-7.
Nedelska Z, Senjem ML, Przybelski SA, et al. Regional cortical perfusion on arterial spin labeling MRI in dementia with Lewy bodies: associations with clinical severity, glucose metabolism and tau PET. Neuroimage Clin. 2018;19:939–47. https://doi.org/10.1016/j.nicl.2018.06.020.
Wolk DA, Detre JA. Arterial spin labeling MRI: an emerging biomarker for Alzheimer’s disease and other neurodegenerative conditions. Curr Opin Neurol. 2012;25:421–8. https://doi.org/10.1097/WCO.0b013e328354ff0a.
Oz G, Alger JR, Barker PB, et al. Clinical proton MR spectroscopy in central nervous system disorders. Radiology. 2014;270:658–79. https://doi.org/10.1148/radiol.13130531.
Kantarci K, Jack CR Jr, Xu YC, et al. Regional metabolic patterns in mild cognitive impairment and Alzheimer’s disease: a 1H MRS study. Neurology. 2000;55:210–7.
Sturrock A, Laule C, Decolongon J, et al. Magnetic resonance spectroscopy biomarkers in premanifest and early Huntington disease. Neurology. 2010;75:1702–10. https://doi.org/10.1212/WNL.0b013e3181fc27e4.
Kantarci K, Knopman DS, Dickson DW, et al. Alzheimer disease: postmortem neuropathologic correlates of antemortem 1H MR spectroscopy metabolite measurements. Radiology. 2008;248:210–20. https://doi.org/10.1148/radiol.2481071590.
Oz G, Hutter D, Tkac I, et al. Neurochemical alterations in spinocerebellar ataxia type 1 and their correlations with clinical status. Mov Disord. 2010;25:1253–61. https://doi.org/10.1002/mds.23067.
Ross BD, Bluml S, Cowan R, et al. In vivo MR spectroscopy of human dementia. Neuroimaging Clin N Am. 1998;8:809–22.
Godbolt AK, Waldman AD, MacManus DG, et al. MRS shows abnormalities before symptoms in familial Alzheimer disease. Neurology. 2006;66:718–22. https://doi.org/10.1212/01.wnl.0000201237.05869.df.
Kantarci K, Boeve BF, Wszolek ZK, et al. MRS in presymptomatic MAPT mutation carriers: a potential biomarker for tau-mediated pathology. Neurology. 2010;75:771–8. https://doi.org/10.1212/WNL.0b013e3181f073c7.
Miller BL, Moats RA, Shonk T, et al. Alzheimer disease: depiction of increased cerebral myo-inositol with proton MR spectroscopy. Radiology. 1993;187:433–7. https://doi.org/10.1148/radiology.187.2.8475286.
Oz G, Iltis I, Hutter D, et al. Distinct neurochemical profiles of spinocerebellar ataxias 1, 2, 6, and cerebellar multiple system atrophy. Cerebellum. 2011;10:208–17. https://doi.org/10.1007/s12311-010-0213-6.
Rupsingh R, Borrie M, Smith M, et al. Reduced hippocampal glutamate in Alzheimer disease. Neurobiol Aging. 2011;32:802–10. https://doi.org/10.1016/j.neurobiolaging.2009.05.002.
Krishnan KR, Charles HC, Doraiswamy PM, et al. Randomized, placebo-controlled trial of the effects of donepezil on neuronal markers and hippocampal volumes in Alzheimer’s disease. Am J Psychiatry. 2003;160:2003–11. https://doi.org/10.1176/appi.ajp.160.11.2003.
Bartha R, Smith M, Rupsingh R, et al. High field (1)H MRS of the hippocampus after donepezil treatment in Alzheimer disease. Prog Neuro-Psychopharmacol Biol Psychiatry. 2008;32:786–93. https://doi.org/10.1016/j.pnpbp.2007.12.011.
Lim SM, Katsifis A, Villemagne VL, et al. The 18F-FDG PET cingulate island sign and comparison to 123I-beta-CIT SPECT for diagnosis of dementia with Lewy bodies. J Nucl Med. 2009;50:1638–45. https://doi.org/10.2967/jnumed.109.065870.
Graff-Radford J, Murray ME, Lowe VJ, et al. Dementia with Lewy bodies: basis of cingulate island sign. Neurology. 2014;83:801–9. https://doi.org/10.1212/WNL.0000000000000734.
Shankar GM, Walsh DM. Alzheimer’s disease: synaptic dysfunction and Abeta. Mol Neurodegener. 2009;4:48. https://doi.org/10.1186/1750-1326-4-48.
Scheff SW, Price DA, Schmitt FA, et al. Hippocampal synaptic loss in early Alzheimer’s disease and mild cognitive impairment. Neurobiol Aging. 2006;27:1372–84. https://doi.org/10.1016/j.neurobiolaging.2005.09.012.
Hou Z, Lei H, Hong S, et al. Functional changes in the frontal cortex in Parkinson’s disease using a rat model. J Clin Neurosci. 2010;17:628–33. https://doi.org/10.1016/j.jocn.2009.07.101.
Cai Z, Li S, Matuskey D, et al. PET imaging of synaptic density: a new tool for investigation of neuropsychiatric diseases. Neurosci Lett. 2019;691:44–50. https://doi.org/10.1016/j.neulet.2018.07.038.
Bajjalieh SM, Frantz GD, Weimann JM, et al. Differential expression of synaptic vesicle protein 2 (SV2) isoforms. J Neurosci. 1994;14:5223–35.
Finnema SJ, Nabulsi NB, Eid T, et al. Imaging synaptic density in the living human brain. Sci Transl Med. 2016;8:348–96. https://doi.org/10.1126/scitranslmed.aaf6667.
Jack CR Jr, Lowe VJ, Senjem ML, et al. 11C PiB and structural MRI provide complementary information in imaging of Alzheimer’s disease and amnestic mild cognitive impairment. Brain. 2008;131:665–80. https://doi.org/10.1093/brain/awm336.
Klunk WE, Engler H, Nordberg A, et al. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B. Ann Neurol. 2004;55:306–19. Comparative Study Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, P.H.S. https://doi.org/10.1002/ana.20009.
Chetelat G, Arbizu J, Barthel H, et al. Amyloid-PET and (18)F-FDG-PET in the diagnostic investigation of Alzheimer’s disease and other dementias. Lancet Neurol. 2020;19:951–62. https://doi.org/10.1016/S1474-4422(20)30314-8.
Jansen WJ, Ossenkoppele R, Knol DL, et al. Prevalence of cerebral amyloid pathology in persons without dementia: a meta-analysis. JAMA. 2015;313:1924–38. https://doi.org/10.1001/jama.2015.4668.
Mallik A, Drzezga A, Minoshima S. Clinical amyloid imaging. Semin Nucl Med. 2017;47:31–43. https://doi.org/10.1053/j.semnuclmed.2016.09.005.
Johnson KA, Minoshima S, Bohnen NI, et al. Appropriate use criteria for amyloid PET: a report of the Amyloid Imaging Task Force, the Society of Nuclear Medicine and Molecular Imaging, and the Alzheimer’s Association. J Nucl Med. 2013;54:476–90. https://doi.org/10.2967/jnumed.113.120618.
Buee L, Bussiere T, Buee-Scherrer V, et al. Tau protein isoforms, phosphorylation and role in neurodegenerative disorders. Brain Res Brain Res Rev. 2000;33:95–130. https://doi.org/10.1016/s0165-0173(00)00019-9.
Chien DT, Bahri S, Szardenings AK, et al. Early clinical PET imaging results with the novel PHF-tau radioligand [F-18]-T807. J Alzheimers Dis. 2013;34:457–68. https://doi.org/10.3233/JAD-122059.
Groot C, Villeneuve S, Smith R, et al. Tau PET imaging in neurodegenerative disorders. J Nucl Med. 2022;63:20S–6S. https://doi.org/10.2967/jnumed.121.263196.
Nelson PT, Alafuzoff I, Bigio EH, et al. Correlation of Alzheimer disease neuropathologic changes with cognitive status: a review of the literature. J Neuropathol Exp Neurol. 2012;71:362–81. https://doi.org/10.1097/NEN.0b013e31825018f7.
Graebner AK, Tarsy D, Shih LC, et al. Clinical impact of 123I-Ioflupane SPECT (DaTscan) in a movement disorder center. Neurodegener Dis. 2017;17:38–43. https://doi.org/10.1159/000447561.
Palermo G, Ceravolo R. Molecular imaging of the dopamine transporter. Cells. 2019;8:872. https://doi.org/10.3390/cells8080872.
Kerstens VS, Fazio P, Sundgren M, et al. Reliability of dopamine transporter PET measurements with [(18)F]FE-PE2I in patients with Parkinson’s disease. EJNMMI Res. 2020;10:95. https://doi.org/10.1186/s13550-020-00676-4.
Postuma RB, Berg D, Stern M, et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov Disord. 2015;30:1591–601. https://doi.org/10.1002/mds.26424.
Sakakibara S, Hashimoto R, Katayama T, et al. Longitudinal change of DAT SPECT in Parkinson’s disease and multiple system atrophy. J Parkinsons Dis. 2020;10:123–30. https://doi.org/10.3233/JPD-191710.
Nandhagopal R, Kuramoto L, Schulzer M, et al. Longitudinal evolution of compensatory changes in striatal dopamine processing in Parkinson’s disease. Brain. 2011;134:3290–8. https://doi.org/10.1093/brain/awr233.
Ibrahim N, Kusmirek J, Struck AF, et al. The sensitivity and specificity of F-DOPA PET in a movement disorder clinic. Am J Nucl Med Mol Imaging. 2016;6:102–9.
Morrish PK, Sawle GV, Brooks DJ. Clinical and [18F] dopa PET findings in early Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1995;59:597–600. https://doi.org/10.1136/jnnp.59.6.597.
Pavese N, Brooks DJ. Imaging neurodegeneration in Parkinson’s disease. Biochim Biophys Acta. 2009;1792:722–9. https://doi.org/10.1016/j.bbadis.2008.10.003.
Berger-Sweeney J. The cholinergic basal forebrain system during development and its influence on cognitive processes: important questions and potential answers. Neurosci Biobehav Rev. 2003;27:401–11. https://doi.org/10.1016/s0149-7634(03)00070-8.
Roy R, Niccolini F, Pagano G, et al. Cholinergic imaging in dementia spectrum disorders. Eur J Nucl Med Mol Imaging. 2016;43:1376–86. https://doi.org/10.1007/s00259-016-3349-x.
Bohnen NI, Kaufer DI, Hendrickson R, et al. Cognitive correlates of alterations in acetylcholinesterase in Alzheimer’s disease. Neurosci Lett. 2005;380:127–32. https://doi.org/10.1016/j.neulet.2005.01.031.
Kuhl DE, Minoshima S, Fessler JA, et al. In vivo mapping of cholinergic terminals in normal aging, Alzheimer’s disease, and Parkinson’s disease. Ann Neurol. 1996;40:399–410. https://doi.org/10.1002/ana.410400309.
Kaasinen V, Nagren K, Jarvenpaa T, et al. Regional effects of donepezil and rivastigmine on cortical acetylcholinesterase activity in Alzheimer’s disease. J Clin Psychopharmacol. 2002;22:615–20. https://doi.org/10.1097/00004714-200212000-00012.
Klein JC, Eggers C, Kalbe E, et al. Neurotransmitter changes in dementia with Lewy bodies and Parkinson disease dementia in vivo. Neurology. 2010;74:885–92. https://doi.org/10.1212/WNL.0b013e3181d55f61.
Versluis MJ, van der Grond J, van Buchem MA, et al. High-field imaging of neurodegenerative diseases. Neuroimaging Clin N Am. 2012;22:159–71, ix. https://doi.org/10.1016/j.nic.2012.02.005.
Zhang N, Song X, Bartha R, et al. Advances in high-field magnetic resonance spectroscopy in Alzheimer’s disease. Curr Alzheimer Res. 2014;11:367–88. https://doi.org/10.2174/1567205011666140302200312.
Barker PB, Hearshen DO, Boska MD. Single-voxel proton MRS of the human brain at 1.5T and 3.0T. Magn Reson Med. 2001;45:765–9. https://doi.org/10.1002/mrm.1104.
Tkac I, Andersen P, Adriany G, et al. In vivo 1H NMR spectroscopy of the human brain at 7 T. Magn Reson Med. 2001;46:451–6. https://doi.org/10.1002/mrm.1213.
Hohenfeld C, Werner CJ, Reetz K. Resting-state connectivity in neurodegenerative disorders: is there potential for an imaging biomarker? Neuroimage Clin. 2018;18:849–70. https://doi.org/10.1016/j.nicl.2018.03.013.
Smitha KA, Akhil Raja K, Arun KM, et al. Resting state fMRI: a review on methods in resting state connectivity analysis and resting state networks. Neuroradiol J. 2017;30:305–17. https://doi.org/10.1177/1971400917697342.
Filippi M, Spinelli EG, Cividini C, et al. Resting state dynamic functional connectivity in neurodegenerative conditions: a review of magnetic resonance imaging findings. Front Neurosci. 2019;13:657. https://doi.org/10.3389/fnins.2019.00657.
Seeley WW, Crawford RK, Zhou J, et al. Neurodegenerative diseases target large-scale human brain networks. Neuron. 2009;62:42–52. https://doi.org/10.1016/j.neuron.2009.03.024.
Mills E, Dong XP, Wang F, et al. Mechanisms of brain iron transport: insight into neurodegeneration and CNS disorders. Future Med Chem. 2010;2:51–64. https://doi.org/10.4155/fmc.09.140.
Morris G, Berk M, Carvalho AF, et al. Why should neuroscientists worry about iron? The emerging role of ferroptosis in the pathophysiology of neuroprogressive diseases. Behav Brain Res. 2018;341:154–75. https://doi.org/10.1016/j.bbr.2017.12.036.
Ndayisaba A, Kaindlstorfer C, Wenning GK. Iron in neurodegeneration - cause or consequence? Front Neurosci. 2019;13:180. https://doi.org/10.3389/fnins.2019.00180.
Langkammer C, Schweser F, Krebs N, et al. Quantitative susceptibility mapping (QSM) as a means to measure brain iron? A post mortem validation study. Neuroimage. 2012;62:1593–9. https://doi.org/10.1016/j.neuroimage.2012.05.049.
Haacke EM, Liu S, Buch S, et al. Quantitative susceptibility mapping: current status and future directions. Magn Reson Imaging. 2015;33:1–25. https://doi.org/10.1016/j.mri.2014.09.004.
Ravanfar P, Loi SM, Syeda WT, et al. Systematic review: quantitative susceptibility mapping (QSM) of brain iron profile in neurodegenerative diseases. Front Neurosci. 2021;15:618435. https://doi.org/10.3389/fnins.2021.618435.
Chen Q, Boeve BF, Forghanian-Arani A, et al. MRI quantitative susceptibility mapping of the substantia nigra as an early biomarker for Lewy body disease. J Neuroimaging. 2021;31:1020–7. https://doi.org/10.1111/jon.12878.
Werry EL, Bright FM, Piguet O, et al. Recent developments in TSPO PET imaging as a biomarker of neuroinflammation in neurodegenerative disorders. Int J Mol Sci. 2019;20:3161. https://doi.org/10.3390/ijms20133161.
Tournier BB, Tsartsalis S, Ceyzeriat K, et al. In vivo TSPO signal and neuroinflammation in Alzheimer’s disease. Cells. 2020;9:1941. https://doi.org/10.3390/cells9091941.
Hamelin L, Lagarde J, Dorothee G, et al. Early and protective microglial activation in Alzheimer’s disease: a prospective study using 18F-DPA-714 PET imaging. Brain. 2016;139:1252–64. https://doi.org/10.1093/brain/aww017.
Lavisse S, Goutal S, Wimberley C, et al. Increased microglial activation in patients with Parkinson disease using [(18)F]-DPA714 TSPO PET imaging. Parkinsonism Relat Disord. 2021;82:29–36. https://doi.org/10.1016/j.parkreldis.2020.11.011.
Sucksdorff M, Matilainen M, Tuisku J, et al. Brain TSPO-PET predicts later disease progression independent of relapses in multiple sclerosis. Brain. 2020;143:3318–30. https://doi.org/10.1093/brain/awaa275.
Kantarci K. 2021 marks a new era for Alzheimer’s therapeutics. Lancet Neurol. 2022;21:3–4. https://doi.org/10.1016/S1474-4422(21)00412-9.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Zeydan, B., Kantarci, K. (2023). What Is Neurodegeneration?. In: Cross, D.J., Mosci, K., Minoshima, S. (eds) Molecular Imaging of Neurodegenerative Disorders. Springer, Cham. https://doi.org/10.1007/978-3-031-35098-6_1
Download citation
DOI: https://doi.org/10.1007/978-3-031-35098-6_1
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-35097-9
Online ISBN: 978-3-031-35098-6
eBook Packages: MedicineMedicine (R0)