Abstract
Autism spectrum disorders (ASD) are a collection of neurodevelopmental disorders. Even though ASD has no cure, early detection and intervention can help developing language, behavior, and communication skills. Research shows that ASD can be diagnosed from brain signals, particularly electroencephalography (EEG) where the brain activity is recorded over time as an EEG signal from humans’ scalp and then used to study neuropsychiatric disorders. This study investigates the classification performance of the Neural Networks (NN) model with Hjorth features namely activity, mobility, and complexity extracted from EEG signals through selected channels using the XGBoost algorithm. The classification accuracy is 80% using all 19 channels of EEG data whereas the accuracy of the model using activity, mobility and complexity reached 100% with selected channels. Hjorth parameter-based NN model with channel selection seems to be promising that improves the computation complexity significantly.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Autism spectrum disorders (ASD) [1] are a collection of neurological diseases marked by a lack of verbal and nonverbal communication as well as repetitive stereotypical behaviors. Children with ASD experience symptoms like social communication and social interaction deficiencies. Very often autistic children show repetitive patterns of behavior or activities [1]. Children with ASD may not look when someone points to a thing like a flying airplane because they lack joint attention. Many autistic children experience language difficulties and echolalia, in which they repeat words or phrases instead of speaking normally [2].
In roughly 20% of children with ASD, inattention, impulsivity, and hyperactivity are important symptoms [3]. In South Asia, one out of every 160 children is thought to have ASD [4]. ASD affects about 2 out of 1000 children in Bangladesh, according to the Bangabandhu Sheikh Mujib Medical University (BSMMU). In metropolitan areas, the disease is more prevalent than in rural areas. There is mounting evidence that these early interventions can assist children with ASD in improving their living.
Nowadays, detecting ASD is a major consideration. There are various methods for detecting ASD. A screening strategy [5] is ideal for ASD detection since it is a cost-effective way for primary health care providers to identify patients that require additional specialist treatment. Screening aids in determining whether further specialized study by doctors is required. As ASD is a neurodevelopmental disorder usually reflected in brain signals, therefore, detecting ASD directly from brain signals would be more authentic. Among the widely used non-invasive neuroimaging techniques for brain function analysis are Magnetic Resonance Imaging (MRI) [6], functional MRI (fMRI) [6, 7], electroencephalography (EEG) [8,9,10], and magnetoencephalography (MEG) [11]. Because EEG has a high temporal resolution and is more accessible than other brain signals, it is being identified as a potential diagnostic tool for assessing brain activity.
EEG [12] is regarded as a realistic and effective signal for ASD detection among various brain signals since it has numerous advantages over other approaches for examining brain activity. They are also effective for diagnosing and monitoring brain activity because they exhibit pathological, physiological, and metabolic alterations in the brain and possibly other sections of the body, in addition to functional changes [13]. Therefore, EEG is employed as a useful indicator of ASD.
Several studies reported in the literature used EEG signals for ASD detection [14,15,16]. EEG signal was used as a clinical tool in assessing abnormal brain growth where an SVM was deployed as a classifier [14]. The prognosis of the clinical diagnosis of ASD using the EEG signals was accurate for children as early as 3 months old. Pearson's Correlation Coefficient (PCC) was used in [15] where EEG data from the 19 channels were converted into two-dimensional images to employ CNN. An accuracy of 100% was achieved using Resnet of CNN. Alturki et al. [16] employed discrete wavelet transform to decompose EEG signal into its sub-bands, extracted five statistical features from the sub-bands, and used four classifiers for diagnosing neurological disorders.
Research shows that the accuracy of classifiers largely depends on the quality of the features extracted from the EEG signals. A brief account of feature descriptors and extraction methods is provided in [17]. In pursuit of such quality features, new feature descriptors based on Hjorth parameters to be extracted from EEG signals are proposed [18]. The first three derivatives of EEG signal, i.e., mobility, activity, and complexity, are the most commonly utilized Hjorth parameters. Banerjee et al. [19] used the Hjorth parameters as features of electrooculogram signals and classified them with ensemble classifiers, which achieved an average accuracy of 90.96%. Elamir et al. [20] used Hjorth parameters as features extracted from three physiological signals, namely EEG, EMG, and GSR, and employed three supervised classifiers (KNN, SVM, Decision Tree) for emotion classification (arousal and valence) that achieved an accuracy of 93.2%. Mehmood et al.[21] used Hjorth parameters as features extracted from EEG signals and classified them by SVM for emotion recognition. This method showed an accuracy of 70%. Prakash et al. [22] has also used Hjorth parameters as features for automatic detection of sleep from single channel EEG signals. Bagging Classifier is used in this work for classification.
This research will investigate the pertinence of Hjorth parameters as features to be used for ASD detection from EEG signals. EEG data were collected from 19 channels for 25 patients at resting-state. The three main Hjorth parameters, i.e., activity, mobility, and complexity, values were calculated for all channels of EEG signals. The feature extracted from EEG data were fed into the Neural Network (NN) model, which allowed classification for ASD and control participants. A channel selection has been applied using XGBoost algorithm to select a subset of channels with higher information content suitable for classification. Finally obtained classification accuracies of Hjorth parameters were compared with and without channel selection.
The remaining part of the paper is structured as follows: The methodology is described in detail in Sect. 2. Sections 3 and 4 present the experimental outcomes and discussion, respectively. The conclusion is presented in Section 5.
2 ASD Diagnosis Using Hjorth Parameters from EEG
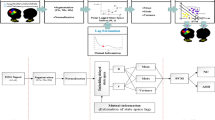
This study aims to investigate NN based ASD detection method from EEG signals. Hjorth parameter-based feature extraction is employed in this research to exploit the performance of the NN-model. Three features defined by activity, mobility, and complexity are extracted from EEG signals. Another facet of the EEG signal-based ASD detection method is channel selection. Hence, the XGBoost algorithm is used to select the information-rich channels out of 19 channels. The proposed methodology is presented in Fig. 1. The different steps in the methodology are described in the following sections.
2.1 EEG Data Preparation
EEG is a powerful modality representing brain signals corresponding to various mental states and activities. Therefore, EEG signals are used for the diagnosis and monitoring of neuropsychiatric disorders. The standard 10–20 scheme of electrode placement on the scalp is used for EEG signal recording [23]. The layout of the standard 10–20 electrode placement is shown in Fig. 2.
2.2 Feature Extraction Using Hjorth Parameters
Hjorth parameters are time-domain parameters that are often utilized to construct feature descriptors from physiological signals such as EEG. It was first introduced by Hjorth [18]. The Hjorth Parameters are called activity, mobility, and complexity. Since they may be specified using first and second derivatives, these are also known as normalized slope descriptors.
Activity:
Activity is the first parameter of the Hjorth parameter set. It is a measurement of the mean power of the signal that also represents the frequency domain surface of the power spectrum. Activity is defined by the following equation:
where y(t) is the signal itself, activity is the variance (mean power) of that signal and var(y(t)) denotes the biased variance of signal y(t) of length N with the mean value of t and it is given by
Mobility:
The second parameter mobility is an estimate of the mean frequency representing the proportion of standard deviation of the power spectrum. Mobility is defined by the following equation.
Complexity:
The third parameter of the Hjorth parameter set is complexity. It provides an estimate of the signal's bandwidth. The value of complexity converges to 1 if the signal is similar. Complexity is defined by the following equation:
These three parameters are calculated for the dataset. Each channel contains one value of activity, mobility, and complexity individually. So, each subject has 19 values of activity, 19 values of mobility, and 19 values of complexity.
2.3 Channel Selection Using XGboost Algorithm
XGBoost [24] stands for extreme Gradient Boosting and represents the premier machine learning toolkit for regression, classification, and ranking tasks. In this research, XGBoost is used to select the more information-rich channels from 19 channels of the dataset. At first, XGBoost is applied to the activity feature set. After applying this channel number is reduced to 10 channels from 19 channels. Similarly, after applying XGBoost to the mobility and complexity feature sets, 14 channels and 12 channels are selected respectively.
2.4 Classification Using Neural Network
Neural networks (NN) [25, 26] are layered interconnected neural computing models that function similarly to neurons in the human brain. An NN model with a single hidden layer with a sufficient number of neurons is capable of modeling any non-linear system. In this study, a simple three layered NN is considered for classification. Extracted features from EEG signals (i.e., activity, mobility, and complexity) with and without selected channels are passed into the NN model. Table 1 shows the parameters of the NN architecture used in this study.
3 Experimental Studies
The experimental results of the proposed ASD detection system are presented here. Classification performance was analyzed with and without channel selection.
3.1 Experimental Data
This study uses an EEG dataset collected from Villa Santa Maria Institute, Italy [27]. The 19-channel EEG data system was used to collect EEG signals from 15 (3 female, 12 male) ASD subjects and 10 (6 female, 4 male) controlled subjects. The age ranges of the subjects were 7 to 14 years and 7 to 12 years for the ASD and control subjects respectively. A bandpass filter of 0.3–70 Hz was applied to filter the EEG signals.
3.2 Experimental Setup
The algorithm is implemented using Anaconda. A Windows 10 OS platform-based PC (Processor of 8th Generation Intel® Core™ i5 – 8265U CPU @ 1.60 GHz, 1.80 GHz, 4 GB RAM) was used for the simulation experiments. For the training-testing protocol, 20 cases were used for the training set and 5 cases were used for the testing set. The available set of libraries such as Keras, Pandas, Sklearn, NumPy, Os, Tesorflow, Matplotlib and pyplot was used for the Neural Networks.
3.3 Experimental Result and Analysis
For each participant's channels, activity, mobility, and complexity features were calculated. Then feature extracted EEG data was passed to NN for classifying the ASD and control subjects. XGBoost algorithm was applied to the features for selecting the important channels individually. From 19 channels, it selects10 channels for activity features. Similarly, 14 channels and 12 channels are picked separately after applying XGBoost for the mobility and complexity feature sets. Figure 3 shows the accuracy comparison between activity, mobility, and complexity before channel selection. According to the figure, for activity, train accuracy reached 95% after 400 epochs and test accuracy reached 80% after 100 epochs. For mobility, train accuracy fluctuates up to 200 epochs and then reached 100%. On the other hand, test accuracy reached 80% after 200 epochs. For complexity, train accuracy reached 95% after 400 epochs and test accuracy reached 80% after 200 epochs. So, the performance of mobility feature is better than activity and complexity. Figure 4 shows the accuracy comparison of activity, mobility and complexity after channel selection. After performing channel selection using XGBoost, 10 channels are selected for activity, 14 channels are selected for mobility and similarly, 12 channels are selected for complexity. For activity, test accuracy reaches to 100% with 10 channels since it was 80% before. For mobility and complexity test accuracy reaches 100% with 14 channels and 12 channels respectively since it was 80% before for both cases. So, the accuracy is increased with a reduced number of channels for activity, mobility, and complexity. So, the channel selection method seems to be promising.
Accuracy comparison of activity, mobility and complexity before and after channel selection is shown in Table 2. At first accuracy for 19 channels is computed individually for activity, mobility, and complexity. After channel selection using XGBoost, accuracy is again computed to make a comparison. For activity, using 19 channels the accuracy is 80% and the F1 score is 86%. After channel selection, 10 channels are selected and the accuracy is 100% with F1 score of 86%. Again, for mobility, the accuracy is 80% using 19 channels with F1 score of 86%. After channel selection, 14 channels are picked for accuracy and this time the accuracy is 100% with an F1 score of 100%. So, the accuracy is increased. Then for complexity, the accuracy is gained at 80% using 19 channels with F1 score of 86%. After channel selection, 12 channels are selected and the accuracy is 100% with an F1 score of 100%. This time also, the accuracy is increased. The number of channels and overall data decreased significantly after the channel selection is applied.
4 Discussion
The proposed method detects ASD using the Hjorth parameter and NN. Along with this method, XGBoost is used to reduce the number of channels of EEG data. By using the channel selection method, a significant reduction of number of channels, i.e., approximately 50%–66% is achieved. This method selects 10 channels for activity, 14 channels for mobility, and 12 channels for complexity. The amount of data is huge for 19 channels contributing to the features set with high computational cost. The reduction of the number of channels has direct impact on the data reduction leading to significant reduction of computational cost. This will enable designing a simple and efficient EEG-based ASD detection system. It also ensures an accuracy of 100%. This is a relatively high accuracy for early detection of ASD.
5 Conclusion
The performance of machine learning model-based detection of ASD depends on the technique used for feature extraction from EEG signals. This research presents the use of Hjorth parameters employed for feature extraction from EEG signals, which are used in the machine learning model for ASD detection. Another facet of the EEG signal-based ASD detection approach is channel selection. This research also reports a channel reduction method using XGBoost after feature extraction. Neural Network is used as a classifier in this research. Channel reduction is performed for activity, mobility, and complexity individually. Application of both Hjorth parameters and XGBoost has resulted in channel reduction and accuracy improvement as well as a reduced amount of data, which again improves the overall computational complexity. A future extension of this research would be to apply this approach to large data sets.
References
Johnson, C.P., Myers, S.M.: Identification and evaluation of children with autism spectrum disorders. Pediatrics 120(5), 1183–1215 (2007). https://doi.org/10.1542/peds.2007-2361
Roberts, J.M.A.: Echolalia and comprehension in autistic children. J. Autism Dev. Disord. 19(2), 271–281 (1989). https://doi.org/10.1007/BF02211846
Tonge, B.J., Brereton, A.: Autism spectrum disorders. Aust. Fam. Physician. 40(9), pp. 7–11, 2011, [Online]. Available: https://www.racgp.org.au/download/documents/AFP/2011/September/201109tonge.pdf
Hasan, M.S.: ASD and its Care in Bangladesh. The Daily Star, 25th Aug 2021 (2021)
Vladimir, V.F.: Autism screening in children: using the social communication questionnaire in South Africa”. Gastron. ecuatoriana y Tur. local. 1(69), 5–24 (1967)
Ha, S., Sohn, I.-J., Kim, N., Sim, H.J., Cheon, K.-A.: Characteristics of brains in autism spectrum disorder: structure, function and connectivity across the lifespan. Exp. Neurobiol. 24(4), 273–284 (2015). https://doi.org/10.5607/en.2015.24.4.273
Sólon, A., Rosa, A., Craddock, R.C., Buchweitz, A., Meneguzzi, F.: NeuroImage: Clinical identification of autism spectrum disorder using deep learning and the ABIDE dataset. NeuroImage Clin. 17, 16–23 (2018). https://doi.org/10.1016/j.nicl.2017.08.017
Korik, A., Sosnik, R., Siddique, N., Coyle, D.: 3D hand motion trajectory prediction from EEG mu and beta bandpower. In: Brain-Computer Interfaces: Lab Experiments to Real-World Applications, vol. 228, 1st edn., pp. 71–105. Elsevier B.V. (2016)
Korik, A., Sosnik, R., Siddique, N., Coyle, D.: Imagined 3D hand movement trajectory decoding from sensorimotor EEG rhythms. In: 2016 IEEE International Conference System Man, Cybernatics SMC 2016 – Conference Proceedings, pp. 4591–4596 (2017). https://doi.org/10.1109/SMC.2016.7844955
Korik, A., Sosnik, R., Siddique, N., Coyle, D.: Decoding imagined 3D hand movement trajectories from EEG: evidence to support the use of mu, beta, and low gamma oscillations. Front. Neurosci. 12(MAR), 1–16 (2018). https://doi.org/10.3389/fnins.2018.00130
Aoe, J., et al.: Automatic diagnosis of neurological diseases using MEG signals with a deep neural network. Sci. Rep. 9(1), 1–9 (2019). https://doi.org/10.1038/s41598-019-41500-x
Ibrahim, S., Djemal, R., Alsuwailem, A.: Electroencephalography (EEG) signal processing for epilepsy and autism spectrum disorder diagnosis. Biocybern. Biomed. Eng. 38(1), 16–26 (2018). https://doi.org/10.1016/j.bbe.2017.08.006
Sanei, S.: EEG/MEG signal processing. Hindawi Publ. Corp. Comput. Intell. Neurosci. Artic. ID 97026 2007, 2 (2007). https://doi.org/10.1155/2007/97026
Bosl, W.J., Tager-Flusberg, H., Nelson, C.A.: EEG analytics for early detection of autism spectrum disorder: a data-driven approach. Sci. Rep. 8(1), 1–20 (2018). https://doi.org/10.1038/s41598-018-24318-x
Peya, Z.J., Akhand, M.A.H., Ferdous Srabonee, J., Siddique, N.: EEG based autism detection using CNN through correlation based transformation of channels’ data. In: 2020 IEEE Region 10 Symposium, TENSYMP 2020, 2020, no. June, pp. 1278–1281. https://doi.org/10.1109/TENSYMP50017.2020.9230928
Alturki, F.A., AlSharabi, K., Abdurraqeeb, A.M., Aljalal, M.: Eeg signal analysis for diagnosing neurological disorders using discrete wavelet transform and intelligent techniques. Sensors 20(9), 2505 (2020). https://doi.org/10.3390/s20092505
Rizon, M.M.M.: Feature extraction methods for human emotion recognition using eeg – a study. In: Conference Malaysia-Japan International Symposium Advance Technology, no. July 2017 (2007)
Hjorth, B.: EEG analysis based on time domain properties. Electroencephalogr. Clin. Neurophysiol. 29(3), 306–310 (1970). https://doi.org/10.1016/0013-4694(70)90143-4
Banerjee, A., Pal, M., Datta, S., Tibarewala, D.N., Konar, A.: Eye movement sequence analysis using electrooculogram to assist autistic children. Biomed. Signal Process. Control 14(1), 134–140 (2014). https://doi.org/10.1016/j.bspc.2014.07.010
Elamir, M.M., Al-atabany, W., Eldosoky, M.A.: Emotion recognition via physiological signals using higher order crossing and Hjorth parameter. Res. J. Life Sci. Bioinform. Pharm. Chem. Sci. 5(2), 839–846 (2019). https://doi.org/10.26479/2019.0502.63
Mehmood, R.M., Lee, H.J.: EEG based emotion recognition from human brain using Hjorth parameters and SVM. Int. J. Bio-Science Bio-Technology 7(3), 23–32 (2015). https://doi.org/10.14257/ijbsbt.2015.7.3.03
Prakash, A., Roy, V.: An automatic detection of sleep using different statistical parameters of single channel EEG signals. Int. J. Signal Process. Image Process. Pattern Recognit. 9(11), 335–344 (2016). https://doi.org/10.14257/ijsip.2016.9.10.32
Misra, U.K., Kalita, J.: Clinical Electroencephalography E-Book. Elsevier Health Sciences (2018)
Chen, T., Guestrin, C.: XGBoost: A scalable tree boosting system. In: Proceedings ACM SIGKDD International Conference Knowledge Discovery Data Mining, vol. 13–17-Aug, pp. 785–794 (2016). https://doi.org/10.1145/2939672.2939785
Siddique, N., Adeli, H.: Computational Intelligence: Synergies of Fuzzy Logic, Neural Networks and Evolutionary Computing. John Wiley and Sons, Chichester, UK (2013)
Cross, S., Harrison, R.F., Kennedy, R.L.: Introduction to neural networks. The Lancet 346(8982), 1075–1079 (1995). https://doi.org/10.1016/S0140-6736(95)91746-2
Grossi, E., Olivieri, C., Buscema, M.: Diagnosis of autism through EEG processed by advanced computational algorithms: a pilot study. Comput. Methods Programs Biomed. 142, 73–79 (2017). https://doi.org/10.1016/j.cmpb.2017.02.002
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 ICST Institute for Computer Sciences, Social Informatics and Telecommunications Engineering
About this paper
Cite this paper
Peya, Z.J., Zaman, B., Akhand, M.A.H., Siddique, N. (2023). Autism Spectrum Disorder Detection from EEG Through Hjorth Parameters and Classification Using Neural Network. In: Satu, M.S., Moni, M.A., Kaiser, M.S., Arefin, M.S. (eds) Machine Intelligence and Emerging Technologies. MIET 2022. Lecture Notes of the Institute for Computer Sciences, Social Informatics and Telecommunications Engineering, vol 491. Springer, Cham. https://doi.org/10.1007/978-3-031-34622-4_3
Download citation
DOI: https://doi.org/10.1007/978-3-031-34622-4_3
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-34621-7
Online ISBN: 978-3-031-34622-4
eBook Packages: Computer ScienceComputer Science (R0)