Abstract
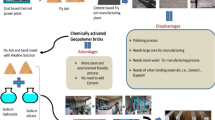
Each year, billions of bricks are produced in the world, but their production is far from environmentally friendly. A growing field of research is focusing on geopolymer bricks constructed with lignocellulosic ashes and calcined clays as a long-lasting, durable, and sustainable binder. This study aims to develop geopolymer bricks utilizing clay from a burned brick factory’s quarry that had been calcined at 700 ℃ for an hour and fly ash (10 to 30%) generated from biomass combustion (olive waste) as an aluminosilicate’s sources. As an alkaline activator, a solution of 8 M NaOH was utilized. According to DRX, while 700 ℃ is inadequate to convert the crystalline phases of clay into more reactive amorphous phases and, as a result, to initiate the geoplymerization reaction, adding potassium and calcium-rich ashes results in stronger bricks.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
In the 1970s, Davidovits used the term “geopolymer” to describe research on how metakaolin reacts with alkaline conditions to form aluminosilicate polymers [1]. Geopolymers are created by activating an aluminosilicate source derived from natural minerals, calcined clays, or industrial by-products [2]. These alumiosilicates degrade rapidly in an extremely alkaline medium, releasing tetrahedral units of SiO4 and AlO4, which hastens the polycondensation process [3]. The most widely used activating agents are hydroxides (NaOH or KOH) and silicate solutions (Na2SiO3 or K2SiO3) [4]. Low temperatures (less than 100 ℃) are required to produce amorphous geopolymers, whereas high-pressure autoclaves can produce crystalline geopolymers at temperatures as high as 200 ℃ [5].The majority of research has been concentrated on creating metakaolin-based geopolymer materials, which are composed primarily of the clay mineral kaolinite, are silica and alumina-rich, and become amorphous when heated to temperatures between 500 ℃ and 700 ℃ [6]. Heat treatment converts crystalline phases into more reactive amorphous phases. These amorphous phases, which are active during the geopolymerization process, regulate the final characteristics of the geopolymers [7]. In fact, a geopolymer with high mechanical properties and a strength range of 20 to 50 MPa is produced when these amorphous phases interact with alkaline solutions. Research on using raw clay as a source of aluminosilicates is currently relatively restricted. In fact, Clay is a fairly complex mineralogical mixture of several distinct minerals, and the origin of the source rocks has a significant impact on this complexity. These substances, on the other hand, can be found in abundance and, when thermally activated, can exhibit some reactivity. Indeed, thermal activation of clay minerals generally results in dehydroxylation, which is associated with mass loss due to the departure of the mineral’s crystalline hydroxyl groups (OH) as water and breakdown in a disordered amorphous state [8]. The ideal activation temperature is significantly influenced by the mineralogical composition of the clay. If the clay is primarily composed of kaolin, a temperature of 700 ℃ is frequently sufficient to produce a dehydroxylated and completely amorphous metakaolin; however, this temperature will not be sufficient to dehydroxylate a clayey material primarily composed of illite [9].
The principles of eco-sustainability now permit the use of waste materials from industrial processes as raw materials. The creation of geopolymeric materials from industrial wastes like fly ash and slag as a partial replacement for clay has received the majority of the study’s attention [10,11,12]. However, the production of geopolymers from lignocellulosic biomass ashes is incredibly rare. These lignocellulosic ashes are very promising because they are rich in potassium oxide, which is the activation solution that forms geopolymerization. They are also rich in silica and alumina, which confirms their use in the geopolymerization reaction by alkaline activators [13]. Geopolymeric materials based on natural clay and lignocellulosic biomass ashes seem to be a practical and eco-friendly option for the preservation of the environment, the reduction of toxicity and landfill issues associated with these ashes, as well as the development of eco-friendly, economical, and sustainable binders that require less energy and have potent qualities like good mechanical properties, low liquid permeability, resistance to high temperatures, etc.
In the current study, calcined natural clay reinforced with olive pomace ash was used as an aluminosilicate source precursor. These substances were activated with an alkaline sodium hydroxide solution to produce geopolymeric materials. To demonstrate the impact of clay minerals’ mineralogical behaviors on calcination temperature as a crucial factor in the creation of geopolymeric binders, natural clay and calcined clay were chemically compared.
2 Materials and Methods
Geopolymer bricks were made using raw clay (RC) that was recovered from a Tunisian brick factory and fly ash from olive pomace (OPFA) that was gathered from the “Zouila Tunisia” olive oil factory. The aforementioned “Zouila” factory uses olive pomace as fuel in gall ovens to generate heat that is then turned to electricity to power the dryers. Fly ash is extracted from the kiln’s electro filter. The raw materials’ chemical compositions and mineral phases were determined using a high-resolution SEM imaging approach with a low vacuum pressure setting of 0.5 “Tm” and an X-ray diffractometer utilizing the D8 Discover Diffractometer.
2.1 Sample Preparation
The raw clay was crushed and sieved through a 200 µm sieve to guarantee that the grains were all the same micrometer size. The clay was then calcined at 700 ℃ for an hour in order to produce more reactive aluminosilicate minerals.
Geopolymer bricks were made in two steps. First, the dry components were combined at low speed for 3 min (calcinated clay (RC/c) and various percentages of OPFA (160 µm)). The water was then added and mixed for one minute on high speed with the dry ingredients. The created geopolymer materials were molded in 7 × 3.5 cm cylindrical molds and allowed to cure for 8 h at 80 ℃ (Table 1).
A second batch of samples was created using NaOH (8 M) to examine how the addition of the alkaline solution affected the growth of the geopolymer bricks.
2.2 Sample Characterization
Mechanical testing was carried out on cylindrical specimens using a mechanical press of the Tinius Olsen H50KS type, which was linked to a 200 KN force transducer and three 50 mm displacement transducers (Fig. 1). The experiments were carried out at loading speeds of 1 mm/min. The crystalline properties of raw clay and calcined clay, including percent crystallinity (Cr%) and FWHM, were investigated using an X-ray diffraction pattern and High Score Plus software. The air peak method was used to calculate the percent crystallinity from X-ray diffraction spectra, according to Eq. (1) [14].
3 Results and Discussion
3.1 Initial Testing of By-Products
This section describes the results and conclusions reached from tests aimed at determining the chemical properties of the study’s elements, raw clay and olive pomace fly ash. First, chemical analyses of various materials used in the design of geopolymeric materials were performed to determine the elemental chemical composition of each component (Figs. 2 and 3).
The raw clay used to make geopolymer bricks is composed of 35.5% crystalline SiO2 and Al2O3, which serve as precursor materials for the geopolymerization reaction (Fig. 2). However, amorphous aluminosilicate minerals are the most reactive minerals. The reason for this is that an hour-long calcination process at 700 ℃ was performed to convert the crystalline phase to more reactive amorphous aluminosilicate minerals.
The components of fly ash made from olive pomace are 2.15% SiO2, Al2O3, and 25% potassium (Fig. 3). The chemical composition is very promising because it is rich in silica and amorphous alumina, which confirms their use in the geopolymerization reaction, it also contains a significant amount of potassium oxide, which is responsible for the generation of geopolymerization.
XRD was used to determine the raw clay’s mineralogical composition. Figure 4 depicts the diffractogram obtained on raw clay powder. X-ray diffraction (XRD) analysis reveals the presence of clay minerals and crystalline phases, primarily in the form of tectosilicate. The latter is reflected as a line of significant intensity relative to quartz observed at the angular position 2θ = 26.62°. Moreover, the reflection at 8.81° in 2θ, corresponds to the presence of illite. Other relatively more intense peaks are observed at 12.33° (7.16 Å) and 20.84° (4.25 Å). These peaks highlight the presence of kaolinite. The diffractogram also reveals the presence of diffraction lines corresponding to clay minerals present respectively at 29.03° and 30.84° which are attributed to calcite and dolomite. The determination of mineralogical compositions was made possible the clay’s X-ray diffraction analysis results. The clay is mostly illito-kaolinite with impurities such as quartz, calcite, and dolomite.
3.2 Initial Testing of Geopolymer Materials
The sections that follow provide details on the tests’ findings that are listed in the material characterization section. The findings of this study’s tests provide preliminary conclusions that allow us to assess the feasibility of a binder made from calcined clay and olive pomace biomass fly ash.
X-ray Diffraction:
An X-ray diffraction analysis was carried out on the calcined clay at a temperature of 700 ℃ for one hour in order to confirm the viability of the calcination temperature used to produce reactive amorphous aluminosilicate minerals in the geopolymer reaction. Figure 5 compares the raw clay’s diffraction pattern to that of the calcined clay.
Analysis of the XRD peak profiles of calcined clay and raw clay indicated the disappearance of peak observed at angular position 2θ = 12. 33° which highlighted the presence of kaolinite as well as an increase in the full width at half maximum (FWHM) of peak observed at angular position 2θ = 20.84° (0.103 [°2θ] for calcined clay versus 0.082[°2θ] for raw clay) highlighting the beginning of kaolinite amorphization. Indeed, a higher FWHM means a less improved crystal quality due to the heat treatment process during the preparation of the calcined clay [14]. No change in the reflections of illite was found, due to its higher thermal stability. Indeed, the reflection at 8.81° in 2θ, corresponding to the presence of illite in the raw clay, is still present in the calcined clay and it still keeps the same FWHM (0.165 [°2θ]). Indeed, the raw clay had a crystallinity percentage of 77.63%, whereas the calcined clay had a crystallinity percentage of 63.85%, indicating that the chosen temperature was unable to convert the clay minerals from crystalline to amorphous phases.
Mechanical Characterization:
The compressive strength of geopolymeric specimens showed that materials elaborated with water had a low strength (Table 2). In fact, geopolymerization takes place in the presence of an alkaline solution and an aluminosilicate precursor. The goal of this solution is to chemically attack the precursor, allowing for its complete dissolution, and releasing Si or Al elements that will be used during the geopolymerization process. However, clays rarely dissolve completely and quickly in an alkaline medium due to their crystalline structure. This explains why all specimens have such low mechanical resistance. Indeed, as was previously shown in the DRX analysis, neither the complete transformation of kaolinite into metakoalin nor the dehydroxylation of illite and its transformation into more reactive illite anydrydes were possible at the calcination temperature used. Furthermore, the use of water and fly ash did not result in geoplymeric bricks with good mechanical properties. In fact, the potassium concentration in the fly ash was insufficient to serve as an alkaline solution. In fact, the effect of fly ash addition on compressive strength values is only noticeable at significant percentages of OPFA (> 25%).
The elaborated materials’ mechanical properties were enhanced by the addition of an alkaline sodium hydroxide solution and potassium from the olive pomace ashes. In fact, adding 25% olive pomace ash increased the developed specimens’ mechanical strength by 122%. However, after 30% by weight of olive biomass fly ash incorporation, compressive strength decreases. The cause is that potash, at more than 30% of its weight, is excessive and does not interfere with the freezing of the geopolymer. Therefore, the geopolymerization process is hampered by a higher proportion of fly ash from olive biomass [15].
4 Conclusion
In this study, geopolymer bricks are produced using calcined clay and fly ash as aluminosilicate precursors and sodium hydroxide as an alkali activation solution.
The following results were obtained:
-
The data presented showed that a calcination temperature of 700 ℃ for one hour was insufficient to produce reactive aluminosilicate minerals, resulting in the specimens’ low mechanical strength. The presence of illite in the mineralogical structure of the clay used will necessitate more thermal activation energy associated with the dehydroxylation of the (OH) group. To determine the optimal calcination temperature for dehydroxylation, a thermogravimetric analysis (TGA) must be performed.
-
The addition of a sodium hydroxide solution and olive pomace fly ash allowed for improved mechanical performances. Indeed, these ashes are high in potassium, present in the form of K2O, as well as alumina and reactive silica, which promotes hydraulic consolidation and the elimination of free water, resulting in higher values of compression resistance.
The most traditional method of creating a geopolymer with good mechanical properties and durability is through the alkaline activation of calcined kaolin, also known as metakaolin. However, this approach costs money and uses a lot of energy. Alkaline activation of clay soils at low temperatures to produce economical and environmentally friendly geopolymer bricks is becoming increasingly researched.
Abbreviations
- RC:
-
Raw Clay
- RC/c:
-
Calcined Raw Clay
- OPFA:
-
Olive Pomace Fly Ash
- FWHM:
-
Full Width at Half Maximum
References
Davidovits, J.: Geopolymers: Inorganic polymeric new materials. J. Therm. Anal. 37, 1633–1656 (1991)
Cong, P., Cheng, Y.: Advances in geopolymer materials: a comprehensive review. J. Traffic Transp. Eng. (English Edition) 8(3), 283–314 (2021). https://doi.org/10.1016/j.jtte.2021.03.004
Davidovits, J.: Geopolymers: ceramic-like inorganic polymers. J. Ceram. Sci. Technol. 8(3), 335–350 (2017). https://doi.org/10.4416/JCST2017-00038
Gómez-Casero, M.A., Moral-Moral, F.J., Pérez-Villarejo, L., Sánchez-Soto, P.J., Eliche-Quesada, D.: Synthesis of clay geopolymers using olive pomace fly ash as an alternative activator. Influence of the additional commercial alkaline activator used. J. Market. Res. 12, 1762–1776 (2021). https://doi.org/10.1016/j.jmrt.2021.03.102
Amran, Y.H.M., Alyousef, R., Alabduljabbar, H., El-Zeadani, M.: Clean production and properties of geopolymer concrete; A review. J. Cleaner Prod. 251, 119679 (2020). https://doi.org/10.1016/j.jclepro.2019.119679
Sore, S.O., Messan, A., Prud’Homme, E., Escadeillas, G., Tsobnang, F.: Comparative study on geopolymer binders based on two alkaline solutions (NaOH and KOH). J. Miner. Mater. Charact. Eng. 8(6), 407–420 (2020). https://doi.org/10.4236/jmmce.2020.86026
Seiffarth, T., Hohmann, M., Posern, K., Kaps, Ch.: Effect of thermal pre-treatment conditions of common clays on the performance of clay-based geopolymeric binders. Appl. Clay Sci. 73, 35–41 (2013)
Shvarzman, A., Kovler, K., Grader, G.S., Shter, G.E.: The effect of dehydroxylation/amorphization degree on pozzolanic activity of kaolinite. Cem. Concr. Res. 33(3), 405–416 (2003). https://doi.org/10.1016/S0008-8846(02)00975-4
Gualtieri, A.F., Ferrari, S.: Kinetics of illite dehydroxylation. Phys. Chem. Miner. 33(7), 490–501 (2006). https://doi.org/10.1007/s00269-006-0092-z
Ahmad, M., Rashid, K., Hameed, R., Haq, E.U., Farooq, H., Ju, M.: Physico-mechanical performance of fly ash based geopolymer brick: influence of pressure − temperature − time. J. Build. Eng. 50, 104161 (2022). https://doi.org/10.1016/j.jobe.2022.104161
Qaidi, S.M.A., Tayeh, B.A., Isleem, H.F., de Azevedo, A.R.G., Ahmed, H.U., Emad, W.: Sustainable utilization of red mud waste (bauxite residue) and slag for the production of geopolymer composites: a review. Case Stud. Const. Mater. 16, e00994 (2022). https://doi.org/10.1016/j.cscm.2022.e00994
Ferone, C., Colangelo, F., Cioffi, R., Montagnaro, F., Santoro, L.: Mechanical performances of weathered coal fly ash based geopolymer bricks. Procedia Eng. 21, 745–752 (2011). https://doi.org/10.1016/j.proeng.2011.11.2073
Labaied, I., Douzane, O., Lajili, M., Promis, Geoffrey: Bricks using clay mixed with powder and ashes from lignocellulosic biomass: a review. Appl. Sci. 12(20), 10669 (2022). https://doi.org/10.3390/app122010669
Segal, L., Creely, J.J., Martin, A.E., Conrad, C.M.: “Opportunity for new developments in all phases of textile manufacturing. ‘Literature Cited An Empirical Method for Estimating the Degree of Crystallinity of Native Cellulose Using the X-Ray Diffractometer.” (1952)
Carrillo-Beltran, R., Corpas-Iglesias, F.A., Terrones-Saeta, J.M., Bertoya-Sol, M.: New geopolymers from industrial by-products: olive biomass fly ash and chamotte as raw materials. Constr. Build. Mater. 272, 121924 (2021). https://doi.org/10.1016/j.conbuildmat.2020.121924
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this paper
Cite this paper
Labaied, I., Douzane, O., Lajili, M., Promis, G. (2023). Bricks Geopolymer Based on Olive Waste Fly Ash: Mechanical Properties. In: Amziane, S., Merta, I., Page, J. (eds) Bio-Based Building Materials. ICBBM 2023. RILEM Bookseries, vol 45. Springer, Cham. https://doi.org/10.1007/978-3-031-33465-8_5
Download citation
DOI: https://doi.org/10.1007/978-3-031-33465-8_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-33464-1
Online ISBN: 978-3-031-33465-8
eBook Packages: EngineeringEngineering (R0)