Abstract
This chapter aims to familiarize the readers with terminology and basic concepts applied to conchological and anatomical characters that have been widely used for the identification of lymnaeid snails. The main diagnostic traits of the lymnaeid shell and soft body anatomy are reviewed and illustrated.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
For a very long time, shell characters served as the sole source of information for the classification and identification of lymnaeid snails. Shell size, shape, proportions, sculpture, coloration were viewed as the main source of taxonomic signal even though all these characters are subject to enormous variation, both at the intra- and interpopulation level. As Falniowski (1980, p. 327) stated, the shell variation among lymnaeids is “probably the largest one in all of the freshwater gastropods.” Today, in the epoch of the molecular taxonomy predominance, the significance of conchological information for the lymnaeid identification is lowered but the shells and their properties did not lose completely their usefulness. This is especially evident in paleontology, where shells represent virtually the only material available to researchers. Another field where conchology still keeps its importance is working with historical (museum) samples, including type specimens of lymnaeid species described in the mid-eighteenth–mid-twentieth centuries. In the majority of cases, the pond snail specimens collected till the dawn of the last century are represented in museum collections by dried shells only. At last, for amateurs and “citizen scientists,” the examination of shells of lymnaeids and other families of freshwater molluscs is virtually the only method of their fast identification, which may be important for monitoring of invasive species. Below, we provide an overview of the lymnaeid shells, with emphasis on structures and characters most frequently used in identification keys (see, for example, Glöer 2002; Kruglov 2005; Khokhutkin et al. 2009; Andreeva et al. 2010; Piechocki and Wawrzyniak-Wydrowska 2016; Vinarski 2019; Pointier and Vàzquez 2020).
This chapter aims to familiarize the readers with terminology and basic concepts applied to conchological and anatomical characters that have been widely used for the identification of lymnaeid snails. One can find similar accounts in works by previous authors (Jackiewicz 1998; Stadnichenko 2004; Kruglov 2005; Andreeva et al. 2010; Vinarski 2019; Pointier and Vàzquez 2020). An overview of molecular techniques used for the taxonomic identification of lymnaeid snails can be found in Alda et al. (2023).
Whereas most of the recent lymnaeid snails possess turbospiral shells, a small group of limpets (sometimes separated as a distinct subfamily, the Lancinae) is known within the family. A turbospiral lymnaeid shell comprises several parts, illustrated in Fig. 4.1. The uppermost point of a shell, where coiling starts, is named apex. The aperture (or mouth), the opening through which the soft body extends, is situated on the opposite part of a shell tube formed during the coiling. In all pulmonate aquatic snails, including Lymnaeidae, the aperture lacks an operculum (characteristic for the gill-breathing freshwater Gastropoda) as well as other protective structures. The spire and the body (ultimate) whorl represent two more shell parts important for identification.
In addition, some other terms related to gastropod shell morphology should be explained here (see the full glossary in Cox 1960):
The shell axis is an imaginary line through the shell apex, about which whorls are coiled (not applicable to limpets). It is surrounded by a solid or hollow pillar, not visible from outside, which is formed by adaxial (this term means “toward shell axis inward”; Cox 1960) walls of whorls. This pillar is called the columella.
The suture is a continuous line adjoining two consequent whorls. This line can be deep or relatively shallow, straight, or oblique. Its state depends on the degree of shell whorl convexity. In most lymnaeid species, shell whorls are slightly convex or flattened, though some pond snails are characterized by strongly convex, sometimes almost stepped, whorls [for example, Galba truncatula (O.F. Müller, 1774); see Fig. 4.2].
(a) A scheme of linear measurements of a turbospiral shell (as exemplified by a shell of the dwarf pond snail, Galba truncatula). Abbreviations: SH—shell height; SW—shell width; SpH—spire height; BWH—body whorl height; AH—aperture height; AW—aperture width. (b) A scheme of linear measurements of an ancyliform (limpet) shell. Abbreviations: H—shell height; L—shell length; W—aperture width
The umbilicus is a cavity or depression formed around the shell axis inside the body whorl and situated at the base of the shell. In most species of the discussed family, it is absent or looks like a very thin slit covered by the columellar lip.
The sculpture is a relief pattern on the shell surface. It may be represented by ribs or riblets, thin striae, small depressions, ridges, and other structures. Some lymnaeid shells (for example, that of the gelatinous pond snail, Myxas glutinosa) virtually lack any prominent sculpture, being smooth and glossy. If sculptural elements are oriented parallel to the shell axis, then these are designated as radial sculpture. The spiral sculpture is formed by sculptural elements oriented transversely to the axis. The latter type of sculpture is quite rare among lymnaeids.
The variability in shell surface sculpture (and coloration) can be very prominent at the intraspecific level but, as a rule, it lacks any taxonomic value though there are data that the pattern of shell sculpture may be species-specific in some lymnaeids (Jackiewicz and Koralewska-Batura 1995; Jackiewicz 1998).
A keel (or carina) is a prominent spiral ridge of a shell. It may be solid or relatively soft, in the latter case, the keel may look like a thin and fragile fringe on the shell edge. This structure is rarely manifested among freshwater pulmonate snails, except for some genera of the family Planorbidae. Within Lymnaeidae, the keeled species are known among extinct species (see Vinarski and Pointier 2023).
A special nomenclature for the designation of parts of the shell aperture has been developed (see Fig. 4.1). The margin of the aperture adjacent to the body whorl is called parietal; the free lateral margin is palatal, and the lower free margin is basal. The columellar margin is adjacent to the shell columella. In some species of the family, there is a more or less prominent depression situated at the junction of the columellar and parietal margins. Often, it is called the columellar fold.
The columellar margin in lymnaeids is usually reflected over the adjacent part of the shell which forms the columellar lip, more or less broad. The coloration of this lip is usually lighter than the body whorl surface. In most lymnaeid species, the columellar lip is rather thin; usually, it completely covers the umbilicus, which makes the latter virtually invisible. In other cases, the umbilicus looks like a very narrow slot.
The absolute shell size is very plastic in most taxa of the Lymnaeidae and therefore is generally avoided in identification keys (though it may be useful for fast identification of some genera). There are many factors influencing shell size, including age (ontogenic variation), ambient temperature, food supply, parasitism, and so on (Abel 1920; Hubendick 1951; Calow 1981; Lam and Calow 1988; Lakowitz et al. 2008; Vinarski 2012, 2013; Whelan 2021; Vinarski and Pointier 2023). The absolute shell size is, thus, of a low significance or taxonomy, however, most genera and subgenera may be characterized by a more or less clear range of body sizes, which may be attained by their representatives. There are genera of large-bodied lymnaeids (Bulimnea, Lymnaea) and genera of much smaller size (Galba, Orientogalba). In some cases, the congeneric species may differ by their absolute shell sizes. For example, shells of Stagnicola corvus (Gmelin, 1791) are generally larger than those of S. palustris (O.F. Müller, 1774) or S. turricula (Held, 1836); shells of Radix auricularia (Linnaeus, 1758) are more voluminous as compared to shells of R. euphratica (Mousson, 1874) and R. natalensis (Krauss, 1848).
The use of the so-called morphometric indices, like the ratio between shell width (SW) and shell height (SH), has been served as a standard means for quantitative expression and analysis of these interspecific differences, though today it is gradually replacing by more advanced statistical techniques such as geometric morphometry. These indices are simple to calculate, and therefore they are still in wide use. Their use aims to give the simplest quantitative characteristics of proportions of a given shell or a particular shell sample.
Shell proportions are thought to be a rather more reliable source of taxonomically relevant information. In order to use shell proportions for species identification, different conchometric indices are calculated based on plain linear measurements. For example, one may use the ratio between shell width and shell height (SW/SH; Fig. 4.2) for characterization of relative slenderness of a snail shell, and so on. These ratios, however, are also prone to change with age, which is explained by the allometric growth (Fig. 4.3). Consequently, the shell habitus is also subject to ontogenic alterations.
Ontogenic changes in shell proportions in a sample of Lymnaea taurica kazakensis (Central Kazakhstan, Sary-Kopa Lake, n = 548). (a) The relationship between shell height and shell width. (b) Ontogenic change in the values of SW/SH index. SH is used as a proxy for snail age. Regression equations and trend lines are given. Based on unpublished data of M. Vinarski
Though the use of conchometric indices has been a long tradition in the lymnaeid studies, we have to stress that only in a minority of cases do these indices represent a reliable way of distinguishing between closely affined species. As a rule, their values greatly overlap even among the most tightly related taxa. Such an overlap of values is characteristic for comparisons based on multivariate statistics as well (Fig. 4.4).
The overlap of three samples of two sister lymnaeid species (Ampullaceana fontinalis, A. lagotis) in the plane of the first two principal components. The analysis is based on the six standard shell measurements with addition of whorl number. The two first PCs explain 96.6% of variation. Red dots—A. fontinalis from Seversky Donets River, Russia. Blue dots—A. fontinalis from Veselovskoye Reservoir, Russia. Violet dots—A. lagotis from the Panj River floodplain, Tajikistan. All three samples were identified genetically. The primary data taken from Vinarski et al. (2020)
The shell measurement scheme (see Fig. 4.2a) includes six basic measurements however it by no means is standard; the number of linear measurements can be increased depending on the task of a particular research (see, for example, Samadi et al. 2000). For example, it may be needed to introduce supplementary measurements like the body whorl width above the aperture, the height and width of the penultimate whorl, and so on. In the case of limpet shells, the measurement scheme undergoes corresponding changes (see Fig. 4.2b).
In addition to linear measurements, it is sometimes helpful to count the whorl number, since many genera and subgenera of the Lymnaeidae differ from each other by the maximum number of shell whorls. The scheme of whorl counting is given in Fig. 4.5. This parameter is frequently used as a proxy of the absolute age of snails. Another useful parameter is the shell tangent line, i.e. an imaginary line that “just touches” the surface of all or several of the whorls constituting the spire. It may be straight or almost straight, convex or concave (see 4.5b). The form of this line may be species-specific and in certain cases allows one to distinguish between closely allied and conchologically similar species.
(a) A scheme of the whorl number counting. (b) Three types of the tangent line. After Vinarski (2019), slightly modified
Ever since Baker (1911, 1915) and Roszkowski (1914a, b, 1922, 1926), it was believed that the internal organs of the lymnaeids, as being not greatly modified by external influences, represent a more reliable criterion for purposes of classification as compared to conchological characters. The structure of the reproductive system was thought to be the most important source of taxonomic and phylogenetic signals. Most consistently, this idea was developed in seminal works of Hubendick (1951), Jackiewicz (1959, 1993, 1998), and Paraense (1976, 1982, 1983, 1984, 1994, 1995), who relied heavily on anatomical data in their search for a “natural” system of the Lymnaeidae and the reliable identification of these snails. The extensive use of the soft body characters for the identification of the species and higher taxa of the Lymnaeidae has also been advocated by Falniowski (1980) and Kruglov (2005).
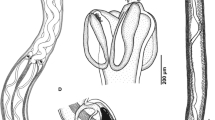
The Lymnaeidae are hermaphroditic snails, with the male and female reproductive organs co-existing in the same animal (see for example Fig. 4.6). Not all of these organs are equally important for taxonomy. The size and proportions of the copulatory organ, the shape of the spermatheca, and the relative length of its duct as well as the internal structure of the prostate are the characters that have most frequently been used by practitioners for the sake of taxonomic identification of lymnaeids.
(a) Anatomy of the reproductive system of Galba truncatula from France: ac = apical chamber; ag = albumen gland; ng = nidamental gland; od = ovispermiduct; ot = ovotestis; ov = oviduct; pr = prostate; pp. = preputium; ps = penis sheath; rm. = retractor muscle of penial complex; sd = spermiduct; sp. = spermatheca; va = vagina; scale bar = 1 mm. (b) Variability of the form of the spermatheca and the length of the spermathecal duct in selected species of radicine snails: 1 = Radix auricularia; 2 = Peregriana peregra; 3 = Ampullaceana balthica; 4 = A. lagotis. Photos are taken from Schniebs et al. (2011) and modified
The copulatory organ in the Lymnaeidae typically consists of two large structures named the praeputium and the penis sheath (Fig. 4.6). The ratio of their lengths (known as the “index of the copulatory organ,” ICA) has been used for species delimitation in different genera of Lymnaeidae (Jackiewicz 1959, 1996, 1998; Falniowski 1980; Kruglov and Starobogatov 1985; Vinarski 2011a; Standley et al. 2013; Pointier 2015). It has been revealed, though, that both intra- and interspecific variation of ICA values is significant that limits its direct use for lymnaeid identification (Schniebs et al. 2011, 2013; Vinarski 2011b). It should also be noted that in certain species of the genus Radix this ratio may be polymorphic within a population, and conchologically indistinguishable individuals may differ drastically from each other in the penis sheath length (Vinarski 2011b). Another source of uncertainty in using this ratio is its ontogenic variation (Beriozkina and Starobogatov 1988); in some species, the spatial (geographical) variation of this index is observed (Vinarski 2009).
A statistical analysis of the intrapopulation variability of ICA has shown that, in most populations, this variability is rather moderate, with the coefficient of variation (Cv) being lower than 20% (Table 4.1). The data of Table 4.1 reveals the phylogenetically allied species show virtually no significant differences in this index (contrary to the opinion of Beriozkina and Starobogatov 1988; Kruglov 2005), which greatly limits the usefulness of this ratio for identification purposes. However, some contrary instances can be reported here. In the genus Stagnicola, two sister and conchologically cryptic species, S. palustris and S. turricula, demonstrate a statistically significant difference in their mean ICA values (Falkner 1985; Glöer 2002, 2019). In S. turricula, the praeputium is nearly twice shorter than the penis sheath, which results in the ICA values between 0.34–0.63 (Falkner 1985; Vinarski, unpublished). In another species, the praeputium length is subequal to the penis sheath length (Fig. 4.7) and sometimes slightly exceeds the latter, and the ICA lies in the range 0.60–1.18 (Vinarski, unpublished data). On the other hand, some overlap in the ICA values in the two species is observed (see Fig. 4.7), therefore the diagnostic significance of this ratio is not absolute. However, in the vast majority of cases, the two stagnicoline species can be distinguished through this index. Though it is almost impossible to differentiate S. palustris and S. turricula based on their shell traits (Jackiewicz 1998), the molecular evidence of their distinct species status has recently been provided (Pieńkowska et al. 2015). There is another stagnicoline species, S. saridalensis (Mozley, 1934), distributed in Siberia, whose ICA ratio is less than 0.25 (a very short praeputium and extremely long and narrow penis sheath; see Lazareva 1967; Kruglov 2005; Vinarski 2014) [see Table 4.1].
The copulatory organs of Stagnicola palustris (a–c) and S. turricula (d–e), with a diagram showing an overlap of the distributions of their ICA values. (a) Germany, Brandenburg, Kesselsee Lake. (b) Russia, Pskov Region, Yasskoye Lake. (c) Russia, Murmansk Region, Gorelyi Island. (d) Germany, Bavaria, Danube River near Oberalteich. (e) Azerbaijan, a lake adjoining the Caspian Sea near Narimanabad Town. Scale bars 1 mm. After Vinarski (unpublished data)
In South America, conchologically cryptic species of the genus Galba can be distinguished based on the proportions of their copulatory organs. The ICA was reported as the only morphological character allowing the distinction between G. viator (d’Orbigny, 1835), G. truncatula, and G. cubensis (L. Pfeiffer, 1839) [Standley et al. 2013; Pointier 2015; Pointier and Vàzquez 2020]. However, as it was cautiously noted, this index “has no practical interest for the identification of a single snail collected in the field” (Pointier 2015, p. 109), which is explained by the overlap in the distribution of the values of this parameter.
A small group of Palearctic Lymnaeidae described as the genus Aenigmomphiscola Kruglov & Starobogatov, 1981 demonstrates a more advanced structure of the copulatory organ with the so-called praeputial organ within the praeputium that is regarded to be asymmetrically enlarged velum (Kruglov and Starobogatov 1981; Vinarski et al. 2011). In all other respects, however, these snails are quite similar to the rest of lymnaeids.
The relative length and proportions (long and narrow vs short and thick) of the spermathecal duct as well as the determination of the number of internal prostate folds (see Fig. 4.8) are useful for discrimination among genera and subgenera of Lymnaeidae. For example, the species of the genus Radix are typically characterized by a compact globose (or sac-like) spermatheca (or bursa copulatrix) sitting on a relatively long and narrow spermathecal duct. The representatives of the genera Ampullaceana and Peregriana, having similar shell proportions, can be distinguished from the genus Radix by the very short spermathecal duct (which sometimes is virtually absent) and oblong spermatheca (see Fig. 4.6b). In most instances, these differences are prominent enough to differentiate between species of Radix and other, conchologically similar, genera. However, as Schniebs et al. (2011, 2013, 2019) reported, there are cases, when this distinguishing character fails to help. Among specimens of Ampullaceana ampla (W. Hartmann, 1821), for example, some individuals with very long spermathecal duct were found (see Fig. 5 in Schniebs et al. 2019), which makes these individuals anatomically indistinguishable from Radix auricularia (note: the identity of these specimens as belonging to A. ampla was confirmed by molecular methods).
It may be concluded, on the basis of the abovementioned facts, that neither particular anatomical character allows unequivocal differentiation between two lymnaeid individuals belonging to phylogenetically related species. The absolutely correct identification of a single snail is possible by means of genetic tools (Schniebs et al. 2011, 2013). Both qualitative and quantitative anatomical characters are, thus, only workable if whole samples, not single snails, are compared (see Vinarski 2011b).
The internal structure of the prostate was thought to provide useful information for distinguishing between the lymnaeid genera (Starobogatov 1976; Jackiewicz 1993, 1998; Kruglov and Starobogatov 1993; Kruglov 2005). Kruglov (2005) delineated the five main types of the prostate (Fig. 4.8): (A) simple prostate without any internal fold (genera Omphiscola, Pseudobulinus, Pseudosuccinea); (B) one (rarely two) internal unbranched folds within the prostate (most recent lymnaeid genera fall to this group); (C) prostate with a single branched fold (genus Racesina); (D) many-folded prostate with 5–10 branched internal folds (genus Lymnaea); (E) many-folded prostate with several branched folds (some representatives of Stagnicola). The distribution of these character states among the living Lymnaeidae reveals that the five structural types lack any phylogenetical meaning, since the same structural type might have been developed independently in different lineages of the family. The loss of the internal folds of the prostate, for instance, has happened at least thrice in the lymnaeid evolution, and the unfolded prostate is found in the European genus Omphiscola, the North American Pseudosuccinea, and in the genus Pseudobulinus, which is endemic to Hawaii (Kruglov and Starobogatov 1993).
The use of molecular techniques has revealed that the Bauplan of the reproductive anatomy within the Lymnaeidae is extremely conservative (except for the subfamily Lancinae having two prostates of different structure; see Vinarski and Pointier 2023). At least several genetically divergent genera and subgenera of the subfamily Amphipepleinae exhibit the same structure of the copulatory apparatus and the prostate (Aksenova et al. 2018; Vinarski et al. 2020). The results of the most recent “integrative” studies have confirmed the enormous level of intra- and interspecific variability in the lymnaeid snails, which makes the genetic methods the solely reliable tool for identification of the pond snails. However, even popular molecular approaches like DNA barcoding can sometimes fail in the lymnaeid identification (Schniebs et al. 2016), therefore we advocate here the integrative approach to taxonomic diagnostic of the lymnaeid snails which combines the data provided by conchological, anatomical, and molecular genetic methods.
References
Abel O (1920) Lehrbuch der Paläozoologie. G. Fischer Verlag, Jena
Aksenova OV, Bolotov IN, Gofarov MY et al (2018) Species richness, molecular taxonomy and biogeography of the radicine pond snails (Gastropoda: Lymnaeidae) in the Old World. Sci Rep 8:11199. https://doi.org/10.1038/s41598-018-29451-1
Alda P, Bonel N, Alba A et al (2023, this volume) Molecular techniques for the study of ecological and evolutionary processes in lymnaeids. In: Vinarski MV, Vázquez A (eds) The Lymnaeidae. A handbook on their natural history and parasitological significance. Springer, Cham, pp 121–146
Andreeva SI, Andreev NI, Vinarski MV (2010) Key to freshwater gastropods of Western Siberia (Mollusca: Gastropoda). V. 1. Gastropoda: Pulmonata. Fasc. 1. Families Acroloxidae and Lymnaeidae. The authors, Omsk [in Russian]
Baker FC (1911) The Lymnaeidae of North and Middle America. Special publication of the Chicago Academy of Sciences 3:1–539. https://doi.org/10.5962/bhl.title.20500
Baker FC (1915) On the classification of the Lymnaeids. The Nautilus 29(2):20–24
Beriozkina GV, Starobogatov YI (1988) Reproductive ecology and egg clusters of freshwater Pulmonata. Trudy Zoologicheskogo Instituta AN SSSR 174:1–306. [in Russian]
Calow P (1981) Adaptational aspects of growth and reproduction in Lymnaea peregra (Gastropoda: Pulmonata) from exposed and sheltered aquatic habitats. Malacologia 21:5–13
Cox LM (1960) Gastropoda – general characteristics of Gastropoda. In: Moore RC (ed) Treatise on invertebrate Paleontology. Part I. Mollusca 1. University of Kansas Press, Lawrence, pp 84–169
Falkner G (1985) Stagnicola turricula (Held) – eine selbständige Art neben Stagnicola palustris (O.F. Müller). Heldia 1(2):47–50
Falniowski A (1980) The anatomical determination of Polish Lymnaeidae (Mollusca, Basommatophora). Acta Hydrobiologica 22(3):327–335
Glöer P (2002) Die Sußwassergastropoden Nord- und Mitteleuropas: Bestimmungschlussel, Lebenweise, Verbreitung. Die Tierwelt Deutschlands. Conchbooks, Hackenheim 73:1–327
Glöer P (2019) The freshwater gastropods of the West-Palaearctis. Volume 1. Fresh- and brakish waters except spring and subterranean snails. Identification key, anatomy, ecology, distribution. The author, Hetlingen
Hubendick B (1951) Recent Lymnaeidae. Their variation, morphology, taxonomy, nomenclature and distribution. Kungliga Svenska Vetenskapsakademiens Handlingar Fjärde Serien 3(1):1–223
Jackiewicz M (1959) Badania nad zmiennością i stanowiskiem systematycznym Galba palustris O. F Müll. Prace Komisji Biologicznej, Poznańskie Towarzystwo Przyjaciół Nauk 19(3):1–86
Jackiewicz M (1993) Phylogeny and relationships within the European species of the family Lymnaeidae. Folia Malacologica 5:61–95. https://doi.org/10.12657/folmal.005.003
Jackiewicz M (1996) Concerning Hungarian populations of “Galba palustris” studied by Kilias (1992) (Gastropoda; Pulmonata: Lymnaeidae). Malakologische Abhandlungen / Museum für Tierkunde Dresden 18:165–172
Jackiewicz M (1998) European species of the family Lymnaeidae (Gastropoda, Pulmonata, Basommatophora). Genus 9(1):1–93
Jackiewicz M, Koralewska-Batura E (1995) The shell-surface sculpture of Lymnaeidae (Gastropoda, Pulmonata, Basommatophora). Malakologische Abhandlungen / Museum für Tierkunde Dresden 17(2):191–197
Khokhutkin IM, Vinarski MV, Grebennikov ME (2009) Molluscs of the Urals and the adjacent areas. The family Lymnaeidae (Gastropoda, Pulmonata, Lymnaeiformes). Fasc. 1. Goshchitskiy Publishers, Yekaterinburg. [in Russian]
Kruglov ND (2005) Lymnaeid snails of Europe and Northern Asia. Smolensk State Pedagogical University Press, Smolensk. [in Russian]
Kruglov ND, Starobogatov YI (1981) A new genus of the Lymnaeidae and taxonomy of the subgenus Omphiscola (Lymnaea) (Pulmonata, Gastropoda). Zool Zh 60(7):965–977. [in Russian]
Kruglov ND, Starobogatov YI (1985) Methods of experimental hybridization and some results of its applications in the taxonomy of Lymnaeidae. Malacol Rev 18:21–35
Kruglov ND, Starobogatov YI (1993) Annotated and illustrated catalogue of species of the family Lymnaeidae (Gastropoda Pulmonata Lymnaeiformes) of Palaearctic and adjacent river drainage areas. Part I Ruthеnica Russian Malacol J 3(1):65–92
Lakowitz T, Brönmark C, Nyström P (2008) Tuning in to multiple predators: conflicting demands for shell morphology in a freshwater snail. Freshw Biol 53:2184–2191. https://doi.org/10.1111/j.1365-2427.2008.02045.x
Lam PKS, Calow P (1988) Differences in the shell shape of Lymnaea peregra (Müller) (Gastropoda: Pulmonata) from lotic and lentic habitats; environmental or genetic variance? J Mollus Stud 54:197–207. https://doi.org/10.1093/mollus/54.2.197
Lazareva AI (1967) On the systematics of freshwater snails of Kazakhstan from the group Lymnaea palustris Müller (Gastropoda, Pulmonata). Zool Zh 46(9):1340–1349. [in Russian]
Paraense WL (1976) Lymnaea viatrix: a study of topotypic specimens (Mollusca: Lymnaeidae). Rev Bras Biol 36(2):419–428
Paraense WL (1982) Lymnaea rupestris sp. n. from southern Brazil (Pulmonata: Lymnaeidae). Mem I Oswaldo Cruz 77(4):437–443. https://doi.org/10.1590/S0074-02761982000400011
Paraense WL (1983) Lymnaea columella in Northern Brazil. Mem I Oswaldo Cruz 78(4):477–482. https://doi.org/10.1590/S0074-02761983000400011
Paraense WL (1984) Lymnaea diaphana: a study of topotypic specimens (Pulmonata: Lymnaeidae). Mem I Oswaldo Cruz 79:75–81. https://doi.org/10.1590/S0074-02761984000100009
Paraense WL (1994) A Mexican population of Lymnaea elodes (Gastropoda: Lymnaeidae). Malacol Rev 27:5–11
Paraense WL (1995) Lymnaea cousini Jousseaume, 1887, from Ecuador (Gastropoda: Lymnaeidae). Mem I Oswaldo Cruz 90(5):605–609. https://doi.org/10.1590/S0074-02761995000500011
Piechocki A, Wawrzyniak-Wydrowska B (2016) Guide to freshwater and marine Mollusca of Poland. Bogucki Wydawnictwo Naukowe, Poznań
Pieńkowska JR, Rybska E, Banasiak J et al (2015) Taxonomic status of Stagnicola palustris (O.F. Müller, 1774) and S. turricula (Held, 1836) (Gastropoda: Pulmonata: Lymnaeidae) in view of new molecular and chorological data. Folia Malacologica 23(1):3–18. https://doi.org/10.12657/folmal.023.003
Pointier JP (ed) (2015) Freshwater molluscs of Venezuela and their medical and veterinary importance. Conchbooks, Harxheim
Pointier JP, Vàzquez AA (2020) Lymnaeoidea, Lymnaeidae. In: Damborenea C, Rogers DC, Thorp JH (eds) Thorp and Covich’s freshwater invertebrates, Volume 5: Keys to Neotropical and Antarctic Fauna. Academic Press, London, pp 281–290
Roszkowski W (1914a) Contribution à l’étude des Limnées du lac Léman. Rev Suisse Zool 22(15):457–539
Roszkowski W (1914b) Note sur l’appareil génital de Limnaea auricularia L. et de Limnaea ovata Drap. Zool Anz 44(4):175–179
Roszkowski W (1922) Przyczynki do poznania rodziny Lymnaeidae. VI. Aparat płciowy Galba truncatula (Müll.). Archivum Nauk Biologicznych Towarzystwa Naukowego Warszawskiego 1(5):1–6
Roszkowski W (1926) Contributions to the study of the family Lymnaeidae. VII. The structure of prostate of the Lymnaeidae. Annales Zoologici Musei Polonici Historiae Naturalis 5(1):1–14
Samadi S, Roumégoux A, Bargues MD et al (2000) Morphological studies of lymnaeid snails from the human fascioliasis endemic zone of Bolivia. J Mol Stud 66:31–44. https://doi.org/10.1093/mollus/66.1.31
Schniebs K, Glöer P, Vinarski M et al (2011) Intraspecific mophological and genetic variability in Radix balthica (Linnaeus 1758) (Gastropoda: Basommatophora: Lymnaeidae) with morphological comparison to other European Radix species. J Conchol 40(6):657–678
Schniebs K, Glöer P, Vinarski M et al (2013) Intraspecific morphological and genetic variability in the European freshwater snail Radix labiata (Rossmaessler, 1835) (Gastropoda: Basommatophora: Lymnaeidae). Contrib Zool 82(1):55–68. https://doi.org/10.1163/18759866-08201004
Schniebs K, Glöer P, Vinarski M et al (2016) A barcode pitfall in Palaearctic Stagnicola specimens (Mollusca: Lymnaeidae): incongruence of mitochondrial genes, a nuclear marker and morphology. North West J Zool 12(2):239–254
Schniebs K, Glöer P, Vinarski M et al (2019) Intraspecific morphological and genetic variability in the Palaearctic freshwater snail Radix ampla (Hartmann, 1821) (Gastropoda: Basommatophora: Lymnaeidae). J Conchol 43(3):245–267
Stadnichenko AP (2004) Pond snails and limpet snails (Lymnaeidae and Acroloxidae) of Ukraine. Tsentr uchebnoi literatury, Kiev. [in Russian]
Standley CJ, Prepelitchi L, Pietrokovsky SM (2013) Molecular characterization of cryptic and sympatric lymnaeid species from the Galba/Fossaria group in Mendoza Province, Northern Patagonia, Argentina. Parasit Vectors 6:304. http://www.parasitesandvectors.com/content/6/1/304
Starobogatov YI (1976) The system and phylogeny of the Lymnaeidae. Problemy Zoologii. Zoologicheskiy Institut AN SSSR: 79–81 [in Russian]
Vinarski MV (2009) Geographical variability in the male genitalia in two stagnicoline species (Gastropoda: Pulmonata: Lymnaeidae). Mollusca (Dresden) 27(2):157–166
Vinarski MV (2011a) A new species of stagnicoline snails (Mollusca: Gastropoda: Lymnaeidae) from the extreme north of Western Siberia. Zootaxa 2817:55–58. https://doi.org/10.11646/zootaxa.2817.1.2
Vinarski MV (2011b) The “index of the copulatory apparatus” and its application to the systematics of freshwater pulmonates (Mollusca: Gastropoda: Pulmonata). Zoosystematica Rossica 20(1):11–27
Vinarski MV (2012) Geographical variability in freshwater mollusks. Biol Bull Rev 2:390–399. https://doi.org/10.1134/S2079086412050088
Vinarski MV (2013) The variation in freshwater pulmonate mollusks (the taxonomic aspect). Omsk State Pedagogical University Press, Omsk. [In Russian]
Vinarski MV (2014) Lymnaea likharevi Lazareva, 1967 is a junior synonym of Lymnaea saridalensis Mozley, 1934 (Gastropoda: Pulmonata: Lymnaeidae). Ruthenica. Russian Malacol J 24(1):35–44
Vinarski MV (2019) 11. Class Gastropoda. In: Rogers DC, Thorp JH (eds) Thorp and Covich’s freshwater invertebrates, vol IV. Keys to Palaearctic Fauna. Academic Press, London, pp 310–345
Vinarski MV, Pointier JP (2023, this volume) General characteristics of the family Lymnaeidae. In: Vinarski MV, Vàzquez AA (eds) The Lymnaeidae: a handbook. Springer, Cham, pp 25–66
Vinarski MV, Schniebs K, Glöer P, Hundsdoerfer A (2011) The taxonomic status and phylogenetic relationships of the genus Aenigmomphiscola Kruglov and Starobogatov, 1981 (Gastropoda: Pulmonata: Lymnaeidae). J Nat Hist 45(33–34):2049–2068. https://doi.org/10.1080/00222933.2011.574800
Vinarski MV, Aksenova OV, Bolotov IN (2020) Taxonomic assessment of genetically-delineated species of radicine snails (Mollusca, Gastropoda, Lymnaeidae). Zoosyst Evol 96(2):577–608. https://doi.org/10.3897/zse.96.52860
Whelan N (2021) Phenotypic plasticity and the endless forms of freshwater gastropod shells. Freshw Mollusk Biol Conserv 24(2):87–103. https://doi.org/10.31931/fmbc-d-20-00015
Acknowledgments
MVV thanks the Russian Scientific Fund for financial support of his research on the lymnaeid taxonomy and phylogeny (project No. 19-14-00066-P).
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Vinarski, M.V., Pointier, JP. (2023). Conchological and Anatomical Identification of the Lymnaeid Snails. In: Vinarski, M.V., Vázquez, A.A. (eds) The Lymnaeidae. Zoological Monographs, vol 7. Springer, Cham. https://doi.org/10.1007/978-3-031-30292-3_4
Download citation
DOI: https://doi.org/10.1007/978-3-031-30292-3_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-30291-6
Online ISBN: 978-3-031-30292-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)