Abstract
Scolecophidia (Squamata, Serpentes), commonly known as blindsnakes, wormsnakes or threadsnakes comprises a group of small snakes, with relatively few systematic and morphological studies when compared to Alethinophidia. Since the external morphology is very conserved amongst scolecophidians, internal morphological studies—such as the hemipenial morphology—are useful to unravel several systematic issues within the group. We aimed to describe the hemipenial morphology of Epictia vellardi (Epictinae, Leptotyphlopidae) based on 16 organs belonging to eight specimens. The organ is unilobed, with the body conspicuously narrower than the base and with the apex slightly expanded, without any macroscopic ornamentation. The comparison of the hemipenial morphology of E. vellardi with other hemipenes of Epictia spp. allowed us to identify two general morphological patterns for the genus, which are proposed and discussed in detail in the present study. The results found herein may be extremely relevant for future hemipenial descriptions. In addition, independent characters found in Types I and II (such as ornamentation, shape of base, body and apex) should be regarded for future systematic and evolutionary morphology studies within Leptotyphlopidae, in addition to assisting in the taxonomy and identification of species of the genus.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Scolecophidia (sensu Miralles et al. 2018), commonly known as blindsnakes, wormsnakes or threadsnakes, consists of a group of snakes with relatively few studies (Francisco et al. 2018) regarding their evolution and natural history when compared to Alethinophidian snakes and, therefore, several issues related to its systematics still remain (Mezzasalma et al. 2016). This taxon currently comprises about 440 species (Uetz et al. 2020) allocated in the family Leptotyphlopidae (approx. 150 species) and in the superfamily Typhlopoidea (approx. 290 species) (Uetz et al. 2020).
Morphological characters are so far the most common source of information applied as diagnostic features for taxa within Leptotyphlopidae (Francisco et al. 2018). However, the reduced size of individuals hampers the conduction of internal morphological studies, since most of those techniques demand invasive procedures to assess anatomical data. In addition, due to their fossorial habit, the representativeness of these snakes in scientific collections is extremely low, with many species currently known exclusively from their holotype or type series (e.g., Epictia rioignis, Koch et al. 2019), adding even more limitations to the conduction of morphological studies that demand destructive approaches.
Considering that scolecophidians exhibit a very conserved external morphology (Martins et al. 2019a), the use of morphological data—such as hemipenial morphology—is particularly useful in the delimitation of species (Arnold 1986; Dowling and Savage 1960) and the distinction of cryptic species (Arnold 1986; Eberhard 1985, 2009; Myers and McDowell 2014; Passos et al. 2018). However, despite studies on hemipenial morphology being widely used in snakes in general, descriptive works on hemipenial morphology in Leptotyphlopidae are still scarce (Martins et al. 2019a).
Representatives of Leptotyphlopidae are distributed in Africa and in the Neotropics (South America, Central America and the Antilles), with a smaller number of species occurring in the south of North America, in Arabia and southwest of Asia (Adalsteinsson et al. 2009; Martins et al. 2019a). Despite its wide distribution in the world, little is known about its systematics and ecology, even though they occur in a variety of habitats such as deserts, forests, swamps, savannas and modified habitats (Adalsteinsson et al. 2009; Martins et al. 2019a). This family is divided into two subfamilies: Leptotyphlopinae, with approximately 50 species (Uetz et al. 2020), and Epictinae, with approximately 90 species (Uetz et al. 2020). The subfamily Leptotyphlopinae is composed of four genera: Epacrophis, Leptotyphlops, Myriopholis and Namibiana; while Epictinae is composed of ten genera: Epictia, Mitophis, Rena, Rhinoguinea, Rhinoleptus, Siagonodon, Tetracheilostoma, Tricheilostoma, Trilepida and Habrophallos. The genus Epictia currently represents the most diverse among the Leptotyphlopidae family (Martins et al. 2019a), with 45 currently known species that occur in Mexico and throughout Central and South America (Martins et al. 2019a; Wallach 2016; Uetz et al. 2020).
Many of the hemipenial morphological studies available for Leptotyphlopidae focus on the New World (NW) taxa (e.g., Bailey and Carvalho 1946; Cei 1993; Fabrezi et al. 1985; Orejas-Miranda 1962; Passos et al. 2005; 2006; Peters and Orejas-Miranda 1970; Pinto et al. 2010; Pinto and Curcio 2011; Pinto and Fernandes 2012; Savage 2002; Scrocchi 1990; Thomas 1975), with even more limited contributions for the leptotyphlopids of the Old World (OW) (e.g., Branch 1986). Almost all the works currently available are focused on the genera Trilepida (NW) and Epictia (NW), with few works available for the genera Rena (NW) and Leptotyphlops (OW); no descriptive works on the hemipenial morphology are available for the genera Tetracheilostoma (NW), Tricheilostoma (OW), Rhinoleptus (OW) and Siagonodon (NW)—in Epictinae—, and Epacrophis (OW), Myriopholis (OW) and Namibiana (OW)—in Leptotyphlopinae (Adalsteinsson et al. 2009; Martins et al. 2019a; Uetz et al. 2020). However, although there are a few available studies on the hemipenial morphology of representatives of Epictia, they are still punctual and scarce to allow an understanding of the dimension and morphological variety of this genus, with only 13 species having their hemipenial morphology described: E. bakewelli Oliver 1937, E. striatula (Smith and Laufe 1945), E. diaplocia (Orejas-Miranda 1969), E. australis (Freiberg and Orejas-Miranda 1968), E. wynni Wallach (2016), E. tenella Klauber 1939, E. resetari Wallach (2016), E. pauldwyeri Wallach (2016), E. martinezi Wallach (2016), E. magnamaculata Taylor 1940, E. albipuncta (Burmeister 1861), E. munoai (Orejas-Miranda 1961) and E. ater Taylor 1940.
Given the insufficiency on the knowledge of threadsnakes morphology, herein we aim to describe in detail the hemipenis of Epictia vellardi (Laurent 1984). This species is currently distributed in Paraguay, Argentina (in Formosa), and Brazil (in Corumbá, in the State of Mato Grosso do Sul; and in the State of Mato Grosso) (Cabral et al. 2016; Costa and Bérnils 2018). We also provide a comprehensive discussion of the hemipenial morphology within the genus Epictia, with the definition of two patterns of hemipenial morphology within this taxon.
Materials and methods
The hemipenes of Epictia vellardi were described based on specimens from Reserva Particular do Patrimônio Natural Acurizal (Serra do Amolar, Corumbá, Mato Grosso, Brazil) housed at Coleção de Répteis of the Universidade Federal do Mato Grosso (UFMT). The morphology of the hemipenes was analyzed in situ from 16 organs belonging to eight specimens. The hemipenial terminology for description followed Dowling and Savage (1960), with modifications by Myers and Campbell (1981) and Branch (1986). Photographs were made with a Leica M205C stereomicroscope microscope, with an attached camera. The photos taken in various focal planes were combined in multifocal images with Leica Application Suite software version 3.6.0. Schematic drawings to illustrations of hemipenial general shape were conducted in Inkscape 1.0. The literature data on the hemipenial morphology of the genus Epictia are based on the citations given in the “Discussion”.
The identification of specimens was based on the species original description (Laurent 1984) and Francisco et al. (2012). We considered Wallach (2016) for species allocation in the following groups: E. gr. albifrons, E. gr. goudotii and E. gr. phenops.
Results
Our results are based on the examination of 2 organs fully everted and partially expanded (UFMT1343) and 14 organs partially everted and partially expanded (UFMT1297; UFMT1301; UFMT1348; UFMT1351; UFMT1355; UFMT1357; UFMT1502).
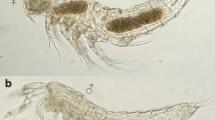
UFMT1343 (examined in situ, fully everted, partially expanded; Fig. 1): the organ is unilobed, approximately 2.6 mm in length from the base to the apex, with no macroscopic ornamentation. It has a wide base, narrowing along its body. The apex is spherical and wider than the body, being differentiated from the body by a conspicuous narrowing in the organ’s upper third. The spermatic groove has raised edges and exhibits a small lateralization in the region adjacent to the cloaca in a counterclockwise direction, but at the base it follows a straight route to the apex.
We found no intraspecific variation in the organ base or body. We were not able to evaluate such variation in the apex given most organs were partially everted, and therefore had their apex inverted.
Discussion
Due to the relatively few studies regarding the scolecophidian systematics, several uncertainties of their representatives’ relationship hypotheses still remain. Even though morphological characters are the most common source of information used to diagnose the Leptotyphlopidae taxa (Francisco et al. 2018), the majority of the systematic studies currently available for such group are based on molecular data (e.g., Adalsteinsson et al. 2009, Pyron and Wallach 2014, and Nagy et al. 2015), with most of the morphological data not yet phylogenetically tested. However, morphological data such as gland morphology (e.g., Martins et al. 2018), skull morphology (e.g., Salazar-Valenzuela et al. 2015; Koch et al. 2019; Martins et al. 2019a), head and neck myology (e.g., Martins et al. 2019b), visceral anatomy (e.g., Broadley and Wallach 2007a, b; Wallach 2016) and hemipenial morphology (e.g., Passos et al. 2006; Graboski et al. 2018; Martins et al. 2019a) have proven to be useful for the scolecophidian systematics.
The lack of studies related to the hemipenial morphology of Scolecophidia is mainly due to two factors: scarcity of specimens available in scientific collections and great difficulty in eversion of the hemipenis in situ, due to its small size and tissue fragility (Martins et al. 2019a; Pesantes 1994). Therefore, most available studies regarding the threadsnakes (Leptotyphlopidae) hemipenial morphology are based on organs previously everted (Martins et al. 2019a) or partially everted upon fixation.
The importance of such characters for diagnosing threadsnakes has already been previously highlighted in the literature. Passos et al. (2006) have suggested that hemipenial characters might be synapomorphic for threadsnakes of the genus Trilepida, and Martins et al. (2019a) have also suggested that the combination of a few hemipenial characters might be exclusive to the genus Habrophallos. Even though such characters have never been phylogenetically tested, the hemipenial morphology of scolecophidians allows us to unravel systematic issues within such group. Although such characters have been widely explored within snakes, those studies are still extremely underexplored within scolecophidians.
The results found herein are in accordance with the basic pattern of hemipenial morphology within scolecophidians, with organs being single and bearing an undivided sulcus spermaticus (Branch 1986; Wallach 1998 Unpubl. Thesis; Passos et al. 2006; Graboski et al. 2018; Martins et al. 2019a). Considering the hemipenis of Epictia vellardi and other Epictia spp., current available papers related to the hemipenial morphology of representatives of Epictia seem to indicate the presence of two morphological patterns within this taxon (Fig. 2): (1) the first pattern (here called Type I) relates to an organ devoid of or has little ornamentation, with a conspicuously broad base and an evident constriction towards the body and apex (Fig. 2a, b); (2) the second pattern (here called Type II) includes organs without a clear distinction between the width of the body and the apex, with the base being the same width or slightly narrower than the body and apex, commonly presenting conspicuous and evident macro-ornamentations (such as flounces, calyces and papillae) in the base and/or body (Fig. 2c, d). Although the shape of the hemipenes may vary due to preparation artifacts (Passos et al. 2016; Zaher and Prudente 2003), we believe that the approximate shapes proposed herein reflect the actual morphology and the patterns for Epictia spp.
Schematic drawing of everted hemipenes of Epictia spp., illustrating the morphological variation between the Type I and Type II patterns. a E. vellardi, Type I, based on original hemipenis description from the present study; b E. resetari, Type I, based on Fig. 3 from Peters and Orejas-Miranda (1970); c E. ater, Type II, based on Fig. 15G from Wallach (2016), with evident flounces at the apex; d E. martinezi, Type II, based on Fig. 15 M from Wallach (2016), with evident calyces at the apex
Available data on hemipenial morphology of Epictia spp. indicate that E. australis (Martins et al. 2019a; Scrocchi 1990), E. magnamaculata (Peters and Orejas-Miranda 1970; Wallach 2016), E. munoai (Branch 1986; Orejas-Miranda 1962), E. pauldwyeri (Wallach 2016), E. resetari (Peters and Orejas-Miranda 1970; Wallach 2016), E. wynni (Wallach 2016), E. tenella (Bailey and Carvalho 1946; Martins et al. 2019a) and E. vellardi (present study) exhibit a Type I hemipenis, while E. ater (Martins et al. 2019a; Savage 2002; Wallach 2016), E. diaplocia (Martins et al. 2019a; Thomas 1975), E. martinezi (Wallach 2016) and E. striatula (Martins et al. 2019a; Peters and Orejas-Miranda 1970) have a Type II hemipenis (Table 1).
Four out of eight species that have a Type I hemipenis are currently allocated in the Epictia gr. phenops and four in the Epictia gr. albifrons; while two out of four Type II species are in the Epictia gr. phenops and two in the Epictia gr. albifrons (Wallach 2016) (Table 1), indicating that both patterns are found in both phenotypic groups known for the genus Epictia. Although hemipenial characters are important to elucidate systematic questions, many of the species for which there is hemipenial description available are not included in recent phylogenetic hypotheses (e.g., Adalsteinsson et al. 2009; Pyron and Wallach 2014; Martins et al. 2019a; McCranie and Hedges 2016; Murphy et al. 2016; Nagy et al. 2015). A larger sample of these taxa in molecular and combined analyzes, as well as evolutionary studies, are essential to elucidate the issues related to hemipenial evolution in this group.
During the literature review carried out in this study, we identified some challenges that hindered the correct allocation of some hemipenes in Type I or Type II patterns. Considering E. magnamaculata, the first descriptive work on its hemipenial morphology was held by Peters and Orejas-Miranda (1970). In this study, two specimens (AMNH 103852 and AMNH 103864) from Providence Island (Colombia) were analyzed, with their hemipenis having a broader and more rounded basal part, followed by an elongated naked body. In the next quarter of the body, calyces are present, which proximally are larger and gradually reduce their size distally. The distal half is gradually larger in diameter, with the apex wider than the hemipenis body. However, this same species was analyzed by Wallach (2016), who described the organ of one specimen (FMNH 282651) from Roatán Island (Honduras). In this study, the hemipenis has a gradual narrowing towards the apex and with a spermatic groove without ornamentation, except for the presence of some papillae at the distal tip. A second specimen described by Wallach (2016), based on a partially everted organ (LACM 127623), presents an error of location, since in the appendix of material examined and in the caption of the hemipenis image (Fig. 15 of the mentioned article) the location mentioned is Cozumel Island (Mexico), but in the description of the hemipenis is actually from Guanaja Island (Honduras). In addition, Wallach (2016) accidentally switches the description of the hemipenes with their respective images (Fig. 15, I and J), considering that the image I presents the partially everted hemipenis and the image J presents the hemipenis completely everted. It is noticeable that both descriptions (Peters and Orejas-Miranda 1970; Wallach 2016) are extremely divergent, and this may indicate that one of these specimens was mistakenly identified, or may even point to the presence of a cryptic species within what it is currently classified as E. magnamaculata. To reach a conclusion, it would be necessary to analyze the specimen examined by Peters and Orejas-Miranda (1970), and, since we do not have access to these specimens, we take into consideration the description made by Wallach (2016) to classify the morphological pattern of the hemipenis of E. magnamaculata.
It was not possible to identify the type of hemipenis of E. bakewelli considering that its description available in Wallach (2016) was made exclusively from a specimen with partially everted hemipenis. Thus, as we do not have the description of the body and the apex of the organ, the species could not be associated with either morphological patterns. Similarly, the hemipenis of E. albipuncta could be associated with neither Types I nor II, since the organs used for description in Fabrezi (1985) were probably partially everted hemipenes. What the authors consider “terminal disk”—a flat structure at the apex of the hemipenis with a depressed central part—presumably represents the apical region partially everted of the organ. Consequently, until there are new descriptions of the organ, we cannot allocate the species in any of the types proposed herein.
Both propositions (Type I and Type II) reflect attempts to accommodate general hemipenial patterns within the genus Epictia. We must highlight that many Epictia spp. show modified patterns, as follows. Among the species classified as Type I, we could identify the following exceptions concerning the apex and ornamentation: E. tenella has its apex laterally expanded and dorsoventrally flattened (Bailey and Carvalho 1946); E. munoai does not have any macroscopic ornamentation, but has microscopic calyces and spinules in the medial portion of the hemipenis (Branch 1986; Orejas-Miranda 1962); E. resetari has spines and spinules in the distal half of the hemipenis terminal portion, which gradually decrease towards its most expanded apex (Peters and Orejas-Miranda 1970; Wallach 2016); E. pauldwyeri presents in its proximal portion a dozen scattered papillae (Wallach 2016); E. wynni holds a series of rugae and papillae on the distal portion of its body, the proximal portion has seven circular folds with transverse rugae, and the distal portion is covered by six columns of papillae arranged in five transverse rows (Wallach 2016).
Considering the available biogeographic hypotheses (i.e., Adalsteinsson et al. 2009) and the available phylogenetic trees that include Epictia spp. (Adalsteinsson et al. 2009; Martins et al. 2019a; McCranie and Hedges 2016; Murphy et al. 2016; Wallach 2016), Types I and II do not seem to be geographically or phylogenetically cohesive or exclusive. Thus, future broader phylogenetic and biogeographic analyses must consider the phenotypic variation seen in Epictia spp. In addition, the recognition of both patterns—and their variation—might be a first step into understanding the hemipenial evolution within this genus, as well as putative synapomorphies based on the organ. Recent molecular studies (Martins et al. 2019a) have recovered Epictia as the sister group of Siagonodon + Habrophallos. Even though the hemipenis of Siagonodon spp. have never been described, the suggested exclusive and diagnostic characters of the hemipenis of Habrophallos seem to be, in fact, synapomorphic. However, the absence of conspicuous macro-ornamentation (such as observed in Type I) seem to be a more typical pattern found in Epictini (sensu Martins et al. 2019a), as Habrophallos, Mitophis, Trilepida and Rena exhibit hemipenis with no evident macro-ornamentation as Type II Epictia spp. Therefore, such hemipenial types might be derived amongst Epictinae, and exclusive to a few taxa. However, this assumption still needs to be further elucidated with the description of additional organs/species.
Given the discussion above, one should also acknowledge that as such morphological types do not seem to be biogeographically or even phylogenetically cohesive, the wide diversity of hemipenial morphological characters may represent a mosaic of characters that evolved independently. The difference in shapes of the base, body and apex of hemipenis (e.g., widened base and narrow apex), the presence or absence of ornamentation (e.g., spines, papillae, calyces and flounces), the orientation of the hemipenis and of the sulcus, among others, should be analyzed independently.
This hypothesis should be tested in the future, considering available data for the taxa in relation to phylogenetic hypotheses available for the genus, also providing valuable data on the ancestral states of those putatively independent characters. Such efforts yet must reckon on additional hemipenial descriptions of Epictia spp. (and other Leptotyphlopidae) as well as the refinement of phylogenetic hypotheses for species of Leptotyphlopidae, and with the addition of more terminals for the genus. So far, we must conjecture that considering available data on the literature and the observation of previous phylogenetic hypotheses (e.g., Martins et al. 2019a), an ancestral for Epictia might have had a narrow and not ornamented base as exhibited in Type I.
In summary, the description of the E. vellardi hemipenis and the establishment of two hemipenial morphological patterns within the Epictia genus may aid in the taxonomy and identification of species pertaining to this genus. In fact, given the relatively conservative external morphology among the representatives of Scolecophidia, data on such organ are extremely relevant to clarify the systematics of Leptotyphlopidae and their representatives, and should be regarded in future integrative phylogenetic studies, as well as comparative phylogenetic methods.
References
Adalsteinsson SA, Branch WR, Trape S, Vitt LJ, Hedges SB (2009) Molecular phylogeny, classification, and biogeography of snakes of the family Leptotyphlopidae (Reptilia, Squamata). Zootaxa 2244:1–50
Arnold EN (1986) Why copulatory organs provide so many useful taxonomic characters: the origin and maintenance of hemipenial differences in lacertid lizards (Reptilia: Lacertidae). Zool J Linn Soci 29:263–281
Bailey JR, Carvalho AL (1946) A new Leptotyphlops from Mato Grosso, with notes on Leptotyphlopstenella Klauber. Boletim do Museu Nacional, Nova série, Zool 52:1–7
Branch WR (1986) Hemipenial morphology of African snakes: a taxonomic review. Part 1. Scolecophidia and Boidae. Journal of Herpetology 20:285–299
Broadley DG, Wallach V (2007a) A revision of the genus Leptotyphlops in northeastern Africa and southwestern Arabia (Serpentes: Leptotyphlopidae). Zootaxa 1(408):1–78
Broadley DG, Wallach V (2007b) A review of East and Central African species of LetheobiaCope, revived from the synonymy of Rhinotyphlops Fitzinger, with descriptions of five new species (Serpentes: Typhlopidae). Zootaxa 1(515):31–68
Cabral H, Netto F (2016) Epictia vellardi. Geogr Distrib Herpetol Rev 47(1):83
Cei JM (1993) Reptiles del noroeste, nordeste y este de la Argentina. Herpetofauna de las selvas subtropicales, Puna y Pampas. Museo Regionale di Scienze Naturali, Torino 14:1–94
Costa HC, Bérnils RS (2018) Répteis do Brasil e suas Unidades Federativas: lista de espécies. Herpetologia Brasileira 7:11–57
Dowling HG, Savage JM (1960) A guide to the snake hemipenis: a survey of basic structure and systematic characteristics. Zoologica 45:17–28
Eberhard WG (1985) Sexual selection and animal genitalia. Harvard University Press, Cambridge, UK
Eberhard WG (2009) Evolution of genitalia: theories, evidence, and new directions. Genetica 138:5–18
Fabrezi M, Marcus A, Scrocchi G (1985) Contribución al conocimiento de los Leptotyphlopidae de Argentina. I. Leptotyphlopsweyrauchi y Leptotyphlopsalbipuncta. Cuadernos de Herpetología 1:1–20
Francisco BCS, Pinto RR, Fernandes DS (2012) Taxonomy of Epictiamunoai (Orejas-Miranda, 1961) (Squamata: Serpentes: Leptotyphlopidae). Zootaxa 3512:42–52
Francisco BC, Pinto RP, Fernandes DS (2018) Taxonomic notes on the Genus SiagonodonPeters, 1881, with a report on morphological variation in Siagonodoncupinensis (Bailey and Carvalho, 1946) (Serpentes: Leptotyphlopidae). Copeia 106:321–328
Graboski R, Arredondo JC, Grazziotin FG, da Silva AAA, Prudente ALC, Rodrigues MT, Bonatto SL, Zaher H (2018) Molecular phylogeny and hemipenial diversity of South American species of Amerotyphlops (Typhlopidae, Scolecophidia). Zoolog Scr. https://doi.org/10.1111/zsc.12334
Koch C, Martins A, Schweiger S (2019) A century of waiting: description of a new EpictiaGray, 1845 (Serpentes: Leptotyphlopidae) based on specimens housed for more than 100 years in the collection of the Natural History Museum Vienna (NMW). PeerJ 7:e7411. https://doi.org/10.7717/peerj.7411
Laurent RF (1984) El genero Leptotyphlops en la coleccion de la Fundacion Miguel Lillo. Acta Zool Lilloana 38(1):29–34
Martins AR, Passos P, Pinto RR (2018) Unveiling diversity under the skin: comparative morphology study of the cephalic glands in threadsnakes (Serpentes: Leptotyphlopidae: Epictinae). Zoomorphology 137:433–443
Martins A, Koch C, Pinto R, Folly M, Fouquet A, Passos P (2019) From the inside out: discovery of a new genus of threadsnakes based on anatomical and molecular data, with discussion of the leptotyphlopid hemipenial morphology. J Zool Syst Evol Res 57:840–863
Martins A, Passos P, Pinto R (2019) Moving beyond the surface: Comparative head and neck myology of threadsnakes (Epictinae, Leptotyphlopidae, Serpentes), with comments on the ‘scolecophidian’ muscular system. PLoS ONE 14(7):e0219661. https://doi.org/10.1371/journal.pone.0219661
McCranie JR, Hedges SB (2016) Molecular phylogeny and taxonomy of the Epictiagoudotii Species complex (Serpentes: Leptotyphlopidae: Epictinae) in Middle America and northern South America. PeerJ 4:e1551. https://doi.org/10.7717/peerj.1551
Mezzasalma M, Andreone F, Glaw F, Petraccioli A, Odierna G, Guarino FM (2016) A karyological study of three typhlopid species with some inferences on chromosome evolution in blindsnakes (Scolecophidia). Zoologischer Anzeiger: A J Comp Zool 264:34–40
Miralles A, Marin J, Markus D, Herrel A, Hedges SB, Vidal N (2018) Molecular evidence for the parapyly of Scolecophidia and its evolutionary implications. J Evol Biol 31:1782–1793
Murphy JC, Rutherford MG, Jowers MJ (2016) The threadsnake tangle: lack of genetic divergence in Epictiatenella (Squamata, Leptotyphlopidae): evidence for introductions or recent rafting to the West Indies. Stud Neotrop Fauna Environ 51:197–205
Myers CW, Campbell JA (1981) A new genus and species of colubrid snake from Sierra Madre del Sul of Guerrero, Mexico. Am Museum Novit 2708:1–20
Myers CW, McDowell SB (2014) New taxa and cryptic species of Neotropical snakes (Xenodontinae), with commentary on hemipenes as generic and specific characters. Bullet Am Museum Nat Hist 385:1–112
Nagy ZT, Marion AB, Glaw F, Miralles A, Nopper J, Vences M, Hedges B (2015) Molecular systematics and undescribed diversity of Madagascan scolecophidian snakes (Squamata: Serpentes). Zootaxa 4040(1):031–047
Orejas-Miranda BR (1962) Descripcion del hemipenis de Leptotyphlopsmunoai Orejas-Miranda, 1961. Comunicaciones Zool del Museo de Hist Nat de Montevideo 7(97):1–9
Passos P, Martins A (2016) Pinto-Coelho D (2016) population morphological variation and natural history of Atractus potschi (Serpentes: Dipsadidae) in Northeastern Brazil. South Am J Herpetol 11(3):188–211
Passos P, Caramaschi U, Pinto R (2005) Rediscovery and redescription of Leptotyphlops salgueiroi Amaral, 1954 (Squamata, Serpentes, Leptotyphlopidae). Boletim do Museu Nacional 520:1–10
Passos P, Caramaschi U, Pinto RR (2006) Redescription of Leptotyphlops koppesi Amaral, 1954, and description of a new species of the Leptotyphlops dulcis group from Central Brazil (Serpentes: Leptotyphlopidae). Amphibia-Repitilia 27:347–357
Passos P, Prudente ALC, Ramos LO, Caicedo-Portilla JR, Lynch J (2018) Species delimitation in the Atractus collaris complex. Zootaxa 4392(3):491–520
Pesantes OS (1994) A method for preparing the hemipenis of preserved snakes. J Herpetol, Oxford: Soci Study Amphib Reptiles 28(1):93–95
Peters JA, Orejas-Miranda BR (1970) Notes on the hemipenis of several taxa in the family Leptotyphlopidae. Herpetologica 26(3):320–324
Pinto RR (2011) Curcio F (2011) On the generic identity of Siagonodon brasiliensis, with the description of a new leptotyphlopid from Central Brazil (Serpentes: Leptotyphlopidae). Copeia 1:53–63
Pinto RR (2012) Fernandes R (2012) A new blind snake species of the genus Tricheilostoma from Espinhaço Range, Brazil and taxonomic status of Rena dimidiata (Jan 1861) (Serpentes: Epictinae: Leptotyphlopidae). Copeia 1:37–48
Pinto RR, Passos P, Portilla JRC, Arredondo JC, Fernandes R (2010) Taxonomy of the threadsnakes of the tribe Epictini (Squamata: Serpentes: Leptotyphlopidae) in Colombia. Zootaxa 2724:1–28
Pyron RA, Wallach V (2014) Systematics of the blindsnakes (Serpentes: Scolecophidia: Typhlopoidea) based on molecular and morphological evidence. Zootaxa 3829: 001–081. Seymour, R. S. (1987). Scaling of cardiovascular physiology in snakes. Am Zool 27:97–109
Salazar-Valenzuela D, Martins A, Amador-Oyola L, Torres-Carvajal O (2015) A new species and country record of threadsnakes. Amphib Reptile Conserv 8(1(special section)):107–120(e89)
Savage JM (2002) The amphibians and reptiles of costa rica: a Herpetofauna between two continents, between two seas. The University of Chicago Press, Chicago, Illinois, United States
Scrocchi G (1990) Contribución al conocimiento de los Leptotyphlopidae de Argentina. II: Nuevos datos sobre Leptotyphlops australis Freiberg y Orejas-Miranda, 1968. Acta Zoológica Lilloana 39:113–114
Thomas R (1975) The hemipenis of Leptotyphlops tenella Klauber (Serpentes: Leptotyphlopidae) and a new distributional record. Journal of Herpetology 9:250–252
Uetz P, Freed P, Hošek J (2020) The Reptile Database. https://www.reptile-database.org. Accessed 2 June 2020
Wallach V (2016) Morphological review and taxonomic status of the Epictia phenops species group of Mesoamerica, with description of six new species and discussion of South American Epictia albifrons, E. goudotii, and E. tenella (Serpentes: Leptotyphlopidae: Epictinae). Mesoam Herpetol 3:215–374
Zaher H, Prudente ALC (2003) Hemipenes of Siphlophis (Serpentes, Xenodontinae) and techniques of hemipenial preparation in snakes: a response to Dowling. Herpetological Review 34(4):302–307
Acknowledgements
We are thankful to F. Curcio (UFMT) for allowing us to examine specimens and to Professor J.R.P. Pujol, from the Department of Zoology of the Instituto de Ciências Biológicas (UnB), for allowing us to use the Leica M205C stereomicroscope microscope available to photograph and analyze specimens. A.C. Ferreira thanks Programa de Iniciação Científica of the Universidade de Brasília (ProIC/UnB) and Fundação de Apoio à Pesquisa do Distrito Federal (FAPDF) for the scholarship granted for this project.
Funding
Ana Carolina Ferreira was supported by Fundação de Apoio à Pesquisa do Distrito Federal (00193.00002054/2018-91).
Author information
Authors and Affiliations
Contributions
ACF has contributed in the study conceptualization, data collection, formal analysis, methodology, project administration and manuscript writing. AM contributed in conceptualization, data collection, formal analysis, methodology, project administration, supervision and manuscript revision. JK contributed with conceptualization, methodology, project administration, supervision and manuscript revision.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest or competing interests.
Ethics approval
This study did not demand any ethics approval.
Consent to participate
This study did not demand any consent to participate.
Consent for publication (include appropriate statements)
All the authors agreed with the content and to submit this manuscript. We reinforced that we obtained consent from the responsible authorities at the institution where the work has been carried out.
Availability of data and material (data transparency)
All specimens analyzed herein are housed at the Coleção de Répteis at the UFMT.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ferreira, A.C., Klaczko, J. & Martins, A. Hemipenial morphology of Epictia vellardi (Laurent, 1984) (Leptotyphlopidae, Serpentes) with the proposition and discussion of two general hemipenial patterns within the genus Epictia. Zoomorphology 140, 143–150 (2021). https://doi.org/10.1007/s00435-020-00506-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00435-020-00506-0