Abstract
Background
Curcumin is a traditional remedy for diseases associated with hyper-inflammatory responses and immune system impairment. Piperine, a bioactive compound in black pepper, has the potential to enhance curcumin bioavailability. 0This study aims to examine the effect of the curcumin-piperine co-supplementation in patients infected with SARS-CoV-2 and admitted to the intensive care unit (ICU).
Material and Methods
In this parallel randomized, double-blind, placebo-controlled trial, 40 patients with COVID-19 admitted to ICU were randomized to receive three capsules of curcumin (500 mg)-piperine (5 mg) or placebo for 7 days.
Results
After 1 week of the intervention, serum aspartate aminotransferase (AST) (p = 0.02) and C-reactive protein (CRP) (p = 0.03) were significantly decreased, and hemoglobin was increased (p = 0.03) in the curcumin-piperine compared to the placebo group. However, compared with the placebo, curcumin-piperine had no significant effects on the other biochemical, hematological, and arterial blood gas and 28-day mortality rate was three patients in each group (p = 0.99).
Conclusion
The study results showed that short-term curcumin-piperine supplementation significantly decreased CRP, AST, and increased hemoglobin in COVID-19 patients admitted to the ICU. Based on these promising findings, curcumin appears to be a complementary treatment option for COVID-19 patients, although some parameters were not affected by the intervention.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
The COVID-19 outbreak began in Wuhan, China, in December 2019 and spread quickly to other countries [1]. Based on its genome similarity of 79% to Coronaviruses, this new strain was called SARS-CoV-2 [2]. Many new SARS-CoV-2 variants have emerged since the first outbreak, despite isolation, lockdown, and other containment measures [3]. Recently, the WHO reported 600 million cases of confirmed COVID-19 and over 6.5 million deaths [4]. Even with rapid advances in public vaccination, the disease remains a major public health concern [5] and has negatively affected people’s lives [6]. Despite early determination of the SARS-CoV-2 structure and the development of some effective treatments and vaccines [7], the virus continued to spread and the pathogenesis is still not entirely clear. However, it appears that a cytokine storm effect caused by alteration of the immune system plays a crucial role in disease effects [8]. The cytokine storm effect can lead to inflammatory responses and changes in hematologic parameters, leading to damaging effects such as severe lung damage, liver injury, and death in some cases [9,10,11,12,13].
A number of traditional compounds have shown some promise in curbing some of these effects and prove effective as well-tolerated alternate therapies for COVID-19 infection. Curcumin is a bioactive polyphenol with a multitude of pharmacological effects [14,15,16,17,18,19,20,21] and a number of recent studies have shown that this compound has beneficial effects on diseases associated with hyperinflammatory responses and immune system impairment, such as COVID-19 [8, 22,23,24,25,26]. Many preclinical and clinical studies have indicated the health benefits and safety (tolerated up to 12 g/day) benefits of this nutraceutical [27, 28]. Additionally, a wide range of pharmacological and biological activities have been attributed to its therapeutic mechanism of action, including immunomodulatory, anti-tumor, anti-microbial, antiviral, antioxidant, and anti-inflammatory properties [29,30,31,32]. However, the poor solubility in aqueous solutions, extensive metabolism in the liver and intestine, and rapid elimination of curcumin result in low bioavailability. To overcome this issue, compounds, such as piperine, a bioactive compound in black pepper, have been used to enhance curcumin absorption, inhibit metabolic enzymes, and limit curcumin clearance through the P glycoprotein efflux pump [33, 34]. Adding piperine to curcumin can significantly increase its bioavailability in humans [34]. Few studies have shown the benefits of curcumin in COVID-19 infection, but none have investigated the impact of curcumin-piperine supplementation in patients in intensive care units (ICUs). Thus, this study aims to examine the effect of the administration of curcumin-piperine supplementation on ICU patients infected with SARS-CoV-2.
2 Material and Methods
2.1 Study Design and Participants
This parallel randomized, double-blind, placebo-controlled trial assessing the efficacy of co-supplementation of curcumin-piperine on COVID-19 patients admitted to ICUs of Alzahra hospital, an academic hospital affiliated with Isfahan University of Medical Sciences, Isfahan, Iran, between June and September in 2021. The summary of the study protocol was published earlier [35]. The protocol was approved by the ethics committee of the Isfahan University of Medical Sciences (ethic code: IR.MUI.RESEARCH.REC.1400.057) and conducted based on the principles of the Declaration of Helsinki. The trial was also registered in the Iranian Registry of Clinical trials (IRCT) with ID: IRCT20121216011763N52. Before starting the study, the objectives and procedures of the trial were explained to patients or their caregivers, and written informed consent was obtained from all participants. Patients with a definitive diagnosis of COVID-19 confirmed via real-time polymerase chain reaction (RT-PCR), 30–70 years-old, and who were admitted to the ICUs, were included. Exclusion criteria were as follows: unstable hemodynamic status, renal or liver disease, undergoing dialysis, cancer patients undergoing chemotherapy, and pregnancy. The other exclusion criteria included use of parenteral nutrition, taking anticoagulant drugs such as warfarin and having a history of sensitivity to herbal products such as turmeric and pepper. Patients were withdrawn from the trial if they were unwilling to continue or showed any adverse effects.
2.2 Randomization and Blinding
A total of 40 patients were randomized in a ratio of 1:1 into two groups. An independent statistician conducted the sequencing of the assignment using a table of random numbering and this was kept in opaque, sealed, numbered envelopes until the end of the assessment of the eligibility criteria. Curcumin-piperine and placebo capsules were provided in identical formats with the same shape, size, color, and odor. Participants, investigators, laboratory staff, outcome assessors, and data analyzers were blinded to treatment assignments until the completion of data analyses.
2.3 Intervention
Patients in the intervention group received three curcumin piperine capsules containing 500 mg curcumin and 5 mg piperine per capsule, amounting to a total of 1500 mg curcumin and 15 mg piperine in a day. Capsules were administered orally or with enteral nutrition (gavage) at 9 am, 3 pm, and 9 pm (6 h apart). The duration of the intervention was 7 days. Patients in the control group received three matched placebo capsules a day, each containing 505 mg maltodextrin (1515 mg maltodextrin/day). All capsules were provided by Sami-Sabinsa Group Limited (Bangalore, India). The intervention was started 24–48 h after admission to the ICU when hemodynamic resuscitation and stabilization were carried out and when patients received at least 70% of their energy requirements based on 25 kcal/kg body weight. All patients continued standard treatment as per the physician’s prescriptions and were allowed to take their usual medications without any limitations.
2.4 Outcome Measures and Data Collection
Acute physiology and chronic health evaluation II (APACHE II) and NUTRIC score were calculated to assess COVID-19 disease severity and nutritional status of the patients, respectively, at the beginning of the study. Blood samples (5 mL) were obtained early in the morning after approximately 6 h fasting before and after the intervention. These were left for 60 min to allow clotting and centrifuged at room temperature for 10 min to isolate serum, which was stored at −80 °C until use. The parameters measured were serum calcium (Ca), magnesium (Mg), sodium (Na), potassium (K), chloride (Cl), alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), albumin (ALB), C-reactive protein (CRP), complete blood count (CBC) including white blood cells (WBCs), red blood cells (RBCs), hemoglobin (Hb), hematocrit, mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), platelets (PLT), blood urea nitrogen (BUN), serum creatinine (Cr), prothrombin time (PT), and partial phromboplastin time (PTT). These parameters were assessed at baseline and end of the study at the laboratory center of Alzahra hospital using enzymatic methods and auto-analyzer with commercial kits (Pars Azmun, Karaj, Iran). Furthermore, arterial blood gas (ABG) was taken while the patient was breathing room air.
2.5 Statistical Analysis
The statistical package for the social sciences (SPSS) software version 16 (SPSS Inc., Chicago, IL, USA) was used to analyze data. Paired sample t and chi-squared tests were used to analyze within-group differences. The differences between the groups were assessed using independent student’s t-test. Data were reported as mean ± standard deviation (SD) or frequency (percentage). Analysis of covariance (ANCOVA) was used to compare the mean values of continuous outcomes at the end of the study between two groups, considering adjustment for baseline values. Chi-squared or Fisher exact tests were used to compare qualitative outcomes between groups. A p-value less than 0.05 was considered statistically significant.
3 Results
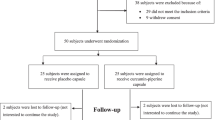
A total of 94 patients were assessed for eligibility, 42 patients were excluded for not meeting inclusion criteria, and 12 persons refused to participate in the study (Fig. 22.1). After this, patients (19 men and 21 women) were randomized to receive the curcumin-piperine (n = 20) or maltodextrin (n = 20) capsules in three divided doses for 7 days. One subject in the curcumin piperine group and one subject in the control group died before the end of the study, and thus analyses were conducted on 38 patients (19 patients in the intervention and 19 samples in the control groups).
The baseline characteristics of patients were comparable between the groups. There was no significant difference between the groups in any of the baseline characteristics, including age, sex, APACHII, or NUTRIC scores (Table 22.1). The effects of curcumin-piperine supplementation on selected metabolic and biochemical parameters are shown in Table 22.2. The intra-group comparison showed a decreasing trend in serum AST in the curcumin-piperine group (p = 0.08) and a significant increase in the level of AST in the placebo group (p = 0.03). Furthermore, compared to the baseline, after 7 days of intervention, a non-significant increase was found in the serum levels of BUN (p = 0.09), Cr (p = 0.07), ALT (p = 0.09) in the placebo and for ALP (p = 0.08) in the curcumin piperine group. Based on the inter-group comparisons, it was found that the AST (p = 0.02) and CRP (p = 0.03) levels significantly decreased in the intervention group in comparison to the placebo group. However, there were no significant differences regarding BUN, Cr, ALT, and ALP between groups.
The effects of curcumin-piperine supplementation on hematological parameters are presented in Table 22.3. Within-group comparisons indicated that one-week supplementation with curcumin piperine led to a significant increase in MCV (p = 0.009) and a significant decrease in platelets (p = 0.02), while there was no significant change regarding other variables. Also, in the placebo group, the lymphocyte count showed a significant increase (p = 0.01), while hemoglobin (p = 0.07) and MCHC (p = 0.08) showed a non-significant decrease. Between-group analysis showed that in comparison to the placebo, curcumin-piperine supplementation significantly increased the serum level of hemoglobin (p = 0.03). Minerals and ABG parameters and their changes are presented in Table 22.4. At the end of the intervention, we observed a significant increase in pCO2 (p = 0.02) and a decrease in pH (p = 0.01) in the curcumin-piperine compared to the placebo group. The only significant finding in the placebo group was a decrease in Cl levels (p = 0.02). There was no significant difference in minerals and ABG gas parameters between the two groups (p for all > 0.05). Finally, the 28-day mortality rate was 3 (15%) patients in each group, with no statistical difference between the groups (p = 0.99).
4 Discussion
The results of this study suggest that curcumin-piperine consumption is efficacious and safe in COVID-19 patients. Recent studies revealed that this polyphenol could positively affect disease symptoms such as sore throat, cough, fever and weakness, O2 saturation, and length of hospital stay [30, 36, 37]. The main findings of our study are that CRP and AST levels decreased, and hemoglobin concentration increased significantly with curcumin-piperine supplementation for 7 days in COVID-19 ICU patients.
A number of prior studies have obtained similar results regarding anti-inflammatory effects of curcumin in COVID-19. A previous randomized-controlled trial on 60 COVID-19 patients revealed that subjects receiving 160 mg of curcuminoids daily had reduced CRP levels than placebo [38], as we found here. It has also been shown that other inflammatory markers such as IL-6 and IL-1β are also reduced due to curcumin supplementation [22, 39, 40]. A systematic review performed in 2022 indicated that curcumin supplementation reduced not only pro-inflammatory cytokines but also was effective in increasing IL-10, IL-35, and TGF-a as anti-inflammatory cytokines [8]. These effects are most likely driven by the curcumin modulation of inflammatory signaling pathways such as the nuclear factor-κB (NF-kB), mitogen-activated protein kinase (MAPK), activator protein 1 (AP-1), and Janus kinase/signal transducer and activator of transcription (JAK/STAT) transcription factors [24].
Higher levels of liver enzymes have been observed in many COVID-19 patients, which is related to the severity of the disease and mortality risk [41, 42]. It has been proposed that elevated levels of ALT and AST indicate a possibility of COVID-19 recurrence [43]. The lowering effects of curcumin on ALT and AST have been shown in some animal and human studies [44,45,46,47]. In the present clinical trial, the administration of curcumin-piperine combination reduced the levels of AST in COVID-19 patients compared to the placebo. In the intervention group, both ALT and AST levels decreased at the end of the study compared to the baseline, although these changes did not reach statistical significance. However, AST was significantly increased in the placebo group which may be an indicator of worsening severity. This could have been caused by uncontrolled inflammation, hypoxia, and potential hepatocyte damage caused by the viral infection and replication process in those patients receiving the placebo [48]. Although BUN and creatinine did not change significantly in the curcumin group, these markers increased in the placebo group compared to the baseline as a potential indicator of impaired renal function [49]. Increased BUN and creatinine levels also serve as risk factors for a more severe disease course and increased mortality [49, 50].
We also found that hemoglobin concentrations were significantly increased in individuals who received curcumin-piperine compared to those in the placebo group. Furthermore, hemoglobin concentrations and MCHC values showed non-significant decreases in the placebo group compared with the baseline values. This is consistent with a study by Huang et al. which found that approximately 38% of COVID-19 patients had decreased levels of hemoglobin [51]. In addition, Fouad et al. concluded that hemoglobin concentration is a helpful indicator of disease severity [52]. The effective transport of oxygen in the blood is directly influenced by the hemoglobin concentration and, when an infection occurs, the peripheral tissues require more oxygen, which may result in disease complications like hypoxia and ischemia [53]. This is also consistent with our finding in the curcumin-piperine group of a significant increase in MCV, which is an indicator of red blood cell volume.
Finally, there was no difference in the 28-day mortality rate between the intervention and control groups. This result is not in line with another study which showed that supplementation curcumin-piperine two times per day over 2 weeks reduced the mortality rate in COVID-19 patients [37]. However, it is possible that the larger sample size and longer treatment used in the above mentioned study accounts for this difference.
Our work has some limitations. First, the sample size was relatively small which may have impacted on our ability to detect some significant changes or differences between the groups. Second, the duration of this study was short, although it is common approach in trials of critically ill patients. Finally, the number of biomarkers and physiological parameters that we measured was small and could be expanded to include other inflammation-related analytes, such as cytokine arrays or multiplex immunoassay panels [54,55,56,57].
In conclusion, the results of the current randomized controlled trial revealed that short-term curcumin-piperine supplementation is well-tolerated and can significantly decrease CRP, AST, and increase hemoglobin levels in COVID-19 patients admitted to ICU. Based on these findings, further larger studies should be conducted over both short and longer time periods to investigate the potential use of this compound as a novel therapeutic option for treatment of COVID-19 disease and potentially other respiratory virus infections.
References
Dourado D, Freire DT, Pereira DT, et al (2021) Will curcumin nanosystems be the next promising antiviral alternatives in COVID-19 treatment trials? Biomed Pharmacother 139111578. https://doi.org/10.1016/j.biopha.2021.111578
Lu R, Zhao X, Li J, et al (2020) Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet 395(10224):565–574
Shah AS, Wood R, Gribben C, et al (2020) Risk of hospital admission with coronavirus disease 2019 in healthcare workers and their households: nationwide linkage cohort study. BMJ 371. https://doi.org/10.1136/bmj.m3582
World Health Organization (WHO) WHO coronavirus disease (COVID-19) dashboard. https://www.who.int/emergencies/diseases/novel-coronavirus-2019. Accessed September 19, 2022
Mathieu E, Ritchie H, Ortiz-Ospina E, et al (2021) A global database of COVID-19 vaccinations. Nat Hum Behav 5(7):947–953
Shek DT (2021) COVID-19 and quality of life: Twelve reflections. Appl Res Qual Life 16(1):1–11
Wojcieszyńska D, Guzik H, Guzik U (2022) Non-steroidal anti-inflammatory drugs in the era of the Covid-19 pandemic in the context of the human and the environment. Sci Total Environ; 834:155317. https://doi.org/10.1016/j.scitotenv.2022.155317
Vahedian-Azimi A, Abbasifard M, Rahimi-Bashar F, et al (2022) Effectiveness of Curcumin on Outcomes of Hospitalized COVID-19 Patients: A Systematic Review of Clinical Trials. Nutrients 14(2). https://doi.org/10.3390/nu14020256
Canoğlu K, Şaylan B, Çalışkan T (2021) COVID-19 and thrombosis: Prophylaxis and management. Tuberk Toraks 69(2):269–278
Hu B, Huang S, Yin L (2021) The cytokine storm and COVID-19. J Med Virol 93(1):250–256
Conti P, Ronconi G, Caraffa A, et al (2020) Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by Coronavirus-19 (COVI-19 or SARS-CoV-2): anti-inflammatory strategies. J Biol Regul Homeost Agents 34(2):327–331
Vitiello A, La Porta R, D’Aiuto V, Ferrara F (2021) The risks of liver injury in COVID-19 patients and pharmacological management to reduce or prevent the damage induced. Egypt Liver J 11(1):11. https://doi.org/10.1186/s43066-021-00082-y
Yuan X, Huang W, Ye B, et al (2020) Changes of hematological and immunological parameters in COVID-19 patients. Int J Hematol 112(4):553–559
Momtazi-Borojeni AA, Haftcheshmeh SM, Esmaeili SA, et al (2018) Curcumin: A natural modulator of immune cells in systemic lupus erythematosus. Autoimmun Rev 17(2):125–135
Iranshahi M, Sahebkar A, Takasaki M, et al (2009) Cancer chemopreventive activity of the prenylated coumarin, umbelliprenin, in vivo. Eur J Cancer Prev 18(5):412–415
Panahi Y, Ghanei M, Bashiri S, et al (2014) Short-term Curcuminoid Supplementation for Chronic Pulmonary Complications due to Sulfur Mustard Intoxication: Positive Results of a Randomized Double-blind Placebo-controlled Trial. Drug Res 65(11):567–573
Panahi Y, Khalili N, Sahebi E, et al (2017) Curcuminoids modify lipid profile in type 2 diabetes mellitus: A randomized controlled trial. Complement Ther Med 33:1–5
Parsamanesh N, Moossavi M, Bahrami A, et al (2018) Therapeutic potential of curcumin in diabetic complications. Pharmacol Res 136:181–193
Shah M, Murad W, Mubin S, et al (2022) Multiple health benefits of curcumin and its therapeutic potential. Environ Sci Pollut Res Int 29(29):43732–43744
Gorabi AM, Kiaie N, Hajighasemi S, et al (2019) The effect of curcumin on the differentiation of mesenchymal stem cells into mesodermal lineage. Molecules 24(22):4029. https://doi.org/10.3390/molecules24224029
Mohajeri M, Bianconi V, Ávila-Rodriguez MF, et al (2020) Curcumin: a phytochemical modulator of estrogens and androgens in tumors of the reproductive system. Pharmacol Res 156:104765. https://doi.org/10.1016/j.phrs.2020.104765
Hassaniazad M, Eftekhar E, Inchehsablagh BR, et al (2021) A triple-blind, placebo-controlled, randomized clinical trial to evaluate the effect of curcumin-containing nanomicelles on cellular immune responses subtypes and clinical outcome in COVID-19 patients. Phytother Res 35(11):6417–6427
Kunnumakkara AB, Rana V, Parama D, et al (2021) COVID-19, cytokines, inflammation, and spices: How are they related? Life Sci 284:119201. https://doi.org/10.1016/j.lfs.2021.119201
Peng Y, Ao M, Dong B, et al (2021) Anti-Inflammatory Effects of Curcumin in the Inflammatory Diseases: Status, Limitations and Countermeasures. Drug Des Devel Ther 15:4503–4525
Zahedipour F, Hosseini SA, Sathyapalan T, et al (2020) Potential effects of curcumin in the treatment of COVID-19 infection. Phytother Res 34(11):2911–2920
Heidari Z, Daei M, Boozari M, et al (2022) Curcumin supplementation in pediatric patients: A systematic review of current clinical evidence. Phytother Res 36(4):1442–1458
Goel A, Kunnumakkara AB, Aggarwal BB (2008)Curcumin as "Curecumin": from kitchen to clinic. Biochem Pharmacol 75(4):787–809
Minassi A, Sánchez-Duffhues G, Collado JA, et al (2013) Dissecting the pharmacophore of curcumin. Which structural element is critical for which action? J Nat Prod 76(6):1105–1112
Boroumand N, Samarghandian S, Hashemy SI (2018) Immunomodulatory, anti-inflammatory, and antioxidant effects of curcumin. J Herbmed Pharmacol 7(4):211–219
Askari G, Sahebkar A, Soleimani D, et al (2022) The efficacy of curcumin-piperine co-supplementation on clinical symptoms, duration, severity, and inflammatory factors in COVID-19 outpatients: a randomized double-blind, placebo-controlled trial. Trials 23(1):472. https://doi.org/10.1186/s13063-022-06375-w
Hassanzadeh S, Read MI, Bland AR, et al (2020) Curcumin: an inflammasome silencer. Pharmacol Res 159:104921. https://doi.org/10.1016/j.phrs.2020.104921
Farhood B, Mortezaee K, Goradel NH, et al (2019) Curcumin as an anti-inflammatory agent: Implications to radiotherapy and chemotherapy. J Cell Physiol 234(5):5728–5740
Rinwa P, Kumar A (2012) Piperine potentiates the protective effects of curcumin against chronic unpredictable stress-induced cognitive impairment and oxidative damage in mice. Brain Res 1488:38–50
Li Q, Zhai W, Jiang Q, et al (2015) Curcumin–piperine mixtures in self-microemulsifying drug delivery system for ulcerative colitis therapy. International Journal of Pharmaceutics 490(1):22–31
Askari G, Alikiaii B, Soleimani D, et al (2021) Effect of curcumin-pipeine supplementation on clinical status, mortality rate, oxidative stress, and inflammatory markers in critically ill ICU patients with COVID-19: a structured summary of a study protocol for a randomized controlled trial. Trials 22(1):434. https://doi.org/10.1186/s13063-021-05372-9
Honarkar Shafie E, Taheri F, Alijani N, et al (2022) Effect of nanocurcumin supplementation on the severity of symptoms and length of hospital stay in patients with COVID-19: A randomizd double-blind placebo-controlled trial. Phytother Res 36(2):1013–1022
Pawar KS, Mastud RN, Pawar SK, et al (2021) Oral Curcumin With Piperine as Adjuvant Therapy for the Treatment of COVID-19: A Randomized Clinical Trial. Front Pharmacol 12:669362. https://doi.org/10.3389/fphar.2021.669362
Ahmadi R, Salari S, Sharifi MD, et al (2021) Oral nano-curcumin formulation efficacy in the management of mild to moderate outpatient COVID-19: A randomized triple-blind placebo-controlled clinical trial. Food Sci Nutr 9(8):4068–4075
Asadirad A, Nashibi R, Khodadadi A, et al (2022) Antiinflammatory potential of nano-curcumin as an alternative therapeutic agent for the treatment of mild-to-moderate hospitalized COVID-19 patients in a placebo-controlled clinical trial. Phytother Res 36(2):1023–1031
Valizadeh H, Abdolmohammadi-Vahid S, Danshina S et al (2020) Nano-curcumin therapy, a promising method in modulating inflammatory cytokines in COVID-19 patients. Int Immunopharmacol 89(Pt B):107088. https://doi.org/10.1016/j.intimp.2020.107088
Lei F, Liu YM, Zhou F, et al (2020) Longitudinal Association Between Markers of Liver Injury and Mortality in COVID-19 in China. Hepatology 72(2):389–398
Wijarnpreecha K, Ungprasert P, Panjawatanan P, et al (2021) COVID-19 and liver injury: a meta-analysis. Eur J Gastroenterol Hepatol 33(7):990–995
Chen LZ, Lin ZH, Chen J, et al (2020) Can elevated concentrations of ALT and AST predict the risk of ’recurrence’ of COVID-19? Epidemiol Infect 148:e218 https://doi.org/10.1017/s0950268820002186
Jalali M, Mahmoodi M, Mosallanezhad Z, et al (2020) The effects of curcumin supplementation on liver function, metabolic profile and body composition in patients with non-alcoholic fatty liver disease: A systematic review and meta-analysis of randomized controlled trials. Complement Ther Med 48:102283. https://doi.org/10.1016/j.ctim.2019.102283
Mansour-Ghanaei F, Pourmasoumi M, Hadi A, Joukar F (2019) Efficacy of curcumin/turmeric on liver enzymes in patients with non-alcoholic fatty liver disease: A systematic review of randomized controlled trials. Integr Med Res 8(1):57–61
Xu G, Gu Y, Yan N, et al (2021) Curcumin functions as an anti-inflammatory and antioxidant agent on arsenic-induced hepatic and kidney injury by inhibiting MAPKs/NF-κB and activating Nrf2 pathways. Environ Toxicol 36(11):2161–2173
Zhong W, Qian K, Xiong J, et al (2016) Curcumin alleviates lipopolysaccharide induced sepsis and liver failure by suppression of oxidative stress-related inflammation via PI3K/AKT and NF-κB related signaling. Biomed Pharmacother 83:302–313
Zhou F, Xia J, Yuan HX, et al (2021) Liver injury in COVID-19: Known and unknown. World J Clin Cases 9(19):4980–4989
Nogueira SÁ R, Oliveira SCS, Carvalho AFM, et al (2020) Renal changes and acute kidney injury in covid-19: a systematic review. Rev Assoc Med Bras (1992) 66Suppl 2(Suppl 2):112–117
Qu J, Zhu HH, Huang XJ, et al (2021) Abnormal Indexes of Liver and Kidney Injury Markers Predict Severity in COVID-19 Patients. Infect Drug Resist 14:3029–3040
Huang Y, Tu M, Wang S, et al (2020) Clinical characteristics of laboratory confirmed positive cases of SARS-CoV-2 infection in Wuhan, China: A retrospective single center analysis. Travel Med Infect Dis 36:101606. https://doi.org/10.1016/j.tmaid.2020.101606
Fouad SH, Allam MF, Taha SI, et al (2021) Comparison of hemoglobin level and neutrophil to lymphocyte ratio as prognostic markers in patients with COVID-19. J Int Med Res 49(7):3000605211030124. https://doi.org/10.1177/03000605211030124
Taneri PE, Gómez-Ochoa SA, et al (2020) Anemia and iron metabolism in COVID-19: a systematic review and meta-analysis. Eur J Epidemiol 35(8):763–773
Simple Plex™; Bio-Techne; Minneapolis, MN, USA. https://www.bio-techne.com/instruments/simple-plex
Cytokine Array – Human Cytokine Antibody Array (Membrane, 42 Targets) (ab133997); Abcam; Cambridge, United Kingdom. https://www.abcam.com/cytokine-array%2D%2Dhuman-cytokine-antibody-array-membrane-42-targets-ab133997.html
LEGENDplexTM Human Inflammation Panel 1 multiplex immunoassay. BioVendor; Brno, Czechia. https://www.biolegend.com/nl-be/products/legendplex-human-inflammation-panel-1-13-plex-with-v-bottom-plate-16929
Multiplex Cytokine Assays with Luminex; Redwood City, CA, USA. https://www.precisionformedicine.com/specialty-lab-services/cytokine-analysis/luminex/
Acknowledgments
This study was approved and funded by Isfahan University of Medical Sciences with grant number 299197.
Competing Interests
MM is the founder of Sami-Sabinsa group of companies.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Askari, G. et al. (2023). Evaluation of Curcumin-Piperine Supplementation in COVID-19 Patients Admitted to the Intensive Care: A Double-Blind, Randomized Controlled Trial. In: Guest , P.C. (eds) Application of Omic Techniques to Identify New Biomarkers and Drug Targets for COVID-19. Advances in Experimental Medicine and Biology(), vol 1412. Springer, Cham. https://doi.org/10.1007/978-3-031-28012-2_22
Download citation
DOI: https://doi.org/10.1007/978-3-031-28012-2_22
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-28011-5
Online ISBN: 978-3-031-28012-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)