Abstract
Background
COVID-19 disease caused by the SARS-CoV-2 virus can lead to an acute respiratory illness with a high hospitalization and mortality risk. Therefore, prognostic indicators are essential for early interventions. As a component of complete blood counts, the coefficient of variation (CV) of red blood cell distribution width (RDW) reflects cellular volume variations. It has been shown that RDW is associated with increased mortality risk in a wide range of diseases. This study aimed to determine the relationship between RDW and mortality risk in COVID-19 patients.
Methods
This retrospective study was performed on 592 patients admitted to hospital between February 2020 and December 2020. Patients were divided into low and high RDW groups and the relationship between RDW and mortality, intubation, admission to intensive care unit (ICU), and need for oxygen therapy was investigated.
Results
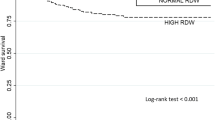
The mortality rate in the low RDW group was 9.4%, while that in the high group was 20% (p < 0.001). Also, ICU admission in the low group was 8%, whereas this was 10% in the high RDW group (p = 0.040). The results of the Kaplan–Meyer curve showed that the survival rate was higher in the low group compared to the high RDW group. Cox results in the crude model showed that higher RDW values were directly related to increased mortality, although this was not significant after adjustment for other covariates.
Conclusion
The results of our study reveal that high RDW is associated with increased hospitalization and risk of death and that RDW may be a reliable indicator of COVID-19 prognosis.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection which causes COVID-19 disease can induce a hyperinflammatory condition that may lead to acute respiratory syndrome (ARDS) in the host [1]. Therefore, early diagnosis and treatment plays an important role in determining patient outcomes and preventing life-threatening complications. To meet these objectives, the use of biomarkers, especially laboratory biomarkers for assessing the prognosis of patients, has a vital role in the management of this disease [2]. The coefficient of variation of red blood cell distribution width (RDW) is a hematologic parameter routinely measured in blood cell count analysis. RDW readings show the size heterogeneity of circulating red blood cells (RBCs), otherwise known as the degree of anisocytosis [3]. Changes in erythropoiesis can cause heterogeneity of RBC size as an indicator of certain pathological conditions [4]. For example, RDW values are known to be higher in malnutrition, tuberculosis, hemolytic anemia, myelodysplastic syndrome, cardiovascular disease, pneumonia, sepsis, influenza, viral hepatitis, and cancer [5, 6]. Increasing levels of RDW can also be indicative of an imbalance in RBC production in the bone marrow or a high turnover rate of these cells. Importantly, high RDW values have also been associated with an increased risk of mortality [7,8,9].
In a prospective study of 240,477 healthy individuals, participants were followed for nine years to investigate the prognostic role of RDW [10]. This showed that the levels of RDW in cardiovascular diseases and cancer (especially colorectal cancer and leukemia) were increased, and this was associated with an increased risk of mortality in patients. Recent studies have reported the prognostic role of RDW in COVID-19 patients (for a meta-analysis on this topic see [8]). Various mechanisms have been suggested for an elevation in RDW in COVID-19 patients. One of these is the potential increase in the levels of inflammatory cytokines such as interleukin (IL)-1, IL-6, and tumor necrosis factor (TNF)-α [11]. These cytokines increase hepcidin (as a negative regulator of iron) and decrease the release of stored iron, leading to an impairment of iron metabolism [12]. In addition, CD147, also known as the OK blood group antigen, is expressed on erythrocyte lineage cells and is known to act as a novel receptor for SARS-CoV-2 binding [13]. It has also been suggested that this binding on RBCs can lead to viral invasion of these cells and their subsequent destruction, an outcome predicted to affect RDW values.
Here, we have investigated the relationship between RDW levels and the outcomes of COVID-19-hospitalized patients. Our main focus was on intubation rate, need for oxygen therapy, intensive care unit (ICU) admission, and length of hospital stay.
2 Materials and Methods
2.1 Study Design
This retrospective single-center study was conducted at Shahid Mostafa Khomeini Hospital in Tabas, Iran. The study population consisted of COVID-19 patients with a positive polymerase chain reaction (PCR) test hospitalized in Shahid Mostafa Khomeini Hospital between February 2020 and December 2020. People under 18 years or with hematologic malignancies were excluded. Finally, 592 patients were included in the study.
This study was approved by the ethics committee of Birjand University of Medical Sciences, Iran (IR.BUMS.REC.1400.006). Patient electronic medical records were reviewed, and demographic characteristics, clinical signs, comorbidities, laboratory test results [complete blood count (CBC) [14], blood urea nitrogen (BUN), creatinine (Cr), and C-reactive protein (CRP)], PO2 (partial pressure of oxygen), computerized tomography (CT) scan results, hospitalization duration time, and body temperature were determined. CBCs were analyzed by Sysmex analyzer model Kx-21. The normal range of the coefficient of variation of RDW in our laboratory was 11.5–14.5. Patients were divided into two groups: those with RDW less than 14.5 (RDW < 14.5) and those with RDW above 14.5 (RDW > 14.5).
The relationship between RDW and mortality, intubation, admission to intensive care unit, and the need for oxygen therapy was assessed in both groups.
2.2 Statistical Analysis
The Stata software (version 14) was used for data analysis. Descriptive data were presented as mean, standard deviation (SD), frequency, and frequency percentage. The Kolmogorov–Smirnov test evaluated the normality of continuous variables, and the Schoenfeld residuals test was performed to check the proportional hazards (PH) assumption in the simple and Cox multiple models. To determine the difference between the means of variable data between the two groups (RDW > 14.5 vs. RDW < 14.5), an independent t-test was used for continuous variables such as PO2 status, temperature, white blood cells (WBC), platelet (PLT), neutrophil, and lymphocyte counts. For nonparametric variables, comparisons between groups were performed with the Mann–Whitney U test. Fisher’s exact test and chi-square test were used for categorical variables.
A Kaplan–Meyer curve was drawn to show the survival time of patients in the RDW > 14.5 and RDW < 14.5 groups, and a log-rank test was performed to check the difference in survival time between the two groups. In addition, a Cox simple regression model was used to determine the factors related to survival time in patients with COVID-19, and a multivariable Cox regression model was performed for variables that were found to have significant effects in the simple Cox regression model. Finally, hazard ratio (HR) and 95% confidence interval (CI) were reported for each variable related to patient survival time in the two simple models and the Cox multivariable model. P-values less than 0.05 were considered as significant.
3 Results
The present study was performed on 592 patients with COVID-19 disease. Of these, 73.3% (n = 434) were classed in the RDW < 14.5 set, and the remaining participants were in the RDW > 14.5 group. The mean age of all subjects was 60.4 ± 21.5 years, 56.7 ± 21.0 years in the RDW < 14.5 group and 70.5 ± 19.7 years in the RDW > 14.5 group. The independent t-test showed that the difference in mean age between the two groups was statistically significant (Table 12.1). The chi-square test results showed that RDW level was not statistically related to gender.
In addition, we found that age was directly related to higher RDW values (p = 0.001). Also, higher RDW levels were related to higher ICU admission rates (RDW < 14.5: 8%, RDW > 14.5: 10%, p = 0.04). Furthermore, the prevalence of cancer and cardiovascular disease was higher in the group with RDW > 14.5 than in the RDW < 14.5 group.
The mortality rate in the RDW < 14.5 group was 9.4% while that in the RDW > 14.5 group was higher at 20.2% (p = 0.001). PO2 was also significantly associated with RDW as 78% of the patients with a PO2 greater than 93% were in the RDW < 14.5 group, compared to only 22% of the RDW > 14.5 patient group (p = 0.004). The group with RDW > 14.5 also had lower hemoglobin and higher BUN levels than the group with RDW < 14.5 (p = 0.001).
The results of the Kaplan–Meyer curve showed that the survival rate was higher in the group with RDW < 14.5 than in the RDW > 14.5 group. In addition, the Cox results showed that the rate of intubation was directly related to mortality. Finally, Cox analyses of the crude and adjusted models showed that higher RDW values were directly related to the increase in death, although this relationship was not significant after adjustment for age, fever, cough, cardiovascular disease, oxygen therapy status, temperature, intubation, PO2, WBC count, and BUN (Fig. 12.1).
4 Discussion
The results of this study showed that elevated RDW was associated with higher ICU admission rates and an increased risk of death. This suggests that RDW can be considered as a negative prognostic indicator of clinical conditions of COVID-19 patient clinical conditions. In line with our study, a retrospective study of 1198 COVID-19 patients found that having an RDW > 14.5 was associated with an increased risk of death at all ages [15]. Also, the Cox model used in this previous study showed that, after adjusting for age, lymphocyte count, and D-dimer levels in patients with RDW > 14.5, the mortality rate was higher. In our study, the results of the Cox model in the crude model showed that increased RDW values were associated with an increased risk of death. However, this association was not significant after adjusting for other covariates including age, which is an important risk factor for severe COVID-19 and ensuing hard outcomes.
We found that patients with higher RDW had higher rates of ICU admission. In line with our analysis, a study conducted in Ankara on 127 COVID-19 patients showed that patients with higher RDW values had higher ICU admissions [16]. In a study on 294 COVID-19 patients in Brooklyn, Ramachandran et al. examined the association with mortality, septic shock, and the need for mechanical ventilation [17]. The results showed elevated RDW was associated with increased mortality and septic shock. However, they found no association between increased RDW and increased need for ventilation, which is in line with the findings of our study.
The pathological mechanisms underlying RDW increase in COVID-19 are unknown. However, previous studies have shown that elevated RDW in COVID-19 is associated with increased inflammatory cytokines such as IL-1 and TNF-α, which can disrupt iron metabolism and cause anemia and increased RBC apoptosis [18]. Also, an increased RDW reflects an imbalance between hematopoiesis and survival of RBCs, and a delay in removing old RBCs from the peripheral blood [18]. Kaufman et al. also reported that elevated RDW was associated with higher CRP and BUN levels along with increased mortality risk [19]. This is in line with our findings as we also found that BUN was higher in the group with higher RDW. High BUN levels are used an indicator of kidney dysfunction which can also be manifested in patients with SARS-CoV-2 infections [20].
One of the limitations of our study is this retrospective design, which limits our access to information. Another limitation of our study is that it was performed in only one center. Lastly, our analysis only considered the effects of a single biomarker (RDW values) on ICU admission and death outcomes in COVID-19 patients admitted to hospital. Future studies should attempt to incorporate additional markers with RDW values such as BUN, CRP, IL-1, IL-6, and TNFα using a multiplex algorithm. These analyte values can be obtained using cytokine arrays or multiplex immunoassay panels [21, 22]. Also, in addition to the current study of inpatients here, future investigations should analyze the effects on outpatients. Thus, further studies are required in multiple centers and on larger population groups.
In conclusion, the results of our study showed that increased RDW is associated with an increase in hospitalization in ICU and an increased risk of death, and can be used as a nonspecific, inexpensive, and accessible indicator to determine the prognosis of COVID-19 patients. Thus, future studies should be carried out to validate and optimize the performance of this biomarker and associated algorithms in prediction of COVID-19 disease outcomes. This will help to stratify patients according to the most appropriate therapeutic options.
References
Dreher M, Kersten A, Bickenbach J, et al (2020) The characteristics of 50 hospitalized COVID-19 patients with and without ARDS. Dtsch Arztebl Int 117(16):271–278
Foy BH, Carlson JC, Reinertsen E, et al (2020) Elevated RDW is associated with increased mortality risk in COVID-19. medRxiv. https://doi.org/10.1101/2020.05.05.20091702
Cohen RM, Franco RS, Khera PK, et al (2008) Red cell life span heterogeneity in hematologically normal people is sufficient to alter HbA1c. Blood 112(10):4284–4291
Higgins JM, Mahadevan L (2010) Physiological and pathological population dynamics of circulating human red blood cells. Proc Natl Acad Sci USA 107(47):20587–20592
Perlstein TS, Weuve J, Pfeffer MA, et al (2009) Red blood cell distribution width and mortality risk in a community-based prospective cohort. Arch Intern Med 169(6):588–594
Salvagno GL, Sanchis-Gomar F, Picanza A, et al (2015) Red blood cell distribution width: a simple parameter with multiple clinical applications. Crit Rev Clin Lab Sci 52(2):86–105
Hammons L, Filopei J, Steiger D, et al (2019) A narrative review of red blood cell distribution width as a marker for pulmonary embolism. J Thromb Thrombolysis 48(4):638–647
Sarkar S, Kannan S, Khanna P, Singh AK (2022) Role of red blood cell distribution width, as a prognostic indicator in COVID-19: A systematic review and meta-analysis. Rev Med Virol 32(2):e2264. https://doi.org/10.1002/rmv.2264
Soni M, Gopalakrishnan R (2021) Significance of RDW in predicting mortality in COVID-19 – An analysis of 622 cases. Int J Lab Hematol 43(4): O221–O223
Pilling LC, Atkins JL, Kuchel GA, et al (2018) Red cell distribution width and common disease onsets in 240,477 healthy volunteers followed for up to 9 years. PLoS One 13(9):e0203504. https://doi.org/10.1371/journal.pone.0203504
Ragab D, Salah Eldin H, Taeimah M, et al (2020) The COVID-19 cytokine storm; what we know so far. Front Immunol 11:1446. https://doi.org/10.3389/fimmu.2020.01446
Alcaino H, Pozo J, Pavez M, et al (2016) Red cell distribution width as a risk marker in patients with cardiovascular diseases. Rev Med Chil 144(5):634–642
Ulrich H, Pillat MM (2020) CD147 as a target for COVID-19 treatment: suggested effects of azithromycin and stem cell engagement. Stem Cell Reviews and Reports 16(3):434–440
Laboratory Procedure Manual; Complete Blood Count. https://www.cdc.gov/nchs/data/nhanes/nhanes_03_04/l25_c_met_complete_blood_count.pdf
Foy BH, Carlson JC, Reinertsen E, et al (2020) Association of red blood cell distribution width with mortality risk in hospitalized adults with SARS-CoV-2 infection. JAMA Netw Open 3(9):e2022058. Significance of RDW in predicting mortality in COVID-19 – An analysis of 622 cases 1001/jamanetworkopen.2020.22058
Ceyhan MA, Tümer M, Selahattin G, et al (2021) Mean platelet volume and red cell distribution width values in patients with COVID-19 admitted to intensive care units or wards from emergency department. Arch Curr Med Res 2(2):88–92
Ramachandran P, Gajendran M, Perisetti A, et al (2022). Red Blood Cell Distribution Width in Hospitalized COVID-19 Patients. Front Med 8:582403. Significance of RDW in predicting mortality in COVID-19 – An analysis of 622 cases 3389/fmed.2021.582403
Lee JJ, Montazerin SM, Jamil A, et al (2021) Association between red blood cell distribution width and mortality and severity among patients with COVID-19: A systematic review and meta-analysis. J Med Virol 93(4):2513–2522
Kaufmann CC, Ahmed A, Brunner U, et al (2021) Red Cell Distribution Width Upon Hospital Admission Predicts Short-Term Mortality in Hospitalized Patients With COVID-19: A Single-Center Experience. Front Med 8:652707. Significance of RDW in predicting mortality in COVID-19 – An analysis of 622 cases 3389/fmed.2021.652707
Fernandez-Botran R, Furmanek S, Ambadapoodi RS, et al (2022) Association and predictive value of biomarkers with severe outcomes in hospitalized patients with SARS-CoV-2 infection. Cytokine 149:155755. https://doi.org/10.1016/j.cyto.2021.155755
Simple Plex™; Bio-Techne; Minneapolis, MN, USA. https://www.bio-techne.com/instruments/simple-plex
Cytokine Array - Human Cytokine Antibody Array (Membrane, 42 Targets) (ab133997); Abcam; Cambridge, United Kingdom. https://www.abcam.com/cytokine-array%2D%2Dhuman-cytokine-antibody-array-membrane-42-targets-ab133997.html
Acknowledgments
This work was supported by a grant from Birjand University of Medical Sciences.
Conflict of Interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Kouhpeikar, H. et al. (2023). Red Cell Distribution Width as a Prognostic Indicator for Mortality and ICU Admission in Patients with COVID-19. In: Guest , P.C. (eds) Application of Omic Techniques to Identify New Biomarkers and Drug Targets for COVID-19. Advances in Experimental Medicine and Biology(), vol 1412. Springer, Cham. https://doi.org/10.1007/978-3-031-28012-2_12
Download citation
DOI: https://doi.org/10.1007/978-3-031-28012-2_12
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-28011-5
Online ISBN: 978-3-031-28012-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)