Abstract
The identification of superspreaders is essential to contain an epidemic, especially when there is not enough information about the disease to develop precautionary measures. Unlike infections caused directly between individuals of the same species, epidemics caused by vectors have well-explored peculiarities. In this direction, we intend to study the networks obtained from the dissemination of dengue to verify, from the results of a simulation of agent based models, if the transmission of this disease follows the 20/80 rule for the proportion of spreaders and infected. We built different transmission networks considering the spread between vectors and humans up to the second generation and we observed that, despite the human-to-human transmission network follow the 20/80 rule, the other networks (human–mosquito, mosquito–mosquito and mosquito–human) did not follow this rule. Varying the density of agents, we show that the phenomenon of superspreading is accentuated with high density of mosquitoes. These characteristics of vector-borne disease networks need to be further explored, as these vectors are highly vulnerable to climate change, and a better understanding of disease spread can help better target dengue epidemic control strategies.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Numerous infectious diseases spread from human to human or human to animal and vice versa, creating infection networks [18]. Research in graph theory has provided a wide variety of tools over time for describing networks, many of them with applications in epidemiological studies [10]. Many epidemic control methods can be modeled with networks, such as contact tracing and vaccination, allowing the study of the effect of different strategies in an artificial environment before their real application [10]. The introduction of many actual elements and better knowledge about the temporal pattern of the network is essential for a better understanding of the epidemic propagation [14].
In the spread of infectious diseases, heterogeneity in the behavior of a population often plays a crucial role in determining whether an epidemic occurs [2]. The social network structure is essential in the dynamics of disease spread [13], and the network topology influences the general spreading behavior of an epidemic and the fluctuations of network connectivity, which can increase the incidence of infection [14].
In the study of disease transmission, including vector-borne parasites and sexually transmitted pathogens, it is estimated that about 20% of those infected are responsible for 80% of infections (20/80 rule) [19]. This rule can be verified through the study of networks. For COVID-19, for example, a study in Hong Kong constructed the disease transmission networks and found that 69% of infected individuals did not spread the disease to anyone, while 17–19% of infectious individuals were responsible for 80% of all transmission events [1].
Regarding dengue, a mosquito-borne viral disease transmitted mainly by Aedes aegypti, a study showed that the disease epidemic in Singapore organized itself in a scale-free network of transmission during outbreaks between 2000 and 2005 [12].
Dengue epidemics have recently increased due to the rapid spread of the disease in many tropical countries, Europe, and the USA [7]. It has already been considered a minor public health problem but has become an important reemerging disease and was considered the most important vector-borne viral disease in the last decade [6].
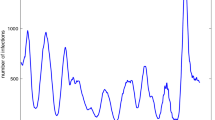
The disease transmission occurs from the interaction between humans, mosquitoes, viruses, and the environment factors [7], and its epidemics usually show periodic patterns related to mosquito population dynamics, which are driven by natural climate variations [15] (Fig. 1). Given the importance of re-emerging diseases and climate change, including mosquito-borne diseases, we aim to investigate whether dengue transmission follows the 20/80 rule using network analysis applied to the results of an agent-based model simulation.
2 Methods
We use an agent-based model (ABM) to simulate the dynamics of dengue in an urban environment. Mosquitoes and humans are agents in the model, and the environment is built using a satellite image of the study area (Belo Horizonte, Brazil), which corresponds to an area of 100 m \(\times \) 100 m. Regular square network cellular automata (each site corresponding to \(\sim 1\,{\text {m}}^2\)) were used to build the environment. Thus, agents interact not only with each other but also with the environment. The total simulation time was two years, and the time step is equivalent to one minute.
Only female mosquitoes were used in the simulation, as they transmit the disease to humans [4, 8]. We use the SIR compartment model dynamics (Susceptible–Infected–Recovered) in our ABM. The statuses of mosquitoes and agents are: “susceptible”, “infected” or “incubated”. Humans can also be “recovered”. Mosquitoes cannot be recovered because once infected, the mosquito remains so until the end of its life, even after repeated meals of human blood [4].
We also evaluated local precipitation and temperature as attributes of the environment. If it rained and if the maximum temperature was less than 30\(\,^{\circ }\textrm{C}\) and the minimum temperature was greater than 20\(\,^{\circ }\textrm{C}\),which are ideal conditions for eggs to hatch [17], we increased the site attribute by 50%. To introduce this meteorological data, we used the average daily temperature and precipitation data from 2000 to 2016 from the Pampulha Meteorological Station, in Belo Horizonte, Minas Gerais, Brazil.
While each mosquito lives approximately 45 d [3], being replaced as soon as it dies, human renovation only occurs with a probability of 5% per year in the model. Mosquitoes and humans are randomly distributed across the sites. Humans can walk (random walk), and mosquitoes can fly, both in two dimensions. Mosquitoes can also bite humans or reproduce. A random walk guides the mosquito’s flight with weight (quenched noise) according to the value of the environment. Mosquitoes and humans walk with a step size of one site. A mosquito decides on its activities at each time step: it can fly (25% probability), reproduce (25% probability, but only happens if weather conditions are favorable; otherwise, it flies), or bite (50% probability, but only happens if there is at least one human in the same location; otherwise, it flies).
The simulation starts with two mosquitoes infected. It was considered that there is a possibility that an egg infected with a randomly selected serotype will hatch during reproduction to simulate the introduction of new dengue serotypes over time.
The model was previously calibrated using data from mosquito traps [16]. We found that the best calibration occurred with approximately 100 mosquitoes and 500 humans per area. However, other simulations were tested with different amounts of mosquitoes and humans to build the infection network in this work. The simulations combined 250, 500, 750 and 1000 humans with 100, 180, and 360 mosquitoes. Each combination refers to a scenario. We ran 30 simulations.
We built the infection networks from the simulation results. The networks are directed, and if a node i infects a node j, there is a connection from i to j. We notice that the greater the number of mosquitoes, the larger and more connected the networks. The greater the number of humans, the smaller the networks since the simulations showed a lower percentage of infected humans.
We measure the degree of network nodes to assess superspreaders, following the same procedure as Lima and Atman [11]. However, as dengue involves a vector in its transmission between humans, we analyzed four types of networks: human-human, mosquito-mosquito, human-mosquito, and mosquito-human.
3 Results
The percentage infected is showed in Fig. 2. We can see that the higher the number of humans and the lower the number of mosquitoes, the lower the percentage infected. This indicates that, at the simulation level, even with many humans in an area, the percentage of infection will be small if the number of mosquitoes is small, highlighting the importance of vector control. In contrast, this number is high when the number of mosquitoes is high (Fig. 2L).
On the other hand, with few humans (Figs. 2A, 2B, and 2C), population saturation occurs rapidly, with almost the entire population infected within the first few days of simulation. This saturation occurs for all cases with high numbers of mosquitoes over time (Figs. 2C, 2F, 2I, and 2L). An example of a network generated at the end of each simulation scenario is shown in Fig. 3.
Percentage of dengue infected in each scenario. Mean of 30 samples and 95% confidence interval. The percentage was calculated considering the number of humans in each simulation. Scenarios are A: 250 humans and 100 mosquitoes; B: 250 humans and 180 mosquitoes; C: 250 humans and 360 mosquitoes; D: 500 humans and 100 mosquitoes; E: 500 humans and 180 mosquitoes; F: 500 humans and 360 mosquitoes; G: 750 humans and 100 mosquitoes; H: 750 humans and 180 mosquitoes; I: 750 humans and 360 mosquitoes; J: 1000 humans and 100 mosquitoes; K: 1000 humans and 180 mosquitoes; L: 1000 humans and 360 mosquitoes.
Figure 2 shows that the growth of dengue cases over the simulation time (2 years) represents some recorded epidemic years, especially epidemic ones (such as 2010, 2013, and 2016) (Fig. 1). Notice that this simulation result should be compared with Fig. 1 considering a time window of two years in the real data.
Examples of directed infection networks in each scenario. Black nodes represent humans and the red ones represent mosquitoes. Two nodes are directly connected from the one who transmits the infection towards the new infected. The scenarios are A: 250 humans and 100 mosquitoes; B: 250 humans and 180 mosquitoes; C: 250 humans and 360 mosquitoes; D: 500 humans and 100 mosquitoes; E: 500 humans and 180 mosquitoes; F: 500 humans and 360 mosquitoes; G: 750 humans and 100 mosquitoes; H: 750 humans and 180 mosquitoes; I: 750 humans and 360 mosquitoes; J: 1000 humans and 100 mosquitoes; K: 1000 humans and 180 mosquitoes; L: 1000 humans and 360 mosquitoes.
Network analysis shows that the human-human network recorded a high degree for some humans in all simulations with 360 mosquitoes (higher degrees ranging from approximately 130–180) (Fig. 4). This indicates that some humans indirectly infected many other humans in the simulation. The same was recorded in the human-mosquito networks, in which some mosquitoes infect a high number of humans (higher degrees ranging from approximately 140–180).
For the human-mosquito network, the maximum degree values were 26 (simulations with 500 humans and 360 mosquitoes) and 28 (simulations with 250 humans and 360 mosquitoes). This indicates that the same human transmitted (indirectly) the disease to 26 and 28 humans, respectively. In mosquito-mosquito networks, the highest degree values are for the network of 250 humans and 360 mosquitoes (maximum degree of 37). It means that 37 mosquitoes were infected by humans who received the infection from the same mosquito.
In the case of the human-mosquito networks, mosquito control can break hubs more easily than in lower-grade networks, where it is necessary to remove many vectors to break a hub. This shows that, at the simulation level, it is easier to control the infection in areas with fewer vectors and humans, highlighting the need for more outstanding efforts by the community and health authorities to contain transmission in overcrowded areas and mosquito-friendly environments. Also, a study that analyzed small dengue networks showed that they allow for in-depth analysis of dengue transmission, which could be used to inform the more effective spatial application of disease control strategies [9].
The percentage of mosquitoes and humans responsible for infections in each scenario (among the infected) is presented in Table 1. It is worth noting that human-human networks followed the 20/80 rule, then approximately 20% of humans are responsible for about 80% of human infections in all scenarios. In the mosquito networks, the values ranged from 16.75 to 39.46% of mosquitoes infecting about 80% of mosquitoes. In human-mosquito and mosquito-human networks, approximately 40–70% of the respective infecting agents were responsible for about 80% of infections. This value is more than double the expected number in the 20/80 rule.
The Shapiro-Wilk normality test showed that the data from Table 1 have a normal distribution. Thus, to compare the means between the groups, the ANOVA test was used (\(\alpha = 0.05\)), which showed no significant difference between the percentages of infected agents when comparing the networks. However, there is a significant difference between all the networks for the percentages of agents transmitting the infections.
4 Discussion
Although the degree of the nodes of the human-mosquito network are high, this transmission occurred in areas with lower population density and high numbers of mosquitoes. Consequently, more mosquitoes tend to bite the same human. This did not happen in areas with many humans and mosquitoes, as there are more humans for the mosquito to bite. For mosquito networks, it is worth noting that as the mosquito population renews itself more frequently (although the population size remains constant), there are always new susceptible individuals. This does not happen in human networks, so the population can quickly become immune, saturating the infection network.
In this work, network analyzes were crucial to investigate the role of agents as superspreaders of dengue. The analysis of superspreaders individuals and superspreading events is essential for emergency public health responses, as one or a few superspreaders can ignite epidemic outbreaks [5]. Large epidemic outbreaks can also occur when the spread of a disease starts in superspreaders [21].
Our findings show that, at the simulation level, although human-human networks have superspreaders agents and obey the 20/80 rule, this does not happen in the others. It may be a characteristic of networks involving vectors and hosts. However, a more extensive study, perhaps involving other vector diseases, is needed to understand how infections occur in these networks.
Although the initial results allow inferences about the structure of infection networks, this model has several limitations, as well as practically all models. The first limitation is that the population is constant and with low renovation. This dynamic does not reflect the reality of urban centers, where there is no frontier for the disease, which spreads temporally and spatially, hardly infecting the entire population. This population variation is one of the reasons why dengue has cyclical outbreaks every three or five years. The same limitation occurs in the mosquito population, which, although renewed more frequently, is restricted to a space and a population.
Despite being programmed to run with the four dengue serotypes circulating in Brazil, the model was used only for one serotype for simplification and because of the short simulation time (two years). If the model is run for a longer time, it is recommended to include all serotypes. The results can even be studied in networks with a layer for each serotype. A multiplex approach can also be adopted for one or more serotypes.
Even though its limitations, this initial study can be used as a starting point for a more extensive and in-depth analysis, as it is impossible to effectively track a mosquito-borne disease. It is worth noting that, unlike an airborne or sexually transmitted disease, it is not feasible to measure the transmissibility of each mosquito. In this context, one of the best ways to understand whether networks involving the vector follow the 20/80 rule is via computer simulations.
The structure of the networks of a vector-borne disease involves agents other than humans and a significant influence of environmental factors. Understanding dengue transmission networks can help in proposing measures to reduce infection by the disease, such as vector control [20].
References
Adam, D.C., Wu, P., Wong, J.Y., Lau, E.H., Tsang, T.K., Cauchemez, S., Leung, G.M., Cowling, B.J.: Clustering and superspreading potential of sars-cov-2 infections in Hong Kong. Nature Med. 26(11), 1714–1719 (2020)
Ball, F., Mollison, D., Scalia-Tomba, G.: Epidemics with two levels of mixing. Ann. Appl. Probab. 46–89 (1997)
Crovello, T.J., Hacker, C.S.: Evolutionary strategies in life table characteristics among feral and urban strains of aedes aegypti (l.). In: Evolution, pp. 185–196 (1972)
Donalísio, M.R., Glasser, C.M.: Vigilância entomológica e controle de vetores do dengue. Revista Brasileira de Epidemiologia 5(3), 259–279 (2002)
Duan, W., Qiu, X., Cao, Z., Zheng, X., Cui, K.: Heterogeneous and stochastic agent-based models for analyzing infectious diseases’ super spreaders. IEEE Intel. Syst. 28(4), 18–25 (2013)
Gubler, D.J.: The economic burden of dengue. Am. J. Tropical Med. Hygiene 86(5), 743 (2012)
Guzman, M.G., Harris, E.: Dengue. Lancet 385(9966), 453–465 (2015)
Halstead, S.B.: Dengue virus-mosquito interactions. Annu. Rev. Entomol. 53, 273–291 (2008)
Hendron, R.W.S., Bonsall, M.B.: The interplay of vaccination and vector control on small dengue networks. J. Theoret. Biol. 407, 349–361 (2016)
Keeling, M.J., Eames, K.T.: Networks and epidemic models. J. R. Soc. Interface 2(4), 295–307 (2005)
Lima, L., Atman, A.: Impact of mobility restriction in covid-19 superspreading events using agent-based model. Plos One 16(3), e0248708 (2021)
Massad, E., Ma, S., Chen, M., Struchiner, C.J., Stollenwerk, N., Aguiar, M.: Scale-free network of a dengue epidemic. Appl. Math. Comput. 195(2), 376–381 (2008)
Moore, C., Newman, M.E.: Epidemics and percolation in small-world networks. Phys. Rev. E 61(5), 5678 (2000)
Moreno, Y., Pastor-Satorras, R., Vespignani, A.: Epidemic outbreaks in complex heterogeneous networks. Eur. Phys. J. B Condensed Matter Complex Syst. 26(4), 521–529 (2002)
Obolski, U., Perez, P.N., Villabona-Arenas, C.J., Thézé, J., Faria, N.R., Lourenço, J.: Mvse: an r-package that estimates a climate-driven mosquito-borne viral suitability index. Methods Ecol. Evol. 10(8), 1357–1370 (2019)
Resende, M.C.D., Ázara, T.M.F.D., Costa, I.O., Heringer, L.C., Andrade, M.R.D., Acebal, J.L., Eiras, Á.E.: Field optimisation of mosquitrap sampling for monitoring aedes aegypti linnaeus (diptera: Culicidae). Memórias do Instituto Oswaldo Cruz 107(3), 294–302 (2012)
Tun-Lin, W., Burkot, T., Kay, B.: Effects of temperature and larval diet on development rates and survival of the dengue vector Aedes aegypti in north Queensland, australia. Med. Veterinary Entomol. 14(1), 31–37 (2000)
Watts, D.J.: The “new” science of networks. Annu. Rev. Sociol. 30, 243–270 (2004)
Woolhouse, M.E., Dye, C., Etard, J.F., Smith, T., Charlwood, J., Garnett, G., Hagan, P., Hii, J., Ndhlovu, P., Quinnell, R., et al.: Heterogeneities in the transmission of infectious agents: implications for the design of control programs. Proc. Nat. Acad. Sci. 94(1), 338–342 (1997)
Ximenes, R., Amaku, M., Lopez, L.F., Coutinho, F.A.B., Burattini, M.N., Greenhalgh, D., Wilder-Smith, A., Struchiner, C.J., Massad, E.: The risk of dengue for non-immune foreign visitors to the 2016 Summer Olympic Games in Rio de Janeiro, Brazil. BMC Infect. Diseases 16(1), 186 (2016)
Zeng, Q., Liu, Y., Tang, M., Gong, J.: Identifying super-spreaders in information-epidemic coevolving dynamics on multiplex networks. Knowl. Syst. 229, 107365 (2021)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this paper
Cite this paper
Lima, L.L., Atman, A.P.F. (2023). Paths for Emergence of Superspreaders in Dengue Fever Spreading Network. In: Cherifi, H., Mantegna, R.N., Rocha, L.M., Cherifi, C., Miccichè, S. (eds) Complex Networks and Their Applications XI. COMPLEX NETWORKS 2016 2022. Studies in Computational Intelligence, vol 1077. Springer, Cham. https://doi.org/10.1007/978-3-031-21127-0_49
Download citation
DOI: https://doi.org/10.1007/978-3-031-21127-0_49
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-21126-3
Online ISBN: 978-3-031-21127-0
eBook Packages: EngineeringEngineering (R0)