Abstract
Microstructures for microelectromechanical system (MEMS) applications are widely fabricated using silicon wet bulk micromachining technique. In this method, potassium hydroxide (KOH) is one of the most used anisotropic etchants. It provides high etch rate when it is modified by the addition of hydroxylamine (NH2OH). Moreover, it shows high undercutting which is a desirable property for the fabrication of overhanging microstructures. In this paper, the effect of isopropyl alcohol (IPA) in NH2OH-added KOH on the etching characteristics of Si{100} and Si{110}) is systematically studied. The results are compared with the etching characteristics of IPA-added pure KOH. The undercutting rate reduces drastically when IPA is added to NH2OH + KOH which protects the convex corners of the structures during etching process. At the same time, the etch rate of {110} plane is suppressed considerable which is exploited to expose {110} plane at the mask edges aligned along <100> direction on {100} surface that makes 45° angle with {100} surface and act as a micromirror.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Wet anisotropic etching is a simple and cheap fabrication process, which is extensively used in silicon micromachining for the fabrication of microstructures for microelectromechanical systems (MEMS) [1,2,3,4,5,6,7,8]. Potassium hydroxide (KOH) and tetramethylammonium hydroxide (TMAH) are most used alkaline solutions in silicon wet anisotropic etching [1]. KOH exhibits higher etch rate and improved etch selectivity between Si{100}/Si{110} and Si{111} in comparison to TMAH. Etch rate is a very important etching parameter that affects the industrial production. To improve the etch rate, various methods such as microwave irradiation, ultrasonic agitation, etching temperature at the boiling point of etchants, etc. are investigated [9]. To obtain smooth etched surface morphology, effects of alcohols and surfactants are investigated [10,11,12,13,14,15]. Recently, we reported the etching characteristics of silicon in various concentrations of NH2OH-added 20 wt% KOH [4, 16,17,18,19]. The addition of NH2OH in 20 wt% KOH considerably increases the etch rate and the undercutting at convex corners. Moreover, it improves etch selectivity between Si and SiO2.
The addition of IPA in KOH suppresses the etch rate of Si{110} planes. Therefore {110} planes expose at <100> mask edge on Si{100} surface that makes an angle of 45° with wafer surface. As 15% NH2OH-added 20 wt% KOH provides very high etch rate and undercutting, the study of the effect of IPA in this etchant composition is required. In this paper, the effect of IPA on the etching characteristics of Si{100} and Si{110} in 20 wt% KOH and 15% NH2OH + 20 wt% KOH is studied.
2 Experimental Details
4-inch Cz-grown {100} and {110} oriented p-type silicon wafers with resistivity of 5–10 Ω cm are used. A thermally grown silicon dioxide of 1 μm thickness is employed as etch mask for the selective etching of silicon. The first step of the experimental process is to pattern the oxide layer by photolithography. Silicon dioxide is etched out in buffered hydrofluoric acid (BHF) followed by cleaning in running DI water. Thereafter, the wafer is diced into small chips (2 × 2 cm2). Diced chips are cleaned in a piranha bath (H2O2:H2SO4::1:1), then thoroughly rinsed in running DI water. Prior to dipping these chips in an etchant, oxide layer grown in piranha bath is removed in 1% hydrofluoric acid (HF) followed by cleaning in DI water. To prepare 20 wt% KOH solution, KOH pellets (99.99%, Alfa Aesar) are dissolved in DI water. Aqueous NH2OH solution is used to prepare 15% NH2OH + 20 wt% KOH. To study the effect of IPA on the etching characteristics of Si{100} and Si{110}, different concentration IPA is added to KOH and NH2OH + KOH solutions. All experiments are performed at a fixed temperature of 75 ± 1 °C. A reflux condenser is used to avoid any change in etchant concentration during the etching process. Etch depth, etched surface roughness, and undercutting at convex corners are measured using a 3D laser scanning microscope (Olympus, OLS4000). Scanning electron microscope (SEM) is used to inspect various kinds of microstructures.
3 Results and Discussion
Etching characteristics of Si{100} and Si{110} (i.e., etch rates, etched surface morphologies, undercutting at convex corners) are investigated in KOH and NH2OH + KOH without and with addition of different concentration IPA. The results obtained in different etchant compositions are compared. Etching characteristics are methodically presented in the following subsections.
3.1 Etch Rate
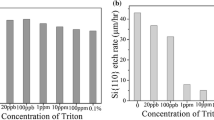
The study of etch rate is very important in the fabrication of MEMS structures such as grooves and cavities. Figure 1 shows the etch rates of {100} and {110} planes in 20 wt% KOH and 15% NH2OH-added 20 wt% KOH without and with the addition of different concentration IPA. It can easily be noticed that the etch rates of both Si{100} and Si{110} drastically decreases as the concentration of IPA increases and attain almost minimum value if the concentration of added IPA increases more than 6%. In the case of Si{110}, the decrease in etch rate significantly higher than that of Si{100}. This property of etching is very useful to fabricate 45° mirror on Si{100} surface by exposing {110} planes at <100> mask edges [11].
In IPA-added KOH solution, IPA molecules form a layer on the silicon surface, which protects the silicon surface from the reactants [12, 13, 20]. Therefore, the etch rate of silicon reduces on the addition of IPA. Recently, we reported the etching mechanism of silicon in NH2OH-added KOH [16,17,18,19]. In NH2OH-added KOH, H2O is the main reactive molecule, while NH2O− and OH− work as catalysts. When IPA is added to NH2OH + KOH, the etch rate of silicon is suppressed as shown in Fig. 1. It may be due to the adsorption of IPA molecules on the silicon surface that reduces the diffusion of reactants and catalysts to silicon surface. This is schematically presented in Fig. 2.
3.2 Etched Surface Roughness and Morphology
Surface roughness is one of the important concerns, especially when it is used for optical and solar cell applications [21, 22]. Figures 3 and 4 show the surface roughness of Si{100} and Si{110}, respectively, planes in 20 wt% KOH and 15% NH2OH-added 20 wt% KOH without and with the addition of different concentration IPA. In both cases, surface roughness is improved when 6% (or more) IPA is added in the etchant. The main cause of the formation of hillocks on the surface is the generation of hydrogen bubbles on the surface being etched and/or the stiction of impurities and byproducts on the surface [1]. The addition of IPA might reduce the surface tension of the etchant, or the IPA layer does not allow the formation of bubbles on the surface [1, 20].
3.3 Undercutting
Undercut rate is an important etching characteristic that is advantageously used to release the suspended structures [23,24,25]. On the other hand, it is an unwanted effect when mesa structures are fabricated with protected convex corners. Figure 5 shows the undercut rate and corresponding 3D laser scanning microscope images of the convex corners on Si{100} etched in pure and NH2OH-added KOH solution without and with addition of different concentration IPA. It can easily be noticed in Fig. 5 that the undercut rate suppressed significantly when IPA is added in pure and NH2OH-added KOH. It is well known that the undercut rate reduces due to the reduction of etch rate of high index planes that are exposed at the convex corner during the wet anisotropic etching process [1, 20]. In this case, IPA layer forms on the high index planes appearing at convex corners during etching process that protect the convex corner from the main reactive elements.
Mesa and cavity structures are successfully fabricated on Si{100} in IPA-added 20 wt% KOH and 15% NH2OH + 20 wt% KOH solutions as presented in Fig. 6. It can easily be observed that the fabrication of mesa/cavity with same height/depth requires less etching time in IPA + NH2OH + KOH in comparison to that in IPA + KOH solution. Thus, the fabrication of mesa and cavity structures with a higher etch rate, is very useful for the fabrication microstructures with 45° sidewalls that act as micromirror [11].
4 Conclusions
The effect of IPA on the etching characteristics of silicon (i.e., etch rate, surface roughness, and undercutting) in 20% KOH and 15% NH2OH + 20 wt% KOH solutions is methodologically investigated. The addition of IPA suppresses the etch rate and improves the etched surface morphology. The etch rate of Si{110} is more drastically reduced in comparison to that of Si{100}. This property is exploited to form 45° mirror on Si{100} surface. Moreover, the undercutting rate in both KOH and NH2OH + KOH is influenced dramatically on the addition of IPA, which is desirable property to form well shaped mesa structures on Si{100}. Surface morphology in IPA + NH2OH + KOH follows the same trend as IPA + KOH. Finally, it can be concluded that the addition of IPA affects the etching characteristics of both KOH and NH2OH + KOH significantly and the selection of the etchant depend upon the requirement of undercutting, etch rate and surface morphology.
References
Pal, P., Sato, K.: Silicon Wet Bulk Micromachining for MEMS. Pan Stanford Publishing, Singapore (2017)
Koo, K.I., Chung, H., Yu, Y., Seo, J., Park, J., Lim, J.M., Paik, S.J., Park, S., Choi, H.M., Jeong, M.J., Kim, G.S.: Fabrication of pyramid shaped three-dimensional 8 × 8 electrodes for artificial retina. Sens. Actuators A Phys. 130, 609–615 (2006)
Narasimha Rao, A.V., Swarnalatha, V., Pandey, A.K., Singh, S.S., Pal, P.: Determination of precise crystallographic directions on Si{111} wafers using self-aligning pre-etched pattern. Micro Nano Syst. Lett. 6(1), 4 (2018)
Menon, P.K., Narasimha Rao, A.V., Murthy, A.L., Pandey, A.K., Pal, P.: High speed etching of silicon in KOH+NH2OH solution at lower temperatures for the fabrication of through holes in silicon wafer. Micro Nano Lett., 1–6 (2020)
Yang, H., et al.: A novel method to fabricate single crystal nano beams with (111)-oriented Si micromachining. Microsyst. Technol. 14(8), 1185–1191 (2008)
Pal, P., Sato, K.: Various shapes of silicon freestanding microfluidic channels and microstructures in one step lithography. J. Micromech. Microeng. 19(5), 055003 (2009)
Rola, K.P., Zubel, I.: Impact of alcohol additives concentration on etch rate and surface morphology of (100) and (110) Si substrates etched in KOH solutions. Microsyst. Technol. 19(4), 635–643 (2013)
Seidel, H., Csepregi, L., Heuberger, A., Baumgärtel, H.: Anisotropic etching of crystalline silicon in alkaline solutions I. Orientation dependence and behavior of passivation layers. J. Electrochem. Soc. 137(11), 3612–3626 (1990)
Pal, P., Swarnalatha, V., Narasimha Rao, A.V., Pandey, A.K., Tanaka, H., Sato, K.: High speed silicon wet anisotropic etching for applications in bulk micromachining: a review. Micro Nano Syst. Lett. 9(1), 1–59 (2021)
Pal, P., Sato, K., Gosalvez, M.A., Kimura, Y., Ishibashi, K., Niwano, M., Hida, H., Tang, B., Itoh, S.: Surfactant adsorption on single crystal silicon surfaces in TMAH solution: orientation-dependent adsorption detected by in-situ infra-red spectroscopy. J.Microelectromech. Syst. 18, 1345–1356 (2009)
Xu, Y.W., Michael, A., Kwok, C.Y.: Formation of ultra-smooth 45° micromirror on (100) silicon with low concentration TMAH and surfactant: techniques for enlarging the truly 45° portion. Sens. Actuators A Phys. 166(1), 164–171 (2011)
Zubel, I., Kramkowska, M.: The effect of alcohol additives on etching characteristics in KOH solutions. Sens. Actuators A Phys. 101(3), 255–261 (2002)
Zubel, I., Rola, K., Kramkowska, M.: The effect of isopropyl alcohol concentration on the etching process of Si-substrates in KOH solutions. Sens. Actuators A Phys. 171, 436–445 (2011)
Pal, P., Sato, K., Gosalvez, M.A., Shikida, M.: Study of rounded concave and sharp edge convex corners undercutting in CMOS compatible anisotropic etchants. J. Micromech. Microeng. 17(11), 2299–2307 (2007)
Narasimha Rao, A.V., Swarnalatha, V., Pal, P.: Effect of surfactant and alcohol additives on etching characteristics in aqueous potassium hydroxide solutions. ECS Trans. 77(11), 1761–1769 (2017)
Narasimha Rao, A.V., Swarnalatha, V., Ashok, A., Singh, S.S., Pal, P.: Effect of NH2OH on etching characteristics of Si{100} in KOH solution. ECS J. Solid State Sci. Technol. 6(9), P609–P614 (2017)
Narasimha Rao, A.V., Pal, P., Pandey, A.K., Swarnalatha, V., Menon, P, K., Tanaka, H., Sato, K.: Aging effects of KOH + NH2OH solution on the etching characteristics of silicon. ECS J. Solid State Sci. Technol. 8(11), P685–P692 (2019)
Narasimha Rao, A.V., Swarnalatha, V., Pal, P.: Etching characteristics of Si {110} in 20 wt% KOH with addition of hydroxylamine for the fabrication of bulk micromachined MEMS. Micro Nano Syst. Lett. 5(1), 1–9 (2017)
Swarnalatha, V., Vismaya, K.T., Narasimha Rao, A.V., Pal, P., Pandey, A.K., Tanaka, H., Sato, K.: Etching mechanism behind the high speed etching of silicon in NH2OH-added alkaline solutions. IEEJ Trans. Sens. Micromach. 140(1), 24–30 (2020)
Zubel, I.: Anisotropic etching of Si. J. Micromech. Microeng. 29(9), 93002 (2019)
Gupta, A., Pal, P., Sharma, C.S.: Surface texturing of Silicon {100} in an extremely low concentration TMAH for minimized reflectivity. ECS J. Solid State Sci. Tech. 8(10), 622–628 (2019)
Rai-Choudhury, P.: MEMS and MOEMS Technology and Applications. SPIE Press (2000)
Pal, P., Sato, K.: A comprehensive review on convex and concave corners in silicon bulk micromachining based on anisotropic wet chemical etching. Micro Nano Syst. Lett. 3(1), 1–42 (2015). https://doi.org/10.1186/s40486-015-0012-4
Pal, P., Sato, K., Chandra, S.: Fabrication techniques of convex corners in (100)-silicon wafer using bulk micromachining: a review. J. Micromech. Microeng. 17(10), R111–R133 (2007)
Pal, P., Sato, K., Shikida, M., Gosalvez, M.A.: Study of corner compensating structures and fabrication of various shapes of MEMS structures in pure and surfactant added TMAH. Sens. Actuators A 154(2), 192–203 (2009)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this paper
Cite this paper
Narasimha Rao, A.V., Pal, P. (2023). Effect of IPA on Micromachining Characteristics of Silicon in KOH-Based Solution. In: Pandey, A.K., Pal, P., Nagahanumaiah, Zentner, L. (eds) Microactuators, Microsensors and Micromechanisms. MAMM 2022. Mechanisms and Machine Science, vol 126. Springer, Cham. https://doi.org/10.1007/978-3-031-20353-4_23
Download citation
DOI: https://doi.org/10.1007/978-3-031-20353-4_23
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-20352-7
Online ISBN: 978-3-031-20353-4
eBook Packages: EngineeringEngineering (R0)