Abstract
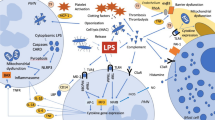
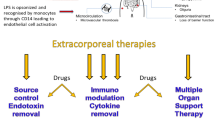
The induction of DIC is multifactorial, but the first critical event is the activation of tissue factor (TF) by different mechanisms. TF is expressed in many cells, as macrophage, monocyte neutrophils, and endothelial cells. During sepsis pro-inflammatory cytokines, damaged associated molecular pattern (e.g., endotoxin) or damage-associated molecular pattern (e.g., cellular lysis products) act on monocyte/macrophage via Toll like receptor and activate lymphocyte T. Extracorporeal blood purification is increasingly used in septic patients with organ failure, including coagulation dysfunction, which may result in disseminated intravascular coagulation. Particularly, pro-inflammatory molecules had a role in clotting the membrane, confirming the link between coagulation and inflammation. All these factors must be carefully evaluated when patients with organ failure need extracorporeal support to ensure an optimal treatment.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
11.1 Introduction
Disseminated intravascular coagulation (DIC) is a severe clinical condition, which involves considerable activation of both coagulation and fibrinolysis in the circulating blood [1, 2]. It is characterized by organ failure and a tendency to bleed. The pathological feature of DIC is characterized by extensive thrombus formation in the microvasculature due to coagulopathy despite differences in underlying causes. Chan et al. revised this definition and proposed a unified theory, which consider DIC during sepsis primarily a result of microthrombogenesis, due to activation of Ultra Large Von Willebrand Factors (ULVWF) and proposed the new definition of an endotheliopathy-associated vascular microthrombotic disease (EA-VMTD) [3, 4].
11.2 Pathophysiology
11.2.1 The Coagulation Cascade
The induction of DIC is multifactorial, but the first critical event is the activation of tissue factor (TF) by different mechanisms. TF is expressed in many cells, as macrophage, monocyte neutrophils, and endothelial cells [5]. During sepsis pro-inflammatory cytokines, pathogen-associated molecular pattern (e.g., endotoxin) or damage-associated molecular pattern (e.g., cellular lysis products) act on monocyte/macrophage via Toll like receptor and activate TF [6]. TF is upregulated as sepsis worsens and induces coagulation through activation of factor VII and thrombin. Yang X et al. demonstrated that TF and VII factor activation depends also on gasdermin, a protein that induces release of calcium to the inner membrane of macrophage through a caspase-dependent reaction [7, 8].
The second important event is platelet activation through ULVWF, which is upregulated during sepsis and it is released from activated endothelium and not inhibited by anti-thrombotic proteins, as ADAMS T3 [8]. These two procoagulant events turn on microvascular thrombosis and the macro, which in conjunction with hemodynamic derangement contributes to multi-organ failure. [9].
These procoagulant events are associated with alteration of normal endothelial cell physiology and normal fibrinolytic processes. The glycocalyx, nitric oxide (NO), thrombomodulin, protein C, tissue factor pathway inhibitor, and antithrombin III maintain the normal vascular homeostasis: during sepsis all these factors are deranged and contribute to a prothrombotic environment [10, 11].
The fibrinolytic process exerts a protective effect through the activation of a prothrombotic and hyperfibrinogenemia state. The initial response to coagulation activation in bacteremia and endotoxemia is an increase of the fibrinolytic capacity, due to enhanced release of tissue plasminogen activator (tPA) from the endothelium and acceleration of tPA-induced plasminogen activation by fibrin [12]. However, levels of plasminogen activator inhibitor 1 (PAI-1), the main inhibitor of fibrinolysis, increase during the course of an inflammatory reaction and a prothrombotic status is inducted through the activation of TAFI [13,14,15,16].
11.2.2 Cytokines Endotoxin and Coagulopathy
This procoagulant activity is exacerbated by the dysfunction of the platelets, which induce the initial coagulant process and then amplify the inflammatory and prothrombotic cascade.
Platelet-derived MPs (microparticles) arise from activated platelets and induce the release of interleukin (IL)-1b, IL-6, IL-8, and monocyte chemoattractant protein-1 (MCP-1) and monocytic (IL-1b, Tumor necrosis factor (TNF), IL-8) cytokines. The procoagulant activity of MPs is much stronger than activated platelets and correlates with a huge release of pro-inflammatory mediators [17]. IL-6 is one of the major mediators involved in coagulation: it induces TF expression. At different cellular levels, it activates endothelial cells and impairs anticoagulant mechanisms [18, 19].
However, also endotoxin interferes with the coagulation. Many studies evaluated this relationship with discordant results. In vitro and in vivo animal models demonstrated the potential of LPS to initiate clotting [20, 21]. Clinical models patients with sepsis demonstrate shorter coagulation time and clot formation times. Neither platelet counts or function nor conventional clotting parameters were influenced by endotoxin concentration [22].
In contrast to these data, Zacharowski et al. observed hematologic parameters characterized by a consumption coagulopathy, with platelets decrease, fibrinogen consumption, and increase of vWF and PAI-1. These data were significantly related to increases of R and K times and decreases in MA evaluated by the thromboelastography [23].
These contradictory data could stem from different animal models, different times of study and not homogeneous laboratory parameters.
Recently, the pandemic Covid-19 storm complicated this issue, as the coagulation response during Covid infection has new aspects in relation to sepsis model, which interferes with a prompt diagnosis and therapy [24].
11.3 Clinical Features of DIC
Depending on the underlying disease, the intensity of coagulation activation, and the deficiency of the natural anticoagulant pathways, DIC may present as either a latent and compensated activation of coagulation with subtle hemostatic dysfunction and overt DIC with both bleeding and thrombotic manifestations. This may include both microvascular thrombosis and thrombosis of larger vessels, first of all in septic patients [25].
11.3.1 Differential Diagnosis in ICU
Whereas many clinical pathological conditions may result in thrombocytopenia, this requires a differential diagnosis and different therapeutic approach [26].
First, primary pseudothrombocytopenia must be excluded. Thereafter, drugs affecting platelet function and therefore blood deficiency should be excluded. In addition, some clinical events, such as increased blood loss and hemodilution, can also reduce the number of platelets. Many extracorporeal treatments can interfere with coagulation and simultaneously activate an inflammatory response. The immune-mediated disorder and post-transfusion purpura should be excluded. Renal and hepatic diseases can induce hypersplenism and hemolytic-uremic syndrome. Finally, myelodysplastic syndrome, cancer, and HTCP must be considered.
11.3.2 Diagnosis
11.3.2.1 Laboratory Findings
Laboratory findings have a prominent role for the diagnosis of DIC in ICU. Levi M et al. revised the most important criteria for the diagnosis of DIC [27].
Today, there are five different diagnostic scoring systems for DIC established by the ISTH, the Japanese Ministry Health and Welfare (JMHW), the Japanese Association for Acute Medicine (JAAM), the British Committee for Standards in Haematology (BCSH), and the Italian Society of thrombosis and Hemostasis. Recently a new score—sepsis induced coagulopathy (SIC)—dedicated to DIC in septic shock has been proposed, considering that delay for diagnosis is a major drawback of the above score system.
SIC is composed of three items: (1) presence of organ dysfunction; (2) decreased platelet count; (3) increase of PT-INR. Some studies reported a high predictive value for this score.
11.3.2.2 Thromboelastography
Thromboelastography (TEG) is a point-of-care test that quickly measures the rate (reaction time®, clot formation speed (K), and alpha angle, strength (maximum amplitude (MA)), and stability (lysis after 30 min (LY30)) of clot formation. It correlates with bleeding and thrombosis in cardiac surgery, neurosurgery, trauma and liver surgery, but is seldom used in septic patients with derangement of coagulation.
Nevertheless, it is useful for quickly identifying patients at increased risk of DIC at admission and also for guiding targeted therapy for coagulopathy. Moreover, during the management of extracorporeal treatment, TEG is essential to titrate the exact dosage of the anticoagulant end/or choose the right one, a light of new clinical scenario (e.g., platelets decrease during heparin). Recently, two studies identified MA decrease (<60 mm) and prolonged K time, decreased angle, and increased R value as predictors of early DIC in septic patients, with a correlation of laboratory findings [28].
The basis of DIC treatment is the removal of the underlying causative factor. However, DIC will most often progress even after appropriate treatment of the underlying disease. Ideally, an effective treatment for DIC would distinguish between hyperfibrinolytic and hypofibrinolytic degradation, in which prothrombotic vs. hypofibrinogenemia should be differentiated. However, laboratory diagnostic means for such distinctions are not universally available and the DIC treatment during sepsis remains controversial [29].
11.4 Extracorporeal Support During Septic DIC and the Coagulation Response
Extracorporeal blood purification is increasingly used in septic patients with organ failure, including coagulation dysfunction, which may result in DIC. Moreover, anticoagulation must be used to avoid clotting of the membrane: either heparin or regional citrate has many effects on platelets and coagulation factors, which may worsen the clinical condition. Only recently, Villa et al. addressed this important point in a multicenter prospective study, aimed to describe the incidence and the associated factors of premature clotting of the oXiris membrane [30]. Interesting, either the hematological factors, the anticoagulant but also the pro-inflammatory molecules had a role in clotting the membrane, confirming the link between coagulation and inflammation. All these factors must be carefully evaluated when patients with organ failure need extracorporeal support to ensure an optimal treatment.
11.4.1 Blood Purification with oXiris Filter: Effect on Endotoxemia and Coagulation
11.4.1.1 Clinical Experience
From January 2012 to September 2020 143 patients with sepsis septic shock (Sepsis III definition) and AKI (AKIN classification) required ICU admission to Aurelia Hospital and European Hospital in Rome. One hundred one patients received CRRT with oXiris filters and completed the study. In these patients Endotoxemia [EAA, Endotoxin Activity Assay; Spectral Diagnostics, Inc., Toronto, Ont., Canada], PAI-1[Human PAI-1 ELISA Kit] SIC, Sepsis–induced coagulopathy, Thromboelastography—[Haemonetic 5–6] was evaluated at T0 (Basal time) and T1 (after 72 h) [31]. Endotoxin was detected in all the patients at T0. Its value at T0 was 0.73 ± 0.14 units.
At T0 8.5% of patients had low EAA activity (<0.39 units), 28% medium EAA activity (0.40–0.59 units), and 63% of patients high EAA activity (>0.60 units), confirming the massive release of endotoxin in septic patients with AKI (Fig. 11.1). At T1, EAA decreased to 0.52 ± 0.17 units (p < 0.01 vs. T0). At this time the percentage of patients with EAA high activity decreased with the changes of EAA (Fig. 11.1). IL-6 changes mirrored this improvement. The number of platelets, in part, correlated with the EAA activity (Fig. 11.1).
At T0 patients with laboratory signs of DIC were 19% at T0 and 22% at T1 (p = NS), but when DIC was assessed by TEG (MA < 60 mm) the percentage of patients with DIC was 10%. This is at variance with a recent study, in which all patients with septic shock had a tendency toward hypocoagulability with alteration of all TEG values.
This confirms the safety of RRT with oXiris filter, when anticoagulation with citrate is used and is in agreement with the course of TEG parameters (Table 11.1).
Patients with DIC assessed by TEG, in comparison to non-DIC patients, had less decrease of IL-6 and EAA, confirming that clearance of pro-inflammatory mediators could improve the coagulation (Fig. 11.2). Finally, we evaluated the course of PAI-1 during RRT with oXiris. PAI-1 is a key inducer of DIC in septic patients, is triggered by many pro-inflammatory mediators and increases the risk of micro-thrombosis in septic patients. Recent evidence suggest that PAI-1 overexpression is the hallmark of sepsis-associated DIC, as hypofibrinogenemia is less present in septic patients and decrease of platelets and PT prolongation is more present [32].
(a, b) indicate the different effect of DIC, evaluated by TEG, on the changes of EAA and IL-6. (c) indicates the different effect of DIC, evaluated by TEG, on the changes of PAI-1. The effect was more prominent for the IL-6. In all the patients, not stratified on MA data, the changes of PAI are shown: the oXiris modulates significantly the prothrombotic effect of PAI-1 (p < 0.01)
PAI-1 decreased during oXiris treatment, probably adsorbed as other pro-inflammatory mediators by the membrane: this may, in part, explain the stability of coagulation and the few episodes of bleeding in the patients (Fig. 11.2).
References
Okabayashi K, Wada H, Ohta S, Shiku H, Nobori T, Maruyama K. Hemostatic markers and the sepsis-related organ failure assessment score in patients with disseminated intravascular coagulation in an intensive care unit. Am J Hematol. 2004;76(3):225–9.
Gando S, Saitoh D, Ogura H, Mayumi T, Koseki K, Ikeda T, et al. Disseminated intravascular coagulation (DIC) diagnosed based on the Japanese Association for Acute Medicine criteria is a dependent continuum to overt DIC in patients with sepsis. Thromb Res. 2009;123(5):715–8.
Chang JC. Hemostasis based on a novel ‘two-path unifying theory’ and classification of hemostatic disorders. Blood Coagul Fibrinolysis. 2018;29(7):573–84.
Chang JC. Thrombogenesis and thrombotic disorders based on ‘two-path unifying theory of hemostasis’: philosophical, physiological, and phenotypical interpretation. Blood Coagul Fibrinolysis. 2018;29(7):585–95.
Chang JC. Disseminated intravascular coagulation: is it fact or fancy? Blood Coagul Fibrinolysis. 2018;29(3):330–7.
Engelmann B, Massberg S. Thrombosis as an intravascular effector of innate immunity. Nat Rev Immunol. 2013;13(1):34–45.
Evavold CL, Ruan J, Tan Y, Xia S, Wu H, Kagan JC. The pore-forming protein gasdermin D regulates interleukin-1 secretion from living macrophages. Immunity. 2018;48(1):35–44.e6.
Yang X, Cheng X, Tang Y, Qiu X, Wang Y, Kang H, et al. Bacterial endotoxin activates the coagulation cascade through gasdermin D-dependent phosphatidylserine exposure. Immunity. 2019;51(6):983–996.e6.
Wada H, Thachil J, Di Nisio M, Mathew P, Kurosawa S, Gando S, et al. Guidance for diagnosis and treatment of DIC from harmonization of the recommendations from three guidelines. J Thromb Haemost. 2013; https://doi.org/10.1111/jth.12155.
Dupuy M. Injections de matière cérébrale dans les veines. Gaz Med Paris. 1834;2:524.
Giles AR, Nesheim ME, Mann KG. Studies of Factors V and VIII:C in an animal model of disseminated intravascular coagulation. J Clin Invest. 1984 Dec;74(6):2219–25. https://doi.org/10.1172/JCI111648.
Gando S, Nanzaki S, Sasaki S, Kemmotsu O. Significant correlations between tissue factor and thrombin markers in trauma and septic patients with disseminated intravascular coagulation. Thromb Haemost. 1998;79(6):1111–5.
Ahamed J, Niessen F, Kurokawa T, Lee YK, Bhattacharjee G, Morrissey JH, Ruf W. Regulation of macrophage procoagulant responses by the tissue factor cytoplasmic domain in endotoxemia. Blood. 2007;109(12):5251–9.
Asakura H. Classifying types of disseminated intravascular coagulation: clinical and animal models. J Intensive Care. 2014;2(1):20.
Madoiwa S. Recent advances in disseminated intravascular coagulation: endothelial cells and fibrinolysis in sepsis-induced DIC. J Intensive Care. 2015;3:8. https://doi.org/10.1186/s40560-015-0075-6.
Hoshino K, Kitamura T, Nakamura Y, Irie Y, Matsumoto N, Kawano Y, et al. Usefulness of plasminogen activator inhibitor-1 as a predictive marker of mortality in sepsis. J Intensive Care. 2017;5:42.
Sinauridze EI, Kireev DA, Popenko NY, Pichugin AV, Panteleev MA, Krymskaya OV, et al. Platelet microparticle membranes have 50- to 100-fold higher specific procoagulant activity than activated platelets. Thromb Haemost. 2007;97(3):425–34.
Nakamura M, Shimizu Y, Sato Y, Miyazaki Y, Satoh T, Mizuno M, et al. Toll-like receptor 4 signal transduction inhibitor, M62812, suppresses endothelial cell and leukocyte activation and prevents lethal septic shock in mice. Eur J Pharmacol. 2007;569(3):237–43.
Chong DLW, Sriskandan S. Pro-inflammatory mechanisms in sepsis. Contrib Microbiol. 2011;17:86–107.
Marshall JC, Foster D, Vincent JL, Cook DJ, Cohen J, Dellinger RP, et al. Diagnostic and prognostic implications of endotoxemia in critical illness: results of the MEDIC study. J Infect Dis. 2004;190(3):527–34.
Levi M, van der Poll T. Inflammation and coagulation. Crit Care Med. 2010;38(2 Suppl):S26–34.
Esmon CT. The interactions between inflammation and coagulation. Br J Haematol. 2005;131(4):417–30.
Zacharowski K, Sucker C, Zacharowski P, Hartmann M. Thrombelastography for the monitoring of lipopolysaccharide induced activation of coagulation. Thromb Haemost. 2006;95(3):557–61.
Iba T, Levy JH, Levi M, Connors JM, Thachil J. Coagulopathy of coronavirus disease 2019. Crit Care Med. 2020;48(9):1358–64.
Taylor FB Jr, Toh CH, Hoots WK, Wada H, Levi M, Scientific Subcommittee on Disseminated Intravascular Coagulation (DIC) of the International Society on Thrombosis and Haemostasis (ISTH). Towards definition, clinical and laboratory criteria, and a scoring system for disseminated intravascular coagulation. Thromb Haemost. 2001;86(5):1327–30.
Hunt BJ. Bleeding and coagulopathies in critical care. N Engl J Med. 2014;370(9):847–59.
Levi M, Toh CH, Thachil J, Watson HG. Guidelines for the diagnosis and management of disseminated intravascular coagulation. British Committee for Standards in Haematology. Br J Haematol. 2009;145(1):24–33.
Kim SM, Kim SI, Yu G, Kim JS, Hong SI, Chae B, et al. Role of thromboelastography as an early predictor of disseminated intravascular coagulation in patients with septic shock. J Clin Med. 2020;9(12):3883.
Papageorgiou C, Jourdi G, Adjambri E, Walborn A, Patel P, Fareed J, et al. Disseminated intravascular coagulation: an update on pathogenesis, diagnosis, and therapeutic strategies. Clin Appl Thromb Hemost. 2018;24(9_suppl):8S–28S.
Villa G, Fioccola A, Mari G, Cecchi M, Pomarè Montin D, Scirè-Calabrisotto C, et al. A role of circuit clotting and strategies to prevent it during blood purification therapy with oXiris membrane: an observational multicenter study. Blood Purif. 2022;51:503–12.
Turani F, Barchetta R, Falco M, Busatti S, Weltert L. Continuous renal replacement therapy with the adsorbing filter oXiris in septic patients: a case series. Blood Purif. 2019;47(Suppl 3):1–5.
39th International symposium on intensive care and emergency medicine. Crit Care. 2019;23:72. https://doi.org/10.1186/s13054-019-2358-0.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
11.1 Electronic Supplementary Material
Data 11.1
(PPTX 449 kb)
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Turani, F., Barettin, G., Busatti, S., Vannicola, F. (2023). Clinical Management of Endotoxemia: Treatment of DIC. In: De Rosa, S., Villa, G. (eds) Endotoxin Induced-Shock: a Multidisciplinary Approach in Critical Care. Springer, Cham. https://doi.org/10.1007/978-3-031-18591-5_11
Download citation
DOI: https://doi.org/10.1007/978-3-031-18591-5_11
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-18590-8
Online ISBN: 978-3-031-18591-5
eBook Packages: MedicineMedicine (R0)