Abstract
The effects of altered gravity on the vestibular system have been studied since the beginning of human space exploration. It is known that on Earth, sensory information transmitted from the vestibular system is important for gaze stability and motor control. In microgravity, aspects of the vestibular system, especially the otolith organs, do not function properly as gravireceptors and cannot provide useful information about static head orientation (tilt). The sudden offloading of gravitoinertial information has significant implications for spatial orientation, postural stability, and oculomotor function, all of which may ultimately influence in-flight operator proficiency. Space motion sickness, vestibular-mediated bone loss, and altered cardio-sympathetic reflexes are additional challenges experienced by some astronauts while adapting to and from microgravity environments. There is incentive to enhance our understanding of vestibular reflex function to reduce the often-debilitating effects of spaceflight and to improve operational performance in challenging environments.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Vestibular
- Otolith
- Microgravity
- Semicircular canal
- Motion sickness
- Space motion sickness
- Utricle
- Saccule
- Centrifuge
Introduction
Humans have evolved to function optimally in the presence of the Earth’s gravity. The vestibular system provides sensory information utilizing the constant pull of gravity, allowing for the perception of verticality, appropriate motor function, and central nervous system integration. The impact of microgravity on the vestibular system significantly influences the ability of humans to maximally perform during spaceflight and may have complex consequences, especially for long-duration missions.
Vestibular System Anatomy and Physiology
The human vestibular system is comprised of peripheral sensory organs, central processing components, and mechanisms for motor output [1]. Information transmitted from the peripheral vestibular end organs leads to appropriate postural stability and stable gaze through numerous reflex pathways. Further integration of vestibular system information throughout the cortex influences other processes, including cognition, spatial awareness, as well as autonomic reflexes and bone maintenance.
The Vestibular Labyrinth
The peripheral vestibular sensory system lies within the inner ear, laterally adjacent to the air-filled middle ear, medially bordered by the temporal bone, posterior to the cochlea (Fig. 6.1) [2]. The bony labyrinth is the osseous outer wall of the inner ear located within the temporal bone. Inside the capsule is the membranous labyrinth which contains the vestibular sensory receptors. The bony and membranous labyrinths each contain a specific fluid. Perilymph provides a cushion between the bony and membranous labyrinths and has a high sodium concentration similar to cerebrospinal fluid. Endolymph is contained within the membranous labyrinth and has a high potassium concentration similar to intracellular fluid [3]. The membranous labyrinth contains two types of sensory end organs, the semicircular canals (SCCs) and the otoliths.
The SCCs detect angular acceleration, such as head turns. These curved tubes each contain an enlargement (ampulla) housing the sensory epithelium (crista ampullaris). The crista ampullaris is covered with the sensory epithelium. A gelatinous structure (cupula) arises from the crista ampullaris, extending across the ampulla to maintain a fluid tight seal. Because the cupula maintains the same specific weight as the surrounding endolymph, it does not respond to linear forces [4,5,6].
The crista ampullaris contains the sensory hair cells responsible for encoding angular acceleration. The vestibular end organs are mechanoreceptors that translate mechanical force into neural potentials. Each sensory epithelium contains bundles of 20–100 stereocilia and one kinocilium. The cilia are linked in a stairstep pattern that allows the bundle to deflect together [7]. There are two types of hair cells—type I and type II. These different hair cells produce irregular and regular firing patterns, respectively [1], allowing for the broad representation of frequency and acceleration information needed to accurately identify acceleration profiles [8,9,10].
Vestibular system afferents produce high spontaneous resting rates which allows each sensory end organ to demonstrate firing patterns encoding both excitation and inhibition of the system [11, 12]. The three SCCs are oriented orthogonally in yaw, pitch, and roll planes. The vertical (anterior and posterior) canals form an approximate 45-degree angle with the sagittal plane [13]. Between ears, the SCCs are coplanar and are inversely excited in a push–pull fashion. For example, excitation of the anterior SCC in one ear corresponds to inhibition of the posterior SCC in the opposite ear. This arrangement allows for three-dimensional representation of rotational acceleration (Fig. 6.2) [14].
Semicircular canal physiology. The semicircular canals encode angular acceleration. When the head is rotated, the endolymph lags, bending the cupula in the opposite direction and deflecting the underlying sensory hair cells to encode the acceleration (With permission from CFCF / Wikimedia Commons / Public Domain / https://commons.wikimedia.org/wiki/File:1410_Equilibrium_and_Semicircular_Canals.jpg)
The two otolith organs, the saccule and utricle, lie within the vestibule in the center part of the bony labyrinth. Sensory neuroepithelium reside in each organ as a single patch of sensory cells, called macula. The maculae are positioned horizontally in the utricle and vertically in the saccule. The sensory hair cell bundles project into a gelatinous membrane which is embedded with calcium carbonate particles (otoconia). The additional weight provided by the otoconia means that the maculae are heavier than the surrounding endolymph. Linear acceleration generates force on the otoconia and gelatinous membrane, resulting in deflection of hair cell bundles. The utricle is stimulated by movement in the horizontal plane (e.g., head tilt sideways; lateral displacement) while the saccule is excited by movement in the vertical plane (e.g., sagittal plane upward, downward; forward, backward) (Fig. 6.3) [15].
Otolith organ physiology. The otolith organs encode linear acceleration induced by head tilt or linear translation. When the head is tipped, the otoconia are pulled downward, deflecting the underlying sensory hair cells and encoding the acceleration (Used with permission of Mayo Foundation for Medical Education and Research, all rights reserved)
While the SCCs and otolith organs are coplanar between ears [16], each otolith organ also encodes both excitation and inhibition for each linear acceleration. The otolith organs are divided into two sections of opposing polarity demarcated by the striola, a curved dividing ridge running through the middle of the macula. Head tilt results in excitation of a distinct subset of hair cells on one side of the striola and reduced afferent discharge from the hair cells on the other side. Additionally, a subset of afferent fibers encodes when the head is upright, increasing or decreasing the discharge rate with head tilt [17]. The otolith organs are limited in the capacity to distinguish between tilt with respect to gravity and linear translation. For example, the set of otolith cells that are activated by head tilt toward the right ear is also activated by translational acceleration toward the left ear [15]. This is resolved by incorporating extra-otolith cues from SCCs, proprioception, and visual system information [18].
From the peripheral end organs, afferent projections travel along the vestibular nerve (cranial nerve eight, CN VIII) [19] and enter the brainstem at the pontomedullary junction. Central processing initiates as CN VIII enters the brainstem, the vestibular nucleus complex, and the cerebellum. These areas facilitate integration of input from each vestibular labyrinth, as well as from somatosensory and visual systems [1]. Otolith and SCC input continues to integrate at all central vestibular areas, from the vestibular nuclei to central vestibular processing centers [20].
Vestibular Reflex Function
The vestibular system is involved in a variety of functions, ranging from postural and oculomotor reflexes to spatial representation and cognition [21]. The vestibulo-ocular reflex (VOR) is the most well-described vestibular-mediated pathway. The VOR functions to stabilize images on the fovea by producing compensatory eye movements in the direction opposite a given head movement [1] at head rotations greater that 1 Hz [22, 23]. The VOR encodes the physical acceleration of the head into neural signals directing eye movement [1]. This reflexive eye movement is elicited at the first level of central vestibular processing through innervation of the vestibular nuclei. The resulting VOR response, or nystagmus, is used as a metric to quantify function.
Vestibular information is transmitted to the trunk and limbs for postural control through the vestibulo-spinal reflex (VSR). Most contralateral VSR inputs are part of the medial vestibulo-spinal tract (MVST) [24]. The MVST originates primarily from the medial vestibular nucleus, descends through the medial longitudinal fasciculus bilaterally, and terminates no lower than the mid-thoracic spinal cord [25]. Most ipsilateral excitatory pathways are part of the lateral vestibulo-spinal tract (LVST). The LVST originates in the lateral vestibular nucleus, descends through the inferior vestibular nucleus, and terminates on the anterior horn cells at various levels of the spinal cord. The MVST mediates head position by controlling neck and shoulder muscles, while the LVST controls postural adjustments to movement. When the head is tilted, both the SCCs and otolith organs are activated, transmitting impulses through the MVST and LVST to the spinal cord to induce extensor activity ipsilaterally and flexor activity contralaterally. An additional pathway originates in the reticular formation, descends to the spinal cord, and influences limb and trunk movement. The vestibular nuclei and reticular formation provide information to the spinal cord to maintain compensatory feedback responses to postural instability [1].
Central Vestibular Processing
Beyond stabilizing gaze and regulating postural control, the vestibular system also contributes to interpreting heading direction, localization of body in space, and distance traveled using inertial information obtained during displacement [26,27,28,29,30,31,32,33,34]. This information is uploaded and cross-referenced with other sources of sensory information.
Higher-order functions, such as spatial memory and self-motion perception, are associated with vestibular projections to the thalamus, which processes and relays sensory information to the cortex [16, 35,36,37]. These projections are multisensory and include convergent motor signals and proprioceptive feedback information [38]. The cerebellum maintains a key role in spatial orientation, motion perception, and vestibular reflex integration [39]. Vestibular system afferents directly project to the cerebellum [40], with afferent projections described from the SCCs to the nodulus and from the saccule to the uvula [41].
Vestibular sensory information ascends throughout the cortex, but unlike other sensory systems, there is no isolated primary vestibular cortex in primates. Instead, there is a network of separate areas in the temporoparietal area—the parietoinsular vestibular cortex (PIVC)—that integrates vestibular, visual, and somatosensory input [42, 43]. There are also projections between the hemispheres, throughout the pontomesencephalic brainstem, and between the PIVC and visual cortex [43].
Vestibular system information becomes multimodal at an early stage of processing [36], as various sensory inputs within the brainstem generate a “best estimate” of body orientation and motion within the environment [44]. This integration is described as both multisensory convergence and multisensory transformation [45]. Convergence occurs as sensory information from various vestibular end organs combines with information from other sensory inputs. Transformation occurs when one sensory modality influences the integration of additional sensory modalities. This is illustrated by the known activation of the PIVC and simultaneous decrease in visual cortex activity with vestibular system stimulation [46]. Inversely, inhibitory vestibular-visual sensory interaction has also been described using large-field optokinetic stimuli to induce self-motion perception, finding increased activity in the occipital cortex and simultaneous decrease in PIVC activity bilaterally [47]. This relationship allows the dominant sensory input to shift from one modality to another, depending on the most reliable mode of stimulation [48]. Sensory integration and transformation are key to understanding central vestibular pathway compensation mechanisms.
Vestibular System Compensation
Reduced vestibular system function following peripheral or central pathology results in symptoms of dizziness and imbalance. These symptoms typically resolve over the following weeks due to the central nervous system’s ability to compensate [49]. When a vestibular reflex pathway is altered, dizziness occurs due to an imbalance in vestibular nuclei resting neural discharge rate. During compensation, the resting rate is “rebalanced” as the commissural inhibitory system linking the vestibular nuclei modifies expectations regarding the current input [50]. Additional factors, such as altered vestibular nucleus neuron excitability, altered inhibition of vestibular networks via the cerebellum, neurogenesis in the ipsilesional vestibular nuclei, and adjustment of synapses in the vestibular pathways likely contribute to this process (for review, see [51]). Compensation allows for recalibration of altered vestibular sensory input and applies, in part, to the adaptation that astronauts experience upon and during exposure to microgravity. Regardless of the underlying cause, disruption of vestibular input leads to a compensatory response to reorganize and rebalance sensory input [52].
History of Vestibular System Evaluation in Spaceflight
Understanding the impact of microgravity on the vestibular system has long been at the forefront of space research. In 1961, Yuri Gagarin became the first man to enter space, completing an orbit in less than 2 h. He reported no significant vestibular concerns during his short exposure. It was GhermanTitov on the subsequent Vostok 2 mission who demonstrated the significance of microgravity on the vestibular system. Once in orbit, Titov described an abrupt onset of nausea and vomiting with lingering illness even after sleeping. Symptoms abruptly resolved nearing the end of his 25-h flight and he described feeling completely functional. Titov was the first human to experience space motion sickness [53, 54].
Initial reports of spatial disorientation experienced on Mercury and Gemini missions in the 1960s were minor and had minimal reported impact on operations. Therefore, initial work evaluating neurovestibular function focused on postural instability and reduced coordination post-flight, well-known challenges documented as early as the Apollo missions. Bedside balance and gait evaluations were completed before and after return from missions [55]. Specialized platform-based postural stability measurements were included as technology advanced [56]. Postural instability continues to challenge returning astronauts and these tools remain a useful metric to guide our understanding of imbalance.
The focus of neurovestibular research expanded as astronauts moved more freely within the capsule and as mission durations increased [57]. Symptoms associated with “space motion sickness” (SMS) or “space adaptation syndrome” were reported by both American and Soviet space programs, heightening concern for reduced operational performance that could endanger the crew as well as the mission [58,59,60]. The prevalence and severity of SMS were unexpected—astronauts were known for high levels of motion tolerance and had significant aviation training [61]. While initial reports described mild symptoms, later crew described symptom severity that could be quite severe [62, 63]. Today, ongoing research endeavors to better understand susceptibility and appropriate countermeasures to reduce SMS. Conclusive results are lacking, and SMS mitigation remains difficult. Astronauts continue to experience these effects. In mild cases, many wait out the symptoms, while nearly half report managing symptoms with vestibular suppressants [64,65,66].
Research describing vestibular system physiology in microgravity began in earnest in the 1970s and 1980s with the Skylab and Salyut space programs [67]. Researchers adapted technology commonly used on Earth to conduct comparable studies on the station, such as the rotational chair used for Skylab Experiment 131 (Fig. 6.4). This study compared SCCs and otolith function on Earth and in microgravity conditions [68]. Later investigations conducted through the 1980s and early 1990s examined visual, vestibular, and visual-vestibular integration function of astronauts on both short- and long-duration missions [69]. Research conducted on the space stations evaluated how atypical vestibular system information altered various domains, including postural stability, motor control and adaptation, and operational proficiency [70, 71].
Skylab’s Human Vestibular Function Experiment 131. This study evaluated coordination function in long-duration spaceflight, focusing on susceptibility to motion sickness as well as otolith and semicircular canal function (With permission from NASA/Marshall Space Flight Center/Public Domain https://archive.org/details/MSFC-0102036)
When the shuttle program began in the 1980s, astronauts were tasked with increased operational control during missions. In higher risk situations, such as the return to Earth, the sudden reintroduction of otolith reflex pathway information led to unique challenges not previously highlighted in capsule landings. The abrupt addition of otolith reflex pathway information led to overestimated perception of translation during and after landing [72]. An unexpectedly high proportion of shuttle landings occurred outside of preferred operational specifications, which was attributed at least in part to the somatogravic illusion. This illusion occurs when otolith reflex information is misinterpreted, resulting in altered attitude perception. Commanders and pilots were forewarned about this illusion, but it could not be replicated during simulation [73]. No significant events occurred during landing that were attributed to this illusion; however, the profound effect of reintroducing otolith information abruptly into overall spatial perception is a significant concern when altered perception may reduce operational performance.
Research continues to expand our understanding of the effects of microgravity on the vestibular system. The long-term presence of astronauts living on space stations has allowed scientists to further study these complex sensory interactions [59].
Techniques Used to Study the Vestibular System in Microgravity
While altered vestibular system function may lead to significant operational concerns, evaluating the vestibular system during spaceflight is challenging. Various methods have been used to evaluate the numerous reflexes associated with the vestibular system; however, these assessments are likely incomplete.
Animal Models
Because of the limited number of astronauts available for testing, along with the less than optimal test methodology, animal models provide an invaluable method for evaluating the effects of altered gravity in meaningful ways. Significant structural changes have been documented in non-human species, particularly related to the otolith organs. Histopathological studies have described increased otoconial mass in adult rodent utricles in as little as 1 week of microgravity exposure [74, 75], while saccular changes have been shown only with embryological or larval exposure in mollusks and newts [76, 77]. Conversely, hypergravity environments, such as prolonged centrifugation, reduced otoconial mass in mollusks, fish, and rodents [78,79,80]. Sensory hair cells and neural synapses have also demonstrated alterations. In rodent models, neurodegeneration has not been observed in short-duration spaceflight [74], but increased perinuclear and intercellular spaces have been found [78, 81]. Interestingly, another study using a rodent model found that there were significant changes in synaptic density for utricular areas associated with encoding low frequency and static changes in linear acceleration [82]. Longer duration missions have shown increased alterations in type I and type II hair cells. Specifically, hair cells have developed significantly more neural synapses in microgravity which reduced to baseline upon return to Earth [83,84,85]. These data suggest that the vestibular system will adapt—often quickly—to altered gravity environments, though the extent to which these changes occur in humans is not yet known.
Vestibular reflex pathway recordings are the standard method for documenting function. SCC-mediated VOR responses are stimulated by angular acceleration and therefore should not show altered function in microgravity. Animal models, however, provide evidence of transient angular VOR alterations. In monkeys, single unit recordings from the medial vestibular nucleus and flocculus have shown significantly reduced neural activation in the first few days of microgravity exposure [86]. Responses to linear acceleration have also demonstrated variability over the first few days, with increased neural activity recorded in the vestibular nucleus within hours of exposure. While activity levels return to baseline over the following day, another increase in neural response to linear acceleration has been recorded on days four and five, again returning to baseline. Responses to linear and angular acceleration have described variable time courses for adaptation and suggest that the otolith organs contribute to the adaptation mechanisms for the angular VOR pathways [86].
Earth-Bound Models
Few humans have been studied in an actual microgravity environment and the data collected from those human studies have often been inconclusive or contradictory. Earth-bound models can be utilized to improve our understanding of the effects of altered gravity conditions in larger groups of subjects.
Parabolic flight has been used consistently to evaluate vestibular reflex pathways. In this paradigm, 20–30 s of actual microgravity can be achieved per parabola. This method provides the only Earth-bound model to achieve actual microgravity, but study methodology is limited to those tasks that can be completed in this short duration [64]. Importantly, parabolic flight provides a method to closely describe vestibular system performance at the initial transition between gravity conditions, capturing the effects of sudden on- and offloading of otolith reflex information. Since this information has not been documented in actual spaceflight, parabolic flight provides valuable insight into this transition. Data collection during parabolic flight should be interpreted during this time course and not used to infer vestibular system function throughout spaceflight. Additional factors such as vestibular system adaptation and compensation, body fluid redistribution, diminished muscle mass, underlying anatomical changes, and prevalence of SMS cannot be replicated with this technique [64]. Parabolic flight continues to be useful in describing function during critical gravity transitions, but also leads to improved research questions and protocol development for missions where more detailed investigation may occur.
Prolonged head-down bed rest is used in various Earth-bound protocols to simulate the reduced sensorimotor input and altered cerebral hemodynamics found in microgravity [87]. This method allows for improved understanding of the somatosensory system and its influence on posture and motor control. Subjects evaluated using this analog demonstrate similarly reduced postural performance as astronauts evaluated after return to Earth [88]. This method not only has shown usefulness in modeling somatosensory changes, but also has been used to evaluate altered central integration. Advanced imaging methods such as resting state functional magnetic resonance imaging (fMRI) have demonstrated altered connectivity between the motor, somatosensory, and vestibular systems when completing spatial orientation tasks [89], and even simple vestibular reflex pathways have shown reduced function [90]. Head-down bed rest provides a useful model to evaluate the effects of microgravity in a larger pool of subjects in controlled conditions that may not be replicated in spaceflight.
While microgravity receives more focus, astronauts are subjected to enhanced gravity during launch (~3.2 g) and upon return (~1.4 g). Enhanced otolith information also influences vestibular reflex pathways and may confound operational performance, as noted with the somatogravic illusion described in section “History of Vestibular System Evaluation in Spaceflight”. Centrifugation has long been used as a method to evaluate the effects of hypergravity. Humans are generally only temporarily exposed to hypergravity conditions; however, this can have significant effects on operations. Atypical orientation perception has been reported as an overestimation of roll-tilt angle during hypergravity conditions, yet an underestimation during centrifuge-created hypogravity conditions [91, 92]. Performance variations have been described. For example, flight simulator performance has been significantly reduced in naïve subjects in hypergravity conditions, but not for trained aviators [93, 94], suggesting that training may assist in managing altered orientation effects.
Methodology
Technologically advanced methods for evaluating vestibular function were introduced during the Skylab missions in the 1970s [95, 96]. While direct assessment of each vestibular end organ is not possible using current techniques, there are numerous methods available to evaluate subsequent reflex pathways. These recordings therefore infer the functionality of the end organs. Vestibular system testing most commonly includes the VOR. Various methods of nystagmography (i.e., eye movement recording) have been used, including video cameras, scleral coils, corneo-retinal dipole potentials (e.g., electronystagmography), and infrared pupil recordings (e.g., videonystagmography) as technology developed and advanced.
VOR testing can be completed using various protocols. Caloric testing is commonly used clinically for the diagnostic evaluation of the vestibular system. It is a well-established technique but was not expected to be reliable in flight. Robert Bárány’s work describing the thermo-conductive mechanism elicited by endolymphatic temperature change in the horizontal SCC [97, 98] suggests that the caloric response should be hindered in microgravity. This was supported by results obtained in parabolic flight, which found reduced nystagmus in microgravity and enhanced nystagmus in hypergravity conditions [99, 100]. Bárány’s theory on the mechanism of this response was further evaluated with work completed on Skylab. These studies found no significant change in the nystagmus response from on-Earth measures [101,102,103], and led to alternative hypotheses for the underlying mechanism of this response [104]. Interestingly, work completed on Skylab-1 found consistent nystagmus responses in flight—except for one recording completed on the first day in orbit [105]. That individual datapoint suggested that there may be variability in VOR function over the course of adaptation to microgravity and was consistent with data collected in parabolic flight. Taken in context, the abrupt offloading of the otolith organs upon entry into microgravity is hypothesized to initially suppress the angular VOR response, returning to typical function over the course of a few days [106]. Therefore, the expected outcomes for VOR comparison will vary depending on the time post-entry into space.
While the caloric response is standard for evaluating the vestibular system on Earth, this test elicits a low frequency response (~0.003 Hz) [107] that is well below typical functional movement. Understanding the compensatory ability of the caloric response may not carry over into interpreting the higher frequency function needed for typical activities. Physiologically, frequencies are encoded differently, with low frequencies encoded by regular vestibular afferents and higher frequencies encoded by irregular afferents [107]. Rotational chair testing offers an ability to evaluate angular VOR function using various frequency and acceleration profiles. This technology has been studied in Earth-bound and microgravity environments [95], providing an understood model of bilateral vestibular system integration. This technology requires equipment capable of precise performance; however, there are limitations. For example, higher frequency oscillations can produce significant artifact in the recordings. Additionally, this method requires substantial equipment, challenging considering weight restrictions and available space on board the craft. Other methods are under evaluation. With advancing technology, higher frequency VOR responses will be evaluated, recording reflexive eye movements during head oscillations at target frequencies above those recorded with previous techniques [108]. Newer methods may prove more helpful in documenting change in angular VOR function over time while also using more compact equipment.
While the otolith system is key to understanding the effects of microgravity on the vestibular system, otolith reflex testing is challenging. Initially, there were no clinical protocols available to easily transition into assessing otolith information in flight. Earth-bound protocols were only available in specialized laboratories. These tools were modified for use in orbit, with one of the first iterations used on Skylab. Early research utilized a “space sled” designed to evaluate the otolith system [109]. This device included a 6-m-long assembly mounted to the floor of Skylab to provide controlled linear acceleration. While this device provided the capability to perform precise experiments, it did require a significant footprint on board the laboratory [110].
Because of the technical limitations associated with linear translation paradigms on board, researchers assessed other possible methods for documenting otolith function. Otolith information is integrated into various additional pathways, including the VOR, and is required for appropriate neural representation of the VOR in pitch and roll. When the head tilts, the otolith reflex pathways induce ocular counter-roll (OCR) or torsional VOR. Absent OCR leads to atypical representation of the environment and contributes to spatial disorientation. OCR can be used to document otolith function using centrifuge [111] or retinal afterimage paradigms [112].
Otolith reflex pathways may also be evaluated using evoked potentials. This technology is newer but shows promise as a simple method to evaluate these reflex pathways. Vestibular evoked myogenic potentials (VEMP) evaluate the sacculo-collic (cervical VEMP) and utriculo-ocular (ocular VEMP) reflex pathways. These potentials can be quickly acquired with minimal equipment and provide information regarding descending vestibulo-spinal pathways not previously well-described. While clinicians have used this technique for years, minimal work has been done in microgravity. In parabolic flight, cervical VEMP responses have demonstrated greater amplitude in hypogravity than in normo- or hypergravity conditions [113], consistent with enhanced neural responses documented in animal models [114]. Further work with this technique is needed to evaluate its usefulness in understanding the otolith-mediated reflex pathways in prolonged microgravity environments.
Returning astronauts continue to experience challenges with postural stability [115] and are evaluated using a combination of bedside measures, computerized balance paradigms, and kinematic analysis of gait. Computerized methods have led to improved ability to understand the contributions of visual, vestibular, and somatosensory cues after exposure to microgravity in order to determine any prevalent sensory preference. Other methods have used objective recording of Hoffmann’s reflex (H-reflex) [116] or electromyography (EMG). These techniques utilize the relationship between the otolith organs and vestibulo-spinal network to incrementally study these reflex pathways in weight-bearing muscles. Responses have been studied during and after exposure to vertical linear translation.
Effects of Microgravity on the Vestibular System
Once in orbit, the otolith organs are immediately offloaded, meaning that they no longer function as gravireceptors. The alteration in expected vestibular system input disrupts orientation, balance, and gaze control, affecting perception of self-orientation and motion [117] and requires the central nervous system to recalibrate and adapt [118].
Otolith Function
Most vestibular-related effects in microgravity occur after the abrupt loss of otolith-specific information. Under Earth-bound conditions, the otolith organs are stimulated by head tilt and translation that depend on head orientation relative to gravity, thereby eliciting the OCR reflex and aiding in the VSR. In microgravity, the otolith organs do not function properly as gravireceptors and cannot provide useful information about static head orientation (i.e., tilt). The microgravity environment does not exclude otolith information entirely as translation is still encoded. Additionally, otolith reflex pathways and the gravitoinertial analyzer are abnormally excited at least during the initial transition to microgravity [113, 114, 119], while low frequency otolith afferent information is suppressed by the central nervous system [120]. This is an important consideration: microgravity and vestibular dysfunction are not the same in terms of central interpretation. In microgravity, otolith information is still transmitted for linear translations, but not for head tilt, while we assume that vestibulopathy impairs both [114].
Initial evaluation of otolith reflex function in altered gravity was described using animal models in parabolic flight. In the frog, utricular neural activity varied closely with the magnitude of gravitational change. During the transition from 1 g to 0 g, there was an initial increase in spontaneous neural firing followed by a subsequent suppression of neural activity at 10 s into weightlessness. A large increase in neural firing was then noted in the hypergravity condition with a final restoration of baseline discharge rate after returning to the 1 g condition. In prolonged microgravity, it is presumed that the otolith organs “float” which overall should lead to decreased excitation [114].
The OCR is absent in microgravity conditions [121] and is reduced following long-duration spaceflight [122]. While those returning from short duration missions may not experience significant reduction [123], OCR may take several weeks to recover [122, 124,125,126]. There does not appear to be a lasting effect, however, and OCR eventually returns to pre-flight values [59].
Semicircular Canal Function
In contrast to the gravity-dependent otolith system, the SCCs should be unaffected by altered gravity environments [127,128,129]. Rotational chair testing has been used to describe angular VOR function and responses induced by trapezoidal acceleration several days into flight have not been significantly different from baseline. In-flight recordings to angular velocity changes have found nystagmus velocity (i.e., SCC response) as independent of linear acceleration [130].
While the SCCs may not be affected physiologically by changes in gravity environment, they are not completely immune to these effects. The otolith organs mediate these pathways. This was described early by evaluating the effect of “cross-coupling,” or simultaneous stimulation of multiple vestibular end organs. Significantly, cross-coupling the SCCs did not lead to motion sickness in microgravity—an unexpected finding as this perception (i.e., Coriolis effect) is quite profound on Earth [95]—and was quickly associated with reduced otolith contribution to this integrated mechanism. Additional VOR responses, such as measures of the vertical SCC VOR pathways and central velocity storage mechanisms that prolong the VOR response to sustained motion, are reduced in microgravity [126, 131,132,133]. These paradigms provide evidence that the otolith-ocular pathway contributes to the integration and interpretation of the angular vestibular reflex pathways.
Research continues to evaluate alterations in vestibular system function. As previously discussed, traditional methods of evaluating SCC function focus on low frequency stimuli due to methodological limitations. Newer techniques are providing access to higher frequency VOR responses that more consistently align with typical head movements. Recent work has evaluated the recovery of angular VOR function using stimulus frequencies up to 1 Hz. Results suggest that there is a frequency effect to the angular VOR compensation process in flight, with higher frequencies requiring longer compensation time [108]. It is not yet known if full compensation can be achieved, even in long-duration flight, and what limitations may continue. As we learn more about these responses, our understanding of the influence of gravity on the angular VOR system will likely change.
Postural Stability and Sensorimotor Responses
Data from various laboratories have suggested that prolonged exposure to microgravity leads to postural instability for various reasons, including:
-
1.
Decreased requirement for postural reflexes in weight-bearing muscles
-
2.
Central nervous system reinterpretation of otolith reflexes
-
3.
Reduced static and dynamic postural inputs from the proprioceptive system
-
4.
Altered tonic activity in soleus and anterior tibialis muscles (for review, see [134])
-
5.
Increased sensory weighting to visual cues.
Some level of disorientation during and after landing has been universally reported, with ataxic gait, inability to correct for postural errors, and concern for falling prevalent among returning astronauts. Furthermore, returning crew have described the need for slow and focused movements to stay upright and noted concern if quick responses were needed during an emergency [73].
Postural stability has been shown to decline with both short- and long-duration spaceflights, although the effects have been more pronounced and persistent with longer exposure [135]. Increased sway when in vision denied and/or disrupted somatosensory conditions has been described [136]. For a week post-return, postural stability tasks that included dynamic head movement on an unstable platform have been too difficult, suggesting a reweighting of balance ability to increased reliance on somatosensory cues [135].
Evoked potential recordings have demonstrated facilitation [137] or early potentiation [138, 139] of the H-reflex as a function of free fall or reduced gravity load. Prolonged free fall has been shown to facilitate sensory-motor rearrangement, and this adaptation may lead to central reinterpretation of otolith input. Similarly, rearrangement and reinterpretation have been proposed as a possible mechanism for muscle proprioceptive signal alterations that occur during prolonged exposure to microgravity [64]. These reflexes return to pre-flight values immediately after flight [138].
The effects of deconditioned otolith-spinal reflexes extend to gait and locomotion. Postural muscles contributing to upright stance have been shown to atrophy in microgravity [140]. Post-flight changes in step-cycle, walking speed, gaze stability, and amount of unrestricted head movement have all been reported [52, 141, 142]. Ataxia, disorientation in unstructured visual environments, illusory movement of the visual field, veered walking path, disruption of head stabilization in response to vertical translation [143], and decreased stability while turning corners have been demonstrated shortly after return [121, 144]. For short duration missions, these effects typically diminish within 12 h; however, for long-duration missions, it may take weeks for gait to return to pre-flight baseline [145].
Oculomotor Function
Although spatial orientation in microgravity shifts to increased dependence on visual and somatosensory cues, vision may be altered in microgravity as well. Detailed information on changes to the ocular system is provided in Chap. 7. While altered VOR pathways in microgravity conditions were expected, research has found significant deficits in other oculomotor domains including gaze stability, saccade and smooth pursuit systems, and gaze fixation ability [146, 147]. Parabolic flight paradigms have described reduced precision and speed for smooth pursuit tasks, as well as prolonged duration for establishing stable gaze [148]. These results are consistent with returning crew who have consistently demonstrated reduced performance in acquiring visual targets, prolonged latency, and reduced eye and head movement velocity. Additionally, returning crew have demonstrated reliance on saccadic eye movement to manage smooth pursuit stimuli [144, 148, 149]. Oculomotor challenges have been described especially for vertical eye movements, consistent with known otolith involvement in these pathways [150]. Overall, recovery time of these metrics is similar to that of the OCR, with return to baseline over days to weeks [146].
Visual perception is another area of concern. Judgment of size and distance of objects is altered during [151] and following [152] several months of microgravity exposure, suggesting that mental depiction of three-dimensional space may be altered. Both close (<60 cm) and long (180 m, 1500 m) range distances have been underestimated by as much as 35% when compared to ground-based performance. Specifically, in this environment, the body is used to scale visual space as well as to perceive the size and distance of objects [152]. There may be significant limitations associated with this altered perception. For example, a review of 100 missions found that 20% of landings were above limits for touchdown speed, emphasizing the altered judgment of distance estimates [153]. These perceptual changes have implications for operational tasks and crew safety, especially during critical phases of the mission.
Other Consideration
To reduce the effects of altered otolith information, studies have evaluated the possible benefit of centrifugation while in orbit. This method was designed to stimulate the otolith pathways during the mission to maintain conditioning. Unfortunately, there have been mixed results using this paradigm as a method to significantly improve OCR function [111, 154]. There may be additional reasons to consider stimulating this pathway, however, as the vestibular system does interact with various other systems, especially the sympathetic nervous system. Most significant for this discussion include bone remodeling and autonomic reflex function.
Bone loss is a recognized sequela of spaceflight associated with the effects of prolonged weightlessness on the skeletal system. Bone loss occurs rapidly, within a few days after exposure and can be severe after two to five months in orbit. Upon return, bone is regained, however, bone density generally does not reach pre-flight levels. Therefore, astronauts may be at risk for accelerated bone loss leading to early-onset osteoporosis after a career in spaceflight [155]. Animal models have demonstrated reduced bone formation in microgravity, as well as enhanced bone development in hypergravity conditions [156, 157]. There are additional downstream effects associated with bone loss, including reduced magnesium, vitamin D, and protein available for absorption [158]. The relationship between the vestibular system and sympathetic skeletal projections that influence bone remodeling have been described in animal models noting significantly reduced bone formation and increased bone absorption in weight-bearing bones [156, 159]. While this work has been completed on Earth in animals with peripheral vestibulopathy, further investigating this relationship in altered gravity may lead to additional methods to address spaceflight-induced osteoporosis concerns.
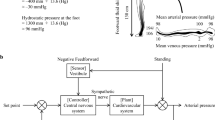
Autonomic function may also be altered with atypical otolith input. Vestibulo-sympathetic reflexes, such as those involved in cardiovascular system regulation, may be impacted [160,161,162]. The otolith organs are especially involved in regulating blood pressure during orthostatic challenge. Carotid heart rate and mean arterial pressure are significantly altered in the various gravity environments obtained in parabolic flight paradigms, emphasizing the relationship of the otolith organs in regulating these responses [163, 164]. There has been a significant association reported between altered OCR during head tilt and reduced blood pressure response in symptomatic astronauts post-flight (Fig. 6.5) [165]. Animal models evaluating this relationship have found that microgravity-associated cardiovascular changes do not occur in those with vestibular end organ lesions [166,167,168], highlighting the likely association between unreliable otolith reflex information and these sympathetic responses. Impaired vestibulo-cardiovascular responses have been measured in humans for up to 4 days after return from long-duration missions, returning to pre-flight levels within 2 months. These data suggest that long-term exposure and deconditioning of otolith-mediated autonomic system reflexes may contribute to spaceflight-induced orthostatic intolerance [160, 169, 170].
Approximated depiction of post-flight recovery timeline for mean postural control after short- and long-duration spaceflight (adapted from [135]), OCR after long-term spaceflight (adapted from [165]), cardiovascular control measured via mean arterial pressure (adapted from [165]), and gait equilibrium measured via amplitude of lateral body displacement during the gait cycle (adapted from [171]). Change in function was approximated based on 100% pre-flight performance. The initial recovery phase is highlighted in the lighter box. Slower recovery phase is depicted in the darker box
Space Motion Sickness
Space motion sickness (SMS) affects nearly 70% of astronauts, developing within an hour after launch and resolving within 3–4 days. The sensation has some characteristics similar to motion sickness experienced on Earth, including nausea, drowsiness, and fatigue. The initiation and resolution of SMS though is quite different than on Earth (Table 6.1) [172,173,174], as most describe abrupt onset and offset of symptoms.
Two theories have been proposed to account for SMS. (1) The fluid shift hypothesis suggests that SMS occurs when intracranial pressure, cerebrospinal fluid pressure, and/or inner ear fluid pressure increases and alters vestibular end organ function. This hypothesis suggests that central volume expansion lowers the threshold for vestibular stimulation, leading to increased motion sensitivity [175, 176]. (2) The sensory conflict hypothesis describes the conflict between actual and anticipated otolith signals, leading to a mismatch between visual and vestibular information [175, 177, 178]. Additionally, sensory feedback pathways also differ from the actual motor commands, enhancing the conflict. Sensory conflict is the most accepted mechanism for understanding SMS. Head movements, especially in the pitch plane [179], unusual visual patterns, and adverse reaction to orientation illusions have also been associated with increased SMS symptoms.
Due to the high prevalence of SMS in astronauts, significant research has been conducted to predict who may be most at risk. Questionnaires [180], laboratory studies including provocative visual and vestibular stimulation [181], and personality trait analysis [182] have been used in attempts to predict the degree of SMS, all without significant success. Standard clinical measures of vestibular end organ function do not predict SMS [183]. Minimal association has been found between individuals who experience motion sensitivity during parabolic flight and those who later develop SMS [120, 184]. Interestingly, parabolic flight paradigms may have found a possible connection between changes in torsional ocular alignment associated with the effects of otolith reflex pathway asymmetry decompensation. When the otolith organs are offloaded, any underlying otolith asymmetry may be recovered [154, 185,186,187,188,189]. This has been documented in spaceflight as well and noted to persist throughout long-duration missions and upon return to Earth [190]. Further work continues to evaluate the predictive value of pre-flight evaluation of torsional ocular alignment as a metric to identify individuals at risk for significant SMS.
Perceptual Changes in Microgravity
Spatial disorientation is common in microgravity due to changes in otolith sensitivity and altered central integration of extra-vestibular inputs. Perceptual illusions were described initially in the 1960s as the “wrong position of the body in space” [192]. These sensations were highly variable, developed abruptly or gradually, and were present regardless of the eyes being open or closed. Illusions consistently resolve with acceleration changes and can be reduced with increased proprioception, such as using footholds to anchor oneself [192, 193]. Approximately 80% of crew members have described illusory sensations of self and the surrounding during active head movements [194], suggesting that internal estimates of verticality are unstable. This is likely to occur only in those with appropriate vestibular function; sensory illusions are not expected in those with vestibular areflexia [192].
Duration of microgravity exposure is important in the formation of perception change; however, nearly all crew members experience at least some disruption of spatial orientation on transitioning to microgravity [195]. In parabolic flight, individuals often have difficulty in determining “up” or “down,” instead deferring to the position of the head as “up” and feet as “down” [196]. This inversion illusion [192] occurs early in the transition to microgravity as the otolith are abruptly offloaded and typically disappears with longer duration exposure as the body becomes the frame of reference for self [196]. Many describe the inversion illusion as a sense of tumbling backward upon entering microgravity, or as a prolonged sense of being upside down [195]. Describing internal perception of verticality can be done using a subjective visual vertical (SVV) task. On Earth, correct verticality estimates depend on visual, proprioceptive, and vestibular cues, weighted in proportion to reliability [197]. The otolith reflexes are heavily involved in this estimate [198, 199]. In microgravity, however, the lack of otolith input leads to a bias in verticality toward the body’s midline, or the idiotropic vector [197]. Upon return, the mean ability to complete this task returns to baseline, however, there is a significant difference in pre-post flight variability or precision. This variability suggests that otolith input may not immediately integrate reliably into maintaining spatial orientation [200].
Post-Spaceflight Vestibular Adaptation
The transition between gravity environments, whether into or return from microgravity leads to significant alterations in coordination between sensory feedback and motor control. These changing environmental demands can be challenging as crew members return to on-Earth gravitational conditions. The significance of understanding these effects was well described by American astronaut Scott Kelly, who stated that after returning from his 340-day mission, “…Every part of my body hurts. All my joints and all of my muscles are protesting the crushing pressure of gravity…I struggle to get up. Find the end of the bed. Feet down. Sit up. Stand up. At every stage I feel like I’m fighting through quicksand. When I’m finally vertical, the pain in my legs is awful, and on top of that pain I feel a sensation that’s even more alarming: it feels as though all the blood in my body is rushing to my legs…” [201].
The effects of abrupt reintroduction of otolith information into the vestibular system can be striking and immediate; however, evaluating vestibular reflex pathways and adaptation mechanisms has been challenging. The vestibular system demonstrates functional changes within the first hours to days following a transition between gravity conditions and therefore likely requires evaluation quickly upon return as well as over the next days to weeks. Additional variables, such as mission duration, are also likely to play a role in the ability of the vestibular system to quickly and adequately compensate (Fig. 6.5) [202].
Post-flight functional decrements have been documented since the Apollo era, and have included reduced postural control and motor coordination, ataxia, oculomotor deficits, and significant lightheadedness [203]. Gait and postural control have been extensively evaluated. Most returning astronauts have described perception of self or environmental motion during the return flight and after landing [57]. While kinematic data have shown that pre-flight coordination between head and trunk are compensatory during locomotion, coordination between angular head movement in the pitch plane and vertical trunk translation and head orientation is moderated after flight (Fig. 6.5) [141, 171]. These post-flight postural changes have been associated with various compensatory strategies for locomotion, including wide-based gait, increased arm use, and shorter step length [204].
Static postural stability is expected to recover in at least 5 days and follows a predictable course. The initial recovery phase is rapid, accounting for approximately 50% of postural stability recovery, followed by a slower recovery phase occurring over the subsequent 100 h [134]. As we further evaluate these recovery profiles more granularly, it is likely that there will be additional variables and time courses to consider. For example, other metrics have suggested that while postural stability may recover quickly, neuromuscular control may take up to 3 weeks to return to baseline [205, 206]. Mission duration and crew member experience likely also contribute to the recovery profile (Fig. 6.5) [135]. Experienced astronauts demonstrate less severe post-flight postural instability than first-time astronauts, suggesting that prior exposure may facilitate learned plasticity for adaptive motor strategies upon return [207]. Problematically, however, functional balance and gait assessments have known high interindividual variability and there are numerous metrics available that may be used to define recovery. Refining these protocols will assist future research identifying difficulties in balance and gait.
SCC and otolith-mediated ocular reflexes have also demonstrated atypical function post-flight. Even after short missions, post-flight visual target acquisition velocity has been described as slowed and gaze stabilization as less accurate than pre-flight function [208]. While some have found no substantial change in angular VOR function [208], others have described significant reductions in the caloric response at 10-days post-flight [209]. Functionally, decreased post-flight dynamic visual acuity has been reported, meaning that astronauts may experience oscillopsia with typical head movement [210].
Studies evaluating the OCR have found 70% reduction in response compared to pre-flight levels. The recovery timeline of the OCR has been associated with mission duration, with longer durations requiring at least 11 days for recovery, while shorter durations require only a few hours. While much adaptation occurs quickly, these data suggest that reintroduction of otolith information may not be immediate, especially for longer duration exposure (Fig. 6.5) [122, 211].
Reinterpretation of vestibular input during landing and immediately post-flight has been associated with increased attention to remaining sensory signals, especially vision [120]. During exposure to altered gravity environments, attenuation of vestibular input leads to “visual dependence” and visual orientation illusions. The increased weighting of visual information experienced during as well as the readaptation upon return has been compared to the sensory reorganization experienced by patients recovering from vestibular pathology [21, 118]. Perception of self-orientation is also altered. Visual and tactile sensory modalities are weighted differently for each individual, and post-flight postural strategies vary from pre-flight strategies, describing a shift in sensory organization [212,213,214,215]. Understanding how these sensory inputs are reweighted to address changes in environment will lead to improved methods for reducing the possible challenges associated with these effects.
Future Directions
Vestibular system adaptation has proven challenging to astronauts and requires our attention to fully understanding the long-term consequences of altered gravity environments. There is incentive to enhance our understanding of vestibular reflex function to reduce the often-debilitating effects of SMS and to improve operational performance in challenging environments. Initiating appropriate and timely vestibular system compensation will allow for improved operational performance and reduce symptoms associated with spatial disorientation in critical transitions. Work in this area is promising. Because astronauts with multiple spaceflight exposures demonstrate improved ability to transition between these environments [207], it is possible that astronauts could be habituated to various gravity conditions pre-flight. Essentially, crew would be trained to maintain various adaptation profiles depending on the gravity input available [216]. Establishing a training paradigm to allow for fluid transition between gravity conditions may reduce concerns regarding operational performance, at least to some degree.
Exposure to otolith-mediated illusions pre-flight may also reduce concerns for high-risk transitions. Developing appropriate simulations so that the crew can recognize when to expect altered perception is key to improving performance. Methods such as galvanic vestibular stimulation (GVS) may be useful in various conditions. Disruptive GVS applied in training paradigms may lead to reduced perceptual errors and improved functional performance upon reentry [217,218,219]. While the current use of capsules may reduce the level of precise performance expected by the crew, understanding these sensory illusions will lead to overall safer returns, especially if emergencies arise. In orbit, GVS may provide a method for recoupling the VOR pathways to mimic those provided in 1 g environments with the goal of reducing spatial disorientation and perhaps severity of SMS [220].
More broadly, maintaining appropriate otolith reflex pathway conditioning may also lead to improved vestibulo-sympathetic reflex function, reducing the impact on bone remodeling or orthostatic challenge. Other concerns may also be addressed by regulating vestibular reflexes. For example, sleep can be a considerable issue for astronauts. While there are numerous contributors to disrupted sleep in orbit, such as altered hemodynamics, reduced motor activity, environmental noise, and overall discomfort [221], vestibular-mediated autonomic alterations may also contribute. Recent research suggests that the vestibulo-sympathetic reflex pathways may contribute to reported challenges transitioning between sleep states [160] and may also be implicated in reduced sleep duration due to increased vigilance regarding altered gravity and continued effort to maintain appropriate posture [222,223,224]. More work in this area is needed to better understand how to improve sleep quality for crew members. Adaptation or management of altered vestibular system information may provide improved quality of on-board experience, especially with long-duration missions.
Human space exploration is advancing and understanding the significant impact of altered gravity is key to our success in these endeavors. With goals of long-duration missions to the moon or to Mars, or even the ability for civilians to enter space, understanding and mediating the effects of the vestibular system will continue to play a role in future exploration.
References
Baloh R, Honrubia V. Clinical neurophysiology of the vestibular system. 3rd ed. Philadelphia, PA: FA Davis; 2001.
Dieterich M, Brandt T. Vestibular system: anatomy and functional magnetic resonance imaging. Neuroimaging Clin N Am. 2001;11(2):263–73.
Smith C, Lowry O, Wu M. The electrolytes of the labyrinthine fluids. Laryngoscope. 1965;64:141–53.
Hillman D, McLaren J. Displacement configuration of semicircular canal cupulae. Neuroscience. 1979;4(12):1989–2000.
Lysakowski A, McCrea R, Tomlinson R. Anatomy of vestibular end organs and neural pathways. In: Cummings C, Fredrickson J, Harker L, Krause C, Richardson M, Schuller D, editors. Otolaryngology head and neck surgery. 3rd ed. St. Louis, MO: Mosby; 1998.
Scherer R. On the role of the ampulla in disturbances of vestibular function. Biol Sci Space. 2001;15(4):350–2.
Lowenstein O, Wersall J. A functional interpretation of the electron microscopic structure of the sensory hair cells in the cristae of the elasmobranch Raja clavita. Nature. 1954;184:1807–10.
Fernández C, Goldberg J. Physiology of peripheral neurons innervating otolith organs of the squirrel monkey. II. Directional selectivity and force-response relations. J Neurophysiol. 1976;39:985–95.
Goldberg J. Afferent diversity and the organization of the central vestibular pathways. Exp Brain Res. 2000;130:277–97.
Goldberg J, Fernández C. Vestibular mechanisms. Annu Rev Physiol. 1975;37:129–62.
Goldberg J, Fernández C. Physiology of peirpheral neurons innervating semicircular canals of the squirrel monkey. I. Resting discharge and response to constant angular accelerations. J Neurophysiol. 1971;34:635–60.
Lysakowski A, Minor L, Fernández C, Goldberg J. Physiological identification of morphologically distinct afferent classes innervating the cristae ampullares of the squirrel monkey. J Neurophysiol. 1995;73:1270–81.
Romer A, Parsons TS. The vertebrate body. Philadelphia: Saunders; 1977.
Graf W. Spatial coordination of compensatory eye movements in vertebrates: form and function. Acta Biol Hung. 1988;39(2–3):279–90.
Wong A. Eye movement disorders. New York: Oxford University Press; 2008.
Cullen KE. The vestibular system: multimodal integration and encoding for self-motion for motor control. Trends Neurosci. 2012;35(3):185–96.
Goldberg JM, Fernández C. Physiology of peripheral neurons innervating otolith organs of the squirrel monkey, Parts 1, 2, 3. J Neurophysiol. 1976;39:970–1008.
Zupan LH, Merfeld DM. Neural processing of gravito-inertial cues in humans. IV. Influence of visual rotational cues during roll optokinetic stimuli. J Neurophysiol. 2003;89(1):390–400.
Gacek RR. Neuroanatomical correlates of vestibular function. Ann Otol Rhinol Laryngol. 1980;89(1 Pt 1):2–5.
Kingma H. Function tests of the otolith or statolith system. Curr Opin Neurol. 2006;19(1):21–5.
Borel L, Lopez C, Peruch P, Lacour M. Vestibular syndrome: a change in internal spatial representation. Neurophysiol Clin. 2008;38(6):375–89.
Angelaki DE, Hess BJ, Arai Y, Suzuki J. Adaptation of primate vestibuloocular reflex to altered peripheral vestibular inputs. I. Frequency-specific recovery of horizontal VOR after inactivation of the lateral semicircular canals. J Neurophysiol. 1996;76(5):2941–53.
Schweigart G, Mergner T, Becker W. Eye stabilization by vestibulo-ocular reflex (VOR) and optokinetic reflex (OKR) in macaque monkey: which helps which? Acta Otolaryngol. 1995;115(1):19–25.
Goldberg JM, Cullen KE. Vestibular control of the head: possible functions of the vestibulocollic reflex. Exp Brain Res. 2011;210(3–4):331–45.
Ten Donkelaar HJ. Descending pathways from the brain stem to the spinal cord in some reptiles. II. Course and site of termination. J Comp Neurol. 1976;167(4):443–63.
Barlow JS. Inertial navigation as a basis for animal navigation. J Theor Biol. 1964;6(1):76–117.
Beritoff JS. Neural mechanisms of higher vertebrate behavior. Boston: Little, Brown and Co; 1965.
Guedry FE. Psychophysics of vestibular sensation. In: Kornhuber HH, editor. Handbook of sensory physiology. V1/2. Berlin: Springer; 1974. p. 3–154.
Ivanenko YP, Grasso R, Israel I, Berthoz A. The contribution of otoliths and semicircular canals to the perception of two-dimensional passive whole-body motion in humans. J Physiol. 1997;502:223–33.
Miller S, Potegal M, Abraham L. Vestibular involvement in a passive transport and return task. Physiol Psychol. 1983;11:1–10.
Mittelstaedt ML, Glasauer S. Idiothetic navigation in gerbils and humans. Zool Jahrb Physiol. 1991;95:427–35.
Potegal M. Vestibular and neostratal contribution to spatial orientation. In: Potegal M, editor. Spatial abilities. London: Academic Press; 1982. p. 361–87.
Wiener S, Berthoz A. Forebrain structures mediating the vestibular contribution during navigation. In: Berthoz A, editor. Multisensory control of movement. Oxford: Oxford University Press; 1993. p. 427–55.
Worchel P. The role of vestibular organs in space orientation. J Exp Psychol. 1952;44(1):4–10.
Liu S, Angelaki DE. Vestibular signals in macaque extrastriate visual cortex are functionally appropriate for heading perception. J Neurosci. 2009;29(28):8936–45.
Angelaki DE, Cullen KE. Vestibular system: the many facets of a multimodal sense. Annu Rev Neurosci. 2008;31:125–50.
Lopez C, Blanke O. The thalamocortical vestibular system in animals and humans. Brain Res Rev. 2011;67(1-2):119–46.
Marlinski V, McCrea RA. Self-motion signals in vestibular nuclei neurons projecting to the thalamus in the alert squirrel monkey. J Neurophysiol. 2009;101(4):1730–41.
Yakusheva T, Blazquez PM, Angelaki DE. Frequency-selective coding of translation and tilt in macaque cerebellar nodulus and uvula. J Neurosci. 2008;28(40):9997–10009.
Walker MF, Tian J, Shan X, Tamargo RJ, Ying H, Zee DS. The cerebellar nodulus/uvula integrates otolith signals for the translational vestibulo-ocular reflex. PLoS One. 2010;5(11):e13981.
Newlands SD, Perachio AA. Central projections of the vestibular nerve: a review and single fiber study in the Mongolian gerbil. Brain Res Bull. 2003;60(5-6):475–95.
Shinder ME, Newlands SD. Sensory convergence in the parieto-insular vestibular cortex. J Neurophysiol. 2014;111(12):2445–64.
Dieterich M, Brandt T. The parietal lobe and the vestibular system. In: Vallar G, Coslett HB, editors. The parietal lobe. Handbook of clinical neurology. 151: Elsevier; 2018. p. 119–40.
Carriot J, Jamali M, Brooks AX, Cullen KE. Integration of canal and otolith inputs by central vestibular neurons is subadditive for both active and passive self-motion: Implication for perception. J Neurosci. 2015;35(8):3555–65.
Ferre ER, Walther LE, Haggard P. Multisensory interactions between vestibular, visual and somatosensory signals. PLoS One. 2015;10(4):e0124573.
Brandt T, Bartenstein P, Danek A, Dieterich M. Reciprocal inhibitory visual vestibular interaction: visual motion stimulation deactivates the parieto-insular vestibular cortex. Brain. 1998;121:1749–58.
Dieterich M, Bense S, Stephan T, Yousry TA, Brandt T. fMRI signal increases and decreases in cortical areas during small field optokinetic stimulation and central fixation. Exp Brain Res. 2003;148(1):117–27.
Brandt T, Dieterich M. The vestibular cortex. Its locations, functions, and disorders. Ann N Y Acad Sci. 1999;28(871):293–312.
Curthoys IS, Halmagyi GM. Vestibular compensation: a review of the oculomotor, neural, and clinical consequences of unilateral vestibular loss. J Vestib Res. 1995;5(2):67–107.
Precht W, Shimazu H, Markham CH. A mechanism of central compensation of vestibular function following hemilabyrinthectomy. J Neurophysiol. 1966;29(6):996–1010.
Dutia MB. Mechanisms of vestibular compensation: recent advances. Curr Opin Otolaryngol Head Neck Surg. 2010;18(5):420–4.
Clément GR, Boyle RD, George KA, Nelson GA, Reschke MF, Williams TJ, et al. Challenges to the central nervous system during human spaceflight missions to Mars. J Neurophysiol. 2020;123(5):2037–63.
Siddiqi AA. Challenge to Apollo: the Soviet Union and the space race. NASA; 2000. p. 1945–74.
Oman CM, Lichtenburg BK, Money KE. Symptoms and signs of space motion sickness on Spacelab-1. In: Crampton G, editor. Motion and space sickness. Boca Raton, FL: CRC Press; 1990. p. 218.
Homick JL, Reschke MF, Miller EF. Effects of prolonged exposure to weightlessness on postural equilibrium. Washington, DC: NASA; 1977.
Black FO, Paloski WH. Computerized dynamic posturography: what have we learned from space? Otolaryngol Head Neck Surg. 1998;113(3 Pt 2):S45–51.
Reschke MF, Bloomberg JJ, Harm DL, Paloski WH, Layne C, McDonald V. Posture, locomotion, spatial orientation, and motion sickness as a function of space flight. Brain Res Brain Res Rev. 1998;28(1-2):102-17.
Young LR. Space and the vestibular system: what has been learned? J Vestib Res. 1992;3(3):203–6.
Clément G, Ngo-Anh JT. Space physiology II: adaptation of the central nervous system to space flight - past, current, and future studies. Eur J Appl Physiol. 2013;113(7):1655–72.
Bloomberg JJ, Kozlovskaya IB, Lange G, Layne CS, McDonald VP, Melton SS, et al. The effects of long-duration space flight on eye, head and trunk coordination during locomotion. NASA: Johnson Space Center. Available from: http://lsda.jsc.nasa.gov/scripts/experiment/exper.cfm?exp_index=747
Mixed up in space: NASA Science; 2001. Available from: https://science.nasa.gov/science-news/science-at-nasa/2001/ast07aug_1
Reschke MF, Wood SJ, Clément G. A case study of severe space motion sickness. Aerosp Med Hum Perform. 2018;89(8):749–53.
Michel EL, Johnston RS, Dietlein LF. Biomedical results of the Skylab program. Life Sci Space Res. 1976;14:3–18.
Lackner JR, DiZio P. Motor function in microgravity: movement in weightlessness. Curr Opin Neurobiol. 1996;6:744–50.
Wood CD, Graybiel A. Evaluation of sixteen anti-motion sickness drugs under controlled laboratory conditions. Aerosp Med. 1968;39:1341–4.
Putcha L, Berens KL, Marshburn TH, Ortega HJ, Billica RD. Pharmaceutical use by U.S. astronauts on space shuttle missions. Aviat Space Environ Med. 1999;70:705–8.
Chladek J. Outposts on the frontier: a fifty-year history of space stations. Omaha, NE: University of Nebraska Press; 2017.
Evans JS, Zitterkopf DL, Konigsberg R, Blackburn CM. Skylab experiment M131: rotating little chair. NASA; 1977.
Kornilova LN, Grigorova V, Bodo G. Vestibular function and sensory interaction in space flight. J Vestib Res. 1993;3:219–30.
Young LR, Oman CM, Merfeld D, Watt D, Roy S, DeLuca C, et al. Spatial orientation and posture during and following weightlessness: human experiements on Spacelab Life Sciences 1. J Vestib Res. 1993;3:231–9.
Moore ST, Dilda V, Morris TR, Yungher DA, MacDougall HG, Wood SJ. Long-duration spaceflight adversely affects post-landing operator proficiency. Sci Rep. 2019;9.
Clément G, Wood SJ. Rocking or rolling - perception of ambiguous motion after returning from space. PLoS One. 2014;9(10):1–8.
Bloomberg JJ, Reschke MF, Clément GR, Mulavara AP, Taylor LC. Evidence report: risk of impaired control of spacecraft/associated systems and decreased mobility due to vestibular/sensorimorot alterations associated with space flight. NASA; 2006.
Ross MD, Donovan K, Chee O. Otoconial morphology in space flown rats. Physiologist. 1985;28:219–20.
Lychakov DV, Pashchinin AN, Bojajieva-Mikhailova A, Khristov I. Study of receptor organs of the vestibular apparatus of Cosmos-1667 rats (in Russian). Kosm Biol Aviakosm Med. 1989;23:27–33.
Weiderhold ML, Pedrozo HA, Harrison JL, Hejl R, Gao W. Development of gravity-sensing organs in altered gravity conditions: opposite conclusions from an amphibian and molluscan preparation. J Gravit Physiol. 1997;4:51–4.
Weiderhold ML, Harrison JL, Parker K, Nomura H. Otoliths developed in microgravity. J Gravit Physiol. 2000;7:39–42.
Krasnov IB. The otolith apparatus and cerebellar nodulus in rats developed under 2-G gravity. Physiologist. 1991;34:S206–S7.
Anken R, Kappel T, Rahmann H. Morphometry of fish inner ear otoliths after development at 3g hypergravity. Acta Otolaryngol. 1998;118:534–9.
Pedrozo HA, Weiderhold ML. Effects of hypergravity on statocyst development in embryonic Aplysia californica. Hear Res. 1994;79:137–46.
Krasnov IB. Increase in the sensitivity of otolith apparatus in weightlessness: morphological evidence. Intercosmos Space Biol Med Grp Mtng. 1987;10:127.
Sultemeier DR, Choy KR, Schweizer FE, Hoffman LF. Spaceflight-induced synaptic modifications within hair cells of the mammalian utricle. J Neurophysiol. 2017;117:2163–78.
Ross MD, Tomko DL. Effect of gravity on vestibular neural development. Brain Res Rev. 1998;28:44–51.
Ross MD. Changes in ribbon synapses and rough endoplasmic reticulum of rat utricular macular hair cells in weightlessness. Acta Otolaryngol. 2000;120(4):490–9.
Graydon CW, Manor U, Kindt KS. In vivo ribbon mobility and turnover of ribeye at zebrafish hair cell synapses. Sci Rep. 2017;7:7467.
Cohen B, Yakushin SB, Holstein GR, Dai M, Tomko DL, Badakva AM, et al. Vestibular experiments in space. Adv Space Biol Med. 2005;10:105–64.
Reschke MF, Bloomberg JJ, Paloski WH, Mulavara AP, Feiveson AH, Harm DL. Postural reflexes, balance control, and functional mobility with long-duration head-down bed rest. AviatSpace Environ Med. 2009;80:A45–54.
Mulavara AP, Batson CD, Buxton RE, Feiveson AH, Kofman IS, Lee SMC, et al. Vestibular and somatosensory convergence in postural equilibrium control: insights from spaceflight and bed rest studies. Society for Neuroscience 2014 Annual Meeting; Washington, DC; 2014.
Cassady K, Koppelmans V, Reuter-Lorenz P, De Dios Y, Gadd N, Wood S, et al. Effects of a spaceflight analog environment on brain connectivity and behavior. Neuroimage. 2016;141:18–30.
Burgeat M, Toupet M, Loth D, Ingster I, Guell A, Coll J. Status of vestibular function after prolonged bedrest. Acta Astronaut. 1981;8(9-10):1019–27.
Galvan-Garza RC, Clark TK, Sherwood D, Diaz-Artiles A, Rosenberg M, Natapoff A, et al. Human perception of whole body roll-tilt orientation in a hypogravity analog: underestimation and adaptation. J Neurophysiol. 2018;120(6):3110–21.
Clark TK, Newman MC, Oman CM, Merfeld DM, Young LR. Human perceptual overestimation of whole body roll tilt in hypergravity. J Neurophysiol. 2015;113(7):2062–77.
Dalecki M, Bock O, Guardiera S. Simulated flight path control of fighter pilots and novice subjects at +3 Gz in a human centrifuge. Aviat Space Environ Med. 2010;81(5):484–8.
Guardiera S, Dalecki M, Bock O. Stability of simulated flight path control at +3 Gz in a human centrifuge. Aviat Space Environ Med. 2010;81(4):394–8.
Graybiel D, Miller EF, Homick I. Experiments: M131. Human vestibular function. NASA; 1977.
Johnston RS. Skylab: medical program. Overview. NASA; 1977. Contract No.: SP 377.
Bárány R. Untersuchungen uber den vom Vestibularapparat des Obres Reflektorisch Ausgelosten Rhytmischen Nystagmus und seine Begleitertscheinungen. Monatsschr Ohrenheilkd. 1906;40:193–297.
Bárány R. Physiologie und Pathologie (Funktionsprufung) des Bogengangapparatus beim Menschen. Leipzig: Franz Deuticke; 1907.
Oosterveld WJ, Greven AJ, Gürsel AO, de Jong HA. Caloric vestibular test in the weightless phase of parabolic flight. Acta Otolaryngol. 1985;99(5-6):571–6.
Oosterveld WJ, de Jong HAA, Kortschot HW. The caloric vestibular nystagmus during short lasting microgravity. Acta Astronaut. 1991;23:69–77.
Scherer H, Brandt U, Clarke AH, Merbold U, Parker R. European vestibular experiments on the Spacelab-1 mission: 3. caloric nystagmus in microgravity. Exp Brain Res. 1980;64:255–63.
Scherer H, Clarke AH. The caloric vestibular reaction in space. Acta Otolaryngol (Stockh). 1985;100:328–36.
von Baumgarten R, Benson A, Berthoz A, Brandt T, Brand U, Bruzek W, et al. Effects of rectilinear acceleration and optokinetic and caloric stimulations in space. Science. 1984;225:208–12.
Kassemi M, D. D, Oas JG. Effect of gravity on the caloric stimulation of the inner ear. Ann N Y Acad Sci. 2004;1027:360–70.
Scherer H, Brandt U, Clarke AH, Merbold U, Parker R. European vestibular experiments on the Spacelab-1 mission: 3. caloric nystagmus in microgravity. Exp Brain Res. 1986;64(2):255–63.
Viéville G, Clément F, Lestienne F, Berthoz A. Adaptive modifications of the optokinetic vestibulo-ocular reflexes in microgravity. In: Keller EL, Zee DS, editors. Adaptive processes in visual and oculomotor systems. New York: Pergamon Press; 1986. p. 111–20.
Halmagyi GM, Curthoys IS, Cremer PD, Henderson CE, Todd MJ, Staples MJ, et al. The human horizontal vestibulo-ocular reflex in response to high acceleration stimuli before and after unilateral vestibular neurectomy. Exp Brain Res. 1990;81(3):479–90.
Clément G, Wood SJ, Paloski WH, Reschke MF. Changes in gain of horizontal vestibulo-ocular reflex during spaceflight. J Vestib Res. 2019;29:241–51.
Steinz JA. The sled programs. 1980. Contract No.: ESA Bulletin No 22.
Harry NA, Benson AJ. Space sled – a device for the investigation of the physiological effects of weightlessness. Proc Inst Mech Eng G J Aerosp Eng. 1989;203:1–10.
Moore ST, Clément G, Raphan T, Cohen B. Ocular counterrolling induced by centrifugation during orbital space flight. Exp Brain Res. 2001;137:323–35.
Reschke MF, Wood SJ, Clément G. Ocular counter rolling in astronauts after short- and long-duration spaceflight. Sci Rep. 2018;8:7747.
Shojaku H, Watanabe Y, Tsubota M, Katayama N. Evaluation of the vestibular evoked myogenic potential during parabolic flight in humans. Exp Brain Res. 2008;187(3):477–81.
Gerathewohl SJ. Otolith functions in weightlessness. Life Sci Space Res. 1975;13:33–40.
Paloski WH, Reschke MF, Black FO. Recovery of postural equilibrium control following space flight. Washington, DC: NASA; 1999.
Hugon M. Methodology of the Hoffmann reflex in man. In: Desmedt JE, editor. Human reflexes, pathophysiology of motor systems, methodology of human reflexes. 3. Basel: Karger; 1973. p. 277–93.
Baloh RW, Halmagyi GM. Disorders of the vestibular system. 1st ed. North York: Oxford University Press Canada; 1996.
Lawson BD, Rupert AH, McGrath BJ. The neurovestibular challenges of astronauts and balance patients: some past countermeasures and two alternative approaches to elicitation, assessment and mitigation. Front Syst Neurosci. 2016;10(96):1–12.
Sirota MG, Babayev BM, Beloozerova IB, Nyrova AN, Yakushin SB, Kozlovskaya IB. Characteristics of vestibular reactions to canal and otolith stimulation an at early stage of exposure to microgravity. Physiologist. 1987;30:S82–S4.
Oman CM, Lichtenberg BK, Money KE, McCroy RK. MIT/Canadian vestibular experiments on the Spacelab-1 mission: 4. Space motion sickness: symptoms, stimuli, and predictability. Exp Brain Res. 1986;64(2):316–34.
Reschke MF, Clément G. Verbal reports of neurovestibular symptoms in astronauts after short-duration space flight. Acta Astronaut. 2018;152:229–34.
Hallgren E, Kornilova L, Fransen E, Glukhikh D, Moore ST, Clément G, et al. Decreased otolith-mediated vestibular response in 25 astronauts induced by long-duration spaceflight. J Neurophysiol. 2016;115:3045–51.
Clément G, Denise P, Reschke MF, Wood SJ. Human ocular counter-rolling and roll tilt perception during off-vertical axis rotation after spaceflight. J Vestib Res. 2007;17(5,6):209–15.
Kornilova LN, Temnikova VV, Sagalovich SV, Aleksandrov VV, Iakushev AG. Effects of otoliths upon function of the semicircular canals after long-term stay under conditions of microgravitation. Rossiiskii fiziologicheskii zhurnal imeni IM Sechenova. 2007;93(2):128–40.
Clément G. Alteration of eye movements and motion perception in microgravity. Brain Res Rev. 1998;28:161–72.
Clarke AH, Grigull J, Mueller R, Scherer H. The three-dimensional vestibulo-ocular reflex during prolonged microgravity. Exp Brain Res. 2000;134:322–34.
Benson AJ, Viéville T. European vestibular experiments on the Spacelab-1 mission: 6. Yaw axis vestibulo-ocular reflex. Exp Brain Res. 1986;64(2):279–83.
Correia MJ. Neuronal plasticity: adaptation and readaptation to the environment of space. Brain Res Rev. 1998;28:61–5.
Wilson VJ, Melville JG. The mammalian vestibular system. New York: Plenum Press; 1979.
DiZio P, Lackner JR, Evanoff JN. The influence of gravitoinertial force level on oculomotor and perceptual responses to sudden stop stimulation. Aviat Space Environ Med. 1987;58(9 Pt 2):A224–A30.
Cohen B, Kozlovskaya I, Raphan T, Solomon D, Helwig D, Cohen N, et al. Vestibuloocular reflex of rhesus monkeys after spaceflight. J Appl Physiol. 1985;1992(73):121S–31S.
Lackner J, DiZio P. Gravitational effects of nystagmus and on perception of orientation. Ann N Y Acad Sci. 1988;545:93–104.
Oman CM, Kulbaski MJ. Spaceflight affects the 1-g post rotatory vestibulo-ocular reflex. Adv Otorhinolaryngol. 1988;42:5–8.
Paloski WH, Reschke MF, Black FO, Doxey DD, Harm DL. Recovery of postural equilibrium control following spaceflight. Ann N Y Acad Sci. 1992;656(1):747–54.
Wood SJ, Paloski WH, Clark JB. Assessing sensorimotor function following ISS with computerized dynamic posturography. Aerosp Med Hum Perform. 2015;86(12):A45–53.
Paloski WH, Wood SJ, Feiveson AH, Black FO, Hwang EY, Reschke MF. Destabilization of human balance control by static and dynamic head tilts. Gait Posture. 2006;23(3):315–23.
Greenwood R, Hopkins A. Monosynaptic reflexes in falling man. J Neurol Neurosurg Psychiatry. 1977;40(5):448–54.
Reschke MF, Anderson DJ, Homick JL. Vestibulo-spinal response modification as determined with the H-reflex during the Skylab-1 flight. Exp Brain Res. 1986;64(2):367–79.
Reschke MF, Anderson DJ, Homick JL. Vestibulospinal reflexes as a function of microgravity. Science. 1984;225(4658):212–4.
LeBlanc A, Rowe R, Schneider V, Evans H, Hedrick T. Regional muscle loss after short duration spaceflight. AviatSpace Environ Med. 1995;66:1151–4.
Bloomberg JJ, Peters BT, Smith SL, Huebner WP, Reschke MF. Locomotor head-trunk coordination strategies following space flight. J Vestib Res. 1997;7(2-3):161–77.
Layne CS, McDonald PV, Bloomberg JJ. Neuromuscular activation patterns during treadmill walking after space flight. Exp Brain Res. 1997;113(1):104–16.
Moore ST, Hirasaki E, Cohen B, Raphan T. Effect of viewing distance on the generation of vertical eye movements during locomotion. Exp Brain Res. 1999;129:347–61.
Reschke MF, Good EF, Clément G. Neurovestibular symptoms in astronauts immediately after space shuttle and International Space Station missions. OTO Open. 2017;1(4):2473974X17738767.
Mulavara AP, Ruttley T, Cohen HS, Peters BT, Miller C, Brady R, et al. Vestibular-somatosensory convergence in head movement control during locomotion after long-duration space flight. J Vestib Res. 2012;22:153–66.
Kornilova LN, Naumov IA, Azarov KA, Sagalovich SV. Gaze control and vestibular-cervical-ocular responses after prolonged exposure to microgravity. Aviat Space Environ Med. 2012;83(12):1123–34.
Kornilova LN, Sagalovitch SV, Temnikova VV, Yakushev AG. Static and dynamic vestibulo-cervico-ocular responses after prolonged exposure to microgravity. J Vestib Res. 2007;17(5-6):217–26.
Kornilova LN. The role of gravitation-dependent systems in visual tracking. Neurosci Behav Physiol. 2004;34(8):773–81.
Tomilovskaya ES, Berger M, Gerstenbrand F, Kozlovskaya IB. Effects of long-duration space flights on characteristics of the vertical gaze fixation reaction. J Vestib Res. 2013;23:3–12.
Clément G, Andre-Deshays C, Lathan CE. Effects of gravitoinertial force variations on vertical gaze direction during oculomotor reflexes and visual fixation. AviatSpace Environ Med. 1989;60(12):1194–8.
Clément G, Lathan C, Lockerd A. Perception of depth in microgravity during parabolic flight. Acta Astronaut. 2008;63(7-10):828–32.
Clément G, Skinner A, Lathan C. Distance and size perception in astronauts during long-duration spaceflight. Life. 2013;3(4):524–37.
Moore ST, MacDougall HG, Lesceu X, Speyer JJ, Wuyts F, Clark JB. Head-eye coordination during simulated orbiter landing. Aviat Space Environ Med. 2008;79(9):888–98.
Buytaert KI, MacDougall HG, Moore ST, Clément G, Pattyn N, Migeotte P-F, et al. Validation of centrifugation as a countermeasure for otolith deconditioning during spaceflight: preliminary data of the ESA SPIN study. J Vestib Res. 2013;23:23–31.
Sibonga JD, Evans HJ, Sung HG, Spector ER, Lang TF, Organov VS, et al. Recovery of spaceflight-induced bone loss: bone mineral density after long-duration missions as fitted with an exponential function. Bone. 2007;41(6):973–8.
Vignaux G, Besnard S, Ndong J, Philoxène B, Denise P, Elefteriou F. Bone remodeling in regulated by inner ear vestibular signals. J Bone Miner Res. 2013;28(10):2136–44.
Kawao N, Morita H, Obata K, Tamura Y, Okumoto K, Kaji H. The vestibular system is critical for the changes in muscle and bone induced by hyperactivity in mice. Physiol Rep. 2016;4(19):e12979.
Gibson DC. Terrestrial and extraterrestrial space dangers: outer space perils, rocket risks and the health consequences of the space environment. Potomac, MD: Bentham Science Publishers; 2015.
Vignaux G, Ndong JDLC, Perrien DS, Elefteriou F. Inner ear vestibular signals regulate bone remodeling via the sympathetic nervous system. J Bone Miner Res. 2015;30(6):1103–11.
Yates BJ. Autonomic reaction to vestibular damage. Otolaryngol Head Neck Surg. 1998;119(1):106–12.
Yates BJ, Bolton PS, Macefield VG. Vestibulo-sympathetic responses. Compr Physiol. 2014;4(2):851–87.
Deschpande N, Laurie SS, Lee SMC, Miller CA, Mulavara AP, Peters BT, et al. Vestibular and cardiovascular responses after long-duration spaceflight. Aerosp Med Hum Perform. 2020;91(8):621–7.
Iwata C, Abe C, Tanaka K, Morita H. Role of the vestibular system in the arterial pressure response to parabolic-flight-induced gravitational changes in human subjects. Neurosci Lett. 2011;495:121–5.
Ogoh S, Marais M, Lericollais R, Denise P, Raven PB, Normand H. Interaction between graviception and carotid baroreflex function in humans during parabolic flight-induced microgravity. J Appl Physiol. 1985;2018(125):634–41.
Hallgren E, Migeotte P-F, Kornilova L, Delière Q, Fransen E, Glukhikh D, et al. Dysfunctional vestibular system causes a blood pressure drop in astronauts returning from space. Sci Rep. 2015;5(17627):1–8.
Jian BJ, Cotter LA, Emanuel BA, Cass SP, Yates BJ. Effects of bilateral vestibular lesions on orthostatic tolerance in awake cats. J Appl Physiol (1985). 1999;86(5):1552–60.
Gotoh TM, Fujiki N, Matsuda T, Gao S, Morita H. Roles of baroreflex and vestibulosympathetic reflex in controlling arterial blood pressure during gravitational stress in conscious rats. Am J Physiol Regul Integr Comp Physiol. 2004;286(1):R25–30.
Abe C, Tanaka K, Awazu C, Morita H. Impairment of vestibular-mediated cardiovascular response and motor coordination in rats born and reared under hypergravity. Am J Physiol Regul Integr Comp Physiol. 2008;295(1):R173–80.
Morita H, Abe C, Tanaka K. Long-term exposure to microgravity impairs vestibulo-cardiovascular reflex. Sci Rep. 2016;6(33405):1–10.
Yates BJ, Kerman IA. Post-spaceflight orthostatic intolerance: possible relationship to microgravity-induced plasticity in the vestibular system. Brain Res Rev. 1998;28:73–82.
Courtine G, Pozzo T. Recovery of the locomotor function after prolonged microgravity exposure. I. Head-trunk movement and locomotor equilibrium during various tasks. Exp Brain Res. 2004;158(1):86–99.
Johnson WH. Motion sickness, Part 1. Etiology and autonomic effects. In: Kornhuber HH, editor. Handbook of sensory physiology. VI/2. Berlin: Springer; 1974.
Johnson WH, Sunahara FA, P. LJ. Motion sickness, vascular changes accompanying pseudo-coriolis-induced nausea. Aviat Space Environ Med. 1993;64:367–70.
Thornton WE, Moore TP, Pool SL, Vanderploeg J. Clinical characteristics and etiology of space motion sickness. Aviat Space Environ Med. 1987;58:A1–8.
Heer M, Paloski WH. Space motion sickness: incidence, etiology, and countermeasures. Auton Neurosci. 2006;129:77–9.
Simanonok KE, Charles JB. Space sickness and fluid shifts: a hypothesis. J Clin Pharmacol. 1994;34:652–63.
Reason JT. Motion sickness: a special case of sensory rearrangement. Adv Sci. 1970;26:386–93.
Lackner J, DiZio P. Space motion sickness. Exp Brain Res. 2006;175:377–99.
Graybiel A. Space motion sickness: Skylab revisted. Aviat Space Environ Med. 1980;51:814.
Lentz JM. Nystagmus, turning sensations, and illusory movement in motion sickness susceptibility. Aviat Space Environ Med. 1976;47(9):931–6.
Miller EFI, Graybiel A. The semicircular canals as a primary etiological factor in motion sickness. Aerosp Med. 1972;43:1065–74.
Dobie TG. Airsickness in aircrew. London; 1974. Contract No.: AGARDograph No. 177.
Kubiczkowa J. Vestibular tests in the selection of cosmonauts. Acta Astronaut. 1981;8(9-10):1029–34.
Homick JL. Space motion sickness. Acta Astronaut. 1979;6:1259–72.
Cheung BS, Money KE, Howard IP. Human gaze instability during brief exposure to reduced gravity. J Vestib Res. 1994;4:17–27.
Markham CH, Diamond SG. A predictive test for space motion sickness. J Vestib Res. 1993;3:289–95.
Markham CH, Diamond SG, Stoller DF. Parabolic flight reveals independent binocular control of otolith-induced eye torsion. Arch Ital Biol. 2000;138:73–86.
von Baumgarten RJ, Thumler R. A model for vestibular function in altered gravitational states. Life Sci Space Res. 1979;17:161–70.
Clarke AH, Schönfeld U. Modification of unilateral otolith responses following spaceflight. Exp Brain Res. 2015;233:3613–24.
Diamond SG, Markham CH. The effect of space missions on gravity-responsive torsional eye movements. J Vestib Res. 1998;8:217–31.
Thornton WE, Bonato F. Space motion sickness and motion sickness: symptoms and etiology. Aviat Space Environ Med. 2013;84(7):716–21.
Graybiel A, Kellogg RS. The inversion illusion in parabolic flight: its probable dependence on otolith function. US Naval Aerospace Medical Institute; 1966.
Gazenko O. Medical investigations on spaceships ‘Vostok’ and ‘Vosh kod’. United States Air Force, Aerospace Medicine Division, Brooks AFB; 1965.
Paloski WH, Oman CM, Bloomberg JJ, Reschke MF, Wood SJ, Harm DL, et al. Risk of sensory-motor performance failures affecting vehicle control during space missions: a review of the evidence. J Gravit Physiol. 2008;15(2):1–29.
Young LR. Vestibular reactions to spaceflight: human factors issues. Aviat Space Environ Med. 2000;71(9):A100–A4.
Glasauer S, Mittelstaedt H. Determinants of orientation in microgravity. Acta Astronaut. 1992;27:1–9.
Dyde RT, Jenkin MR, Harris LR. The subjective visual vertical and the perceptual upright. Exp Brain Res. 2006;173(4):612–22.
Tarnutzer AA, Bockisch C, Straumann D. Head roll dependent variability of subjective visual vertical and ocular counterroll. Exp Brain Res. 2009;195(4):621–6.
Vibert D, Safran AB. Subjective visual vertical in peripheral unilateral vestibular diseases. J Vest Res. 1999;9(2):145–52.
Harris LR, Jenkin M, Jenkin H, Zacher JE, Dyde RT. The effect of long-term exposure to microgravity on the perception of upright. NPJ Microgravity. 2017;3:3.
Kelly S. Astronaut Scott Kelly on the devastating effects of a year in space. Sydney Morning Herald. 2017 10/06/2017;Sect. Lifestyle.
Clément G. Fundamentals of space medicine. 2nd ed. New York, NY: Microcosm Press, El Segundo and Springer; 2011.
Bacal K, Billica R, Bishop S. Neurovestibular symptoms following space flight. J VestibRes. 2003;13:93–102.
Chekirda IF, Bogdashevskiv AV, Yeremin AV, Kolosov IA. Coordinated structure of walking of Soyuz-9 crew members before and after flight. Kosmicheskaya Biologiya i Meditsina. 1971;5:48–52.
Edgerton VR, Roy RR. Neuromuscular adaptations to actual and simulated spaceflight. In: Handbook of physiology environmental physiology. III. Bethesda, MD: American Physiology Society; 1996. p. 721–63.
Antonutto G, Capelli C, Girardis M, Zamparo P, di Prampero PE. Effect of microgravity on maximal power of lower limbs during very short-efforts in humans. J Appl Physiol. 1985;1999(86):85–92.
Bloomberg JJ, Layne CS, McDonald PV, Peters BT, Huebner WP, Reschke MF, et al. Effects of space flight on locomotor control. NASA Johnson Space Center; 1999. Contract No.: NASA SP-1999-534.
Glasauer S, Reschke M, Berthoz A. The effect of spaceflight on gaze control strategy. Life Sci Res Space. 1994;366:339.
Clarke AH, Teiwes W, Scherer H. Vestibulo-oculomotor testing during the course of a spaceflight mission. Clin Investig. 1993;71(9):740–8.
Bloomberg JJ, Mulavara AP. Changes in walking strategies after spaceflight. IEEE Eng Med Biol Mag. 2003;22(2):58–62.
Dai M, McGarvie L, Kozlovskaya I, Sirota M, Raphan T, Cohen B. Reduction of ocular counter-rolling by adaptation to space. NASA; 1993. Contract No.: CR-196279.
Clément G, Berthoz A, Lestienne F. Adaptive changes in perception of body orientation and mental image rotation in microgravity. Aviat Space Environ Med. 1987;58(9 Pt 2):A159–A63.
Glasauer S, Mittelstaedt H. Perception of spatial orientation in microgravity. Brain Res Rev. 1998;28(1-2):185–93.
Watt DGD, Landolt LR, Young LR, editors. Effects of long-term weightlessness on roll-circular vection. 7th CASI Conference on Astronautics; 1992; Ottawa.
Black FO, Paloski WH, Doxey-Gasway DD, Reschke MF. Vestibular plasticity following orbital spaceflight: recovery from postflight postural instability. Acta Otolaryngol. 1995;115:450–4.
Shelhamer M, Zee DS. Context-specific adaptation and its significance for neurovestibular problems of space flight. J Vestib Res. 2003;13:345–62.
Dilda V, Morris TR, Yungher DA, MacDougall HG, Moore ST. Central adaptation to repeated galvanic vestibular stimulation: implications for pre-flight astronaut training. PLoS One. 2014;9(11):1–7.
Moore ST, MacDougall HG, Peters BT, Bloomberg JJ, Curthoys IS, Cohen HS. Modeling locomotor dysfunction following spaceflight with galvanic vestibular stimulation. Exp Brain Res. 2006;174(4):647–9.
Moore ST, Dilda V, MacDougall HG. Galvanic vestibular stimulation as an analogue of spatial disorientation after spaceflight. Aviat Space Environ Med. 2011;82(5):535–42.
Cevette MJ, Stepanek J, Cocco D, Galea AM, Pradhan GN, Wagner LS, et al. Oculo-vestibular recoupling using galvanic vestibular stimulation to mitigate simulator sickness. Aviat Space Environ Med. 2012;83(6):549–55.
Myasnikov VI. Characteristics of the sleep of men in simulated space flights. Aviat Space Environ Med. 1975;46(4 Sec 1):401–8.
Gonfalone A. Sleep on manned space flights: zero gravity reduces sleep duration. Pathophysiology. 2016;23(4):259–63.
Gonfalone A. Hypothetical role of gravity in rapid eye movements during sleep. Med Hypotheses. 2019;127:63–5.
Dharani NE. The role of vestibular system and the cerebellum in adapting to gravitoinertial, spatial orientation and postural challenges of REM sleep. Med Hypotheses. 2005;65(1):83–9.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Bogle, J.M., Zaleski-King, A. (2022). Vestibular System. In: Michael, A.P., Otto, C., Reschke, M.F., Hargens, A.R. (eds) Spaceflight and the Central Nervous System. Springer, Cham. https://doi.org/10.1007/978-3-031-18440-6_6
Download citation
DOI: https://doi.org/10.1007/978-3-031-18440-6_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-18439-0
Online ISBN: 978-3-031-18440-6
eBook Packages: MedicineMedicine (R0)