Abstract
Humankind has always focused on outer space, especially the planetary system of our solar system. Space exploration exposes travelers to a variety of gravitational stresses. Examples include increased acceleration forces during launch and landing, partial gravity on extra-terrestrial locations such as the Moon or Mars, and microgravity during orbital missions and flights between planetary bodies. Space flight environments usually have additional stressors associated with them in addition to the lack of a gravitational vector. Isolation, noise, radiation, toxin buildup, and operational pressures all combine to create a uniquely stressful environment.
Short-duration spaceflight onboard the space shuttle is a unique experience that includes a unique set of stressors that contribute to the dysregulation of nervous system. Astronauts on ISS missions experience many similar stressors for a much longer duration. In this chapter, we have discussed the physiological effects of microgravity and space radiations on the nervous system. Emphasis is laid on understanding the neurological alterations that could occur in outer space. It gives an overview of how nervous system that evolved in a gravitational field changes its function upon exposure to microgravity and space radiations. Furthermore, accessible countermeasures will be deliberated, including a description of microgravity analogs. Eventually, the knowledge of changes in the nervous system due to microgravity and radiation will provide vision into the functioning of nervous system on our planet earth.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
As early as 1609, Galileo became the first human to see Mars through a telescope. With the advancement in technology and human’s curiosity for interplanetary travel, NASA recently sent the largest and most advanced rover to Mars, after a 203-day journey covering approximately 293 million miles. The mission itself personifies the human ideal of persevering toward the future and will help us prepare for human exploration of the Red Planet. This will set the stage for future robotic and crewed missions. The Mars 2020 mission is part of a larger program that includes missions to the Moon to prepare for human exploration of the Red Planet. This has renewed interest of international community in space exploration, with planned man missions to asteroids, Moon, Mars, and beyond in the future. Nonetheless, the effects of long-term spaceflight on human health remains a significant perturbation. One crucial challenge to astronauts on future space missions is extended exposure to environments of microgravity (μg) and radiations [1,2,3]. Past studies have shown adverse effects of μg and radiations on several physiological systems, including notable deleterious effects on the nervous system. Because planning and management and cost are vital limitations to spaceflight studies of nervous tissue, it is important to use alternate models that simulate μg to test hypotheses, design experimental parameters, and augment spaceflight experiments.
During both short- and long-duration spaceflight, astronauts are exposed to cosmic radiation and microgravity, resulting in changes across multiple neurological domains including alterations in sensation, movement, cognition, and coordination [4]. Spaceflight-associated changes to the brain are complex as microgravity itself affects brain by different mechanisms such as cephalic fluid shift, vestibular dysfunction, and weightlessness [5]. In addition, they also endure some common stressors including but not limited to social separation, confinement, sleep deprivation, circadian rhythm disruption, and anxiety. Maintaining the probity of the central nervous system during long duration space flights is a high priority, since proper cognition and somatosensory function are important for many mission critical tasks.
Experiments in real microgravity conditions are rather rare, which is why simulation devices such as Rotary Cell Culture System (RCCS) and Random Positioning Machine (RPM) are used (Fig. 3.1a, b). These devices make use of the principle of microgravity, which is a continuous free-fall. Cells are cultivated in a chamber, which rotates around the horizontal axis, thus counteracting the sedimentation process and keeping the cells in a constant state of free-fall. The Random Positioning Machine (Fig. 3.1a) or, by some referred to as the 3-D clinostat, is a micro weight (‘microgravity’) simulator that is based on the principle of ‘gravity-vector-averaging’ [6]. The system may be compared with a classic 2D clinostat although such a clinostat has only a two-dimensional averaging of the g vector while the RPM provides a functional volume, which is ‘exposed’ to simulated micro weight. Gravity is a vector, i.e., it has a magnitude and a direction. During an experiment run in this two axis RPM the sample’s position about the Earth’s gravity vector direction is constantly changing. The sample may experience this as a zero-gravity environment. The principle of an RPM is to randomly rotate. As with other rotating systems this will generate acceleration. Since we want to simulate microgravity, we are to avoid any additional g forces. The level of simulation within this RPM depends very much on the speed of rotation and the distance of the sample to the center of rotation. In principle only the exact center of the RPM i.e., the center of rotation provides you the ultimate microgravity simulation.
The Rotary Cell Culture System (Fig. 3.1b) is a bioreactor technology that produces3D cultures. It is a dynamic system which suspends cells in a low-shear stress, microgravity-like environment allowing anchorage dependent cells to readily aggregate into 3D spheroids while simultaneously producing high mass transport of nutrients and oxygen. Unlike spinner flasks, the RCCS suspends cells without cell damaging mechanical force. The RCCS can also be used with a variety of scaffolds. The RCCS was originally developed at NASA, Johnson Space Center to simulate the microgravity conditions in space [7]. It was based on the principle of clinorotation, defined as the nullification of the force of gravity by slow rotation around one or two axes. The clinostat developed at NASA is a single axis device known as the Rotating Wall Vessel (RWV). The RCCS is the commercial version of this device.
Effects of Microgravity on Neurobiology
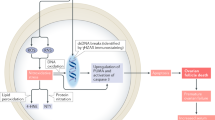
Microgravity has been implicated as a major initiator in space-related neurologic dysfunction. Microgravity in addition with hypobaric exposure during space travel can cause various neurophysiological changes. These encompass decompression sickness, altered central nervous system, and peripheral nervous system symptoms such as memory loss, visual changes, headache, seizures, vertigo, unconsciousness, dysesthesias, paresthesia’s, bowel, and bladder incontinence, fasciculations and paresthesias. Microgravity has been observed to affect cells cytoskeleton [8,9,10]. The delicate interconnection of the intracellular organelles and cytoskeletal structures is maintained by gravity which when altered can affect biochemical and biosynthetic pathways, ultimately negatively effecting DNA replication, microgravity on RNA transcription, and protein transport [11].
Experiments performed by He et al. analyzed the cytoskeletal effects of simulated microgravity in the slime mold Physarum polycephalum. Actin cytoskeletal changes were observed after 40 h of simulated microgravity exposure. Actin fibers appeared to be shortened, disordered, and depolymerized [9]. Another study performed by Mann et al. examined changes in cell morphology due to alterations in cytological architecture in human fetal osteoblasts (hFOB). Following simulated microgravity RPM exposure, a decrease in F-actin filaments was observed. A shift in the microfilament distribution toward F-actin accumulation at the cell boundaries was clearly noticeable in both the 7 days and 14 days RPM samples [12].
As reported by Sarkar et al., microgravity can cause oxidative stress within the hippocampus. Their study in mice hippocampi subjected to microgravity environments showed decreased presence of pyruvate dehydrogenase (PDK-1) and Synuclein β. The decreased presence of Synuclein β could be due to the increased incidence of abnormal protein aggregations seen in microgravitational states [11]. Another study conducted by Demertzi et al. has reported the instance of cortical restructuring in an astronaut’s brain post long-duration spaceflight. The authors reported decreased intrinsic connectivity in right insula and ventral posterior cingulate cortex and diminished integration between right motor cortex and left cerebellum. These outcomes underline the cardinal neural basis for the noted physiological deconditioning due to the spaceflight [13]. Kohn et al. have described structural loss of muscle and bone mass due to continuous adjustments in the sensory and motor systems [14]. According to Fujii et al., a better knowledge of mechanisms of microgravity-induced adverse effects on the nervous system will lead to more effective treatments [15].
Spaceflight-Induced Intracranial Hypertension
Astronauts participating in spaceflight missions are exposed to microgravity which has adverse effects on various organs including eyes. The risk of visual impairment/intracranial pressure (VIIP syndrome) is therefore one of the leading health concerns for NASA. It has been more recently renamed as Spaceflight Associated Neuro-ocular Syndrome (SANS). Intracranial hypertension post spaceflight is now received as a recognizable clinical phenomenon. Although, the key physiological mechanisms causing an increase in intracranial pressure are not well known yet [16]. The most plausible mechanisms of increased intracranial pressure due to microgravity involve a cephalic shift of body fluids, venous outflow blockage, blood–brain barrier malfunction, and disturbance to the cerebrospinal fluid flow. Wostyn and Devyn concur that the response of optic nerve sheath to changes in intracranial pressure may be a potential predictive biomarker for optic disc edema in astronauts [17].
The postflight study of 300 astronauts found that approximately 29% and 60% of astronauts on short-duration and long-duration missions, respectively, reported paucity in distant and near-visual acuity [18].
A retrospective review of data in astronauts post long-duration spaceflight revealed that after 6 months of spaceflight, seven astronauts had ophthalmic findings consisting of optic disc edema in five, globe flattening in five, choroidal folds in five, cotton-wool spots in three, nerve fiber layer thickening detected by optical coherence tomography in six and decreased near vision in six. Five of seven astronauts with near-vision complaints had a hyperopic shift [19].
In another study, Corydon et al. investigated the influence of simulated microgravity using a Random Positioning Machine (Fig. 3.2) on human adult retinal pigment epithelium (ARPE-19) cells. The finding of this study revealed that simulated microgravity causes significant changes in the cytoskeletal (F-actin) and cytoskeletal-related proteins ARPE-19, along with cell development behavior and gene expression patterns involved in cell morphology, migration, adhesion, and angiogenesis [20].
Astronauts participating in spaceflight missions are exposed to microgravity which has adverse effects on various organs including eyes. The risk of visual impairment/intracranial pressure (VIIP syndrome) is therefore one of the leading health concerns for NASA. Here the influence of simulated microgravity using a Random Positioning Machine on human adult retinal pigment epithelium (ARPE-19) cells is represented. Following exposure to simulated microgravity for 5 and 10 days a subset of ARPE-19 cells formed multicellular spheroids (MCS), whereas most of the cells remained adherent (AD) as shown by phase contrast microscopy (a) and confocal laser scanning microscopy (b, c). (Open Source: Reprinted with permission)
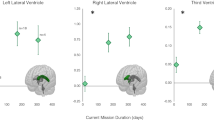
According to Kramer et al. there is enlargement of total brain and cerebrospinal fluid volumes after long distance spaceflight which can be attributed to microgravity-induced intracranial hypertension [21]. The authors reported from a study conducted on 14 astronaut subjects that the increased postflight CSF production rate. This concludes a decrease in CSF production in a microgravity environment, which is upregulated upon return to conventional gravity [22].
Space Motion Sickness
Motion sickness occurs when brain gets mixed signals from various sensory organs including eyes, ears, and body. Motion sickness can begin quickly, and the person might break out in cold sweat and feel nauseated. Space motion sickness symptoms are like those in other forms of motion sickness; they include cold sweating, malaise, loss of appetite, nausea, fatigue, vomiting, and anorexia. Within first 2–3 days in microgravity up to 60%–80% astronauts experience space motion sickness which can affect their operational performance. Spaceflight appears to precipitate headaches without other space motion sickness symptoms in otherwise excellent health status male subjects. Space motion sickness can be due to cranial shifting of body fluids resulting from the loss of hydrostatic pressure gradients in the lower body when entering microgravity. Also, loss of tilt-related otolith signals upon entry into microgravity can cause a conflict between actual and anticipated signals from sense organs discharging spatial orientation inducing space motion sickness.
According to the point of view of Vein et al., headaches are a common, but rarely expressed, complaint during space travel [23]. International Classification of Headache Disorders, second edition (ICHD-II) criteria questionnaire has been used to classify secondary headaches. In a study conducted on 17 astronaut subjects, 12 reported to have experienced at least one headache event while in space. A total of 21 space headache incidents of moderate to severe intensity in 71% of sample subjects was also reported. Majority of headache experiences (76%) were not related with symptoms of space motion sickness. In another post spaceflight study, Penchenkova et al. have reported a diminished association between the vestibular nuclei and sensory/motor regions due to central adaptation which downregulates vestibular input during space flight lessening sensory discord, mitigating space motion sickness [24, 25].
Radiological Changes (Magnetic Resonance Imaging) in Brain Tissue After Microgravity Exposure
Using MRI scans, doctors, scientists, and researchers are now able to examine the inside of the human body in high details. MRI uses a strong magnetic field and radio waves to create detailed images of the organs and tissues within the body (e.g., anomalies of the brain and spinal cord). Nervous system works because information flows from neuron to neuron. The structural characteristics of central nervous system chambers have been examined by Hasan et al. in a retrospective study of 10 healthy astronauts, using multimodal quantitative magnetic resonance imaging (qMRI) [26]. The study reported definitive attributes, indicative of structural neuroplasticity, and adjusting neurogenesis. The brain is made up of gray and white matter. Gray matter consists of short, nonmyelinated neurons and cell bodies, whereas white matter consists of myelinated neurons. The basic pattern of distribution of white and gray matter found in CNS includes a central cavity surrounded by gray matter, with white matter external to the gray matter. The spinal cord exhibits this basic pattern; however, pattern changes with ascent into the brain stem. Brain stem has additional gray matter nuclei scattered within the white matter. Cerebrum and cerebellum contain outer layer of gray matter called the cortex, and they also have scattered areas of gray matter nuclei amid white matter. Several studies have been conducted utilizing MRI to see changes of brain structure following spaceflights, including brains of astronauts before and after long/short-duration missions on the international space station (ISS) and from the Space Shuttle Program [27]. Multiple studies have shown no notable changes in total volume of gray and white matter in astronauts after spaceflights. However, a recent study conducted by Kramer et al. has reported noteworthy augmentation of white matter volume in astronauts (5.5%) post long-duration spaceflight. Pre- and postflight MRI scans after long-duration flights and short-duration flights showed constriction of the central sulcus occurred in 17 of 18 astronauts after long-duration flights (mean flight time, 164.8 days) and in three of 16 astronauts after short-duration flights (mean flight time, 13.6 days) [28]. In another study, Koppelmans et al. have reported increase in gray matter volume in sensorimotor and motor areas of the brain in astronauts post spaceflight [29]. According to study conducted by Jillings et al. MRI scans in cosmonauts post spaceflight showed chiefly changes in gray matter due to volume shifts and white matter volume expansion in the motor and coordination regions of the brain [30]. Also, post flight studies done by Lee et al. have demonstrated focal changes in white matter microstructure within multiple sensory regions including vestibular and proprioceptive processing [31].
Effects of Microgravity on the Vestibular System
The vestibular system is a highly physics-dependent system which exists to aid with proprioception and the ability to adapt the body to optimal position during movement. This process revolves around small movements of endolymph within the semicircular canals for rotatory acceleration adjustments and calcium oxalate crystals on the saccule and utricle for vertical and horizontal acceleration adjustments. These movements are translated and transmitted via the vestibular nerves and associated nerve tracts/nuclei which helps not only the brain send impulses on position but also fires various reflexes to adjust multiple body positioning such as the eyes, head, and torso. Gravity plays a big role in this as it is a major physical force contributing to the speed and acceleration at which the fluid and crystals mentioned move. What happens if gravity is removed from the equation such as with microgravity in outer space? This is an important question to ask as the possibility of space tourism requires a better understanding of space travel and return to gravity as a whole.
Normally the vestibular system works in congruence with the cerebellum and eyes to maintain spatial awareness. In microgravity, these facets are increasingly challenged when compared to the rest of the central nervous system [32]. Specifically, for the vestibular system, the functionality of the otolith organs are more affected than the semicircular canals due to their specialized role in detecting linear accelerations [33] such as gravity, which in turn creates a mismatch in vestibular input as the semicircular canals now provide the majority of signaling [34]. This creates a sort of space sickness, as the body now thinks there is much more angular acceleration than linear, which can cause nausea, vomit, etc. just as motion sickness would. Therefore, when microgravity is introduced, the human vestibular system undergoes a variable adaptation [35]. Functional connectivity of sensorimotor and special working memory has been shown to be increased in microgravity simulations indicating a potential increase in neuroplasticity with a particular focus on spatial adaptation [33, 35]. The contributors to the speed at which adaptation occur likely remains multifactorial and are difficult to quantify [32]. Currently there is no reliable data on countermeasures to take during the adaptation period to minimize space sickness [36].
Research on this subject has been rather generalized with advancements being made every day. Originally, much of the research on the effects of microgravity on vestibular system were done on Earth utilizing microgravity-like methods such as dry immersion, head-down bed rest, and parabolic flights [32]. Now there are more studies being conducted in space, and this is likely to yield more accurate results. Many data-gathering methods such as functional MRI and EEG studies have been utilized, but a combination is likely to provide more accurate results than any one method alone [32]. There is also difficulty in assessing how quickly the vestibular system readapts or potentially maladapts to gravity upon return to Earth as this seems to happen variably and might hold another key into understanding more on this topic. With the increasing prevalence of humans in space, it is also important to look at microgravity effects on human development. Studies currently being done with animal models on this show mixed results and require further exploration [37]. As more studies are being done in space and as technology develops further, it is likely the data will change, and there will be more insight into the exact effects of microgravity on the vestibular system as well as into possible maneuvers to help improve adaptation.
Effects of Space Radiation on the Nervous System
Examination of the health risks associated with long-term deep space missions necessitates an understanding of possible tissue damage resulting from prolonged exposure to HZE radiation. Whereas any type of tissue damage from this radiation is undesirable, CNS injury would be especially devastating to the individual and would be expected to be relatively permanent.
It is known that an astronaut on a 6-month journey to Mars—the time required with conventional propulsion—would be exposed to about 0.3 Sieverts (1 Sievert = 1 Gray = 1 Joule/kg = 100 rad = 100 rems for X-rays, but = Qx1 Gray = 100 rems = Qx100 rads for high-LET radiation, where Q > 1 is the biological quality of radiation), or even to 0.6 on a round-trip. Eighteen months on the surface (if it takes so long to get there, you might as well stay awhile!) would bring another 0.4 Sieverts, for a total exposure of 1 Sievert. Limits set by NASA vary with age and gender but range from 1 to 3 Sieverts. Among the least well-understood health risks for long-term deep space flights is neurological damage induced by HZE particles and secondary nuclei. Exposure to GCR’s will be chronic, ≈1% neurons hit per month. During a 3-year mission to Mars at solar minimum (worst case for GCR exposure), 46% of brain neurons might be hit by a HZE particle (with the electric charge Z > 15), with 13% hit by an iron particle (Z = 26). Therefore, there is a low probability of two hits by iron particles on the same neuron, but a significant likelihood of a hit by an iron particle and another high-LET particle. For nuclei only, hit frequencies are 4–8 times lower. Every cell nucleus in the brain would also be traversed by a proton twice a week, and an alpha particle once a month [38]. Particle fluences may be more relevant than radiation-absorbed doses from GCR to the brain, which will be a few tens of cGy. For low-LET radiation, this would not have severe consequences, but HZE radiation-induced neurological damage could jeopardize mission success and/or induce early onset of neurodegenerative diseases such as Parkinsonism. The existing neurochemical, histological, and behavioral literature on HZE radiation is not comprehensive. First, effects measured at short times after single doses of 100 cGy or higher, which correspond to several hits per cell, may overestimate astronaut’s risk because of DNA repair and/or compensation for lost function by other neurons. Repair and adaptation mechanisms that can counteract effects of particles delivered chronically may be overwhelmed by delivery of radiation in a single dose. Second, other conditions during spaceflight may modify responses to HZE radiation. We believe this is particularly likely for oxidative stress. A major source of indirect damage to the CNS will be oxidative stress caused by free radicals generated during radiation of brain tissue. Spaceflight is known to downregulate antioxidant defense systems [39] which could amplify the impact of free radical generation. Additionally, inflammatory cytokines and other mediators of inflammation are released in response to oxidative stress and amplify the effects of oxidative stress. Oxidative stress and inflammation both activate the hypothalamic–pituitary–adrenal axis, increasing brain exposure to glucocorticoids. Glucocorticoids are known to impair hippocampus-mediated cognitive functions and to suppress hippocampal neurogenesis and reduce hippocampal synaptic density.
Energy deposition from GCRs is largely vconfined to a thin cylinder of tissue which receives a high local dose, especially at the end of the particle range, within a few nanoseconds [40]. One can calculate that for a 1 GeV/n iron particle, the average dose to a cell in the irradiated cylinder will be <40 cGy or that the dose to a nucleus that would be traversed would be about 200 cGy. These doses would be of little consequence for low-LET irradiation of post mitotic cells. However, recent studies have demonstrated active neurogenesis in the hippocampus, a brain structure critically involved in memory, so that effects on mitotic cells have to be considered. Furthermore, effects of ionizing radiation on tissues stem primarily from damage to DNA, and the precise ways in which particular types of radiation interact with matter and break and/or otherwise alter DNA structures govern the potential consequences of irradiation, modulated by the ability of cells to repair damage. A passage of a HZE particle through the nucleus of a cell should cause multiple, intense, and essentially instantaneous ionization events, which induce complex patterns of DNA damage that cannot be fully repaired. Little is known about the effects of charged particles at the cellular and molecular level in mitotic cells and even less about the situation in neurons. Mitochondrial as well as nuclear DNA may be a radiobiological target. Since the mitochondrial electron transport chain is the main endogenous source of reactive oxygen species that cause oxidative stress, damage to mitochondrial DNA may be particularly relevant during spaceflight. Mitochondria have multiple copies of their genome and even possess DNA repair enzymes. However, studies of the effects of HZE radiation in this area are lacking.
The likely nature and extent of brain damage is likely to include necrosis, apoptotic loss of neurons and functionally impaired surviving neurons, as well as impaired neurogenesis. Neuronal apoptosis is an important component of brain ontogeny [41] and is important in the progression of neuropathological conditions such as stroke and neurodegenerative disease [42]. Previous work by both us and other researchers demonstrated that DNA damage activates the apoptotic process in neurons. For example, irradiation [43], cytosine arabinoside [44], cisplatin [45], topoisomerase-II inhibitors [46], and the topoisomerase-I inhibitor camptothecin [47] all induce apoptotic neuronal cell death. A number of these agents cause peripheral neuropathies and neurodegeneration [48]. DNA damage may also participate in initiating cell death in neuropathological conditions such as stroke [49]. Given these observations, it has become increasingly important to understand the downstream signaling events that control DNA damage-evoked neuronal cell death. Several molecular events that mediate death in some neuronal apoptosis paradigms have been described. For example, it has been suggested previously that proteins that normally function to control cell-cycle progression in actively dividing cells may play required roles in the death of terminally differentiated postmitotic neurons [50]. Specific to DNA damage, CDK inhibition, by both pharmacological and molecular means, prevents the death of sympathetic and/or cortical neurons evoked by UV irradiation, AraC, and/or camptothecin [47]. Furthermore, studies that use of camptothecin has demonstrated an increase in cyclin D1-associated kinase activity and protection by the expression of dominant-negative CDK4/6 [47]. These studies indicate that CDK4/6 activity plays a required role in DNA damage-evoked neuronal apoptosis. At least three other molecular events have been suggested to be required for the neuronal death that follows DNA damage. These include the tumor suppressor p53 [43], the proapoptotic Bcl2-related Bax [51], and the various death effector protease enzymes, caspases [51]. The obligate nature of p53 in some neuronal death paradigms is evidenced by significant neuroprotection in p53-deficient neurons exposed to excitotoxic injury [52], ischemia [53], and DNA damage [54].
Studies of retinal cells as surrogates for CNS neurons have suggested a loss of several percent of neurons per 100 cG of iron particles [55]. Older studies demonstrated changes in histological appearance and size of areas of the rabbit brain at doses as low as 100 cGy at times up to 5 years post irradiation [56, 57]. Effects were in the following order: Fe > Ar > Ne > gamma. Dose- and particle-dependent effects were also documented in mouse olfactory tubercle [56]. In other CNS models, HZE radiation induces acute and chronic neuroanatomic changes with lower doses. Philpott et al. (1985) claimed a decrease in the synaptic density in the CA1 area of hippocampus at both 6 and 12 months after exposure to 40Ar particle radiation (0.5–50 cGy). As noted above, effects at the lower dose are very hard to credit. CA1 plays an important role in working memory. Other neuroanatomic effects of high-LET neon particle radiation include neuronal necrosis and altered glial morphology. The neuronal and glial alterations were maintained for at least 35 days after exposure to 4 cGy 84 Kr particle radiation. Some in vivo studies suggest that chronic low-dose exposure to HZE particles might produce effects like aging and neurodegeneration [58]. The retina is part of the central nervous system, and Krebs et al. (1990) [55] found that densities of rat photoreceptors cells or bipolar cells were unaffected by 100 cGy at times up to 185 days. After 250 cGy, photoreceptors and bipolar cells densities were decreased by 20%–50% at 15 days, and this decrease persisted at 185 days. Vazquez and colleagues (1994, 2000) studied effects of 1 GeV/a iron particles in retinal explant cultures and observed dose-dependent reduction of neurite outgrowth 3 days after exposure to varying doses of iron particles (LET 148 keV/μm), with a maximal effect achieved with a dose of 100 cGy. Doses as low as 10 cGy were able to reduce neurite outgrowth by 20% as compared to the control group. In the past, several reports claimed the existence of microlesions expressed as morphological detectable “holes” in the cell surface, as well as tracks in tissues resulting from the passage of high-energy heavy particles with a charge of 20 or more, and with an LET of 200 keV/μm or greater [59, 60]. This purported lesion was considered one of the most harmful for the CNS. The neural retina, as an extension of the CNS, has been used in several studies to first corroborate and later reject the microlesion concept [61]. While the evidence for tunnel lesions has been shown to be inconclusive [55], the data does not exclude the possibility of functional expressions of discrete particle traverses or “microlesions.” A “microlesion“is now generally envisioned as a discrete injury, which need not be reflected by morphological evidence of damage. It could simply represent transient or chronic molecular changes that may alter the cellular/tissue integrity. In the case of neurons, this may in turn impair the neural functions at the integrative level [62]. HZE irradiation on the brain has also been addressed in behavioral and biochemical studies, where alterations in, e.g., conditioned taste avoidance, conditioned place preference, and drug self-administration have been reported [58, 63,64,65,66,67,68,69,70,71]. The data suggest that neurological functions may be impaired in rats at doses of 100–200 cGy. An advantage of mice over rats for these studies is that it allows use of transgenic models to investigate the role of particular enzymes in facilitating or ameliorating effects of toxic insults. A drawback of animal experiments is that subtle aspects of human behavior (e.g., reasoning) could be more sensitive to HZE radiation than easily quantified rodent behaviors. Effects on neurochemistry that underlie behavior and cognition could be more sensitive than behavior itself and may lead to realistic models of human vulnerability.
Conclusion
In conclusion, various studies and data recommend that multiple central nervous system regions are affected during spaceflight, and these alterations probably result from the combinatorial effects of numerous spaceflight associated stressors. The expansion of tedious and lengthy manned space missions as well as future planned travel to Moon, Mars, and beyond will affect human health especially nervous system, and its knowledge has become a relevant subject of study. The vocational risks for astronauts are great, but research into the causes and mechanics of nervous system disorders will not only benefit the astronauts but also the general patient population. Eventually, the knowledge gathered from these space studies will structure the way we prepare for and design exploration class missions, beyond the moon and mars, where nervous system disorders could result in increased risk of wide ranging adverse medical events. Countermeasures to safeguard the astronauts from microgravity and space radiations will require further research and these are essential components in making certain safe and reliable journey to outer deep space.
References
Crucian B, Stowe R, Mehta S, Uchakin P, Quiriarte H, Pierson D, Sams C. Immune system dysregulation occurs during short duration spaceflight on board the space shuttle. J Clin Immunol. 2013;33:456–65.
Grenon SM, Saary J, Gray G, Vanderploeg JM, Hughes-Fulford M. Can I take a space flight? Considerations for doctors. BMJ. 2012;345:e8124.
Sonnenfeld G. The immune system in space and microgravity. Med Sci Sports Exerc. 2002;34:2021–7.
Moore ST, et al. Long-duration spaceflight adversely affects post-landing operator proficiency. Sci Rep. 2019;9:2677.
De la Torre GG. Cognitive neuroscience in space. Life (Basel). 2014;4:281–94.
van Loon JJWA. Some history and use of the random positioning machine, RPM, in gravity related research. Adv Space Res. 2007;39:1161–5.
Schwarz RP, Goodwin TJ, Wolf DA. Cell culture for three-dimensional modeling in rotating-wall vessels: an application of simulated microgravity. J Tissue Cult Methods. 1992;14(2):51–7.
Gaboyard S, Blanchard MP, Travo C, Viso M, Sans A, Lehouelleur J. Weightlessness affects cytoskeleton of rat utricular hair cells during maturation in vitro. Neuroreport. 2002;13:2139–42.
He J, Zhang X, Gao Y, Li S, Sun Y. Effects of altered gravity on the cell cycle, actin cytoskeleton and proteome in Physarum polycephalum. Acta Astronaut. 2008;63:915–22.
Huang Y, Dai ZQ, Ling SK, Zhang HY, Wan YM, Li YH. Gravity, a regulation factor in the differentiation of rat bone marrow mesenchymal stem cells. J Biomed Sci. 2009;16:87.
Sarkar P, Sarkar S, Ramesh V, Hayes BE, Thomas RL, Wilson BL, et al. Proteomic analysis of mice hippocampus in simulated microgravity environment. J Proteome Res. 2006;5:548–53.
Mann V, Grimm D, Corydon TJ, Krüger M, Wehland M, Riwaldt S, Sahana J, Kopp S, Bauer J, Reseland JE, Infanger M, Mari Lian A, Okoro E, Sundaresan A. Changes in human foetal osteoblasts exposed to the random positioning machine and bone construct tissue engineering. Int J Mol Sci. 2019;20(6):1357.
Demertzi A, et al. Cortical reorganization in an astronaut’s brain after long-duration spaceflight. Brain Struct Funct. 2016;221:2873–6.
Kohn FPM, Ritzmann R. Gravity and neuronal adaptation, in vitro and in vivo–from neuronal cells up to neuromuscular responses: a first model. Eur Biophys J. 2018;47:97–107.
Fujii MD, Patten BM. Neurology of microgravity and space travel. Neurol Clin. 1992;10:999–1013.
Lee AG, Mader TH, Gibson CR, Tarver W. Space flight-associated neuro-ocular syndrome. JAMA Ophthalmol. 2017;135(9):992–4.
Wostyn P, De Deyn PP. Intracranial pressure-induced optic nerve sheath response as a predictive biomarker for optic disc edema in astronauts. Biomark Med. 2017;11:1003–8.
Mader TH, Gibson CR, Pass AF, Kramer LA, Lee AG, Fogarty J, et al. Optic disc edema, globe flattening, choroidal folds, and hyperopic shifts observed in astronauts after long-duration space flight. Ophthalmology. 2011;118(10):2058–69.
Alexander DJ, Gibson CR, Hamilton DR, Lee SMC, Mader TH, Otto C, et al. Risk of spaceflight-induced intracranial hypertension and vision alterations. Lyndon B. Houston, Texas: National Aeronautics and Space Administration, Johnson Space Center; 2012.
Corydon TJ, Mann V, Slumstrup L, Kopp S, Sahana J, Askou AL, Magnusson NE, Echegoyen D, Bek T, Sundaresan A, Riwaldt S, Bauer J, Infanger M, Grimm D. Reduced expression of cytoskeletal and extracellular matrix genes in human adult retinal pigment epithelium cells exposed to simulated microgravity. Cell Physiol Biochem. 2016;40(1–2):1–17.
Kramer LA, Hasan KM, Sargsyan AE, Wolinsky JS, Hamilton DR, Riascos RF, et al. MR-derived cerebral spinal fluid hydrodynamics as a marker and a risk factor for intracranial hypertension in astronauts exposed to microgravity. J Magn Reson Imaging. 2015;42:1560–71.
Kramer LA, Sargsyan AE, Hasan KM, Polk JD, Hamilton DR. Orbital, and intracranial effects of microgravity: findings at 3-T MR imaging. Radiology. 2012;263:819–27.
Vein AA, Koppen H, Haan J, Terwindt GM, Ferrari MD. Space headache: a new secondary headache. Cephalalgia. 2009;29:683–6.
Pechenkova E, et al. Alterations of functional brain connectivity after long-duration spaceflight as revealed by fMRI. Front Physiol. 2019;10:761.
Block HJ, Bastian AJ. Sensory weighting and realignment: independent compensatory processes. J Neurophysiol.
Hasan KM, Mwangi B, Keser Z, Riascos R, Sargsyan AE, Kramer LA. Brain quantitative MRI metrics in astronauts as a unique professional group. J Neuroimaging. 2018;28:256–68.
Manzey D, Lorenz B, Poljakov V. Mental performance in extreme environments: results from a performance monitoring study during a 438-day spaceflight. Ergonomics. 1998;41:537–59.
Kramer LA, et al. Intracranial effects of microgravity: a prospective longitudinal MRI study. Radiology. 191413.
Koppelmans V, Bloomberg JJ, Mulavara AP, Seidler RD. Brain structural plasticity with spaceflight. NPJ Microgravity. 2016;2:2.
Jillings S, et al. Macro- and microstructural changes in cosmonauts’ brains after long-duration spaceflight. Sci Adv. 6.
Lee JK, et al. Spaceflight-associated brain white matter microstructural changes and intracranial fluid redistribution. JAMA Neurol. 2019;76:412–9.
Van Ombergen A, Demertzi A, Tomilovskaya E, et al. The effect of spaceflight and microgravity on the human brain. J Neurol. 2017;264:18–22.
Cassady K, Koppelmans V, Reuter-Lorenz P, De Dios Y, Gadd N, Wood S, RiascosCastenada R, Kofman I, Bloomberg J, Mulavara A, Seidler R. Effects of a spaceflight analog environment on brain connectivity and behavior. NeuroImage. 2016;141:18–30.
Vestibular function in microgravity. Lancet. 1984;324, 8402, 561.
Cebolla A, Petieau M, Dan B, et al. Cerebellar contribution to visuo-attentional alpha rhythm: insights from weightlessness. Sci Rep. 2016;6:37824.
Tanaka K, Nishimura N, Kawai Y. Adaptation to microgravity, deconditioning, and countermeasures. J Physiol Sci. 2017;67:271–81.
Walton KD, Harding S, Anschel D, Harris YT, Llinás R. The effects of microgravity on the development of surface righting in rats. J Physiol. 2005;565(Pt 2):593–608. https://doi.org/10.1113/jphysiol.2004.074385.
Curtis SB, Vazquez ME, Wilson JW, Atwell W, Kim M, Capala J. Cosmic ray hit frequencies in critical sites in the central nervous system. Adv Space Res. 1998;22(2):197–207.
Stein TP, Leskiw MJ, Schluter MD, Hoyt RW, Lane HW, Gretebeck RE, LeBlanc AD. Energy expenditure and balance during spaceflight on the space shuttle. Am J Phys. 1999;276(6 Pt 2):R1739–48.
Cohen S, Janicki-Deverts D, Doyle WJ, et al. Chronic stress, glucocorticoid receptor resistance, inflammation, and disease risk. Proc Natl Acad Sci U S A. 2012;109(16):5995–9.
Oppenheim RW. Cell death during development of the nervous system. Annu Rev Neurosci. 1991;14:453–501.
Dirnagl U, Iadecola C, Moskowitz MA. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci. 1999;22:391–7.
Enokido Y, Araki T, Tanaka K, Aizawa S, Hatanaka H. Involvement of p53 in DNA strand break-induced apoptosis in postmitotic CNS neurons. Eur J Neurosci. 1996;8(9):1812–21.
Martin LJ, Kaiser A, Yu JW, Natale JE, Al-Abdulla NA. Injury-induced apoptosis of neurons in adult brain is mediated by p53-dependent and p53-independent pathways and requires Bax. J Comp Neurol. 2001;433:299–311.
Gill JS, Windebank AJ. Activation of the high affinity nerve growth factor receptor by two polyanionic chemotherapeutic agents: role in drug induced neurotoxicity. J Neuro-Oncol. 1998;40(1):19–27.
Nakajima M, Kashiwagi K, Ohta J, Furukawa S, Hayashi K, Kawashima T, Hayashi Y. Nerve growth factor and epidermal growth factor rescue PC12 cells from programmed cell death induced by etoposide: distinct modes of protection against cell death by growth factors and a protein-synthesis inhibitor. Neurosci Lett. 1994;176:161–4.
Park IS, Ahn MR, Suh SK, Choi HS, Sohn SJ, Yang JS, Yoo TM, Kuh HJ. In vitro pharmacodynamics of CKD-602 in HT-29 cells. Arch Pharm Res. 2002;25:718–23.
Mansfield SH, Castillo M. MR of cis-platinum-induced optic neuritis. AJNR Am J Neuroradiol. 1994;15:1178–80.
Chen J, Jin K, Chen M, Pei W, Kawaguchi K, Greenberg DA, Simon RP. Early detection of DNA strand breaks in the brain after transient focal ischemia: implications for the role of DNA damage in apoptosis and neuronal cell death. J Neurochem. 1997;69(1):232–45.
Farinelli SE, Greene LA. Cell cycle blockers mimosine, ciclopirox, and deferoxamine prevent the death of PC12 cells and postmitotic sympathetic neurons after removal of trophic support. J Neurosci. 1996;16:1150–62.
Keramaris E, Stefanis L, MacLaurin J, Harada N, Takaku K, Ishikawa T, Taketo MM, Robertson GS, Nicholson DW, Slack RS, Park DS. Involvement of caspase 3 in apoptotic death of cortical neurons evoked by DNA damage. Mol Cell Neurosci. 2000;15(4):368–79.
Xiang H, Hochman DW, Saya H, Fujiwara T, Schwartzkroin PA, Morrison RS. Evidence for p53-mediated modulation of neuronal viability. J Neurosci. 1996;16:6753–65.
Crumrine RC, Thomas AL, Morgan PF. Attenuation of p53 expression protects against focal ischemic damage in transgenic mice. J Cereb Blood Flow Metab. 1994;14:887–91.
Johnson MD, Kinoshita Y, Xiang H, Ghatan S, Morrison RS. Contribution of p53-dependent caspase activation to neuronal cell death declines with neuronal maturation. J Neurosci. 1999;19:2996–3006.
Krebs W, Krebs I, Worgul BV. Effect of accelerated iron ions on the retina. Radiat Res. 1990;123:213–9.
Cox AB, Kraft LM. Quantitation of heavy ion damage to the mammalian brain: some preliminary findings. Adv Space Res. 1984;4(10):247–50.
Cox AB, Keng PC, Lee AC, Lett JT. Effects of heavy ions on rabbit tissues: damage to the forebrain. Int J Radiat Biol Relat Stud Phys Chem Med. 1982;42:355–67.
Joseph JA, Erat S, Rabin BM. CNS effects of heavy particle irradiation in space: behavioral implications. Adv Space Res. 1998;22:209–16.
Nelson AC, Tobias CA. Rapid development of corneal lesions in rats produced by heavy ions. Adv Space Res. 1983;3:195–209.
Todd P. Stochastics of HZE-induced microlesions. Adv Space Res. 1989;9:31–4.
Nelson AC, Hayes TL, Tobias CA, Yang TC. Some indications of structural damage in retina by heavy ion radiation. Scan Electron Microsc. 1981;4:79–85.
Worgul BV, Krebs W, Koniarek JP. Microlesions: theory and reality. Adv Space Res. 1989;9:315–23.
Denisova NA, Shukitt-Hale B, Rabin BM, Joseph JA. Brain signaling and behavioral responses induced by exposure to (56)Fe-particle radiation. Radiat Res. 2002;158:725–34.
Hunt WA, Dalton TK, Joseph JA, Rabin BM. Reduction of 3-methoxytyramine concentrations in the caudate nucleus of rats after exposure to high-energy iron particles: evidence for deficits in dopaminergic neurons. Radiat Res. 1990;121:169–74.
Joseph JA, Shukitt-Hale B, McEwen J, Rabin B. Magnesium activation of GTP hydrolysis or incubation in S-adenosyl-l-methionine reverses iron-56-particle-induced decrements in oxotremorine enhancement of K+evoked striatal release of dopamine. Radiat Res. 1999;152:637–41.
Joseph JA, Shukitt-Hale B, et al. CNS-induced deficits of heavy particle irradiation in space: the aging connection. Adv Space Res. 2000;25(10):2057–64.
Kandasamy SB, Rabin BM, Hunt WA, Dalton TK, Joseph JA, Harris AH. Exposure to heavy charged particles affects thermoregulation in rats. Radiat Res. 1994;139:352–6.
Rabin BM, Joseph JA, Shukitt-Hale B, McEwen J. Effects of exposure to heavy particles on a behavior mediated by the dopaminergic system. Adv Space Res. 2000;25:2065–74.
Rabin BM, Shukitt-Hale B, Joseph JA, Denissova N. Effects of exposure to 56Fe particles on the acquisition of a conditioned place preference in rats. Phys Med. 2001;17(Suppl 1):196–7.
Rabin BM, Shukitt-Hale B, Szprengiel A, Joseph JA. Effects of heavy particle irradiation and diet on amphetamine- and lithium chloride-induced taste avoidance learning in rats. Brain Res. 2002;953:31–6.
Rabin BM, Joseph JA, et al. Long-term changes in amphetamine-induced reinforcement and aversion in rats following exposure to 56Fe particle. Adv Space Res. 2003;31(1):127–33.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Mann, V., Sundaresan, A., Doursout, MF.J., Devakottai, S. (2022). Effects of Microgravity and Space Radiation on the Nervous System. In: Michael, A.P., Otto, C., Reschke, M.F., Hargens, A.R. (eds) Spaceflight and the Central Nervous System. Springer, Cham. https://doi.org/10.1007/978-3-031-18440-6_3
Download citation
DOI: https://doi.org/10.1007/978-3-031-18440-6_3
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-18439-0
Online ISBN: 978-3-031-18440-6
eBook Packages: MedicineMedicine (R0)