Abstract
Despite significant achievements in cancer treatment, it remains a challenging burden, and there is limited success in the clinical therapy. In recent years, progress in nanotechnology provides plenty of tools to counteract cancer with innovative nanomedicines that can be exploited in intracellular drug delivery. Specifically, the design and development of nanomaterials, such as nanoparticles and hydrogels, aim at achieving smart nanosystems with great multifunctionality and therapeutic potential. In this context, advances in tailored biomaterials for drug delivery as cancer treatment include new strategies to overcome the obstacles and limitations usually encountered with traditional therapeutic agents, thereby reducing the lack of selectivity and side effects. Hence, a big effort is being invested in designing and developing more accurate strategies toward personalized medicine, which has emerged as a promising therapeutic approach with a wide potential to increase treatment outcomes and patient survival. In this chapter, we provide a comprehensive analysis and discuss the development of advanced nanocarriers involving chemotherapeutic agents in clinical trials against multiple types of cancer. We also focus on some reasons that could explain why some treatments fail in clinics.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Cancer is a general term for a large group of diseases, whose causes, characteristics, and occurrence can vary. All of them are characterized by the development of abnormal cells that divide uncontrollably and infiltrate and disrupt normal body tissue. Cancer has a major impact on society across the world, and, in fact, there were 19.3 million new cases in 2020 worldwide (Fig. 1) (https://www.iarc.who.int/). Among these statistics, breast, lung, colorectal, prostate, and stomach highlight as the most common cancer types, with more than 1 million cases each. Moreover, according to the International Agency for Research on Cancer (IARC), the number of new cases per year is expected to rise to 29.5 million by 2040 (https://www.iarc.who.int/).
Cancer statistics across the world. Number of new cases in 2020 for both gender and all ages (a). Estimated number of new cases from 2020 to 2040 for both gender and all ages classified by type of cancer (b) or geographical continent (c). (Data source: GLOBOCAN. Adapted from (https://www.iarc.who.int/))
Current medicine takes advantage of traditional approaches for cancer therapy: surgery, radiotherapy, chemotherapy, phototherapy, immunotherapy, and hormonal therapy (Jabir et al., 2018). Unfortunately, although the available treatments have improved patient survival and treatment outcomes (Ferlay et al., 2021), these clinical approaches can cause nonspecific effects in normal tissues, such as chemical toxicity, radiotoxicity, or phototoxicity, thereby provoking serious issues, namely, nausea, kidney damage, neutropenia, hair loss, loss of appetite, peripheral neuropathy, diarrhea, and skin damage (Koo et al., 2020; Liang et al., 2010). Chemoresistance, and multidrug resistance (MDR) in particular, is another challenge when treating cancer patients. MDR consists on cross-resistance to a wide amount of unrelated chemotherapeutic drugs after exposure to a single anticancer agent (Baguley, 2010; Bukowski et al., 2020). Therefore, cancer research is focused on the discovery and development of biomedical tools to improve the specificity of cancer therapies aiming to achieve therapeutic effect only at the tumor sites.
Although the administration of free chemotherapeutic drugs remains as the gold standard for cancer treatment, this therapeutic strategy still presents inherent challenges (Gonzalez-Valdivieso et al., 2021a, b). One of the most important problems of current medicine resides in the lack of specific treatments and poor drug accumulation in the tumors (Creixell & Peppas, 2012). As a consequence, undesired side effects in healthy tissues occur, especially in the heart (Octavia et al., 2012), bone marrow (Daniel & Crawford, 2006), gastrointestinal tract (Mitchell, 2006), and nervous system (Grothey, 2003). For this reason, novel approaches are needed to overcome these issues and improve the action of unspecific chemotherapeutic agents.
Nanomedicine is one of these recent strategies for cancer therapy (Awasthi et al., 2018; Bobo et al., 2016; Cao et al., 2020; Shreyash et al., 2021). Nanomedicine has emerged as a new discipline combining biology, engineering, chemistry, and physics, among others, with multiple biomedical applications in the screening, diagnosis, and treatment of diseases (Bayda et al., 2019; Caballero et al., 2022; Gonzalez-Valdivieso et al., 2021a, b; Lammers et al., 2011; Man et al., 2018). The therapeutic potential of nanomedicine aims to use sophisticated systems toward a more personalized medicine, in which each patient could take advantage of tailored approaches (Fenton et al., 2018; Park et al., 2021; Sanchez-Moreno et al., 2018). Thus, recent progress in nanotechnology has achieved the development of novel nanomaterials, whose physicochemical characteristics make them excellent candidates to be applied in the biomedical science, with high impact in the pharmaceutical industry (Norouzi et al., 2020; Park et al., 2021; van der Meel et al., 2019; Wicki et al., 2015). Drug delivery, tissue engineering, viral infections, or pathogenic bacteria are some of the biomedical applications in which nanomedicine highlights as an effective and promising tool (Das & Ali, 2021; Girotti et al., 2020a; Gonzalez-Valdivieso et al., 2020; Peres et al., 2021; Qiao et al., 2021; Yacoby & Benhar, 2008). In this work, we will focus on nanomedicine for cancer therapy because, even if drug delivery purposes have been explored for diverse diseases, cancer is undoubtedly the main target of drug delivery research (Davis et al., 2008; Shi et al., 2017) and, in fact, multiple drug delivery nanosystems based on these concepts have been translated into clinical products for chemotherapy, such as Abraxane®, DaunoXome®, Doxil®/Caelyx®, Marqibo®, Myocet®, and Onivyde® (Gonzalez-Valdivieso et al., 2021b; Han et al., 2017; Kushwah et al., 2018; Saw et al., 2017).
2 Cancer Physiology
Cancer is characterized by a challenging physiology which is a huge hurdle for biomedical research and demands therapeutic agents to have special features. Therefore, nanomedicine is able to explore multiple features of cancer that provoke low outcome rates and poor drug accumulation. The aberrant proliferation of cancer cells stimulates the fast formation of new blood vessels, also known as angiogenesis, thereby resulting in leaky vasculature with aberrant tortuosity, abnormal basement membrane, poor lymphatic drainage, high interstitial pressure, dense extracellular matrix (ECM) network, or extensive stromal cells, namely, tumor-associated macrophages (TAMs) and cancer-associated fibroblasts (CAFs) (Matsumoto et al., 2016; Shi et al., 2020). Furthermore, the tumor microenvironment traps many nanocarriers on the tumor vasculature periphery and avoids penetration into the tumor core (Matsumoto et al., 2016).
In addition, cancer cells are characterized by higher expression of multiple proteins, not only cytoplasmic proteins but also anchored receptors to cell membrane (Byrne et al., 2008; Jain & Stylianopoulos, 2010). These cancer markers have huge interest as different targets can be used depending on the type of tumor (Baron, 2012; Sethi et al., 2013). Indeed, cancer markers allow us to even differentiate primary tumors from distance metastasis (Byrne et al., 2008; Quail & Joyce, 2013). Nanocarriers surface can be decorated with molecules (peptides, DNA or RNA aptamers) as targeting systems to specifically drive these devices to cancer cells in specific locations within the body, thereby reducing the amount of drug needed to achieve therapeutic effect and avoiding undesired effects in healthy cells (Agrawal et al., 2020; Girotti et al., 2020a, b; Hwang et al., 2020; Liu et al., 2010; Mitchell et al., 2021). Thus, nanotechnology takes advantages of cancer markers to develop advanced targeted nanocarriers toward personalized biomedical therapeutics (Aguado et al., 2018; Blanco et al., 2015; Cao et al., 2020; Ho et al., 2020).
Beside cancer features and special physiology, the development of accurate systems for controlled release of therapeutics is key when working in drug delivery. Bionanomaterials have been designed for use in advanced drug delivery systems to improve the delivery and efficacy of multiple pharmaceutical agents, such as peptides, antibodies, enzymes, drugs, and vaccines (Caliceti & Matricardi, 2019; Fenton et al., 2018; Yun et al., 2015). Therefore, designing biomaterials for drug delivery purposes is challenging and has to take into account multiple parameters to achieve the maximum therapeutic benefit: (i) biocompatibility of materials themselves and their degradation products, (ii) physicochemical properties of host materials, (iii) adequate drug for prolonged release, (iv) protection of therapeutic agent from breakdown while maintaining biological activity, (v) predictable release profile, (vi) route of administration, and (vii) cost of material synthesis and production (Helary & Desimone, 2015; Mitchell et al., 2017; Yun et al., 2015).
3 Nanocarriers for Drug Delivery
As a consequence of special tumor physiology, Matsumura and Maeda reported the enhanced permeability and retention (EPR) in 1986 (Matsumura & Maeda, 1986). Their research showed that solid tumors have defective architecture within the blood vessels and enhanced vascular permeability, thereby receiving high amounts of nutrients and oxygen for rapid growth. Thus, the EPR effect considers that this nature of tumor blood vessels facilitates transport of molecules (proteins, drug-polymer conjugates, micelles, liposomes) into tumor tissues: molecules larger than the threshold of renal clearance (40 kDa) showed longer circulation times and slow clearance from the body, thereby being accumulated and retained in tumor tissues for long periods (Fang et al., 2011; Islam et al., 2021; Matsumura & Maeda, 1986; Shi et al., 2020). In contrast, this EPR effect does not occur in normal tissues. Thus, the EPR effect is considered a landmark in tumor-targeted chemotherapy.
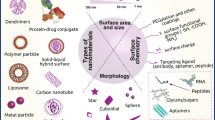
As most chemotherapeutic drugs used in clinics are highly hydrophobic, the development of nanomaterials has explored over the past several decades different approaches and origins with different intrinsic and extrinsic properties to achieve better encapsulation and higher concentrations within tumor cells to achieve better therapeutic effect (Figs. 2 and 3) (Howes et al., 2014; Kushwah et al., 2018; Luginbuhl et al., 2017; Minelli et al., 2010; Yousefpour et al., 2019).
Application of engineered nanomaterials in cancer. Multidisciplinary research results in a wide pool of tailor-made tools for cancer detection, imaging, and therapy, thereby improving survival rates and treatment outcomes. (Reproduced with permissions from (Caballero et al., 2022))
Types of nanoparticles reviewed in this chapter with different origins: polymeric, inorganic, and lipid-based nanomaterials. (Adapted from (Mitchell et al., 2021))
3.1 Types of Nanoparticles
3.1.1 Lipid-Based Nanocarriers
Lipid-based nanomaterials offer many advantages, such as simple formulation, self-assembling, biocompatibility, high bioavailability, or the ability to carry large cargo (Sercombe et al., 2015). These advantages make them very attractive for drug delivery purposes, thereby being the most common class of FDA-approved nanomedicines (Anselmo & Mitragotri, 2019; Fenton et al., 2018). There are different types of lipid-based nanomaterials:
-
(i)
Liposomes, which are typically composed of phospholipids, thereby allowing the liposome to carry hydrophilic, hydrophobic, and lipophilic drugs (Sarfraz et al., 2018). Liposome’ surface is usually modified to extend their circulation times within the body to overcome the fast uptake by the reticuloendothelial system (Alyautdin et al., 2014).
-
(ii)
Lipid nanoparticles (LNPs), which form micellar structures within the particle core. LNPs are typically composed of four major components: phospholipids for particle structure, cationic lipids to complex with negatively charged genetic material, cholesterol for stability and membrane fusion, and PEGylated lipids to enhance longer circulation times (Kulkarni et al., 2019; Leung et al., 2015). LNPs have high efficacy of nucleic acid delivery, simple synthesis, small size, and serum stability as main advantages for gene therapy, but their high uptake in the liver and spleen is an important limitation for translation into the clinics (Cheng et al., 2020; Fenton et al., 2018).
3.1.2 Polymeric Nanocarriers
Polymeric nanocarriers can be synthesized from natural or synthetic materials by emulsification (Brown et al., 2020), nanoprecipitation (Le et al., 2018), ionic gelation (He et al., 2020), or microfluidics (Zhang et al., 2020), among others. Polymeric nanocarriers highlight due to their high biocompatibility, simple formulation, biodegradability, water solubility, stability over time, and wide potential to modify their surfaces for specific targeting (Fenton et al., 2018; Valcourt et al., 2020). Furthermore, this nanomaterial offers many different ways to carry the therapeutic agents, such as binding to the nanoparticle’ surface, chemical conjugation to the polymer, entrapping in the polymer matrix, or encapsulation in the core (Mitchell et al., 2021). This wide versatility allows delivery of hydrophobic and hydrophilic compounds, as well as cargos with different molecular weights, ranging from small molecules to proteins and vaccines (Caldorera-Moore et al., 2019; Knight et al., 2019; Liu et al., 2020, 2010; Zhang et al., 2020). However, despite their advantages, polymeric nanocarriers have some limitations, such as particle aggregation and toxicity. There are multiple subtypes of polymeric nanoparticles, such as nanocapsules (cavities surrounded by a polymeric membrane), nanospheres (solid matrix systems), polymersomes (vesicles with membranes composed of amphiphilic block copolymers), micelles (composed of a hydrophilic core and hydrophobic coating), and dendrimers (hyperbranched polymers with complex 3D architecture and active functional groups on the external part to conjugate biomolecules) (Rideau et al., 2018; Shae et al., 2019; Zelmer et al., 2020).
3.1.3 Inorganic Nanocarriers
Inorganic nanomaterials (gold, iron, and silica) have been widely studied for diagnostics, drug delivery, photothermal therapy, and imaging purposes in biomedicine and cancer research due to their physical, electrical, magnetic, and optical properties (Bobo et al., 2016). Therefore, inorganic nanoparticles present the advantage of a great ability to be engineered into tailored nanocarriers with precise physicochemical properties (size, structure, and geometry). Despite their good biocompatibility and stability, inorganic nanoparticles are limited in the clinical application by their low solubility and toxicity (Bobo et al., 2016; Manshian et al., 2017). There are multiple forms of gold nanoparticles (AuNPs), such as nanospheres, nanorods, nanostars, nanoshells, and nanocages (Quazi et al., 2021). AuNPs can be easily functionalized, thereby allowing researchers to design and develop nanocarriers specifically targeted to different tissues (Bobo et al., 2016; Quazi et al., 2021). Another example of inorganic nanoparticles is magnetic iron oxide NPs, composed of magnetite (Fe3O4) or maghemite (Fe2O3) (Arias et al., 2018). These nanocarriers present superparamagnetic properties especially useful for various applications as contrast agents, drug delivery vehicles, and thermal-based therapies (Arias et al., 2018; Bobo et al., 2016). Calcium phosphate and mesoporous silica nanoparticles are also inorganic nanocarriers typically used for gene and drug delivery (Huang et al., 2020; Xu et al., 2019), while quantum dots are widely used for in vitro imaging applications (Wagner et al., 2019).
Hence, in this chapter, we will focus on a comprehensive analysis of the clinical application of chemotherapy-based drug delivery nanosystems as advanced tools for cancer treatment.
3.2 Mechanism of Action of Classic Chemotherapeutic Agents
In the mid-1900s, the birth of the chemotherapy entailed a whole revolution in cancer treatment. Before that, the only options available were mainly radical surgical methods, with low success rates, that aimed at the complete eradication of the disease before it could spread and metastasize throughout the organism (Falzone et al., 2018).
Classic chemotherapeutic agents, also referred to as antineoplastic agents, are used to directly or indirectly inhibit the uncontrolled growth and proliferation of cancer cells. Their main disadvantages are related to their low specificity toward cancer cells, generating acute toxicity also to healthy tissues, and the drug resistance mechanisms that lower their efficacy.
In the last decades, new discoveries in the field of immunology, cell biology, and molecular biology allowed researchers to investigate the molecular mechanisms responsible for the neoplastic transformation of cells and to redirect the path toward more specific and personalized therapies, including monoclonal antibodies or immunotherapies, among others. However, the classic chemotherapy, alone or in combination with new treatments, is still a key pharmacological option, despite its notable adverse effects (Falzone et al., 2018; Ferlay et al., 2021).
Classic chemotherapeutic agents are classified according to their mechanism of action and include alkylating agents, antimetabolites, topoisomerase inhibitors, antibiotics, and mitotic inhibitors, among others (Malhotra & Perry, 2003).
Alkylating agents impair cell function by alkylating the DNA molecule. They depend on proliferation for activity, but are not cell phase-specific, and are classified according to their chemical structures and mechanisms (Ralhan & Kaur, 2007). Alkylating agents include nitrogen mustards (More et al., 2019), nitrosoureas (Mitchell & Schein, 1986), platinum complexes (Bai et al., 2017), oxazaphosphorines (Giraud et al., 2010), imidazotetrazines (temozolomide) (Moody & Wheelhouse, 2014), alkyl sulfates (busulfan, treosulfan, mannosulfan) (Lawson et al., 2021), and hydrazines (procarbazine) (Tweedie et al., 1987), among others.
Oxazaphosphorines (Zhang et al., 2005), such as cyclophosphamide and ifosfamide, are a type of alkylating agent that induce cross-linking at guanine.
Nitrogen mustards are powerful local vesicants. Their metabolites are highly reactive in alkylating the DNA molecule. The hematopoietic system is especially susceptible to these compounds, and dose-limiting toxicity includes myelosuppression. Severe nausea and vomiting are common side effects and, in some cases, alopecia, sterility, diarrhea, and thrombophlebitis. Examples are chlorambucil and melphalan (Diethelm-Varela et al., 2019).
Nitrosoureas (Brandes et al., 2016), for example, carmustine, lomustine, and streptozocin, are very instable and rapidly and spontaneously decompose into highly reactive intermediates. Their lipophilic nature enables free passage across membranes, including the blood-brain barrier. Therefore, these agents are used for a variety of brain tumors, but their dose-limiting toxicity is related to myelosuppression.
Platinum agents that are still widely used as first- and second-line treatments of various tumors produce intra-strand and interstrand DNA cross-links and form DNA adducts that inhibit their replication. Cisplatin, carboplatin, and oxaliplatin are examples of these compounds. Carboplatin shows greater water solubility, slower hydrolysis, and a different toxicity profile. Dose-limiting toxicities for cisplatin are renal insufficiency, peripheral sensory neuropathy, and ototoxicity. For carboplatin, the dose-limiting toxicity is myelosuppression, especially thrombocytopenia (Chen et al., 2013; Dasari & Tchounwou, 2014).
Antimetabolites’ major effect is interfering with the building blocks of DNA synthesis, and they are therefore most active in the S phase of the cell cycle and have little effect on the cells in G0. Consequently, these drugs are most effective in tumors that have a high growth fraction. Most of them are structural analogs of the naturally occurring metabolites involved in DNA and RNA synthesis. The antimetabolites can be divided into antifolates, purine antagonists, pyrimidine antagonists, and ribonucleotide reductase inhibitors. These include methotrexate, fluorouracil, cytarabine, gemcitabine, mercaptopurine, pemetrexed, pentostatin, hydroxyurea, fludarabine, and cladribine. They can induce myelosuppression and other severe adverse effects, such as hepatotoxicity or neurotoxicity, among others. Among these, 6-mercaptopurine and 5-fluorouracil, analogs of purines and pyrimidines, respectively, are widely used in clinical practices for the treatment of both hematological malignances and solid tumors (Kaye, 1998; Peters et al., 1993; Peters et al., 2000).
Topoisomerase inhibitors interrupt the DNA unbinding during the S and G2 phases of the cell cycle, by blocking topoisomerases I and II. Irinotecan and topotecan, two water-soluble analogs of the camptothecin, bind to topoisomerase I and are used to treat ovarian, colorectal, and small cell lung cancer. Their main adverse effects include severe myelosuppression and acute diarrhea. In particular, irinotecan demonstrated to have much more effective antitumor activity than first-generation camptothecins and less renal toxicity. On the other hand, etoposide and teniposide inhibit topoisomerase II, which leads to DNA double-strand breaks and increased DNA degradation. They are used to treat solid tumors, such as testicular and small cell lung cancer, leukemias, and lymphomas, and their adverse effects include myelosuppression and alopecia (Binaschi et al., 1995; Sinha, 1995; Wang & Tse-Dinh, 2019).
Antitumor antibiotics (Galm et al., 2005) can also be used for cancer treatment. First, bleomycin (Froudarakis et al., 2013), which has a cytotoxic effect on nondividing tumor cells, intercalates DNA, resulting in spontaneous oxidation and formation of free oxygen radicals that cause strand breakage. It is effective in the treatment of lymphomas, germ cell tumors, head and neck cancers, and squamous cell carcinoma, but the dose can be limited by the pulmonary toxicity that occurs in 10–40% of the treated patients. Dermatologic toxicity, fever, and anorexia are also frequently seen.
Other antibiotics, such as the anthracyclines doxorubicin, daunorubicin, and idarubicin, do not depend on the cell cycle and have multiple mechanisms of action, including the inhibition of topoisomerase II and the inhibition of DNA and RNA synthesis by intercalation with DNA, DNA strand excision, and generation of free radicals. They are effective in treating leukemias, lymphomas, and breast, ovarian, and bone cancer, and their adverse effects include cardiomyopathy and cardiotoxicity (Bhagat & Kleinerman, 2020; Carvalho et al., 2009; Greene & Hennessy, 2015).
Actinomycin D and mitomycin are also antibiotics with chemotherapeutic activity whose mechanism of action does not depend on the cell cycle. The first one intercalates into DNA and prevents DNA, RNA, and protein synthesis. It is used to treat some childhood cancers and rhabdomyosarcoma, among others, with a dose-limiting myelosuppression and dermatologic toxicity. On the other hand, mitomycin is used to treat gastric and pancreatic cancers. It alkylates DNA and inhibits DNA and RNA synthesis, also causing myelosuppression, hemolytic uremic syndrome, thrombotic thrombocytopenic purpura, and fever (Bradner, 2001).
Mitotic inhibitors include vinca alkaloids, taxanes, and nontaxane microtubule inhibitors (Jiang et al., 2006). Vinca alkaloids include vincristine, vinblastine, and vinorelbine. Upon entering the cell, vinca alkaloids bind rapidly to the tubulin and inhibit its assembly, during the S phase. Thus, polymerization of microtubules is blocked, resulting in cell cycle arrest in the M phase. They are used to treat many solid tumors, leukemias, and Hodgkin and non-Hodgkin lymphoma, but peripheral neurotoxicity can limit their dose (Duflos et al., 2002; Martino et al., 2018; Moore & Pinkerton, 2009; Moudi et al., 2013).
Taxanes, paclitaxel, and docetaxel, unlike the vinca alkaloids which cause microtubule disassembly, promote microtubule assembly and stability, therefore blocking the cell cycle in mitosis. Docetaxel is more potent in enhancing microtubule assembly and also induces apoptosis. These compounds have revolutionized the treatment of several solid tumors including metastatic breast cancer, metastatic pancreatic adenocarcinoma (in association with gemcitabine), NSCLC (in association with carboplatin), head and neck cancer, and gastric and prostate cancer. In particular, these drugs are used when the first-line treatment failed in metastatic patients and therefore represent the only therapeutic option for patients who show drug resistance mechanisms or are not candidates for curative surgical interventions (Mosca et al., 2021; Muggia & Kudlowitz, 2014; Zhang et al., 2019; Zhu & Chen, 2019). Adverse effects include peripheral neuropathy, interstitial pneumonitis, myelosuppression, cardiotoxicity, alopecia, and skin changes (Brewer et al., 2016; Sibaud et al., 2016).
Nontaxane microtubule inhibitors disrupt microtubule stability by blocking mitotic spindles without affecting depolymerization and thus stop the process of cell division at the G2/M phases. They are commonly used in the treatment of metastatic breast cancer and unresectable liposarcoma. Adverse effects include myelosuppression, peripheral neuropathy, and QT prolongation. Eribulin, ixabepilone, and epothilone are included in this group (Shetty & Gupta, 2014; Swami et al., 2017).
There are other compounds that are also worth mentioning, for example, the L-asparaginase, mostly used in acute lymphoblastic leukemia, an enzyme that breaks down the amino acid L-asparagine to aspartic acid and ammonia, reducing the source of asparagine for leukemic cells and inhibiting protein synthesis in tumor cells. During the treatment, allergic reactions, hepatotoxicity, hyperglycemia, pancreatitis, and blood clotting are frequently observed (Costa-Silva et al., 2020).
3.3 Marketed Chemotherapy-Loaded Nanoparticles for Cancer Treatment
As potent and effective cytotoxic drugs, these classic chemotherapeutic agents would benefit notably from a technology that could improve their specificity toward cancer cells, decreasing their toxicity and adverse effects and thus allowing for the administration of higher doses directed to the tumor. Nanotechnology could be the answer to the specific formulation needs of some of the abovementioned drugs. For example, doxorubicin is known to cause cumulative dose-dependent cardiotoxicity that can be severe, life-threatening, and dose-limiting (Zhao & Zhang, 2017). Changing its pharmacokinetic profile by encapsulating it into nanoparticles has demonstrated to significantly improve this aspect. Meanwhile, the mitotic inhibitor paclitaxel is very insoluble in water and is generally formulated using Cremophor EL, which generates the need for premedication and notably increases its side effects (Gelderblom et al., 2001). Figure 4 summarizes the main formulation problems that can be improved using nanoparticles.
With this idea in mind, for decades, hundreds of scientific groups worldwide have tried to improve the pharmaceutical profile of these antineoplastic agents, encapsulating them in nanoparticles of lipid, polymeric, or even inorganic nature, but it was not until 1995 when this approach finally reached the clinic (Anselmo & Mitragotri, 2019; Kemp & Kwon, 2021; Mitchell et al., 2021) (Table 1).
Doxil® (in Europe Caelyx®), a doxorubicin-loaded liposomal formulation, was the first FDA-/EMA-approved liposomal chemotherapeutic agent (Barenholz, 2012). Its success was based on three key elements: the liposome lipid bilayer was composed of high-T(m) phosphatidylcholine (PC) and cholesterol (in liquid state inside the body), the surface of the liposomes was modified with polyethylene glycol (PEG) to prolong drug circulation time and avoid the uptake by the reticuloendothelial system (RES), and a high drug-loading was achieved with a remote doxorubicin-loading ammonium sulfate-based transmembrane gradient.
With a prolonged circulation time, clearance and volume of distribution are drastically reduced, when compared to free doxorubicin (at least 250-fold and 60-fold, respectively), and the tumor cells are more exposed to the drug, for longer periods. Doxil not only has a better therapeutic effect but also significantly reduces the side effects of doxorubicin, such as myelosuppression, hair loss, vomiting, and diarrhea and, most importantly, the dose-limiting cumulative dose-dependent cardiotoxicity. However, Doxil® causes another characteristic side effect, desquamative dermatitis, which is called palmar-plantar erythrodysesthesia (PPE) or “hand-foot syndrome,” and an infusion-related reaction characterized by flushing and shortness of breath (von Moos et al., 2008). This symptom can be alleviated by slowing down the infusion rate and appropriate medication. Moreover, due to the long circulation time of the PEGylated drug, stomatitis (inflammation of mucus lining) became the new dose-limiting toxicity.
The US FDA approved the first generic version of Doxil® (doxorubicin hydrochloride liposome injection), LipoDox®, made by Sun Pharma Global FZE, in 2013, to ease drug shortage (Pillai & Ceballos-Coronel, 2013).
Just a few months after the approval of Doxil®, DaunoXome®, a liposomal formulation of another anthracycline, daunorubicin, was first licensed in the UK and later approved by the FDA (Petre & Dittmer, 2007). Its liposomes were composed mainly of two lipids, distearoylphosphatidylcholine (DSPC) and cholesterol, with a reduced size and neutral charge that minimized RES uptake, leading to prolonged drug circulation. A citrate salt was used for the active loading of daunorubicin into the nanoparticles.
DaunoXome® was approved for the treatment of AIDS-associated Kaposi sarcoma (KS), in the years where HIV was emerging as a serious threat, and it allowed for the administration of higher cumulative chemotherapeutic doses without significant cardiotoxicity or other adverse effects. Daunorubicin® plasma AUC levels were more than 35fdd greater than those reported for comparable doses of free drug, with responses above 50% for the treatment of KS (Forssen & Ross, 1994; Gill et al., 1996).
There is also a second liposomal doxorubicin approved, in Europe and Canada, for the first-line treatment of metastatic breast cancer, in combination with cyclophosphamide: Myocet® (Batist et al., 2002; Leonard et al., 2009). This formulation consists of doxorubicin encapsulated in non-PEGylated liposomes, made of PC and cholesterol, and its pharmacokinetics differs from both conventional doxorubicin and PEGylated liposomal doxorubicin. The clearance of this formulation is slower than free doxorubicin, with higher plasma levels, but faster than the PEGylated liposomes (Baselga et al., 2014).
Regarding the adverse effects, Myocet® has demonstrated to be substantially less cardiotoxic than doxorubicin and PPE occurs rarely, with an incidence of <0.5% in metastatic breast cancer patients treated in phase III clinical trials. Thus, this formulation has a particular role in patients previously treated with anthracyclines in the adjuvant setting and those with cardiac risk factors (Safra, 2003).
The last anthracycline-based liposomal formulation approved for cancer treatment is actually a combination of daunorubicin with cytarabine, at a cytarabine/daunorubicin 5:1 molar ratio (Blair, 2018). The liposome is composed of DSPC, 1,2-distearoyl-sn-glycero-3-phosphoglycerol (DSPG), and cholesterol. Vyxeos® (CPX-351) efficiently encapsulates both drugs into the same liposome, exploiting the synergies of these two drugs for the treatment of acute myeloid leukemia, providing a survival benefit with acceptable tolerability. In addition, it allows for relatively simple administration versus conventional 7 + 3 chemotherapy. Compared to standard of care treatment, Vyxeos® demonstrated superior median overall survival (3.61 months longer), event-free survival (1.22 months longer), and remission rate (14.4% higher) without increasing treatment-related mortality and toxicities (Lancet et al., 2016; Lancet et al., 2018).
Another sustained-release formulation encapsulating just cytarabine for the treatment of neoplastic meningitis is DepoCyt® (Mantripragada, 2002), prepared by a proprietary technology called DepoFoam®, that comprises tens-of-microns-in-diameter multivesicular particles formed by compartments separated by lipid bilayers. It is composed of palmitoyloleoylphosphatidylcholine (DOPC) and 1,2-Dipalmitoyl-sn-glycero-3-phosphoglycerol (DPPG), and its structure allows encapsulation of large quantities of drugs and ensures prolonged release. It is the only liposomal drug for intrathecal administration.
The terminal half-life of the formulation was 40 times longer than that of standard cytarabine (Chamberlain et al., 1995), notably improving its pharmacokinetic profile. The incidence and severity of chemical arachnoiditis, a common adverse event following administration of DepoCyt, can be reduced by the coadministration of dexamethasone.
The first nanoparticulate system for cancer treatment based in polymeric nanoparticles was approved in 2004, with the name of Eligard®. Polymeric nanoparticles represent very versatile vehicles that can be designed to improve the solubility of the encapsulated drug, the release profile, or the specific target, among others. Eligard® is composed of leuprolide (a testosterone inhibiting drug) incorporated into a polylactide-co-glycolic acid (PLGA) nanoparticle and is indicated as an effective treatment for the symptoms of prostate cancer. PLGA (Makadia & Siegel, 2011) is a widely used hydrophobic and biodegradable polymer that slowly decomposes into the constituent monomeric units over time, generating sustained-release profiles of the nanoencapsulated drug.
Oncaspar® (Dinndorf et al., 2007), by Servier Pharmaceuticals, is another approved nanoparticulate polymeric formulation for cancer treatment, which is composed of asparaginase and PEG. By covalently conjugating the native asparaginase to the hydrophilic polymer PEG, it is possible to increase its circulation and retention time, decrease proteolysis, and hide antigenic determinants from the immune system, thus avoiding hypersensitivity associated to the administration of free asparaginase (Jarrar et al., 2006). Oncaspar® was first approved for use in patients with acute lymphoblastic leukemia (ALL) who developed hypersensitivity to asparaginase. Later, it was approved as first-line treatment for ALL, as part of a multiagent thermotherapy regimen.
Abraxane® (Desai, 2016; Green et al., 2006; Lee et al., 2020) by Celgene is an albumin-bound formulation of another chemotherapy, paclitaxel, which is approved by the FDA for the treatment of metastatic breast cancer, NSCLC, and pancreatic adenocarcinoma. Conjugating the drug with albumin eliminated the need for an organic solvent, usually required for the delivery of the highly water-insoluble free paclitaxel, thus notably decreasing medication-associated side effects.
Another Cremophor-free paclitaxel formulation approved by the EMA is Apealea® (Vergote et al., 2020), which is also the newest nanoparticle formulation for cancer treatment in the market (approved in Europe in 2018). It is indicated in adult patients with a first relapse of platinum-sensitive epithelial ovarian cancer, primary peritoneal cancer and fallopian tube cancer, in combination with carboplatin. The formulation is based on the proprietary XR17 micelle platform technology, composed of two novel micelle-forming excipients, N-(all-trans-retinoyl)-L-cysteic acid methyl ester sodium salt and N-(13-cis-retinoyl)-L-cysteic acid methyl ester sodium salt. Apealea® showed non-inferior efficacy results and improved safety profile in phase III clinical trials against Taxol® (paclitaxel with Cremophor).
Marqibo® (Silverman & Deitcher, 2013), another mitotic inhibitor based formulation, is also approved by the FDA. In this case, vincristine sulfate, a semisynthetic chemotherapeutic agent, was encapsulated in sphingomyelin (SM) and cholesterol liposomes to overcome the dosing, pharmacokinetic, and pharmacodynamic limitations of free vincristine. In clinical trials, alone or in combination, Marqibo® was well tolerated and showed higher activity than standard vincristine treatment, probably due to the pharmacokinetic optimization and enhanced delivery. Currently it is indicated for the treatment of adult patients with Philadelphia chromosome-negative (Ph-) ALL, in second or greater relapse, or whose disease has progressed following two or more antileukemia therapies.
In 2015, based on the encouraging preclinical and clinical data available for the treatment of a variety of solid tumors, Onivyde® (Zhang, 2016), the nanoliposomal formulation of irinotecan, was approved by the FDA, as a combination regimen with 5-fluorouracil (5-FU) and leucovorin, for patients with gemcitabine-based chemotherapy-resistant metastatic pancreatic cancer. In advanced clinical trials, patients who received the combination of this PEGylated liposome formulation and 5-FU/leucovorin gained on average 2 months of survival and showed an average delay in the time to tumor growth of 3.1 months when compared to those who received only 5-FU/leucovorin (FDA Approves Onivyde Combo Regimen for Advanced Pancreatic Cancer, 2015).
Finally, Mepact® was the first drug approved for the management of high-grade, resectable, nonmetastatic bone tumors combined with postoperative combination chemotherapy in children, adolescents, and young adults who have gone through full macroscopic surgical resection. It is made of non-PEGylated liposomes loaded with muramyl tripeptide phosphatidylethanolamine (L-MTP-PE), a fabricated lipophilic derivative of muramyl dipeptide (MDP) (a naturally occurring constituent of bacterial cell walls) that activates monocytes, macrophages, and some cytokines, producing an immune response against osteosarcoma lung metastases. In clinical trials, it demonstrated a very good safety profile, both in patients and healthy volunteers, and given in addition to the usual combination chemotherapy conducted in children and young adults with osteogenic sarcoma showed an increase in 6-year net survival from 70% to 78% (Kager et al., 2010; Meyers et al., 2008).
3.4 Clinical Development of Nanoparticulate Systems for Cancer Treatment
Despite the few nanoparticle-based drugs approved for cancer treatment, many different formulations have reached clinical trials during the last decades. Alkylating agents, antimetabolites, topoisomerase inhibitors, and enzymes, but especially antitumor antibiotics and mitotic inhibitors, have been encapsulated mainly into PEGylated or non-PEGylated liposomes or polymeric micelles, sometimes functionalized for active targeting, but heavily relying just in the EPR effect (Anselmo & Mitragotri, 2021) (Table 2).
Regarding antitumor antibiotics, doxorubicin is by far the most commonly selected drug for its encapsulation into targeted and nontargeted nanoparticles, and, apart from the already mentioned successfully marketed formulations, many others have been tested in clinical trials. In one example from more than 20 years ago, Mitsubishi Pharma Corporation produced MCC-465, a liposome containing doxorubicin, with PEG and anti-GAH mAb that binds specifically to a molecule on the cell surface of gastric cancer cells. The expectations were high, as the results obtained in xenografts were promising, but the phase I trial in patients with gastric cancer revealed no clinical response, and no more clinical trials were performed with the formulation (Matsumura et al., 2004). HER2-targeted PEGylated liposome MM-302, from Merrimack Pharmaceuticals, experienced a similar fate and, besides the promising safety results obtained in the first phase I clinical trial in breast cancer patients, failed to show improvements in efficacy in more advanced studies (Miller et al., 2016; Munster et al., 2018). The two different formulations of doxorubicin-loaded epidermal growth factor receptor (EGFR)-targeting nanoparticles, from EnGeneIC (Whittle et al., 2015) and the Swiss Group for Clinical Cancer Research of the University Hospital of Basel (Mamot et al., 2012), were not successful in reaching the market either. EnGeneIC is now testing its technology, based on the EDV® Nanocell Platform (bacterially derived minicell) with other cytotoxic drugs, and the Swiss Group for Clinical Cancer Research has just started a new phase I clinical trial with a doxorubicin-loaded PEGylated liposome.
2B3-101 from 2-BBB Therapeutics – that later was sold to Oncology Venture, changing its name to 2X-111 – is a glutathione-containing PEGylated liposome loaded with doxorubicin, for the treatment of solid tumors and especially designed to cross the blood-brain barrier (BBB). The first phase I clinical trial started in 2011, and the results showed a good safety profile (Brandsma et al., 2014). A second phase II clinical trial is registered, but its status is “unknown” since a decade ago.
Worth mentioning is also the case of doxorubicin-loaded ThermoDox® system, the first and only thermosensitive liposome formulation to reach clinical trials, based on lipids that enable the temperature triggered release of their encapsulated content. The initial phase III clinical trial on ThermoDox® (i.e., HEAT trial) evaluating the drug in combination with the interventional oncology technique radiofrequency ablation (RFA), in comparison with RFA alone, for treatment of inoperable hepatocellular carcinoma (HCC) failed to meet its primary endpoint in progression-free survival (PFS). However, analysis of patient subgroups revealed a therapeutic benefit for ThermoDox® in patients who received prolonged RFA treatments, and thus Celsion Corporation decided to start a second phase III clinical trial, OPTIMA, exploring this condition, but it demonstrated that the addition of ThermoDox® to RFA does not provide a measurable survival benefit (Dou et al., 2017; Regenold et al., 2021).
To date, liposomal annamycin (semisynthetic analog of doxorubicin) has been tested in clinical trials, with different formulations, by three companies. The products by NYU Langone Health (Booser et al., 2002) and Callisto Pharmaceuticals (Wetzler et al., 2013) failed to show efficacy in patients and are no longer actively being studied. On the contrary, Moleculin Biotech has just announced updated preliminary safety data for annamycin in its three phase I clinical trials for acute myeloid leukemia and metastases of soft-tissue sarcoma, reporting a promising safety profile, with no cardiotoxicity and reduced alopecia (Gil et al., 2019).
Most of the evaluated antibiotics have been encapsulated in the inner aqueous phase of the liposomes, both by passive or active loading, but there are also examples of lipophilic drugs retained in the lipid bilayer of these nanoparticles. This is the case of Promitil® (Gabizon et al., 2020), a mitomycin-C lipidic prodrug loaded in PEGylated liposomes for the treatment of solid tumors that has already completed two phase I clinical trials showing a favorable safety profile and reduced toxicity as compared to equivalent doses of mitomycin-c. The product is currently being evaluated in a third phase I clinical trial.
Two companies selected mitoxantrone as the drug to be encapsulated into liposomes for the treatment of various cancers. The formulation of NeoPharm Labs Ltd. was evaluated 20 years ago, in a phase I clinical trial, but the results did not encourage the continuation of the studies (Ahmad et al., 2005). The mitoxantrone hydrochloride liposome from CSPC ZhongQi Pharmaceutical Technology has been tested in a total of 23 clinical trials, alone or in combination with other chemotherapeutic drugs, for the treatment of very different cancers, such as malignant lymphoma, metastatic breast cancer, acute myeloid leukemia, advanced pancreatic cancer, etc. In general, the shown safety profile is good, and the technology will continue being evaluated in clinical trials to determine its efficacy (Wang et al., 2021).
Antibiotics have also been encapsulated into polymeric nanoparticles, such as the NC-6300 epirubicin-loaded polymeric micelles that showed to be well tolerated, with a manageable side effect profile, in a phase Ib dose escalation trial in patients with advanced solid tumors or advanced, metastatic, or unresectable soft-tissue sarcoma (Chawla et al., 2020; Riedel et al., 2021). Another example is the PE-PEG-composed IMX-110 system, from Immix Biopharma, presented as monotherapy for soft-tissue sarcoma, that just a few weeks ago announced encouraging safety results for their ongoing phase Ib/IIa clinical trial.
Mitotic inhibitors have also been extensively studied in nanoformulations for cancer treatment, especially docetaxel and paclitaxel. Paclitaxel, very insoluble in water, is generally formulated using Cremophor EL. Docetaxel, more soluble in water, is formulated using Tween 80 and ethanol. Tween 80, albeit less toxic than Cremophor EL, may be responsible of some toxic effects. Thus, nanoparticles are a key technology to eliminate these vehicles and improve the drug’s antitumor efficacy.
Merrimack Pharmaceuticals tested a second formulation in a phase I clinical trial – apart from the previously described doxorubicin-loaded MM-302 – the docetaxel-loaded MM-310 anti-EphA2 receptor immunoliposome for the treatment of solid tumors (Kirpotin et al., 2016). The last safety update showed inability to reach optimal therapeutic index due to continued observation of cumulative peripheral neuropathy, and the formulation was discarded (Ernstoff et al., 2018).
The ATI-1123 product from Azaya Therapeutics, now acquired by Cytori Therapeutics, was also tested in a phase I clinical trial with encouraging safety results (Mahalingam et al., 2014). Now, based on the FDA feedback, the company plans to proceed with a follow-on phase II trial in platinum-sensitive small cell lung cancer that have progressed at least 60 days after initiation of first-line chemotherapy. The formulation is composed of phospholipids, cholesterol, human serum albumin (HSA), and sucrose, with the aim of removing the need for solvents, reducing hypersensitivity reactions, eliminating the requirement for premedications, and enhancing systemic docetaxel exposure.
The case of the BIND Therapeutics company is also well known. They developed prostate-specific membrane antigen (PSMA)-targeted polymeric nanoparticles, based on their Accurin® technology, loaded with chemotherapeutics, for the treatment of various cancers (Autio et al., 2018). Specifically, the BIND-014 product was loaded with docetaxel and evaluated in five phase I and II clinical trials for the treatment of prostate, metastatic, non-small cell lung, cervical, head and neck, or Kirsten Rat Sarcoma Viral Oncogene Homologue (KRAS)-positive lung cancers. Despite all the collaborations with the big pharmaceutical companies, the acquired funding, and the high expectations, their products failed to show efficacy in the clinic, and the company declared bankruptcy in 2016.
Cristal Therapeutics relies in polymeric micelles for sustained release of chemotherapeutics too (Braal et al., 2018). Their CriPec® platform is composed of tuneable polymers, biodegradable drug linkers, and optional target motives and has been evaluated, loaded with docetaxel, in three phase I and II clinical trials for the treatment of solid tumors and ovarian cancer. Phase I clinical trials showed well-tolerated safety profile, but in the phase II clinical trial, the efficacy endpoint was not met.
Docetaxel was also one of the chosen molecules for the cyclodextrin-based nanoparticle system of Cerulean, formed by covalently conjugating docetaxel to a linear, cyclodextrin-polyethylene glycol (CD-PEG) copolymer (Piha-Paul et al., 2021). Once again, the safety profile was acceptable, but the company decided to terminate clinical trials fearing lack of efficacy.
Samyang Biopharmaceuticals (South Korea) developed two polymeric micelle formulations loaded with docetaxel and paclitaxel, Docetaxel-PM (also DOPNP201/Nanoxel®) (Lee et al., 2011) and Genexol-PM (Kim et al., 2004; Madamsetty et al., 2019), respectively. These two monomethoxy PEG-b-poly(D,L, lactic acid) (PLA) formulations were specifically designed to improve the solubility of the chemotherapeutic drugs and to avoid the need to use toxic solubilizing agents such as Cremophor EL or Tween 80. Docetaxel-PM is commercialized in South Korea, and it is under clinical evaluation for pharmacokinetic equivalence with docetaxel injection concentrate as well as for safety and antitumor efficacy. Paclitaxel-PM is also available in South Korea and other Asian countries for the treatment of breast, non-small cell lung, and ovarian cancer and is currently undergoing bioequivalence testing to gain marketing approval in the US and European markets, under the name of Cynviloq IG-001, but the process is being long and highly controversial, with even legal accusations between the companies involved.
In addition, there are other four paclitaxel-loaded nanoparticle formulations approved in the Asian market. The first one, called LIPUSU® (Xu et al., 2013; Zhang et al., 2022), is a liposomal formulation, composed of lecithin and cholesterol, that was approved in China for the treatment of non-small cell lung cancer, breast cancer, and ovarian cancer, and it has been administered to over 2 million patients in the last 17 years. The second one is Nanoxel®, by Fresenius Kabi Oncology Ltd., that was approved in India in 2006 (Madaan et al., 2013; Ranade et al., 2013), allowing patients to receive Cremophor and premedication free paclitaxel, with equivalent efficacy. The third, Liporaxel®/DHP107 (Kim et al., 2020; Rugo et al., 2021; Yang et al., 2020), has the peculiarity of being intended for oral administration. The formulation, which is elaborated by mixing up the paclitaxel chemotherapeutic drug with monoolein, tricaprylin, and Tween 80, was approved in South Korea, in 2016, for the treatment of advanced, metastatic, and local recurrent gastric cancer and is currently in clinical trials in patients with other cancers. The last one, the Paclitaxel Injection Concentrate for Nanodispersion (PICN), by Sun Pharma Advanced Research Company Ltd. (SPARC), was approved in India, in 2014, for the treatment of metastatic breast cancer. In a phase II/III clinical study in patients with metastatic breast cancer (Jain et al., 2016; Ma et al., 2021), it was found to be equally effective and safe when compared to Abraxane®. Clinical studies are still ongoing.
Nippon Kayaku and Nanocarrier evaluated a paclitaxel-loaded polymeric micelle, NK105 (Hamaguchi et al., 2005; Hamaguchi et al., 2007; Kato et al., 2012), in a late-stage clinical trial against paclitaxel reference treatment too, but the formulation failed to meet its primary endpoint. Nanocarrier decided to continue clinical trials with a second-generation micelle pipeline in which the drug was chemically conjugated to the polymers inside the nanoparticles. We have already mentioned the epirubicin-loaded NC-6300, and another two, NC-6004 (Subbiah et al., 2018) and NC-4016 (Ueno et al., 2014), encapsulating cisplatin and oxaliplatin, respectively, are also being evaluated in clinical trials. NC-6004, in phase II clinical trials, is administered as a combination therapy, for the treatment of pancreatic, head, or neck cancer, among others. On the other hand, a phase I dose-escalation and pharmacokinetic study of NC-4016 in patients with advanced solid tumors or lymphoma has been completed in 2017, but no results have been published so far.
Finally, two more paclitaxel-loaded liposomal formulations have reached clinical testing: Endotag-I and LEP-ETU. The novelty of Endotag-I, from Medigene, is its positive charge, due to the presence of 1,2-Dioleoyl-3-trimethylammonium propane (DOTAP) in the formulation. It is generally accepted that nanoparticles of neutral or slightly negative charge more efficiently scape removal by the immune system, but positive charges augment the interaction between the nanoparticles and the negatively charged cellular membranes (Mitchell et al., 2021). The hypothesis behind Endotag-I (Fasol et al., 2012) is that because of the positively charged lipids, it interacts with newly developed, negatively charged endothelial cells, which are particularly required for the generation of tumor blood vessels. The nanoparticles attack the endothelial cells as they divide, thus targeting the blood supply to tumors without affecting the blood supply to healthy tissue. However, preclinical studies and clinical trials conducted on different types of cancer such as breast cancer, adenocarcinoma, or pancreatic cancer have shown limited efficacy and sometimes notable adverse events. There are still phase III clinical trials ongoing, with Endotag-I as a second-line treatment for pancreatic cancer.
On the other hand, the paclitaxel-loaded LEP-ETU (Slingerland et al., 2013), from NeoPharm Labs Ltd., is based on a similar formulation to the already mentioned mitoxantrone-loaded LEM-ETU, and the company evaluated a third composition in clinical trials too: the SN-38-loaded LE-SN-38 (Zhang et al., 2004). The three liposome formulations are based on similar combinations incorporating cholesterol and cardiolipin. LEP-ETU entered clinical evaluation to treat ovarian, breast, and lung cancers and completed its last phase II clinical trial in 2012. Since then, it received the Orphan Drug Designation from the FDA, but no updated information has been released. On the other hand, SN-38 is the active metabolite of irinotecan, and the LE-SN-38 liposomal formulation was tested for the treatment of small cell lung cancer and metastatic colorectal cancer in phase II clinical trials, where the formulation showed to be well tolerated but failed to meet efficacy endpoints.
With a slightly different concept, NanOlogy developed NanoDoce® and NanoPac® (Maulhardt et al., 2021, 2020; Mullany et al., 2020; Verco et al., 2021), two formulations of pure drug, docetaxel and paclitaxel, respectively, composed of large surface area microparticle (LSAM) therapeutic platforms, based on a proprietary supercritical precipitation technology that converts taxane API crystals into stable LSAMs, for tumor-directed therapy and sustained drug release. The administration for both products is local/intratumoral, and they are being tested in phase I and II clinical trials for the treatment of different cancers, such as urothelial carcinoma, pancreatic adenocarcinoma, and lung cancer.
Worth mentioning are two other mitotic inhibitors that have been tested in clinical trials in nanoparticulate formulations for cancer treatment: eribulin mesylate and the thiocolchicine analog IDN 5405. Eribulin mesylate, Halaven®, synthesized by Eisai, got FDA approval in 2010, and the same company is now testing eribulin mesylate-loaded liposomal formulation (Halaven E7389-LF) in clinical trials. Results from the first phase I clinical trial showed the formulation was well tolerated in patients with advanced solid tumors (https://www.annalsofoncology.org/article/S0923-7534(19)58570-2/fulltext#relatedArticles). Two more clinical trials, in phase I and phase Ib/II, are now ongoing in Japan, with the liposomal formulation alone or in combination with nivolumab. On the other hand, IDN 5405, the thiocolchicine analog, was formulated bound to albumin to develop ABI-011 – later NTB-011, in collaboration with Celgene – with cytotoxic and vascular disrupting properties (D’Cruz et al., 2009). The expectations were high as the inventors of Abraxane®, the successful albumin-paclitaxel nanoparticle, were involved in the project; however, the first clinical trial was terminated and the second one withdrawn even before starting patient enrollment.
One of the successful stories that ended up in the commercialization of one of the few approved nanoparticle-based chemotherapeutic formulations started with the testing of various sphingosomes by Inex Pharmaceuticals. The nanoparticles composed of SM and cholesterol were loaded with vincristine (Onco TCS) (vincristine liposomal-INEX: lipid-encapsulated vincristine, Onco TCS, transmembrane carrier system-vincristine, vincacine, vincristine sulfate liposomes for injection, VSLI, 2004), vinorelbine (INX-0125) (Semple et al., 2005), or topotecan (INX-0076), among others, and evaluated in clinical trials for the treatment of advanced solid tumors and non-Hodgkin lymphoma (Bulbake et al., 2017). A few years later, Onco TCS changed its name to Marqibo® and was approved by the FDA for the treatment of Philadelphia chromosome-negative ALL and commercialized by Spectrum Pharmaceuticals. This company also tested another formulation in a phase I clinical trial, Alocrest, that resulted to be generally well tolerated (Deitcher et al., 2007).
INX-0076 and LE-SN-38 were not the only nanoparticulate formulation based on topoisomerase inhibitors that reached clinical testing. The therapeutic potential of camptothecins (including irinotecan and topotecan) is limited because they rapidly undergo hydrolysis at physiological pH, changing from their active form (lactone ring structure) to their inactive form (carboxylate structure), leading to a short circulation lifetime. Liposomal formulations of these molecules can be designed to overcome these stability issues.
The previously mentioned company, Cerulean, developed a formulation based on camptothecin (apart from the docetaxel-loaded CRLX301), called CRLX101 (Pham et al., 2015; Svenson et al., 2011; Young et al., 2011) (formerly IT-101), developed by covalently conjugating camptothecin to a linear, cyclodextrin-PEG (CD-PEG) copolymer that self-assembles into nanoparticles. The formulation seemed promising at the preclinical level, as it was expected to address solubility, formulation, toxicity, and pharmacokinetic challenges, improving the efficacy. However, in 2013, it failed to show a benefit in lung cancer, causing a strategy change to drug combinations, but 3 years later, the company reported disappointing results for another phase II clinical trial, in combination with bevacizumab, in renal cell carcinoma patients.
Other clinical stage attempts to encapsulate topoisomerase inhibitors in nanoparticles for cancer treatment including OSI-211, IT-141, and S-CKD602. The non-PEGylated liposomal form of lurtotecan, OSI-211 (Duffaud et al., 2004; Tomkinson et al., 2003), from OSI Pharmaceuticals, composed of hydrogenated soy phosphatidylcholine (HSPC) and cholesterol, was evaluated in a total of six clinical trials that finished more than a decade ago, and there are no updates since then. IT-141 (Carie et al., 2011) was composed of SN-38-loaded polymeric micelles and was evaluated in a phase I clinical trial that was terminated by the sponsor. Lastly, the phase I clinical trial testing the PEGylated liposomal formulation S-CKD602 (Zamboni et al., 2009), from Alza Corporation, finished in 2006, and, besides the company qualifying the results as “promising,” there have been no news since then.
Regarding the use of alkylating agents, we have already mentioned NC-6004 Nanoplatin and NC-4016 DACH-Platin from Nanocarrier, but there are more examples in clinical trials. The most evaluated drug has been cisplatin, in formulations including lipoplatin/nanoplatin, SPI-77, SLIT®, and LiPlaCis®, among others. Cisplatin is one of the most widely used chemotherapies due to its efficacy against multiple cancer types but has severe side effects, demonstrating the critical need for specificity and reformulation.
Lipoplatin® (also known as Nanoplatin®) (Boulikas et al., 2005) is a proprietary PEGylated liposome formulation of cisplatin, by Regulon, Inc. The product has been introduced as Lipoplatin® for the treatment of pancreatic cancer and Nanoplatin® for lung cancer. This liposomes, composed of lipids including DPPG, soy PC, MPEG-distearoyl-sn-glycero-phosphoethanolamine (DSPE) lipid conjugate, and cholesterol, have been tested in phase I trials for malignant pleural effusion, phase II trials for breast and gastric cancer, phase II/III trials for pancreatic cancer, and phase III trials for NSCL ((Mylonakis et al., 2010; Stathopoulos et al., 2005; Stathopoulos et al., 2006a, b). In clinical trials, the company announced good safety profiles with reduced adverse effects associated with CPT including renal toxicity, peripheral neuropathy, ototoxicity, and myelotoxicity (Boulikas et al., 2005; Boulikas, 2009). In 2007, the EMA granted Orphan Drug Designation to this product for pancreatic cancer treatment, while clinical trials were still ongoing; however, no results have been published in years, and the company has not clarified if the drug is still being evaluated.
Formulations of cisplatin (SPI-77) (Seetharamu et al., 2010; Vokes et al., 2000; White et al., 2006) or analogs, developed by ALZA Pharmaceuticals, formerly Sequus Pharmaceuticals, were based on stealth liposomes. Results obtained in phase I and II clinical trials demonstrated a good safety profile but very limited efficacy. These findings were attributed to the low loading capacity and insufficient release of the free drug.
LiPlaCis®, developed for treatment of advanced solid tumors, is a liposomal formulation, incorporating cisplatin, which is composed of lipids with degradation properties controlled by the phospholipase A2 (PLA2) enzyme, highly expressed in a multitude of human solid tumors including prostatic, pancreatic, colorectal, gastric, and breast cancers for a tumor-triggered release mechanism. In clinical trials, LiPlaCis® has demonstrated an enhanced therapeutic window compared to cisplatin, with superior PK properties, greater potency, and an increased maximum tolerated dose. However, severe renal toxicity and an acute infusion reaction were observed in patients in phase I study. Thus, LiPlaCis® clinical studies were halted.
SLIT® (Sustained Release Lipid Inhalation Target) (Chou et al., 2013), the liposomal formulation from Transave (later Inhaled Lipid Cisplatin, ILC, from Insmed Incorporated), was composed of dipalmitoylphosphatidylcholine (DPPC) and cholesterol and presented a key novelty: it was an aerosolized formulation for pulmonary administration. In a phase I/II clinical study in patients with osteosarcoma metastatic to the lung, adverse effects associated to the IV administration of cisplatin were not reported, but changes in the pulmonary function were detected in some patients. Major benefits were described in patients with operable and small tumors (<2 cm), but more studies are needed to determine the efficacy and safety of the treatment.
On the other hand, oxaliplatin has also been nanoencapsulated and tested in clinical trials. As a third-generation water-soluble platinum drug, it is different from cisplatin and carboplatin in that it presents free amino groups linked to platinum and has lower toxicity and tumor resistance. MBP-426 (Sankhala et al., 2009; Senzer et al., 2009) is an oxaliplatin-encapsulated transferrin-conjugated N-glutaryl phosphatidylethanolamine (NGPE)-liposome that targets the transferrin receptor, which is upregulated in many types of cancer. After a phase I clinical trial in patients with advanced or metastatic solid tumors, the formulation entered a phase I/II trial for second-line gastric, gastroesophageal, or esophageal adenocarcinoma in 2009, but results have not been posted yet.
Regulon, Inc., the company that developed the cisplatin-loaded Lipoplatin®, also developed an oxaliplatin-based liposomal formulation, LipoXal® (Stathopoulos et al., 2006a; Tippayamontri et al., 2014). In a phase I study, reduction respect to free oxaliplatin of myelotoxicity, nausea, and peripheral neuropathy was observed, but further clinical tests will be needed to demonstrate the improvement of antitumor activity of LipoXal® over free oxaliplatin.
Aroplatin (L-NDDP) (Dragovich et al., 2006) is a liposome encapsulating a cis-bis-neodecanoato-trans-R,R-1,2-diaminocyclohexane platinum II (NDDP), an oxaliplatin derivative. The multi-lamellar liposomes were formed from 1,2-dimyristoyl-sn-glycero-3-phospho-(1′-racglycerol) (DMPG) and 1,2-dimyris-toyl-sn-glycero-3-phosphocholine (DMPC) lipids in acidified saline solution. In phase II study, Aroplatin was tested in refractory metastatic colorectal cancer, and, besides the acceptable safety profile, in general the response was modest. To date, there is no report of any ongoing phase III study. Two decades ago, the same company, Aronex Pharmaceuticals (now Antigenics), tried to commercialize another liposomal formulation, loaded with tretinoin and named Atragen (Bernstein et al., 1998), but the FDA rejected the approval.
Apart from the cytarabine-containing marketed formulations, nanomedicines based on antimetabolites for the treatment of cancer have been nearly anecdotic, with only one formulation reaching clinical trials: gemcitabine-loaded FF-10832 (Matsumoto et al., 2021), by Fujifilm Pharmaceuticals. The PEGylated formulation is now being evaluated in a phase I clinical trial, for the treatment of solid tumors, and last year, Fujifilm Pharmaceuticals signed an agreement with Merck to start a new clinical study for advanced solid tumors in combination therapy with KEYTRUDA® (pembrolizumab).
Finally, worth mentioning are two strategies that are not based in traditional chemotherapy: LipoCurc® (Bolger et al., 2019) and 188Re-BMEDA-liposome. LipoCurc®, by SignPath Pharma, is composed of curcumin-loaded nanoparticles. Historically, development of curcumin as a pharmaceutical product has been hampered by its poor absorption and cardiac side effects. Thus, LipoCurc® was designed to improve curcumin bioavailability and toxicological profile. First reported results were encouraging, with a very good safety profile despite the high blood concentrations. They are planning new clinical trials in different cancer types.
188Re-BMEDA-liposome (Chang et al., 2007; Lepareur et al., 2019), from the Institute of Nuclear Energy Research of Taiwan, was the only formulation incorporating radioactive isotopes to reach clinical trials for the treatment of primary solid tumors in advanced or metastatic stage. However, the phase I trial was terminated due to concerns of accumulation of radioactivity in both the liver and spleen
4 Challenges in Nanomedicine Clinical Translation
Despite the uncountable attempts to develop targeted nanoparticulate therapies for drug delivery to tumors, few anticancer nanomedicines have been approved by regulatory agencies, thus generating a debate regarding the real effectiveness of these systems for cancer treatment. Most anticancer medicines follow the same two basic criteria when trying to design effective and safe sustained drug delivery systems based on lipid or polymeric nanoparticles: (1) the EPR effect, caused by the leaky vasculature next to the tumor, increases drug accumulation in the affected area, and (2) long systemic circulation of drug-loaded nanoparticles avoids the uptake by the RES, decreasing drug accumulation in the normal organs and reducing toxicity (Sun et al., 2020). The EPR effect influencing nanomedicines has repeatedly been confirmed, both in animal xenografts and in human cancer patients, using nanoparticle-encapsulated imaging agents (Gaillard et al., 2014; Greish, 2010; Hamaguchi et al., 2004; Koukourakis et al., 2000; Torchilin, 2011), but it is difficult to conclude if this EPR effect is different to the one observed for the free drugs. Free drugs, as small molecules with high plasma protein binding, also accumulate in tumors due to this phenomenon (Tang et al., 2014; Torchilin, 2011), and, due to ethical concerns, clinical trials with a free drug control arm are not possible in most cases; thus, there are very few direct comparisons between the free drug and the nanoparticle formulation.
When Doxil® reached the market, the accumulation of doxorubicin in patient tumors was found to be an order of magnitude higher than with free drug, and pathogenic analysis of KS revealed notably leaky vasculature (Northfelt et al., 1998; Uldrick & Whitby, 2011). However, in a later study, the evaluation of the tumor uptake of radiolabeled liposomes, with the same lipid composition as Doxil®, demonstrated considerable heterogeneity between patients with the same and different cancer types (Harrington et al., 2001). Since then, a few studies have demonstrated significantly higher drug concentrations in the tumors when administering liposomal formulations (Gabizon et al., 1994), but limited improvements have been the reason of failure and cancelation of many clinical trials (Dragovich et al., 2006; Kraut et al., 2005; White et al., 2006).
Recent studies increasingly downplay the EPR effect. An interesting analysis by Wilhelm et al., surveying the literature from the past 10 years, concluded that only 0.7% (median) of the administered nanoparticle dose is found to be delivered to a solid tumor (Wilhelm et al., 2016). Another meta-analysis found no significant difference in clinical anticancer efficacy between liposomal and conventional chemotherapeutics in terms of objective response rate, overall survival, and PFS (Petersen et al., 2016).
Another key aspect is the validity of the animal xenograft models to mimic the biological phenomena observed in human cancers. In the available animal models, the EPR effect is notably exaggerated, resulting in a poor clinical translation (Greish, 2010). Thus, there is an urgent necessity to develop new models for in vivo and in silico testing.
Regarding the long systemic circulation and the high plasma concentration, it can increase tumor accumulation if there is a strong EPR effect or decrease drug accumulation in normal organs to reduce toxicity. However, it can also reduce efficacy or alter drug distribution to different organs, generating new adverse events (Harrington et al., 2001; Ngan & Gupta, 2016; Northfelt et al., 1998).
In addition, even if nanoparticles are able to avoid clearance from blood circulation (by the mononuclear phagocytic systems or the RES, among others) and the shear stress caused by varying flow rates and extravasate next to the tumor, the complex extracellular matrix surrounding malignant cells will notably limit their penetration (Yuan et al., 1994). Furthermore, lack of drug release from the vehicles can significantly decrease drug availability (Laginha et al., 2005; White et al., 2006).
Furthermore, after hundreds of preclinical and a few clinical studies with actively targeted nanoparticles incorporating specific motifs directed to molecules that are usually overexpressed on cancer cells, none of the tested strategies have reached the market (Ernstoff et al., 2018; Mamot et al., 2012; Matsumura et al., 2004). This is probably linked to the fact that actively targeted nanosystems also rely on the same principles as the passive targeting until they reach the microenvironment of the tumor where they can match with the specific molecules on the cancer cell membranes, thus dealing with the same challenges.
In general, most of the marketed nanomedicines failed to show improved efficacy, in comparison with the reference treatment, but they significantly and consistently improved the toxicity profile of classic chemotherapeutic agents, allowing for the administration of higher doses and better patient quality of life (Batist et al., 2002; Drummond et al., 1999; Farokhzad & Langer, 2006).
5 Conclusions
Cancer continues to be unstoppable worldwide, and there will be more than 30 million new cases by 2040, according to the International Agency for Research on Cancer. Thus, novel diagnostic and treatment tools are needed to beat this global challenge. Among the approaches explored by scientists, nanomedicine highlights due to its ability to develop an endless variety of accurate nanomaterials to provide a new landscape in cancer research. Thus, different scientific disciplines, such as engineering, chemistry, physics, nanotechnology, materials science, or medicine, work together to achieve precision systems and also enhance the translation to the clinics and pharmaceutical market. However, even though standardization, stability, and reproducibility are required for this goal, tailored features are mandatory for the successful application of the personalized medicine.
In this chapter, we have evidenced the encouraging potential of advanced nanoparticles as smart drug delivery systems to improve the therapeutic effect of current standard drugs and increased patient survival rates. Undoubtedly, there is still a long journey from the nanocarrier design to translation to the pharmaceutical market as viable products. Although thousands of research articles describe great outcomes of drug delivery systems with different nature and properties in multiple in vitro and in vivo cancer models, only a small fraction has successfully reached the translation to clinical level. This limited clinical translation of new nanoparticles is mainly due to incomplete therapeutic efficacy and off-target toxicity in vital organs. Nonetheless, results and evidences from previous clinical trials should guide not only the optimization of nanocarrier formulations but also setting clinical studies taking into account the tumor heterogeneity through the introduction of stratified populations instead of broad cancer patients.
Abbreviations
- AIDS:
-
Acquired immune deficiency syndrome;
- ALL:
-
Lymphoblastic leukemia;
- ARC:
-
International Agency for Research on Cancer;
- AUC:
-
Area under the curve;
- AuNPs:
-
Gold nanoparticles;
- BBB:
-
Blood-brain barrier;
- CAFs:
-
Cancer-associated fibroblasts
- CD:
-
Cyclodextrin
- CD-PEG:
-
Cyclodextrin-polyethylene glycol
- Chol:
-
Cholesterol
- DMPC:
-
1,2-Dimyris-toyl-sn-glycero-3-phosphocholine
- DMPG:
-
1,2-Dimyristoyl-sn-glycero-3-phospho-(1’-racglycerol)
- DNA:
-
Deoxyribonucleic acid
- DOPC:
-
Palmitoyloleoylphosphatidylcholine
- DOPS:
-
Dioleoylphosphatidylserine
- DOTAP:
-
1,2-Dioleoyl-3-trimethylammonium propane
- DPPC:
-
Dipalmitoylphosphatidylcholine
- DPPG:
-
1,2-Dipalmitoyl-sn-glycero-3-phosphoglycerol;
- DSPC:
-
Distearoylphosphatidylcholine
- DSPE:
-
Distearoyl-sn-glycero-phosphoethanolamine
- DSPG:
-
1,2-Distearoyl-sn-glycero-3-phosphoglycerol
- ECM:
-
Extracellular matrix
- EGFR:
-
Epidermal growth factor receptor
- EMA:
-
European Medicines Agency
- EPC:
-
Egg phosphatidylcholine
- EPR:
-
Enhanced permeability and retention
- FDA:
-
US Food and Drug Administration
- HCC:
-
Hepatocellular carcinoma
- HER2:
-
Human epidermal growth factor receptor 2
- HIV:
-
Human immunodeficiency virus
- HSA:
-
Human serum albumin
- HSPC:
-
Hydrogenated soy phosphatidylcholine
- KRAS:
-
Kirsten rat sarcoma viral oncogene homolog
- KS:
-
Kaposi sarcoma
- L-MTP-PE:
-
Muramyl tripeptide phosphatidylethanolamine
- LNPs:
-
Lipid nanoparticles
- LSAM:
-
Large surface area microparticle
- MDP:
-
Muramyl dipeptide
- MDR:
-
Multidrug resistance
- MPEG:
-
Methoxy polyethylene glycol
- MPPE:
-
Maleimidated palmitoyl phosphatidylethanolamine
- MSPC:
-
1-Myristoyl-2-stearoyl-sn-glycero-3-phosphocholine
- NGPE:
-
N-glutaryl phosphatidylethanolamine
- NIPAM:
-
Poly(N-isopropylacrylamide)
- NPs:
-
Nanoparticles
- NSCLC:
-
Non-small cell lung cancer
- PC:
-
Phosphatidylcholine
- PE:
-
Polyethylene
- PEG:
-
Polyethylene glycol
- PEG2000-DSPE:
-
PEGylated distearoyl-sn-glycero-phosphoethanolamine
- PFS:
-
Progression-free survival
- PICN:
-
Paclitaxel injection concentrate for nanodispersion
- PLA:
-
Polylactic acid
- PLA2:
-
Phospholipase A2
- PLGA:
-
Polylactide-co-glycolic acid
- POPC:
-
Palmitoyloleoylphosphatidylcholine
- PPE:
-
Palmar-plantar erythrodysesthesia
- PSMA:
-
Prostate-specific membrane antigen
- PVP:
-
Polyvinyl-pyrrolidone
- RES:
-
Reticuloendothelial system
- RFA:
-
Radiofrequency ablation
- RNA:
-
Ribonucleic acid
- SM:
-
Sphingomyelin
- SPARC:
-
Sun Pharma Advanced Research Company, Ltd.
- TAMs:
-
Tumor-associated macrophages
References
Agrawal, N. K., Allen, P., Song, Y. H., Wachs, R. A., Du, Y., Ellington, A. D., & Schmidt, C. E. (2020). Oligonucleotide-functionalized hydrogels for sustained release of small molecule (aptamer) therapeutics. Acta Biomaterialia, 102, 315–325.
Aguado, B. A., Grim, J. C., Rosales, A. M., Watson-Capps, J. J., & Anseth, K. S. (2018). Engineering precision biomaterials for personalized medicine. Science Translational Medicine, 10, eaam8645.
Ahmad, A., Wang, Y. F., & Ahmad, I. (2005). Separation of liposome-entrapped mitoxantrone from nonliposomal mitoxantrone in plasma: Pharmacokinetics in mice. Methods in Enzymology, 391, 176–185.
Alyautdin, R., Khalin, I., Nafeeza, M. I., Haron, M. H., & Kuznetsov, D. (2014). Nanoscale drug delivery systems and the blood-brain barrier. International Journal of Nanomedicine, 9, 795–811.
Anselmo, A. C., & Mitragotri, S. (2019). Nanoparticles in the clinic: An update. Bioengineering & Translational Medicine, 4, e10143.
Anselmo, A. C., & Mitragotri, S. (2021). Nanoparticles in the clinic: An update post COVID-19 vaccines. Bioengineering Translational Medicine, 6, e10246.
Arias, L. S., Pessan, J. P., Vieira, A. P. M., Lima, T. M. T., Delbem, A. C. B., & Monteiro, D. R. (2018). Iron oxide nanoparticles for biomedical applications: A perspective on synthesis, drugs, antimicrobial activity, and toxicity. Antibiotics, 7(2), 46.
Autio, K. A., Dreicer, R., Anderson, J., Garcia, J. A., Alva, A., Hart, L. L., Milowsky, M. I., Posadas, E. M., Ryan, C. J., Graf, R. P., Dittamore, R., Schreiber, N. A., Summa, J. M., Youssoufian, H., Morris, M. J., & Scher, H. I. (2018). Safety and efficacy of BIND-014, a docetaxel nanoparticle targeting prostate-specific membrane antigen for patients with metastatic castration-resistant prostate cancer: A phase 2 clinical trial. JAMA Oncology, 4, 1344–1351.
Awasthi, R., Roseblade, A., Hansbro, P. M., Rathbone, M. J., Dua, K., & Bebawy, M. (2018). Nanoparticles in cancer treatment: Opportunities and obstacles. Current Drug Targets, 19, 1696–1709.
Baguley, B. C. (2010). Multidrug resistance in cancer. Methods in Molecular Biology, 596, 1–14.
Bai, L., Gao, C., Liu, Q., Yu, C., Zhang, Z., Cai, L., Yang, B., Qian, Y., Yang, J., & Liao, X. (2017). Research progress in modern structure of platinum complexes. European Journal of Medicinal Chemistry, 140, 349–382.
Barenholz, Y. (2012). Doxil®-the first FDA-approved nano-drug: Lessons learned. Journal of Controlled Release, 160, 117–134.
Baron, J. A. (2012). Screening for cancer with molecular markers: Progress comes with potential problems. Nature Reviews Cancer, 12, 368–371.
Baselga, J., Manikhas, A., Cortés, J., Llombart, A., Roman, L., Semiglazov, V. F., Byakhov, M., Lokanatha, D., Forenza, S., Goldfarb, R. H., Matera, J., Azarnia, N., Hudis, C. A., & Rozencweig, M. (2014). Phase III trial of nonpegylated liposomal doxorubicin in combination with trastuzumab and paclitaxel in HER2-positive metastatic breast cancer. Annals of Oncology, 25, 592–598.
Batist, G., Barton, J., Chaikin, P., Swenson, C., & Welles, L. (2002). Myocet (liposome-encapsulated doxorubicin citrate): A new approach in breast cancer therapy. Expert Opinion on Pharmacotherapy, 3, 1739–1751.
Bayda, S., Adeel, M., Tuccinardi, T., Cordani, M., & Rizzolio, F. (2019). The history of nanoscience and nanotechnology: From chemical-physical applications to nanomedicine. Molecules, 25(1), 112.
Bernstein, Z. P., Rios, A., Scadden, D., Groopman, J., Northfelt, D., Lang, W., Fischl, M., Cohen, P., Bock, A., & Gill, P. (1998). A multicenter, phase II/III study of Atragen™ (Tretinoin Liposomal) in patients with AIDS-associated Kaposi’s sarcoma. JAIDS Journal of Acquired Immune Deficiency Syndromes, 17, A24.
Bhagat, A., & Kleinerman, E. S. (2020). Anthracycline-induced cardiotoxicity: Causes, mechanisms, and prevention. Advances in Experimental Medicine and Biology, 1257, 181–192.
Binaschi, M., Zunino, F., & Capranico, G. (1995). Mechanism of action of DNA topoisomerase inhibitors. Stem Cells, 13, 369–379.
Blair, H. A. (2018). Daunorubicin/Cytarabine liposome: A review in acute myeloid leukaemia. Drugs, 78, 1903–1910.
Blanco, E., Shen, H., & Ferrari, M. (2015). Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nature Biotechnology, 33, 941–951.
Bobo, D., Robinson, K. J., Islam, J., Thurecht, K. J., & Corrie, S. R. (2016). Nanoparticle-based medicines: A review of FDA-approved materials and clinical trials to date. Pharmaceutical Research, 33, 2373–2387.
Bolger, G. T., Licollari, A., Tan, A., Greil, R., Vcelar, B., Greil-Ressler, S., Weiss, L., Schönlieb, C., Magnes, T., Radl, B., Majeed, M., & Sordillo, P. P. (2019). Pharmacokinetics of liposomal curcumin (Lipocurc™) infusion: Effect of co-medication in cancer patients and comparison with healthy individuals. Cancer Chemotherapy and Pharmacology, 83, 265–275.
Booser, D. J., Esteva, F. J., Rivera, E., Valero, V., Esparza-Guerra, L., Priebe, W., & Hortobagyi, G. N. (2002). Phase II study of liposomal annamycin in the treatment of doxorubicin-resistant breast cancer. Cancer Chemotherapy and Pharmacology, 50, 6–8.
Boulikas, T. (2009). Clinical overview on Lipoplatin: A successful liposomal formulation of cisplatin. Expert Opinion on Investigational Drugs, 18, 1197–1218.
Boulikas, T., Stathopoulos, G. P., Volakakis, N., & Vougiouka, M. (2005). Systemic Lipoplatin infusion results in preferential tumor uptake in human studies. Anticancer Research, 25, 3031–3039.
Braal, C. L., de Bruijn, P., Atrafi, F., van Geijn, M., Rijcken, C. J. F., Mathijssen, R. H. J., & Koolen, S. L. W. (2018). A new method for the determination of total and released docetaxel from docetaxel-entrapped core-crosslinked polymeric micelles (CriPec®) by LC-MS/MS and its clinical application in plasma and tissues in patients with various tumours. Journal of Pharmaceutical and Biomedical Analysis, 161, 168–174.
Bradner, W. T. (2001). Mitomycin C: A clinical update. Cancer Treatment Reviews, 27, 35–50.
Brandes, A. A., Bartolotti, M., Tosoni, A., & Franceschi, E. (2016). Nitrosoureas in the management of malignant gliomas. Current Neurology and Neuroscience Reports, 16, 13.
Brandsma, D., Milojkovic Kerklaan, B., Diéras, V., Altintas, S., Anders, C. K., Arnedos Ballester, M., Gelderblom, H., Soetekouw, P. M. M. B., Gladdines, W., Lonnqvist, F., Jager, A., van Linde, M. E., Schellens, J., & Aftimos, P. (2014). Phase 1/2A study of glutathione pegylated liposomal doxorubicin (2b3-101) in patients with brain metastases (bm) from solid tumors or recurrent high grade gliomas (HGG). Annals of Oncology, 25, iv157.
Brewer, J. R., Morrison, G., Dolan, M. E., & Fleming, G. F. (2016). Chemotherapy-induced peripheral neuropathy: Current status and progress. Gynecologic Oncology, 140, 176–183.
Brown, S. B., Wang, L., Jungels, R. R., & Sharma, B. (2020). Effects of cartilage-targeting moieties on nanoparticle biodistribution in healthy and osteoarthritic joints. Acta Biomaterialia, 101, 469–483.
Bukowski, K., Kciuk, M., & Kontek, R. (2020). Mechanisms of multidrug resistance in cancer chemotherapy. International Journal of Molecular Sciences, 21(9), 3233.
Bulbake, U., Doppalapudi, S., Kommineni, N., & Khan, W. (2017). Liposomal formulations in clinical use: An updated review. Pharmaceutics, 9(2), 12.
Byrne, J. D., Betancourt, T., & Brannon-Peppas, L. (2008). Active targeting schemes for nanoparticle systems in cancer therapeutics. Advanced Drug Delivery Reviews, 60, 1615–1626.
Caballero, D., Abreu, C. M., Lima, A. C., Neves, N. N., Reis, R. L., & Kundu, S. C. (2022). Precision biomaterials in cancer theranostics and modelling. Biomaterials, 280, 121299.
Caldorera-Moore, M., Vela Ramirez, J. E., & Peppas, N. A. (2019). Transport and delivery of interferon-α through epithelial tight junctions via pH-responsive poly(methacrylic acid-grafted-ethylene glycol) nanoparticles. Journal of Drug Targeting, 27, 582–589.
Caliceti, P., & Matricardi, P. (2019). Advances in drug delivery and biomaterials: Facts and vision. Pharmaceutics, 11(1), 48.
Cao, J., Huang, D., & Peppas, N. A. (2020). Advanced engineered nanoparticulate platforms to address key biological barriers for delivering chemotherapeutic agents to target sites. Advanced Drug Delivery Reviews, 167, 170–188.
Carie, A., Rios-Doria, J., Costich, T., Burke, B., Slama, R., Skaff, H., & Sill, K. (2011). IT-141, a polymer micelle encapsulating SN-38, induces tumor regression in multiple colorectal cancer models. Journal of drug delivery, 2011, 869027.
Carvalho, C., Santos., R. X., Cardoso, S., Correia, S., Oliveira, P. J., Santos, M. S., & Moreira, P. I. (2009). Doxorubicin: The good, the bad and the ugly effect. Current Medicinal Chemistry, 16, 3267–3285.
Chamberlain, M. C., Kormanik, P., Howell, S. B., & Kim, S. (1995). Pharmacokinetics of intralumbar DTC-101 for the treatment of leptomeningeal metastases. Archives of Neurology, 52, 912–917.
Chang, Y. J., Chang, C. H., Chang, T. J., Yu, C. Y., Chen, L. C., Jan, M. L., Luo, T. Y., Lee, T. W., & Ting, G. (2007). Biodistribution, pharmacokinetics and microSPECT/CT imaging of 188Re-bMEDA-liposome in a C26 murine colon carcinoma solid tumor animal model. Anticancer Research, 27, 2217–2225.
Chawla, S. P., Goel, S., Chow, W., Braiteh, F., Singh, A. S., Olson, J. E. G., Osada, A., Bobe, I., & Riedel, R. F. (2020). A phase 1b dose escalation trial of NC-6300 (nanoparticle epirubicin) in patients with advanced solid tumors or advanced, metastatic, or unresectable soft-tissue sarcoma. Clinical Cancer Research, 26, 4225–4232.
Chen, X., Wu, Y., Dong, H., Zhang, C. Y., & Zhang, Y. (2013). Platinum-based agents for individualized cancer treatment. Current Molecular Medicine, 13, 1603–1612.
Cheng, Q., Wei, T., Farbiak, L., Johnson, L. T., Dilliard, S. A., & Siegwart, D. J. (2020). Selective organ targeting (SORT) nanoparticles for tissue-specific mRNA delivery and CRISPR-Cas gene editing. Nature Nanotechnology, 15, 313–320.
Chou, A. J., Gupta, R., Bell, M. D., Riewe, K. O., Meyers, P. A., & Gorlick, R. (2013). Inhaled lipid cisplatin (ILC) in the treatment of patients with relapsed/progressive osteosarcoma metastatic to the lung. Pediatric Blood & Cancer, 60, 580–586.
Costa-Silva, T. A., Costa, I. M., Biasoto, H. P., Lima, G. M., Silva, C., Pessoa, A., & Monteiro, G. (2020). Critical overview of the main features and techniques used for the evaluation of the clinical applicability of L-asparaginase as a biopharmaceutical to treat blood cancer. Blood Reviews, 43, 100651.
Creixell, M., & Peppas, N. A. (2012). Co-delivery of siRNA and therapeutic agents using nanocarriers to overcome cancer resistance. Nano Today, 7, 367–379.
D’Cruz, O., Piacente, M., Huang, T., Faxon, S., Trieu, V., & Desai, N. (2009). Sequence-dependent enhancement of antitumor activity of the vascular disrupting agent ABI-011 by paclitaxel and bevacizumab. Cancer Research, 69, 5638–5638.
Daniel, D., & Crawford, J. (2006). Myelotoxicity from chemotherapy. Seminars in Oncology, 33, 74–85.
Das, A., & Ali, N. (2021). Nanovaccine: An emerging strategy. Expert Review of Vaccines, 20, 1273–1290.
Dasari, S., & Tchounwou, P. B. (2014). Cisplatin in cancer therapy: Molecular mechanisms of action. European Journal of Pharmacology, 740, 364–378.
Davis, M. E., Chen, Z. G., & Shin, D. M. (2008). Nanoparticle therapeutics: An emerging treatment modality for cancer. Nature Reviews Drug Discovery, 7, 771–782.
Deitcher, S., Cullis, P., Wong, M., & Choy, G. (2007). Vinorelbine liposomes injection results in greater tumor drug exposure compared to conventional vinorelbine in tumor-bearing nude mice. Molecular Cancer Therapeutics, 6, 109.
Desai, N. (2016). Nanoparticle albumin-bound paclitaxel (Abraxane®). In M. Otagiri & V. T. G. Chuang (Eds.), Albumin in medicine: Pathological and clinical applications (pp. 101–119). Springer.
Diethelm-Varela, B., Ai, Y., Liang, D., & Xue, F. (2019). Nitrogen mustards as anticancer chemotherapies: Historic perspective, current developments and future trends. Current Topics in Medicinal Chemistry, 19, 691–712.
Dinndorf, P. A., Gootenberg, J., Cohen, M. H., Keegan, P., & Pazdur, R. (2007). FDA drug approval summary: Pegaspargase (Oncaspar®) for the first-line treatment of children with acute lymphoblastic leukemia (ALL). The Oncologist, 12, 991–998.
Dou, Y., Hynynen, K., & Allen, C. (2017). To heat or not to heat: Challenges with clinical translation of thermosensitive liposomes. Journal of Controlled Release, 249, 63–73.
Dragovich, T., Mendelson, D., Kurtin, S., Richardson, K., Von Hoff, D., & Hoos, A. (2006). A phase 2 trial of the liposomal DACH platinum L-NDDP in patients with therapy-refractory advanced colorectal cancer. Cancer Chemotherapy and Pharmacology, 58, 759–764.
Drummond, D. C., Meyer, O., Hong, K., Kirpotin, D. B., & Papahadjopoulos, D. (1999). Optimizing liposomes for delivery of chemotherapeutic agents to solid tumors. Pharmacological Reviews, 51, 691–743.
Duffaud, F., Borner, M., Chollet, P., Vermorken, J. B., Bloch, J., Degardin, M., Rolland, F., Dittrich, C., Baron, B., Lacombe, D., & Fumoleau, P. (2004). Phase II study of OSI-211 (liposomal lurtotecan) in patients with metastatic or loco-regional recurrent squamous cell carcinoma of the head and neck. An EORTC New Drug Development Group study. European Journal of Cancer, 40, 2748–2752.
Duflos, A., Kruczynski, A., & Barret, J. M. (2002). Novel aspects of natural and modified vinca alkaloids. Current Medicinal Chemistry Anti-Cancer Agents, 2, 55–70.
Ernstoff, M. S., Ma, W. W., Tsai, F. Y.-C., Munster, P. N., Zhang, T., Kamoun, W., Pipas, J. M., Chen, S., Santillana, S., & Askoxylakis, V. (2018). A phase 1 study evaluating the safety, pharmacology and preliminary activity of MM-310 in patients with solid tumors. Journal of Clinical Oncology, 36, TPS2604.
Falzone, L., Salomone, S., & Libra, M. (2018). Evolution of cancer pharmacological treatments at the turn of the third millennium. Frontiers in Pharmacology, 9, 1300.
Fang, J., Nakamura, H., & Maeda, H. (2011). The EPR effect: Unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Advanced Drug Delivery Reviews, 63, 136–151.
Farokhzad, O. C., & Langer, R. (2006). Nanomedicine: Developing smarter therapeutic and diagnostic modalities. Advanced Drug Delivery Reviews, 58, 1456–1459.
Fasol, U., Frost, A., Büchert, M., Arends, J., Fiedler, U., Scharr, D., Scheuenpflug, J., & Mross, K. (2012). Vascular and pharmacokinetic effects of EndoTAG-1 in patients with advanced cancer and liver metastasis. Annals of Oncology, 23, 1030–1036.
FDA Approves Onivyde Combo Regimen for Advanced Pancreatic Cancer. (2015). Oncology Times, 37, 8.
Fenton, O. S., Olafson, K. N., Pillai, P. S., Mitchell, M. J., & Langer, R. (2018). Advances in biomaterials for drug delivery. Advanced Materials, 30(29), e1705328.
Ferlay, J., Colombet, M., Soerjomataram, I., Parkin, D. M., Piñeros, M., Znaor, A., & Bray, F. (2021). Cancer statistics for the year 2020: An overview. International Journal of Cancer, 149(4), 778–789.
Forssen, E. A., & Ross, M. E. (1994). Daunoxome® treatment of solid tumors: Preclinical and clinical investigations. Journal of Liposome Research, 4, 481–512.
Froudarakis, M., Hatzimichael, E., Kyriazopoulou, L., Lagos, K., Pappas, P., Tzakos, A. G., Karavasilis, V., Daliani, D., Papandreou, C., & Briasoulis, E. (2013). Revisiting bleomycin from pathophysiology to safe clinical use. Critical Reviews in Oncology/Hematology, 87, 90–100.
Gabizon, A., Catane, R., Uziely, B., Kaufman, B., Safra, T., Cohen, R., Martin, F., Huang, A., & Barenholz, Y. (1994). Prolonged circulation time and enhanced accumulation in malignant exudates of doxorubicin encapsulated in polyethylene-glycol coated liposomes. Cancer Research, 54, 987–992.
Gabizon, A., Shmeeda, H., Tahover, E., Kornev, G., Patil, Y., Amitay, Y., Ohana, P., Sapir, E., & Zalipsky, S. (2020). Development of Promitil®, a lipidic prodrug of mitomycin c in PEGylated liposomes: From bench to bedside. Advanced Drug Delivery Reviews, 154–155, 13–26.
Gaillard, P. J., Appeldoorn, C. C., Dorland, R., van Kregten, J., Manca, F., Vugts, D. J., Windhorst, B., van Dongen, G. A., de Vries, H. E., Maussang, D., & van Tellingen, O. (2014). Pharmacokinetics, brain delivery, and efficacy in brain tumor-bearing mice of glutathione pegylated liposomal doxorubicin (2B3-101). PLoS One, 9, e82331.
Galm, U., Hager, M. H., Van Lanen, S. G., Ju, J., Thorson, J. S., & Shen, B. (2005). Antitumor antibiotics: Bleomycin, enediynes, and mitomycin. Chemical Reviews, 105, 739–758.
Gelderblom, H., Verweij, J., Nooter, K., & Sparreboom, A. (2001). Cremophor EL: The drawbacks and advantages of vehicle selection for drug formulation. European Journal of Cancer, 37, 1590–1598.
Gil, L., Shepard, R. C., Silberman, S. L., Zak, E. M., & Priebe, W. (2019). Clinical efficacy of L-annamycin, a liposomal formulated non-cross-resistant and non-cardiotoxic anthracycline in relapsed/refractory AML patients. Blood, 134, 5147–5147.
Gill, P. S., Wernz, J., Scadden, D. T., Cohen, P., Mukwaya, G. M., von Roenn, J. H., Jacobs, M., Kempin, S., Silverberg, I., Gonzales, G., Rarick, M. U., Myers, A. M., Shepherd, F., Sawka, C., Pike, M. C., & Ross, M. E. (1996). Randomized phase III trial of liposomal daunorubicin versus doxorubicin, bleomycin, and vincristine in AIDS-related Kaposi’s sarcoma. Journal of Clinical Oncology, 14, 2353–2364.
Giraud, B., Hebert, G., Deroussent, A., Veal, G. J., Vassal, G., & Paci, A. (2010). Oxazaphosphorines: New therapeutic strategies for an old class of drugs. Expert Opinion on Drug Metabolism & Toxicology, 6, 919–938.
Girotti, A., Escalera-Anzola, S., Alonso-Sampedro, I., González-Valdivieso, J., & Arias, F. J. (2020a). Aptamer-functionalized natural protein-based polymers as innovative biomaterials. Pharmaceutics, 12(11), 1115.
Girotti, A., Gonzalez-Valdivieso, J., Santos, M., Martin, L., & Arias, F. J. (2020b). Functional characterization of an enzymatically degradable multi-bioactive elastin-like recombinamer. International Journal of Biological Macromolecules, 164, 1640–1648.
Gonzalez-Valdivieso, J., Borrego, B., Girotti, A., Moreno, S., Brun, A., Bermejo-Martin, J. F., & Arias, F. J. (2020). A DNA vaccine delivery platform based on Elastin-Like recombinamer nanosystems for Rift Valley fever virus. Molecular Pharmaceutics, 17, 1608–1620.
Gonzalez-Valdivieso, J., Garcia-Sampedro, A., Hall, A. R., Girotti, A., Arias, F. J., Pereira, S. P., & Acedo, P. (2021a). Smart nanoparticles as advanced anti-Akt kinase delivery systems for pancreatic cancer therapy. ACS Applied Materials & Interfaces, 13, 55790–55805.
Gonzalez-Valdivieso, J., Girotti, A., Schneider, J., & Arias, F. J. (2021b). Advanced nanomedicine and cancer: Challenges and opportunities in clinical translation. International Journal of Pharmaceutics, 599, 120438.
Green, M. R., Manikhas, G. M., Orlov, S., Afanasyev, B., Makhson, A. M., Bhar, P., & Hawkins, M. J. (2006). Abraxane, a novel Cremophor-free, albumin-bound particle form of paclitaxel for the treatment of advanced non-small-cell lung cancer. Annals of Oncology, 17, 1263–1268.
Greene, J., & Hennessy, B. (2015). The role of anthracyclines in the treatment of early breast cancer. Journal of Oncology Pharmacy Practice, 21, 201–212.
Greish, K. (2010). Enhanced permeability and retention (EPR) effect for anticancer nanomedicine drug targeting. Methods in Molecular Biology, 624, 25–37.
Grothey, A. (2003). Oxaliplatin-safety profile: Neurotoxicity. Seminars in Oncology, 30, 5–13.
Hamaguchi, T., Matsumura, Y., Nakanishi, Y., Muro, K., Yamada, Y., Shimada, Y., Shirao, K., Niki, H., Hosokawa, S., Tagawa, T., & Kakizoe, T. (2004). Antitumor effect of MCC-465, pegylated liposomal doxorubicin tagged with newly developed monoclonal antibody GAH, in colorectal cancer xenografts. Cancer Science, 95, 608–613.
Hamaguchi, T., Matsumura, Y., Suzuki, M., Shimizu, K., Goda, R., Nakamura, I., Nakatomi, I., Yokoyama, M., Kataoka, K., & Kakizoe, T. (2005). NK105, a paclitaxel-incorporating micellar nanoparticle formulation, can extend in vivo antitumour activity and reduce the neurotoxicity of paclitaxel. British Journal of Cancer, 92, 1240–1246.
Hamaguchi, T., Kato, K., Yasui, H., Morizane, C., Ikeda, M., Ueno, H., Muro, K., Yamada, Y., Okusaka, T., Shirao, K., Shimada, Y., Nakahama, H., & Matsumura, Y. (2007). A phase I and pharmacokinetic study of NK105, a paclitaxel-incorporating micellar nanoparticle formulation. British Journal of Cancer, 97, 170–176.
Han, W., Chilkoti, A., & López, G. P. (2017). Self-assembled hybrid elastin-like polypeptide/silica nanoparticles enable triggered drug release. Nanoscale, 9, 6178–6186.
Harrington, K. J., Mohammadtaghi, S., Uster, P. S., Glass, D., Peters, A. M., Vile, R. G., & Stewart, J. S. (2001). Effective targeting of solid tumors in patients with locally advanced cancers by radiolabeled pegylated liposomes. Clinical Cancer Research, 7, 243–254.
He, C., Yue, H., Xu, L., Liu, Y., Song, Y., Tang, C., & Yin, C. (2020). siRNA release kinetics from polymeric nanoparticles correlate with RNAi efficiency and inflammation therapy via oral delivery. Acta Biomaterialia, 103, 213–222.
Helary, C., & Desimone, M. F. (2015). Recent advances in biomaterials for tissue engineering and controlled drug delivery. Current Pharmaceutical Biotechnology, 16, 635–645.
Ho, D., Quake, S. R., McCabe, E. R. B., Chng, W. J., Chow, E. K., Ding, X., Gelb, B. D., Ginsburg, G. S., Hassenstab, J., Ho, C. M., Mobley, W. C., Nolan, G. P., Rosen, S. T., Tan, P., Yen, Y., & Zarrinpar, A. (2020). Enabling technologies for personalized and precision medicine. Trends in Biotechnology, 38, 497–518.
Howes, P. D., Chandrawati, R., & Stevens, M. M. (2014). Bionanotechnology. Colloidal nanoparticles as advanced biological sensors. Science, 346, 1247390.
https://www.annalsofoncology.org/article/S0923-7534(19)585702/fulltext#relatedArticles. Accessed 12 Dec 2021.
https://www.iarc.who.int/. Accessed 27 Nov 2021.
Huang, K. W., Hsu, F. F., Qiu, J. T., Chern, G. J., Lee, Y. A., Chang, C. C., Huang, Y. T., Sung, Y. C., Chiang, C. C., Huang, R. L., Lin, C. C., Dinh, T. K., Huang, H. C., Shih, Y. C., Alson, D., Lin, C. Y., Lin, Y. C., Chang, P. C., Lin, S. Y., & Chen, Y. (2020). Highly efficient and tumor-selective nanoparticles for dual-targeted immunogene therapy against cancer. Science Advances, 6, eaax5032.
Hwang, J., Sullivan, M. O., & Kiick, K. L. (2020). Targeted drug delivery via the use of ECM-mimetic materials. Frontiers in Bioengineering and Biotechnology, 8, 69.
Islam, R., Maeda, H., & Fang, J. (2021). Factors affecting the dynamics and heterogeneity of the EPR effect: Pathophysiological and pathoanatomic features, drug formulations and physicochemical factors. Expert Opinion on Drug Delivery, 1–14.
Jabir, N. R., Anwar, K., Firoz, C. K., Oves, M., Kamal, M. A., & Tabrez, S. (2018). An overview on the current status of cancer nanomedicines. Current Medical Research and Opinion, 34, 911–921.
Jain, R. K., & Stylianopoulos, T. (2010). Delivering nanomedicine to solid tumors. Nature Reviews Clinical Oncology, 7, 653–664.
Jain, M. M., Gupte, S. U., Patil, S. G., Pathak, A. B., Deshmukh, C. D., Bhatt, N., Haritha, C., Govind, B. K., Bondarde, S. A., Digumarti, R., Bajpai, J., Kumar, R., Bakshi, A. V., Bhattacharya, G. S., Patil, P., Subramanian, S., Vaid, A. K., Desai, C. J., Khopade, A., Chimote, G., Bapsy, P. P., & Bhowmik, S. (2016). Paclitaxel injection concentrate for nanodispersion versus nab-paclitaxel in women with metastatic breast cancer: A multicenter, randomized, comparative phase II/III study. Breast Cancer Research & Treatment, 156, 125–134.
Jarrar, M., Gaynon, P. S., Periclou, A. P., Fu, C., Harris, R. E., Stram, D., Altman, A., Bostrom, B., Breneman, J., Steele, D., Trigg, M., Zipf, T., & Avramis, V. I. (2006). Asparagine depletion after pegylated E. coli asparaginase treatment and induction outcome in children with acute lymphoblastic leukemia in first bone marrow relapse: A Children’s Oncology Group study (CCG-1941). Pediatric Blood & Cancer, 47, 141–146.
Jiang, N., Wang, X., Yang, Y., & Dai, W. (2006). Advances in mitotic inhibitors for cancer treatment. Mini Reviews in Medicinal Chemistry, 6, 885–895.
Kager, L., Pötschger, U., & Bielack, S. (2010). Review of mifamurtide in the treatment of patients with osteosarcoma. Therapeutics and Clinical Risk Management, 6, 279–286.
Kato, K., Chin, K., Yoshikawa, T., Yamaguchi, K., Tsuji, Y., Esaki, T., Sakai, K., Kimura, M., Hamaguchi, T., Shimada, Y., Matsumura, Y., & Ikeda, R. (2012). Phase II study of NK105, a paclitaxel-incorporating micellar nanoparticle, for previously treated advanced or recurrent gastric cancer. Investigational New Drugs, 30, 1621–1627.
Kaye, S. B. (1998). New antimetabolites in cancer chemotherapy and their clinical impact. British Journal of Cancer, 78, 1–7.
Kemp, J. A., & Kwon, Y. J. (2021). Cancer nanotechnology: Current status and perspectives. Nano Convergence, 8, 34.
Kim, T.-Y., Kim, D.-W., Chung, J.-Y., Shin, S. G., Kim, S.-C., Heo, D. S., Kim, N. K., & Bang, Y.-J. (2004). Phase I and pharmacokinetic study of Genexol-PM, a Cremophor-free, polymeric micelle-formulated paclitaxel, in patients with advanced malignancies. Clinical Cancer Research, 10, 3708–3716.
Kim, S.-B., Zhang, Q., Sun, T., Seo, J. H., Lee, K. S., Kim, T.-Y., Tong, Z., Park, K. H., Moon, Y. W., Wang, S., Li, W., Yang, Y., Wang, J., Wang, X., Choi, J., Lee, J. E., Yoon, K. E., Chung, S., Xu, B., & Sohn, J. (2020). [OPTIMAL 3] A phase III trial to evaluate the efficacy and safety of DHP107 (Liporaxel, oral paclitaxel) compared to Taxol (IV paclitaxel) as first line therapy in patients with recurrent or metastatic HER2 negative breast cancer (BC) (NCT03315364). Journal of Clinical Oncology, 38, TPS1106.
Kirpotin, D. B., Tipparaju, S., Huang, Z. R., Kamoun, W. S., Pien, C., Kornaga, T., Oyama, S., Olivier, K., Marks, J. D., Koshkaryev, A., Schihl, S. S., Fetterly, G., Schoeberl, B., Noble, C., Hayes, M., & Drummond, D. C. (2016). Abstract 3912: MM-310, a novel EphA2-targeted docetaxel nanoliposome. Cancer Research, 76, 3912–3912.
Knight, F. C., Gilchuk, P., Kumar, A., Becker, K. W., Sevimli, S., Jacobson, M. E., Suryadevara, N., Wang-Bishop, L., Boyd, K. L., Crowe, J. E., Joyce, S., & Wilson, J. T. (2019). Mucosal immunization with a pH-responsive nanoparticle vaccine induces protective CD8(+) lung-resident memory T cells. ACS Nano, 13, 10939–10960.
Koo, M. M., Swann, R., McPhail, S., Abel, G. A., Elliss-Brookes, L., Rubin, G. P., & Lyratzopoulos, G. (2020). Presenting symptoms of cancer and stage at diagnosis: Evidence from a cross-sectional, population-based study. The Lancet Oncology, 21, 73–79.
Koukourakis, M. I., Koukouraki, S., Giatromanolaki, A., Kakolyris, S., Georgoulias, V., Velidaki, A., Archimandritis, S., & Karkavitsas, N. N. (2000). High intratumoral accumulation of stealth liposomal doxorubicin in sarcomas–rationale for combination with radiotherapy. Acta Oncologica, 39, 207–211.
Kraut, E. H., Fishman, M. N., Lorusso, P. M., Gordon, M. S., Rubin, E. H., Haas, A., Fetterly, G. J., Cullinan, P., Dul, J. L., & Steinberg, J. L. (2005). Final results of a phase I study of liposome encapsulated SN-38 (LE-SN38): Safety, pharmacogenomics, pharmacokinetics, and tumor response. Journal of Clinical Oncology, 23, 2017–2017.
Kulkarni, J. A., Witzigmann, D., Leung, J., Tam, Y. Y. C., & Cullis, P. R. (2019). On the role of helper lipids in lipid nanoparticle formulations of siRNA. Nanoscale, 11, 21733–21739.
Kushwah, V., Katiyar, S. S., Agrawal, A. K., Gupta, R. C., & Jain, S. (2018). Co-delivery of docetaxel and gemcitabine using PEGylated self-assembled stealth nanoparticles for improved breast cancer therapy. Nanomedicine, 14, 1629–1641.
Laginha, K. M., Verwoert, S., Charrois, G. J., & Allen, T. M. (2005). Determination of doxorubicin levels in whole tumor and tumor nuclei in murine breast cancer tumors. Clinical Cancer Research, 11, 6944–6949.
Lammers, T., Aime, S., Hennink, W. E., Storm, G., & Kiessling, F. (2011). Theranostic nanomedicine. Accounts of Chemical Research, 44, 1029–1038.
Lancet, J. E., Uy, G. L., Cortes, J. E., Newell, L. F., Lin, T. L., Ritchie, E. K., Stuart, R. K., Strickland, S. A., Hogge, D., Solomon, S. R., Stone, R. M., Bixby, D. L., Kolitz, J. E., Schiller, G. J., Wieduwilt, M. J., Ryan, D. H., Hoering, A., Chiarella, M., Louie, A. C., & Medeiros, B. C. (2016). Final results of a phase III randomized trial of CPX-351 versus 7+3 in older patients with newly diagnosed high risk (secondary) AML. Journal of Clinical Oncology, 34, 7000–7000.
Lancet, J. E., Uy, G. L., Cortes, J. E., Newell, L. F., Lin, T. L., Ritchie, E. K., Stuart, R. K., Strickland, S. A., Hogge, D., Solomon, S. R., Stone, R. M., Bixby, D. L., Kolitz, J. E., Schiller, G. J., Wieduwilt, M. J., Ryan, D. H., Hoering, A., Banerjee, K., Chiarella, M., Louie, A. C., & Medeiros, B. C. (2018). CPX-351 (cytarabine and daunorubicin) liposome for injection versus conventional cytarabine plus daunorubicin in older patients with newly diagnosed secondary acute myeloid leukemia. Journal of Clinical Oncology, 36, 2684–2692.
Lawson, R., Staatz, C. E., Fraser, C. J., & Hennig, S. (2021). Review of the pharmacokinetics and pharmacodynamics of intravenous busulfan in paediatric patients. Clinical Pharmacokinetics, 60, 17–51.
Le, Z., Chen, Y., Han, H., Tian, H., Zhao, P., Yang, C., He, Z., Liu, L., Leong, K. W., Mao, H. Q., Liu, Z., & Chen, Y. (2018). Hydrogen-bonded tannic acid-based anticancer nanoparticle for enhancement of oral chemotherapy. ACS Applied Materials & Interfaces, 10, 42186–42197.
Lee, S. W., Yun, M. H., Jeong, S. W., In, C. H., Kim, J. Y., Seo, M. H., Pai, C. M., & Kim, S. O. (2011). Development of docetaxel-loaded intravenous formulation, Nanoxel-PM™ using polymer-based delivery system. Journal of Controlled Release, 155, 262–271.
Lee, H., Park, S., Kang, J. E., Lee, H. M., Kim, S. A., & Rhie, S. J. (2020). Efficacy and safety of nanoparticle-albumin-bound paclitaxel compared with solvent-based taxanes for metastatic breast cancer: A meta-analysis. Scientific Reports, 10, 530.
Leonard, R. C. F., Williams, S., Tulpule, A., Levine, A. M., & Oliveros, S. Y. (2009). Improving the therapeutic index of anthracycline chemotherapy: Focus on liposomal doxorubicin (Myocet). Breast, 18(4), 218–224.
Lepareur, N., Lacœuille, F., Bouvry, C., Hindré, F., Garcion, E., Chérel, M., Noiret, N., Garin, E., & Knapp, F. F. R. (2019). Rhenium-188 labeled radiopharmaceuticals: Current clinical applications in oncology and promising perspectives. Frontiers in Medicine, 6, 132.
Leung, A. K., Tam, Y. Y., Chen, S., Hafez, I. M., & Cullis, P. R. (2015). Microfluidic mixing: A general method for encapsulating macromolecules in lipid nanoparticle systems. The Journal of Physical Chemistry B, 119, 8698–8706.
Liang, X. J., Chen, C., Zhao, Y., & Wang, P. C. (2010). Circumventing tumor resistance to chemotherapy by nanotechnology. Methods in Molecular Biology, 596, 467–488.
Liu, Y., Li, K., Liu, B., & Feng, S. S. (2010). A strategy for precision engineering of nanoparticles of biodegradable copolymers for quantitative control of targeted drug delivery. Biomaterials, 31, 9145–9155.
Liu, X., Li, C., Lv, J., Huang, F., An, Y., Shi, L., & Ma, R. (2020). Glucose and H2O2 dual-responsive polymeric micelles for the self-regulated release of insulin. ACS Applied Biomaterials, 3, 1598–1606.
Luginbuhl, K. M., Mozhdehi, D., Dzuricky, M., Yousefpour, P., Huang, F. C., Mayne, N. R., Buehne, K. L., & Chilkoti, A. (2017). Recombinant synthesis of hybrid lipid-peptide polymer fusions that self-assemble and encapsulate hydrophobic drugs. Angewandte Chemie, 56, 13979–13984.
Ma, W. W., Zhu, M., Lam, E. T., Diamond, J. R., Dy, G. K., Fisher, G. A., Goff, L. W., Alberts, S., Bui, L. A., Sanghal, A., Kothekar, M., Khopade, A., Chimote, G., Faulkner, R., Eckhardt, S. G., Adjei, A. A., & Jimeno, A. (2021). A phase I pharmacokinetic and safety study of Paclitaxel Injection Concentrate for Nano-dispersion (PICN) alone and in combination with carboplatin in patients with advanced solid malignancies and biliary tract cancers. Cancer Chemotherapy and Pharmacology, 87, 779–788.
Madaan, A., Singh, P., Awasthi, A., Verma, R., Singh, A. T., Jaggi, M., Mishra, S. K., Kulkarni, S., & Kulkarni, H. (2013). Efficiency and mechanism of intracellular paclitaxel delivery by novel nanopolymer-based tumor-targeted delivery system, Nanoxel(TM). Clinical & Translational Oncology, 15, 26–32.
Madamsetty, V. S., Mukherjee, A., & Mukherjee, S. (2019). Recent trends of the bio-inspired nanoparticles in cancer theranostics. Frontiers in Pharmacology, 10, 1264.
Mahalingam, D., Nemunaitis, J. J., Malik, L., Sarantopoulos, J., Weitman, S., Sankhala, K., Hart, J., Kousba, A., Gallegos, N. S., Anderson, G., Charles, J., Rogers, J. M., Senzer, N. N., & Mita, A. C. (2014). Phase I study of intravenously administered ATI-1123, a liposomal docetaxel formulation in patients with advanced solid tumors. Cancer Chemotherapy and Pharmacology, 74, 1241–1250.
Makadia, H. K., & Siegel, S. J. (2011). Poly Lactic-co-Glycolic Acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers, 3, 1377–1397.
Malhotra, V., & Perry, M. C. (2003). Classical chemotherapy: Mechanisms, toxicities and the therapeutic window. Cancer Biology & Therapy, 2, S2–S4.
Mamot, C., Ritschard, R., Wicki, A., Stehle, G., Dieterle, T., Bubendorf, L., Hilker, C., Deuster, S., Herrmann, R., & Rochlitz, C. (2012). Tolerability, safety, pharmacokinetics, and efficacy of doxorubicin-loaded anti-EGFR immunoliposomes in advanced solid tumours: A phase 1 dose-escalation study. The Lancet Oncology, 13, 1234–1241.
Man, F., Lammers, T., & de Rosales, R. T. M. (2018). Imaging nanomedicine-based drug delivery: A review of clinical studies. Molecular Imaging and Biology, 20, 683–695.
Manshian, B. B., Jiménez, J., Himmelreich, U., & Soenen, S. J. (2017). Personalized medicine and follow-up of therapeutic delivery through exploitation of quantum dot toxicity. Biomaterials, 127, 1–12.
Mantripragada, S. (2002). A lipid based depot (DepoFoam technology) for sustained release drug delivery. Progress in Lipid Research, 41, 392–406.
Martino, E., Casamassima, G., Castiglione, S., Cellupica, E., Pantalone, S., Papagni, F., Rui, M., Siciliano, A. M., & Collina, S. (2018). Vinca alkaloids and analogues as anti-cancer agents: Looking back, peering ahead. Bioorganic & Medicinal Chemistry Letters, 28, 2816–2826.
Matsumoto, Y., Nichols, J. W., Toh, K., Nomoto, T., Cabral, H., Miura, Y., Christie, R. J., Yamada, N., Ogura, T., Kano, M. R., Matsumura, Y., Nishiyama, N., Yamasoba, T., Bae, Y. H., & Kataoka, K. (2016). Vascular bursts enhance permeability of tumour blood vessels and improve nanoparticle delivery. Nature Nanotechnology, 11, 533–538.
Matsumoto, T., Komori, T., Yoshino, Y., Ioroi, T., Kitahashi, T., Kitahara, H., Ono, K., Higuchi, T., Sakabe, M., Kori, H., Kano, M., Hori, R., Kato, Y., & Hagiwara, S. (2021). A liposomal gemcitabine, FF-10832, improves plasma stability, tumor targeting, and antitumor efficacy of gemcitabine in pancreatic cancer xenograft models. Pharmaceutical Research, 38, 1093–1106.
Matsumura, Y., & Maeda, H. (1986). A new concept for macromolecular therapeutics in cancer chemotherapy: Mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Research, 46, 6387–6392.
Matsumura, Y., Gotoh, M., Muro, K., Yamada, Y., Shirao, K., Shimada, Y., Okuwa, M., Matsumoto, S., Miyata, Y., Ohkura, H., Chin, K., Baba, S., Yamao, T., Kannami, A., Takamatsu, Y., Ito, K., & Takahashi, K. (2004). Phase I and pharmacokinetic study of MCC-465, a doxorubicin (DXR) encapsulated in PEG immunoliposome, in patients with metastatic stomach cancer. Annals of Oncology, 15, 517–525.
Maulhardt, H. A., Marin, A. M., & diZerega, G. S. (2020). Intratumoral submicron particle docetaxel inhibits syngeneic Renca renal cancer growth and increases CD4+, CD8+, and Treg levels in peripheral blood. Investigational New Drugs, 38, 1618–1626.
Maulhardt, H., Marin, A., Hesseltine, H., & diZerega, G. (2021). Submicron particle docetaxel intratumoral injection in combination with anti-mCTLA-4 into 4T1-Luc orthotopic implants reduces primary tumor and metastatic pulmonary lesions. Medical Oncology, 38, 106.
Meyers, P. A., Schwartz, C. L., Krailo, M. D., Healey, J. H., Bernstein, M. L., Betcher, D., Ferguson, W. S., Gebhardt, M. C., Goorin, A. M., Harris, M., Kleinerman, E., Link, M. P., Nadel, H., Nieder, M., Siegal, G. P., Weiner, M. A., Wells, R. J., Womer, R. B., & Grier, H. E. (2008). Osteosarcoma: The addition of muramyl tripeptide to chemotherapy improves overall survival–a report from the Children’s Oncology Group. Journal of Clinical Oncology, 26, 633–638.
Miller, K., Cortes, J., Hurvitz, S. A., Krop, I. E., Tripathy, D., Verma, S., Riahi, K., Reynolds, J. G., Wickham, T. J., Molnar, I., & Yardley, D. A. (2016). HERMIONE: A randomized phase 2 trial of MM-302 plus trastuzumab versus chemotherapy of physician’s choice plus trastuzumab in patients with previously treated, anthracycline-naïve, HER2-positive, locally advanced/metastatic breast cancer. BMC Cancer, 16, 352.
Minelli, C., Lowe, S. B., & Stevens, M. M. (2010). Engineering nanocomposite materials for cancer therapy. Small, 6, 2336–2357.
Mitchell, E. P. (2006). Gastrointestinal toxicity of chemotherapeutic agents. Seminars in Oncology, 33, 106–120.
Mitchell, E. P., & Schein, P. S. (1986). Contributions of nitrosoureas to cancer treatment. Cancer Treatment Reports, 70, 31–41.
Mitchell, M. J., Jain, R. K., & Langer, R. (2017). Engineering and physical sciences in oncology: Challenges and opportunities. Nature Reviews Cancer, 17, 659–675.
Mitchell, M. J., Billingsley, M. M., Haley, R. M., Wechsler, M. E., Peppas, N. A., & Langer, R. (2021). Engineering precision nanoparticles for drug delivery. Nature Reviews Drug Discovery, 20, 101–124.
Moody, C. L., & Wheelhouse, R. T. (2014). The medicinal chemistry of imidazotetrazine prodrugs. Pharmaceuticals, 7, 797–838.
Moore, A., & Pinkerton, R. (2009). Vincristine: Can its therapeutic index be enhanced? Pediatric Blood & Cancer, 53, 1180–1187.
More, G. S., Thomas, A. B., Chitlange, S. S., Nanda, R. K., & Gajbhiye, R. L. (2019). Nitrogen mustards as alkylating agents: A review on chemistry, mechanism of action and current USFDA status of drugs. Anti-Cancer Agents in Medicinal Chemistry, 19, 1080–1102.
Mosca, L., Ilari, A., Fazi, F., Assaraf, Y. G., & Colotti, G. (2021). Taxanes in cancer treatment: Activity, chemoresistance and its overcoming. Drug Resistance Updates, 54, 100742.
Moudi, M., Go, R., Yien, C. Y., & Nazre, M. (2013). Vinca alkaloids. International Journal of Preventive Medicine, 4, 1231–1235.
Muggia, F., & Kudlowitz, D. (2014). Novel taxanes. Anti-Cancer Drugs, 25, 593–598.
Mullany, S., Miller, D. S., Robison, K., Levinson, K., Lee, Y. C., Yamada, S. D., Walker, J., Markman, M., Marin, A., Mast, P., & diZerega, G. (2020). Phase II study of intraperitoneal submicron particle paclitaxel (SPP) plus IV carboplatin and paclitaxel in patients with epithelial ovarian cancersurgery. Gynecologic Oncology Reports, 34, 100627.
Munster, P., Krop, I. E., LoRusso, P., Ma, C., Siegel, B. A., Shields, A. F., Molnár, I., Wickham, T. J., Reynolds, J., Campbell, K., Hendriks, B. S., Adiwijaya, B. S., Geretti, E., Moyo, V., & Miller, K. D. (2018). Safety and pharmacokinetics of MM-302, a HER2-targeted antibody-liposomal doxorubicin conjugate, in patients with advanced HER2-positive breast cancer: A phase 1 dose-escalation study. British Journal of Cancer, 119, 1086–1093.
Mylonakis, N., Athanasiou, A., Ziras, N., Angel, J., Rapti, A., Lampaki, S., Politis, N., Karanikas, C., & Kosmas, C. (2010). Phase II study of liposomal cisplatin (Lipoplatin) plus gemcitabine versus cisplatin plus gemcitabine as first line treatment in inoperable (stage IIIB/IV) non-small cell lung cancer. Lung Cancer, 68, 240–247.
Ngan, Y. H., & Gupta, M. (2016). A comparison between liposomal and nonliposomal formulations of doxorubicin in the treatment of cancer: An updated review. Archives of Pharmacy Practice, 7(1), 1–13.
Norouzi, M., Amerian, M., Amerian, M., & Atyabi, F. (2020). Clinical applications of nanomedicine in cancer therapy. Drug Discovery Today, 25, 107–125.
Northfelt, D. W., Dezube, B. J., Thommes, J. A., Miller, B. J., Fischl, M. A., Friedman-Kien, A., Kaplan, L. D., Du Mond, C., Mamelok, R. D., & Henry, D. H. (1998). Pegylated-liposomal doxorubicin versus doxorubicin, bleomycin, and vincristine in the treatment of AIDS-related Kaposi’s sarcoma: Results of a randomized phase III clinical trial. Journal of Clinical Oncology, 16, 2445–2451.
Octavia, Y., Tocchetti, C. G., Gabrielson, K. L., Janssens, S., Crijns, H. J., & Moens, A. L. (2012). Doxorubicin-induced cardiomyopathy: From molecular mechanisms to therapeutic strategies. Journal of Molecular and Cellular Cardiology, 52, 1213–1225.
Park, H., Otte, A., & Park, K. (2021). Evolution of drug delivery systems: From 1950 to 2020 and beyond. Journal of Controlled Release, 342, 53–65.
Peres, C., Matos, A. I., Moura, L. I. F., Acúrcio, R. C., Carreira, B., Pozzi, S., Vaskovich-Koubi, D., Kleiner, R., Satchi-Fainaro, R., & Florindo, H. F. (2021). Preclinical models and technologies to advance nanovaccine development. Advanced Drug Delivery Reviews, 172, 148–182.
Peters, G. J., Schornagel, J. H., & Milano, G. A. (1993). Clinical pharmacokinetics of anti-metabolites. Cancer Surveys, 17, 123–156.
Peters, G. J., van der Wilt, C. L., van Moorsel, C. J., Kroep, J. R., Bergman, A. M., & Ackland, S. P. (2000). Basis for effective combination cancer chemotherapy with antimetabolites. Pharmacology & Therapeutics, 87, 227–253.
Petersen, G. H., Alzghari, S. K., Chee, W., Sankari, S. S., & La-Beck, N. M. (2016). Meta-analysis of clinical and preclinical studies comparing the anticancer efficacy of liposomal versus conventional non-liposomal doxorubicin. Journal of Controlled Release, 232, 255–264.
Petre, C. E., & Dittmer, D. P. (2007). Liposomal daunorubicin as treatment for Kaposi’s sarcoma. International Journal of Nanomedicine, 2, 277–288.
Pham, E., Birrer, M. J., Eliasof, S., Garmey, E. G., Lazarus, D., Lee, C. R., Man, S., Matulonis, U. A., Peters, C. G., Xu, P., Krasner, C., & Kerbel, R. S. (2015). Translational impact of nanoparticle–drug conjugate CRLX101 with or without bevacizumab in advanced ovarian cancer. Clinical Cancer Research, 21, 808–818.
Piha-Paul, S. A., Thein, K. Z., De Souza, P., Kefford, R., Gangadhar, T., Smith, C., Schuster, S., Zamboni, W. C., Dees, C. E., & Markman, B. (2021). First-in-human, phase I/IIa study of CRLX301, a nanoparticle drug conjugate containing docetaxel, in patients with advanced or metastatic solid malignancies. Investigational New Drugs, 39, 1047–1056.
Pillai, G., & Ceballos-Coronel, M. L. (2013). Science and technology of the emerging nanomedicines in cancer therapy: A primer for physicians and pharmacists. SAGE Open Medicine, 1, 2050312113513759.
Qiao, D., Chen, Y., & Liu, L. (2021). Engineered therapeutic nanovaccine against chronic hepatitis B virus infection. Biomaterials, 269, 120674.
Quail, D. F., & Joyce, J. A. (2013). Microenvironmental regulation of tumor progression and metastasis. Nature Medicine, 19, 1423–1437.
Quazi, M. Z., Lee, U., Park, S., Shin, S., Sim, E., Son, H., & Park, N. (2021). Cancer cell-specific enhanced Raman imaging and photothermal therapeutic effect based on reversibly pH-responsive gold nanoparticles. ACS Applied Biomaterials, 4, 8377–8385.
Ralhan, R., & Kaur, J. (2007). Alkylating agents and cancer therapy. Expert Opinion on Therapeutic Patents, 17, 1061–1075.
Ranade, A. A., Joshi, D. A., Phadke, G. K., Patil, P. P., Kasbekar, R. B., Apte, T. G., Dasare, R. R., Mengde, S. D., Parikh, P. M., Bhattacharyya, G. S., & Lopes, G. L. (2013). Clinical and economic implications of the use of nanoparticle paclitaxel (Nanoxel) in India. Annals of Oncology, 24, 6–12.
Regenold, M., Bannigan, P., Evans, J. C., Waspe, A., Temple, M. J., & Allen, C. (2021). Turning down the heat: The case for mild hyperthermia and thermosensitive liposomes. Nanomedicine: Nanotechnology, Biology, and Medicine, 40, 102484.
Rideau, E., Dimova, R., Schwille, P., Wurm, F. R., & Landfester, K. (2018). Liposomes and polymersomes: A comparative review towards cell mimicking. Chemical Society Reviews, 47, 8572–8610.
Riedel, R. F., Chua, V. S., Kim, T., Dang, J., Zheng, K., Moradkhani, A., Osada, A., & Chawla, S. P. (2021). Results of NC-6300 (nanoparticle epirubicin) in an expansion cohort of patients with angiosarcoma. Journal of Clinical Oncology, 39, 11543–11543.
Rugo, H. S., Pluard, T. J., Sharma, P., Melisko, M., Al-Jazayrly, G., Vidula, N., Ji, Y., Weng, D., Lim, H.-S., Yoon, K. E., & Cho, H. J. (2021). Abstract PS13-16: Pharmacokinetic evaluation of an oral paclitaxel DHP107 (Liporaxel®) in patients with recurrent or metastatic breast cancer (MBC): Phase II study (OPERA, NCT03326102). Cancer Research, 81, 13–16.
Safra, T. (2003). Cardiac safety of liposomal anthracyclines. The Oncologist, 8, 17–24.
Sanchez-Moreno, P., Ortega-Vinuesa, J. L., Peula-Garcia, J. M., Marchal, J. A., & Boulaiz, H. (2018). Smart drug-delivery systems for cancer nanotherapy. Current Drug Targets, 19, 339–359.
Sankhala, K. K., Mita, A. C., Adinin, R., Wood, L., Beeram, M., Bullock, S., Yamagata, N., Matsuno, K., Fujisawa, T., & Phan, A. (2009). A phase I pharmacokinetic (PK) study of MBP-426, a novel liposome encapsulated oxaliplatin. Journal of Clinical Oncology, 27, 2535.
Sarfraz, M., Afzal, A., Yang, T., Gai, Y., Raza, S. M., Khan, M. W., Cheng, Y., Ma, X., & Xiang, G. (2018). Development of dual drug loaded nanosized liposomal formulation by a reengineered ethanolic injection method and its pre-clinical pharmacokinetic studies. Pharmaceutics, 10(3), 151.
Saw, P. E., Yu, M., Choi, M., Lee, E., Jon, S., & Farokhzad, O. C. (2017). Hyper-cell-permeable micelles as a drug delivery carrier for effective cancer therapy. Biomaterials, 123, 118–126.
Seetharamu, N., Kim, E., Hochster, H., Martin, F., & Muggia, F. (2010). Phase II study of liposomal cisplatin (SPI-77) in platinum-sensitive recurrences of ovarian cancer. Anticancer Research, 30, 541–545.
Semple, S. C., Leone, R., Wang, J., Leng, E. C., Klimuk, S. K., Eisenhardt, M. L., Yuan, Z. N., Edwards, K., Maurer, N., Hope, M. J., Cullis, P. R., & Ahkong, Q. F. (2005). Optimization and characterization of a sphingomyelin/cholesterol liposome formulation of vinorelbine with promising antitumor activity. Journal of Pharmaceutical Sciences, 94, 1024–1038.
Senzer, N. N., Matsuno, K., Yamagata, N., Fujisawa, T., Wasserman, E., Sutherland, W., Sharma, S., & Phan, A. (2009). Abstract C36: MBP-426, a novel liposome-encapsulated oxaliplatin, in combination with 5-FU/leucovorin (LV): Phase I results of a Phase I/II study in gastro-esophageal adenocarcinoma, with pharmacokinetics. Molecular Cancer Therapeutics, 8, C36.
Sercombe, L., Veerati, T., Moheimani, F., Wu, S. Y., Sood, A. K., & Hua, S. (2015). Advances and challenges of liposome assisted drug delivery. Frontiers in Pharmacology, 6, 286.
Sethi, S., Ali, S., Philip, P. A., & Sarkar, F. H. (2013). Clinical advances in molecular biomarkers for cancer diagnosis and therapy. International Journal of Molecular Sciences, 14, 14771–14784.
Shae, D., Becker, K. W., Christov, P., Yun, D. S., Lytton-Jean, A. K. R., Sevimli, S., Ascano, M., Kelley, M., Johnson, D. B., Balko, J. M., & Wilson, J. T. (2019). Endosomolytic polymersomes increase the activity of cyclic dinucleotide STING agonists to enhance cancer immunotherapy. Nature Nanotechnology, 14, 269–278.
Shetty, N., & Gupta, S. (2014). Eribulin drug review. South Asian Journal of Cancer, 3, 57–59.
Shi, J., Kantoff, P. W., Wooster, R., & Farokhzad, O. C. (2017). Cancer nanomedicine: Progress, challenges and opportunities. Nature Reviews Cancer, 17, 20–37.
Shi, Y., van der Meel, R., Chen, X., & Lammers, T. (2020). The EPR effect and beyond: Strategies to improve tumor targeting and cancer nanomedicine treatment efficacy. Theranostics, 10, 7921–7924.
Shreyash, N., Sonker, M., Bajpai, S., & Tiwary, S. K. (2021). Review of the mechanism of nanocarriers and technological developments in the field of nanoparticles for applications in cancer theragnostics. ACS Applied Biomaterials, 4, 2307–2334.
Sibaud, V., Lebœuf, N. R., Roche, H., Belum, V. R., Gladieff, L., Deslandres, M., Montastruc, M., Eche, A., Vigarios, E., Dalenc, F., & Lacouture, M. E. (2016). Dermatological adverse events with taxane chemotherapy. European Journal of Dermatology, 26, 427–443.
Silverman, J. A., & Deitcher, S. R. (2013). Marqibo® (vincristine sulfate liposome injection) improves the pharmacokinetics and pharmacodynamics of vincristine. Cancer Chemotherapy and Pharmacology, 71, 555–564.
Sinha, B. K. (1995). Topoisomerase inhibitors. Drugs, 49, 11–19.
Slingerland, M., Guchelaar, H. J., Rosing, H., Scheulen, M. E., van Warmerdam, L. J., Beijnen, J. H., & Gelderblom, H. (2013). Bioequivalence of Liposome-Entrapped Paclitaxel Easy-To-Use (LEP-ETU) formulation and paclitaxel in polyethoxylated castor oil: A randomized, two-period crossover study in patients with advanced cancer. Clinical Therapeutics, 35, 1946–1954.
Stathopoulos, G. P., Boulikas, T., Vougiouka, M., Deliconstantinos, G., Rigatos, S., Darli, E., Viliotou, V., & Stathopoulos, J. G. (2005). Pharmacokinetics and adverse reactions of a new liposomal cisplatin (Lipoplatin): Phase I study. Oncology Reports, 13, 589–595.
Stathopoulos, G. P., Boulikas, T., Kourvetaris, A., & Stathopoulos, J. (2006a). Liposomal oxaliplatin in the treatment of advanced cancer: A phase I study. Anticancer Research, 26, 1489–1493.
Stathopoulos, G. P., Boulikas, T., Vougiouka, M., Rigatos, S. K., & Stathopoulos, J. G. (2006b). Liposomal cisplatin combined with gemcitabine in pretreated advanced pancreatic cancer patients: A phase I-II study. Oncology Reports, 15, 1201–1204.
Subbiah, V., Grilley-Olson, J. E., Combest, A. J., Sharma, N., Tran, R. H., Bobe, I., Osada, A., Takahashi, K., Balkissoon, J., Camp, A., Masada, A., Reitsma, D. J., & Bazhenova, L. A. (2018). Phase Ib/II trial of NC-6004 (nanoparticle cisplatin) plus gemcitabine in patients with advanced solid tumors. Clinical Cancer Research, 24, 43–51.
Sun, D., Zhou, S., & Gao, W. (2020). What went wrong with anticancer nanomedicine design and how to make it right. ACS Nano, 14, 12281–12290.
Svenson, S., Wolfgang, M., Hwang, J., Ryan, J., & Eliasof, S. (2011). Preclinical to clinical development of the novel camptothecin nanopharmaceutical CRLX101. Journal of Controlled Release, 153, 49–55.
Swami, U., Shah, U., & Goel, S. (2017). Eribulin in non-small cell lung cancer: Challenges and potential strategies. Expert Opinion on Investigational Drugs, 26, 495–508.
Tang, L., Yang, X., Yin, Q., Cai, K., Wang, H., Chaudhury, I., Yao, C., Zhou, Q., Kwon, M., Hartman, J. A., Dobrucki, I. T., Dobrucki, L. W., Borst, L. B., Lezmi, S., Helferich, W. G., Ferguson, A. L., Fan, T. M., & Cheng, J. (2014). Investigating the optimal size of anticancer nanomedicine. Proceedings of the National Academy of Sciences, 111, 15344–15349.
Tippayamontri, T., Kotb, R., Sanche, L., & Paquette, B. (2014). New therapeutic possibilities of combined treatment of radiotherapy with oxaliplatin and its liposomal formulation, Lipoxal™, in rectal cancer using xenograft in nude mice. Anticancer Research, 34, 5303–5312.
Tomkinson, B., Bendele, R., Giles, F. J., Brown, E., Gray, A., Hart, K., LeRay, J. D., Meyer, D., Pelanne, M., & Emerson, D. L. (2003). OSI-211, a novel liposomal topoisomerase I inhibitor, is active in SCID mouse models of human AML and ALL. Leukemia Research, 27, 1039–1050.
Torchilin, V. (2011). Tumor delivery of macromolecular drugs based on the EPR effect. Advanced Drug Delivery Reviews, 63, 131–135.
Tweedie, D. J., Erikson, J. M., & Prough, R. A. (1987). Metabolism of hydrazine anti-cancer agents. Pharmacology & Therapeutics, 34, 111–127.
Ueno, T., Endo, K., Hori, K., Ozaki, N., Tsuji, A., Kondo, S., Wakisaka, N., Murono, S., Kataoka, K., Kato, Y., & Yoshizaki, T. (2014). Assessment of antitumor activity and acute peripheral neuropathy of 1,2-diaminocyclohexane platinum (II)-incorporating micelles (NC-4016). International Journal of Nanomedicine, 9, 3005–3012.
Uldrick, T. S., & Whitby, D. (2011). Update on KSHV epidemiology, Kaposi Sarcoma pathogenesis, and treatment of Kaposi Sarcoma. Cancer Letters, 305, 150–162.
Valcourt, D. M., Dang, M. N., Scully, M. A., & Day, E. S. (2020). Nanoparticle-mediated co-delivery of Notch-1 antibodies and ABT-737 as a potent treatment strategy for triple-negative breast cancer. ACS Nano, 14, 3378–3388.
van der Meel, R., Sulheim, E., Shi, Y., Kiessling, F., Mulder, W. J. M., & Lammers, T. (2019). Smart cancer nanomedicine. Nature Nanotechnology, 14, 1007–1017.
Verco, S., Maulhardt, H., Baltezor, M., Williams, E., Iacobucci, M., Wendt, A., Verco, J., Marin, A., Campbell, S., Dorman, P., & diZerega, G. (2021). Local administration of submicron particle paclitaxel to solid carcinomas induces direct cytotoxicity and immune-mediated tumoricidal effects without local or systemic toxicity: Preclinical and clinical studies. Drug Delivery and Translational Research, 11, 1806–1817.
Vergote, I., Bergfeldt, K., Franquet, A., Lisyanskaya, A. S., Bjermo, H., Heldring, N., Buyse, M., & Brize, A. (2020). A randomized phase III trial in patients with recurrent platinum sensitive ovarian cancer comparing efficacy and safety of paclitaxel micellar and Cremophor EL-paclitaxel. Gynecologic Oncology, 156, 293–300.
Vincristine liposomal-INEX: Lipid-encapsulated vincristine, onco TCS, transmembrane carrier system--vincristine, vincacine, vincristine sulfate liposomes for injection, VSLI. (2004). Drugs in R&D, 5, 119–123.
Vokes, E. E., Gordon, G. S., Mauer, A. M., Rudin, C. M., Krauss, S. A., Szeto, L., Golomb, H. M., & Hoffman, P. C. (2000). A phase I study of STEALTH cisplatin (SPI-77) and vinorelbine in patients with advanced non small-cell lung cancer. Clinical Lung Cancer, 2, 128–132.
von Moos, R., Thuerlimann, B. J., Aapro, M., Rayson, D., Harrold, K., Sehouli, J., Scotte, F., Lorusso, D., Dummer, R., Lacouture, M. E., Lademann, J., & Hauschild, A. (2008). Pegylated liposomal doxorubicin-associated hand-foot syndrome: Recommendations of an international panel of experts. European Journal of Cancer, 44, 781–790.
Wagner, A. M., Knipe, J. M., Orive, G., & Peppas, N. A. (2019). Quantum dots in biomedical applications. Acta Biomaterialia, 94, 44–63.
Wang, W., & Tse-Dinh, Y. C. (2019). Recent advances in use of topoisomerase inhibitors in combination cancer therapy. Current Topics in Medicinal Chemistry, 19, 730–740.
Wang, L., Cao, J., Li, C., Wang, X., Zhao, Y., Li, T., Du, Y., Tao, Z., Peng, W., Wang, B., Zhang, J., Zhang, S., Wang, Z., & Hu, X. (2021). Efficacy and safety of mitoxantrone hydrochloride liposome injection in Chinese patients with advanced breast cancer: A randomized, open-label, active-controlled, single-center, phase II clinical trial. Investigational New Drugs, 40, 330–339.
Wetzler, M., Thomas, D. A., Wang, E. S., Shepard, R., Ford, L. A., Heffner, T. L., Parekh, S., Andreeff, M., O'Brien, S., & Kantarjian, H. M. (2013). Phase I/II trial of nanomolecular liposomal annamycin in adult patients with relapsed/refractory acute lymphoblastic leukemia. Clinical Lymphoma, Myeloma & Leukemia, 13, 430–434.
White, S. C., Lorigan, P., Margison, G. P., Margison, J. M., Martin, F., Thatcher, N., Anderson, H., & Ranson, M. (2006). Phase II study of SPI-77 (sterically stabilised liposomal cisplatin) in advanced non-small-cell lung cancer. British Journal of Cancer, 95, 822–828.
Whittle, J. R., Lickliter, J. D., Gan, H. K., Scott, A. M., Simes, J., Solomon, B. J., MacDiarmid, J. A., Brahmbhatt, H., & Rosenthal, M. A. (2015). First in human nanotechnology doxorubicin delivery system to target epidermal growth factor receptors in recurrent glioblastoma. Journal of Clinical Neuroscience, 22, 1889–1894.
Wicki, A., Witzigmann, D., Balasubramanian, V., & Huwyler, J. (2015). Nanomedicine in cancer therapy: Challenges, opportunities, and clinical applications. Journal of Controlled Release, 200, 138–157.
Wilhelm, S., Tavares, A. J., Dai, Q., Ohta, S., Audet, J., Dvorak, H. F., & Chan, W. C. W. (2016). Analysis of nanoparticle delivery to tumours. Nature Reviews Materials, 1, 16014.
Xu, X., Wang, L., Xu, H. Q., Huang, X. E., Qian, Y. D., & Xiang, J. (2013). Clinical comparison between paclitaxel liposome (Lipusu®) and paclitaxel for treatment of patients with metastatic gastric cancer. Asian Pacific Journal of Cancer Prevention, 14, 2591–2594.
Xu, C., Nam, J., Hong, H., Xu, Y., & Moon, J. J. (2019). Positron emission tomography-guided photodynamic therapy with biodegradable mesoporous silica nanoparticles for personalized cancer immunotherapy. ACS Nano, 13, 12148–12161.
Yacoby, I., & Benhar, I. (2008). Antibacterial nanomedicine. Nanomedicine, 3, 329–341.
Yang, J. I., Jin, B., Kim, S. Y., Li, Q., Nam, A., Ryu, M. O., Lee, W. W., Son, M. H., Park, H. J., Song, W. J., & Youn, H. Y. (2020). Antitumour effects of Liporaxel (oral paclitaxel) for canine melanoma in a mouse xenograft model. Veterinary and Comparative Oncology, 18, 152–160.
Young, C., Schluep, T., Hwang, J., & Eliasof, S. (2011). CRLX101 (formerly IT-101)-a novel nanopharmaceutical of camptothecin in clinical development. Current Bioactive Compounds, 7, 8–14.
Yousefpour, P., Ahn, L., Tewksbury, J., Saha, S., Costa, S. A., Bellucci, J. J., Li, X., & Chilkoti, A. (2019). Conjugate of doxorubicin to albumin-binding peptide outperforms aldoxorubicin. Small, 15, e1804452.
Yuan, F., Leunig, M., Huang, S. K., Berk, D. A., Papahadjopoulos, D., & Jain, R. K. (1994). Microvascular permeability and interstitial penetration of sterically stabilized (stealth) liposomes in a human tumor xenograft. Cancer Research, 54, 3352–3356.
Yun, Y. H., Lee, B. K., & Park, K. (2015). Controlled drug delivery: Historical perspective for the next generation. Journal of Controlled Release, 219, 2–7.
Zamboni, W. C., Ramalingam, S., Friedland, D. M., Edwards, R. P., Stoller, R. G., Strychor, S., Maruca, L., Zamboni, B. A., Belani, C. P., & Ramanathan, R. K. (2009). Phase I and pharmacokinetic study of pegylated liposomal CKD-602 in patients with advanced malignancies. Clinical Cancer Research, 15, 1466–1472.
Zelmer, C., Zweifel, L. P., Kapinos, L. E., Craciun, I., Güven, Z. P., Palivan, C. G., & Lim, R. Y. H. (2020). Organelle-specific targeting of polymersomes into the cell nucleus. Proceedings of the National Academy of Sciences, 117, 2770–2778.
Zhang, H. (2016). Onivyde for the therapy of multiple solid tumors. Oncotargets and Therapy, 9, 3001–3007.
Zhang, J. A., Xuan, T., Parmar, M., Ma, L., Ugwu, S., Ali, S., & Ahmad, I. (2004). Development and characterization of a novel liposome-based formulation of SN-38. International Journal of Pharmaceutics, 270, 93–107.
Zhang, J., Tian, Q., Yung, C. S., Chuen, L. S., Zhou, S., Duan, W., & Zhu, Y. Z. (2005). Metabolism and transport of oxazaphosphorines and the clinical implications. Drug Metabolism Reviews, 37, 611–703.
Zhang, E., Xing, R., Liu, S., & Li, P. (2019). Current advances in development of new docetaxel formulations. Expert Opinion on Drug Delivery, 16, 301–312.
Zhang, L., Beatty, A., Lu, L., Abdalrahman, A., Makris, T. M., Wang, G., & Wang, Q. (2020). Microfluidic-assisted polymer-protein assembly to fabricate homogeneous functional nanoparticles. Materials Science & Engineering C, Materials for Biological Applications, 111, 110768.
Zhang, J., Pan, Y., Shi, Q., Zhang, G., Jiang, L., Dong, X., Gu, K., Wang, H., Zhang, X., Yang, N., Li, Y., Xiong, J., Yi, T., Peng, M., Song, Y., Fan, Y., Cui, J., Chen, G., Tan, W., Zang, A., Guo, Q., Zhao, G., Wang, Z., He, J., Yao, W., Wu, X., Chen, K., Hu, X., Hu, C., Yue, L., Jiang, D., Wang, G., Liu, J., Yu, G., Li, J., Bai, J., Xie, W., Zhao, W., Wu, L., & Zhou, C. (2022). Paclitaxel liposome for injection (Lipusu) plus cisplatin versus gemcitabine plus cisplatin in the first-line treatment of locally advanced or metastatic lung squamous cell carcinoma: A multicenter, randomized, open-label, parallel controlled clinical study. Cancer Communications, 42, 3–16.
Zhao, L., & Zhang, B. (2017). Doxorubicin induces cardiotoxicity through upregulation of death receptors mediated apoptosis in cardiomyocytes. Scientific Reports, 7, 44735.
Zhu, L., & Chen, L. (2019). Progress in research on paclitaxel and tumor immunotherapy. Cellular & Molecular Biology Letters, 24, 40.
Acknowledgments
Marco Cordani is currently a recipient of a Maria Zambrano research contract from the Spanish Ministry of Universities and Complutense University of Madrid (call of grants for the requalification of the Spanish university system 2021–2023). Marco Cordani acknowledges support of the Recovery, Transformation, and Resilience Plan “Next generation EU”. Raffaele Strippoli acknowledges a grant form Ministry for Health of Italy (Ricerca Corrente).
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Lopez-Mendez, T.B., Strippoli, R., Trionfetti, F., Calvo, P., Cordani, M., Gonzalez-Valdivieso, J. (2023). Clinical Trials Involving Chemotherapy-Based Nanocarriers in Cancer Therapy: State of the Art and Future Directions. In: Almeida de Sousa, Â.M., Pienna Soares, C., Chorilli, M. (eds) Cancer Nanotechnology. Springer, Cham. https://doi.org/10.1007/978-3-031-17831-3_12
Download citation
DOI: https://doi.org/10.1007/978-3-031-17831-3_12
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-17830-6
Online ISBN: 978-3-031-17831-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)