Abstract.
Oil reserves present in Alberta, Canada, in the form of oil sands, are estimated to contain 169 billion barrels of recoverable bitumen [1]. The extraction process of bitumen involves the use of large volumes of caustic hot water, that even with recycling efforts in place, have continued to accumulate for decades in large settling basins, known as tailings ponds [2]. As of a 2019 report given by the Alberta Energy Regulator, there is an estimated 1302 Mm3 of fluid tailings being stored in tailings ponds [3]. The high toxicity of this wastewater commonly known as oil sands process affected waters (OSPW) has impeded its release resulting in a zero-discharge policy. Although many different contaminates have been identified to contribute to this toxicity, evidence indicates naphthenic acids (NAs) are the main toxic component present in OSPW and are therefore of major concern [4, 5].
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords:
Oil reserves present in Alberta, Canada, in the form of oil sands, are estimated to contain 169 billion barrels of recoverable bitumen [1]. The extraction process of bitumen involves the use of large volumes of caustic hot water, that even with recycling efforts in place, have continued to accumulate for decades in large settling basins, known as tailings ponds [2]. As of a 2019 report given by the Alberta Energy Regulator, there is an estimated 1302 Mm3 of fluid tailings being stored in tailings ponds [3]. The high toxicity of this wastewater commonly known as oil sands process affected waters (OSPW) has impeded its release resulting in a zero-discharge policy. Although many different contaminates have been identified to contribute to this toxicity, evidence indicates naphthenic acids (NAs) are the main toxic component present in OSPW and are therefore of major concern [4, 5].
The term naphthenic acid represents a very broad distribution of carboxylic acid species that are ubiquitous in the Athabasca region and the oil sands [1, 5]. NAs are a diverse and complex class of compounds with potentially thousands of different components, which makes their removal from OSPW challenging. A promising remediation strategy that has gained more attention recently is the use of activated carbon adsorbents [6,7,8]. However, there is a need to better characterize the NA adsorption system while using activated carbon. A few studies have focused on model NA adsorption recently using activated carbon [9,10,11,12,13], but given the diversity of NA species within OSPW, characterizing the adsorption of a broader range of model NA species is warranted.
In this study, we characterized the adsorption of seven model NA species onto three different activated carbons. Two of the ACs were produced in our laboratory with a focus on commercial viability and waste recycling. They are a petroleum coke-sourced activated carbon made in our lab (KOHAC) and a phosphoric acid activated waste-wood-based AC (PAAC). These were compared with a commercial AC (COMAC). Adsorption profiles of each model NA in terms of kinetics and isotherms were used to characterize each activated carbon as discussed below.
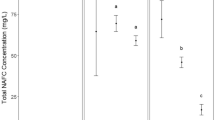
Adsorption kinetics results are shown in Fig. 1 for adsorption on the three ACs. This shows that for each of the ACs, the uptake of NAs is very dependent on the NA itself. Diphenylacetic acid (DPA) and dicyclohexylacetic acid (DHA) clearly are most adsorbed, while heptanoic acid (HA), cyclohexane acetic acid (CHA), and 2-methyl-cyclohexane acetic acid (2-MCH) all had lower percent adsorption, and 1,4 cyclohenxanedicarboxylic acid (1,4-CHA) and succinic acid (SA) had very little adsorption. Although these species represent a very small cross-section of the possible NA species, what this demonstrates is that optimal performance for AC removal of adsorption will require more species-specific adsorption evaluation than total NA adsorbed.
The rate of adsorption for each of the evaluated model NAs is also dependent on the method of activation, surface functionality, and textural features of the activated carbon. For these model NAs, the rate of adsorption is fastest on the COMAC, in which adsorption equilibrium was achieved between 30 and 60 minutes. Equilibrium on the KOHAC is achieved between 8 and 24 hours depending on the model NA. This difference in kinetics may be a result of the differences in pore size distribution between the COMAC and KOHAC. However, the PAAC which has the highest mesoporosity also exhibits a slower approach to equilibrium of 2–8 hours. The influence of particle size is considered a confounding factor in these evaluations.
The interplay of surface functionality as generated by method of activation and feedstock makes it challenging to evaluate only the effect of textural material properties. However, the use of multiple activation cycles gives rise to a convenient method for increasing mesoporosity without significantly altering the surface composition or surface area while using the same feedstock and activation chemistry. This approach was evaluated for a range of model NAs on singly, doubly, and triply activated KOHAC.
As shown in Table 1, the multiple heating cycles resulted in a systematic modification of the textural properties of the KOHAC with an approximately 20% increase in mesoporosity percent surface area per activation cycle.
The multiple activations demonstrated faster kinetics for each model NA as shown in Table 2 for DPA, CHA, and HA. The adsorption kinetics were modeled using both pseudo-first-order kinetics and multiple exponential kinetics. The half-life of adsorption is shown for the multiple exponent kinetics models. X-ray photoelectron spectroscopy (not shown) demonstrates no significant change in surface functionality. This increase in mesoporosity is expected to improve the internal diffusion of adsorbate within the AC pores to facilitate adsorbate access to the microporous space.
Conveniently, with minimal chemical input, the adsorption kinetics for a range of model NAs can be improved by thermal cycling allowing for tailoring of the textural properties of the material and their resulting potential application. The evaluation of species-specific NA removal from both synthetic and industrial water samples containing a broad mixture of NAs is in progress using electrospray ionization mass spectroscopy (ESI) to provide further insights into the optimization of AC surface chemistry and textural properties for oil sands process water remediation.
References
Brown LD, Ulrich AC. Oil sands naphthenic acids: a review of properties, measurement, and treatment. Chemosphere. 2015;127:276–90. https://doi.org/10.1016/j.chemosphere.2015.02.003. Elsevier Ltd.
Allen EW. Process water treatment in Canada’s oil sands industry: II. A review of emerging technologies. J Environ Eng Sci. 2008;7(5):499–524. https://doi.org/10.1139/S08-020. ICE Publishing.
A. Energy Regulator. State of fluid tailings management for mineable oil sands. 2019. [Online]. Available: www.aer.ca
Li C, Fu L, Stafford J, Belosevic M, Gamal El-Din M. The toxicity of oil sands process-affected water (OSPW): a critical review. Sci Total Environ. 2017;601–602:1785–802. https://doi.org/10.1016/j.scitotenv.2017.06.024. Elsevier B.V.
Clemente JS, Fedorak PM. A review of the occurrence, analyses, toxicity, and biodegradation of naphthenic acids. Chemosphere. 2005;60(5):585–600. https://doi.org/10.1016/j.chemosphere.2005.02.065. Elsevier Ltd.
Mohamed MH, Wilson LD, Headley JV, Peru KM. Novel materials for environmental remediation of tailings pond waters containing naphthenic acids. Process Saf Environ Prot. 2008;86(4):237–43. https://doi.org/10.1016/j.psep.2008.04.001.
Islam MS, Zhang Y, McPhedran KN, Liu Y, Gamal El-Din M. Mechanistic investigation of industrial wastewater naphthenic acids removal using granular activated carbon (GAC) biofilm based processes. Sci Total Environ. 2016;541:238–46. https://doi.org/10.1016/j.scitotenv.2015.09.091.
Iranmanesh S, Harding T, Abedi J, Seyedeyn-Azad F, Layzell DB. Adsorption of naphthenic acids on high surface area activated carbons. J Enviro Sci Health A. 2014;49(8):913–22. https://doi.org/10.1080/10934529.2014.894790.
Martinez-Iglesias A, Niasar HS, Xu CC, Ray MB. Adsorption of model naphthenic acids in water with granular activated carbon. Adsorpt Sci Technol. 2015;33(10):881–94. https://doi.org/10.1260/0263-6174.33.10.881.
Niasar HS, Li H, Kasanneni TVR, Ray MB, Xu CC. Surface amination of activated carbon and petroleum coke for the removal of naphthenic acids and treatment of oil sands process-affected water (OSPW). Chem Eng J. 2016;293:189–99. https://doi.org/10.1016/j.cej.2016.02.062.
Kawano T, Kubota M, Onyango MS, Watanabe F, Matsuda H. Preparation of activated carbon from petroleum coke by KOH chemical activation for adsorption heat pump. Appl Therm Eng. 2008;28(8–9):865–71. https://doi.org/10.1016/j.applthermaleng.2007.07.009.
Niasar HS, Li H, Das S, Kasanneni TVR, Ray MB, (Charles) Xu C. Preparation of activated petroleum coke for removal of naphthenic acids model compounds: Box-Behnken design optimization of KOH activation process. J Environ Manag. 2018;211:63–72. https://doi.org/10.1016/j.jenvman.2018.01.051.
Seyedy Niasar H, (Chunbao) Xu C, Ray M. Treatment of oil sands process-affected water using activated and surface modified petroleum coke for organic compounds recovery. Chem Biochem Eng. 2017;Doctor of. 190.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this paper
Cite this paper
Nazari, E., Roy, T.M., Strong, O.K.L., Vreugdenhil, A.J. (2023). Adsorption of Naphthenic Acids from Oil Sand Process-Affected Water (OSPW) Using Commercially Viable Petcoke-Sourced Activated Carbon. In: Proceedings of the 61st Conference of Metallurgists, COM 2022. COM 2022. Springer, Cham. https://doi.org/10.1007/978-3-031-17425-4_35
Download citation
DOI: https://doi.org/10.1007/978-3-031-17425-4_35
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-17424-7
Online ISBN: 978-3-031-17425-4
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)