Abstract
Diabetic distal symmetric polyneuropathy (diabetic DSP) has variable clinical presentation that can complicate the diagnostic process. It is primarily identified by asymptomatic annual screening or from neuropathic symptoms. In this chapter, we present key considerations for findings on screening or clinical evaluation. First, identification of risk factors for diabetic DSP establishes a general pre-assessment probability. Second, identification of the other component causes (foot deformity, vascular impairment) of foot complications along with identifying the impaired protective sensation that is part of diabetic DSP is essential for preventing foot outcomes. Third, the clinician must recognize that there is heterogeneity in manifestations, involving small and large nerve fiber types. As in any process of diagnosis, a clinical evaluation considers each symptom or sign’s contribution to incrementally revising the clinician’s estimates of disease probability and it reduces clinical uncertainty. Simple screening methods are valid, as are clinical scales, adopted into research cohorts and trials, that can be implemented into practice. Once a chronically-progressive distal symmetric pattern of polyneuropathy is confidently identified, alternate causes can generally be accomplished by simple clinical considerations and simple laboratory testing. While uncommon, a typical features such as asymmetry, nonlength dependence, acute or subacute rather than chronic onset and progression, and motor predominance call for specialized testing and clinical expertise from a neurologist. Depending on the number and severity of deformity, vascular insufficiency, and diabetic DSP’s impairment in protective sensation, interventions are initiated including self-foot care education and professionally-fitted therapeutic footwear to referral for wound management and surgical consultation.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Diabetic distal symmetric polyneuropathy
- Diagnosis and screening
- Clinical scales
- Michigan neuropathy screening instrument
- Toronto clinical neuropathy score
1 Introduction and Context
The variability in clinical presentation and complexities in measurement of diabetic neuropathy have not only challenged the clear understanding of epidemiology, impact, and the clinical trial development of therapies, but these often complicate the diagnostic process. However, with a clear understanding of these obstacles and the relevant clinical tools, clinicians from all fields of specialty can develop an efficient approach to diagnosis. This includes more simplified approaches suitable to primary care and diabetes management clinics that screen for and diagnose diabetic neuropathy, to the specialty neurology clinics that may use more refined diagnostic methods required for confirmation of neuropathy and the evaluation of more atypical cases. Certainly, the vast majority of cases of diabetic neuropathy do not require referral to a neurology specialist for confirmation. This places a responsibility on the diabetes care clinician to be able to confidently make a working diagnosis on clinical evidence and to initiate management, consider other causes of polyneuropathy, while identifying atypical features that warrant specialist referral. This chapter discusses these considerations and the approaches to the clinical diagnosis of diabetic neuropathy, while chapter “Clinical Features Diabetic Neuropathies” details clinical features, and chapter “Diagnostic Techniques for Diabetic Peripheral Neuropathy” details specialized diagnostic techniques.
1.1 Diabetic Distal Symmetric Polyneuropathy (Diabetic DSP): A Brief Overview
The most common form—and the one usually referred to as “diabetic peripheral neuropathy” or even more simply as “diabetic neuropathy”—is the diffuse, symmetrical, slowly-progressive length-dependent damage to the peripheral and autonomic nervous system classified technically by the term “diabetic distal symmetrical polyneuropathy” (diabetic DSP) [1]. It typically remains asymptomatic for years, may first present clinically with symptoms of abnormal sensation symmetrically at the tips of the toes and may over time spread to the stocking-and-glove distribution. It involves injury to different anatomical nerve types that show variable clinical manifestations between individuals. These can be classified as small fibers (thinly-myelinated autonomic B-fibers, and thinly- or un- myelinated autonomic and sensory C-fibers) that make up autonomic and pain and temperature sensory fibers. Damage to large fibers (myelinated Aα, Aβ, Aδ sensory, and Aγ motor) are responsible for other sensory and skeletal muscle impairments [2, 3]. At the symptomatic stages, some have a large fiber-predominant pattern and experience numbness, tingling, or imbalance owing to sensory ataxia, while others have small fiber-predominant symptoms that may instead present with burning and stabbing pain, impairment in sensing heat and cold, or a propensity to clinical autonomic abnormalities like postural hypotension and gastroparesis [1]. Some experience combined manifestations of large and small fiber types, and, regardless of pattern, people with diabetes can be asymptomatic for extended periods even though their physical examination or specialized testing can reveal subtle or even marked impairments in nerve structure and function. With progression to foot muscle weakness (motor weakness represents another large fiber dysfunction), or clinical autonomic manifestations like dry feet from limited sweat production (sudomotor dysfunction), the subsequent risk of ulcer, infection, and amputation intensify. Complications like these are feared more than death itself [4], and of great concern is the recent evidence in certain parts of the world of a resurgence in the risk of amputations [5,6,7].
1.2 Staging of Diabetic DSP
For the purpose of understanding the face value of symptoms and signs and their measurement scales, we center the discussion of clinical diagnosis in this chapter around the conventional staging system described in Table 1 [9]. In simple terms, in practice diabetic DSP (Stage 1b or higher) is identified through two possible points in clinical care: Asymptomatic screening, leading to a clinical diagnostic workup if screening physical examination results are abnormal, or alternatively through complaints of neuropathic symptoms on a clinical encounter, leading similarly to a clinical workup involving history, physical examination, simple laboratory tests, and consideration in a small subset of people for neurologist referral for specialized testing and evaluation.
2 Key Clinical Considerations FOR Diabetic DSP Diagnosis
2.1 Clinical Risk Factors for Diabetic DSP Are Typically Present
Elevated cumulative glycemic exposure is the fundamental risk factor for diabetic DSP onset [10,11,12]. As such, age, diabetes duration, and glycemic exposure measured by an inability to maintain glycated hemoglobin A1c at target levels, generally considered to be 7.0% or less, are related risk factors [13]. Additionally, cardiovascular metabolic risk factors including presence of hypertension, abdominal obesity, hypertriglyceridemia, and low high-density lipoprotein, as well as taller height, alcohol abuse, and chronic kidney disease have been identified, in addition to putative racial and genetic factors that may be clinically identifiable in future [13,14,15,16]. However, specific threshold levels of risk factors and their duration have not been proposed. Generally, an individual with short duration of type 1 diabetes is extremely unlikely to have diabetic DSP unless exceedingly high glycemic exposure has occurred and other risk factors are present. However, in type 2 diabetes, the existence of pre-diabetes may lead to polyneuropathy and therefore it may be present at diagnosis even without substantial elevations in glycemic exposure [17].
2.2 The Impaired Protective Sensation of Diabetic DSP Represents Only One Component Cause of Foot Complications
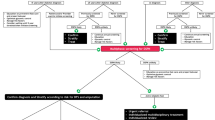
While diabetic DSP itself is sufficient to cause pain and imbalance as complications, it represents only a component cause, neither necessary nor sufficient, to cause ulcer, infection, the Charcot deformity, or amputation. Tissue ischemia from peripheral vascular disease, and foot deformity or the minor trauma to skin that incites ulceration and infection induced by inadequacies in footwear and general foot care represent the other component causes. The implication is that if a clinician is to meaningfully help to prevent foot complications, the annual foot evaluation must not focus only on the identification of the loss of protective sensation representing diabetic DSP alone. It must include evaluation for arterial patency (pedal pulses and skin changes), and foot inspection for presence of abnormalities such as callouses or deformity that may indicate the presence of repetitive minor trauma (Fig. 1 and Table 2).
A simplified view of the core component causes of foot complications and amputation. While sufficient for pain and sensory ataxia, the presence of loss of protective sensation from diabetic neuropathy alone is not considered sufficient for advanced foot complications without the presence of peripheral vascular disease or minor trauma to skin. The implication is that clinical evaluation focused only on diabetic neuropathy, without consideration of the other component causes through simple inspection and palpation for pulses, is insufficient
2.3 Impaired Protective Sensation Shows Heterogeneity Between People with Diabetes
Impaired protective sensation may occur asymptomatically (in approximately half of those with objective evidence of diabetic DSP) [21] or symptomatically. Whether symptomatic or not, the symptoms and signs may represent small or large fiber sensory modalities, as well as autonomic and motor modalities. Severity can be determined by the extent of sensory modality impairment, and by the presence and extent of motor weakness in the muscles of the feet. Furthermore, while autonomic dysfunction can be observed at early stages by specialized testing, clinical autonomic manifestations generally occur at more advanced stages. Finally, many may present with mixed findings representing both small- and large-fiber modalities [1, 11]. The implication of this heterogeneity of clinical presentation is that the evaluating clinician must consider these varied attributes, that theoretically the selection of individual screening tests may not be sufficient, and that scales that consider multiple attributes may have advantages over other investigative tests.
In summary, evaluation of the feet in people with diabetes represents consideration of risk factors for diabetic DSP, evaluation of a composite of the varied components of diabetic DSP, as well as a composite of the features of neuropathy, peripheral vascular disease, and foot deformity and foot care. The diagnosis of diabetic DSP itself requires a process of clinical assessment, either initiated by asymptomatic screening or in response to a symptomatic complaint, that considers the results of various clinical history and examination tests. As in any process of diagnostic medicine, each symptom or sign incrementally revises the clinician’s estimates of disease probability and reduces clinical undertainty [22].
3 Asymptomatic Clinical Presentation: Screening for Diabetic DSP
It is estimated that approximately half of people with objective evidence by physical examination or specialized testing have no symptoms of neuropathy [1, 21]. Asymptomatic screening for neuropathy has therefore generally been justified based on “disease principles” criteria for screening [23]. For example, the epidemiology of diabetic DSP is generally understood, its natural history includes a latent phase, and the target population for screening—including type 2 diabetes at diagnosis and type 1 diabetes with 5 or more years of diabetes duration—is well defined [1]. Screening is also supported by “test principles.” For example, screening test performance characteristics and their interpretation and thresholds are generally well understood [23]. This justification exists even if “system principles” are not uniformly met. While a diabetes multiprofessional care infrastructure exists, screening is acceptable to people with diabetes and carries little harm, some aspects are not yet well understood. These include how valid specific tests are when implemented in different settings, the economic evaluation of screening, the lack of neuropathy-specific, and the implications of misclassifying neuropathy as present when it is not (false positive) or falsely negative. Despite these limitations, the diabetes community has consistently supported screening for diabetic DSP [1, 24, 25].
In practice, the annual screening examination recommended by diabetes organizations can be accomplished by way of very simple tests for loss of protective sensation. Examples include testing pressure sensation with a monofilament, testing vibration sensation with a tuning fork, or frankly an even simpler “Touch the Toes” approach adopted by Diabetes UK in which the feet are simply exposed, inspected, and touched with the fingertip [24, 26, 27]. Some organizations, like the American Diabetes Association through its neuropathy position statement, recommend a more extensive evaluation. This recommendation calls for an annual careful neuropathic history, along with a small fiber function test (such as temperature or pinprick sensation), a large fiber test (vibration sensation using a 128-Hz tuning fork), as well as a simplified version of the 10 g monofilament test to assess for feet at risk of ulceration or non-traumatic amputation [1].
While we discuss physical examination maneuvers below under Neuropathic Signs, we wish to discuss a particular aspect of screening for impaired protective sensation using common maneuvers. Most maneuvers have been justified by their face validity: They make sense, they are available in clinical practice, they are well recognized by diverse clinicians, and represent a key attribute of diabetic DSP. Several common examples exist that have more extensive research evidence in that they not only have demonstrated face validity and reasonable test reproducibility, but also intrinsic diagnostic accuracy for concurrent validity [28, 29]. Concurrent validity represents the ability of a test to reasonably classify, in a cross-sectional study design, who in the study sample has neuropathy defined by a reference standard test (including nerve conduction studies) and who does not. Several examples of tests with this level of evidence exist [28, 29]. However, we highlight a particular quantitative variation of the 10 g-monofilament examination that has even further evidence as a screening test for the prediction of future onset of diabetic DSP. This scoring system has been created in order to measure more subtle degrees of abnormality in protective sensation [8, 24]. First, it is applied only to a distal, non-callousing site (proximal to the nail bed as shown in Fig. 2) in order to identify earlier, distal length-dependent sensory impairment as compared to the more traditional multi-site testing that includes more proximal sites on the soles of the feet. Second, rather than simply grading a 10g monofilament test as a binary “normal” or “abnormal” result as in the traditional multi-site testing, in the test variation the monofilament is applied four times to each great toe in a random, arrhythmic manner to generate a quantitative score. For each stimulus, a score of 0 is assigned if it is not perceived, 0.5 if it is substantially less than that perceived on the forehead or sternum, and 1 if it is perceived normally, resulting in a total score from 0 (completely insensate) to 8 (completely sensate). Unlike the binary score, this allowed determination of two specific thresholds: The concurrent validity threshold for identifying the presence of neuropathy in cross-sectional study (this was determined to be a score of 0–3 out of 8), as well as a predictive validity threshold not associated with the current presence of neuropathy but instead with future onset in a 4-year longitudinal cohort structure (this was determined in a longitudinal cohort to be a score of 3.5–5.5). A score of 6–8 is interpreted as ruling out current neuropathy and extremely low 4-year risk of onset [8, 24]. In summary, while frank loss of sensation to the 10g monofilament helps to predict future ulcer and amputation risk among those with neuropathy, a quantitative variation of this examination seeking more subtle changes in sensation that would otherwise be called “normal” can be used as a screening tool, such that asymptomatic individuals (Stage 0 and 1a) can be reasonably stratified into those at low and high future risk. This, after all, is the intention of asymptomatic screening for disease: to identify at the earliest stages when interventions, such as improvement in glycemic control, are more likely to be effective. The identification of other tests, including objective measures, that could have sensitive thresholds to identify individuals at future risk of neuropathy onset represents an urgent clinical research need [30]. While the more sensitive, quantitative variation of the monofilament examination provides an approach to screening for protective sensation that has been evaluated in meta-analysis [28], we readily acknowledge that the predictive validity findings arise form a single-center longitudinal cohort and that it is not known with certainty if there are advantages over other simple [27] or complex [1] screening approaches.
Quantitative variation of the 10 g monofilament test suitable for diabetic dsp screening for the presence of neuropathy (impaired protective sensation) or its future risk. The 10g monofilament is also known as the 10 g Semmes-Weinstein monofilament. Adapted from Bril et al. [24] The testing procedure: (1) Show the 10 g monofilament to the person with diabetes. (2) Touch it first to the person’s forehead or sternum so that the sensation is understood. (3) Instruct the person to say “less” or “same” every time the monofilament stimulus is perceived. (4) With the person’s eyes closed, apply the monofilament to the dorsum of the great toe proximal to the nail bed as shown in the illustration. Use a smooth motion to touch the skin, bend the filament for a full second, then lift from the skin. (5) Perform this stimulus four times per foot in an arrhythmic manner so the person does not anticipate when the stimulus is to be applied. (6) For each of the 8 stimuli, assign a score of 0 if it is not perceived, 0.5 if it is substantially less than that perceived on the forehead or sternum, and 1 if it is perceived the same. A score of 3 out of 8 correct responses means that the presence of neuropathy is likely. A score of 3.5–5 means that the risk of new-onset neuropathy in the next 4 years is high. A score of 5.5 or greater indicates that there is a low risk of neuropathy onset in the next 4 years
4 Neuropathic Symptoms
The common symptoms of diabetic DSP are the large and small fiber sensory manifestations. Historically classified as “positive” symptoms, indicating nerve hyper-function creating a sensation normally absent, or “negative” symptoms, that represent hypo-function creating loss of a sensation normally present, this distinction is not clinically informative as there is a subtlety to the lived experience of polyneuropathy [31, 32]. Some recommend classification by small and large fiber sensory modalities [1], and we describe clinical features according to this classification throughout this chapter (summarized in Table 2). Regardless of classification, common symptoms are pain, tingling, numbness, unsteadiness, and a feeling of weakness. Symptoms typically have a slow, insidious progression, beginning bilaterally at the tips of the toes moving proximally over months or years to involve the lower thighs at which point the hands may become involved. This “stocking and glove” distribution of sensory symptoms can even progress to involve the midline of the abdomen, and symptom descriptions are variable between people. Unequivocally, symptoms present in the hands that are not accompanied by clinical features first in the lower limbs at or below the knee are not consistent with a diagnosis of diabetic DSP. Among the small fiber symptoms, common pain descriptors are “burning,” “stinging,” “shock-like,” lancinating pain [33]. Less common descriptors are a “squeezing,” pressure sensation. Pain can be present during the daytime or nighttime, and often have paroxysmal patterns. Small fiber dysfunction also includes impaired temperature discrimination, often described as a loss of ability to identify hot or cold water with one’s feet in the bathtub. Large fiber sensory symptoms include tingling, “pins and needles,” or numbness. Numbness can represent a lack of sensation, and is often used variably by people with diabetes to describe lack of thermal sensitivity, light touch, lack of pain sensation from minor traumas that would normally be present, such as the finding of a stone in one’s shoe or a cut to the skin without feeling pain or discomfort. People frequently describe a relative feeling of unsteadiness, which can be a manifestation of pain, tingling, numbness, or instead from a sensory ataxia in which either foot proprioception is affected or the stabilizing effect of feeling the ground. Similarly, a nonspecific feeling of weakness is a very common descriptor, likely as a consequence of abnormal sensory symptoms, as frequently this is described even when motor testing is completely normal. Motor symptoms resulting from muscle weakness can occur in more advanced diabetic DSP with impairment of foot dorsiflexion and plantar flexion that may be described as an inability to perform activities that require standing on the forefoot or on extended toes or heal-walking. Hyperalgesia and allodynia as manifestations of abnormal sensory processing are less common. Hyperalgesia refers to a painful stimulus that feels exaggerated, while allodynia refers to a normal stimulus that is perceived as painful such as severe pain induced by rubbing the toes along the bedsheets. While uncommon in diabetic DSP without first experiencing sensory symptoms, symptoms of autonomic dysfunction such as dryness of skin from sudomotor neuropathy, or the symptoms of gastroparesis, enteropathy, or clinical cardiac autonomic neuropathy with postural lightheadedness and syncope may occur. Finally the symptoms of foot complications like infection, ulceration and Charcot deformity may be present symptoms in advanced neuropathy.
5 Neuropathic Signs
A directed neurological examination for diabetic sensorimotor polyneuropathy (DSP) requires examination of the lower limbs starting distally at the toes [33]. As with symptoms and depending on stage and severity, the signs may be a reflexion of small fiber dysfunction, large fiber sensory dysfunction, large fiber motor weakness, presence of clinical autonomic abnormalities such as sudomotor neuropathy, and the manifestations of foot complications (Table 2). Appearance of the skin and feet can reveal dryness and color changes, loss of hair, clawing and deformity of the toes, and ulcerations at late stages. Clinical sensory function is assessed for small (thermal sensitivity, pin prick), large (vibration, proprioception), and mixed (light touch) fiber modalities. These sensory tests are performed by first applying the stimuli to the sternum or forehead so that the person can appreciate the normal sensation, and then distally at the first toe, or adjacent toe, in case of amputation. To determine severity, the stimuli are then moved proximally to provide a level at which the sensory function becomes normal. For vibration, a 128 Hz tuning fork is sufficient and must be applied on bony surfaces. Alternatively, small pocket-sized battery-operated electronic devices that standardize vibration can be used [34]. For proprioception, the distal phalanx is held on the lateral surfaces and moved upwards and downwards in small movements. For pinprick, a disposable sterile sharp metal tip can be used [35]. For thermal sensitivity, cold sensation can be assessed with a steel tuning fork cooled under running cold water, or alternatively, with a simple pen-like device that has a cool metallic end and a warmer plastic end for comparison [36]. For light touch, a cotton wisp may be used. The 10 g monofilament is a nylon thread affixed to a handle that bends at a standardized pressure of 10 g, representing a combination of touch and pressure sensation. Other physical examination tests such as 2-point discrimination, or the tactile circumferential discriminator to evaluate a person’s ability to differentiate stimuli of different diameters, are not commonly used for assessment of diabetic DSP.
Motor function is evaluated by muscle bulk and power of the small foot muscles, and specific strength on foot dorsiflexion and plantarflexion are generally tested. Strength in other lower limb groups should also be assessed (knee and hip movements). The deep tendon reflexes need to be performed with the person fully relaxed and knee and ankle reflexes should be assessed. Gait and ability to perform a tandem gait, Romberg test for sensory ataxia, and also ability to walk on heels and toes would complete the lower limb examination. Upper limbs should be examined depending on lower limb findings [33].
From a diagnostic perspective, the principal purpose of the physical examination is to determine, through the process of clinical assessment, if the various signs are in keeping with the clinical pattern of diabetic DSP through a process, with each finding, of incrementally revising clinical estimates of disease probability. For example, as discussed later, signs such as motor predominance over abnormal sensory tests, asymmetry, and lack of length-dependence are findings that substantially decrease the probability of diabetic DSP. This is of particular importance because it is known that individual physical examination signs are known to have very poor inter- and intra-rater reproducibility, even in the hands of expert neurologists [37]. This can be partially overcome by the clinician having a broad, systematic physical examination including multiple physical examination maneuvers as described, as well as by the performance of objective confirmatory tests if diagnostic uncertainty remains (chapter “Diagnostic Techniques for Diabetic Peripheral Neuropathy”).
Though research into implementation is limited, we make brief mention of certain point-of-care devices, defined as simple, rapid, lab-quality diagnostic devices for use by clinicians at the moment of clinical care for diagnosis. Some are an extension of the physical examination (battery-operated vibrating device in place of a tuning fork, temperature discrimination devices, or the “Neuropad” paper to identify sweat function) [34,35,36, 38, 39], and, like sensory physical examination, many are heavily dependent on subjective patient responses. Objective sudomotor function assessment by way of electrochemical skin conductance devices have shown conflicting evidence in the literature [40,41,42,43], but may be amenable to implementation into practice for screening [44]. Simplified point-of-care nerve conduction devices for sural nerve amplitude and conduction velocity are reproducible and valid, and in future may have impact on clinical decision-making as well as implementation into practice [45,46,47,48,49].
6 Composite Symptom Scales, Sign Scales, and Combined Scales
We have introduced thus for in this chapter the essential components of clinical evaluation, mostly representing symptoms and signs that represent effective clinical practice but that have not individually been evaluated in investigative test research for their diagnostic performance. However, we then introduced some specific screening maneuvers that have concurrent validity for identifying the presence of DSP such as the Ipswich Touch Test adopted by Diabetes UK [27] and versions of pain, vibration, light touch sensation [28, 29], as well as the example of a variation of the 10-g monofilament examination that may permit prediction of future onset of DSP [8]. Investigative test research is broad, though, as these tests can include single symptoms, signs, laboratory parameters, as well as composite scoring systems [50]. As individual symptoms or signs are likely not sensitive or specific enough to serve as a biomarker response, for example, to a therapeutic intervention in a clinical trial, one strategy is to implement a composite score, such as a self-reported symptom questionnaire or the clinical scales that make up a focus of this Chapter [51]. While these have been developed primarily as a way to summarize the proportions and severity of diabetic DSP in trial or cohort study populations, these composite scales can serve four key purposes to the clinician [22, 50]. First, they incorporate (and remind the clinician of the) multiple aspects of the manifestations of diabetic DSP, rather than focusing on one in isolation. Second, they can provide a diagnostic threshold for concurrent diagnosis, and, though they have not yet been evaluated in this way, for prediction of future onset of diabetic DSP or its related foot complications. Third, the quantitative scores allow a measure of diabetic DSP severity that is typically not conferred by the result of a single symptom or sign. Finally, many identify pain and its severity and can therefore be used to guide therapy for painful diabetic DSP.
7 The Michigan Neuropathy Screening Instrument (MNSI)
First published in 1994 [52], the Michigan Neuropathy Screening Instrument (MNSI) has had major impact on the identification of diabetic DSP, as well as providing a measure of its severity, and, potentially also an ability to track progression and regression in many observational studies and clinical trials [21, 53,54,55,56]. The MNSI is, in fact, made up of two separate assessments. First, a questionnaire (symptom scale) component, has major value for screening as it is a self-administered questionnaire made up of 15 items. These include questions such as having received a diagnosis by a physician in the past, or a history of amputation or ulceration, dry skin, but also about neuropathic symptoms such as numbness, pain, temperature sensation, and allodynia. Furthermore, there are two questions that are unscored, including generalized weakness and cramping, that can help the rater understand other features that may not relate directly or specifically to DSP and foot complications risk factors. The second component is a five-part lower extremity physical examination made up of examination for abnormal appearance including presence of callus or deformity, examination for ulceration, ankle reflexes, and simple 3-level scores using vibration sensation and the monofilament (present, reduced, absent).
7.1 Scale Face Validity
Both the MNSI Questionnaire and MNSI Examination scales incorporate attributes that relate specifically to polyneuropathy, however, they test the presence of other attributes including the presence of foot deformities. Whether the person has polyneuropathy or not, the presence of foot abnormalities such as callus, fissures, ulceration likely places them at high risk of foot complications. Though less specific to diabetic DSP, these features increase its clinical relevance as an outcome measure. For polyneuropathy-specific research, the manifestations especially on foot examination may not be sensitive enough to identify those at earlier stages of polyneuropathy alone, given that the sensory tests are graded in three simple categories.
7.2 Test Quality and Reliability
The MNSI itself has not been tested formally using standard methods and metrics for inter- and intra-rater reproducibility. Studies on variations of the MNSI show suitably high reliability [57, 58].
7.3 Diagnostic Accuracy: Concurrent
The original publication, representing analysis of a small cross-sectional group of type 1 and type 2 participants who had diabetic DSP confirmed by nerve conduction studies, undertook a simple tabulation suggesting that an MNSI Questionnaire score of 7 or greater, and an MNSI Examination score exceeding 2, are associated with extremely high predictive values positive, good sensitivity and extremely high specificity [52]. However, a diagnostic study design conducted in a type 1 diabetes observational cohort that also operationalizing nerve conduction studies to define diabetic DSP cases, suggested that these particular thresholds were associated with substantially lower diagnostic performance, and suggested alternate thresholds [51]. First, a threshold score of 4 or greater on the MNSI Questionnaire, such a test result would carry 40% sensitivity and 92% specificity. Second, an MNSI Examination result exceeding a score of 2 carried 61% sensitivity and 79% specificity. However, on inspection of the receiver operating characteristic curves from this publication, we observe that an MNSI Questionnaire score (such as a score of 1 or greater) could reasonably be chosen to maximize sensitivity to nearly 60%.
7.4 Diagnostic Accuracy: Predictive
While many longitudinal designs have been conducted, and even some cohort designs (in which those with neuropathy at baseline have been excluded), we were unable to find in the literature analyses that explored whether a certain test score could predict future onset of diabetic DSP. While this does not make inherent sense for the MNSI examination scores, which measure manifestations of established diabetic DSP (such as presence of ulcer or complete reduction or abnormality—rather than subtle impairments—of vibration or monofilament sensitivity), such a design could be applied to the MNSI Questionnaire that has components that are suited to earlier, pre-diagnostic stages of diabetic DSP.
7.5 Effect on Treatment Decisions, Impact on Patient Outcomes, Economic Analysis
The results of MNSI testing have not been studied on their effect on the treatment decisions made by clinicians, or whether MNSI testing is associated with improved outcomes relative to current standards of screening and diagnosis. However, it has been frequently operationalized as a neuropathy outcome in large-scale clinical trials including those evaluating cardiovascular and microvascular outcomes in type 1 and type 2 diabetes [21, 53,54,55,56]. As many of these studies required a measure of more advanced diabetic foot disease, the MNSI has provided a successful proof of concept for applying neuropathy scales in trials.
8 Toronto Clinical Neuropathy Score (TCNS)
The Toronto Clinical Neuropathy Score (TCNS), first validated and reported in 2002, was developed specifically for use in a study of simple screening measures for DSP that could be used in the family physician’s office or the diabetes clinic [29, 59]. Spectrum bias is a very common design flaw (a source of selection bias) in investigative test research. It represents inappropriate recruitment of many participants for whom there is no diagnostic uncertainty, and recruitment of participants with limited variation in disease characteristic [22]. The original purpose of the TCNS was to create a quantitative score that ranged across all diabetic DSP Stages (Table 1) that would allow for stratified accrual across the full spectrum of neuropathy to appropriately determine the diagnostic performance of simple screening measures [29]. The scale comprises six symptoms (present/absent), knee and ankle reflexes (normal, reduced, or absent scored bilaterally) and sensory tests (normal/abnormal) as shown in Table 3, Panel A and ranges from a perfectly normal score of 0 without clinical evidence of DSP to a maximum abnormal score of 19 points. The symptoms are: foot pain, numbness, tingling, weakness, unsteadiness, weakness, and presence of upper limb symptoms. The sensory signs include sensory tests of pinprick, temperature, light touch, vibration, and proprioception. The deep tendon reflexes include the knee and ankle reflexes. Out of a total score of 19, the grades are defined as follows: 0–5 = no neuropathy; 6–8 = mild neuropathy; 9–11 = moderate neuropathy; ≥ 12 = severe neuropathy.
8.1 Scale Face Validity
The TCNS has attributes that relate specifically to manifestations of small and large fiber dysfunction of polyneuropathy, excluding other components such as foot deformity that raise risk for foot complications. The broad scoring range that includes six points for symptoms and 13 for signs may permit greater sensitivity for earlier stages, though individual test components are assigned binary or three-level ordinal scores (Table 3).
8.2 Test Quality and Reliability
Formal evaluation of reliability reported internal consistency of attributes, and very good to excellent inter- and intra-rater reproducibility [60]. While in opposition to the findings of poor reproducibility of individual physical examination [37], reproducibility is likely greater with investigative tests that make up a composite of multiple individual tests.
8.3 Diagnostic Accuracy: Concurrent
Diagnostic accuracy has been evaluated extensively, including relationship with nerve conduction studies, diabetic DSP classified by signs, symptoms, and nerve conduction studies, and even relative to the results of fiber density on sural nerve biopsies [59, 60]. Furthermore, its validity has been tested in non-diabetic forms of polyneuropathy [61]. In a type 1 diabetes cohort, TCNS had receiver operating characteristic curve area under the curve of 0.86 for identification of Stage 2 diabetic DSP, with an optimal threshold value of 6 or greater identifying DSP with sensitivity and specificity each approximating 80% [51]. Additionally, very good overall accuracy was achieved even for the identification of subclinical Stage 1a neuropathy, with an area under the curve of 0.74 [62]. However, its diagnostic performance in type 2 diabetes, along with objective tests, was lower [52].
8.4 Diagnostic Accuracy: Predictive
A pilot longitudinal study that included baseline TCNS indicated subtle baseline differences in scores between those with future incident diabetic DSP and those who remained neuropathy-free, but it did not appear to have diagnostic predictive validity [63]. Though not conclusive, this implies that the main role of the TCNS, like other scales, is optimized for identification rather than on prediction of future neuropathy incidence.
8.5 Effect on Treatment Decisions, Impact on Patient Outcomes, Economic Analysis
Similar to the MNSI, the TCNS has not been studied for effect on the treatment decisions made by clinicians or screening and diagnosis outcomes, but it has frequently been operationalized as a neuropathy outcome in clinical trials and cohort studies [44, 64,65,66,67,68]. The trials, however, are studies of neuropathy therapies as opposed to the large-scale cardiovascular outcome trials that the MNSI has been used as a neuropathy outcome measure.
9 Modified Toronto Clinical Neuropathy Score (mTCNS)
With the intention of creating a scale that is more sensitive for earlier stages of neuropathy, the TCNS has been adapted to the modified TCNS (mTCNS). The modified scale omits the deep tendon reflex assessment, which was a somewhat less reproducible part of the TCNS [60], and that additionally represented later (motor) dysfunction on the spectrum of diabetic DSP severity. The mTCNS also adds greater quantitative levels to both symptoms and sensory signs to create a larger measurement range across the earlier DSP stages, and a more granular scale. The symptoms of the TCNS are graded according to patient-reported outcome principles as absent (0), present but not interfering with activities or sense of well-being (1), present and interfering with sense of well-being but not with activities (2), and present and interfering with activities (3). The signs of the TCNS are graded as absent (0), present at the toes (1), present up to the ankles (2), and present proximal to the ankles (3) in a stocking distribution. The mTCNS score ranges from 0 to 33, as detailed in Table 3, Panel B.
9.1 Scale Face Validity
The mTCNS has modifications that, compared to the TCNS, exclude motor manifestations that confers a measure of earlier stages of diabetic DSP, and expands the scoring of symptoms and sensory signs to include test components that are assigned four-level ordinal scores each (Table 3, Panel B).
9.2 Test Quality, Reliability, and Accuracy
The very good to excellent inter- and intra-rater reproducibility of the mTCNS was formally evaluated and reported along with a new evaluation of the original TCNS in a multicenter study [60]. Furthermore, as hypothesized, scores shared lower correlation with the TCNS as they measure different stages of diabetic DSP (Pearson coefficient 0.56), but validity for identification of Stage 2 neuropathy remains similar [60]. However, a specific threshold has not been independently assessed for the concurrent validity of diabetic DSP, and also ranges of severity have not been assigned as for the TCNS.
9.3 Effect on Treatment Decisions, Impact on Patient Outcomes, Economic Analysis
Similar to the MNSI and the TCNS, the mTCNS has not been studied for effect on the treatment decisions made by clinicians or screening and diagnosis outcomes, but it has frequently been operationalized as a neuropathy outcome in clinical trials and cohort studies that by design focus on earlier stages of diabetic DSP [69, 70]. Furthermore, it is being implemented in contemporary studies of populations at risk of earlier stages of neuropathy (https://clinicaltrials.gov/ct2/show/NCT04664426).
10 Neuropathy Impairment Score of Lower Limbs (NIS-LL)
The NIS-LL is an extensive composite score of neurological signs and objective tests in the lower limbs, excluding symptoms, that was designed primarily for implementation in clinical trials [71]. This physical examination score was developed by investigators at the Mayo Clinic as a simplification of an even more extensive score, termed the NIS-LL+7 that incorporated the results of seven objective tests including vibration perception testing, heart rate variability with deep breathing as a measure of autonomic dysfunction, and five nerve conduction study parameters. This extensive version is considered to have 100% sensitivity for Stage 1a diabetic DSP [72]. The NIS-LL clinical scale version (excluding the seven additional objective tests) incorporates extensive motor, sensory, and reflex physical examination maneuvers with granular scoring systems. For example, grading of muscle power involves eight discrete levels ranging from 0 to 4, including quarter points. The overall scale is graded from a perfectly normal score of 0 to a maximum of 88 points [71].
10.1 Scale Face Validity
The NIS-LL assigns 64 of the 88 points to muscle strength attributes that are frequently absent when first clinically identified by clinicians, and is therefore at face value a measure of later stage (generally Stage 1b). The potential score for sensory testing is 16/88 points with a maximal loss of sensory modalities of touch pressure, pinprick, vibration (using the 165Hz tuning fork), and joint position. The reflexes at knees and ankles are considered as present, reduced or absent, and defined according to age limits adding potential confusion to non-expert examiners. Both in terms of the later stage diabetic DSP identification and the expertise required for examination, the NIS-LL is not seen as a tool that can be translated to clinical diagnosis, but rather as a specialize tool for interval cohort studies and clinical trials. Given its wide spectrum of motor scores, at face value it might represent a sensitive measure for neuropathic change in clinical trials examining the effect of interventions on more advanced neuropathy stages.
10.2 Test Quality, Reliability, and Accuracy
The NIS-LL has not been evaluated specifically for reproducibility or concurrent validity for a diagnosis of diabetic DSP. However, it was evaluated in a comparative study of neuropathy associated with impaired glucose tolerance subjects and in this setting—as with the other scales evaluated—its overall diagnostic accuracy was excellent [73].
10.3 Effect on Treatment Decisions, Impact on Patient Outcomes, Economic Analysis
Similar to the previous composite scores, the NIS-LL has not been studied for effect on the treatment decisions made by clinicians or screening and diagnosis outcomes as it primarily represents a research tool. However, it has been extensively applied as a neuropathy outcome in clinical trials and cohort studies that by design focus on later stages of diabetic DSP [74,75,76,77].
11 Utah Early Neuropathy Scale
This physical examination scale was developed specifically to serve the need for measurement scales that could detect early stages of sensory-predominant polyneuropathies such as DSP at Stage 1b [78]. The scale includes limited scores for motor function (great toe extension and ankle reflexes), and greater proportional contribution to sensation for pin and vibration as well as allodynia. The scale varies from 0 to 42 with 24/42 points for pin sensation, 8/42 for vibration, and 2/42 for allodynia. To demonstrate an example of a score for earlier sensory manifestations and how it is operationalized, we include the scoring for the Utah Early Neuropathy Scale in Fig. 3.
Performing the Utah Early Neuropathy Scale (UENS) Examination. The UENS requires a number 2 (13/4 inch) safety pin and a 128 Hz tuning fork. Pin sensation is tested by first reviewing normal sharp sensation to pin on an unaffected portion of the skin. Once this is established, touch the dorsal surface of the foot and leg with the pin, working centripetally from the great toe in 1–2 cm increments while asking the subject to respond when they first feel “any sharpness,” and again more proximally when the pin feels “as sharp as they would expect.” Repeat to firmly establish these levels. On each side, two points are scored for each region in which the patient fails to feel any sharpness. One additional point is scored for each additional region in which the pin feels less sharp than expected. Only distal sensory loss is scored. So, for instance, a person who reported absent pin sensation to the mid foot dorsum (four points) and reduced sensation to the low ankle (1 point) bilaterally would score a total of ten points for this portion of the UENS. Vibration is tested by first acquainting the subject with vibration (as opposed to pressure) sensation, then holding the maximally vibrated tuning fork to the dorsum of the great toe at the distal interphalangeal joint. Extinction of vibration in less than 10 s is considered “diminished,” while “absent” requires that the patient cannot detect the maximally vibrating tuning fork at the toe. The motor examination is limited to great toe dorsiflexion. Other aspects are as typically performed in neurological examination. Figure courtesy of A. Gordon Smith, co-creator of the Utah Early Neuropathy Scale. The Scale is further detailed in [78]
11.1 Scale Face Validity
The scale does not include symptoms, and therefore in the context of diabetic DSP would be amenable for use as a screening tool to identify Stage 1b disease. Furthermore, the relative weight of sensory signs is high, including small fiber function, such that variation of scores likely represent earlier-stage neuropathy when interventions and therapies are most likely to be of benefit.
11.2 Test Quality, Reliability, and Accuracy
Reproducibility has not been evaluated cross-sectionally within and between examiners, but the 1-year change in score showed very high consistency and has been reported as an indication of excellent reproducibility [78]. Additionally, it correlated with other scoring systems. For Stage 2 polyneuropathy confirmed by nerve conduction studies, in a study sample with a very high proportion of polyneuropathy participants it showed very good overall concurrent validity by area under the receiver operating characteristics curce [78]. This level of accuracy appeared to be similar to other scales [78], and it has been examined in other causes of polyneuropathy [79, 80]. However, it is our view that this scale may show advantage in study samples that reflect general diabetes practice, with lower prevalence of advanced polyneuropathy and higher prevalence of early disease [81].
11.3 Effect on Treatment Decisions, Impact on Patient Outcomes, Economic Analysis
Similar to the previous composite scores, the Utah Early Neuropathy Scale has not been studied for effect on the treatment decisions made by clinicians or screening and diagnosis outcomes as it primarily represents a research tool. However, it has been extensively applied as a neuropathy outcome in cohort studies and clinical trials that by design focus on earlier stages of diabetic DSP [82,83,84].
12 Neuropathy Symptom Score (NSS)
First described in 1993 in a descriptive study of the prevalence of the hospital-based clinic diabetes population in the United Kingdom [85], the Neuropathy Symptom Score was an interviewer-based questionnaire meant to evaluate the presence and severity of specific polyneuropathy manifestations, with a particular emphasis on painful diabetic DSP. In the interview, patients are asked about their experience of pain or discomfort in the legs. Characteristics are evaluated such that burning, numbness or tingling in the legs provides two points, and fatigue, cramping or aching 1 point. Location provides 2, 1, and 0 points for feet, calves, and elsewhere, respectively. Nocturnal symptoms provide 2 further points, 1 if also daytime, 0 if only daytime. An additional point was assigned if patients are awoken at night from symptoms. Finally, alleviating factors are scored: 2, 1, and 0 if alleviated by walking, standing, or laying down. With a maximum of 9, it is implied that symptoms are present if a minimum score of 3 is achieved. Severity was graded as mild (score of 3–4), moderate (score of 5–6), or severe (score of 7–9).
12.1 Face Validity
The score, at face value, weighs heavily on painful diabetic DSP and its consequences, including night-time symptoms and awakening, and the alleviating symptoms in the context of such awakening. Consequently, its primary role is in the classification of the presence and severity of painful diabetic DSP.
12.2 Test Quality and Reliability
While not tested formally for reproducibility, the NSS has served as a historical benchmark for the development of updated symptom scores that correlate highly with it, and that themselves have excellent reproducibility in small studies [86].
12.3 Diagnostic Accuracy: Concurrent
During the development of the NSS, studies of its intrinsic validity were not conducted. However, it has been evaluated by independent groups primarily in combination with a physical examination scale termed the Neuropathy Disability Scale that is discussed below [87, 88].
13 The Revised Neuropathy Disability Score (NDS)
Developed as a simplification of the NIS-LL and also first described in 1993 along with the Neuropathy Symptom Score in a descriptive study of neuropathy prevalence, the score incorporates small fiber sensory attribute, and large fiber sensory and motor attributes. It involves examination of the ankle reflex, vibration, pin-prick and temperature (cold tuning fork) sensation at the great toe. The sensory modalities are scored as either present = 0 or reduced/absent = 1 for each side, and reflexes as normal = 0, present with reinforcement = 1 or absent = 2 per side. With a maximum abnormal score of 10, classification of severity is graded as mild (score of 3–5), moderate (score of 6–8), and severe (score of 9 or 10). Generally, a score of 6 or more has been used to define presence of signs for identification of DSP [89, 90].
13.1 Face Validity
This score includes small fiber sensory attributes that could identify pre-symptomatic or symptomatic early-stage DSP, as well as later large-fiber stages owing to sampling of vibration sensation and reflexes.
13.2 Test Quality and Reliability
There are no published studies examining intra- and inter-rater reproducibility of the revised NDS.
13.3 Diagnostic Accuracy: Concurrent
During the development of the revised NDS, studies of its intrinsic validity were not conducted. However, it has been partially evaluated by independent groups [87, 88]. While there are no cross-sectional studies evaluating the optimal threshold for the identification of diabetic DSP, a score of 6 or more has traditionally been used in studies in which it is the reference standard [89, 90].
13.4 Diagnostic Accuracy: Predictive
While many longitudinal designs have been conducted, we were unable to find in the literature analyses that explored whether a certain test score in those without DSP could predict future onset of diabetic DSP. However, among those followed in a diabetic foot clinic (and likely had presence of diabetic DSP), the revised NDS was generally associated with short-term risk of incident ulceration, though in this study population the best prediction was associated with abnormality in foot pressure sensation measurement [91]. In combination with the NSS, the revised NDS has been used by several research groups for the identification of higher risk patients for diabetic DSP, and as the clinical sign and symptom measures to define presence of diabetic DSP [92, 93].
14 Perspectives on the Comparison of Scales
Each of the scales chosen for review in this chapter has been designed with specific research purposes in mind. At face value they reflect different aspects of diabetic DSP (from early sensory signs in the Utah Early Neuropathy Scale, to those that include advanced foot deformity and ulceration like the Michigan Neuropathy Screening Instrument Examination). As summarized in the above sections and in Table 4, they have different levels of research published on the hierarchy of investigative test research principles, studies of validity include different study samples, study sizes, prevalence of neuropathy, spectrum of disease, variable reference standard definitions. Even with harmonization of such procedures, systematic review and meta-analysis of diagnostic tests are complex as they require Bayesian techniques like clinical trial network metanalysis methods [95]. Though we present their attributes and critical appraisal in detail, for these methodological reasons we intentionally do not present a quantitative side-by-side comparison of their diagnostic operating characteristics. One research group has undertaken a direct comparison of many of these scales in the setting of pre-diabetic neuropathy and, while they imply that some are more valid than others (the modified TCNS), uniformly in that study most scales had unexpectedly high levels of concurrent diagnostic accuracy [73]. We challenge clinicians to consider operationalizing these scales in clinical setting to suit specific needs: For example, the self-administered Michigan Neuropathy Screening Instrument questionnaire, the Neuropathy Symptom Scale, or the symptom subscales of other composite scores like the modified Toronto Clinical Neuropathy Score, can be applied for virtual diabetes care as has been required during the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic, while others could be selected for physical examination screening and evaluation in the clinical setting.
15 Differential Diagnosis: A Diagnosis of Diabetic DSP Requires Consideration of Other Causes of Polyneuropathy
A clinical evaluation consistent with the features of diabetic DSP requires consideration of other etiologies for distal and symmetric polyneuropathies. First, the presence of atypical features listed in Table 2 should be identified, as they may further indicate other causes of peripheral nerve damage other than polyneuropathy, such as focal, multifocal, or nonlength dependent forms of neuropathies. These include focal nerve entrapments and other non-distal and non-symmetric forms (chapters “The Epidemiology of Diabetic Neuropathy” and “Treatment Induced Neuropathy of Diabetes (TIND)”), rare conditions such as hereditary neuropathy with propensity to pressure palsies (HNPP), and polyneuropathies without sensory predominance such as common forms of chronic inflammatory demyelinating polyneuropathy (CIDP) and its variants. Furthermore, consideration should be made for factors associated with potentially similar polyneuropathy to diabetic DSP such as alcohol, hypothyroidism, toxins, and drugs. With this in mind, a simple laboratory panel should be undertaken: comprehensive metabolic panel to determine current metabolic risk factors such as glycemic exposure, presence of hypertriglyceridemia, renal dysfunction; thyroid hormone to indicate hypothyroidism; complete blood cell count may indicate macrocytosis associated with alcohol or nutritional deficiencies and infectious causes; the B12 level itself; erythrocyte sedimentation rate potentially associated with inflammatory causes; and serum protein immune-electrophoresis to suggest malignancy, monoclonal gammopathy, or gammopathy associated with rare syndromes like Polyneuropathy, Organomegaly, Endocrinopathies, Monoclonal protein, and Skin lesions (including hemangioma, hyperpigmentation, hypertrichosis or Raynaud’s phenomenon as part of the “POEMS” Syndrome). Testing for infectious causes of polyneuropathy should be guided by history, as would consideration of familial forms of DSP. It is important for the clinician to recognize that the patient with a consistent chronic, insidious, sensory-predominant distal and symmetric clinical history and physical examination for diabetic DSP typically only requires this focused laboratory work-up, and that the most common causes of DSP other than diabetes are B12 deficiency, alcohol, and hypothyroidism, and diabetic DSP exacerbated by renal failure. Referral to specialist neurology should be strongly considered in the uncommon case of atypical features (Table 2), or if rare causes are suspected (Table 5) that require confirmatory work-up that specialized testing might clarify (chapter “Diagnostic Techniques for Diabetic Peripheral Neuropathy”).
16 Concluding Overview
The clinical diagnosis of diabetic DSP, initiated either by a screening test or a symptomatic complaint, requires careful consideration of the historical features, symptoms, and signs, where each finding revises for the clinician an estimate of disease probability. Composite scores, reviewed in detail in the chapter, help to remind the clinician of key supportive symptoms and signs for diagnosis and are commonly used in cohort studies and clinical trials. Even if the clinical evaluation is consistent with typical features of diabetic DSP, simple laboratory tests are likely to identify the presence of other causes. It is essential that the clinician identify atypical features (listed in Table 2) or the suspicion of other causes (listed in Table 5) to seek confirmatory tests and expert opinion through neurology specialist referral.
References
Pop-Busui R, Boulton AJ, Feldman EL, et al. Diabetic neuropathy: a position statement by the American Diabetes Association. Diabetes Care. 2017;40(1):136–54. https://doi.org/10.2337/dc16-2042.
Manzano GM, Giuliano LM, Nobrega JA. A brief historical note on the classification of nerve fibers. Arq Neuropsiquiatr. 2008;66(1):117–9. https://doi.org/10.1590/s0004-282x2008000100033.
Carmichael J, Fadavi H, Ishibashi F, Shore AC, Tavakoli M. Advances in screening, early diagnosis and accurate staging of diabetic neuropathy. Front Endocrinol. 2021;12:671257. https://doi.org/10.3389/fendo.2021.671257.
Wukich DK, Raspovic KM, Suder NC. Patients with diabetic foot disease fear major lower-extremity amputation more than death. Foot Ankle Spec. 2018;11(1):17–21. (comparative study) (in eng). https://doi.org/10.1177/1938640017694722.
Gregg EW, Li Y, Wang J, et al. Changes in diabetes-related complications in the United States, 1990-2010. N Engl J Med. 2014;370(16):1514–23. (Research support, U.S. Gov’t, P.H.S.) (in eng). https://doi.org/10.1056/NEJMoa1310799.
Geiss LS, Li Y, Hora I, Albright A, Rolka D, Gregg EW. Resurgence of diabetes-related nontraumatic lower-extremity amputation in the young and middle-aged adult U.S. population. Diabetes Care. 2019;42(1):50–4. (In eng). https://doi.org/10.2337/dc18-1380.
Hussain MA, Al-Omran M, Salata K, et al. Population-based secular trends in lower-extremity amputation for diabetes and peripheral artery disease. CMAJ. 2019;191(35):955–61. https://doi.org/10.1503/cmaj.190134.
Perkins BA, Orszag A, Ngo M, Ng E, New P, Bril V. Prediction of incident diabetic neuropathy using the monofilament examination: a 4-year prospective study. Diabetes Care. 2010;33(7):1549–54. https://doi.org/10.2337/dc09-1835.
Dyck PJ, Albers JW, Andersen H, et al. Diabetic polyneuropathies: update on research definition, diagnostic criteria and estimation of severity. Diabetes Metab Res Rev. 2011;27(7):620–8. https://doi.org/10.1002/dmrr.1226.
Tesfaye S, Stevens LK, Stephenson JM, et al. Prevalence of diabetic peripheral neuropathy and its relation to glycaemic control and potential risk factors: the EURODIAB IDDM Complications Study. Diabetologia. 1996;39(11):1377–84. https://doi.org/10.1007/s001250050586.
Feldman EL, Callaghan BC, Pop-Busui R, et al. Diabetic neuropathy. Nat Rev Dis Primers. 2019;5(1):41. https://doi.org/10.1038/s41572-019-0092-1.
Andersen ST, Witte DR, Dalsgaard EM, et al. Risk factors for incident diabetic polyneuropathy in a cohort with screen-detected type 2 diabetes followed for 13 years: ADDITION-Denmark. Diabetes Care. 2018;41(5):1068–75. https://doi.org/10.2337/dc17-2062.
Tesfaye S, Chaturvedi N, Eaton SE, et al. Vascular risk factors and diabetic neuropathy. N Engl J Med. 2005;352(4):341–50. https://doi.org/10.1056/NEJMoa032782.
Callaghan BC, Gao L, Li Y, et al. Diabetes and obesity are the main metabolic drivers of peripheral neuropathy. Ann Clin Transl Neurol. 2018;5(4):397–405. https://doi.org/10.1002/acn3.531.
Prabodha LBL, Sirisena ND, Dissanayake VHW. Susceptible and prognostic genetic factors associated with diabetic peripheral neuropathy: a comprehensive literature review. Int J Endocrinol. 2018;2018:8641942. https://doi.org/10.1155/2018/8641942.
Lu B, Hu J, Wen J, et al. Determination of peripheral neuropathy prevalence and associated factors in Chinese subjects with diabetes and pre-diabetes - ShangHai Diabetic neuRopathy Epidemiology and Molecular Genetics Study (SH-DREAMS). PLoS One. 2013;8(4):e61053. https://doi.org/10.1371/journal.pone.0061053.
Kirthi V, Perumbalath A, Brown E, et al. Prevalence of peripheral neuropathy in pre-diabetes: a systematic review. BMJ Open Diabetes Res Care. 2021;9(1):40. https://doi.org/10.1136/bmjdrc-2020-002040.
Kraiwong R, Vongsirinavarat M, Hiengkaew V, von Heideken WP. Effect of sensory impairment on balance performance and lower limb muscle strength in older adults with type 2 diabetes. Ann Rehabil Med. 2019;43(4):497–508. https://doi.org/10.5535/arm.2019.43.4.497.
Callaghan BC, Price RS, Feldman EL. Distal symmetric polyneuropathy in 2020. JAMA. 2020;324(1):90–1. https://doi.org/10.1001/jama.2020.0700.
Maltese G, Tan SV, Bruno E, Brackenridge A, Thomas S. Peripheral neuropathy in diabetes: it’s not always what it looks like. Diabet Med. 2018;35(10):1457–9. https://doi.org/10.1111/dme.13701.
Martin CL, Albers JW, Pop-Busui R. Neuropathy and related findings in the diabetes control and complications trial/epidemiology of diabetes interventions and complications study. Diabetes Care. 2014;37(1):31–8. https://doi.org/10.2337/dc13-2114.
Scott IA, Greenberg PB, Poole PJ. Cautionary tales in the clinical interpretation of studies of diagnostic tests. Intern Med J. 2008;38(2):120–9. https://doi.org/10.1111/j.1445-5994.2007.01436.x.
Dobrow MJ, Hagens V, Chafe R, Sullivan T, Rabeneck L. Consolidated principles for screening based on a systematic review and consensus process. CMAJ. 2018;190(14):422–9. https://doi.org/10.1503/cmaj.171154.
Bril V, Breiner A, Perkins BA, Zochodne D, Diabetes Canada Clinical Practice Guidelines Expert C. Neuropathy. Can J Diabetes. 2018;42(1):217–21. https://doi.org/10.1016/j.jcjd.2017.10.028.
Perez-Panero AJ, Ruiz-Munoz M, Cuesta-Vargas AI, Gonzalez-Sanchez M. Prevention, assessment, diagnosis and management of diabetic foot based on clinical practice guidelines: a systematic review. Medicine. 2019;98(35):e16877. https://doi.org/10.1097/MD.0000000000016877.
Embil JM, Albalawi Z, Bowering K, Trepman E, Diabetes Canada Clinical Practice Guidelines Expert C. Foot care. Can J Diabetes. 2018;42(1):S222–7. (In eng). https://doi.org/10.1016/j.jcjd.2017.10.020.
Sharma S, Kerry C, Atkins H, Rayman G. The Ipswich Touch Test: a simple and novel method to screen patients with diabetes at home for increased risk of foot ulceration. Diabet Med. 2014;31(9):1100–3. (Randomized Controlled Trial Research Support, Non-U.S. Gov’t Validation Studies) (In eng). https://doi.org/10.1111/dme.12450.
Kanji JN, Anglin RE, Hunt DL, Panju A. Does this patient with diabetes have large-fiber peripheral neuropathy? JAMA. 2010;303(15):1526–32. https://doi.org/10.1001/jama.2010.428.
Perkins BA, Olaleye D, Zinman B, Bril V. Simple screening tests for peripheral neuropathy in the diabetes clinic. Diabetes Care. 2001;24(2):250–6. https://doi.org/10.2337/diacare.24.2.250.
Bruce A, LEL P, Lewis EJH, Bril V, Ferdousi M, Orszag A, Edwards K, Pritchard N, Russell A, Dehghani C, Pacaud D, Romanchuk K, Mah JK, Jeziorska M, Marshall A, Shtein RM, Pop-Busui R, Lentz SI, Tavakoli M, Boulton AJM, Efron N, Malik RA. Corneal confocal microscopy predicts the development of diabetic neuropathy: a longitudinal diagnostic multinational consortium study. Diabetes Care. 2021;44(9):2107–14.
Kirk JK, Hunter JC, Mihalko SL, Danhauer SC, Shumaker SA. Perspectives of pain in patients with type 2 diabetes. Expert Rev Endocrinol Metab. 2019;14(3):215–9. https://doi.org/10.1080/17446651.2019.1592674.
Bai JW, Lovblom LE, Cardinez M, et al. Neuropathy and presence of emotional distress and depression in longstanding diabetes: results from the Canadian study of longevity in type 1 diabetes. J Diabetes Complicat. 2017;31(8):1318–24. https://doi.org/10.1016/j.jdiacomp.2017.05.002.
Callaghan BC, Cheng HT, Stables CL, Smith AL, Feldman EL. Diabetic neuropathy: clinical manifestations and current treatments. Lancet Neurol. 2012;11(6):521–34. https://doi.org/10.1016/S1474-4422(12)70065-0.
Bracewell N, Game F, Jeffcoate W, Scammell BE. Clinical evaluation of a new device in the assessment of peripheral sensory neuropathy in diabetes. Diabet Med. 2012;29(12):1553–5. https://doi.org/10.1111/j.1464-5491.2012.03729.x.
Nather A, Keng Lin W, Aziz Z, Ong C, McFeng B. Assessment of sensory neuropathy in patients with diabetic foot problems. Diabetes Foot Ankle. 2011;2:6367. https://doi.org/10.3402/dfa.v2i0.6367.
Gylfadottir SS, Weeracharoenkul D, Andersen ST, Niruthisard S, Suwanwalaikorn S, Jensen TS. Painful and non-painful diabetic polyneuropathy: clinical characteristics and diagnostic issues. J Diabetes Investig. 2019;10(5):1148–57. https://doi.org/10.1111/jdi.13105.
Dyck PJ, Overland CJ, Low PA, et al. Signs and symptoms versus nerve conduction studies to diagnose diabetic sensorimotor polyneuropathy: Cl vs. NPhys trial. Muscle Nerve. 2010;42(2):157–64. https://doi.org/10.1002/mus.21661.
Zografou I, Iliadis F, Sambanis C, Didangelos T. Validation of neuropad in the assessment of peripheral diabetic neuropathy in patients with diabetes mellitus versus the michigan neuropathy screening instrument, 10g monofilament application and biothesiometer measurement. Curr Vasc Pharmacol. 2020;18(5):517–22. https://doi.org/10.2174/1570161117666190723155324.
Rodriguez-Sanchez B, Pena-Longobardo LM, Sinclair AJ. Cost-effectiveness analysis of the Neuropad device as a screening tool for early diabetic peripheral neuropathy. Eur J Health Econ. 2020;21(3):335–49. https://doi.org/10.1007/s10198-019-01134-2.
Ang L, Jaiswal M, Callaghan B, Raffel D, Pop-Busui R. Sudomotor dysfunction as a measure of small fiber neuropathy in type 1 diabetes. Auton Neurosci. 2017;205:87–92. https://doi.org/10.1016/j.autneu.2017.03.001.
Novak P. Electrochemical skin conductance: a systematic review. Clin Auton Res. 2019;29(1):17–29. https://doi.org/10.1007/s10286-017-0467-x.
Rajan S, Campagnolo M, Callaghan B, Gibbons CH. Sudomotor function testing by electrochemical skin conductance: does it really measure sudomotor function? Clin Auton Res. 2019;29(1):31–9. https://doi.org/10.1007/s10286-018-0540-0.
Vinik AI, Casellini CM, Parson HK. Electrochemical skin conductance to measure sudomotor function: the importance of not misinterpreting the evidence. Clin Auton Res. 2019;29(1):13–5. https://doi.org/10.1007/s10286-018-0562-7.
Binns-Hall O, Selvarajah D, Sanger D, Walker J, Scott A, Tesfaye S. One-stop microvascular screening service: an effective model for the early detection of diabetic peripheral neuropathy and the high-risk foot. Diabet Med. 2018;35(7):887–94. https://doi.org/10.1111/dme.13630.
Shibata Y, Himeno T, Kamiya T, et al. Validity and reliability of a point-of-care nerve conduction device in diabetes patients. J Diabetes Investig. 2019;10(5):1291–8. https://doi.org/10.1111/jdi.13007.
Scarr D, Lovblom LE, Cardinez N, et al. Validity of a point-of-care nerve conduction device for polyneuropathy identification in older adults with diabetes: results from the Canadian study of longevity in type 1 diabetes. PLoS One. 2018;13(4):e0196647. https://doi.org/10.1371/journal.pone.0196647.
Lee JA, Halpern EM, Lovblom LE, Yeung E, Bril V, Perkins BA. Reliability and validity of a point-of-care sural nerve conduction device for identification of diabetic neuropathy. PLoS One. 2014;9(1):e86515. https://doi.org/10.1371/journal.pone.0086515.
Perkins BA, Orszag A, Grewal J, Ng E, Ngo M, Bril V. Multi-site testing with a point-of-care nerve conduction device can be used in an algorithm to diagnose diabetic sensorimotor polyneuropathy. Diabetes Care. 2008;31(3):522–4. https://doi.org/10.2337/dc07-1227.
Poulose S, Cheriyan E, Poulose A, Cheriyan R, Vadakkanezath B, Ziemer P. Usefulness of the NC-stat DPNCheck nerve conduction test in a community pharmacy as an educational tool for patients with diabetes. Can Pharm J. 2015;148(1):17–20. https://doi.org/10.1177/1715163514561055.
Zhou X, Obuchowski NA, McClish DK. Basic concepts and methods, introduction. In: Statistical methods in diagnostic medicine. 2nd ed. Hoboken: Wiley; 2011. p. 1–11.
Herman WH, Pop-Busui R, Braffett BH, et al. Use of the Michigan Neuropathy Screening Instrument as a measure of distal symmetrical peripheral neuropathy in type 1 diabetes: results from the diabetes control and complications trial/epidemiology of diabetes interventions and complications. Diabet Med. 2012;29(7):937–44. https://doi.org/10.1111/j.1464-5491.2012.03644.x.
Feldman EL, Stevens MJ, Thomas PK, Brown MB, Canal N, Greene DA. A practical two-step quantitative clinical and electrophysiological assessment for the diagnosis and staging of diabetic neuropathy. Diabetes Care. 1994;17(11):1281–9. https://doi.org/10.2337/diacare.17.11.1281.
Seferovic JP, Pfeffer MA, Claggett B, et al. Three-question set from Michigan Neuropathy Screening Instrument adds independent prognostic information on cardiovascular outcomes: analysis of ALTITUDE trial. Diabetologia. 2018;61(3):581–8. https://doi.org/10.1007/s00125-017-4485-y.
Mather KJ, Bebu I, Baker C, et al. Prevalence of microvascular and macrovascular disease in the glycemia reduction approaches in diabetes - a comparative effectiveness (GRADE) study cohort. Diabetes Res Clin Pract. 2020;165:108235. https://doi.org/10.1016/j.diabres.2020.108235.
Hansen CS, Jensen TM, Jensen JS, et al. The role of serum methylglyoxal on diabetic peripheral and cardiovascular autonomic neuropathy: the ADDITION Denmark study. Diabet Med. 2015;32(6):778–85. https://doi.org/10.1111/dme.12753.
Pop-Busui R, Lu J, Brooks MM, et al. Impact of glycemic control strategies on the progression of diabetic peripheral neuropathy in the bypass angioplasty revascularization investigation 2 diabetes (BARI 2D) cohort. Diabetes Care. 2013;36(10):3208–15. https://doi.org/10.2337/dc13-0012.
Barbosa M, Saavedra A, Severo M, Maier C, Carvalho D. Validation and reliability of the Portuguese version of the Michigan neuropathy screening instrument. Pain Pract. 2017;17(4):514–21. https://doi.org/10.1111/papr.12479.
Bax G, Fagherazzi C, Piarulli F, Nicolucci A, Fedele D. Reproducibility of Michigan neuropathy screening instrument (MNSI). A comparison with tests using the vibratory and thermal perception thresholds. Diabetes Care. 1996;19(8):904–5. https://doi.org/10.2337/diacare.19.8.904.
Bril V, Perkins BA. Validation of the Toronto clinical scoring system for diabetic polyneuropathy. Diabetes Care. 2002;25(11):2048–52. https://doi.org/10.2337/diacare.25.11.2048.
Bril V, Tomioka S, Buchanan RA, Perkins BA. Reliability and validity of the modified Toronto clinical neuropathy score in diabetic sensorimotor polyneuropathy. Diabet Med. 2009;26(3):240–6. https://doi.org/10.1111/j.1464-5491.2009.02667.x.
Abraham A, Barnett C, Katzberg HD, Lovblom LE, Perkins BA, Bril V. Toronto clinical neuropathy score is valid for a wide spectrum of polyneuropathies. Eur J Neurol. 2018;25(3):484–90. https://doi.org/10.1111/ene.13533.
Lysy Z, Lovblom LE, Halpern EM, et al. Measurement of cooling detection thresholds for identification of diabetic sensorimotor polyneuropathy in type 1 diabetes. PLoS One. 2014;9(9):e106995. https://doi.org/10.1371/journal.pone.0106995.
Lovblom LE, Halpern EM, Wu T, et al. In vivo corneal confocal microscopy and prediction of future-incident neuropathy in type 1 diabetes: a preliminary longitudinal analysis. Can J Diabetes. 2015;39(5):390–7. https://doi.org/10.1016/j.jcjd.2015.02.006.
Lewis EJH, Perkins BA, Lovblom LE, Bazinet RP, Wolever TMS, Bril V. Effect of omega-3 supplementation on neuropathy in type 1 diabetes: A 12-month pilot trial. Neurology. 2017;88(24):2294–301. https://doi.org/10.1212/WNL.0000000000004033.
Sekiguchi K, Kohara N, Baba M, et al. Aldose reductase inhibitor ranirestat significantly improves nerve conduction velocity in diabetic polyneuropathy: a randomized double-blind placebo-controlled study in Japan. J Diabetes Investig. 2019;10(2):466–74. https://doi.org/10.1111/jdi.12890.
Zinman LH, Ngo M, Ng ET, Nwe KT, Gogov S, Bril V. Low-intensity laser therapy for painful symptoms of diabetic sensorimotor polyneuropathy: a controlled trial. Diabetes Care. 2004;27(4):921–4. https://doi.org/10.2337/diacare.27.4.921.
Abraham A, Katzberg HD, Lovblom LE, Perkins BA, Bril V. Uric acid levels correlate with sensory nerve function in healthy subjects. Can J Neurol Sci. 2019;46(3):337–41. https://doi.org/10.1017/cjn.2019.9.
Dunnigan SK, Ebadi H, Breiner A, et al. The characteristics of chronic inflammatory demyelinating polyneuropathy in patients with and without diabetes–an observational study. PLoS One. 2014;9(2):e89344. https://doi.org/10.1371/journal.pone.0089344.
Ghavami H, Radfar M, Soheily S, Shamsi SA, Khalkhali HR. Effect of lifestyle interventions on diabetic peripheral neuropathy in patients with type 2 diabetes, result of a randomized clinical trial. Agri. 2018;30(4):165–70. https://doi.org/10.5505/agri.2018.45477.
Wahren J, Foyt H, Daniels M, Arezzo JC. Long-acting C-peptide and neuropathy in type 1 diabetes: a 12-month clinical trial. Diabetes Care. 2016;39(4):596–602. https://doi.org/10.2337/dc15-2068.
Bril V. NIS-LL: the primary measurement scale for clinical trial endpoints in diabetic peripheral neuropathy. Eur Neurol. 1999;41(Suppl 1):8–13. https://doi.org/10.1159/000052074.
Dyck PJ, Davies JL, Litchy WJ, O'Brien PC. Longitudinal assessment of diabetic polyneuropathy using a composite score in the rochester diabetic neuropathy study cohort. Neurology. 1997;49(1):229–39. https://doi.org/10.1212/wnl.49.1.229.
Zilliox LA, Ruby SK, Singh S, Zhan M, Russell JW. Clinical neuropathy scales in neuropathy associated with impaired glucose tolerance. J Diabetes Complicat. 2015;29(3):372–7. https://doi.org/10.1016/j.jdiacomp.2015.01.011.
Lin X, Yarlas A, Vera-Llonch M, et al. Rate of neuropathic progression in hereditary transthyretin amyloidosis with polyneuropathy and other peripheral neuropathies: a systematic review and meta-analysis. BMC Neurol. 2021;21(1):70. https://doi.org/10.1186/s12883-021-02094-y.
Ziegler D, Edmundson S, Gurieva I, Mankovsky B, Papanas N, Strokov I. Predictors of response to treatment with actovegin for 6 months in patients with type 2 diabetes and symptomatic polyneuropathy. J Diabetes Complicat. 2017;31(7):1181–7. https://doi.org/10.1016/j.jdiacomp.2017.03.012.
Ziegler D, Low PA, Freeman R, Tritschler H, Vinik AI. Predictors of improvement and progression of diabetic polyneuropathy following treatment with alpha-lipoic acid for 4 years in the NATHAN 1 trial. J Diabetes Complicat. 2016;30(2):350–6. https://doi.org/10.1016/j.jdiacomp.2015.10.018.
Tesfaye S, Tandan R, Bastyr EJ, et al. Factors that impact symptomatic diabetic peripheral neuropathy in placebo-administered patients from two 1-year clinical trials. Diabetes Care. 2007;30(10):2626–32. https://doi.org/10.2337/dc07-0608.
Singleton JR, Bixby B, Russell JW, et al. The Utah Early Neuropathy Scale: a sensitive clinical scale for early sensory predominant neuropathy. J Peripher Nerv Syst. 2008;13(3):218–27. https://doi.org/10.1111/j.1529-8027.2008.00180.x.
Boger MS, Hulgan T, Haas DW, et al. Measures of small-fiber neuropathy in HIV infection. Auton Neurosci. 2012;169(1):56–61. https://doi.org/10.1016/j.autneu.2012.04.001.
Andreasson M, Lagali N, Badian RA, et al. Parkinson’s disease with restless legs syndrome-an in vivo corneal confocal microscopy study. NPJ Parkinsons Dis. 2021;7(1):4. https://doi.org/10.1038/s41531-020-00148-5.
Fernandez-Torres R, Ruiz-Munoz M, Perez-Panero AJ, Garcia-Romero JC, Gonzalez-Sanchez M. Clinician assessment tools for patients with diabetic foot disease: a systematic review. J Clin Med. 2020;9(5):487. https://doi.org/10.3390/jcm9051487.
Smith AG, Singleton JR. Obesity and hyperlipidemia are risk factors for early diabetic neuropathy. J Diabetes Complicat. 2013;27(5):436–42. https://doi.org/10.1016/j.jdiacomp.2013.04.003.
Smith AG, Lessard M, Reyna S, Doudova M, Singleton JR. The diagnostic utility of Sudoscan for distal symmetric peripheral neuropathy. J Diabetes Complicat. 2014;28(4):511–6. https://doi.org/10.1016/j.jdiacomp.2014.02.013.
Gonzalez-Duarte A, Lem M, Diaz-Diaz E, Castillo C, Cardenas-Soto K. The efficacy of pregabalin in the treatment of prediabetic neuropathic pain. Clin J Pain. 2016;32(11):927–32. https://doi.org/10.1097/AJP.0000000000000339.
Young MJ, Boulton AJ, MacLeod AF, Williams DR, Sonksen PH. A multicentre study of the prevalence of diabetic peripheral neuropathy in the United Kingdom hospital clinic population. Diabetologia. 1993;36(2):150–4. https://doi.org/10.1007/BF00400697.
Meijer JW, Smit AJ, Sonderen EV, Groothoff JW, Eisma WH, Links TP. Symptom scoring systems to diagnose distal polyneuropathy in diabetes: the Diabetic Neuropathy Symptom score. Diabet Med. 2002;19(11):962–5. https://doi.org/10.1046/j.1464-5491.2002.00819.x.
Asad A, Hameed MA, Khan UA, Ahmed N, Butt MU. Reliability of the neurological scores for assessment of sensorimotor neuropathy in type 2 diabetics. J Pak Med Assoc. 2010;60(3):166–70.
Jia WP, Shen Q, Bao YQ, Lu JX, Li M, Xiang KS. Evaluation of the four simple methods in the diagnosis of diabetic peripheral neuropathy. Zhonghua Yi Xue Za Zhi. 2006;86(38):2707–10.
Abbott CA, Carrington AL, Ashe H, et al. The North-West Diabetes Foot Care Study: incidence of, and risk factors for, new diabetic foot ulceration in a community-based patient cohort. Diabet Med. 2002;19(5):377–84. https://doi.org/10.1046/j.1464-5491.2002.00698.x.
Weintrob N, Amitay I, Lilos P, Shalitin S, Lazar L, Josefsberg Z. Bedside neuropathy disability score compared to quantitative sensory testing for measurement of diabetic neuropathy in children, adolescents, and young adults with type 1 diabetes. J Diabetes Complicat. 2007;21(1):13–9. https://doi.org/10.1016/j.jdiacomp.2005.11.002.
Pham H, Armstrong DG, Harvey C, Harkless LB, Giurini JM, Veves A. Screening techniques to identify people at high risk for diabetic foot ulceration: a prospective multicenter trial. Diabetes Care. 2000;23(5):606–11. https://doi.org/10.2337/diacare.23.5.606.
Dhage S, Ferdousi M, Adam S, et al. Corneal confocal microscopy identifies small fibre damage and progression of diabetic neuropathy. Sci Rep. 2021;11(1):1859. https://doi.org/10.1038/s41598-021-81302-8.
Ferdousi M, Kalteniece A, Azmi S, et al. Corneal confocal microscopy compared with quantitative sensory testing and nerve conduction for diagnosing and stratifying the severity of diabetic peripheral neuropathy. BMJ Open Diabetes Res Care. 2020;8(2):e001801. https://doi.org/10.1136/bmjdrc-2020-001801.
Cornblath DR, Chaudhry V, Carter K, et al. Total neuropathy score: validation and reliability study. Neurology. 1999;53(8):1660–4. https://doi.org/10.1212/wnl.53.8.1660.
Menten J, Lesaffre E. A general framework for comparative Bayesian meta-analysis of diagnostic studies. BMC Med Res Methodol. 2015;15:70. https://doi.org/10.1186/s12874-015-0061-7.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Perkins, B.A., Bril, V. (2023). Clinical Diagnosis of Diabetic Peripheral Neuropathy. In: Tesfaye, S., Gibbons, C.H., Malik, R.A., Veves, A. (eds) Diabetic Neuropathy. Contemporary Diabetes. Humana, Cham. https://doi.org/10.1007/978-3-031-15613-7_5
Download citation
DOI: https://doi.org/10.1007/978-3-031-15613-7_5
Published:
Publisher Name: Humana, Cham
Print ISBN: 978-3-031-15612-0
Online ISBN: 978-3-031-15613-7
eBook Packages: MedicineMedicine (R0)