Abstract
In recent years, there have been numerous breakthroughs in the treatment of diseases in aquaculture. Progressive developments in nanotechnology have unlocked new approaches to producing more potent vaccines. Nanovaccines are a new generation of vaccines using nanoparticles as carriers or adjuvants. Various antigens can be encapsulated or adsorbed on the surface of these nanoparticles for administration. The nanoformulations can be prepared with several materials such as poly lactic-co-glycolic acid (PLGA), chitosan, gold nanoparticles, liposomes, and more of the same, with each one of them having its advantages and disadvantages. Nanovaccines have several benefits over conventional vaccines. The nanoparticles serve as a potential vehicle for vaccine delivery. In aquaculture, oral administration is a more convenient and relatively more economical way to achieve antigen delivery since it eliminates the laborious and tedious injection technique. For this purpose, nanoparticles protect the antigens from degradative enzymes and provide a sustained release. Due to the smaller size of the particle, it can be very efficient at triggering cellular and humoral immune responses. This chapter aims to provide an insight into the promising possibilities of using nanotechnology to reform aquaculture healthcare. The introduction of emerging technologies is expected to accelerate the production of appropriate nanoparticles for pharmaceutical applications. Despite their widespread use, there are questions about a few nanoparticles that exhibit varying degrees of toxicity.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
3.1 Introduction
Disease prevention by vaccination is one of the landmarks of modern medicine. Vaccination is a method of producing an active immune response in an organism against a targeted pathogen. It is one of the most influential and sustainable methods to treat bacterial and viral diseases in humans as well as in veterinary medicine. Aquaculture produces ∼80 million tonnes of aquatic animals with a first-sale value of $232 billion. It represents the fastest-growing animal production sector in the world, making aquaculture the fastest primary food-producing sector globally. However, the major hindrance faced in the industry is the incidence of diseases, which amounts to more than $10 billion in losses annually on a global scale (http://www.fao.org/3/i9540en/I9540EN.pdf). The disease outbreaks may be due to various pathogenic organisms like viruses, bacteria, fungi, and parasites, which are generally tackled with the help of chemical agents, antibiotics, and vaccines. Lately, nanotechnology is being applied in many fields of science, including vaccine development. Nanotechnology refers to the study and use of structures between 1 and 100 nm in size. The technology has been applied and is undergoing clinical trials to deliver many therapeutics such as antibiotics and prophylactic treatments. The increasing significance of nanotechnology-based drug delivery systems has been an essential aspect of researching and developing various novel vaccine formulations.
Nanovaccines are a new generation of vaccines consisting of nanoparticles (NPs) and a pathogen-specific antigen that elicit a controlled immune response. Conventionally, in aquaculture, the antigens used in the vaccine formulations are peptides, nucleic acids, toxoids, and other biomolecules. But due to the selective permeability of the cell membranes, most of these biological macromolecules cannot enter the cells to activate an effective immune response. As they have a large surface-to-volume ratio, nanoparticles can overcome this limitation and enhance circulation time, promote bioaccumulation in lymphoid organs, and efficiently target immune cells (Bharadwaj et al. 2020). The physio-chemical properties of the nano-carriers can be altered to give optimal antigen presentation, biodistribution, and cellular trafficking (Vartak and Sucheck 2016). In the past decade, there has been a high demand for nanoparticle-based vaccines due to increasing diseases in aquaculture globally. Nanovaccines are emerging as an improved and novel construct of vaccines.
3.2 Controlling the Disease Burden in Aquaculture Through Vaccinations
Aquaculture production peaked at 82.1 million tonnes in 2018, up by 3.2% from 2017 (http://www.fao.org/3/i9540en/I9540EN.pdf). In most countries around the world, the diseases in aquaculture were conventionally treated using antiviral drugs, antibiotics, antifungal agents, and other chemicals. This type of treatment can lead to various other undesirable effects like environmental pollution and multidrug resistance in bacteria. On the other hand, vaccines seem to be more effective and provide a long-term measure to treat diseases.
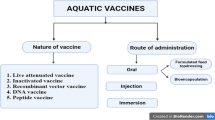
Vaccines may be defined as biological preparations that contribute to active immunity and long-term protection against a particular disease. The first vaccine in aquaculture was licensed in 1976 against enteric redmouth disease caused by Yersinia ruckeri. Currently, there are more than 20 commercially available licensed vaccines for aquaculture. These include whole killed vaccines, subunit vaccines, recombinant protein vaccines, DNA vaccines, and live attenuated vaccines. These vaccines are administered to fishes orally, by immersion, or by injection.
Live attenuated vaccines contain the pathogen in its weakened (attenuated) form. Earlier, the pathogens were passed through in vitro systems multiple times to attain random mutations (Adams 2019), which would weaken the pathogen. But in recent times, this attenuation is obtained through the genetic modification of the organism. Generally, a single dose of this vaccine is sufficient to elicit a robust immune response. Live attenuated vaccines are more beneficial in the case of diseases caused by intracellular pathogens. However, safety and reversion into the virulent form are always major concerns.
In the case of salmonids, vaccines have been used for almost 30 years. Most of them are oil-adjuvant-based injectable vaccines. In the United States, there are currently two live attenuated vaccines available commercially for enteric septicaemia of catfish (caused by Edwardsiella ictaluri) and columnaris disease (caused by Flavobacterium columnare). There is another licensed vaccine against enteric septicaemia of catfish in Vietnam that is manufactured by PHARMAQ AS. Inactivated vaccines are available against photobacteriosis caused by Photobacterium damselae subspecies piscicida (by Merck). Inactivated vaccines are also available against vibriosis caused by Vibrio anguillarum, Vibrio ordalii, and Vibrio salmonicida. A virulent live culture is used as a vaccine against bacterial kidney disease caused by Renibacterium salmoninarum. An inactivated vaccine for Tenacibaculosis caused by Tenacibaculum maritimum in Spain (ICTHIOVAC® TM – HIPRA) is available.
Vaccines are also present for viral diseases such as koi herpesvirus (CyHV-3) – there is a subunit vaccine (peptide-VP2) against infectious pancreatic necrosis in Norway (Merck) – viral haemorrhagic septicaemia. The first DNA vaccine to be licensed for use in aquaculture was Apex-IHN (Elanco) against infectious haematopoietic necrosis virus (IHNV) in Atlantic salmon (Salonius et al. 2007). There are other vaccines against salmon pancreatic disease (Merck), such as a recombinant vaccine against infectious salmon anaemia caused by salmon isavirus (Centrovet) in Chile.
Vaccines in aquaculture are mainly administered by the immersion method, oral administration, and intraperitoneal injections. Most of the vaccines available currently are of the whole-cell killed pathogen type, which is injected intraperitoneally. However, this type of vaccine might not be feasible in all countries; as a result, most of them settle with antibiotics. Therefore, there is a significant need for more knowledge and research in this field. The more feasible oral administration of drugs essentially requires protection as the antigenic components may be subjected to gastric digestion or digestion before absorption (Aklakur et al. 2016). Vaccines activate an immune response against the respective diseases but can have restrictions such as compromised effectiveness, reduced immunogenic responses, poor constancy, and the need for booster doses. These limitations in conventional vaccines have surfaced a need for an improved form of vaccines. With the recent advancements in nanotechnology, science has paved the way to explore the blend of nanotechnology and immunotherapy through nanovaccines.
3.3 Choice of Antigens as Nanovaccines
Nanovaccines mainly consist of two components, i.e. a synthetic or natural nanomaterial that functions as a carrier/adjuvant and an antigen. The antigens can be of various subunits of the pathogen types such as peptides, proteins, polysaccharides, capsules, and toxins, but ultimately, all of them have the collective objective of eliciting an immune response against the targeted pathogen. There are inactivated or killed forms of the pathogens which are used to activate an immune response. Formalin-killed cells are primarily used in aquaculture. Although these cells can activate both humoral and cell-mediated immunity, they sometimes do not attain an optimal level of protection (Collins et al. 2019). Subunit vaccines do not contain any whole bacteria or viruses; instead, they have one or more specific antigens of the pathogen that function as ‘flags’ for the immune system. Subunit vaccines are mainly designed through reverse vaccinology, which involves identifying a suitable vaccine candidate through in silico analysis.
Outer membrane proteins (OMPs) present on the membrane of gram-negative bacteria have become an important topic of research. They are present in the outermost region of the bacteria and are the first to come into contact with the host and help in adhesion, invasion, and contributing to pathogenesis. They are conserved among serotypes and are highly immunogenic in nature; hence, they would serve as an efficient vaccine candidate (Maiti et al. 2012, 2020).
DNA molecules have tremendous potential as vaccine candidates. DNA vaccines elicit both humoral and cell-mediated immune responses. Still, they sometimes lose their integrity before they reach the target cell due to degradation by endogenous nucleases, and also due to the net negative charge present on the cell surface, the DNA molecules can be repelled away from the cell membrane (Bhavsar and Amiji 2007). Hence, nanoparticles can ensure this integrity for effective delivery of the polynucleotide to the target site, followed by cellular internalization and processing. It is more beneficial to deliver DNA vaccines with the help of polymer-based nanoparticles (Bhavsar and Amiji 2007). Similarly, RNA-based vaccines are also safe and effective. As the name suggests, virus-like particles are particles that resemble the external structure of a virus but do not contain any genetic material; hence, they cannot replicate or mutate. They have the ability to self-assemble into the full tertiary structure that mimics the virus (Jeong et al. 2020). The antigen-presenting cells (APCs) recognize the epitopes on the particle to generate an immune response. They will require booster doses as the particles cannot replicate.
3.4 Nanoformulation of Vaccines and Delivery Systems
A revolutionization in the food and aquaculture sectors due to the huge potential of nanotechnology has provided innovative tools for disease prevention. Despite the tremendous application of traditional vaccines, lack of control over vaccine release, intrinsic instability, indiscriminate distribution, and a need for multiple administrations in aquaculture are various concerns governing the health sector today. To overcome these limitations, a nanoparticle-based drug delivery system provides a tool for targeting the desired site and enhancing the immune response of the host against the invading pathogens. Generally, the generation of vaccines with nanoparticles as adjuvants is formulated by optimizing the size, loading capacity, and surface charge of the nanoparticles. Further, an optimized dosage of nanovaccine, in turn, stimulates the immune system, thereby triggering the adaptive immune response during pathogen invasion. Previous findings suggest that the application of nanovaccines compared to traditional vaccines showed maximal antibody production in animal models (Gheibi Hayat and Darroudi 2019). Hence, novel nanovaccines are enormously necessary for sustaining aquatic health, preserving endangered species, and inhibiting the passage of pathogens in the food chain (food-borne illness).
Nanoparticles have been used as drug delivery vehicles for fish immunization studies. The use of polymeric nanoparticles has the advantage of allowing bioactive molecules to be encapsulated and protected from hydrolytic and enzymatic degradation. Due to their rapid escape from the degradative endo-lysosome, nanoparticles containing plasmid DNA provide sustained release. The use of a nanoparticle vaccine delivery system can also help to improve the immune response to synthetic peptide vaccines (Salvador et al. 2011). Biologics, polymers, carbon-based materials, silicon-based materials, and metals are commonly used in alternatives for drug delivery (nanoscale). Protein and gene delivery are being investigated using biodegradable polymer nanoparticles made of polylactic acid (PLA), polyglycolic acid (PGA), or polylactic-co-glycolic acid (PLGA). Some polymers under investigation for nanoscale drug carriers consist of poly (3-hydroxybutanoic acid), polyglycolic acid, poly (ethylene glycol), poly (ethylene oxide), and copolymers such as PLA-PEG. Solid nanoparticles have been engineered using a variety of materials, both with and without surface functionality. The aliphatic polyesters, specifically the hydrophobic PLA, the hydrophilic PGA, and their copolymer PLGA, can make up the majority of polymers. Polymeric NPs are made up of a polymer that has gained much deliberation due to advancements in polymer science and technology advancements. Biocompatibility and biodegradability are critical characteristics for tissue engineering, drug and gene delivery, and novel vaccine strategies. Antigen/drug delivery using polymeric nanoparticles has many benefits over conventional delivery methods, including the ability to target drug delivery to a particular site, such as an intracellular infection (Abdelghanyet al. 2012), reducing systemic toxicity, and facilitating the sustained release of a drug.
3.5 Different Types of Nanoparticles and Their Potential Applications for the Delivery of Vaccines
Today, different nanostructures are applied as delivery vehicles for vaccine efficacy and to explore newer avenues in vaccine administration in aquaculture (Fig. 3.1). The most applied nanoparticles as adjuvants include polymeric nanoparticles like alginate, chitosan, PLGA, dendrimers, and liposomes. However, conjugating the vaccine molecules with appropriate nano-carriers is essential to improve vaccine’s characteristics and its delivery potential. Conjugation of vaccine molecules with a suitable nano-carrier is generally performed by surface conjugation, encapsulation, and surface adsorption. The presence of a charge or hydrophobic interaction between nanoparticles and antigen molecules provides a medium for antigen adsorption on the surface of a nano-carrier. Usually, due to the non-covalent nature of their interaction, a sudden dissociation of antigens from the nano-carriers owing to the effect of external factors like pH, ionic strength, and temperature makes surface adsorption less popular. Other methods like encapsulation by embedding the antigen of interest and surface conjugation are universally applied in nanovaccine preparations due to strong intermolecular bonding, surface interaction, and the ability to release the antigen after partial or complete degradation of the nano-carrier (Pati et al. 2018). The nanovaccines used against various fish pathogens are summarized in Table 3.1.
Once the desired vaccine is designed and formulated, effective administration of the immunostimulant to control the infectious diseases in fish is necessary. Techniques may be many; however, there are three popular methods for vaccine administration in fish: oral, immersion, and injectable administration. Nowadays, vaccination by injection is the most reliable and effective method in fish immunization when compared to immersion and oral means, owing to easier administration, stress-free handling, and dose determination ability. Nanoparticles as delivery systems provide a medium for administering vaccines in a controlled manner to achieve the desired therapeutic effect. This is because cell or tissue targeted delivery, bioavailability, enhanced solubilization of hydrophobic drugs, controlled release, and therapeutic agent dissemination from degradation are protected (Ji et al. 2015). In this chapter, a summarization of different nano delivery systems for fish vaccination is further described.
3.5.1 Polylactic-Co-Glycolic Acid
A biodegradable polymer, PLGA, is a copolymer of lactic acid and glycolic acid. The characteristics like degradation rate, the strength of the nano-carrier, and loading capacity are altered by adjusting the monomeric ratio (Badekila et al. 2021). The biological compatibility and biodegradable nature of PLGA are helpful for human use and are approved by the Food and Drug Administration (FDA, USA) and the European Medicines Agency. This is due to the hydrolysis of PLGA into glycolic acid and lactic acid monomers, which are easily excreted (citric acid cycle) from the fish. It is one of the most commonly used biodegradable synthetic polymer nano-carriers, with a long history of biomedical application and a high safety profile (Semete et al. 2010). These particles are attractive for oral vaccination because they are easy to manufacture and relatively inexpensive. In the body, it undergoes non-enzymatic hydrolysis, yielding biodegradable metabolites such as lactic acid and glycolic acid. These are generally present in the body and participate in several biochemical and physiological pathways. As a result, there is very little systemic toxicity associated with PLGA use. PLGA and PLA can be made in various sizes and forms, and they can be used to encapsulate a wide range of molecules.
The size of the PLGA particles can be modified, and surface modifications can be added to vaccine formulations for oral, mucosal, and systemic administration. PLGA can easily be degraded in the body since, in the aqueous environment, its ester bond is hydrolyzed, allowing the easy release of antigens. The release kinetics of the system can be easily manipulated by varying the ratio of PLA/PLGA and is faster in acidic conditions. The particle size, surface alteration, and release profile of the PLGA nanoparticle all influence the immunogenicity of the entrapped antigen. PLA, unlike PGA, has a methyl group, making the copolymer more hydrophobic at higher PLA proportions. Similarly, the PGA/PLA ratio affects other particle physicochemical properties, including mechanical force, hydration power (swelling behaviour), gelation temperature, and charge.
PLGA is commonly prepared by the double emulsion method by dissolving it in an organic solvent like chloroform, dichloromethane, or ethyl acetate (McCall and Sirianni 2013). Hydrophobic compounds (antigens) are directly added to the organic phase, while hydrophilic compounds are emulsified with PLGA polymer solution prior to particle formation. Emulsification of the solution is performed by adding a surfactant or emulsifying agent like polyvinyl alcohol. The solid nanoparticles containing the antigen of interest are obtained by evaporating the solvent by continuous stirring or pressure reduction. However, the uptake mechanism of PLGA-loaded antigens is not well understood in aquatic culture (Ji et al. 2015). Recently, a study encapsulated an inactivated virus in PLGA nanoparticles and evaluated its efficacy against viral haemorrhagic septicaemia virus (VHSV) in the marine fish, Paralichthys olivaceus (12 g) by oral/immersion route of administration. A relative percent survival (RPS) greater than 60% was observed after 4 weeks in this study with an upregulation of immunity genes like IgM, IgT, pIgR, MHC-I, MHC-II, IFN-γ, and Caspase 3 (Kole et al. 2019). Another study investigated a PLGA encapsulated vaccine for innate and adaptive immune responses in kelp grouper, Epinephelus bruneus (31 ± 2 g), against an opportunistic pathogen in marine fishes, Uronema marinum, at different time intervals of 1–4 weeks. The cumulative mortality was less than 20% in PLGA-based vaccinated groups during scuticociliatosis disease. The acquired protection of the marine fishes against the protozoans was significantly due to the PLGA-based vaccination that comparatively enhanced respiratory burst activity, complement activity, and α-2-microglobulin (Harikrishnan et al. 2012a, b, c). Till today, high survival and serum antibody content suggested both direct and indirect involvement of PLGA vaccine in stimulating immune protective factors. A further mechanism of long-term protection needs to be evaluated for the future enhancement of aquatic organisms.
PLGA particles may also serve as adjuvants (Katare and Panda 2006), and the FDA has approved their use in human and veterinary medicine. Multiple antigens can be released simultaneously with PLGA particles, and antigens can be transported to intracellular compartments. To activate APCs, they may be engineered to be the same size as. Furthermore, biological degradation can take months to years depending on their characteristics (Prokop and Davidson 2008). Many researchers have studied the feasibility of using PLGA as a drug carrier. In a study, PLGA nanoparticles were filled with the anti-mycobacterial agent rifampicin and then injected into zebrafish embryos. Since the zebrafish embryos are transparent, it showed that the treatment had a significant impact on Mycobacterium marium infection. The rifampicin-PLGA nanoparticle showed an increased therapeutic effect against M. marium and higher embryo survival compared to rifampicin alone (Danhier et al. 2012).
3.5.2 Polylactic Acid
PLA is a biocompatible and biodegradable polymer. It is non-toxic and can be metabolized into monomeric units of lactic acid in the body. Since 1980, PLA polymer has been extensively studied in surgical implants, sutures, and drug delivery.
3.5.3 Alginate
Alginate is a natural polymer derived from brown seed weed/algae that comprises copolymers containing D-mannuronic acid and -L-guluronic acid found in brown algae cell walls. It is biodegradable, biocompatible, non-toxic, acid-resistant, mucoadhesive, and well-suited for oral vaccine administration (Rivas-Aravena et al. 2013). Alginate is commonly used in antigen encapsulation for a variety of reasons: it has low toxicity and mucoadhesiveness, it allows interaction of the alginate particle with the epithelial mucus walls; it is acid and protease-resistant, and it is inexpensive. The size, antigenic composition, production strategy, alginate selection, and antigen concentration influence the alginate particle’s characteristics. Alginate has been used in the delivery of fish vaccines in the form of microparticles. More often than nanoformulations, alginate nanoparticles were evaluated for oral vaccine delivery against Ichthyophytirius multifiliis in rainbow trout for booster vaccination.
The mechanical property of alginate polymer is dependent on the G units in its block formation. Alginate as cargo for vaccine delivery is attractive. It is stable at low pH during its release in the foregut and midgut of fish and can be applied in the oral administration of nanovaccines (Ji et al. 2015). A study combined alginate and chitosan by ionic gelation for oral vaccination of Oncorhynchus mykiss (10 g) against pathogenic bacteria like Lactococcus garvieae and Streptococcus iniae. The challenge test results showed 76% survival (against S. iniae) and 66% survival (against L. garvieae) for the polymer-based vaccine after 10 days of the challenge. Thus, indicating the effectiveness of the oral bivalent Streptococcus-Lactococcus vaccine (Halimi et al. 2019). Many studies have applied alginate in microform; however, studies of alginate as a nanovaccine are still in their nascent stages. Hence, further improvement in the application of alginate-based vaccines in nanoparticle form needs to be performed.
3.5.4 Chitosan
Chitosan is a linear polysaccharide composed of (1–4)-linked D-glucosamine and N-acetyl-D-glucosamine, which is present in the exoskeletons of crustaceans, insects, and some microorganisms. Chitosan nanoparticles are used for drug delivery and have excellent properties. They are made of a biocompatible, non-toxic, and biodegradable polymer that is easily excreted by the kidneys. Because of their mucoadhesive properties, they can be modified for slow and sustainable drug release. In reality, chitosan activates immune cells such as macrophages, natural killer cells, APCs, and T lymphocytes by stimulating the production of cytokines (Foged et al. 2005). Purity, molecular weight, degree of deacetylation, quality, and viscosity all affect the characteristics of chitosan particles. Fish vaccines have been developed using chitosan nanoparticles, such as the inactivated virus vaccine against infectious salmon anaemia virus (ISAV), which includes the DNA coding for ISAV replicase as an adjuvant. The outer membrane protein K-encoding gene of Vibrio parahaemolyticus was loaded onto chitosan nanoparticles to create an oral DNA vaccine. In the black sea bream Acanthopagrus schlegelii, this recombinant nanovaccine elicited a defensive immune response against V. parahaemolyticus (Li et al. 2013). Recently, a reddish body iridovirus oral DNA vaccine based on chitosan nanoparticles was created (Zheng et al. 2016). Chitosan and chitosan/triopoly phosphate nanoparticles were used to produce an oral DNA vaccine against V. anguillarum in Asian sea bass, Lates calcarifer. The nanovaccine conferred only moderate protection against the pathogen (Vinay et al. 2016). Similarly, in Asian sea bass (L. calcarifer), chitosan nanoparticles were tested for their ability to deliver plasmid DNA encoding V. anguillarum OMPs (Poobalane et al. 2010). Using immunohistochemistry, Omp38 was subsequently found in the liver, kidney, spleen, and intestine. Fish were partly safe from homologous challenge 21 days after vaccination, with an RPS of 46%. In a study on rainbow trout (O. mykiss), vitamin C was found to be conjugated with chitosan nanoparticles (O. mykiss). Because of the potent synergism between chitosan and vitamin C, the vitamin was released up to 48 h after oral administration, and the fish’s innate immune system was stimulated. It also has the potential to induce a robust adaptive immune response against the conjugated antigen, both cellular and humoral (Arca et al. 2009). The use of chitosan nanoparticles in fish vaccines has several advantages, including the ability to boost mucosal immunity through the oral route of vaccination. Several oral DNA vaccination studies in fish have shown that chitosan nanoparticles are more effective than other formulations against antigens derived from turbot reddish body iridovirus, nodavirus, V. parahaemolyticus, and V. anguillarum. Vimal et al. (2012) and Rivas-Aravena et al. (2015) also demonstrated the efficacy of chitosan nanoformulations against inactivated ISAV. A recent study found that chitosan was effective in the intraperitoneal administration of a vaccine against VHSV recombinant glycoprotein (rgpG) in zebrafish (Kavaliauskis et al. 2016). According to the findings, the use of chitosan nanoparticles improves vaccine-mediated protection against infection in fish. It also contributes to the immune response by modulating leukocyte trafficking. It is biocompatible, biodegradable, hydrophilic, and abundant in nature, making it one of the most appealing candidate nanoparticles.
3.5.5 Dendrimers
Dendrimers are symmetric nanoparticles constituting a central core, an inner shell, and an outer shell with treelike arms and branches. The synthesis of the dendrimer is generally performed by convergent (addition of monomers from the chain end) and divergent (synthesis starting from the core) methods followed by size and branch optimization (Klajnert and Bryszewska 2001). The presence of multiple functional groups in the dendrimer surface is advantageous in coupling biologically relevant molecules. With a need for efficient nanovaccines, dendrimers provide molecularly defined multivalent scaffolds to fabricate conjugates with antigens. The biocompatibility, predictable biodistribution, and ligand-receptor interacting characteristics are dependent on the size and surface charge of dendrimers (Heegaard et al. 2010). Hence, an optimized synthesis of dendrimers based on size and surface charge provides feasibility for efficient nanovaccine preparation in conjunction with delivery systems. Previously, few studies explored the suitability of amine−/amide-based dendrimers like polypropylene imine and polyamido amine (PAMAM) for antigen delivery to produce an immune response in the host against contagious viruses (Chahal et al. 2016). Currently, chlamydial infection in fish is emerging as a cause for concern in aquaculture industries (Stride et al. 2014). In this regard, the dendrimer-conjugated peptide vaccine is suitable for the clearance of the infection. The proposed carrier system in a previous study involved a PAMAM dendrimer to which a chlamydial peptide mimic, glycolipid antigen-peptide 4, was conjugated through an ester bond (Ganda et al. 2017). Even though nanoformulation is effective in controlling infectious bacterial and viral strains in mouse models, considerable research is not available in aquatic models.
3.5.6 Inorganic Nanoparticles
Inorganic nanoparticles have a measurable impact on modern material science research due to their possible technological importance, particularly in the field of bio-nanotechnology, and their unique physical properties including size-dependent magnetic, optical, electronic, and catalytic properties. Thus, spreading their possible applications in fluorescence labelling, magnetic resonance imaging, and stimulus-responsive drug delivery is crucial to the diagnosis and treatment of diseases (Kobayashi et al. 2014). It is a viable alternative to organic forms that could be used for vaccine delivery. Many inorganic nanoparticles have been explored for their application in vaccine delivery. Despite their non-biodegradable nature, inorganic nanoparticles have advantages like a unique rigid structure for controllable synthesis (Kalkanidis et al. 2006). The four most commonly used inorganic NPs are (1) noble metal, (2) magnetic, (3) fluorescence, and (4) multifunctional, e.g. luminescent magnetic.
3.5.7 Gold Nanoparticles
There is a great interest in investigating the antimicrobial effect of gold nanoparticles due to their low toxicity to eukaryotic cells. It can be used in vaccine delivery systems and can be easily manipulated into different sizes and shapes. Gold nanoparticles were produced by green synthesis and showed antibacterial activity against fish bacterial isolates.
3.5.8 Silver Nanoparticles
Silver nanoparticles are one of the most investigated nano-antibacterial agents in the research literature. It is synthesized using citrus (lemon) juice as a reducing agent. It has shown antibacterial activity against Staphylococcus aureus and Edwardsiella tarda and anti-cyanobacterial activity towards Anabaena and Oscillatoria species; there is very little published work on the antifungal and antiviral effect of silver nanoparticles in fish medicine.
3.5.9 Zinc Oxide Nanoparticles (ZnO-NPs)
Zinc oxide nanoparticles have drawn more attention because of their antibacterial and antifungal effects. In the field of fish medicine, ZnO-NPs can inhibit the growth of Aeromonas hydrophila, E. tarda, Flavobacterium branchiophilum, Citrobacter spp., S. aureus, Vibrio species, Bacillus cereus, and Pseudomonas aeruginosa. Ramamoorthy et al. investigated the antibacterial effects of ZnO-NPs against the pathogenic Vibrio harveyi and observed higher bactericidal effects of nanoparticles compared to bulk ZnO. In another interesting study, ZnO nanoparticles were synthesized biologically using A. hydrophila. These nanoparticles exhibited antibacterial activity against the same bacterium and other species like P. aeruginosa, E. coli, Enterococcus faecalis, Aspergillus flavus, and Candida albicans.
3.5.10 Titanium Dioxide Nanoparticles (TiO2−NPs)
TiO2-NPs, when doped with magnetic Fe3O4-NPs, had a bactericidal effect against S. iniae, E. tarda, and P. damselae after activation by light (Abdelghany et al. 2012). These particles can be used to disinfect water, as the fish pathogens bind with the nanoparticles, which can then be easily extracted from the water using a magnet (Abdelghany et al. 2012). However, Jovanovic et al. (2011) concluded that TiO2-NPs influence the immune system of fish by decreasing the antimicrobial activity of fish neutrophils, rendering the fish more susceptible to infection and hence increasing mortality, particularly during disease outbreaks.
3.5.11 Nano-selenium
Nano-selenium has a substantial impact on the physiology of fish by improving the animal’s physiological and immunological systems. Selenium supplementation protects cells from damage and is important for fish development, fertilization, and immunological function. By increasing lysozyme activity and red blood cell count, selenium supplementation boosts fish immunity. The nano form of selenium has the most favorable effect, as it is more effective than the bulky version. The nano form of selenium is a unique kind that draws greater interest than inorganic and organic forms due to its high bioavailability and lower toxicity (Khurana et al. 2019), whereas inorganic compounds are more harmful than organic compounds. The biological properties of selenium nanoparticles are dependent on their size; smaller particles have more activity. In comparison to other organic and inorganic oxidation states, nano-selenium (nano-Se) advantages from the capacity to utilize selenium at zero oxidation, which has low toxicity and excellent bioavailability. It’s highly unstable, and it can readily revert to a dormant state. On the other hand, encapsulation with chitosan can help to stabilize it (Nasr-Eldahan et al. 2021).
3.5.12 Liposome
For nearly four decades, liposomes have been recognized as possible drug delivery vehicles (Nasr-Eldahan et al. 2021). The size is between 100 and 400 nm. Some liposome nanoparticle formulations include liposome-polycation-DNA nanoparticles and interlayered, crosslinked multilamellar vesicles. A liposome is a spherical vesicle made artificially from biologically inert lipids that are non-toxic and biodegradable and composed of a lamellar lipid bilayer. The antigen can be encapsulated within the core of liposomes, which are made up of biodegradable and harmless phospholipids (Giddam et al. 2012). The lipid bilayer structure facilitates the loading of both hydrophobic and hydrophilic compounds. Liposomes are phospholipid vesicles that develop spontaneously in aqueous solutions and have the ability to capture dissolved particles. They are biodegradable, slowly releasing the charged molecule as they decompose in the body. Depending on their size, the number of lamellae that make them up, and their ability to trap molecules in solution, they have diverse features. Given the high quantity of mucin in fish gills, the liposome charge must be taken into account while giving chemicals to fish in liposomes. The pH of the water deprotonates the mucin’s sialic acid, enabling its interaction with cationic liposomes of <100 nm that contain DNA, for example, enhancing the residence time and uptake of the load. Because the interaction with the gills can produce hypoxia in the fish, this interaction results in large quantities of cationic liposomes, which are lethal to the fish. Similar amounts of anionic or neutral liposomes, on the other hand, are not lethal.
At present, liposomes are widely used as vaccine delivery vehicles in nanomedicine. Oral nanoliposomes have been used for fish vaccine administration with liposome nanoparticle-entrapping A. salmonicida and koi herpesvirus, which provided a better immune response than other formulations. A study was published on the effectiveness of nanoliposomes in the intraperitoneal injection of a Vibrio harveyi vaccine (Harikrishnan et al. 2012a, b, c). Phosphatidylcholine liposomes encapsulating inactivated A. salmonicida with formalin, as well as lipopolysaccharide (LPS) and inactivated toxin, were given to rainbow trout via immersion, which gave minimal protection against furunculosis, being slightly more efficient than the free antigen. Fernandez-Alonso et al. (1999) showed that when liposomes are given to fish, they can enable DNA expression. The green fluorescent protein (GFP) was detected in the fins of 0.2–0.5 g rainbow trout after immersion in 10–20 μm of DOTAP liposomes carrying codifying DNA for GFP (Fernandez-Alonso et al. 1999). There is no direct relationship between the size of liposomes and the organs in which they concentrate, according to studies, but there is a relationship in terms of their ability to lodge in specific organs. When rainbow trout were given large unilamellar phosphatidylcholine liposomes (LUV, 250 nm), they accumulated in larger proportions in their organs than multilamellar liposomes (MLV, 1–5 μm). They were collected (in decreasing order) in the spleen, head kidney, posterior kidney, visceral fat, and liver 24 h after treatment; liposome accumulation in hematopoietic organs has also been seen. Because liposomes are easily destroyed in the stomach, trials of fish vaccines using liposomes have been used for intraperitoneal injection or immersion delivery. More research into different forms of liposomes is needed to establish their use in the encapsulation of antigens for fish. Oral vaccinations are now possible thanks to modified liposomes that are resistant to stomach digestion.
Certain factors like net charge, lipid composition, particle size, and amount of loaded compound determine the vaccine delivery potential of the liposomes. Especially, the net charge of liposomes indirectly contributes to acute toxicity in fish after treatment due to the presence of high levels of mucin in fish gills, resulting in an unintended interaction between cationic liposomes and anionic mucin. However, a study investigated the effect of oral immunization with liposome-entrapped bacterial antigen on protection against A. hydrophila (a bacterial pathogen majorly attacking mucosal surfaces in the fish intestine and causing furunculosis disease). For the nanovaccine formulation, the liposome carrier was synthesized by combining dipalmitoylphosphatidylcholine, dipalmitoylphosphatidylserine, and cholesterol, followed by entrapping the A. hydrophila antigen in saline solution. The liposome-entrapped A. hydrophila was orally administered in Cyprinus carpio (25–30 g). A comparison of immunized with non-immunized fishes showed an 83.5% survival rate during pathogen invasion (Choi and Oh 2007). Similarly, another study conducted liposome (made of dipalmitoylphosphatidylcholine, dipalmitoylphosphatidylserine, cholesterol)-based oral vaccine administration in E. bruneus (29.5 ± 2.1 g) against Vibrio harveyi in kelp grouper and showed 90% mortality in non-immunized fish during V. harveyi infection (Harikrishnan et al. 2012a, b, c). Thus, the potential of the liposomal conjugated bacterial vaccine showed tremendous potential in fish survival. Further, a newer strategy to protect fish against viral/bacterial infection by immunostimulation of Danio rerio (0.6 ± 0.12 g) with nanoliposome co-encapsulating poly (Inosinic: Cytidylic) (a synthetic analogue of viral double-stranded RNA) and bacterial LPS to protect against lethal virus spring viraemia of carp virus and bacteria P. aeruginosa (PAO1) showed promising avenues in fish immunization (Ruyra et al. 2014).
3.6 Nanoemulsion
Nanoemulsions usually have a diameter of 20–200 nm and are an isotropic system made up of two immiscible liquids (water and oil) that stabilize with the addition of a surfactant. They can be in the form of water in oil or oil in water, with vaccinations in their cores, or simply mixed with antigens for delivery. Some emulsion-based nanoparticles including MF59 and Montanide are tailorable nano-sized emulsions. Polymeric chitosan and PLGA nanoparticles have been the most studied nanoparticles in fish vaccination research so far.
3.6.1 Immunostimulating Complex (ISCOM)
Immunostimulating complexes contain immune-stimulating qualities, and they are commonly employed as a vaccination adjuvant to boost the immunological response and provide extended protection. The ISCOMs are spherical structures with an open cagelike structure (40 nm in diameter) that developed spontaneously when cholesterol, phospholipids, and quillaia saponins were mixed in a certain stoichiometry (Aguila et al. 2006). They have excellent adjuvant activity against a wide spectrum of bacterial and viral antigens. Virus-like particles are biocompatible capsid proteins that self-assemble into nanoparticles. They are great nanovaccines because they lack infectious nucleic acids but retain the virus’s developed shape, which induces immunity. The size of nanoparticles utilized in vaccine development is usually between 20 and 800 nm. Immunostimulant complexes have been studied for more than three decades and are only available for veterinary use due to their low toxicity and haemolytic qualities (Smith et al. 2015). Although different forms of saponin have been examined as adjuvants in fish vaccines, there is just one research on the nano form (ISCOMs) for vaccine administration. In the study, the main OMPs of A. hydrophila were encapsulated in ISCOMs and administered intraperitoneally to eels, which provided good protection.
3.7 Immune Responses Due to Nanovaccines
The immune system of fish is divided into two types: innate and adaptive. Surface barriers (mucus, skin, gills, gastrointestinal tract), growth inhibitors (transferrin, interferon), enzyme inhibitors, and other innate defence mechanisms in fish are activated rapidly after infection. Nonspecific cellular factors are phagocytes, lysins (complement, antimicrobial peptides, lysozyme), precipitins and agglutinins (pentraxins, lectins), macrophages and neutrophils, phagocyte-activating chemicals (opsonins, cytokines), natural cytotoxic cells, eosinophils, basophils, mast cells, and inflammation. Adaptive immune responses take several days to become active, after which they provide basic memory cells, which are required for full pathogen elimination. Humoral immunity, cell-mediated immunity, and immunological memory are three facets of the adaptive immune system mediated by lymphocytes. Humoral immunity is characterized by the production of immunoglobulins (Ig) by B-cells, and there are three types of Igs identified in fish to date (IgM, IgD, and IgT) (Ballesteros et al. 2013). Cytotoxic T-lymphocytes are an integral part of the cellular immune system. Pathogens are recognized by adaptive immunity by molecules generated by somatic pathways, which are then followed by humoral and cellular reactions mediated by B- and T-lymphocytes. Dendritic cells, for example, are APCs that play a key role in both innate and adaptive immune responses. The APCs mature in response to microbial surface determinants, resulting in the transfer of MHC molecules (MHC I and MHC II) from intracellular compartments to the cell surface, the secretion of cytokines, morphological changes in dendritic cells, and cytoskeleton reorganization. The antigens are either internalized through the endocytic or non-endocytic pathways. The endocytic pathways, for example, entail the phagocytosis of antigen by APCs and the subsequent degradation of the antigen by proteolytic enzymes and reactive oxygen species. The degradation products (peptides) are then displayed on MHC class II molecules and identified by CD4+ T cells, causing antibody production and memory T-cell development. Non-endocytic pathways: Pathogen antigens are digested by the proteasome, which then displays peptides on MHC class I molecules (Shen et al. 2006). The CD8+ T cells that have cytotoxic activity against infected host cells identify the displayed antigen. The expression of co-stimulatory molecules (maturation markers) increased in a dose-dependent manner after these dendritic cells (DC) were exposed to nanoparticles (Uto et al. 2009). For the induction of DC maturation, however, both the absorption of nanoparticles and the characteristics of the polymers that shape the nanoparticles are critical. Despite their smaller size, nanoparticles have a greater influence on DC activation. As a result, surface interactions between nanoparticles and DCs influence DC maturation. When DCs were matured by PLGA nanoparticles, the expression of MHC class II and CD86 increased slightly compared to controls (Elamanchili et al. 2004). Nanoparticles elicit a variety of immune responses when administered, but they are not immunogenic unless they have been conjugated with an antigen. Pattern recognition receptor activation, cytotoxic T-lymphocyte induction, T-helper (Th) activation, cytokine development in different forms, B-cell activation, and antibody production are all involved in the induction of immune responses through various nanoparticles (Najafi-Hajivar et al. 2016). The size of the particles may play a role in the type of immunity that is caused. The APCs pick up nanoparticles depending on their size (Fifis et al. 2004). Smaller particles induce stronger immune responses than larger particles, according to several studies (Manolova et al. 2008).
3.7.1 Nanoparticle-Antigen Interaction
Nanoparticles can deliver antigens to the immune cell in two ways: (a) immune cell co-ingestion of antigen and nanoparticle and (b) transient transmission, i.e. protecting the antigen and controlling its release at the target site. Nanoparticles engage certain immunological pathways in immune potentiator techniques, accelerating antigen processing and increasing immunogenicity (Mody et al. 2013). Simple physical adsorption or more nuanced methods such as encapsulation or chemical conjugation have been used to bind antigens. The charge or hydrophobic interaction is used to physically adsorb antigen onto a nanoparticle (Wendorf et al. 2006), where the contact between the nanoparticle and antigen is rather weak, resulting in quick disassociation in vivo. Encapsulation and chemical conjugation of antigens to nanoparticles provide the strongest interactions. During formulation, antigens are combined with nanoparticle precursors, resulting in antigen encapsulation in nanoparticles (Zhao et al. 2014). The antigen, on the other hand, is chemically cross-linked to the surface of a nanoparticle, and after being taken up by the nanoparticle, it is released inside the cell (Slütter et al. 2010). Immune potentiator techniques do not require antigen attachment or interaction with nanoparticles, and in some cases, they may be unfavourable in circumstances where the antigenic structure at the nanoparticle contact is altered. Several studies have shown that unique antibodies can be generated against nanoparticles, which is not a desirable trait because it could reduce the efficacy of nanovaccines (Zolnik et al. 2010; Zaman et al. 2013). Nanoparticles are not antigenic by themselves, but they do have antigenic properties when conjugated with antigens (proteins) due to their larger size (Zolnik et al. 2010; Zaman et al. 2013). T cells’ activity in combating infections and cellular components is called cell-mediated immunity, and it’s critical for protecting against a variety of pathogens. A significant cellular immune response can be induced by giving nanoparticle-based vaccinations in non-fish models, according to several studies (Zaman et al. 2013). Immunological memory is an essential feature of a specific immune response, which includes adaptive changes in lymphoid cells. The immune system identifies and kills a pathogen when it is exposed, which is the basis for an effective vaccine strategy (Fig. 3.2). The production of a vaccine necessitates the activation of both the innate and adaptive immune systems, which function in tandem. Furthermore, adjuvants and delivery systems are needed to enhance and prolong the immune response, and various nanoparticles have emerged as frontrunners due to their unique properties. Nanoparticle-based vaccines help to close the gap by inducing the upregulation of many inflammatory, innate, and basic immune-sensitive genes (Zhu et al. 2014; Zheng et al. 2016). In fish, nanovaccines can elicit cellular and humoral immune responses, but cellular responses remain elusive. Based on the identification of the antigen by different methods, it has been suggested that oral nanovaccines can efficiently deliver purified antigens or DNA vaccines in the intestine, gills, liver, muscle, heart, blood, spleen, and kidney (Vimal et al. 2012). Several studies have shown that NPs can target the liver and other organs. Some nanovaccines can alter cell junction integrity during the permeation process, a mechanism that has been linked to the positive charges of NPs and is proposed as a useful property for enhancing antigen or DNA vaccine delivery (Liu et al. 2016). Fish innate immune cells respond to oral nanovaccines by increasing respiratory burst activity and immune-related enzymatic activities such as lysozyme, myeloperoxidase, and superoxide dismutase (Kole et al. 2018). Since systemic and mucosal (skin)-specific antibodies have been identified, B cells are directly involved (Rivas-Aravena et al. 2015). Except for TLR22 and NOD1 receptors, the function of receptors in vaccinated fish has been studied in depth (Liu et al. 2016; Kole et al. 2018). Immune-related gene expression research has revealed new information about the immunological pathways linked to nanovaccines. The upregulation of genes linked to innate and adaptive immune responses, such as iNOS, IL-1β, TNF-α, Mxs, IFNs, IL-10, IL-12, TGF-β, MHC, CD4, and CD8, was discovered during the evaluation of oral nanovaccines in fish (Rivas-Aravena et al. 2015; Liu et al. 2016). These genes were chosen based on the predicted immunological responses following the administration of a particular nanovaccine.
3.7.2 Nanoparticle-Antigen-Presenting Cells
The encapsulation of antigenic cells in a nanoparticle has sparked research into the mechanism for efficient antigen transmission to APCs, which leads to antigen maturation and then cross-presentation to induce an immune response. The DCs prefer virus particles with a diameter of 20–200 nm, while macrophages prefer particles with a diameter of 0.5–5 nm. When the particle size was smaller, a higher proportion of DCs interacted with the polystyrene spheres. Similarly, macrophages ingested PLA nanoparticles with a diameter of 200–600 nm more effectively than microparticles (Kanchan and Panda 2007). Particle shape, size, and surface charge are all essential particulate physicochemical factors that influence how particles interact with APCs. In contrast to hydrophilic particles, hydrophobic particles have been shown to elicit a stronger immune response (Hillaireau and Couvreur 2009).
3.8 Benefits of Nanovaccines
In recent years, rapid advances in nano-sciences and nanotechnologies have opened up new frontiers for several industrial and aquaculture areas. Nanotechnology is undeniably a significant prospect for the economic and long-term development of aquatic resources in many countries. In aquaculture, nanotechnology has evolved into a comprehensive tool for addressing a wide range of issues. Nano-vaccination is a novel approach to improving vaccine immunogenicity by employing nanoparticles as a carrier or adjuvant. There are a variety of materials available today that can be employed in an antigen delivery system for oral fish immunization. The nanomaterial-based vaccine’s advantages include targeted antigen delivery, antigen stability, improved release kinetics, higher immune protectiveness, and immunogenicity. Because they are biodegradable and biocompatible, as well as less toxic, they are regarded as a viable alternative to standard vaccines. Nanoparticles also serve as adjuvants, assisting in the stimulation of immune responses while also protecting the antigen from degradation and allowing for regulated antigen release at the targeted site. It provides gastrointestinal stability, which is a key requirement for oral vaccination. Nanoparticles can imitate a natural illness, reducing the requirement for a booster dose while also improving vaccine efficacy. Various biodegradable polymeric particles, such as PLGA or PLA, operate as adjuvants and aid in the establishment of long-lasting immunity following a single injection. Biodistribution is aided by surface modification. The NPS can also be combined with targeting ligands, allowing particles to be directed to specific cells or regions. Nanoparticle-based delivery of DNA vaccines to APCs is one of the most promising delivery technologies for optimizing DNA vaccine formulation for immunotherapy (Rauta and Nayak 2015). The advantages and disadvantages of various types of nanoparticles are summarized in Table 3.2.
Vaccination with nanoparticles has several advantages over traditional vaccines.
-
1.
A nanoparticle’s size and shape can be modified to imitate a pathogen, allowing for efficient lymphatic drainage and subsequent internalization in APCs.
-
2.
In physiological settings, nanoparticles effectively prevent the encapsulated antigen from destruction.
-
3.
The charge and size of the nanoparticle influence the particle’s biodistribution and retention in lymph nodes and spleens, encouraging memory immune responses.
-
4.
Antigen and adjuvant co-delivery through nanoparticles to specific APCs, resulting in optimum antigen presentation and immune activation.
-
5.
NP systems designed to facilitate endosomal escape can transfer antigens to the cytosol of APCs, enabling efficient antigen cross-presentation and the production of cytotoxic CD8+ T lymphocyte responses.
-
6.
Antigen and adjuvant co-delivery through nanoparticles to specific APCs, resulting in optimum antigen presentation and immune activation.
-
7.
In aquaculture, oral administration is the easier and less expensive way to achieve antigen delivery since it eliminates the labour-intensive, costly, and inconvenient injection technique. The synthesis of nanoparticles costs much less than any other adjuvant, and because they are thermostable, they do not require a cold chain for storage.
Recent research has found that encapsulating A. hydrophila OMPs in PLGA and PLA nanoparticles improved the severity and duration of the immune response. In common carp, zebrafish, and rohu, even single-walled carbon nanotubes improve the immune-protectiveness of DNA and act as a delivery route for recombinant proteins targeting specific diseases. Because nanotubes may infiltrate APCs and carry and translocate bioactive molecules, they are useful carriers of antigens (Liu et al. 2016).
3.9 Current and Future Challenges
In many countries, nanotechnology presents a significant possibility for the economy and the long-term development of aquatic resources. Although the use of nanotechnology in aquaculture is still in its early stages, it has the potential to solve the majority of the problems in the aquaculture and fisheries sectors with improved technical innovation at various levels. Nanoparticles have certain unique properties and have shown promising use in fish vaccine administration, but they also have the drawback of being able to cross the blood-brain barrier, which could cause major problems (Joyappa et al. 2009). The NP-based targeting and delivery system has a small size, and a large surface area can cause aggregation, making physical handling problematic. Other difficulties with employing these nanoparticles include a lack of knowledge about NP distribution and the unpredictability of the process. Nanoparticle toxicity raises biosafety problems. If nanoparticles are made of innocuous chemicals, they are not always hazardous. The principal targets of nanoparticles for immunotoxicity have been identified as cell-mediated immunity and phagocytic cells. Lysosomal instability, frustrated phagocytosis, and changes in phagocytic cell function are all signs of toxicity. Although the humoral immune system is less susceptible to direct nanoparticle immunotoxicity, it is essential for the nanoparticles’ dispersion throughout the body and presentation to phagocytic cells. However, there is a lot of scientific confirmation and research that must be done in this area. Despite the wide range of nanomaterials available, most fish nanovaccines have been produced using polymeric nanoparticles (PLGA, chitosan, and nano-polyplexes). An investigation is required on a wide range of nanomaterials, which can lead to the field’s expansion. As a delivery vehicle, a variety of materials, including metallic and other organic NPs, are being considered. Other NPs currently being developed for vaccines and tested in fish via parenteral or immersion methods include fundamental investigations in fish that are required to establish relationships between the physicochemical qualities of NPs and their stability, biodistribution, destiny, and ultimately efficacy. Although most vaccine prototypes have low toxicity, this area deserves more research, taking into account particle size, bio-inertness, biodegradability, and safe excretion. High concentrations of chitosan (20 g/ml) and PLGA (1.25 mg/ml) NPs in water, for example, cause lead toxicity in zebrafish (D. rerio) embryos, causing impairment of hatching or survival (Nikapitiya et al. 2018). These results may be linked to the agglomeration of NPs on the surface of the chorion and the subsequent induction of hypoxia. Biocompatibility, permeation potential, and interaction-mediated mechanisms of nanovaccines have all been studied in vitro using fish cell lines. Surprisingly, in vivo follow-up experiments have shown the biodistribution of oral nanovaccines, revealing that they end up in the stomach, blood, gills, kidney, spleen, and muscle. Studies on the antigen release rate, cellular uptake dynamics, and intracellular destinies should be added to this information (Dubey et al. 2016a, b). Despite their widespread use, there are questions about a few nanoparticles that exhibit varying degrees of toxicity. Inorganic carbon nanotubes are non-biodegradable and have been confirmed to be toxic (Mutlu et al. 2010). Unregulated applications and toxicity reports from a small number of in vitro studies can skew the public’s perception of nanoparticles, causing unnecessary concern and casting doubt on the science of nanomedicine (Yildirimer et al. 2011). With the increasing number of nanoparticle applications in recent years, the mechanisms will become clearer, and perceptions can shift in either direction. Oral nanovaccine prototypes have been tested in fish against viral and bacterial diseases, with evidence demonstrating their defensive ability against six viruses and five bacterial organisms. As a result, the list of diseases for which this technology can be used must be expanded. There were no studies on fish nanovaccines for parasitic diseases. Vaccine candidates can be developed using plasmid DNA or purified antigens with proven efficacy. Nanoparticles‘antifungal and antiviral properties against fish diseases must be investigated. Only a few studies have looked at the use of nanoparticles in the diagnosis of bacterial and fungal diseases in aquaculture. Given nanoparticles’ demonstrated ability, more focused investigations of their use in many fish medicine research topics are needed to encourage more effective fish disease diagnostics and therapy (Danhier et al. 2012). Since the former mimics a natural infection, it would better reflect vaccine efficacy in a real scenario; challenge studies focused on experimental infection rather than intraperitoneal pathogenic challenges are required to test nanovaccines more accurately (Rombout et al. 2011) and extend the challenge duration after mucosal vaccination. This will allow researchers to determine whether the defence is a result of innate or adaptive immune responses. When developing mucosal vaccines for fish, it’s important to remember that a variety of factors influence the induction of long-lasting adaptive immune responses, including age, genetics, and climate (Embregts and Forlenza 2016). Mechanistic experiments on oral fish nanovaccines show that they boost respiratory blast, myeloperoxidase, lysozyme, and superoxide dismutase activities, as well as humoral responses, as measured by total and specific antibody output in serum and skin mucus (Zheng et al. 2016). To better understand the behaviour of oral nanovaccines, a study of encapsulation efficiency and release of encapsulated antigens should be done on a case-by-case basis, and induced immune responses should be compared among fish species. The latest progress on oral nanovaccine prototypes for use in fish has shown that they are feasible for use in aquaculture. The following scientific breakthroughs were discovered: (i) Only organic NPs were used, with chitosan and PLGA serving as the most popular nanomaterials; (ii) plasmid DNA and purified recombinant antigen were chosen; (iii) an encapsulated strategy was chosen over a surface-displayed strategy; (vii) most oral nanovaccines improved survival relative to other vaccines and routes of administration; (v) nano-polyplexes have only been tested against a bacterial disease, while (vi) viruslike particles have only been investigated against viral pathogens; (vii) most oral nanovaccines improve survival as compared to other vaccines and administration methods; and (viii) nanovaccines must be manufactured and tested on a large scale. These breakthroughs pave the way for new research into the use of oral nanovaccines in fish aquaculture, which would benefit both the scientific community and the industry (Benezra et al. 2011). Several obstacles remain, including the difficulty of synthesizing non-aggregated nanoparticles with consistent and desirable properties, a lack of understanding of how the physical properties of nanoparticles affect their biodistribution and targeting, and how these properties influence their interactions with the biological system at all levels, from the cell to the tissue. As a result, rational design combined with the reproducible production of nanoparticles with desirable properties, functionalities, and efficacy will become increasingly necessary, and it is expected that the introduction of new technologies, such as microfluidics, for the regulated synthesis of nanoparticles, will speed the creation of suitable nanoparticles for pharmaceutical applications. Novel vaccine systems for unmet needs, such as single-dose and needle-free delivery, will become feasible soon by combining some other appealing properties, such as slow-release, targeting, and alternative administration methods and delivery pathways (Zhao et al. 2014). Concerns about the toxicity of the particles, as well as difficulties in producing the materials and presenting antigens in their native form, are all drawbacks of using NPs for vaccine delivery. As a result, rational design combined with a repeatable synthesis of nanoparticles with desirable properties, functionalities, and efficacy is becoming increasingly important. The introduction of emerging technologies is expected to accelerate the production of appropriate nanoparticles for pharmaceutical applications. This sector, however, is still in its early stages. It’s important to dig deeper into the physical-chemical properties of the nanoparticles used in vaccine production, as well as the properties of the antigens after they’ve been encapsulated. Finally, to better understand the effects of oral nanovaccines, a study of the mediated immune responses should be conducted across fish species. There is still a significant research gap, and new types of efficient nanoparticles, such as dendrimer nanocapsules, mesoporous nanoparticles, and others that are now accessible, must be investigated to develop effective vaccine delivery systems for aquaculture organisms. Since the precise mechanism of action of nanoparticles has yet to be fully known, there is still concern about toxicity. Recent developments and additional research into the biocompatibility of these nanoparticles can alter perceptions, opening up new avenues for combating deadly pathogens in aquaculture.
3.10 Conclusions
Most biological macromolecules that are used as antigens in vaccines are not able to get through the biological membranes to elicit a potent immune response. Hence, nanovaccines can ensure effective vaccine delivery. Immunization through nanovaccines provides better targeting and stimulates an antibody response at a cellular level. Nanoparticle-encapsulated vaccines aid in a sustained release of the antigen, reducing the dosing frequency. By designing an effective oral feed formulated with nano-carriers, the stress induced by the injection system can be reduced. A controlled and sustained release of the antigen will ensure a good immunogenic memory. With these properties, nanovaccines have shown promising potential for the prevention of diseases in aquaculture. Hence, exploring new possibilities to increase nanovaccine safety and efficacy should be the chief objective for further research in the field to enable aquaculture industries all around the world to use nanovaccines routinely in the future.
References
Abdelghany SM, Quinn DJ, Ingram RJ et al (2012) Gentamicin-loaded nanoparticles show improved antimicrobial effects towards Pseudomonas aeruginosa infection. Int J Nanomedicine 7:4053
Adams A (2019) Progress, challenges and opportunities in fish vaccine development. Fish Shellfish Immunol 90:210–214
Adomako M, St-Hilaire S, Zheng Y, Eley J, Marcum RD, Sealey W, Donahower BC, LaPatra S, Sheridan PP (2012) Oral DNA vaccination of rainbow trout, Oncorhynchus mykiss (Walbaum), against infectious haematopoietic necrosis virus using PLGA [poly (D, L-lactic-co-glycolic acid)] nanoparticles. J Fish Dis 35:203–214
Aguila A, Donachie AM, Peyre M et al (2006) Induction of protective and mucosal immunity against diphtheria by a immune stimulating complex (ISCOMS) based vaccine. Vaccine 24:5201–5210
Aklakur M, Asharf Rather M, Kumar N (2016) Nanodelivery: an emerging avenue for nutraceuticals and drug delivery. Crit Rev Food Sci Nutr 56:2352–2361
Arca HC, Günbeyaz M, Senel S (2009) Chitosan-based systems for the delivery of vaccine antigens. Expert Rev Vaccines 8:937–953
Badekila AK, Kini S, Jaiswal AK (2021) Fabrication techniques of biomimetic scaffolds in three-dimensional cell culture: a review. J Cell Physiol 236:741–762
Ballesteros NA, Castro R, Abos B et al (2013) The pyloric caeca area is a major site for IgM+ and IgT+ B cell recruitment in response to oral vaccination in rainbow trout. PLoS One 8:e66118
Behera T, Swain P (2011) Antigen adsorbed calcium phosphate nanoparticles stimulate both innate and adaptive immune response in fish, Labeo rohita H. Cell Immunol 271:350–359
Benezra M, Penate-Medina O, Zanzonico PB et al (2011) Multimodal silica nanoparticles are effective cancer-targeted probes in a model of human melanoma. J Clin Invest 121:2768–2780
Bhardwaj P, Bhatia E, Sharma S et al (2020) Advancements in prophylactic and therapeutic nanovaccines. Acta Biomater 108:1–21
Bhavsar MD, Amiji MM (2007) Polymeric nano-and microparticle technologies for oral gene delivery. Expert Opin Drug Deliv 4:197–213
Chahal JS, Khan OF, Cooper CL et al (2016) Dendrimer-RNA nanoparticles generate protective immunity against lethal Ebola, H1N1 influenza, and toxoplasma gondii challenges with a single dose. Proc Natl Acad Sci U S A 113:E4133–E4142
Choi SH, Oh CH (2007) Effect of oral immunization with liposome-entrapped bacterial antigen on protection against experimental Aeromonas hydrophila. Int J Integr Biol 11:33–38
Collins C, Lorenzen N, Collet B (2019) DNA vaccination for finfish aquaculture. Fish Shellfish Immunol 85:106–125
Danhier F, Ansorena E, Silva JM et al (2012) PLGA-based nanoparticles: an overview of biomedical applications. J Control Release 161:505–522
Dong CF, Lin TL, Gong H, Ou YSD, Yang S. (2005) Major outer membrane protein (MOMP) of Aeromonas hydrophila induced protective immunity to European eels (Anguilla anguilla). Acta Hydrobiol Sin 29:285–290
Dubey S, Avadhani K, Mutalik S et al (2016a) Aeromonas hydrophila OmpW PLGA nanoparticle oral vaccine shows a dose-dependent protective immunity in rohu (Labeo rohita). Vaccine 4:21
Dubey S, Avadhani K, Mutalik S et al (2016b) Edwardsiella tarda OmpA encapsulated in chitosan nanoparticles shows superior protection over inactivated whole cell vaccine in orally vaccinated fringed-lipped peninsula carp (Labeo fimbriatus). Vaccine 4:40
Elamanchili P, Diwan M, Cao M, Samuel J (2004) Characterization of poly (D, L-lactic-co-glycolic acid) based nanoparticulate system for enhanced delivery of antigens to dendritic cells. Vaccine 22:2406–2412
Embregts CW, Forlenza M (2016) Oral vaccination of fish: lessons from humans and veterinary species. Dev Comp Immunol 64:118–137
Fernandez-Alonso M, Alvarez F, Estepa A et al (1999) A model to study fish DNA immersion vaccination by using the green fluorescent protein. J Fish Dis 22:237–241
Fifis T, Mottram P, Bogdanoska V et al (2004) Short peptide sequences containing MHC class I and/or class II epitopes linked to nano-beads induce strong immunity and inhibition of growth of antigen-specific tumour challenge in mice. Vaccine 23:258–266
Foged C, Brodin B, Frokjaer S, Sundblad A (2005) Particle size and surface charge affect particle uptake by human dendritic cells in an in vitro model. Int J Pharm 298:315–322
Fredriksen BN, Grip J (2011) PLGA/PLA micro-and nanoparticle formulations serve as antigen depots and induce elevated humoral responses after immunization of Atlantic salmon (Salmo salar L.). Vaccine 30:656–667
Fredriksen BN, Sævareid K, McAuley L et al (2011) Early immune responses in Atlantic salmon (Salmo salar L.) after immunization with PLGA nanoparticles loaded with a model antigen and β-glucan. Vaccine 29:8338–8349
Ganda IS, Zhong Q, Hali M et al (2017) Dendrimer-conjugated peptide vaccine enhances clearance of chlamydia trachomatis genital infection. Int J Pharm 527:79–91
Gheibi Hayat SM, Darroudi M (2019) Nanovaccine: A novel approach in immunization. J Cell Physiol 234:12530–12536
Giddam AK, Zaman M, Skwarczynski M, Toth I (2012) Liposome-based delivery system for vaccine candidates: constructing an effective formulation. Nanomed J 7:1877–1893
Halimi M, Alishahi M, Abbaspour MR et al (2019) Valuable method for production of oral vaccine by using alginate and chitosan against Lactococcus garvieae/streptococcus iniae in rainbow trout (Oncorhynchus mykiss). Fish Shellfish Immunol 90:431–439
Harikrishnan R, Balasundaram C, Heo MS (2012a) Effect of Inonotus obliquus enriched diet on hematology, immune response, and disease protection in kelp grouper, Epinephelus bruneus against Vibrio harveyi. Aquac Res 344:48–53
Harikrishnan R, Balasundaram C, Heo MS (2012b) Poly d, l-lactide-co-glycolic acid (PLGA)-encapsulated vaccine on immune system in Epinephelus bruneus against Uronema marinum. Exp Parasitol 131:325–332
Harikrishnan R, Kim JS, Balasundaram C, Heo MS (2012c) Vaccination effect of liposomes entrapped whole cell bacterial vaccine on immune response and disease protection in Epinephelus bruneus against Vibrio harveyi. Aquac Res 342:69–74
Heegaard PM, Boas U, Sorensen NS (2010) Dendrimers for vaccine and immunostimulatory uses. A review. Bioconjug Chem 21:405–418
Heidarieh M, Moodi S, Katuli KK, Unger H (2015) Biochemical effects of encapsulated radiovaccine via alginate nanoparticles as useful strategy for booster in immunized rainbow trout against Ichthyophytirius multifiliis. Acta Sci Vet 43:1330
Hillaireau H, Couvreur P (2009) Nanocarriers’ entry into the cell: relevance to drug delivery. Cell Mol Life Sci 66:2873–2896
Irie T, Watarai S, Iwasaki T, Kodama H (2005) Protection against experimental Aeromonas salmonicida infection in carp by oral immunization with bacterial antigen entrapped liposomes. Fish Shellfish Immunol 18:235–242
Jeong KH, Kim HJ, Kim HJ (2020) Current status and future directions of fish vaccines employing virus-like particles. Fish Shellfish Immunol 100:49–57
Ji J, Torrealba D, Ruyra À, Roher N (2015) Nanodelivery systems as new tools for immunostimulant or vaccine administration: targeting the fish immune system. Biology 4:664–696
Jovanovic B, Anastasova L, Rowe EW et al (2011) Effects of nanosized titanium dioxide on innate immune system of fathead minnow (Pimephales promelas Rafinesque, 1820). Ecotoxicol Environ Saf 74:675–683
Joyappa DH, Kumar CA, Banumathi N et al (2009) Calcium phosphate nanoparticle prepared with foot and mouth disease virus P1-3CD gene construct protects mice and Guinea pigs against the challenge virus. Vet Microbiol 139:58–66
Kalkanidis M, Pietersz GA, Xiang SD et al (2006) Methods for nano-particle based vaccine formulation and evaluation of their immunogenicity. Methods 40:20–29
Kanchan V, Panda AK (2007) Interactions of antigen-loaded polylactide particles with macrophages and their correlation with the immune response. Biomaterials 28:5344–5357
Katare YK, Panda AK (2006) Influences of excipients on in vitro release and in vivo performance of tetanus toxoid loaded polymer particles. Eur J Pharm Sci 28:179–188
Kavaliauskis A, Arnemo M, Speth M et al (2016) Protective effect of a recombinant VHSV-G vaccine using poly (I: C) loaded nanoparticles as an adjuvant in zebrafish (Danio rerio) infection model. Dev Comp Immunol 61:248–257
Khurana A, Tekula S, Saifi MA et al (2019) Therapeutic applications of selenium nanoparticles. Biomed Pharmacother 111:802–812
Klajnert B, Bryszewska M (2001) Dendrimers: properties and applications. Acta Biochim Pol 48:199–208
Kobayashi K, Wei J, Iida R et al (2014) Surface engineering of nanoparticles for therapeutic applications. Polym J 46:460–468
Kole S, Kumari R, Anand D et al (2018) Nanoconjugation of bicistronic DNA vaccine against Edwardsiella tarda using chitosan nanoparticles: evaluation of its protective efficacy and immune modulatory effects in Labeo rohita vaccinated by different delivery routes. Vaccine 36:2155–2165
Kole S, Qadiri SSN, Shin SM et al (2019) PLGA encapsulated inactivated-viral vaccine: formulation and evaluation of its protective efficacy against viral haemorrhagic septicaemia virus (VHSV) infection in olive flounder (Paralichthys olivaceus) vaccinated by mucosal delivery routes. Vaccine 37:973–983
Li L, Lin SL, Deng L, Liu ZG (2013) Potential use of chitosan nanoparticles for oral delivery of DNA vaccine in black seabream Acanthopagrus schlegelii Bleeker to protect from Vibrio parahaemolyticus. J Fish Dis 36:987–995
Liu Y, Wang FQ, Shah Z et al (2016) Nano-polyplex based on oleoyl-carboxymethy-chitosan (OCMCS) and hyaluronic acid for oral gene vaccine delivery. Colloids Surf 145:492–501
Maiti B, Shetty M, Shekar M et al (2012) Evaluation of two outer membrane proteins, Aha1 and OmpW of Aeromonas hydrophila as vaccine candidate for common carp. Vet Immunol Immunopathol 149:298–301
Maiti B, Dubey S, Munang’andu HM et al (2020) Application of outer membrane protein-based vaccines against major bacterial fish pathogens in India. Front Immunol 11:1362
Manolova V, Flace A, Bauer M et al (2008) Nanoparticles target distinct dendritic cell populations according to their size. Eur J Immunol 38:1404–1413
McCall RL, Sirianni RW (2013) PLGA nanoparticles formed by single-or double-emulsion with vitamin E-TPGS. J Vis Exp 82
Mody KT, Popat A, Mahony D, Cavallaro AS, Yu C, Mitter N (2013) Mesoporous silica nano-particles as antigen carriers and adjuvants for vaccine delivery. Nano 5:5167–5179
Munang’andu HM, Fredriksen BN, Mutoloki S et al (2012) Comparison of vaccine efficacy for different antigen delivery systems for infectious pancreatic necrosis virus vaccines in Atlantic salmon (Salmo salar L.) in a cohabitation challenge model. Vaccine 30:4007–4016
Mutlu GM, Budinger GS, Green AA et al (2010) Biocompatible nanoscale dispersion of single-walled carbon nanotubes minimizes in vivo pulmonary toxicity. Nano Lett 10:1664–1670
Najafi-Hajivar S, Zakeri-Milani P, Mohammadi H et al (2016) Overview on experimental models of interactions between nanoparticles and the immune system. Biomed Pharmacother 83:1365–1378
Nasr-Eldahan S, Nabil-Adam A, Shreadah MA et al (2021) A review article on nanotechnology in aquaculture sustainability as a novel tool in fish disease control. Aquac Int 29:1–22
Nikapitiya C, Dananjaya SHS, De Silva BCJ et al (2018) Chitosan nanoparticles: a positive immune response modulator as display in zebrafish larvae against Aeromonas hydrophila infection. Fish Shellfish Immunol 76:240–246
Pati R, Shevtsov M, Sonawane A (2018) Nanoparticle vaccines against infectious diseases. Front Immunol 9:2224
Poobalane S, Thompson KD, Ardó L et al (2010) Production and efficacy of an Aeromonas hydrophila recombinant S-layer protein vaccine for fish. Vaccine 28:3540–3547
Prokop A, Davidson JM (2008) Nanovehicular intracellular delivery systems. J Pharm Sci 97:3518–3590
Rajesh Kumar S, Ishaq Ahmed VP, Parameswaran V et al (2008) Potential use of chitosan nanoparticles for oral delivery of DNA vaccine in Asian sea bass (Lates calcarifer) to protect from vibrio (Listonella) anguillarum. Fish Shellfish Immunol 25:47–56
Rauta PR, Nayak B (2015) Parenteral immunization of PLA/PLGA nanoparticle encapsulating outer membrane protein (Omp) from Aeromonas hydrophila: evaluation of immunostimulatory action in Labeo rohita (rohu). Fish Shellfish Immunol 44:287–294
Rivas-Aravena A, Sandino AM, Spencer E (2013) Nanoparticles and microparticles of polymers and polysaccharides to administer fish vaccines. Biol Res 46:407–419
Rivas-Aravena A, Fuentes Y, Cartagena J et al (2015) Development of a nanoparticle-based oral vaccine for Atlantic salmon against ISAV using an alphavirus replicon as adjuvant. Fish Shellfish Immunol 45:157–166
Rombout JH, Abelli L, Picchietti S et al (2011) Teleost intestinal immunology. Fish Shellfish Immunol 31:616–626
Ruyra A, Cano-Sarabia M, García-Valtanen P et al (2014) Targeting and stimulation of the zebrafish (Danio rerio) innate immune system with LPS/dsRNA-loaded nanoliposomes. Vaccine 32:3955–3962
Salonius K, Simard N, Harland R, Ulmer JB (2007) The road to licensure of a DNA vaccine. Expert Opin Investig Drugs 8:635
Salvador A, Igartua M, Hernández RM, Pedraz JL (2011) An overview on the field of micro-and nanotechnologies for synthetic peptide-based vaccines. J Drug Deliv
Semete B, Booysen L, Lemmer Y et al (2010) In vivo evaluation of the biodistribution and safety of PLGA nanoparticles as drug delivery systems. Nanomedicine 6:662–671
Shen H, Ackerman AL, Cody V et al (2006) Enhanced and prolonged cross-presentation following endosomal escape of exogenous antigens encapsulated in biodegradable nanoparticles. Immunology 117:78–88
Slütter B, Bal SM, Que I et al (2010) Antigen − adjuvant nanoconjugates for nasal vaccination: an improvement over the use of nanoparticles? Mol Pharm 7:2207–2215
Smith JD, Morton LD, Ulery BD (2015) Nanoparticles as synthetic vaccines. Curr Opin Biotechnol 34:217–224
Stride MC, Polkinghorne A, Nowak BF (2014) Chlamydial infections of fish: diverse pathogens and emerging causes of disease in aquaculture species. Vet Microbiol 170:19–27
Tian J, Yu J (2011) Poly (lactic-co-glycolic acid) nanoparticles as candidate DNA vaccine carrier for oral immunization of Japanese flounder (Paralichthys olivaceus) against lymphocystis disease virus. Fish Shellfish Immunol 30:109–117
Uto T, Akagi T, Hamasaki T et al (2009) Modulation of innate and adaptive immunity by biodegradable nanoparticles. Immunol Lett 125:46–52
Vartak A, Sucheck SJ (2016) Recent advances in subunit vaccine carriers. Vaccine 4:12
Vimal S, Taju G, Nambi KN et al (2012) Synthesis and characterization of CS/TPP nanoparticles for oral delivery of gene in fish. Aquaculture 358:14–22
Vimal S, Majeed SA, Nambi KSN et al (2014) Delivery of DNA vaccine using chitosan–tripolyphosphate (CS/TPP) nanoparticles in Asian sea bass, Lates calcarifer (Bloch, 1790) for protection against nodavirus infection. Aquaculture 420:240–246
Vinay TN, Tanmoy GC, Anutosh P et al (2016) Nanovaccines: a possible solution for mass vaccination in aquaculture. World Aquacult 31
Wang Y, Liu GL, Li DL et al (2015) The protective immunity against grass carp reovirus in grass carp induced by a DNA vaccination using single-walled carbon nanotubes as delivery vehicles. Fish Shellfish Immunol 47:732–742
Wendorf J, Singh M, Chesko J et al (2006) A practical approach to the use of nanoparticles for vaccine delivery. J Pharm Sci 95:2738–2750
Yasumoto S, Kuzuya Y, Yasuda M et al (2006) Oral immunization of common carp with a liposome vaccine fusing koi herpesvirus antigen. Fish Pathol 41:141–145
Yildirimer L, Thanh NT, Loizidou M, Seifalian AM (2011) Toxicology and clinical potential of nanoparticles. Nano Today 6:585–607
Zaman M, Good MF, Toth I (2013) Nanovaccines and their mode of action. Methods 60:226–231
Zhang L, Zeng Z, Hu C et al (2016) Controlled and targeted release of antigens by intelligent shell for improving applicability of oral vaccines. Biomaterials 77:307–319
Zhao L, Seth A, Wibowo N et al (2014) Nanoparticle vaccines. Vaccine 32:327–337
Zheng F, Liu H, Sun X et al (2016) Development of oral DNA vaccine based on chitosan nanoparticles for the immunization against reddish body iridovirus in turbots (Scophthalmus maximus). Aquaculture 452:263–271
Zhu B, Liu GL, Gong YX et al (2014) Single-walled carbon nanotubes as candidate recombinant subunit vaccine carrier for immunization of grass carp against grass carp reovirus. Fish Shellfish Immunol 41:279–293
Zhu B, Liu GL, Gong YX et al (2015) Protective immunity of grass carp immunized with DNA vaccine encoding the vp7 gene of grass carp reovirus using carbon nanotubes as a carrier molecule. Fish Shellfish Immunol 42:325–334
Zolnik BS, González-Fernández Á, Sadrieh N, Dobrovolskaia MA (2010) Minireview: nanoparticles and the immune system. Endocrinology 151:458–465
Acknowledgments
This study was supported by the Department of Science and Technology (DST), Government of India, through the Indo-Norway joint project (INT/NOR/RCN/BIO/P-01/2018) under Bioeconomy.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Maiti, B., Harshitha, M., Disha, S., Badekila, A.K., Kini, S., Rai, P. (2023). Nanovaccine. In: Kirthi, A.V., Loganathan, K., Karunasagar, I. (eds) Nanotechnological Approaches to the Advancement of Innovations in Aquaculture. Nanotechnology in the Life Sciences. Springer, Cham. https://doi.org/10.1007/978-3-031-15519-2_3
Download citation
DOI: https://doi.org/10.1007/978-3-031-15519-2_3
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-15518-5
Online ISBN: 978-3-031-15519-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)