Abstract
Soybean is an important leguminous crop because its seed is a rich source of protein and oil. It is necessary to increase soybean production to meet the needs of the rapidly increasing human population in the world. Pythium root rot (PRR) caused by Pythium spp. is a major seedling disease of soybean. The breakdown of PRR resistance is the reason for yield uncertainty in many soybean-producing regions. Host plant resistance is the most effective, economical, and environment-friendly method to cope with this disease. Conventional breeding strategies, which rely solely on phenotypic selection, have not successfully produced new PRR-resistant cultivars with long-lasting and broad spectrum of resistance. Over recent decades, substantial progress has been made to overcome the conventional breeding limitations through molecular breeding. Molecular breeding strategies such as quantitative trait locus (QTL) analysis, marker-assisted selection (MAS), and genetic transformation have helped to enhance the resistance levels and developed new disease-resistant cultivars in different crops, including soybean in a short span of time. So far, many QTLs and genes conferring resistance to PRR of soybean have been identified through various molecular breeding approaches. This chapter briefly reviews molecular breeding approaches for improving PRR resistance in soybean.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
8.1 Introduction
Soybean (Glycine max (L.) Merr.) is an important legume crop with numerous uses for food, feed, and industrial materials. The crop is the predominant source of high-quality vegetable protein and oil for food products. The protein content in soybean seed is approximately 40%, and the oil content is about 20% (Clemente and Cahoon 2009). Soybean is not only a source of food for humans but also a universally acceptable animal feed and has numerous industrial applications (i.e., pharmaceuticals, cosmetics, and biodiesel) (Song et al. 2011; Candeia et al. 2009; Ko et al. 2013). Soybeans are becoming a more popular crop plant as a result of their many uses, which is driving up demand. The amount of soybean production is severely threatened by various biotic and abiotic stresses, like other economically important crops (Hartman et al. 2015). Among the biotic stresses, diseases are detrimental to flourishing soybean production. Pythium root rot (PRR) caused by Pythium spp. is a destructive seedling disease found in all soybean-producing regions of the world. Therefore, addressing this issue is important to ensure soybean production profitability while ensuring global food/feed security.
Management practices of PRR disease include fungicide applications and agronomic practices such as crop rotation and ploughing the field for better drainage (Dorrance et al. 2004; Broders et al. 2007; Radmer et al. 2017). However, the effect of fungicide on seed and the emerging seedling is temporary (1–2 weeks after planting). Therefore, it cannot efficiently prevent the emerging root. Aegerter et al. (2002) reported that extensive use of fungicides to disease control has resulted in ineffectiveness to the targeted pathogen. On the other hand, fungicide application has a detrimental effect on public health, farmer’s health, and the environment (Paulsrud and Montgomery 2005). In this context, host plant resistance (HPR) is an effective, economical, and environment-friendly method to cope with this disease (Rupe et al. 2011; Lin et al. 2013). At the same time, due to the evolution of new isolates, a breakdown of PRR resistance has occurred, and resistance remains non-susceptible for a limited duration. Therefore, new methods offering long-standing defense over extensive geographical areas must be developed to build a robust resistance. Existing studies related to plant disease resistance mechanism have revealed that more than one gene contributes to the directive of pathogen-triggered defense responses (Gordon et al. 2007; Kou and Wang 2010; Zhang et al. 2013). Traditional breeding, which depends solely on phenotypic selection, is not successful in developing disease-resistant cultivars due to the environmental effects, genotype × environment interactions, and observation blunders. Therefore, researchers have shown attention to advanced technologies that can make this procedure more effective. Recent developments in molecular breeding have opened the door to various novel methods to enhance breeding selection strategies.
In the last two decades, we have witnessed the success of molecular breeding approaches to discover the genetic and molecular bases of disease resistance and ultimately to generate genotypes enhanced for disease resistance in different crops, including soybean (Rosso et al. 2008; Ali and Yan 2012; Li et al. 2020). Breeders have made increasing use of molecular breeding approaches in soybean breeding programs. For soybean molecular breeding programs, quantitative trait locus (QTL) analysis, marker-assisted selection (MAS), and genetic transformation are the most frequently used techniques. These techniques allow researchers to make enhancements to a soybean plant’s genetic composition with a view to improving disease resistance. To date, many QTLs and genes associated with resistance to PRR of soybean have been identified through various molecular breeding approaches (Stasko et al. 2016; Clevinger et al. 2021). The present chapter discusses the molecular breeding approaches for improving PRR resistance in soybean.
8.2 Pythium Root Rot: A Threat to Soybean Production
PRR caused by Pythium spp. is one of the severe seedling diseases that attack soybean. Pythium is a soil-borne oomycete pathogen that belongs to the class of Oomycota and the family Pythiaceae. Generally, the documentation of Pythium spp. has been based on the visual examination of their morphological characteristics. The primary criteria used by researchers to identify the species are the occurrence of sexual reproductive structures, sporangia type, oogonial wall, and antheridia characters (Matsumoto et al. 1999; Li et al. 2019). Saturated soil is the primary reason for Pythium infection, and more than 50 described Pythium spp. are accountable for PRR disease in soybean (Papa et al. 1967; Kirkpatrick et al. 2006; Rojas et al. 2017). Experimental studies showed that Pythium sp. infection and damage are specific to temperature. For instance, P. debaryanum, P. torulosum, and P. ultimum cause infection and damage to soybean at lower temperatures (20 °C or less) and early planted soybean. In contrast, P. aphanidermatum and P. myriotylum cause infection to soybean at high temperatures (30 °C or higher) and prevail to damage the late-planted soybeans (Yang 1999).
PRR is a persistent issue in places that are over-irrigated or inadequately drained or have just had a lot of rain. PRR likely disturbs soybeans before the germination stage and continues through the seedling stage. The primary signs of pythium infection in the field are generally pre- and post-emergence damping-off (Coffua et al. 2016; Wei et al. 2011). In the case of pre-emergence, seeds are collapsed and fail to germinate. For post-emergence damping-off, lesions and discoloration occur in the root, and following infection, roots will start to disintegrate and rot. The seedling will finally collapse because of the decaying root system. In comparison to older and larger plants, young and small plants are highly susceptible to PRR. This is due to the fact that each plant’s root tissue thickness is different. Diseased plants are pulled from the soil because of rotted roots (Hartman et al. 1999). Considerable reduction and overall yield loss is a result of this disease in large soybean-producing areas under favorable environmental conditions. The main obstacle of controlling disease incited by several Pythium spp. is difficult to accurately identifying all the species associated with the disease. The challenge is correctly differentiating Pythium spp. and figuring out how many diverse Pythium spp. occur in the field, which species more often occur in the field, and which are the most pathogenic (Broders et al. 2007). Attempts are being made to generate soybean genotypes that are resistant to PRR. Continuous research investigations on PRR disease are vital to overcoming this disease issue and ensuring soybean production in the future.
8.3 Notable PRR-Resistant Soybean Accessions Reported
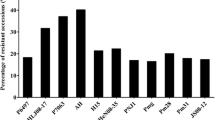
There have been limited studies on resistance to individual or more than one Pythium spp. in soybean. So far, soybean accessions with complete resistance to Pythium spp. have been reported. Several soybean accessions may have a moderate resistance to Pythium spp. (Klepadlo et al. 2019). Details on PRR-resistant soybean accessions are summarized in Table 8.1. Earlier, Keeling (1974) described that the soybean cultivar (cv.) ‘Semmes’ was more resistant than ‘Hood’. Grifen (1990) reported the soybean cv ‘Dare’ had significantly higher emergence in P. ultimum-infested soil than the cultivar ‘Essex’, suggesting a greater level of resistance in Dare than in Essex. Soybean cultivar Archer and breeding line V81-141 were resistant to P. ultimum (Kirkpatrick et al. 2006; Bates et al. 2008). Also, Archer has exhibited a resistance to different Pythium spp. including P. aphanidermatum, P. irregulare, and P. vexans. There were 1289 soybean accessions obtained from the USDA soybean germplasm collection assessed by Bernard et al. (1998), for resistance to P. ultimum. In this study, 60 soybean accessions with resistance to P. ultimum were identified. PI 424354 showed the highest levels of partial resistance against P. irregulare isolates (Ellis et al. 2013). Among the 298 soybean genotypes, Dennison, Williams, Kottman, Streeter, and Wyandot expressed a moderate level of resistance to P. ultimum (Balk et al. 2014). Researchers evaluated 90 North American ancestral soybean genotypes and reported several genotypes, including PI 84637, Maple Isle, Fiskeby III, and Fiskeby 840-7-3, had moderate levels of resistance to P. ultimum, P. irregulare, and P. sylvaticum (McLachlan, 2016; Rod et al. 2018). In another study, soybean genotypes E05226-T and E09014 from Michigan State University were reported to be moderately resistant to P. sylvaticum (Lin et al. 2018). Primary screening results of Lerch-Olson et al. (2020) found that soybean accessions including PI 424237A, PI 424237B, PI 408097, and PI 408029 had higher levels of resistance to P. sylvaticum, P. irregulare, P. oopapillum, and P. torulosum.
8.4 Breeding Soybean for PRR Control
The most desirable method of controlling PRR disease is the breeding of resistant cultivars. It can reduce fungicide use, successively controlling agrochemical pollution in the soybean fields and decreasing production costs. Plant breeders, geneticists, and farmers are continuously paying attention and seeking new techniques for resistance breeding in soybean to reduce the damages incited by PRR. Dissecting genetic resistance is an important strategy to manage this disease. Earlier, traditional breeding methods are successful in dissecting the genetics of resistance and developing disease-resistant cultivars in soybean (Wilcox 1983; Ma et al. 1995; Chen et al. 2001; Rosso et al. 2008). They can generate novel genetic variants, preserving wild germplasm, and hybridization among diverse parents and mutation. Different methods, including pedigree, backcrossing, recurrent selection, and mutant breeding, are widely used in traditional breeding programs to develop disease-resistant genotypes in soybean (Wilcox 1983; Poehlman 1987). However, disease resistance breeding via traditional methods has acceptable flaws, including prolonged breeding cycle, difficulties in the appropriate selection of genotypes, the challenge in distant crossing resulting in a lag among the generation of novel resistant cultivars, and the evolution of virulent isolates of the pathogen, and labor-intensive task. Besides, traditional breeding is influenced by linkage drag, which has resulted in the transferring of loci having possibly unwished agronomic traits because of its close linkage with resistance loci (Pratap et al. 2021). Current advances in biotechnology have offered promising techniques for plant breeders to improve the soybean cultivars of the future that is referred to as molecular breeding. Molecular breeding approaches are successful in tracing the resistance and introducing genes that provide resistance against diseases.
8.5 Molecular Breeding Approaches for PRR Resistance
Over recent decades, we have seen a knowledge explosion in soybean molecular genetics. Soybean is considered a fruitful crop in biotechnological approaches leading to crop improvements. The soybean genome has been sequenced (Schmutz et al. 2010), and the whole genome sequence (WGS) has helped to know the genome composition. Deciphering WGS has the potential to transform soybean breeding by making polymorphism detection, gene expression, and genotyping of populations more convenient and achievable. Therefore, soybean research aimed to know the function of soybean genes and pinpoint the governing mechanisms related to major agronomic traits, including disease and insect pest resistance, and successive conversion of genomic information into agricultural production by different molecular breeding approaches and better agronomic practices. So far, a variety of molecular breeding approaches (i.e., QTL analysis, MAS, and genetic transformation techniques) have been implied to attain disease resistance (i.e., durable and/or broad-spectrum resistance) in soybean (Shi et al. 2008; Gao et al. 2015; Stasko et al. 2016). Several QTLs and genes associated with various diseases, including PRR, have been identified and sequenced. This knowledge assists in understanding the interaction between the host and disease for breeding programs. Besides, diverse genes and mechanisms associated with soybean defense response have been identified and discussed.
DNA markers are one of the important genomics tools assisted to soybean breeding for cultivar development, evaluation, and selection of genotypes. The use of DNA markers allows us to quickly test the existence of more than one disease resistance gene without examining the progeny or relying on rough phenotype evaluation. The SSR-based marker system (producing a high level of polymorphism and repetition compared to other marker systems) based on PCR is more accurate, consistent, and inexpensive. SSRs have been used to detect plants having disease resistance genes in soybean (Cregan et al. 1999; Li et al. 2017; Ren et al. 2018). Next-generation sequencing (NGS) technologies created the opportunities to develop a set of new markers and genotyping platforms. Therefore, information about millions of simple sequence repeats (SSR) and single nucleotide polymorphism (SNP) markers are available in the public domain (Hyten et al. 2010; Song et al. 2010, 2013). Geneticists and breeding scientists effectively used these markers to discover the QTLs and candidate genes associated with resistance to various diseases, including PRR (Rosso et al. 2008; Sun et al. 2014; Karthikeyan et al. 2018). To date, several major and minor QTLs and candidate genes responsible for PRR resistance have been identified, and they were located on chromosomes 6, 8, 10, 13, 18, and 20 in soybean (Ellis et al. 2013; Klepadlo et al. 2019; Lin et al. 2020; Clevinger et al. 2021). However, all of these QTLs and genes are not equally effective to different Pythium spp., which is the causal agent for PRR. The reported QTLs and genes are specific to one or more than one Pythium spp.
As a molecular tag, MAS uses markers that are closely linked to a target gene and can be employed for quick indirect selection of the target gene. The benefits of MAS in soybean improvement are well-known. MAS decreases the phenotypic selection duration and lowers the cost to choose a preferred trait. It assists in accelerating breeding research via permitting to choose plants based on the genotype rather than on the phenotype (Cobb et al. 2019). MAS is a useful and more precise strategy to introduce novel soybean cultivars having disease resistance at the early level. One of the popular applications of MAS is gene pyramiding. By this approach, multiple genes are pyramiding into a single genotype. Marker-aided backcross breeding (MABC) is a popular approach used in soybean disease resistance breeding to incorporate resistance genes (one or more than one genes) into widely adapted popular cultivars. MABC involves employing markers (tightly linked to QTL/gene) to tag the target loci, reducing the length of the donor segment having a target locus, and speeding up the recovery of the recurrent parent genome during backcrossing. MABC’s main goal is to transfer the targeted gene/desired trait together with the background of recurrent parent characters/genome/genes. MAS and MABC are successfully used for the development of soybean disease-resistant lines. There have been some success stories of MAS and MABC in soybean breeding programs to improve disease resistance (i.e., Soybean mosaic virus, rust, Phytophthora sojae, and powdery mildew) (Saghai Maroof et al. 2008; Yamanaka et al. 2015; Ramalingam et al. 2020). However, no such attempts were made to improve the PRR resistance in soybean. Therefore, more research is needed in the future to trace the precise QTLs and genes and their linked markers to PRR resistance in soybean. It will assist in introgression or pyramiding the genes via MABC or MAS.
Genetic engineering has demonstrated to be a notable advancement for soybean breeding programs and permits to produce novel and genetically diverse plant materials. It is also a potential tool in managing plant diseases, and considerable efforts have been done to fix disease resistance in soybean. The question over the stable transformation handicaps the genetic transformation technique in soybean. Compared to MAS, genetic engineering in soybean takes less time and is an efficient as well as a direct approach to enhancing disease resistance. The yield losses caused by diseases have prompted scientists to make better efforts to develop soybean genotypes with improved resistance to diseases by genetic modification. Several transgenic soybean plants, resistant to various diseases, have been reported to date (Gao et al. 2015; Zhou et al. 2018; Yang et al. 2019). For instance, Zhou et al. (2018) detailed that the soybean type II CHI gene (GmCHI1A) shows a positive regulatory role in soybean resistance to P. sojae. In this study, overexpression of GmCHI1A in soybean hairy roots increased the level of daidzein and exhibited resistance to P. sojae. Identifying and using the transgene to engineering the disease resistance is a successful strategy for producing PRR-resistant soybean lines, but no such efforts to increase PRR resistance in soybean have been made. Therefore, even more, research is vital in these areas.
8.6 A Brief Account of PRR Resistance QTLs and Genes
So far, resistance to Pythium spp. in soybean has been categorized into two types, including Rpa1 (Rosso et al. 2008), a monogenic resistance gene to P. aphanidermatum that was considered to be an R-gene type, and several QTLs that are associated with tolerance or partial resistance. Reported QTLs have contributed 4.5–17.8% of the phenotypic variation and are detected on almost all the chromosomes of the soybean genome. The phenotype data and linkage analysis test from Rosso et al. (2008) suggested that P. aphanidermatum resistance in Archer was controlled by a single dominant gene designated as Rpa1. It was located 10.6 cM from Satt510 and 26.6 cM from Satt114. In addition, this study also confirmed that Rpa1 was different from the Rps1k gene in Archer, which is responsible for P. sojae resistance. The first reported QTL for resistance to P. irregulare was discovered in PI 424354 by Ellis et al. (2013). For root weights and root rot scores, resistance QTL with alleles from PI 424354 were identified on chromosomes 1 and 6 in OHS 303× (Williams ×PI 424354). Also, using Dennison × (Williams × PI 424354) population, resistance QTLs showing 7.9 to 17.8% of the phenotypic variation for root weights and root rot scores were mapped on chromosomes 8, 11, and 13. In another study, two QTLs associated with resistance to P. irregulare were detected using a recombinant inbred line (RIL) population developed from a cross between Conrad and Sloan (Stasko et al. 2016). Two QTLs explaining 6.6 and 5.5% phenotypic variation were located in chromosomes 14 and 19–2. Urrea et al. (2017) mapped the two unique QTLs resistance to P. aphanidermatum from soybean cv Archer at chromosomes 4 and 7. These QTLs explained 4.85–13.85 of phenotypic variation, but they could not precisely be mapped on the chromosomes.
Two QTLs (qRRW11 and qRRW20) for tolerance to P. irregulare were detected from E09088 and E05226-T. The QTL qRRW11 was found to account for 15.4% of the phenotypic variation detected on chromosome 13, and its favorable allele was from E09088. Another QTL qRRW20 explained 12.7–13.3% of phenotypic variation located at chromosome 20, and the favorable allele of this QTL was from E05226-T (Lin et al. 2018). In another study, the same research group, by QTL mapping and GWAS approach, identified the loci associated with partial resistance to P. sylvaticum (Lin et al. 2020). QTL mapping revealed five QTLs, namely, q10.1, q10.2, q18.1, q18.2, and q20.1 on soybean chromosomes of 10, 18, and 20. These QTLs accounted for 9.7–16.6% of phenotypic variation. GWAS analysis unveiled the seven SNP markers from chromosomes 10, 18, and 20 were associated with the partial resistance to P. sylvaticum and exhibited phenotypic variations ranged from 8.1% to 10.2%. Of these, three SNP markers were linked with q10.1 (<50 Kb). One SNP marker from Glyma.18 g081700 was co-localized with q18.2. Another one SNP marker co-localized with the previously identified QTL qRRW20 (Lin et al. 2018), for partial resistance to P. irregulare.
Klepadlo et al. (2019) used a RIL population of a cross of Magellan and PI 438489B to map QTLs for resistance to P. ultimum. In this study, two genomic regions (350 kbp region on chromosome 6 and 260 kbp region on chromosome 8) which accounted for 7.5–13.5% and 6.3–16.8% of the phenotypic variance linked to P. ultimum resistance were identified. Besides, genes associated with disease resistance, such as genes encoding leucine-rich repeat (LRR) domain-containing resistance protein kinases, ring/zinc-finger proteins, MYB transcription factors, and receptor-like proteins, were proposed. In another study, six NAM RIL populations were used to map and compare the QTLs for resistance to P. irregulare, P. ultimum, and P. sojae. Only three of the 33 QTLs were associated with resistance to more than one Pythium spp. The major QTL for resistance to P. ultimum var. ultimum and P. ultimum var. sporangiiferum detected at chromosome 3, and two QTLs found on chromosomes 13 and 17 shared a flanking marker for both P. irregulare and P. ultimum var. ultimum (Scott et al. 2019). PI 424237A, PI 424237B, PI 408097, and PI 408029 expressed a high level of resistance to four prevalent Pythium spp. (P. sylvaticum, P. irregulare, P. oopapillum, and P. torulosum) and were used to construct the four independent RIL populations, and several minor and large effect QTLs were detected, including one large-effect QTL for three different Pythium spp. on chromosome 8, and another large effect QTL for two different Pythium spp. on chromosome 6 (Clevinger et al. 2021). Collectively, identified QTLs responsible for resistance to different Pythium spp. might be combined into popular soybean genotypes to build a partial resistance to Pythium spp. Table 8.2 shows the PRR-resistant QTLs detected in the soybean genome.
8.7 Breeding Methods in the Modern Genomic Era to Improve the PRR Resistance
Advances in biotechnological approaches are game-changers in soybean breeding, offering innovative tools for the breeders to understand the disease resistance mechanism and develop disease-resistant cultivars (Li et al. 2020). Site-directed mutagenesis of target genes to alter the gene function or silencing and the targeted insertion or deletion into the soybean genome are among the most recent breeding strategies. Direct transgene insertion is also a modern breeding technique that can effectively obtain homozygous parental lines from heterozygous-type plants. Several groundbreaking breeding technologies developed in the last decade or so which may relate to genome editing include zinc finger nucleases, TALENs, and CRISPR/Cas9 (Wad et al. 2020). The availability of soybean whole genome sequence and accessibility of massive genomic resources in the public platform make existing genome editing technologies and their new developments easier. Also, soybean researchers would like to use a hairy-root transformation system to assess the genome editing tool’s efficacy quickly. However, in soybean research, the use of genome editing technologies in disease resistance studies is limited. There are few reports on CRISPR/Cas9-mediated genome editing technology application in soybean disease resistance (Zhang et al. 2020; Qiu et al. 2021). Increasing resistance to PRR in soybean still awaits investigation by genome editing technologies. Figure 8.1 shows the applications of molecular breeding approaches and genome editing to generate PRR-resistant plants.
8.8 Conclusion and Perspectives
The use of resistant soybean cultivars is the best option to manage the PRR disease, decrease fungicide use, protect the environment, and increase grower returns in the long run. Early research done using the convention methods helps to know the Pythium spp. and their response to soybean. The development of new molecular techniques urges soybean breeders to find the easiness of these techniques and their cost-effectiveness to combine them with traditional breeding. Soybean researchers have taken considerable efforts to understand the disease resistance mechanism and identify the genotypes and QTL and genes associated with PRR resistance. Nevertheless, several research areas remain focused on identifying a broad spectrum of resistant genes, tracing these genes with DNA markers, pyramiding these genes or QTL regions by MAS, and genetic engineering for PRR resistance. Eventually, considering the current success of molecular breeding approaches for combating PRR disease in soybean is encouraged because of the need for increased production of soybean in a fast-growing population.
References
Aegerter BJ, Greathead AS, Pierce LE Davis RM (2002) Mefenoxam-resistant isolates of Pythium irregulare in an ornamental greenhouse in California. Plant Dis 86:692–692
Ali F, Yan J (2012) Disease resistance in maize and the role of molecular breeding in defending against global threat. J Integr Plant Biol 54:134–151
Balk C, Fitzgibbon T, Novakowiski JH, Erye M, Dorrance AE (2014) Assessment of resistance in soybean towards Pythium ultimum var. ultimum and Pythium ultimum var. sporangiiferum. Phytopathology 104:166–166
Bates GD, Rothrock CS, Rupe JC (2008) Resistance of the soybean cultivar archer to Pythium damping-of and root rot caused by several Pythium spp. Plant Dis 92:763–766
Bernard RL, Cremeens CR, Cooper RL, Collins FI, Krober OA, Athow KL, Laviolette FA, Coble CJ, Nelson RL (1998) Evaluation of the USDA soybean germplasm collection: maturity groups 000–IV, USDA Technical Bulletins 1871. US Government Print. Office, Washington, DC
Broders KD, Lipps PE, Paul PA, Dorrance AE (2007) Characterization of Pythium spp. associated with corn and soybean seed and seedling disease in Ohio. Plant Dis 91:727–735
Candeia RA, Silva MCD, Filho J, Brasilino RC, Bicudo MGA, Santos TC, Souza AG (2009) Influence of soybean biodiesel content on basic properties of biodiesel-diesel blends. Fuel 88:738–743
Chen P, Ma G, Buss GR, Gunduz I, Roane CW, Tolin SA (2001) Inheritance and allelism tests of Raiden soybean for resistance to soybean mosaic virus. J Hered 92:51–55
Clemente TE, Cahoon EB (2009) Soybean oil: genetic approaches for modification of functionality and total content. Plant Physiol 151:1030–1040
Clevinger EM, Biyashev R, Lerch-Olson E, Yu H, Quigley C, Song Q, Dorrance AE, Robertson AE, Saghai Maroof MA (2021) Identification of quantitative disease resistance loci toward four Pythium species in soybean. Front Plant Sci 12:644746
Cobb JN, Biswas PS, Platten JD (2019) Back to the future: revisiting MAS as a tool for modern plant breeding. Theor Appl Genet 132:647–667
Coffua LS, Veterano ST, Clipman SJ, Mena-Ali JI, Blair JE (2016) Characterization of Pythium spp. associated with asymptomatic soybean in southeastern Pennsylvania. Plant Dis 100:1870–1879
Cregan PB, Mudge J, Fickus ED, Marek LF, Danesh D, Denny R, Shoemaker RC, Matthews BF, Jarvik T, Young ND (1999) Targeted isolation of simple sequence repeat markers through the use of bacterial artificial chromosomes. Theor Appl Genet 98:919–928
Dorrance AE, Berry SA, Bowen P, Lipps PE (2004) Characterization of Pythium spp. from three Ohio fields for pathogenicity on corn and soybean and metalaxyl sensitivity. Plant. Health Prog 5:10
Ellis ML, McHale LK, Paul PA, St Martin SK, Dorrance AE (2013) Soybean germplasm resistant to Pythium irregulare and molecular mapping of resistance quantitative trait loci derived from the soybean accession PI 424354. Crop Sci 53:1008–1021
Gao L, Ding X, Liao LKW, Zhong Y, Ren R, Liu Z, Adhimoolam K, Zhi H (2015) Characterization of soybean mosaic virus resistance derived from inverted repeat-SMV-HC-Pro genes in multiple soybean cultivars. Theor Appl Genet 128:1489–1505
Gordon SG, Kowitwanich K, Pipatpongpinyo W, Martin SKS, Dorrance AE (2007) Molecular marker analysis of soybean plant introductions with resistance to Phytophthora sojae. Phytopathology 97:113–118
Grifn GJ (1990) Importance of Pythium ultimum in a disease syndrome of cv. Essex soybean. Can J Plant Pathol 12:135–114
Hartman GL, Sinclair JB, Rupe JC (1999) Compendium of soybean diseases and pests. American Phytopathological Society, St. Paul
Hartman GL, Rupe JC, Sikora E, Domier LL, Davis JA, Steffey KL (2015) Compendium of soybean diseases and pests. American Phytopathological Society, St. Paul
Hyten DL, Choi IY, Song Q, Specht JE, Carter TE, Shoemaker RC, Hwang EY, Matukumalli LK, Cregan PB (2010) A high density integrated genetic linkage map of soybean and the development of a 1536 universal soy linkage panel for quantitative trait locus mapping. Crop Sci 50:960–968
Karthikeyan A, Li K, Li C, Yin J, Li N, Yang Y, Song Y, Ren R, Zhi H, Gai J (2018) Fine-mapping and identifying candidate genes conferring resistance to Soybean mosaic virus strain SC20 in soybean. Theor Appl Genet 131:461–476
Keeling BL (1974) Soybean seed rot and the relation of seed exudate to host susceptibility. Phytopathology 64:1445–1447
Kirkpatrick MT, Rothrock CS, Rupe JC, Gbur EE (2006) The effect of Pythium ultimum and soil flooding on two soybean cultivars. Plant Dis 90:597–602
Klepadlo M, Balk CS, Vuong TD, Dorrance AE, Nguyen HT (2019) Molecular characterization of genomic regions for resistance to Pythium ultimum var. ultimum in the soybean cultivar Magellan. Theor Appl Genet 132:405–417
Ko KP, Park SK, Yang JJ, Ma SH, Gwack J, Shin A (2013) Intake of soy products and other foods and gastric cancer risk: a prospective study. J Epidemiol 23:337–343
Kou Y, Wang S (2010) Broad-spectrum and durability: understanding of quantitative disease resistance. Curr Opin Plant Biol 13:181–185
Lerch-Olson ER, Dorrance AE, Robertson AE (2020) Resistance of the SoyNAM parents to seed and root rot caused by four Pythium species. Plant Dis 104:2489–2497
Li C, Adhimoolam K, Yuan Y, Yin J, Ren R, Yang Y, Zhi H (2017) Identification of candidate genes for resistance to soybean mosaic virus strain SC3 by using fine mapping and transcriptome analyses. Crop Pasture Sci 68:156–166
Li N, Zhou Q, Chang KF, Yu H, Hwang SF, Conner RL, Strelkov SE, McLaren DL, Turnbull GD (2019) Occurrence, pathogenicity and species identification of Pythium causing root rot of soybean in Alberta and Manitoba, Canada. Crop Prot 118:36–43
Li MW, Wang Z, Jiang B, Kaga A, Wong FL, Zhang G, Han T, Chung G, Nguyen H, Lam HM (2020) Impacts of genomic research on soybean improvement in East Asia. Theor Appl Genet 133:1655–1678
Lin F, Zhao M, Ping J, Johnson A, Zhang B, Abney TS, Hughes TJ, Ma J (2013) Molecular mapping of two genes conferring resistance to Phytophthora sojae in a soybean landrace PI 567139B. Theor Appl Genet 126:2177–2185
Lin F, Wani SH, Collins PJ, Wen Z, Gu C, Chilvers MI, Wang D (2018) Mapping quantitative trait loci for tolerance to Pythium irregulare in soybean (Glycine max L.). Genes Genomes Genetics 8:3155–3161
Lin F, Wani SH, Collins PJ, Wen Z, Li W, Zhang N, McCoy AG, Bi Y, Tan R, Zhang S, Gu C (2020) QTL mapping and GWAS for identification of loci conferring partial resistance to Pythium sylvaticum in soybean (Glycine max (L.) Merr). Mol Breed 40:1–11
Ma G, Chen PY, Buss GR, Tolin SA (1995) Genetic characteristics of two genes for resistance to Soybean mosaic virus in PI486355 soybean. Theor Appl Genet 91:907–914
Matsumoto C, Kageyama K, Suga H, Hyakumachi M (1999) Phylogenetic relationships of Pythium species based on ITS and 5.8S sequences of the ribosomal DNA. Mycoscience 40:321–331
McLachlan KS (2016) Evaluation of Pythium root rot and damping of resistance in the ancestral lines of North American soybean cultivars and chemical control of the active ingredient ethaboxam in seed treatments. Master thesis. University of Illinois
Papa KE, Campbell WA, Hendrix FF Jr (1967) Sexuality in Pythium sylvaticum: heterothallism. Mycologia 59:589–595
Paulsrud BE, Montgomery M (2005) Characteristics of fungicides used in field crops. Report on Plant disease 1002
Poehlman JM (1987) Breeding field crops, 3rd edn. Van Nostrand Reinhold, New York, pp 421–450
Pratap A, Das A, Kumar S, Gupta S (2021) Current perspectives on introgression breeding in food legumes. Front Plant Sci 11:589189
Qiu M, Li Y, Ye W, Zheng X, Wang Y (2021) A CRISPR/Cas9-mediated in situ complementation method for Phytophthora sojae mutants. Mol Plant Pathol 22:373–381
Radmer L, Anderson G, Malvick DM, Kurle JE, Rendahl A, Mallik A (2017) Pythium, Phytophthora, and Phytopythium spp. associated with soybean in Minnesota, their relative aggressiveness on soybean and corn, and their sensitivity to seed treatment fungicides. Plant Dis 101:62–72
Ramalingam J, Alagarasan G, Savitha P, Lydia K, Pothiraj G, Vijayakumar E, Sudhagar R, Singh A, Vedna K, Vanniarajan C. (2020) Improved host-plant resistance to Phytophthora rot and powdery mildew in soybean (Glycine max (L.) Merr.). Sci Rep. 10(1):13928. https://doi.org/10.1038/s41598-020-70702-x.
Ren R, Yin J, Zheng H, Wang T, Liu S, Karthikeyan A, Yang Q, Gao L, Zhi H, Li K (2018) Characterization of broad-spectrum resistance to Soybean mosaic virus in soybean [Glycine max(L.) Merr.] cultivar ‘RN-9’. Plant Breed 137:605–613
Rod KS, Walker DR, Bradley C (2018) Evaluation of major ancestors of North American soybean cultivars for resistance to three Pythium species that cause seedling blight. Plant Dis 102(11):2241–2252
Rojas JA, Jacobs JL, Napieralski S, Karaj B, Bradley CA, Chase T, Esker PD, Giesler LJ, Jardine DJ, Malvick DK, Markell SG (2017) Oomycete species associated with soybean seedlings in North America—Part II: diversity and ecology in relation to environmental and edaphic factors. Phytopathology 107:293–304
Rosso ML, Rupe JC, Chen P, Mozzoni LA (2008) Inheritance and genetic mapping of resistance to pythium damping-off caused by Pythium aphanidermatum in ‘Archer’ Soybean. Crop Sc 48:2215–2222
Rupe JC, Rothrock CS, Bates G, Rosso ML, Avanzato MV, Chen P (2011) Resistance to Pythium seedling disease in soybean, Soybean molecular aspects of breeding. InTech. https://doi.org/10.5772/15301
Saghai Maroof MA, Jeong SC, Gunduz I, Tucker DM, Buss GR, Tolin SA (2008) Pyramiding of soybean mosaic virus resistance genes by marker-assisted selection. Crop Sci 48:517–526
Schmutz J, Cannon SB, Schlueter J, Ma J, Mitros T, Nelson W, Hyten DL, Song Q, Thelen JJ, Cheng J (2010) Genome sequence of the palaeopolyploid soybean. Nature 463:178–183
Scott K, Balk C, Veney D, McHale LK, Dorrance AE (2019) Quantitative disease resistance loci towards Phytophthora sojae and three species of Pythium in six soybean nested association mapping populations. Crop Sci 59:605–623
Shi A, Chen P, Zheng C, Hou A, Zhang B (2008) A PCR-based marker for the R sv1 locus conferring resistance to soybean mosaic virus. Crop Sci 48:262–268
Song Q, Jia G, Zhu Y, Grant D, Nelson RT, Hwang E-Y, Hyten DL, Cregan PB (2010) Abundance of SSR motifs and development of candidate polymorphic SSR markers (BARCSOYSSR_1. 0) in soybean. Crop Sci 50:1950–1960
Song F, Tang DL, Wang XL, Wang YZ (2011) Biodegradable soy protein isolate-based materials: a review. Biomacromolecules 12:3369–3380
Song Q, Hyten DL, Jia G, Quigley CV, Fickus EW, Nelson RL, Cregan PB (2013) Development and evaluation of SoySNP50K, a high-density genotyping array for soybean. PLoS One 8:e54985
Stasko A, Wickramasinghe D, Nauth B, Acharya B, Ellis M, Taylor C, McHale L, Dorrance A (2016) High density mapping of resistance QTL towards Phytophthora sojae, Pythium irregulare, and Fusarium graminearum in the same soybean population. Crop Sci 56:2476–2492
Sun J, Li L, Zhao J, Huang J, Yan Q, Xing H, Guo N (2014) Genetic analysis and fine mapping of RpsJS, a novel resistance gene to Phytophthora sojae in soybean [Glycine max (L.) Merr.]. Theor Appl Genet 127:913–919
Urrea K, Rupe J, Chen P, Rothrock CS (2017) Characterization of seed rot resistance to Pythium aphanidermatum in soybean. Crop Sci 57:1394–1403
Wad N, Ueta R, Osakabe Y, Osakabe K (2020) Precision genome editing in plants: state-of-the-art in CRISPR/Cas9-based genome engineering. BMC Plant Biol 20:1–12
Wei L, Xue AG, Cober ER, Babcock C, Zhang J, Zhang S, Li W, Wu J, Liu L (2011) Pathogenicity of Pythium species causing seed rot and damping-off in soybean under controlled conditions. Phytoprotection 91:3–10
Wilcox JR (1983) Breeding soybeans resistant to diseases. Plant Breed Rev 1:183–235
Yamanaka N, Morishita M, Mori T, Lemos NG, Hossain MM, Akamatsu H, Yamaoka Y (2015) Multiple Rpp-gene pyramiding confers resistance to Asian soybean rust isolates that are virulent on each of the pyramided genes. Trop Plant Pathol 40:283–290
Yang XB (1999) Pythium damping-off and root rot, 4th edn. APS Press, St. Paul, pp 42–44
Yang X, Niu L, Zhang W, He H, Yang J, Xing G, Guo D, Zhao Q, Zhong X, Li H, Li Q, Dong Y (2019) Increased multiple virus resistance in transgenic soybean overexpressing the double-strand RNA-specific ribonuclease gene PAC1. Transgenic Res 28:129–140
Zhang Y, Lubberstedt T, Xu ML (2013) The genetic and molecular basis of plant resistance to pathogens. J Genet Genomics 40:23–35
Zhang P, Du H, Wang J, Pu Y, Yang C, Yan R, Yang H, Cheng H, Yu D (2020) Multiplex CRISPR/Cas9-mediated metabolic engineering increases soya bean isoflavone content and resistance to soya bean mosaic virus. Plant Biotechnol J 18:1384–1395
Zhou Y, Huang JL, Zhang XL, Zhu LM, Wang XF, Guo N, Zhao JM, Xing H (2018) Overexpression of Chalcone isomerase (CHI) increases resistance against Phytophthora sojae in soybean. J Plant Biol 61:309–319
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Karthikeyan, A., Sarankumar, C., Senthil, N. (2022). Molecular Breeding for Resistance against Pythium Root Rot (PRR) in Soybean. In: Wani, S.H., Sofi, N.u.R., Bhat, M.A., Lin, F. (eds) Soybean Improvement. Springer, Cham. https://doi.org/10.1007/978-3-031-12232-3_8
Download citation
DOI: https://doi.org/10.1007/978-3-031-12232-3_8
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-12231-6
Online ISBN: 978-3-031-12232-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)