Abstract
Since the 1990s, laparoscopic surgery has undergone unprecedented change and expansion. The benefit and attraction of minimally invasive surgery to both patients and surgeons alike forced this growth and a necessity to perform more and more complex operations laparoscopically.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
10.1 Introduction
Since the 1990s, laparoscopic surgery has undergone unprecedented change and expansion. The benefit and attraction of minimally invasive surgery to both patients and surgeons alike forced this growth and a necessity to perform more and more complex operations laparoscopically [1, 2]. Predictably, a threshold was reached, and surgical advancement plateaued. In response, robotic surgery was introduced, and since then its evolution has transformed minimally invasive surgery (MIS) worldwide [3]. This bourgeoning field of robotics has redefined the gold standard of surgical care for many staple uro-oncological procedures. The introduction of the da Vinci Surgical System in 2000 changed the face of modern MIS [4].
Distinct advantages of the robotic approach, compared to laparoscopic surgery, include a surgeon-controlled camera, three-dimensional high-definition magnified surgical vision, and EndoWrist enhanced manoeuvrability with seven degrees of freedom and 90° articulation [5]. Moreover, providing natural movements consequently enhances dexterity and dissection, precise coordination of hands and eyes, filtration of physiological tremor, and motion scaling [6] which allows for precise tissue dissection and suturing [7, 8]. These advantages enable surgeons to perform more complex MIS procedures and extend the feasibility, and therefore benefits, of MIS to more specialists by reducing the learning curve [9, 10]. Consequently, robotic surgery continues to be rapidly adopted in renal and prostate surgery worldwide [11].
10.2 Background
10.2.1 The Origin of Robotic Surgery and Intuitive Surgical
Although Intuitive Surgical, Inc. was founded in 1995, the current Da Vinci platform is an amalgamation of research and innovation that originated prior in the late 1980s at the non-profit Stanford Research Institute (SRI) International. Through combined efforts, Phil Green and Richard Satava pioneered the prototype robotic surgical system. During its evolution, the “telepresence surgery system” caught the attention of the Defence Advanced Research Projects Agency (DARPA), whose focus under the Advanced Biomedical Technologies (ABMT) program was directed toward improving emergency surgical care to combat casualties [12, 13]. The incorporation of telepresence into medical forward advanced surgical treatment (MEDFAST), in conjunction with key technologies from IBM and MIT, would revolutionise the idea of specialised remote operating and was a landmark inspiration for John Freund, Frederick Moll, and Rob Younge to collectively form Intuitive Surgical, Inc. [12,13,14].
After several prototypes, the landmark da Vinci Surgical System was created in 1999, and by 2000 it was approved by the U.S. Food and Drug Administration (FDA) for use in general laparoscopic surgery. In 2001, the FDA approved use of the system for prostate surgery; and since then has revolutionised Uro-oncological surgical procedures and completely changed operative techniques in renal cancer surgery [15].
Shortly before its public release, Intuitive Surgical was sued for patent infringement by Computer Motion, Inc. Computer Motion had already released the ZEUS Robotic Surgical System (ZRSS), which was approved in Europe although not yet so by the FDA. After generating uncertainty for several years and stifling each company's growth, Intuitive Surgical and Computer Motion agreed to merge in 2003, and the ZEUS system was subsequently phased out in favour of the da Vinci system [12, 13] (see Fig. 10.1).
Timeline of selected company milestones [14]
10.3 The Da Vinci Robotic Surgical System
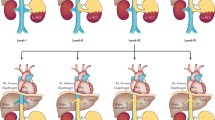
Since the initial public release of the da Vinci Surgical System in 2000, four further generations of da Vinci systems have been introduced to the market: the da Vinci S platform, da Vinci Si platform, da Vinci Xi platform, and da Vinci X platform (see Fig. 10.2). Each generation platform has distinct technological upgrades to optimise surgical techniques and performance. In addition, with each design, the Intuitive market has expanded with rapid succession of innovation from instruments and accessories to systems and services, heralding with it global dissemination, acceptance, and integration of robotic-assisted surgery. At present, 5989 da Vinci systems are in use across 67 different countries, performing over 8.5 million procedures through 2020 [16].
Five generations of the da Vinci Surgical System [14]
At its foundation, compared to prototypes and the ZEUS platform, the original ‘Classic’ da Vinci Surgical System displayed significant enhancements. The robotic system was composed of 3 components—a surgeon console, a patient cart, and a vision cart. All robotic arms originated from a single patient cart, alleviating the need to mount individual arms to the operating table, while providing a solution for optimal table position. The surgeons console provided an innovative three-dimensional visual display with the trademark binocular visualisation which allowed greater optical accommodation and focus, resulting in improved concentration, and reducing surgical fatigue. Additionally, complimentary EndoWrist instrumentation with seven degrees of freedom and two degrees of axial rotation combined with intuitive motion and superior ergonomics culminated in advanced surgical precision. The Classic platform patient cart was originally composed of one endoscope port and two instruments. However, before long, in 2003 a fourth arm was added to overcome exposure limitations. The fourth arm allowed the surgeon greater control of retraction, improved exposure of the surgical field, and reduced dependence on surgical assistance [17].
By 2006, Intuitive Surgical introduced their first generational upgrade in the form on the da Vinci S platform. The new platform offered modest improvements in the form of high-definition (HD) camera vision with an interactive touch display and a more streamlined set-up. In 2009, not satisfied with the previous model, the da Vinci Si platform was released, offering a dual console to optimise collaborative operating and training. Additionally, the incorporation of TilePro software modernised the imaging system, allowing real-time fluorescence imaging with Firefly technology. The Si would become one of the most worldwide distributed platforms for Intuitive since creation. Although remarkable in its time, the Si system had distinct structural limitations. A single, large, vertical column exoskeleton meant that reachable workspace was highly dependent on the orientation of the cart and, combined with bulky robotic arms, frequent external clashing was highly troublesome. Furthermore, multi-quadrant surgery required complete repositioning of the patient cart and redocking of the robotic arms intraoperatively, increasing overall surgical and anaesthetic time.
It wouldn’t be until 5 years later that Intuitive Surgical developed the da Vinci Xi system, which currently still resides today as the flagship platform and most capable system yet. In 2014, the Xi model reinvented the concept of the patient cart design with tremendous mobility, flexibility, and versatility. It introduced new, advanced instrumentation, vision, cart design, table motion and setup automation which almost completely resolved patient cart and arm limitations in previous prototypes. The Xi’s boom-mounted architectural design allows complete rotational all-quadrant access with docking from any angle. This remodelled gantry positions instrument arms directly over operating table, making positioning of the cart base largely independent from workspace orientation, providing overall greater internal range of motion, improving patient access, and minimising external collisions. Additionally, the redesigned flex joints permit robotic arms to be slimmer and compact, unlike earlier da Vinci system generations which required widely spaced external arms to maximise working space. Furthermore, docking of the Xi is streamlined and semi-automated, simple targeting of the surgical field with the endoscope disposes the robotic arms effortlessly into optimal position with appropriate patient clearance. In conjunction with significant upgrades to the patient cart, the surgeon console was modernised with ergonomic refinement, precision control, and improved visualisation technology. Endoscope size was reduced to an 8 mm from previous models, making it less bulky, while providing higher resolution three-dimensional high-definition view, brighter and more immersive images, and longer scope length. Additionally, the Xi 30° camera could be inverted from the surgeon console without bedside assistance. The four now identical robotic arms allowed for versatility and positioning of any instrument in any port at any given time. Moreover, integrated FireFly fluorescence imaging technology, with the administration of indocyanine green, allowed for real-time intraoperative decision-making (e.g., tissue perfusion). Finally, additional instruments were made available (e.g., robotic suction, irrigation, and clip application) and current energy device performance were amplified (e.g., Vessel Sealer Extend) [18, 19].
Most recently, in 2017, Intuitive Surgical released the da Vinci X Surgical System in an expansion bid to provide a financial economical solution to global customers in which cost was a limiting factor. This lower-fee platform offered several key innovative developments taken from the da Vinci Xi system. Although the patient cart is structurally more similar to the Si platform, and despite the lack of a gantry, it boasts a 1.5× greater workspace field than the Si—as compared to 3× greater workspace of the Xi (see Fig. 10.3). This enables the X to provide a more optimised, quadrant-focused surgery (e.g., prostatectomy, partial nephrectomy) and allows the use of finer surgical instruments. Furthermore, the X uses the same vision cart, surgeon console, many of the same advanced instruments and accessories as the Xi, thus providing customers with an upgrade pathway should they desire [14].
10.4 New Robots in Renal Cancer Surgery
Robot-assisted procedures have become a staple in renal surgery, gaining robust clinical status reflected by the current literature. However, the focus and favour of robotic renal surgery lies in technically demanding procedures (i.e., complex and partial nephrectomy, nephroureterectomy, with or without vena cava thrombus, complex transplant surgery, and difficult anatomy as in patients with obesity or adhesions) rather than simple uncomplicated nephrectomies. As described above, the latest generations of leading surgical robots (i.e., da Vinci Xi Surgical System) offer countless mechanical advantages to aid these technical demands, such as (1) three-dimensional high-definition stereoscopic vision, (2) fine-motor tissue manipulation with higher quality instrumentation, (3) ability to perform multi-quadrant surgical procedures (i.e., nephroureterectomy) without the need to re-dock the patient console and thus reduce operative time, (4) easily accessible and integrated FireFly fluorescence imaging technology to assess perfusion and assist with tumour resection [19]. These advancements have been clearly shown to accelerate the learning curve for non-laparoscopic surgeons [20].
Laparoscopic approaches are limited by the challenges of tumour dissection and intracorporal suturing, and in non-robotic institutions open surgery, which carries a longer hospital stay and increased estimated blood loss, may be the only alternative [21]. At present, cost, longer set-up time, and longer overall operative time remain the greatest criticisms for robotic surgery. The role of routine robotic-assisted radical nephrectomy (RARN) is still debatable, primarily due these disparagements of cost and time, versus laparoscopic nephrectomy (LN) [22]. In contrast, a review of 150 nephrectomies revealed that costs of RARN are comparable to LN when a robot is already present [23]. Furthermore, a retrospective single centre review demonstrated that costs of disposable instruments used in LN were comparable to the disposables used in RARN, concluding that robotic surgery for nephrectomy does not always correlate with greater costs [24]. Whether or not these studies are currently widely applicable is unclear; however, if issues of cost are somewhat mitigated in future, then robotic surgery for renal cancer will undeniably become the baseline gold standard approach.
In addition, the Versius Surgical Robotic System (CMR Surgical, Cambridge, UK) is a new tele-operated robotic surgical system designed to assist surgeons in performing MIS and overcome some of the challenges associated with available surgical robots mentioned above (see Fig. 10.4). The Versius System mimics the articulation of the human arm, and with V-wrist technology, the wristed instrument tip provides seven degrees of freedom inside the patient, allowing for even greater surgical access. Instruments and visualisation arms are attached to their own discrete wheeled cart to form a compact and mobile bedside unit. The surgeon interacts with the system via the “game controller” handgrip and visual feedback from the surgeon console. The consoles head-up display relays the three-dimensional video from the endoscopic camera together with a display overlay. Its open design allows surgeons to sit or stand for optimal ergonomics, ensures patient accessibility at all times, and permits easier communication between the surgeon and the team, facilitating training and teaching. The operating room team accesses controls and feedback on the visualisation bedside unit and up to three instrument bedside units, while viewing a two-dimensional version of the endoscope feed and display overlay on an auxiliary display. The systems modular design increases its potential for flexible use, as the bedside units are small enough to be used in a standard operating room and can easily be moved within a single operating room or between operating rooms. The safety and effectiveness of the system in renal cancer surgery have been demonstrated in a feasibility study of 24 procedures successfully completed in cadavers [25].
The main clinical advantages of the robot, compared to a laparoscopic approach, remain the shorter learning curve and more efficient renorrhaphy. Rapid suturing shortens ischemia time associated with renal artery clamping and crucially maximises preservation of renal parenchyma. Hence, the robotic approach sanctions operations of larger and more complex renal masses, especially in the presence of a solitary kidney [26,27,28].
Notably, refinement and development of the da Vinci systems have facilitated optimisation of robotic arm positioning, allowing for a more flexible port placement in renal surgery. This positioning permits multi-quadrant surgery with a wider range of motion of the robotic instruments, while preventing clashing and making several surgical steps such as bowel mobilisation easier. These advantages become even more evident in complex renal cancer surgery (e.g., when performing retroperitoneal lymph node dissection for upper tract urothelial cancer, or more complex surgeries such as cava thrombectomy or renal transplants).
With regards to port placement for renal surgery, the da Vinci X and Xi systems allow for a straight line (‘in-line’) placement of the ports rather than a L-shaped line. In comparison, the S and Si systems narrow range of port placement configurations may make access to the bedside difficult or uncomfortable. Furthermore, the newer da Vinci generation allows for camera targeting, camera hopping, better spatial awareness, and therefore more flexibility to expose the operative field.
Below is a review of the current literature evidence for comparing clinical outcomes of robot-assisted RN and PN versus open and laparoscopic techniques.
10.5 Current Evidence
10.5.1 Radical Nephrectomy (RN)—Robotic Versus Laparoscopic Approach
Data from a large retrospective cohort study on robot-assisted RN (RARN) versus laparoscopic RN (LRN) revealed that RARN was not associated with increased risk of any or major complications; but, had longer operative times and higher hospital costs as compared to LRN [29]. While a systematic review on RARN versus LRN showed no substantial differences in local recurrence rates, or all-cause cancer-specific mortality [30]. The improved dexterity of the robotic nephroureterectomy has clear benefit compared to the laparoscopic approach by improving distal ureteric dissection, excision of bladder cuff and bladder closure. However, the advantages of the robotic approach for nephrectomy, compared to the laparoscopic procedure, are not evident from a pure surgical perspective. This is because the extirpative procedure is technically less challenging. Despite the lack of proven benefit for robotic compared to laparoscopic radical nephrectomy, use of the robot has increased over the last decade. This trend primarily results from the surgeon endeavour to gain experience in hilar dissection during radical nephrectomy to complement the learning curve for partial nephrectomies. Additionally, these skills are essential before adopting more complex renal surgery such as donor nephrectomies or radical nephrectomy with inferior vena cava thrombectomy.
10.5.2 Partial Nephrectomy (PN)—Robotic Versus Open Approach
Data from a prospective, single-surgeon study which compared peri-operative outcomes of robot-assisted PN versus open PN reported lower estimated blood loss and shorter hospital stay in the RAPN group. Complications, operative time, warm ischaemia time, variation in creatinine levels and positive margins were similar in both groups [31]. While a multicentre French prospective database compared outcomes of 1800 patients who underwent RAPN and OPN, and found that the RAPN cohort had lower morbidity with less transfusions, less major complications, less overall complications, and a much shorter hospital stay [32].
10.5.3 Partial Nephrectomy (PN)—Robotic Versus Laparoscopic Approach
Data from a retrospective propensity-score-matched study, comparing RAPN, LPN and OPN demonstrated similar rates of local recurrence, distant metastasis, and cancer-related death rates after 5-year median follow-up [33]. While a meta-analysis compared peri-operative outcomes of RAPN versus LPN, and found that the RARP arm had significantly lower rate of conversion to open surgery and to radical surgery, shorter warm ischaemia time, smaller change in eGFR post-operatively, and shorter length of hospital stay. No significant differences were observed between the two groups regarding complications, change of serum creatinine post-operatively, operative time, estimated blood loss and positive surgical margins [34].
10.5.4 Surgical Volume, Positive Margins and RAPN
Data from a retrospective US study of 18,724 patients which the evaluated prognostic impact of hospital volume on outcomes post-RAPN revealed that undergoing higher-volume hospitals may have better peri-operative outcomes (conversion to open and length of hospital stay) and lower positive surgical margin rates [35]. While a French study of 1222 RAPN patients showed that hospital volume was the main predictive factor of the trifecta achievement (warm ischaemia time <25 min, no complications, and a negative surgical margins) [36].
10.6 Future Challenges
Of course, future challenges remain. Improved identification of key anatomic structures remains paramount for a successful outcome. This can be facilitated by technological advancements offered by robotics such as Indocyanine green (ICG) [administered intravenously or through an extracorporeal access point (i.e., percutaneous nephrostomy or indwelling catheter)] to identify vascular perfusion [37].
The next very challenging step in robotic surgery, which is facilitated by refinement of da Vinci systems, will be the standardised implementation of radical nephrectomy with inferior vena cava thrombectomy. In highly specialised centres this operation has been performed up to a level III thrombus [38]. Given the large incision required for the open approach the robotic management is assumed to significantly reduce morbidity, length of hospital stay and hence, economic burden.
Further procedures which are assumed to be adopted robotically include renal transplant surgeries. The evolution of this technique is in progress. Importantly, not only kidney transplantation but also donor nephrectomy, which require optimal operative conditions, can be performed safely. Evidence in the literature show that robotic kidney transplantation is feasible, reducing complications while maintaining the functional results achieved by the open approach [39].
It is expected that both urologic surgeons and robotic systems will steadily continue to advance as experience evolves. Consequently, technically challenging robotic procedures will likely be reserved for tertiary referral centres, whereas lower-volume and less experienced centres will perform common and less complex procedures.
10.7 Conclusion
The current robotic era has already shown huge impact in the field of Uro-oncology and renal cancer surgery. It’s worldwide dissemination and integration has made it clear that robotic surgery will continue to shape and play a significant role in the natural evolution of future minimally invasive surgery. Latest generation da Vinci Surgical Systems, and potentially other innovations such as the Versius Surgical System, have marched forth as the pinnacle of surgical technology, having overcome the intrinsic limitations of laparoscopy years ago, they now provide enticement for experienced surgeons to push the barrier for more and more complex upper tract procedures. Cost remains the rate-limiting factor for these devices, and likely will continue for several years to come; however, similar to any previous innovation or technological advancement, initially thought to be unaffordable, it is possible that further analysis reports will prove cost-effective.
References
Rukstalis DB, Chodak GW. Laparoscopic retroperitoneal lymph node dissection in a patient with stage 1 testicular carcinoma. J Urol. 1992;148:1907–9; discussion 1909–1910.
Berkman DS, Taneja SS. Laparoscopic partial nephrectomy: technique and outcomes. Curr Urol Rep. 2010;11:1–7.
Yates DR, Vaessen C, Roupret M: From Leonardo to da Vinci: the history of robot-assisted surgery in urology. BJU Int. 2011;108:1708–13; discussion 1714.
McGuinness LA, Prasad Rai B. Robotics in urology. Ann R Coll Surg Engl. 2018;100:38–44.
Lanfranco AR, Castellanos AE, Desai JP, Meyers WC. Robotic surgery: a current perspective. Ann Surg. 2004;239:14–21.
Hanna T, Imber C. Robotics in HPB surgery. Ann R Coll Surg Engl. 2018;100:31–7.
Cwach K, Kavoussi L. Past, present, and future of laparoscopic renal surgery. Investig Clin Urol. 2016;57:S110–3.
Babbar P, Hemal AK. Robot-assisted urologic surgery in 2010—advancements and future outlook. Urol Ann. 2011;3:1–7.
Pal RP, Koupparis AJ. Expanding the indications of robotic surgery in urology: a systematic review of the literature. Arab J Urol. 2018;16:270–84.
Finkelstein J, Eckersberger E, Sadri H, Taneja SS, Lepor H, Djavan B. Open versus laparoscopic versus robot-assisted laparoscopic prostatectomy: the European and US experience. Rev Urol. 2010;12:35–43.
Ishii H, Rai BP, Stolzenburg JU, Bose P, Chlosta PL, Somani BK, Nabi G, Qazi HA, Rajbabu K, Kynaston H, et al. Robotic or open radical cystectomy, which is safer? A systematic review and meta-analysis of comparative studies. J Endourol. 2014;28:1215–23.
George E, Brand T, LaPorta A, Marescaux J, Satava R. Origins of robotic surgery: from Skepticism to standard of care. JSLS: J Soc Laparoendosc Surg. 2018;22:e2018.00039.
Satava RM. Robotic surgery: from past to future–a personal journey. Surg Clin North Am. 2003;83(1491–1500):xii.
Azizian M, Liu M, Khalaji I, Sorger J, Oh D, Daimios S. 3—The da Vinci Surgical System. In: Abedin-Nasab, editor. Handbook of robotic and image-guided surgery. MH: Elsevier;2020. p. 39–55. https://doi.org/10.1016/B978-0-12-814245-5.00003-7.
Satava RM. Surgical robotics: the early chronicles: a personal historical perspective. Surg Laparosc Endosc Percutan Tech. 2002;12:6–16.
Intuitive History on World Wide Web. https://www.intuitive.com/en-us/about-us/company/history.
Takács Á, Nagy D, Rudas I, Haidegger T. Origins of surgical robotics: from space to the operating room. 2016;13:13–30.
Damle A, Damle RN, Flahive JM, Schlussel AT, Davids JS, Sturrock PR, Maykel JA, Alavi K. Diffusion of technology: trends in robotic-assisted colorectal surgery. Am J Surg. 2017;214:820–4.
Wirth GJ, Hauser J, Caviezel A, Schwartz J, Fleury N, Tran SN, Iselin CE. Roboterassistierte Operationen in der Urologie. Urologe. 2008;47:960–3.
Hanzly M, Frederick A, Creighton T, Atwood K, Mehedint D, Kauffman EC, Kim HL, Schwaab T. Learning curves for robot-assisted and laparoscopic partial nephrectomy. J Endourol. 2015;29:297–303.
Gill IS, Kavoussi LR, Lane BR, Blute ML, Babineau D, Colombo JR Jr, Frank I, Permpongkosol S, Weight CJ, Kaouk JH, et al. Comparison of 1,800 laparoscopic and open partial nephrectomies for single renal tumors. J Urol. 2007;178:41–6.
Yang DY, Monn MF, Bahler CD, Sundaram CP. Does robotic assistance confer an economic benefit during laparoscopic radical nephrectomy? J Urol. 2014;192:671–6.
Roos FC, Thomas C, Neisius A, Nestler S, Thüroff JW, Hampel C. Robot-assisted laparoscopic partial nephrectomy: functional and oncological outcomes. Urologe A. 2015;54:213–8.
Petros FG, Angell JE, Abaza R. Outcomes of robotic nephrectomy including highest-complexity cases: largest series to date and literature review. Urology. 2015;85:1352–8.
Thomas BC, Slack M, Hussain M, Barber N, Pradhan A, Dinneen E, Stewart GD. Preclinical evaluation of the versius surgical system, a new robot-assisted surgical device for use in minimal access renal and prostate surgery. Eur Urol Focus. 2021;7:444–52.
Laviana AA, Hu JC. Current controversies and challenges in robotic-assisted, laparoscopic, and open partial nephrectomies. World J Urol. 2014;32:591–6.
Gill IS, Kamoi K, Aron M, Desai MM. 800 Laparoscopic partial nephrectomies: a single surgeon series. J Urol. 2010;183:34–41.
Leow JJ, Heah NH, Chang SL, Chong YL, Png KS. Outcomes of robotic versus laparoscopic partial nephrectomy: an updated meta-analysis of 4,919 patients. J Urol. 2016;196:1371–7.
Jeong IG, Khandwala YS, Kim JH, Han DH, Li S, Wang Y, Chang SL, Chung BI. Association of robotic-assisted vs laparoscopic radical nephrectomy with perioperative outcomes and health care costs, 2003 to 2015. JAMA. 2017;318:1561–8.
Asimakopoulos AD, Miano R, Annino F, Micali S, Spera E, Iorio B, Vespasiani G, Gaston R. Robotic radical nephrectomy for renal cell carcinoma: a systematic review. BMC Urol. 2014;14:75.
Masson-Lecomte A, Yates DR, Hupertan V, Haertig A, Chartier-Kastler E, Bitker MO, Vaessen C, Rouprêt M. A prospective comparison of the pathologic and surgical outcomes obtained after elective treatment of renal cell carcinoma by open or robot-assisted partial nephrectomy. Urol Oncol. 2013;31:924–9.
Peyronnet B, Seisen T, Oger E, Vaessen C, Grassano Y, Benoit T, Carrouget J, Pradère B, Khene Z, Giwerc A, et al. Comparison of 1800 robotic and open partial nephrectomies for renal tumors. Ann Surg Oncol. 2016;23:4277–83.
Chang KD, Abdel Raheem A, Kim KH, Oh CK, Park SY, Kim YS, Ham WS, Han WK, Choi YD, Chung BH, et al. Functional and oncological outcomes of open, laparoscopic and robot-assisted partial nephrectomy: a multicentre comparative matched-pair analyses with a median of 5 years’ follow-up. BJU Int. 2018;122:618–26.
Choi JE, You JH, Kim DK, Rha KH, Lee SH. Comparison of perioperative outcomes between robotic and laparoscopic partial nephrectomy: a systematic review and meta-analysis. Eur Urol. 2015;67:891–901.
Xia L, Pulido JE, Chelluri RR, Strother MC, Taylor BL, Raman JD, Guzzo TJ. Hospital volume and outcomes of robot-assisted partial nephrectomy. BJU Int. 2018;121:900–7.
Peyronnet B, Tondut L, Bernhard JC, Vaessen C, Doumerc N, Sebe P, Pradere B, Guillonneau B, Khene ZE, Nouhaud FX, et al. Impact of hospital volume and surgeon volume on robot-assisted partial nephrectomy outcomes: a multicentre study. BJU Int. 2018;121:916–22.
Diana P, Buffi NM, Lughezzani G, Dell’Oglio P, Mazzone E, Porter J, Mottrie A. The role of intraoperative indocyanine green in robot-assisted partial nephrectomy: results from a large. Multi-inst Ser Eur Urol. 2020;78:743–9.
Gill IS, Metcalfe C, Abreu A, Duddalwar V, Chopra S, Cunningham M, Thangathurai D, Ukimura O, Satkunasivam R, Hung A, et al. Robotic level III inferior Vena Cava Tumor Thrombectomy: initial series. J Urol. 2015;194:929–38.
Territo A, Mottrie A, Abaza R, Rogers C, Menon M, Bhandari M, Ahlawat R, Breda A. Robotic kidney transplantation: current status and future perspectives. Minerva Urol Nefrol. 2017;69:5–13.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Soliman, C., Furrer, M.A., Lawrentschuk, N. (2022). New Robots and How this has Changed Operative Technique in Renal Cancer Surgery. In: Goonewardene, S.S., Persad, R., Albala, D. (eds) Robotic Surgery for Renal Cancer. Management of Urology. Springer, Cham. https://doi.org/10.1007/978-3-031-11000-9_10
Download citation
DOI: https://doi.org/10.1007/978-3-031-11000-9_10
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-10999-7
Online ISBN: 978-3-031-11000-9
eBook Packages: MedicineMedicine (R0)