Abstract
An analytical study of the results of physical and chemical modeling of natural objects of various hydrogeochemical provinces was performed. The HCh software package was used for modeling (Shvarov Yu.V., MSU). V. I. Vernadsky’s position on the diversity of chemical types of natural waters in the Earth’s crust is substantiated. The main components of the “water–rock–gases” system transformation are identified. The high convergence of numerical thermodynamic models based on the fundamental laws of physical chemistry with direct analytical studies is shown. From a detailed analysis of the step-by-step phase changes hydrogeochemical system on the background of the four main factors that determine the chemical composition of natural waters, it was found that the leading parameter of the diversity of waters at the regional scale is the rate of water exchange (the model parameter is the mass ratio of rock/water), canvas serves as the mineral composition of water-bearing rocks.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Hydrogeochemical type of natural waters
- Physical and chemical simulation
- Transformation factors
- Water exchange
1 Introduction
Studying analyzes of the chemical composition of natural waters, Vernadsky expressed the opinion about the many possible types of water in the Earth’s crust [1], which are formed in natural water–rock systems due to various combinations of water and rocks.
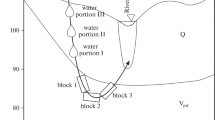
We consider this process from the position of phase equilibria. The water–rock–gas system can be represented schematically as a trihedral pyramid with a base in the form of an equilateral triangle, the top of which is H2O, the left corner of the base of the triangle is the composition of well-soluble minerals H (salts, carbonates, secondary silicates), the right corner of the base is the composition of poorly soluble minerals L (oxides, diasporas, goethite), the composition of the gas phase (O2, CO2) vertically (Fig. 1). The compositions of the aqueous solution are points in the field between the top of the triangle and the phase equilibrium curve (thick line). Points h and l are the solubilities of phases H and L, respectively. The ratio of the mass of the rock and water R/W (water exchange parameter) is the ray from the top of the triangle to the base. The point at which the ray of the parameter R/W cuts the curve separating the field of the aqueous solution and the field of mineral phases is the composition of the aqueous solution and the equilibrium mineral association.
From a consideration of the phase diagram of the water–rock–gas system and model calculations performed in the HCh software environment [2, 3] (Tables 1 and 2), the reason for the variety (quite natural) of the observed compositions of natural waters and the reliability of the mechanisms of composition change in various climatic zones of mountain-folded are clear regions of Central Eurasia [4], which can be considered as an illustration of the position of Vernadsky [1] on the variety of chemical types of natural waters in the Earth crust.
2 Research Methods
The efforts of many experts were directed to elucidating the causes and mechanism of the formation of the chemical composition of natural waters [5, 6]. The chemical and mineral composition of rocks, water exchange, landscape, climatic and tectonic conditions were considered by them as basic, determining factors. But the establishment of the dependence of the chemical composition of waters on these factors was usually not quantitative (numerically expressed). Only with the development of methods of mineral and isotope equilibria [2, 7,8,9,10,11] and the development of computer modeling of water–rock–gas systems [2, 3, 6, 10, 12,13,14,15] it became possible to quantitatively consider the processes of formation of the chemical composition of Earth crust natural waters.
In [12] it was shown that the chemical composition of natural waters is determined by:
-
(a)
the chemical potentials of inert and perfectly mobile components (PMC, according to Korzhinsky) of the water–rock–gas system, i.e. chemical and mineral composition of reacting rocks and water, and partial pressure (volatility) of gases;
-
(b)
the mass ratio of reacting rocks and water (R/W), i.e. water exchange;
-
(c)
temperature and pressure.
As can be seen, the necessary natural characteristics can be obtained by studying the object, and the thermodynamic characteristics of the components can be taken from the thermodynamic literature [13, 16].
3 Computer Simulation
In [1], the results of studying the chemical composition of natural waters and corresponding mineral associations in various landscape and climatic zones are presented and the mechanisms of their formation are proposed. It seems appropriate to simulate the water–rock–gas systems studied on natural material and compare the results of a traditional study of natural objects and their thermodynamic models.
A study of the regions of the Polar Urals and Lake Poyanhu (China) in [4] are established the dissolution of rocks and the transition in to an aqueous solution the hydrolyzed elements Fe, Al, Mn, as well as rare earth elements (REE) and silicon. The formation of the chemical composition of water is due to the combined action of dissolution of rock minerals and complex formation of dissolved forms of the elements.
Under permafrost conditions, the geochemical environment contributes to the accumulation of elements in an aqueous solution, and in a subtropical climate, the geochemical groundwater environment helps to remove these elements from the solution and their accumulation in the secondary mineral phase. In the studied areas, bicarbonate calcium, calcium-magnesium waters are common. A similar ionic composition and low water salinity is determined by the short time of interaction with rocks. Only in the conditions of permafrost this is due to the presence of water in the solid state for most of the year, and in the conditions of the subtropical climate-with active water exchange.
It is interesting to compare the results of the field study with the model of formation of the composition of natural waters proposed by Kraynov [12]. For this purpose, equilibrium model concentrations of chemical elements and mineral phases were calculated in the system “water-clay-rock-atmospheric gases”, thermodynamically open by O2 (0.2 bar) and CO2 (0.0004 bar) of the atmosphere at 5 °C (Table 1) and graphs were constructed “Model concentrations as a function of the R/W parameter (water exchange characteristic)” (Fig. 2). Analytically established minimum and maximum concentrations of dissolved elements are plotted on the same graphs (Table 3, Fig. 2).
It should be noted that the accumulation of macrocomponents in the aqueous phase of the thermodynamic model, corresponding to the observed concentrations in natural objects of the active water exchange zone, occurs in the range of R/W values from 0.01 to 0.9 (Table 3). As can be seen, the model concentrations are consistent with the analytical concentrations of the dissolved elements; on the other hand, the range of model R/W values indicated in Table 3 corresponds to the real groundwater flow velocities of the active water exchange zone, 10−2–10−3 cm/s [6]. Thermodynamic calculations show decrease the number of minerals for which the model solution is saturated with the growth of runoff (decrease of R/W value), which increases the potential diversity of hydrochemical type of natural water [17].
At the initial stages of the evolution of the water–rock–gas system (Table 1), the accumulation of Fe, Al, Mn hydrolyzates, as well as REE and Si, occurs in the natural waters of the humid regions. Under the conditions of a subtropical climate, the formation of colloidal compounds Fe and Al, as a result of the deposition of which these elements are removed from the solution and accumulate in the secondary mineral phase. A type of water is formed in equilibrium with the hydroxides of aluminum and iron at R/W = 0.00014–0.00071. With an increase in the R/W parameter, the iron–aluminum type of water reaches equilibrium with kaolinite and turns into the aluminum–silicon type [4] (R/W > 0.000713).
In a system open to atmospheric oxygen, the manganese content is negligible due to the formation of pyrolusite (R/W > 0.001). With a further increase (R/W > 0.011) in an aqueous solution, saturation with fluoroapatite (R/W > 0.057) is achieved and stilbite precipitates (at R/W > 0.01), and further accumulation of K, Ca, Mg, Mn, Si, precipitation of calcite (at R/W > 0.14), clinochlor (at R/W > 0.29) and muscovite (at R/W > 3.76). Curves of changes in the analytical concentrations of dissolved elements (Fig. 2) indicate a change in water types: siliceous Ca-Mg-Na type of water is replaced by aluminum-siliceous, then a siliceous carbonate-calcium type of water is formed in equilibrium with calcite, kaolinite, stilbite (at R/W = 0.141).
In water–rock systems of the main composition-gases (Table 2) goethite, pyrolusite and kaolinite precipitate at R/W > 0.00014, a further increase in R/W > 0.00034 leads to the dissolution of kaolinite. Stilbite is stable in the mineral association at R/W > 0.00034, quartz–at R/W > 0.00071, clinochlore–at R/W > 0.00123, F-apatite–at R/W > 0.00916, rutile–at R/W > 0.118.
As Ca, Mg, Na, K accumulates in the aqueous phase at R/W > 0.00442, Ca-saponite and calcite are formed, siliceous Ca, Mg, Na type water forms in equilibrium with Ca-saponite. As sodium and potassium accumulate in the aqueous phase, Na-saponite (R/W > 7.789) and K-saponite (R/W > 57.87) are formed. The formation of minerals of the saponite group is favored by an increase in the pH of the aqueous phase.
In orogenic regions with carbon dioxide releases [4], the considered water–rock–gas systems are retained as thermodynamically open in CO2, but at a partial pressure of CO2 substantially higher than atmospheric value, the type of aqueous solution changes to carbon dioxide-siliceous-carbonate-calcium in equilibrium with calcite, at an increased partial pressure of CO2, saponites are not formed.
Oxidation of sulfides adds sulfates to the composition of water, and leaching of sodium chloride-chloride ion, converting water to siliceous-sulfate-sodium in equilibrium with calcite and clays and siliceous sodium chloride in equilibrium with calcite and clays. Aqueous solutions of the above compositions do not produce significant changes in the composition of the equilibrium mineral association, except for the appearance of sulfate minerals.
4 Conclusion
Based on physicochemical factors [12] and using modern computer programs for calculating the equilibrium state of water–rock–gas systems [3, 9, 14, 18], as well as the rate of reaching the equilibrium state [16, 19], it is possible to predict the equilibrium compositions of water phase and mineral associations, and evaluate the time to reach equilibrium. Materials on the study of natural waters and mineral associations of Central Asia published in [4] confirm the conclusion of Vernadsky that many mineral associations and types of water are formed in the Earth crust. Also water and time are responsible for countenance of the rock planets.
References
Shvartsev, S.L., Yanshina, F.T. (eds.): Vernadsky, V.I: History of natural waters. Nauka, Moscow (2003)
Shvarov, Yu.V.: Algorithmization of the numeric equilibrium modeling of dynamic geochemical processes. Geochem. Int. 37(6), 571–576 (1999)
Shvarov, Yu.V.: HCh: New potentialities for the thermodynamic simulation of geochemical systems offered by windows. Geochem. Int. 46(8), 834–839 (2008)
Guseva, N.V.: Features of the formation of geochemical types of natural waters in certain regions of central Eurasia. Geochem. Int. 58(13), 1443–1576 (2020)
Zaitsev, I.K. (ed.): Hydrogeochemical studies (hypergenesis zone). Nedra, Moscow (1985)
Zverev, V.P.: The role of underground waters in the migration of chemical elements. Nedra, Moscow (1982)
Naumov, G.B., Ryzhenko, B.N., Khodakovsky, I.L.: Handbook of thermodynamic data US department of commerce. Natl Tech. Inf. Serv., Washington (1974)
Garrels, R.M., Christ, C.L.: Solutions, minerals, and equilibria. Harper & Row, New York (1965)
Borisov, M.V., Shvarov, Yu.V.: Thermodynamics of geochemical processes. Moscow State University Publishing House, Moscow (1992)
Shvartsev, S.L.: Hydrogeochemistry of the hypergenesis zone. Nedra, Moscow (1998)
Karpov, I.K., Kiselev, A.I., Letnikov, F.A.: Chemical thermodynamics in geochemistry and petrology. Publishing house of the USSR Academy of Sciences, Irkutsk (1971)
Kraynov, S.R., Ryzhenko, B.N., Shvets, V.M.: Geochemistry of underground waters: theoretical, applied and ecological aspects. CentrLitNefteGas, Moscow (2012)
Ovchinnikov, A.M.: Hydrogeochemistry. Nedra, Moscow (1970)
Charykova, M.V., Charykov, N.A.: Thermodynamic modeling of evaporite sedimentation processes. Nauka, Saint-Petersburg (2003)
Chudnenko, K.V.: Thermodynamic modeling in geochemistry: theory, algorithms, software, applications. GEO, Novosibirsk (2010)
Kraynov, S.R., Shvarov, Yu.V., Grichuk, D.V., et al.: Methods of geochemical modeling and forecasting in hydrogeology. Nedra, Moscow (1988)
Limantseva, O.A.: The role of groundwater saturation in formation of hydrochemical water type with reference to the minerals of water-bearing rocks. In: Proceedings of higher educational establishments. Geol. Explor. 2, 46–53 (2007)
Nordstrom, D.K., Plummer, L.N., Parkhurst D.L. et al.: Chemical modeling in aqueous systems: a comparison of computerized chemical models for equilirium calculations in aqueous systems. Symp. Ser. 93, 857–892. American Chemical Society (1979)
Mironenko, M.V., Zolotov, M.Yu.: Equilibrium–kinetic model of water–rock interaction. Geochem. Int. 50(1), 1–7 (2012)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Limantseva, O.A., Ryzhenko, B.N., Shvarov, Y.V. (2023). Influence of Water Exchange on the Equilibrium Composition of Aqueous Phase and Mineral Association. In: Kolotov, V.P., Bezaeva, N.S. (eds) Advances in Geochemistry, Analytical Chemistry, and Planetary Sciences. Springer, Cham. https://doi.org/10.1007/978-3-031-09883-3_9
Download citation
DOI: https://doi.org/10.1007/978-3-031-09883-3_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-09882-6
Online ISBN: 978-3-031-09883-3
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)