Abstract
In this chapter, we describe how a new trend of materials “nano” and its composites preferential grown as different sizes of small crystallites of structures using varied synthesis routes for photocatalytic dye degradation. Here, we will discuss about the different synthesis methods “traditional and trending” for the purification of water by degradation of dyes using photocatalytic techniques. To support the main outline of this chapter, we have supported various characterizations done to confirm the presence of nanomaterials like XRD for its crystal structure, SEM/TEM for morphology and XPS for elemental analysis; confirming with some functional groups using FTIR and Raman analysis. The modulation of parameters for the purification of water/effluents is pivotal in such work. So, we have compared the new novel techniques and materials trending all over research community as compared to the conventional ones. Certain nanomaterials synthesized are even doped with metals like rare-earth ions and transition metal ions to bring an effective output when illuminated to UV or visible light. Photocatalytic degradation has grown in last 20 years because of the impurities added to water bodies as source from industries and sewage, mainly comprising of waste like chemical dyes. This has paved way for lot of research in the fields of materials science, chemistry, environmental science and energy. Majorly synthetic dyes used in clothes, plastic, leather and other accessories are biggest motif behind this research. Our aim in this chapter is to summarize the whole scenario in compact but covering all relevant information. Usually, the procedure for photocatalytic dye degradation is step-wise process, involving choice of catalyst, concentration of dyes, light illumination and time taken for degradation of dye. Time taken for degradation of dye and choice of materials are inter-related, however efficiency of catalyst for certain dyes is better than other dyes. To make it clearer we will first give a brief introduction on the dyes, photocatalysis process, materials used as catalysts and purification.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
3.1 Introduction
From the last 20 years, the on-going trend in the field of photocatalytic degradation of dyes has increased relatively and gradually showing pace in its extensive usage; synthetic dyes are most prominently used in textile and plastic industries. As, it has become a major issue of textile, leather and plastic industries that the waste “effluents” generated are polluting the water bodies (Sacco et al. 2012; Mukhtish et al. 2013; Khan et al. 2017a; Khataee et al. 2010; Akpan and Hameed; Rauf and Salman). Most of the dyeing process effluents enter our environment in a large amount, approximately 20%; thus, hampering our natural resources (Rauf et al. 2011). So, to easily remove this issue, one such method adapts to tackle it involves the degradation of dyes. Where large molecules of dyes are oxidized down into smaller molecules like water, carbon dioxide, hydrogen and other by-products. But we are still struggling to find way to completely utilize all the dye molecules for degradation, as most of it released in water remains unused; making it difficult for researchers.
New modern methods now revolve around heterogenous photocatalysis for the dye’s degradation; hetero here means more than one material. Spintronics of the charge carriers or the charge dipole at different energy levels gives rise to its transfer from the valence band to the conduction band when illuminated by light of particular wavelength (occurs in semiconducting material oxides) (Pandit et al. 2015). The exciting electrons generated at the energy levels react with the water molecules or oxygen molecules thus producing hydroxide radicals and oxide anions. Such high oxidizing species have better efficiency to degrade n number of molecules; effluents of industries and others. Advanced Oxidation Process (AOP) is well known type of Fenton processes in the scientific world which is actually the decontamination process using reactive oxygen species and other molecular species. So, advanced Oxidation Process has become an important research area in the common history of photocatalytic degradation but in this chapter, we will limit our discussions to the semiconducting medium types of catalysts (Elmorsi et al. 2010; Gul and Yildlrln 2009; AlHamedi et al. 2009).
It is compulsory at this stage to detail about the demand of this topic as a review. Some of the cause for this to happen have been listed below (Xie and Li 2006):
-
1.
Several groups in the field of science and technology are working and collaborating in this realm. So, it’s good to compile the complete literature and its ongoing trend at uniform recurring.
-
2.
This method which is known for its various advantages over the years has major benefits using it in a bigger realm; as it is economically plausible thus making the photocatalytic procedure of degradation of dyes as demanding research.
-
3.
The by-products evolved after degradation are mostly non-toxic components like oxygen, carbon dioxide and water.
-
4.
The efficiency of degradation of the pollutants could be priorly calculated using the reagents like the hydroxide (OH group) to the potential applied for reduction of the pollutant and numerical counts of the pollutant’s oxidation potential (Odling and Robertson 2017).
Variety of chemical dyes are used in coloring things in the industries and markets for our daily necessities. These dye materials have been classified in accordance with its molecular structures, optical properties, color, wavelength and applications. Major difference in dyes is done through its chromophoric group (having colour) in the molecular species such as azo dyes, nitro dyes, quinine-amine dyes, acridine dyes, xanthene dyes, anthraquinone dyes and so on (Rauf et al. 2011). The photocatalytic dye degradation studies reported mainly dealing with the variables such as the number of catalysts used, intensity of the light irradiated, concentration of dye, time taken for effect to occur when irradiated by light evolving oxygen and other species. The pseudo first order reaction with kinetic data fitting to the equation −ln(C/C0) = kt is nothing but the kinetics of photocatalytic degradation of dyes. The importance of kinetic data fit has been shown separately in other sub-section.
Although humongous studies have been reported for degradation of dyes using photocatalytic technique but still there are several areas which have not been attentively checked. Now we will point out those issues lying in this field that need to be tackled. As the Fig. 3.1 shows in the bar graph that there is a sharp increase of research in the field of catalysis in the last 15 years; five times in the 10 years of span thus the publication number have risen double in last 5 years (Akpan and Hameed; Rauf and Salman; Rauf et al. 2011). As we have told in the outline of this chapter that mostly research done in this area are metal oxides or its composites (hybrid) varying the crystallites size to tune the overall performance. Before we start with the ongoing trends in the research of photocatalysis and its limitations, we would briefly illustrate about the literature (comprehensive history).
We have tried to summarize some of the important research done in the field of photocatalysis in the last few years in Table 3.1. Most of the research works that have been reported in literature state the degradation effect of dyes that depends on variety of stuff like concentration of dye, amount of catalyst taken, pH of solution, time taken for light illumination, source of illumination etc. The first order system is followed in the study of the kinetics of photocatalytic dye degradation (Khan et al. 2017b). Traditional wet chemical, physical and bio-friendly synthesis routes have been used extensively for dye degradation present in effluents. These synthesis processes have shown certain disadvantages like high energy, high cost, by-products formed end up in other types of secondary pollutants. Therefore, Advanced Oxidation Process i.e., AOP has gained considerable notice for the degradation of such harmful dyes in recent trends (Xiong et al. 2010).
3.2 Literature
This is one of the research fields that is done all over the world when compared with other research areas. To validate more about our above point that the research in this particular area “photocatalytic degradation” has always been in trend since more than 10 years as compared to other realms of science and technology; we have shown it in Table 3.1. The pictorial representation of dye degradation phenomena happening in the spintronic level of molecules is clearly shown in Fig. 3.2. The most common of all materials used for the degradation of dyes as photocatalysts is titanium dioxide. But unfortunately, the main flaws of using titanium dioxide (TiO2) are its wide bandgap i.e., 3.2 eV. Out of all the crystal phases of TiO2, in reports it is researched that the anatase gives better performance in dye degradation in photocatalysis due to its high absorption intensity for photons. This shows that the crystal phase in TiO2 plays a major role for dyes degradation (Rajesh et al. 2007; Dnyaneshwar 2017; Avasarala et al. 2016; Mahadwad et al. 2011; Mohammed et al. 2016; Nagaraja et al. 2012; Mehra and Sharma 2012).
Photocatalytic degradation of dyes in semiconducting materials (ref. Birte et al. (2017))
Out of the three most prominent phases of titanium dioxide such as anatase, rutile and brookite; anatase and rutile phases have been studied more as shown in Table 3.1. The oxygen ions positioned in the anatase crystal structure of TiO2 is in such an arrangement that allows maximum absorption of organic pollutants, even the orientation of titanium ions creates a condition favorable for reaction condition with those absorbed dyes (Mo and Ching 1995; Iakandar et al. 2007; Bavykin et al. 2006). But unfortunately, such beneficial crystal structural arrangements of molecules are not present in rutile phase of titanium dioxide. As reviewed, the researchers have found out that the pure form of anatase with small percent of rutile phase is more conductive for porosity and favoring better dye degradation. Spintronically, the charge carriers are excited by illumination of light thus hovering from lower energy level “valence band” to higher energy levels “conduction band” thus creating electron–hole pair, this generates radical ions which are an important reason for the degradation of dyes into water, carbon dioxide and some other species (Koelsch et al. 2002; Morgan and Watson 2010; Qamar et al. 2008; Sclafani and Herrmann 1996; Bakardiieva et al. 2005; Ohno et al. 2004; Colon et al. 2006; Li 2011; Ohtani et al. 1997).
For the dyes degradation using photocatalytic effect needs a material “catalyst” with larger surface area to have good adsorption of the effluents waste thus increasing the kinetics of the reaction. It will be considered slowly. In the standard P-25 TiO2, which is the mixture of 20% rutile and 80% anatase phase, making the overall material as a core–shell system with high productivity and active in most of the cases, that’s the reason it is used as standard material in comparison. For the treatment of effluents, various ways have been tried in history but dye removal using photodegradation has implied a pivotal role. Due to environmental and aesthetic concerns, the dye degradation from the waste in water bodies of industries like textiles, leather, plastic, etc. played a significant role (Elahee 2010). Metal oxides like ZnO and TiO2 have been used in nanospheres, nanorods, thin films, nanowires and nanofibers on a polymeric matrix; they have high performance, low price and eco-friendly. There are other semiconducting materials too like CdS, WO3, ZrO2, etc. for the dye degradation under light irradiation; these materials are already included in Table 3.1. The cons of such materials like titanium dioxide are that it has got high band gap, and need UV light illumination to degrade the dye in effluents. To give a clear picture of photodegradation of dyes, Fig. 3.3. Typically, some other materials and their composites show such photodegradation which is shown in Fig. 3.4a, b using pictorial presentation. Brookite has also been served for photocatalysis phenomena in dye degradation as one of the phases of titanium dioxide. Commonly, the studies carried out on photocatalytic dye degradation as checked by chemical oxygen demand measurements. Still, it is said to check the product concentration while formation and clear various routes through which these products are formed. There has always been a limitation to using such studies in large scale as it is important to see if the degradation material products are non-toxic to living beings and environment (Singh et al. 2013; Hoffman et al. 1995; Meng and Juan 2008; Hernandez-Alonso et al. 2009; Mills et al. 1993; Hashimoto et al. 2005). Optimization of parameters is pivotal as like the choice of photocatalyst material for successful technique implementation in purifying effluents in this new trending technology. So, the trend in this field has shown that with increase in light intensity of irradiating source, the degradation of dyes percent can also be increased. High value of irradiation light, more will be the recombination (electron–hole pairs) and vice-versa.
Energy band diagram of photocatalytic degradation of dyes in semiconducting materials (ref. Elena et al. (2017))
a Diffusion behavior of charge carriers in layered nanosheets and b transport pathway of the excited electron in the mixed photocatalyst (ref. Thuy-Duong Nguyen et al. (2011))
The photocatalytic response mainly depends on the chemical nature and annealing temperature of the semiconductor. Most of our choices of semiconducting materials and its crystal structures are based on several parameters like the physical form of the semiconductor and its stability under adverse the conditions of chemical reactions (Neppolian et al. 1999; Ye et al. 2010; Colmenares et al. 2009; Anpo et al. 1987; Lin et al. 2006; Sarkhanpour et al. 2017; Ajmal et al. 2014). Environmentally friendly, low cost or economically viable, less toxic are pivotal characteristics for good photocatalytic materials and out of this titanium dioxide has been reported and most trending material of all in this area of research. As the last research has been drafted and reviewed, it is seen that the standard material for comparison of photocatalytic performance of any new material is compared with P-25 TiO2 (anatase has performance better than rutile but less than standard material). Although, various parameters are optimized to get best result but the amount of photocatalyst material has been used mainly depends on the chemical behavior of the semiconducting property of it; thus, photocatalytic reaction has been easily tuned with certain modifications in literature (Tesfay et al. 2015; Paola et al. 2013; Nguyen-Phan et al. 2011). The changes have been done with various levels like modulating wavelengths of light, and spin-energy level coupling in semiconduction for efficient usage of the charge pair or e− hole pair. Recently a new trend has emerged in materials used as catalysts for dye degradation using photocatalysis phenomena i.e., use of graphitic carbon nitride (g-C3N4) as shown in Fig. 3.5. The V.B. level (valence band level) in the system could be shifted to better value which enhances the oxidation ability. Simultaneously, using the photoexcitation in the dye degradation, the transfer of electrons to the higher energy levels i.e., conduction band. This is predominant in the presence of visible light. Other than this many researchers have tried doping the semiconductor catalysts for better in large realm of wavelengths and degradation performance (Ioannis and Triatafyllos 2004; Kurny and Fahmida 2017; Fox and Dulay 1993).
3.3 Photocatalytic Dye Degradation Chemical Phenomena in Core–Shell
Due to progressively increasing textiles, paper and other coloring industries around the world, the residues sank out of them are a major issue of chemical pollutants/hazards of damaging our routine life-cycle by contaminating water bodies and badly affecting the living beings (both aquatic and non-aquatic). Color dyes have been classified into various classes in the literature, which are often non-biodegradable of a source of badly affecting our eco-system of a green nature. Out of all this, a crystal violet (CV) dye is one of the cationic dyes being used in inks that is majorly polluting our green nature throughout the globe. To control an environmental pollution, it is thus highly required to degrade it (before it is damped as a waste in nature) in an ecofriendly method, such as its photocatalytic degradation in a water host, which is the mostly easily available in plenty in the nature. Usually, a CV dye strongly absorbs visible light with absorption maximum lying at 570 or 590 nm, which can be explored in degrading it using a suitable photocatalyst, for example, C-CaIn2O4 we synthesized in small core–shell crystallites in this work. As a result, in this investigation, we analyze how a CV dye can be degraded using a core–shell C-CaIn2O4 as a photocatalyst as follows (Tiwari et al. 2018).
A photocatalytic experiment was carried out under UV irradiation (24 W power, 400 lx intensity, 300–420 nm range with its average peak position lying at λmax = 365 nm) of a CV dye (5 × 10–5 ML−1 concentration in water) charged on a sample C-CaIn2O4 in water while stirring. Typically, a 1.0 gL−1 of a C-CaIn2O4 sample was dispersed in each solution to let the pollutant adsorb over its surfaces to the equilibrium in a deep dark for a sufficient time-period of 30 min. Then, the sample was degraded in parts by irradiating for equal intervals of 15 min followed by centrifuged at 6000 rpm for 5 min to separate the dye from the C-CaIn2O4 catalyst. Recovered solutions in this way were studied in terms of UV–visible absorption spectra to determine any residual dye left-out after degradation in the solutions. An average of three consecutive measurements was used in analyzing the degradation process and its kinetic parameters. A good catalyst offers its photocatalytic features in four major factors (i) how it uploads a given pollutant in a liquid carrier, (ii) how the uploaded species absorb light in a wide band, (iii) how the “e− − h+” ion pairs decouple in transient interfaces formed on the catalyst by irradiating so obtained sample and (iv) how degraded species after the reaction ultimately get separated from the dye and disperse back into the solution of a reservoir. Small C-CaIn2O4 core-shells and tiny pores of a sample we used here promptly adsorb a dye over large surfaces having large charge carriers. The whole reaction process occurs in successive steps that can be put in a simple model reaction scheme as follows. Model molecular diagrams given in Fig. 3.6 describe how (a) core–shell C-CaIn2O4 crystallites uploads (b) a crystal violet dye (when adding in a solution) on (c) its reactive C-sp2 modified surfaces so that the dye degrades thereon in a light induced reaction and (d) ultimately the degraded dye species segregate and disperse into the solution as a separate phase (Tiwari and Ram 2019).
As a result, as soon as the dye degrades in this process it proportionally loses its absorbance of its characteristic light absorption band as shown in Fig. 3.7. In this figure, only selective absorption bands, which were recorded in a regular interval of 30 min of UV-irradiating (over 300–420 nm wavelengths) an as-prepared C-CaIn2O4 sample-1 dispersed along with a CV dye in water, are compiled in order to determine how the irradiation causing the dye to be progressively degrading over a time scale, which is prolonged up to 240 min in a continuous UV irradiation. No any measurable degradation appears in absence of any UV–visible light in a complete dark medium ensuring that it is only the UV-irradiation that it leads the dye to degrade on the CaIn2O4 sample-1 in the photocatalytic reactions with its reactive surfaces. The reaction is mostly complete in a 240 min of a regular irradiation after which only a small residual absorption band lasts and it does not change any more (Mills et al. 1993; Hashimoto et al. 2005; Neppolian et al. 1999).
As usual, excited photons in the UV-irradiation lead to excite an electron from the valence band to the conduction band, leaving behind a hole “h+” in the valence band in a decoupled “e− − h+” ion-pair. These holes could readily oxidize the donor dye molecules so as to promptly react with H2O forming refined hydroxyl species (Ye et al. 2010; Colmenares et al. 2009; Anpo et al. 1987; Lin et al. 2006). We optimized adsorption of the dye on the different C-CaIn2O4 samples-1, 2, 3 dispersed in water in a fixed volume in a completely dark room in terms of its absorbance in its characteristic light-absorption band. Both as-prepared and annealed C-CaIn2O4 samples readily adsorb the CV dye on its multiple surfaces of small core–shell crystallites. A dispersion of the dye on C-CaIn2O4 in water has been equilibrated in its maximum adsorption on its surfaces over an extended exposure time to nearly 90 min until it becomes stable. As soon as the adsorption completed and the final sample becomes stable in absorbing light, we started measuring its degradation by irradiating it by UV-light over predetermined intervals of time of 30 min as shown in the so obtained results, which are plotted in Fig. 3.8. Here, C0 (which is proportional to the maximum absorbance in the dye in the sample) describes an initial dye concentration before any irradiation and C/C0 is its normalized residual value after a photocatalytic degradation performed in this experiment. To find out a critical extent of a recurring degradation achieved in this experiment, the intermediate samples were centrifuged at 6000 rpm for 10 min in which so far degraded dye desorbs off the catalyst and disperses in the water.
In the plot in Fig. 3.8a, as the UV-light irradiating time t → 25 min, the CV dye in sample-1 is decayed rapidly till a minimum C/Co ≅ 40%. A nearly four times larger dye lasts in Fig. 3.8d in decaying much slowly in a standard P25-TiO2. A reasonably large porosity of ultrafine pores and a reasonably thick C-sp2 surface layer in this sample-1 promptly favor its catalytic features. A reasonably larger degradation rate constant k = 0.065 min−1 (against 0.013 min−1 in the P25-TiO2) thus prolongs in a pseudo first order reaction in a non-linear regression of ln(C/Co) = −kt over t as plotted in Fig. 3.9a for this sample. Other samples involve much lower k-values as can be determined assuming linear plots in Fig. 5.9b, c. Further, the degradation plots in Fig. 3.10 describe a non-linear degradation of the CV dye (a) before and (b) after dispersing in a photocatalytic C-CaIn2O4 sample-3 in water and irradiating over UV-light. In a degradation plot made in Fig. 3.10a, a bare CV dye hardly degrades up to 12% in a very slowly reaction with H2O molecules by UV irradiation when dispersed in water. This result clearly shows a crucial role a photocatalyst is playing in degrading it reasonably faster as observed in this experiment in the presence of a photocatalyst of a C-CaIn2O4 sample.
Further, in absence of an irradiation, the dye dispersed in a catalytic sample-3 decays much slowly (hardly by nearly up to 25%) in a marked microscopic effect of a light induced photochemical reaction in this example. A maximum of 81% degradation efficiency, η = (C0 − C∞) C0−1, of degrading a CV-dye thus has been observed in our photocatalytic C-CaIn2O4 sample-3 in water after a long exposure of UV irradiation up to 210 min. Here, C = C∞ as t → ∞ (taken as 210 min of the degradation reaction) has been used according to the degradation plots given in Fig. 3.8. Nevertheless, an as-prepared C-CaIn2O4 sample-1 serves to be a faster photocatalyst in degrading the CV-dye over early periods, t ≤ 60 min, of the UV irradiation in the present experiments. An annealed C-CaIn2O4 sample-2 at 400 °C for 2 h in air exhibits a reasonably lower η = 40% in accounts of its enhanced electrical conductivity on forming highly regular and stable conductive C-sp2 surface channels (of a critical δ = 1–2 nm thickness of a conjoint network of a surface layer) as will be described in the following section in this chapter. In this case, a decoupled “e− − h+” ion-pair mostly takes part in the electrical conduction rather than in a desired photocatalytic degradation in this experiment. A standard P25-TiO2 photocatalyst as used over here yields a still inferior η = 20% in degrading a CV-dye in water in the present experimental conductions.
A bar chart given in Fig. 3.11 compares η-values observed in selective samples studied in this work. A few other authors have studied photocatalytic properties of pure and C-sp2 modified CaIn2O4 in degrading crystal violet dye dispersed in water and in visible irradiations, with similar degradation results of η ≤ 80 values. Also in these experiments, a surface C-sp2 modified CaIn2O4 had duly improved photocatalytic properties according to its local structure.
3.4 Optimization of Variables in Photocatalysis of Dye Degradation
In this we have explained that while optimization of dye degradation chemical phenomena, we not just check chemical synthesis methods for the materials employed but even the wavelength of light used as source for the energy bandgap of semiconducting materials. So, the dye degradation has effects of several variables used in experiment that can be varied and optimized for best result. We have tried to give collective bandgap information on the last 20 years and ongoing semiconducting materials in Table 3.2. Unfortunately, most of the time researchers don’t report the light source intensity and it’s monitoring; LEDs having good energy efficiency have also been employed, temperature of chemical reaction, light intensity, source of light and the catalyst particles size, surface area of catalyst and varied forms of materials affecting the dye degradation rate.
3.4.1 pH Variable Effect on the Dye’s Degradation Chemical Phenomena
As shown in Table 3.1, that we have mentioned so beautifully about materials and its dye degradation phenomena is dependent on pH value. The change in oxidation potential as per pH values for the molecular species used in the chemical solution for dye degradation is on pivotal observation. As the hole oxidation potential and electron reduction power that is generated when light is irradiated on the semiconducting catalysts for dye degradation are even dependent on the positions of the high energy level of the valence band and low energy level of conduction band. Thus, pH has greater effect even when adsorption of dye is done on the photocatalyst materials, a change in pH and surface characteristics is noticed. OH− ions (hydroxyl groups) could be easily formed in these reactions by hydroxide ions having positive holes. At neutral of high pH values, the majorly considered species are hydroxyl radicals whereas the holes (positive charges) are predominantly considered at low values of pH. OH− ions are produced easier in the alkaline solution by oxidizing the hydroxide species available at the surface of semiconducting materials, thus count to increase in efficiency of dye degradation; mostly dyes have similar behavior like this. According to trends in this research field, dyes have been considered as a function of pH like bromo-cresol purple dye degrade better in acidic medium than in alkaline medium. The synthesis methods for the photo-degradation depends on the formation of products on the way molecules of dyes get adsorbed to the surface of catalysts, thus altering electronic properties.
It has been researched by the scientific teams that in dye degradation using photocatalytic technique the advent of adsorption on unmodified TiO2 shows better for cationic dyes with positive charges than anionic dyes with negative charges. Therefore, explicitly the behavior of a dye and its pH give a huge impact on photocatalytic reaction efficiency. Azo dyes are cationic dyes having positive charge with low pH of around 6.8 and at higher pH value it gives anionic charges (negatively charged), hence effecting the adsorption of dye on the surface of materials. Lucidly, the effluents treatment should take into consideration two important points that are:
-
1.
pH of the waste should not be neutral
-
2.
Surface behavior of semiconducting materials gets influenced by the substances that will be mixed inside the water.
The dyes adsorbed on the semiconducting materials surface and the electron and hole charge pair separation state which will happen when electrical double layer forms due to charged species. The rate at which dye degrades in the photocatalysis phenomena mostly depends on pH of that dye and the reaction rate for that particular dye is maximum on the nature of pH, whether its acid or alkaline. Like OH− radicals are formed in alkaline medium that increases the reaction rate of catalysis for dye degradation. We have given trends in materials for dye degradation showing influence of pH value of solution in Table 3.3. So, it is pivotal for us to understand the chemical behavior of these dyes and pH value for proper degradation using photocatalysis. As we change the annealing temperatures, pH value varies thus changing the phase or crystal structure of the semiconducting materials. As compared with few examples given in Table 3.3 that TiO2 degrades faster with good efficiency when at acidic medium. Hence, it degrades the chemically toxic dyes by absorbing H+ ions on the surface of semiconducting materials; ultimately giving a new trend for wide range of dyes degraded alone with a single material. Based on the mechanism of chemical reaction, effects of pH on the dye degradation should be rationalized. Possibly, there are only 3 ways through which dyes can photocatalytically degraded in relation to pH value:
-
i.
Involvement of positive charges “holes” directly in the oxidation reaction
-
ii.
Hydroxyl species are directly attacked
-
iii.
When electrons are excited to the higher energy bands (conduction bands) get directly reduced.
A detailed collection of the optimized values of pH for different photocatalysts and varied dyes is given in Table 3.2 with references.
3.4.2 Issues Still to Be Handled with New Trends
In the research literature most of the covered portions of this field as variables; source of light for dye degradation, its light intensity, the quantity and concentration of catalyst used, pH of the solution medium, initial dye concentration used for the detailed study of its degradation, time taken for irradiation in the whole dye degradation along with other cations present in the chemical reaction medium. Since ages, literature shows that research community has been following these variables avidly. So, it is considered that it is important to study these variables for getting the correct information for the effluents treatment using photocatalytic phenomenon in the dye industries like textile, paint and plastic. The main purpose of this chapter is to briefly compile and present all the past and ongoing trends in photocatalytic dye degradation for the waste treatment “effluents” and cleaning water bodies that are getting polluted by the industries, considering all parameters.
3.4.2.1 Kinetics of Dye Degradation
The kinetics of dye degradation using photocatalytic phenomenon by these semiconducting catalysts have been mostly using the first order kinetics. In Table 3.4, we have clearly given detailed information of so many catalysts used in past and present that are trending for dye degradation with references along with its kinetic value “first order rate constant value”. The purpose of this analysis is to determine some of the outcomes of treating these first order kinetic data in the degradation of the dye “pollutants” and other toxic effluents in the form of molecular species under the irradiation of light and semiconducting catalysts. The equation used for the first order kinetic in these chemical reaction phenomena could be written as
where C is quoted as the concentration, at t time in seconds, Co is the initial dye concentration and k represents the rate constant value, and adsorption constant, so on. Typical data of kinetic analysis is given for C-CaIn2O4 in Fig. 3.9 for dye degradation using photocatalytic phenomenon. This study could be concluded with pivotal points that the innate rate of catalytic reaction is source dependent and self-degradation in first order kinetics (Gouvea et al. 2000; Rong et al. 2015; Rochkind et al. 2014; Eyasu et al. 2013; Raji and Palanivelu 2016; Foletto et al. 2012; He et al. 2015). The lucid rate constant in the kinetic equation is a lumped parameter mostly consisting of those rates constant value, the other parallel chemical reaction rates that took place over semiconducting catalyst surface and several other unclear rates of dye degradation. So, the rate constant for catalytic reaction cannot be taken as a lucid measure for comparing this process that is taking place between two or more catalysts that are not similar; it will have some very serious misconceptions when doing comparative study. In these examples given in Table 3.4, the researchers have reported the chemical kinetic rate constant for the best catalyst as around 24 min−1 whereas the value for the degradation of chlorophenol rate constant of around 3.47 min−1 (here photon irradiated degradation is not present) (Li et al. 2017; Paridwala et al. 2017; Liu et al. 2015, 2016; Kumar and Sahare 2013).
Some of the precautions have been exercised when comparing two or more semiconducting catalytic system phenomenon on the basis of the chemical kinetic constant rate values when used first order for catalysts reactions when dye gets adsorbed on the surface following its degradation. This may apply to certain reactions generally involving multiple steps that may succeed or precede the surface chemical reactions that are sometimes taken as first order reaction kinetics. Degradation of dye can foster several preconditions, out of which one is the adsorption of dyes on the surface of semiconducting catalyst surface and the equilibrium constant must be shown in the rate constant value calculated in data collected. There are so many preconditions that have been reported for the dye degradation, like the surface adsorption of organic dyes on catalyst and rate constant value. The surface values are capable of giving different orders of reactivity for adsorbents, and it should be considered when choosing the materials for treatment of effluents. BET adsorption isotherms are very famous Brunauer, Emmett, Teller isotherms as shown in Fig. 3.12 for determining surface area of the catalyst using adsorption curve; Table 3.5 shows the reported surface areas and pore sizes of annealed samples of core–shell C-CaIn2O4 nanostructures (This is one of my recent works). Keeping in mind the surface characteristics of these catalysts and nature of organic dyes have always been considered in this research field, not just this but even pH can change the surface charge carriers thus effecting the adsorption of dyes. Dyes are mostly chosen with surface as acidic or basic characteristics, showing anionic or cationic property in charged dyes. This aspect has not yet been tackled when treating the chemical kinetics of photocatalytic dye degradation on semiconducting materials. In recent studies, the details on adsorption of dyes and its consequences on the kinetic behavior of dye degradation have not been touched or linked yet; this comparison will benefit for future.
3.4.2.2 Loading of Semiconducting Catalyst
Some of the observations that have been checked and recorded in the recent trends of photocatalytic degradation of dyes is that as the weight of dyes load increases, the overall efficiency to degrade dye via adsorption on the catalyst surface decreases. So, it’s not an easy one to find out as the surface area exposed to dye adsorption of the catalyst will sometimes not be equivalent to quantity of catalyst present in the solution. The saturation point for dye adsorption on the surface of the semiconducting catalyst is also checked, as beyond certain extent of limit it will stop the photocatalytic phenomenon; most of the research studies report only few kg of around 3–4 g per liter of dye in solution. Industries should now put a control on the effluents that are possibly destroying water bodies and environment, as already mentioned that the weight extent of dye adsorption on the surface of catalyst is still an ongoing limitation. This could be marked as the control of pollution and effluents by the authorities for our environmental safety and protection.
3.4.2.3 Light Wavelength and Its Intensity of Irradiation Effect
The usage of solar light radiation has always been less expensive and less toxic to our lives and environment. The effect of photo light irradiation on the chemical photocatalytic phenomenon has been demonstrated in three ranges:
-
i.
At low intensity, the rate of reaction increases with the light irradiation
-
ii.
At medium and high intensities, the kinetic rate is not dependent on the light intensity.
Overall, the degradation rate for UV–visible light as a source of irradiation is better than the solar light radiation. Secondly, the charge pairs i.e., electron–hole formation & its recombination would be differently done under UV–visible light source. Thus, light wavelength is another major point in affecting the charge pair formation and recombination rate.
3.4.2.4 Dye Degradation Mechanism
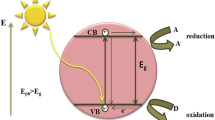
Photocatalyst is one of the best methods that can be applied for several applications for example, organic pollutants degradation from wastewater, production of hydrogen gas, purifying of air and other antibacterial activity. In 1972, Fujishima and Honda discovered the beginning of a new era in heterogeneous photocatalysis as a photocatalytic splitting of water on a TiO2 electrode as noticeable by the phenomena. The wavelength or light (photon) energy and the catalyst are main characteristics on which photocatalytic reaction are dependent. In general, as a catalyst which performs as sensitizer especially, semiconducting materials due to their electronic structure for the irradiation of light stimulated redox process, which is defined by a filled valence band and a vacant conduction band as shown in Fig. 3.13.
When photons in terms of the light energy falls (or interacts) on the surface of a material of energy of incident radiation if equivalent or more than the bandgap energy of the semiconducting material, the electrons present in valence band are excited to the conduction band of the semiconductor. Holes would be charges left in the valence band; these holes can oxidize donor molecules and react with water molecules to generate hydroxyl radicals. The conduction band electrons react with dissolved oxygen species to form superoxide ions. These electrons induce the redox reactions. These holes and electrons could undergo successive oxidation and reduction reactions with any species, which might be adsorbed on the surface of the semiconductor to give the necessary products. The photocatalytic performance of C-CaIn2O4 powders (as-prepared and annealed at 400 and 600 °C) were studied along with the benchmark Degussa P25 TiO2 powder by considering crystal violet (CV) as the target dye. Photocatalytic studies were done under UV (24 W, intensity 400 lx, λ < 400 nm) irradiation at the sample surface under stirring condition.
Aqueous solutions of crystal violet with a concentration 5 × 10–5 M with a photocatalyst loading of 1 g/L were taken. The dispersions were equilibrated for dye adsorption on the photocatalyst surface for 30 min. During the degradation period, the dispersions were collected at regular intervals of thirty minutes in case of UV light irradiation as shown in Fig. 3.14. To note down the extent of the degradation, the samples were centrifuged at 6000 rpm for 10 min to collect the so far degraded dye solution. Absorption spectra of all the collected dye solutions were taken using Perkin-Elmer UV–visible spectrophotometer Lambda 750.
3.5 Summary
In this chapter, we clearly explained the recent developments in the photocatalytic performance of different semiconducting materials for varied dyes that have been thoroughly studied by researchers in terms of optimized parameters like, pH, concentration of solution, quantity of catalyst employed, cationic and anionic form of dye, bandgap of semiconducting materials and source of light. The research in this field has increased immensely in recent years and is only going to grow as trend says. The reports of these studies mentioned in our literature mainly deal with some common variables as given above. In end the summarization of the results in this detailed study, this work deals with several aspects which we can explore in following way:
-
1.
Insufficiently treating the chemical kinetic information collected in terms of the pseudo first order reaction rate. Hence, we have to priorly take in consideration the adsorption characteristics in this phenomenon.
-
2.
The natural effect in this research is the adequate quantity of catalyst, and identification of those active sites and surface area number density that will be adsorbed on the surface of semiconductor.
-
3.
The dye degradation is partially or sometimes completely dependent on the behavior of oxidizing agent that has been incorporated; choice of oxidizing agent is under our control for the redox potential of the species.
-
4.
The dye adsorption on the semiconducting surface decides that whether the pH of the solution is acidic or basic; thus, the process of degradation gets clear. This variation in pH was been demonstrated for the semiconducting surface in response to the pH value.
We can conclude in the end of this book chapter, that all studies done in this photocatalytic field of dye degradation for waste water treatment or effluents treatment could be appropriately be extended to other detailed studies in future thus covering more economical and global significances.
References
Abo-Farha SA (2010) Photocatalytic degradation of monoazo and diazo dyes in waste water on nanometer sized TiO2. Researcher 2(7):1–20
Ajmal A, Majeed I, Malik RN, Idriss H, Nadeem MA (2014) Principles and mechanism of photocatalytic dye degradation on TiO2 based photocatalysts: a comparative overview. RSC Adv 4:370003–437026
Akpan UG, Hameed BH Parameters affecting the photocatalytic degradation of dyes using TiO2-based photocatalysts: a review. J Hazard Mater 170:520–529
Alaton IA, Balcioglu IA (2001) Photochemical and heterogeneous photocatalytic degradation of waste vinylsulphonate dyes: a case study with hydrolyzed reactive black 5. J Photochem Photobiol A Chem 141:247–254
AlHamedi FH, Rauf MA, Asraf SS (2009) Degradation of Rhodamine B in the presence of UV/H2O2. Desalination 239:159–166
Ameta R, Sharma S, Sharma S, Gorana Y (2015) Visible light induced photocatalytic degradation of toluidine blue-O using molybdenum doped titanium dioxide. Eur J Adv Engineer Technol 2:95–99
Anpo M, Shima T, Kodama S, Kubokawa Y (1987) Photocatalytic hydrogenation of propyne with water on small particle titania: size quantization effects and reaction intermediates. J Phys Chem 91:4305–4310
Apostolescu GA, Cernatescu C, Cobzaru C, Tataru-Farmus RE, Apostolescu N (2015) Studies of the photocatalytic degradation of organic dyes using CeO2-ZnO mixed oxides, 14:415–420
Avasarala BK, Tirukkovalluri SR, Bojja S (2016) Magnesium doped titania for photocatalytic degradation of dyes in visible light. J Environ Anal Toxicol 6:1–8
Bakardiieva S, Subrt J, Stengl V, Dianez MJ, Sayagues MJ (2005) Photoactivity of anatase-rutile TiO2 nanocrystalline mixtures obtained by heat treatment of homogeneously precipitated anatase. Appl Catal B 58:193–202
Baran W, Makowski A, Wardas W (2003) The influence of FeCl3 on the photocatalytic degradation of dissolved azo dyes in aqueous TiO2 suspensions. Chemosphere 53:87–95
Baran W, Makowski A, Wardas W (2008) The effect of UV radiation adsorption of cationic and anionic dye solutions on their photocatalytic degradation in the presence of TiO2. Dyes Pigm 76:226–230
Bauer C, Jacques P, Kalt A (2001) Photooxidation of an azo dye induced by visible light incident on the surface of TiO2. J Photochem Photobiol A Chem 140:87
Bavykin DV, Friedrich JM, Walsh FC (2006) Protonated titanates and TiO2 nanostructured materials: synthesis, properties and applications. Adv Mater 18:2807–2824
Bhati I, Pinki BP, Suresh CA (2010) Photocatalytic degradation of fast green using nanosized CeCrO3. Maced J Chem Chem Eng 29:195–202
Birte M, Lennart M, Olaf W (2017) Photocatalytic degradation of toluene, butyl acetate a limonene under UV and visible light with titanium dioxide-graphene oxide as photocatalyst. Environs 4:1–9
Bubacz K, Choina J, Dolat D, Morawski AW (2010) Methylene blue and phenol photocatalytic degradation on nanoparticles of anatase TiO2 Pol. J Environ Stud 19:685–691
Colmenares JC, Luque R, Campelo JM, Colmenares F, Karpinski Z, Romero AA (2009) Nanostructured photocatalysts and their applications in the photocatalytic transformation of lignocellulosic biomass: an overview. Materials (basel) 2:2228–2258
Colon G, Hidalgo MC, Munuera G, Ferino I, Cutrufello MG, Navio JA (2006) Structural and surface approach to the enhanced photocatalytic activity of sulfated TiO2 photocatalyst. Appl Catal B 63:45–59
Dharmarajan P, Sabastiyan A, K< SM, Titus S, Muthukumar C (2013) Photocatalytic degradation of reactive dyes in effluents employing copper doped titanium dioxide nanocrystals and direct sunlight. Chem Sci Trans 2:1450–1458
Dhatshabamurthi P, Subhash B, Shanthi M (2015) Investigation on UVA light photocatalytic degradation of azo dye in the presence of CdO/TiO2 coupled semiconductor. Mater Sci Semicond Process 35:22–29
Di Paola A, Bellardita M, Palmisano L (2013) Brookite, the least known TiO2 photo-catalyst. Catalysts 3:36–73
Dnyaneshwar R (2017) Photocatalytic degradation of dyes in water by analytical reagent grade photocatalysts—a comparative study. Drink Water Eng Sci 10:109
Elahee K (2010) Heat recovery in the textile dyeing and finishing industry: lessons from developing economies. J Energy Southern Africa 21:9–15
Elena CP, Felix E, Fernando V (2017) Computational nanotechnology to predict photocatalysis of titania nanoparticles in cement-based materials. IEEE Conf Proc 17:208–123
Elmorsi TM, Riyad YM, Mohamed ZH, Abd El Bary HM, Bary E (2010) Decolorization of mordant red 73 azo dye in water using H2O2/UV and photo-Fenton treatment. J Hazard Mater 174:352–358
Eyasu A, Yadav OP, Bachheti RK (2013) Photocatalytic degradation of methyl orange dye using Cr-doped ZnS nanoparticles under visible radiation. Int J Chemtech Res 5:1452–1461
Foletto EL, Battiston S, Collazzo GC, Bassaco MM, Mazutti MA (2012) Degradation of leather dye using CeO2-SnO2 nanocomposite as photocatalyst under sunlight. Water Air Soil Pollution 223:5773–5779
Fox MA, Dulay MT (1993) Heterogeneous photocatalysis. Chem Rev 93:341
Gouvea CA, Wypych F, Moreaes SG, Duran N, Nagat N, Peralta-Zamora P (2000) Semiconductor-assisted photo-catalytic degradation of reactive dyes in aqueous solution. Chemosphere 40:433–440
Guettai N, Amar HA (2005) Photocatalytic oxidation of methyl orange in presence of titanium dioxide in aqueous suspension Part II Kinetic study. Desalination 185:439–448
Guillard C, Diadier J, Monnet C, Dussaud J, Malato S, Blanco J, Maldonano MI, Herrmann JM (2003) Solar efficiency of new deposited titania photo-catalyst: Chlorophenol, pesticide and dye removal applications. Appl Catal b: Environ 46:319–332
Gul S, Yildlrln O (2009) Degradation of reactive red 194 and reactive yellow 145 azo dyes by O3 and H2O2/UV-C process. Chem Eng J 155:684–690
Hashimoto K, Irie H, Fujishima A (2005) TiO2 photocatalysis: a historical overview future prospect. Jpn J Appl Phys 44:8269–8285
He Y, Zhang L, Fan M, Wang X, Walbridge ML, Nong Q, Wu Y, Zhao L (2015) Z-scheme SnO2-x/g-C3N4 composite as an efficient photocatalyst for dye degradation and photocatalytic CO2 reduction. Sol Energy Mater Sol Cells 137:175–184
Hernandez-Alonso MD, Fresno F, Suareza S, Coronado JM (2009) Development of alternative photocatalysts to TiO2: challenges and opportunities. Energy Environ Sci 2:1231–1257
Hoffman MR, Martin ST, Choi W, Bahnemannt DW (1995) Environmental applications of semiconductor photocatalysis. Chem Rev 95:69–96
Huang M, Xu C, Wu Z, Huang Y, Lin J, Wu J (2008) Photocatalytic decolorization of methyl orange solution by Pt modified TiO2 loaded on natural zeolite. Dyes Pigm 2:327–334
Iakandar F, Nandiyanto ABD, Yun KM, Hogan CJ, Biswas P (2007) Enhanced photocatalytic performance of brookite TiO2 macroporous particles prepared by spray drying with colloidal templating. Adv Mater 19:1408–1412
Ioannis KK, Triatafyllos A (2004) TiO2-assisted photocatalytic degradation of azo dyes in aqueous solution: kinetic and mechanistic investigations: a review. Appl Catal B 49:1–14
Kamal S, Afshin M, Behzad S, Borhan M, Fardin G (2014) Investigation of photocatalytic degradation of reactive balck 5 dyes using ZnO-CuO nano-composite. Zanki J Med Sci 15:66–74
Karunakaran C, Magesan P, Gomathisankar P, Vinayagamoorthy P (2013) Photocatalytic degradation of dyes by Al2O3-TiO2 and ZrO2-TiO2 nanocomposites. Mater Sci Forum 734:325–333
Khan MR, Kurney ASW, Fahmida G (2017a) Parameters affecting the photocatalytic degradation of dyes using TiO2: a review. Appl Water Sci 7:1569–1578
Khan MR, Kurny ASW, Eahmida G (2017b) Parameters affecting the photo-catalytic degradation of dyes using TiO2: a review. Appl Water Sci 7:1569–1578
Khataee AR, Pons MN, Zahraa O (2009) Photocatalytic degradation of three azo dyes using immobilized TiO2 nanoparticles on glass plates activated by UV light irradiation: influence of dye molecular structure. J Hazard Mater 168:451–457
Khataee AK, Kasiri MB (2010) Photocatalytic degradation of organic dyes in the presence of nanostructured titanium dioxide: influence of the chemical structure dyes. J Mol Catal Chem 328:8–26
Kirupavasam EK, Allen GR (2012) Photocatalytic degradation of amido black-10B catalyzed by carbon doped TiO2 photocatalyst. Int J Green Chem Bioprocess 2(3):20–25
Koelsch M, Cassaignon S, Guillemoles JF, Jolivet JP (2002) Comparison of optical and electrochemical properties of anatase and brookite TiO2 synthesized by the sol-gel method. Thin Solid Films 403:312–319
Kumar S, Sahare PD (2013) Photocatalytic activity of bismuth vanadate for the degradation of organic compounds. NANO 8:1350007
Kurny ASW, Fahmida G (2017) Parameters affecting the photocatalytic degradation of dyes using TiO2: a review. Appl Water Sci 7:1569–1578
Li H (2011) Enhanced photocatalytic activity of electrospun TiO2 nanofibers with optimal anatase/rutile ratio. J Am Ceramic Soc 94:3184–3187
Li Q, Guan Z, Wu D, Zhao X, Bao S, Tian B, Zhang J (2017) Z scheme BiOCl-Au-CdS heterostructure with enhanced sun light driven photocatalytic activity in degrading water dyes and antibiotics. ACS Sustain Chem Eng 5:6958–6968
Lin H, Huang CP, Li W, Ni C, Shah SI, Tseng Y-H (2006) Size dependency of nanocrystalline TiO2 on its optical property and photocatalytic reactivity exemplified by 2-cholorophrnol. Appl Catal B 68:1–11
Liu Y, Wang R, Yang Z, Du H, Jiang Y, Shen C, Liang K, Xu A (2015) Enhanced visible light photocatalytic activity of Z-scheme graphitic carbon nitride/oxygen vacancy-rich zinc oxide hybrid photocatalysts. Chin J Catal 36:2135–2144
Liu P, Liu Y, Ye W, Ma J, Gao D (2016) Flower like N-doped MoS2 for photocatalytic degradation of RhB by visible light irradiation. Nanotechnology 27:225403
Long X, Yan T, Hu T, Gong X, Li H, Chu Z (2017) Enhanced photocatalysis of g-C3N4 thermally modified with calcium chloride. Catal Lett 147:1922–1930
Mahadwad OK, Parikh PA, Jasra RV, Patil C (2011) Photocatalytic degradation of reactive black-5 dye using TiO2 impregnated ZSM-5 Bull. Mater Sci 34:551–556
Mahmoud MA, Poncheri A, Badr Y, Abd El Wahed MG (2009) Photocatalytic degradation of methyl red dye. South Afr. J. Sci. 105:299–303
Marinovic V, Ljubas D, Curkovic L (2017) Effects of concentration and UV radiation wavelengths on photolytic and photocatalytic degradation of azo dyes aqueous solutions by sol-gel TiO2 films. Holistic Approach Environ 7:3–14
Mehra M, Sharma TR (2012) Photo-catalytic degradation of two commercial dyes in aqueous phase using photo catalyst TiO2. Adv Appl Sci Res 3:849–853
Mehta R, Surana M (2013) Photodegradation of dye acid orange 67 by titanium dioxide in the presence of visible light and UV light. Res Rev 2:1216
Meng Z, Juan Z (2008) Wastewater treatment by photocatalytic oxidation of nano-ZnO. Global Environ Policy Jpn 12:1–9
Mezughi K, Tizaoui C, Ma’an FA (2014) effect of TiO2 concentration on photocatalytic degradation of reactive orange 16 dye (RO16). Adv Environ Biol 8:692–695
Mills A, Davies RH, Worsley D (1993) Water purification by semiconductor photocatalysis. Chem Soc Rev 22:417–425
Min OM, Ho LN, Ong SA, Wong YS (2015) Comparison between the photocatalytic degradation of single and binary azo dyes in TiO2 suspensions under solar light irradiation. J Water Reuse Desalin 5:579–591
Mo S-D, Ching WY (1995) Electronic and optical properties of three phases of titanium dioxide: rutile, anatase and brookite. Phys Rev B Condens Matter 51:13023–13032
Mohammed A, Kapoor K, Mobin SM (2016) Improved photocatalytic degradation of organic dyes by ZnO-nanoflowers. Chem Select 1:3483–3490
Morgan BJ, Watson GW (2010) Intrinsic n-type defect formation in TiO2: a comparison of rutile and anatase from GGA plus U calculations. J Phys Chem 114:2321–2328
Mukhtish MZB, Najnin F, Rahman MM, Uddin MJ (2013) Photocatalytic degradation of different dyes using TiO2 with high surface area: a kinetic study. J Sci Res 5(2):301–314
Nagaraja R, Nagaraju KCRG, Nagabhushana BM (2012) Photocatalytic degradation of Rhodamine B dye under UV/solar light using ZnO nano powder synthesized by solution combustion route. Powder Technol 215:91–97
Narde SB, Lanjewar RB, Gadegone SM, Lanjewar MR (2017) Photocatalytic degradation of azo dye congo red using Ni0.6Co0.4Fe2O4 as photocatalyst. Pharma Chem 9:115–120
Neppolian B, Sakthivel S, Arabindo B, Palanichamy M, Murugesan V (1999) Degradation of textile dye by solar light using TiO2 and ZnO photocatalysts. J Environ Sci Health Part A Tox Hazard Subst Environ Eng 34:1829–1838
Nguyen-Phan TD, Kim EJ, Hahn SH, Kim W-J, Shin EW (2011) Synthesis of hierarchical rose bridal bouquet- and humming-top like TiO2 nanostructures and their shape-dependent degradation efficiency of dye. J Colloid Interface Sci 356:138–144
Nosaka Y, Nosaka AY (2017) Generation and detection of reactive oxygen species in photocatalysis. Chem Rev 117:11302–11336
Odling G, Robertson N (2017) Silar BiOI-sensitized TiO2 films for visible-light photocatalytic degradation of Rhodamine B and 4- cholophenol. Chem Phys Chem 18:728–735
Ohno T, Tsubota T, Toyofuku M, Inaba R (2004) Photocatalytic activity of a TiO2 photo-catalyst doped with C4++ and S4++ ions having a rutile phase under visible light. Catal Lett 98:255–258
Ohtani B, Ogawa Y, Nishimoto S-I (1997) Photocatalytic activity of amorphous-anatase mixture of titanium (IV) oxide particles suspended in aqueous solutions. J Phys Chem 101:19
Pandit VK, Arbuj SS, Pandit YB, Naik SD, Rane SB, Mulik UP, Gosavic SW, Kale BB (2015) Solar light driven dye degradation using novel organic-inorganic (6,13-pentacenequinone/TiO2) nanocomposite. RSC Adv 5:10326–10331
Paridwala JM, Patel FJ, Patel SS (2017) Photocatalytic degradation of RB21 dye by TiO2 and ZnO under natural sunlight: microwave irradiation and UV reactor. Int J Adv Res Engineer Technol 8:8–16
Poulios I, Aetopoulou I (1999) Photocatalytic degradation of the textile dye reactive orange 16 in the presence of TiO2 suspensions. Environ Technol 20:479–487
Poulios I, Avrans A, Rekliti E, Zouboulis A (2000) Photocatalytic oxidation of Auramine O in the presence of semiconducting oxides. J. Chem. Biotechnol. 75:205–212
Qamar M, Yoon CR, Oh HJ, Lee NH, Park K, Kim DH (2008) Preparation and photocatalytic activity of nanotubes obtained from titanium dioxide. Catal Today 131:3–14
Quang MD, Nguyen ND, Nguyen QH (2017) Photocatalytic degradation of azo dye (Methyl Red) in water under visible light using AgNi/TiO2 synthesized by and ɣ-irradiation method. Int J Environ Agric Biotechnol 2:529–538
Raheem Z, Hameed AM (2015) Photocatalytic degradation for methylene blue dye using magnesium oxide. Int J Basic Appl Sci 4:81–83
Rajesh JT, Praveen KS, Ramachandra GK, Raksh VJ (2007) Photocatalytic degradation of dyes and organic contaminants in water using nanocrystalline anatase and rutile TiO2. Sci Technol Adv Mater 8:455–462
Raji J, Palanivelu K (2016) Semiconductor coupled solar photo-Fenton’s treatment of dyes and textile effluent. Adv Environ Res 5:61–77
Rauf MA, Meetani MA, Hisaidee S (2011) An overview on the photocatalytic degradation of azo dyes in the presence of TiO2 doped with selective transition metals. Desalination 276:13–27
Rauf MA, Salman A S Fundamental principles and application of heterogenous photocatalytic degradation of dyes in solution. Chem Engineer J 151:10–18
Reutergarth LB, Iangpashuk M (1997) Photocatalytic decolorization of reactive azo dye: a comparison between TiO2 and CdS photocatalysts. Chemosphere 35:585–596
Rochkind M, Pasternak S, Paz Y (2014) Using dyes for evaluating photocatalytic properties: a critical review. Molecules 20:88–110
Rong X, Qiu F, Zhao H, Yan J, Zhu X, Yang D (2015) Fabrication of single-layer graphitic carbon nitride and coupled systems for the phot-catalytic degradation of dyes under visible-light irradiation. Eur J Inorg Chem 8:1359–1367
Rupa AV, Manikandan D, Divakar D, Revathi S, Preethi ME, Shanthi K, Sivakumar T (2015) Photocatalytic degradation of tetrazine dye using TiO2 catalyst: salt effect kinetic studies. Indian J Chem Technol 14:71–78
Saber A, Rasul MG, Wayde M, Richard B, Hashib MA (2011) Advances in heterogeneous photocatalytic degradation of phenols and dyes in waste water: a review. Water Air Soil Pollut 215:3–29
Sacco O, Stoller M, Vincenzo V, Ciambelli P, Chianese A, Sannino D (2012) Photocatalytic degradation of organic dyes under visible light on N-doped TiO2 photo-catalysts. Int J Photoenergy 2012:1–8
Salem MA, Shaban SY, Ismail SM (2015) Photocatalytic degradation of acid green 25 using ZnO and natural sunlight. Int J Energy Technol Adv Eng 5:439–443
Sarkhanpour R, Tavakoli O, Ghiyasi S, Saeb MR, Borja R (2017) Photocatalytic degradation of a chemical industry wastewater: search for higher efficiency. J Residuals Sci Technol 14:44–58
Sclafani A, Herrmann JM (1996) Comparison of the photoelectronic and photocatalytic activities of various anatase and rutile forms of titania in pure liquid organic phases and in aqueous solutions. J Phys Chem 100:13655–13661
Sharma S, Ameta RRKM, Suresh CA (2013) Photocatalytic degradation of rose Bengal using semiconducting zinc sulphide as the photocatalyst. J Serb Chem Soc 78:897–905
Simovic B, Poleti D, Golubovic A, Matkovic A, Scepanovic M, Babic B, Brankovic G (2017) Enhanced photocatalytic degradation of RO16 dye using Ag modified ZnO nano-powders prepared by the solvothermal method Process. Appl Ceramics 11:27–38
Singh P, Mondal K, Sharma A (2013) Reusable electrospun mesoporous ZnO nanofiber mats for photocatalytic degradation of polycyclic aromatic hydrocarbon dyes in wastewater. J Colloid Interface Sci 394:208–215
Sun J, Wang X, Sun R, Sun S, Qiao L (2006) Photocatalytic degradation and kinetics of orange G using nano-sized Sn (IV)/TiO2/AC photocatalyst. J Mol Catal Chem 260:241–246
Sun J, Qiao L, Sun S, Wang G (2008) Photocatalytic degradation of orange G on nitrogen-doped TiO2 catalysts under visible light and sunlight irradiation. J Hazard Mater 155:312–319
Tang WZ, An H (1995) UV/TiO2 photocatalytic oxidation of commercial dyes in aqueous solutions. Chemosphere 31:4158–4170
Tesfay WG, Manjunatha P, Rani MP (2015) Review on the photocatalytic degradation of dyes and antibacterial activities of pure and doped-ZnO. Int J Sci Res 4:2252–2264
Thuy-Duong Nguyen P, Eui Jung K, Sung Hong H, Woo Jae K, Eun Woo S (2011) Synthesis of hierarchical rose bridal bouquet- and humming-top-like TiO2 nanostructures and their shape-dependent degradation efficiency of dye. J Colloid Interface Sci 356:138–144
Tiwari B, Ram S (2019) Biogenic synthesis of graphitic carbon nitride for photocatalytic degradation of organic dyes. ACS Omega 6:16298–16307
Tiwari B, Ram S, Banerji P (2018) Biogenic synthesis of tunable core-shell C-CaIn2O4, interface bonding, conductive network channels and tailored dielectric properties. ACS Sustain Chem Eng 6:16298–16307
Tunesi S, Anderson M (1991) Influence of chemisorption on the photodecomposition of salicylic acid and related compounds using suspended titania ceramic membranes. J Phys Chem 95:3399–3405
Wang K-H, Hsieh Y-H, Wu C-H, Chang C-Y (2000) The pH and anion effects on the heterogeneous photocatalytic degradation of omethylbenzoic acid in TiO2 aqueous suspension. Chemosphere 40:389–394
Wei C-H, Tang X-H, Liang J-R, Tan SY (2007) Preparation, characterization and photocatalytic activities of boron and cerium codoped TiO2. J Environ Sci (china) 19:90–96
Xiaoqing C, Zhansheng W, Dandan L, Zhenzhen G (2017) Preparation of ZnO photocatalyst for the efficient and rapid photo-catalytic degradation of azo dyes. Nanoscale Res Lett 12:143
Xie YB, Li XZ (2006) Interactive oxidation of photo-electro catalysis and electro-Fenton for azo dye degradation using TiO2-Ti mesh and reticulated vitreous carbon electrode. Mater Chem Phys 95:39–50
Xiong Z, Zhang LL, Ma J, Zhao XS (2010) Photocatalytic degradation of dyes over graphene-gold nanocomposites under visible light irradiation. Chem Commun (camb) 46(33):6099–6101
Ye M, Zhang Q, Hu Y, GE j, Lu Z, He L, Chen Z, Yin Y (2010) Magnetically recoverable core-shell nanocomposites with enhanced photocatalytic activity. Chemistry 16:6243–6250
Zangi ZM, Ganjidoust H, Ayati B (2017) Analysis of photocatalytic degradation of zao dyes under sunlight with response surfsce method. Desalination Water Treat 63:262–274
Zhang T, Oyama T, Horikoshi S, Hidaka H, Zhao J, Serpone N (2001) Photocatalysed N-demethylation and degradation of methylene blue in titania dispersions exposed to concentrated sunlight, solar energy. Mater Solar Cells 73:287–303
Zhiyong Y, Bensimon M, Sarria V, Stolitchnov I, Jardim W, Laub D, Mielczarski E, Mielczarski J, Kiwi-Minsker L, Kiwi J (2007) ZnSO4-TiO2 doped catalyst with higher activity in photocatalytic processes. Appl Catal B 76:185–195
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Tiwari, B., Park, H.J. (2022). Recent Developments in Photocatalytic Techniques of Dye Degradation in Effluents. In: Dave, S., Das, J. (eds) Trends and Contemporary Technologies for Photocatalytic Degradation of Dyes. Environmental Science and Engineering. Springer, Cham. https://doi.org/10.1007/978-3-031-08991-6_3
Download citation
DOI: https://doi.org/10.1007/978-3-031-08991-6_3
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-08990-9
Online ISBN: 978-3-031-08991-6
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)