Abstract
Dyes and pigments are released into the environment through various industrial, domestic, and municipal pathways, causing ecological disturbances and serious environmental and health concerns. Although several studies have investigated the removal of the methylene blue (MB) dye from aqueous solutions, comprehensive studies are still required to understand the correlation between dye-based wastewater treatment and sustainable development. Hence, this study represents the preparation of adsorbent from natural material and zero-cost waste to eliminate MB from synthetic solutions, providing an ecofriendly treatment approach. Box Behnken statistical design was employed to find the effects of three process factors, i.e., time, adsorbent dosage and solution pH, on the MB removal efficiency. It was found that bagasse-based carbon (BBC) and orange peel (OP) powder had a high ability to adsorb MB with a removal efficiency of 86.4% under time = 15.4 min, dosage = 1.28 g/L, and pH = 7.63. A quadratic model was developed to predict the MB removal efficiency with a coefficient of determination (R2) of 0.836 and adjusted R2 of 0.737. The study outputs were highly correlated to ecosystem safety (e.g., pollution reduction), resource utilization (e.g., biosorbent preparation from natural substances), and industrialization (e.g., application of BBC and OP powder in the industrial sector). Accordingly, the study outputs would meet multiple environmental, economic, and social related sustainable development goals (SDGs).
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
14.1 Introduction
The synthetic dye manufacturing and textile industries, in addition to several commercial elements (e.g., plastic, clothes, and paper) utilize large amounts of dyes during production (Jawad et al. 2020). Wastewater released from these industries carries various dyes such as methylene blue (MB), azo dyes, and methyl orange recognized as the main pollution source (Cheng et al. 2021). The disposal of dyes-laden wastewater into the environment has been associated with severe impacts on aquatic culture, human health, and terrestrial animals (Gadow and Li 2020). An excessive discharge of dye effluents into water bodies significantly deteriorates the biological quality and characteristics of the ecosystems by increasing the biodegradable oxygen demand (BOD) levels (Georgin et al. 2019). Additionally, dyes, forming a colored top-layer on the water surface, tend to limit light penetration to marine phytoplankton; hence, inhibiting the photosynthetic activities (Jiang et al. 2021). Accordingly, the removal of dyes from textile effluents before reaching the aquatic and land environments is an essential point of research, requiring comprehensive studies.
Previous studies have shown the application of various physical, biological, and chemical-based treatment methods (or their combinations) for eliminating dyes from aqueous solutions (Moosavi et al. 2020). However, a sustainable treatment approach should provide significant advantages such as design simplicity, flexibility, cost-effectiveness, and insensitivity to toxic compounds, whilst avoiding the formation of secondary pollutants. These advantages fit well to the adsorption process, which entails multiple complexation and/or ionic exchange mechanisms to eliminate any anionic, cationic, or organic contaminants from aqueous solutions (Liu et al. 2020). Moreover, the “Sustainability” concept of wastewater treatment by adsorption should be linked to the utilization of adsorbent material prepared from chemical-free additives (Ajayi and Ogunbayio 2012). For instance, various non-living biomass-based material, such as sawdust, eggshell, seed shells, potato and citrus peels, sugarcane bagasse, and rice husk, have found successful applications for treating wastewater laden with dyes and heavy metals (Khaled et al. 2009). This adsorbent material would not cause substantial secondary pollution when discarded into the environment due to its ability to degrade within the soil matrix. Moreover, this adsorbent substance is prepared from almost zero-cost biomass, resulting in a cheap adsorption process towards contaminants removal. Accordingly, employing a wastewater treatment process based on natural adsorbents would meet various environmental, economic, and social related sustainable development goals (SDGs).
Recently, the United Nations (UN) has passed a number of goals to achieve the three pillars of sustainability by 2030 (Ajayi and Ogunbayio 2012). The SDGs cover several aspects relevant to pollution reduction, waste management, utilization of non-toxic substances (natural resources), human health protection, and public awareness towards sustainability (Sawaf and Karaca 2018). Accordingly, providing a highly effective and economical process for dye removal from wastewater effluents to clean up the polluted environment would meet multiple SDGs (Hamdy et al. 2017). However, there is a lack of studies to define the achievable SDGs associated with the removal of dyes from the environment by adsorption process.
Hence, this study attempts to represent a reliable and cost-efficient adsorption process for removing MB dye from aqueous solutions, highlighting the tangible SDGs achieved. The study objectives are fivefold (1) synthesize and prepare an environmentally friendly adsorbent from bagasse-based carbon (BBC) and orange peel (OP), (2) investigate the adsorption performance of the adsorbent material towards MB, (4) represent a predictive model for the MB removal efficiency using three process factors, i.e., adsorption time, adsorbent dosage, and solution pH, and (5) find the interlinkages between the study objectives and the three pillars of sustainability.
14.2 Methodology
14.2.1 Adsorbent Preparation
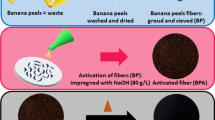
Fresh sugarcane bagasse (SB) was collected from a juice-service store located in Alexandria city, Egypt. The SB biomass was washed and rinsed with tap water, and the resultant clean material was cut into small pieces and dried overnight (100 °C for 24 h). Further, the product was inserted into a muffle furnace operated at 600 °C for 2 h to form bagasse-based carbon (BBC). The orange peels (OP) waste was obtained from a restaurant in Egypt-Japan University of Science and Technology. The raw OP waste was washed to remove any traces of sand particles and other dirt, dried in an oven at 100 °C for 24 h, and then ground by mechanical grinder. Further, the resultant peels were crushed to have a fine powder of 40#-mesh size according to ASTM (American Standards for Testing and Materials). Afterward, the two fine materials (BBC and OP) were mixed in a ratio of 1:1 and treated with NaOH to remove natural fats, waxes, silica, lignin, and hemicelluloses.
14.2.2 Methylene Blue (MB) Solution Preparation
The methylene blue (MB) cationic dye (molecular formula of C16H18N3SCl and molecular weight of 319.85 g/mol) purchased from Oxford Lab Fine Chemicals LLP was selected as a model contaminant in this study. An amount of 1.0 g MB was dissolved in 1 L of distilled water to obtain a stock solution of 1000 ppm. The flask containing MB was placed on a magnetic stirrer for 2 h to establish a homogeneous dye aqueous solution. The absorbance of MB dye was detected at the wavelength of 664 using JASCO V-630 UV–Vis–NIR spectrophotometer.
14.2.3 Experimental Design for Adsorption of Methylene Blue Dye onto OP:BBC Biosorbent
A series of batch scale assays were performed in Erlenmeyer flasks (250 mL-capacity) to examine the performance of the adsorbent towards MB removal (Fig. 14.1). A total of 17 experimental runs, with five center points, were carried out based on Box-Behnken design (BBD). This batch experimentation was conducted to understand the correlation between MB removal efficiency with pH, time, and adsorbent dosage. Generation and evaluation of the experimental design was executed using MATLAB and Design Expert® (Stat-Ease Inc., Minnepolis) software (Tripathi et al. 2009). All experimentations were performed at the room temperature (about 25 °C).
14.3 Results and Discussion
14.3.1 Results of Box–Behnken Design for Optimizing MB Removal
Table 14.1 lists the removal efficiencies of MB under different adsorption conditions, i.e., time (A: 10–30 min), adsorbent dosage (B: 0.5–1.5 g/L), and medium pH (C: 5–9).The matrix of these three factors with three levels was prepared according to the Box–Behnken statistical design. The input–output relationship is further demonstrated by interaction plot (Fig. 14.2a) and main-effects-plot (Fig. 14.2b).
a Interaction plot for the effects of three adsorption factors, i.e., time (min), adsorbent dosage (g/L), and solution pH, on the MB removal efficiencies statistical design b Mean values of MB removal efficiencies estimated at different time (min), adsorbent dosage (g/L), and solution pH proposed by the Box–Behnken
An increase in adsorption time from 10 to 20 min caused a slight improvement in the MB removal efficiency (around 80%), which dropped to about 76%, further prolonging the time to 30 min. During the initial stage, this fast adsorption performance was possibly due to the available number of vacant sites to uptake more MB molecules. Further, the adsorption rate declined at time over 20 min. Moreover, increasing the adsorbent dosage over 1 g/L had insignificant (p > 0.05) impact on MB removal, probably due to the overlap of the pore structure of the BBC: OP powder adsorbent. This pattern would also reduce the effective surface area of adsorbent; hence, the adsorbent access for the cationic dye decreased. A better MB removal efficiency at pH of 7 suggests that the negatively charged sites formed a robust, attractive force with the positively charged MB molecules.
14.3.2 Quadratic Model Development for MB Removal Prediction
Table 14.2 summarizes the results of t-test used to predict MB removal using three adsorption inputs (time, adsorbent dosage, and solution pH). This test revealed an R2: 0.836, Adj-R2: 0.737, SSE: 87.774, DF: 10, F-value: 8.479, P-value < 0.01, and MSE: 8.777. The results of Table 14.2 also revealed that the input “A” had insignificant (p > 0.05) effects hence, the correlation between MB removal-time relationship was a “Flat” curve. Moreover, the plot of MB removal against dosage showed a “Linear Up” curve. The “C” input had significant (p < 0.05) effects for both the linear and quadratic effects. Hence, the plot of MB removal versus solution pH resulted in a “Quadratic concave Linear Up” curve (Tripathi et al. 2009).
An equation of the second-order polynomial model for MB dye removal was derived and described in Eq. (14.1). This quadratic model is significant (F-value: 8.479, P-value < 0.01) and could describe 83.6% of the MB removal efficiency data. A is Time (min), B is Dosage (g/L) and C is pH;
14.3.3 Optimization of Adsorption Factors
Figure 14.4 shows the optimum values for the three inputs, representing time = 15.4 min, adsorbent dosage = 1.28 g/L, and solution pH = 7.63. Under this condition, the highest removal efficiency of the MB dye was 86.53%. This value was confirmed and validated with an experimental efficiency value of 86.4%, hence a reliable model (Vyavahare et al. 2018) (Fig. 14.3).
Figure 14.4 shows the response surface plots as function of time, adsorbent dosage, and medium pH on MB removal, with their mutual interaction. At constant time and pH, a positive relationship between MB removal and adsorbent dosage was manifested. This correlation possibly suggests that the number of adsorbent sites increased with dosage, causing larger specific surface area to capture the MB contaminant. The adsorption rate seems to be fast (around 15 min) to deliver a high MB removal percentage. A comparable trend was reported by Sadaf et al. (2014) to eliminate direct violet 51 dye by adsorption onto lignocellulosic sugarcane bagasse waste.
14.3.4 Sustainable Development Goals (SDGs) Relevant to Dye-Laden Wastewater Treatment
Recently, the exponential increase in demand for textile products has been associated with the release of various organic and inorganic contaminants into the environment. Although there can be improved economic development of the country, the released amounts of wastewater constitute dye compounds. This wastewater affects the aquatic biota and human health; hence, its treatment is essential for (a) ecosystem safety, (b) natural resource utilization, and (c) economic development (Fig. 14.5).
Ecosystem safety
The current study provides a reliable strategy for minimizing the release of elevated dye concentrations into the aquatic environment. This benefit would protect the aquatic animals and plants and maintain their survival and growth rates (Target 15.5 Action against water pollution). The study also showed the preparation of natural-based adsorbent material, which could be further employed to reduce soil pollution (Target 15.3 restoration of polluted soils). For instance, Zubair et al. (2021) was able to reduce cadmium (Cd) mobility in soil using chitosan-coated textile waste biochar. This biochar could be further characterized and evaluated for its application as a soil amendment, enhancing the agricultural sector (Target 2.2.2 Boosting agriculture to serve the hungry). Soil amendments could also enable the cultivation of non-crop plants like flowers for sale. Moreover, the re-utilization of biomass wastes could limit unwise waste disposal (Target 13.1 minimise biomass related emissions).
Resource utilization
The current study represents a sustainable approach for reutilizing waste orange peels and sugarcane bagasse to ensure full exploitation of the natural biomass resource (Target 12.2 Efficient use of natural resources). Whilst there’s a need to utilize resources optimally and efficiently, women leadership in controlling biowaste is key (Target 5.5: women empowerment) (Nasr et al. 2021). A further utilization of natural-based adsorbents for biofuel and biochar productions (Ezz et al. 2021) would enable the achievement of the cleaner energy production agenda (Target 9.4 Clean technologies). The utilization of textile effluents after tertiary treatment and membrane-based technologies for irrigating particular types of crops would be a feasible strategy to mitigate impacts on freshwater ecosystems (Target 1.4.1 Access to basic services).
Socio-economic development
The current study provides a system that could be simply operated by skilled and unskilled laborers (Target 8.3 Job creation). After dye adsorption, the carbonized adsorbent material would be subjected to multiple regeneration cycles to extract the adsorbed dye. This dye is further utilized in the textile factories for printing, coloring, and finishing, providing a great economic value (Meneses et al. 2022).The treatment of dye polluted wastewater provides a well sanitated environment thus allowing the majority to enjoy the comfortable public services.
Conclusion and future aspirations
This study succeeded in providing a reliable and cost-effective adsorbent material prepared from bagasse-based carbon (BBC) and orange peel (OP). A quadratic model was developed and used to predict the MB removal, showing the highest value of 86.4% efficiency under the optimum condition (time = 15.4 min, dosage = 1.28 g/L, and pH = 7.63). The conducted study achieved several UN targets relating to; (ecosystem safety and pollution reduction), economic (e.g., industrialization and trading), and social (e.g., job creation and gender equality) as pillars of sustainability. Further studies are required to synthesize novel adsorbent material for textile wastewater treatment under real scale conditions. Moreover, artificial intelligence tools and internet of things can be incorporated into the adsorption process during industrial wastewaste treatment.
References
Ajayi TO, Ogunbayio AO (2012) Achieving environmental sustainability in wastewater treatment by phytoremediation with water hyacinth (eichhornia crassipes). J Sustain Dev 5:80–90
Al Sawaf MB, Karaca F (2018) Different stakeholders’ opinions toward the sustainability of common textile wastewater treatment technologies in Turkey: A Case study Istanbul province. Sustain. Cities
Cheng H, Yuan M, Zeng Q, Zhou H, Zhan W, Chen H, Mao Z, Wang Y (2021) Efficient reduction of reactive black 5 and Cr(VI) by a newly isolated bacterium of Ochrobactrum anthropi. J Hazard Mater 406:124641
Ezz H, Ibrahim MG, Fujii M, Nasr M (2021) Dual biogas and biochar production from rice straw biomass: a techno-economic and sustainable development approach. Biomas Conver Biorefinery
Gadow SI, Li YY (2020) Development of an integrated anaerobic/aerobic bioreactor for biodegradation of recalcitrant azo dye and bioenergy recovery: HRT effects and functional resilience. Bioresour Technol Reports 9:100388
Georgin J, Alves E, Drumm F, Tonato D, Grassi P, Piccin JS, Oliveira MLS, Dotto GL, Mazutti MA (2019) Application of Beauveria bassiana spore waste as adsorbent to uptake acid red 97 dye from aqueous medium. Environ Sci Pollut Res 26:36967–36977
Hamdy A, Mostafa MK, Nasr M (2018) Zero-valent iron nanoparticles for methylene blue removal from aqueous solutions and textile wastewater treatment, with cost estimation. Water Sci Technol
Jawad AH, Mohammed IA, Abdulhameed AS (2020) Tuning of fly ash loading into chitosan-ethylene glycol diglycidyl ether composite for enhanced removal of reactive red 120 Dye: optimization using the box-behnken design. J Polym Environ 28:2720–2733
Jiang R, Yu G, Ndagijimana P, Wang Y, You F, Xing Z, Wang Y (2021) Effective adsorption of Direct Red 23 by sludge biochar-based adsorbent: adsorption kinetics, thermodynamics and mechanisms study. Water Sci Technol 83:2224–2236
Khaled A, Nemr AE, El-Sikaily A, Abdelwahab O (2009) Removal of Direct N Blue-106 from artificial textile dye effluent using activated carbon from orange peel: adsorption isotherm and kinetic studies. J Hazard Mater 165:100–110
Liu L, Yang C, Tan W, Wang Y, Wang Y (2020) Degradation of acid red 73 by activated persulfate in a heat/Fe3O4@AC system with ultrasound intensification. ACS Omega 5:13739–13750
Meneses IP, Novaes SD, Dezotti RS, Oliveira PV, Petri DFS (2022) CTAB-modified carboxymethyl cellulose/bagasse cryogels for the efficient removal of bisphenol A, methylene blue and Cr(VI) ions: Batch and column adsorption studies. J Hazard Mater 421:0–3
Moosavi S, Li RYM, Lai CW, Yusof Y, Gan S, Akbarzadeh O, Chowhury ZZ, Yue XG, Johan MR (2020) Methylene blue dye photocatalytic degradation over synthesised fe3o4 nano-catalyst: degradation and reusability studies. Nanomaterials 10:1–15
Nasr M, Tawfik A, Awad HM, Galal A, El-Qelish M, Abdul Qyyum M, Mumtaz Ali Khan M, Rehan M, Nizami AS, Lee M (2021) Dual production of hydrogen and biochar from industrial effluent containing phenolic compounds. Fuel 301:121087
Sadaf S, Bhatti HN, Nausheen S, Noreen S (2014) Potential use of low-cost lignocellulosic waste for the removal of direct violet 51 from aqueous solution: equilibrium and breakthrough studies. Arch Environ Contam Toxicol 66:557–571
Tripathi P, Srivastava VC, Kumar A (2009) Optimization of an azo dye batch adsorption parameters using Box-Behnken design. Desalination 249:1273–1279
Vyavahare GD, Gurav RG, Jadhav PP, Patil RR, Aware CB, Jadhav JP (2018) Response surface methodology optimization for sorption of malachite green dye on sugarcane bagasse biochar and evaluating the residual dye for phyto and cytogenotoxicity. Chemosphere 194:306–315
Zubair M, Adnan Ramzani PM, Rasool B, Khan MA, ur-Rahman M, Akhtar I, Turan V, Tauqeer HM, Farhad M, Khan SA, Iqbal J, Iqbal M (2021) Efficacy of chitosan-coated textile waste biochar applied to Cd-polluted soil for reducing Cd mobility in soil and its distribution in moringa (Moringa oleifera L.). J Environ Manage 284
Acknowledgements
The first author acknowledges the Seventh Tokyo International Conference on African Development (TICAD7) for the M.Sc. scholarship. Further, thanks to the Japan International Cooperation Agency (JICA) for providing equipment used in this research. Also, thanks to Egypt-Japan University of Science and Technology (E-JUST).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this paper
Cite this paper
Kalengyo, B.R., Ibrahim, M.G., Fujii, M., Nasr, M. (2022). Synthesis of Carbonized Bagasse and Orange Peel Wastes as Adsorbents for Dye Removal from Aqueous Solution: A Sustainable Approach. In: Jeon, HY. (eds) Sustainable Development of Water and Environment. Environmental Science and Engineering. Springer, Cham. https://doi.org/10.1007/978-3-031-07500-1_14
Download citation
DOI: https://doi.org/10.1007/978-3-031-07500-1_14
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-07499-8
Online ISBN: 978-3-031-07500-1
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)