Abstract
Poly (γ-glutamic acid) (γ-PGA) is a microbial biopolymer composed of D- and L-glutamic acid monomers connected by γ-amide linkage. γ-PGA is considered a promising biopolymer and has broad applications in food, medicine, agriculture, daily chemicals, and environmental protection due to its water-soluble, biodegradable, and nontoxic properties. The production of γ-PGA has already been established on an industrial scale. This chapter provides updated information about strain breeding, biosynthesis, fermentation, purification, and application of γ-PGA. The biosynthetic mechanism of γ-PGA and regulation of molecular weight by synthetic biology are covered in detail. The current and potential applications of γ-PGA have also been reviewed. Finally, future outlooks of microbial γ-PGA production and application are discussed in recent progress, challenges, and trends in this field. This chapter contributes to the further understanding of efficient production of diversified γ-PGA and will serve as valuable references for reducing the cost of production and further development of commercial-scale applications of γ-PGA.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Poly-γ-glutamic acid
- Strain breeding
- Biosynthesis regulation
- Fermentation optimization
- Industrial applications
1 Research Progress of Poly-(γ-Glutamic Acid)-Producing Strains
1.1 Screening and Classification of Poly-(γ-Glutamic Acid)-Producing Strains
Since the 1990s, as a new biopolymer, poly-(γ-glutamic acid) (γ-PGA) has attracted global attention. In 1937, Ivanovics first isolated γ-D-PGA from the capsular component of the disease-causing Gram-positive bacterium Bacillus anthracis (Ivanovics and Bruckner 1937). This compositional synthetic γ-D-PGA is embedded in the membrane, which can enhance the virulence of the strain and help bacteria in different environments to enhance the resistance of the cell, to prevent the damage of adverse factors (Schneerson et al. 2003). In 1942, Bovarnick et al. found that the γ-PGA synthesized by Bacillus subtilis could be transported to the outside of the cell membrane, which belongs to the secretory mode. Most of the current strains applied in fermentation production belong to this type, which opened a new era of the synthesis of γ-PGA by microbial fermentation (Bovarnick 1942). Until now, the industrial production of γ-PGA mainly depends on microbial synthesis. In addition to screening and identification of γ-PGA-producing strains directly through microbial high viscosity phenotypes and physiological characteristics, it can also carry out high-throughput screening based on the electrostatic interaction between basic dyes (neutral red) and γ-PGA polymers which were reported by Zeng et al. (2013). At present, more and more microorganisms have been reported to be able to synthesize γ-PGA, such as Bacillus sp., Fusobacterium nucleatum, archaea and eukaryotes, etc. (Candela et al. 2009; Weber 1989; Hezayen et al. 2001). Among them, the wild-type strains used for γ-PGA production are mainly focus on Bacillus sp., including Bacillus subtilis, Bacillus licheniformis, and Bacillus amyloliquefaciens.
Table 1 summarizes the main strains currently used for γ-PGA fermentation. Based on the differences of glutamic acid precursor requirement, γ-PGA-producing strains can be divided into two categories: the exogenous glutamic acid-dependent strain (type I) and the exogenous glutamic acid-independent strain (type II). The concentration of γ-PGA synthesized by glutamic acid-dependent strains was relatively high and accounted for most of the reported strains. These strains require glutamic acid in the culture medium to produce γ-PGA. Generally, the synthesis efficiency is high, and the product concentration can reach 20–50 g/L. However, due to the high cost caused by the addition of a large amount of glutamate in the medium, γ-PGA is still faced with the limitation of application cost in some fields, such as agriculture and feed.
Glutamate-independent production strain is a new research hotspot in the field of γ-PGA microbiosynthesis because it does not need to add extra glutamate, which greatly reduces the fermentation cost. Zhang et al. isolated a glutamate-independent γ-PGA producer Bacillus subtilis C10 from sauce products, which could use glucose as a carbon source and produce 3.73 g/L of γ-PGA (Zhang et al. 2012a). Bacillus amyloliquefaciens LL3, an independent glutamate-producing bacterium isolated from fermented food, was obtained 4.36 g/L of γ-PGA in a 200 L fermentor when used sucrose as the substrate (Cao et al. 2011). Peng et al. isolated a strain of Bacillus methylotrophicus SK19.001 using glycerol as carbon source from the soil; the γ-PGA production reached 14 g/L without the addition of amino acid precursor (Peng et al. 2015). A novel strain named Bacillus amyloliquefaciens NX-2S was isolated from soil samples of Jerusalem artichoke tubers by Qiu et al. The strain preferred to assimilate inulin as the sole carbon source and could produce 6.85 g/L of γ-PGA without glutamic acid addition, which provides a new strategy for the γ-PGA production from low-cost renewable non-food resources (Qiu et al. 2017). However, the γ-PGA productivity of these strains is low, and there has been no report for industrial production and application so far.
The mechanism of substrate glutamate dependence between the two types of γ-PGA-producing strains above has also been studied. Genomic analysis of glutamate-dependent strains Bacillus subtilis GXA-28 and glutamate-independent strains Bacillus subtilis GXA-5 showed that the genes related to the differences in glutamate dependence were mainly involved in sugar transport metabolism and amino acid metabolism and 13 genes related to γ-PGA biosynthesis (Zeng et al. 2017). In order to study the mechanism of glutamate dependence of Bacillus subtilis NX-2, Sha et al. conducted transcriptome analysis of cultured strains with or without addition of glutamate. Turns out that the overexpression of gltA, gltB, putM, and rocA genes can obviously promote the accumulation of γ-PGA, suggesting that intracellular glutamate synthesis plays a key role in the regulation of γ-PGA production in glutamate-dependent strains (Sha et al. 2019a).
1.2 Mutagenic Breeding of γ-PGA-Producing Strains
In order to select highly efficient γ-PGA-producing strains to meet the needs of industrial production, traditional breeding techniques have been successfully applied to improve the production efficiency of γ-PGA. Using atmospheric and room temperature plasma (ARTP), Qiu et al. successfully improved the γ-PGA productivity of Bacillus amyloliquefaciens NX-2S. Compared with the wild-type strain, the γ-PGA yield of the mutant strain NX-2S154 increased by 58% when used inulin crude extract as the substrate (Qiu et al. 2019). By UV mutagenesis, directed evolution and three rounds of genome shuffling, Zhang et al. obtained a γ-PGA high-yielding strain C2 with high glucose tolerance. Compared with the parent strain W14, the mutant strain increased the γ-PGA production by 3.5 times and the biomass by 2.3 times, respectively (Zhang et al. 2017). However, due to the blindness of traditional mutagenesis breeding and the lack of a fast and effective high-throughput quantitative detection method for γ-PGA concentration in the fermentation broth, the traditional breeding process of γ-PGA strains is still very inefficient.
1.3 Construction and Metabolic Regulation of Efficient Engineered γ-PGA Strains
1.3.1 Research Progress on Engineering of Wild-Strain γ-PGA-Producing Strains
With the development of molecular biology and the establishment of genetic manipulation platform in Bacillus species, it has become a new focus on improving γ-PGA production by using genetic engineering technology. At present, genetic engineering of the wild-type strains to enhance the γ-PGA synthesis mainly revolves around the following aspects:
-
1.
Enhance the substrate utilization pathway: Qiu et al. used the self-developed CRISPR-Cas9 nickase genomic trace-free editing system to modify the substrate inulin hydrolysis module of B. amyloliquefaciens strain by combining the inulinase action module and successfully realized the conversion of substrate inulin and the increase of γ-PGA production (Qiu et al. 2020). Feng et al. transformed the PTS system of the original strain into a non-PTS system in B. amyloliquefaciens LL3 and constructed an energy-saving sucrose metabolism pathway to reduce the energy consumption of carbon source metabolism, ultimately achieving a 38.5% increase in γ-PGA production (Feng et al. 2017). For metabolic regulation by improving utilization rate of industry by-product glycerol in B. licheniformis WX-02, Zhan et al. used the type of promoter (P43), ytzE promoter (PytzE), and bacABC operon promoter (PbacA) to replace natural glpFK promoter, and the glycerol consumption in corresponding mutant strains WX02 P43glpFK, WX02 PytzEglpFK, and WX02-PbacAglpFK is improved by 30.9%, 26.42%, and 18.8%, respectively. And the γ-PGA concentration of the three mutant strains was 33.71%, 23.39%, and 30.05% higher than that of the initial strain WX-02, respectively (Zhan et al. 2017).
-
2.
Enhance the precursor glutamate synthesis pathway: Glutamate is a precursor of γ-PGA synthesis. In B. amyloliquefaciens LL3, Feng et al. knocked out the glutamate-degrading enzyme gene rocG, gudB, and glutamate-inhibiting protein gene rocR. Results showed that both rocG and gudB knockout could increase γ-PGA production by about 38% (Zhang et al. 2015). Furthermore, the glutamate utilization pathway was inhibited by synthetic sRNA against glutamate-degrading gene rocG and glutamine synthetase glnA. The results showed that the yield of anti-glnA sRNA expressing strain NK-anti-glnA and NK-anti-glnA-rocG γ-PGA decreased by 55.9% and 44.3%, respectively, compared with the control NK-E11. Compared with the control NK-E11 strain, the γ-PGA yield of anti-rocG sRNA was increased by 18.5% to 11.04 g/L, and the final γ-PGA yield reached 20.3 g/L by batch fermentation of NK-anti-rocG strain (Feng et al. 2015). In B. amyloliquefaciens NBCSO, Sha et al. optimized γ-PGA synthetic precursor pathway by expression of relevant key genes (citrate synthase citA, glutamate synthetase gltA, proline dehydrogenase ycgM, and Δ1-pyrrolin-5-carboxylic acid dehydrogenase ycgN). At the same time, the shunt pathway of α-ketoglutarate and glutamic acid was weakened, and the final yield of γ-PGA was increased to 22.62 ± 0.41 g/L. These results indicate that glutamate precursors play an important role in further increasing the concentration of γ-PGA (Sha et al. 2020a).
-
3.
Overexpression of γ-PGA synthase and knockdown of γ-PGA degradation pathway: Ashiuchi et al. deleted the γ-PGA synthase gene pgsBCA by using the pKPSD plasmid in B. subtilis ISW 1214 and then replaced pgsBCA under the control of the xylose-inducible promoter. The genetically engineered strain could produce a large amount of γ-PGA in both l-glutamate- and d-glutamate-rich medium (Ashiuchi et al. 2006). Yeh et al. introduced a synthetic expression control sequence (SECS) upstream of pgsBCA gene in B. subtilis DB430 strain to produce 28 g/L γ-PGA in glutamate-free medium (Yeh et al. 2010). In most γ-PGA-producing strains, there existed specific γ-PGA-degrading enzymes which are responsible for the degradation of γ-PGA. The presence of degrading enzyme PgdS can degrade and reduce the γ-PGA production. Therefore, researchers began to try to increase γ-PGA production by knocking out the degrading enzyme gene. Mitsui et al. studied the effects of the knocking out of γ-PGA exonuclease gene ggt, γ-PGA endonuclease gene pgdS, and dl-polypeptide endonuclease family gene on γ-PGA synthesis in B. subtilis (natto). The results showed that only the cwlo gene knockout strain of DL-endopeptidase increased the γ-PGA production by two times compared to the original strain (Mitsui et al. 2011). Scoffone et al. found that double knockout of pgdS and ggt resulted in a twofold increase in γ-PGA production in the strain compared to the Bacillus subtilis strain (Scoffone et al. 2013), while Kimura et al. found that ggt single gene knockout had no effect on γ-PGA synthesis (Kimura et al. 2004a).
-
4.
Removing the by-product synthesis pathway: Most γ-PGA synthetic strains, in addition to the synthesis of γ-PGA, will also synthesize a large number of EPS polysaccharide, levan (fructose oligosaccharides), and other by-products. The existence of these by-products will not only affect the production of γ-PGA by competition of substrate and energy but also interfere with the subsequent separation and purification of γ-PGA. In B. amyloliquefaciens C06, the production of γ-PGA was increased from 3.2 g/L to 6.8 g/L by deleting the polysaccharide biosynthetic gene epsA (Liu et al. 2011). Feng et al. studied the effects of knockout the gene clusters itu, bae, srf, and fen encoding four antibiotic substances synthesis genes on the synthesis of γ-PGA in B. amyloliquefaciens LL3. The γ-PGA yield from the strain with double knockout of itu and srf gene clusters was increased from 3.3 to 4.5 g/L (Gao et al. 2016).
-
5.
Enhanced synthesis of ATP and NADHP cofactors: In B. licheniformis WX-02, overexpression of the glucose-6-phosphate dehydrogenase gene zwf increased the activity of zwf by 9.28 times, thus increasing the production of NADPH and reducing the accumulation of acetone and 2,3-butanediol by products. Finally, the maximum concentration of γ-PGA reached 9.13 g/L, which was 35% higher than that of the original strain (Cai et al. 2017). The supply of ATP plays a vital role in the biosynthesis of γ-PGA. Cai et al. engineered the electron respiration chain by deleting the cytochrome bd oxidase branch, which increased the γ-PGA production by 19.27% in Bacillus licheniformis. Furthermore, the ATP content was increased to 3.53 μmol/g DCW by overexpression of ATP biosynthesis gene adK, resD gene, and Vitreoscilla hemoglobin gene vgb gene, and finally the γ-PGA production of mutant strain WX-BCVAR was 38.64% higher than that of wild-type strain WX-02, reaching 43.81 g/L (Cai et al. 2018).
1.3.2 Application of Synthetic Biology Techniques in γ-PGA-Producing Strains
A great deal of work has been done in γ-PGA high-yielding wild strains. With the rapid development of synthetic biology techniques, some researchers also try to use new model hosts for heterogeneous synthesis of γ-PGA. Ashiuchi et al. heterologously expressed γ-PGA synthase pgsBCA in Escherichia coli and obtained recombinant strains that yield γ-PGA less than 1 g/L (Ashiuchi et al. 1999a). In order to provide sufficient d-glutamate substrate donors, Cao et al. co-express γ-PGA synthase pgsBCA and glutamate racemase racE and finally obtained only 0.645 ± 0.016 g/L of γ-PGA (Cao et al. 2013). After optimizing the expression of γ-PGA synthase by constitutive and inductive regulation, the content of γ-PGA in the recombinant strain reached 3.7 g/L after batch feeding (Jiang et al. 2006). In conclusion, the yield of heterogeneous production of γ-PGA in Escherichia coli is low.
Researchers explore a new chassis for γ-PGA production. Corynebacterium glutamicum can produce high concentrations of glutamate precursor and save the addition of exogenous glutamate, which has been considered as an ideal host for γ-PGA production. The researchers introduced the γ-PGA synthase into Corynebacterium glutamicum 13,032 and obtained 0.5 g/L of γ-PGA (Cao et al. 2010). Xu et al. optimized and induced the expression of γ-PGA synthase gene in a high-yield glutamate-producing strain of glutamate, C. glutamicum F343; 11.4 g/L of γ-PGA was obtained in the engineered strain. By further introduction of the glutamate racemase gene racE from Bacillus subtilis, the yield of γ-PGA (2000–4000 kDa) reached to 21.3 g/L. This is the highest reported yield for the synthesis of γ-PGA by genetically engineered bacteria so far (Xu et al. 2019a).
γ-PGA is a kind of ultrahigh-molecular-weight biological polymer, which makes it has good plasticity in the degree of polymerization. Like most polysaccharides, polypeptides, and other biological polymers, γ-PGA in different degrees of polymerization tend to present different physical and chemical properties and biological activity. With the continuous expansion of γ-PGA application field, the demand for specific molecular weight biopolymer products has been increasingly urgent. Therefore, it is a valuable research direction to find a way to synthesize biopolymers with a controlled molecular weight of γ-PGA. At present, industrial regulation of the molecular weight of γ-PGA depends on acid-base hydrolysis, poor control, and pollution to the environment. So, how to achieve the efficient biosynthesis of different molecular weights γ-PGA is an urgent problem to be solved. The analysis of the synthesis mechanism of γ-PGA polymerase is still in the preliminary stage. The structure characterization of the complex enzyme has not been successfully obtained, due to inability to grasp its enzymatic properties and catalytic mechanism. Therefore, the regulation mechanism of γ-PGA polymerase on the molecular weight of γ-PGA has not been clearly understood, and the regulation of the molecular weight of γ-PGA by γ-PGA polymerase needs further study in the future.
A specific degrading enzyme gene pgdS/ywtD was also found on the γ-PGA synthase gene cluster responsible for the degradation of γ-PGA, which provides a new idea for regulating the molecular weight of γ-PGA products. At present, the research on γ-PGA-degrading enzymes is mainly focused on the production of γ-PGA bacteria. According to enzyme cleavage mechanism, it can be divided into endonucleated and exonucleated degrading enzymes. Suzuki and Tahara cloned a gene encoding a γ-PGA-degrading enzyme, named ywtD, from strain B. subtilis IFO 16449 (Suzuki and Tahara 2003). The gene located on downstream of the γ-PGA synthase gene cluster, ywtABC, is partially in line with the gene encoding d/l-endonuclease, a glycopeptide-degrading enzyme. Heterologous expression of the gene ywtD in E. coli further confirmed that the enzyme has γ-PGA degradation enzyme activity and only specific hydrolysis of the γ-glutamic acid bond between d-glutamic acid and l-glutamic acid in γ-PGA. The resulting hydrolysates were high-molecular-weight γ-PGA (490 kDa, containing 100% l-glutamic acid) and low-molecular-weight γ-PGA (11 kDa, containing 80% d-glutamic acid and 20% l-glutamic acid). Ashiuchi et al. studied the γ-PGA-degrading enzyme gene pgdS from B. subtilis (Chungkookjang) and found that its sequence was basically the same as that of ywtD gene, which was an endonucleotide degrading enzyme. When different configurations of γ-PGA were used as the substrate for hydrolysis, it was found that the γ-PGA specifically degraded the γ-glutamyl bond between d- and l-glutamic acid. The above results indicated that different B. subtilis sources of γ-PGA degradation enzyme hydrolysis substrate have different configurations (Ashiuchi and Misono 2002a). Yao et al. expressed the ywtD gene of γ-PGA-degrading enzyme derived from B. subtilis NX-2 in E. coli and studied its enzymatic properties. γ-PGA with molecular weight distribution in the range of 20–1000 kDa was obtained through enzymatic hydrolysis experiment (Yao et al. 2009). Kimura et al. identified γ-glutamyl transferase GGT from B. subtilis NAFM5, which has exonuclease hydrolytic activity. At the later stage of fermentation, γ-PGA is hydrolyzed from the amino terminal to generate free d- and l-glutamate monomers, which can be used as nutrient components for the growth of bacteria (Kimura and Itoh 2003). King et al. studied the hydrolysis characteristics of γ-PGA-degrading enzyme from B. licheniformis ATCC 9945A and found that the enzyme was tightly bound to γ-PGA and could be activated by metal ions Zn2+ and Ca2+, belonging to the endonucleated γ-PGA-degrading enzyme (King et al. 2001).
In Bacillus anthracis, the gene capD-encoding γ-PGA-degrading enzyme is not only participates in the hydrolysis of γ-PGA but also is responsible for binding γ-PGA to the peptidoglycan on the cell wall. Unlike other γ-PGA-degrading enzymes, capD is immobilized on the surface of spore for subsequent catalytic reactions. DNA sequence alignment showed that capD has low homology with B. subtilis-degrading enzyme YWTD (<15%) and high homology with γ-glutamyl transferase GGT (Candela and Fouet 2005).
γ-PGA-degrading enzyme activity was also found in some microorganisms that did not synthesize γ-PGA. Tanaka et al. isolated and purified a γ-PGA hydrolase from the fermentation liquid of Myrothecium sp.TM-4222. The enzyme has endonuclease activity to γ-PGA and can hydrolyze the γ-amide bond between L-glutamic acid and L-glutamic acid (Tanaka et al. 1993). In addition, degrading enzymes with γ-PGA exonuclease activity were found in the culture medium of Flavobacterium and Micromonospora melanosporea (Volcani and Margalith 1957; Muro et al. 1990). Although there are some reports about γ-PGA-degrading enzymes at present, there is still a lack of commercial γ-PGA-degrading enzymes with high enzyme activity and good stability, and the production cost is high by separating and purifying the enzyme for in vitro enzymatic hydrolysis of γ-PGA. In the process of γ-PGA fermentation, the establishment of “side formation-side degradation” process through rational regulation of degradation enzymes can realize the substrate economy and efficiently obtain γ-PGA with desirable molecular weights, which lays the foundation for industrial application. Sha et al. screened γ-PGA-degrading enzymes from different bacterial sources in B. amyloliquefaciens NB and found that the γ-PGA-degrading enzyme PgdS divided from B. subtilis NX-2 was the most suitable for the synthesis of low-molecular-weight γ-PGA (LMW-γ-PGA) by the NB strain (Sha et al. 2019b).
For further implementation of γ-PGA synthesis with various molecular weight control, the Xu group optimized heterogenous expression of γ-PGA-degrading enzyme in the pre-built γ-PGA efficient synthesis B. amyloliquefaciens cell factory and revealed the correlation between the expression level of NX-2-derived degradation enzyme PgdS and the molecular weight of γ-PGA. On this basis, the CRISPRi system was further designed to regulate the expression level of PgdS, and the dynamic regulation of PgdS was realized by the strategy of multiple sgRNA combinations. Different molecular weights of γ-PGA were obtained in a strain with high (>800 kDa)-, medium (400–600 kDa)-, and low (50–100 kDa)-molecular-weight, and the yield reached 25–27 g/L (Sha et al. 2020a). In addition, the molecular weight of γ-PGA corresponds to the activity of γ-PGA-degrading enzyme, and the stereochemistry of γ-PGA also affects the subsequent molecular weight regulation. In previous reports, glutamate racemase was confirmed to be involved in the regulation of this process (Halmschlag et al. 2019). In order to achieve the regulation of the stereochemical configuration of γ-PGA, Sha et al. further regulated the stereochemical configuration of γ-PGA by using the racemic glutamate gene racE and γ-PGA synthase gene pgsA by coupling γ-PGA-degrading enzyme, realizing that the current industrial production accuracy is difficult to meet the low molecular weight (<10 kDa) γ-PGA (Sha et al. 2020b).
2 Occurrence and Biosynthetic Mechanism of γ-PGA
In recent years, some progress has been made in studies on the metabolic pathway and key enzymes of γ-PGA. For the mechanism of γ-PGA synthesis, the metabolic pathway and related enzymes of γ-PGA synthesis were also different in different strains. Ogawa et al. studied the relationship between glutamate supply of γ-PGA and its precursors, indicating that glutamate required by microorganisms for production of γ-PGA came from their own metabolism or the external environment (Ogawa et al. 1997). At present, it is generally believed that glutamate-independent strains provide l-glutamate monomer through their own metabolic pathways. However, for glutamate-dependent strains, there are different ways to obtain l-glutamate monomer supply; γ-PGA monomer is not all sourced from the conversion of exogenous glutamate.
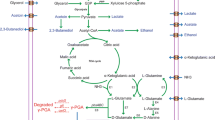
The supply of exogenous glutamate also has different effects. Briefly, the biosynthesis pathways of γ-PGA can be roughly defined as follows. One is the synthesis of glutamic acid monomers, including glycolysis, tricarboxylic acid cycle, and glutamic acid synthesis pathways. The second is the polymerization pathway of glutamic acid monomer to generate γ-PGA (Fig. 1). Wu et al. analyzed the pathway of γ-PGA glutamate-dependent synthesis by 13C isotope tracer method in a γ-PGA strain B. subtilis NX-2, and the results showed that glucose synthesized glutamate precursors through glycolysis, pentose phosphate pathway, tricarboxylic acid cycle, and other pathways, and then the synthesized glutamate precursors were polymerized to γ-PGA. When glucose concentration was 40 g/L in the medium, about 9% of the γ-PGA carbon skeleton was entered by glucose. These results indicated that glucose was mainly used as a growth-limiting substrate for the growth and energy metabolism, which was only a small part of the carbon skeleton of γ-PGA molecules. However, l-glutamic acid was the main source of the carbon skeleton of γ-PGA molecules (Wu et al. 2008).
2.1 Synthesis of γ-PGA Precursors
2.1.1 Synthesis of l-Glutamate
It is well-known that glutamic acid is the precursor of γ-PGA synthesis. l-glutamic acid is mainly synthesized by anabolism and catabolism in Bacillus subtilis. Usually, one molecule of l-glutamine and one molecule of α-ketoglutarate generate two molecules of l-glutamic acid in the coupled reaction system of l-glutamine synthase and l-glutamic synthase (Belitsky et al. 2000). In the presence of glutamine, l-glutamate is formed from α-ketoglutarate through the action of glutamate dehydrogenase. In addition, l-aspartic acid transaminase can also convert l-aspartic acid and α-ketoglutarate to oxaloacetic acid and l-glutamic acid. l-arginine and l-proline can form l-glutamic acid through the catabolic pathway (Belitsky and Sonenshein 1998). Since γ-PGA-producing bacteria can be classified as glutamate-dependent and non-glutamate-dependent, the study of intracellular l-glutamate synthesis is of great significance for the understanding of the synthesis mechanism in γ-PGA-producing strains.
2.1.2 Synthesis of d-Glutamate
For the synthesis of d-glutamate, there are two main confirmed pathways, involving d-aminotransaminase and glutamate racemase. The activity of d-aminotransaminase was higher in γ-D-PGA or γ-DL-PGA-producing bacteria with higher proportion of d-type monomers. The researchers initially detected d-aminotransaminase activity in B. anthracis and B. licheniformis ATCC 9945A (Ashiuchi and Misono 2002b). Although it is generally believed that d-glutamate is catalyzed by d-aminotransferase, the activity of this enzyme was not detected in the cell lysate of B. subtilis IFO3336, and only the highly active glutamate racemase was detected (Ashiuchi et al. 1998). d-aminotransaminase and glutamate racemase activity were studied in B. subtilis subsp. chungkookjang, and the results show that the d-aminotransaminase and glutamate racemase coexist in logarithmic phase. During the stable phase, the activity of d-aminotransaminase decreased sharply, while the activity of glutamate racemase increased (Ashiuchi et al. 2001a). The results indicated that d-aminotransaminase in B. subtilis subsp. chungkookjang probably did not participate in the supply of d-glutamic acid in γ-PGA production and d-glutamic acid was all catalyzed by glutamate racemase.
In general, the activity of glutamate racemase in most bacteria is very low, such as glutamate racemase Mur I in E. coli. If the expression of this enzyme is increased, it will lead to mitotic aberration and cell proliferation inhibition (Balikό and Venetianer 1993). Obviously, the glutamate racemic enzyme in B. subtilis has different properties. This enzyme has high catalytic efficiency and low affinity for d-glutamate, which is very important for maintaining high concentration of d-glutamate when l-glutamate is accumulated in large amounts in the cell. After purifying the glutamate racemase protein (RacE) (Ashiuchi et al. 1998), Ashiuchi et al. isolated and purified the RacE isoenzyme YrpC (Ashiuchi et al. 1999b). YrpC is similar to RacE in its primary structure, substrate specificity, cofactor independence, and other enzymatic characteristics, but its kinetic parameters are completely different. YrpC is similar to the glutamate racemic enzyme Mur I in E. coli, where overexpression of this enzyme also inhibits bacterial growth. Unlike RacE, YrpC has low catalytic efficiency and high affinity for l-glutamate, which is an advantage for providing the limited d-glutamate necessary for cell growth at low intracellular concentrations of l-glutamate. When knockout of gene racE, the growth of the cells does not need to add additional d-glutamic acid. Furthermore, for strains with double knockout of racE and yrpC, it was found exogenous d-glutamic acid must be added to maintain the normal growth of cells. The relationship between yrpC and racE is complementary, which also indicates that in B. subtilis, glutamate racemase provides d-glutamate for the synthesis of γ-PGA (Kimura et al. 2004b).
2.2 Polymerization of γ-PGA Precursors
The γ-PGA synthase gene has been of great interest to the γ-PGA research groups for many years. With the research and development, many strains of γ-PGA synthase operons have been cloned and verified. Ashiuchi and team (Belitsky et al. 2000; Candela and Fouet 2006) cloned and verified γ-PGA synthase genes for the first time, including pgsB, pgsC, and pgsA genes from B. subtilis natto chromosome genome (Ashiuchi et al. 2001a; Ashiuchi et al. 2001b). The pgsBCA knockout strain could not synthesize γ-PGA, but all other traits were the same as those of the wild strain. The results showed that pgsBCA was necessary for the synthesis of γ-PGA. Using newly synthesized protein kinetics data from an in vitro transcription and translation system, the researchers summarized the functions of individual proteins of γ-PGA synthase. The results showed that the binding between pgsB and pgsC was very tight, while the binding between pgsBC and pgsA was relatively loose, and there was little difference in the affinity between pgsBC and pgsBCA for glutamate. However, due to the instability of γ-PGA synthase, the researchers were unable to isolate and purify the enzyme, although the function of each protein in the γ-PGA polymerase system can be elucidated through some experimental data: pgsB is considered to be the main catalytic protein in the γ-PGA polymerase system. The structural characteristics of an amide ligase were found in this enzyme, and the previously identified ligases were all cytoplasmic enzymes. This is the first example of a membrane ligase. In addition, it was found that pgsB can catalyze the hydrolysis of ATP in the presence of glutamic acid monomer, providing energy for the extension of γ-PGA peptide chain (Kimura et al. 2009; Tomosho et al. 2008).
The N-acetyltransferase domain similar to that of N-acetylglutamate synthase was found in pgsC. Interestingly, three series similar N-acetyltransferase domains have been found in the C-terminal of ε-PL synthase, and this domain is important for the enzyme-catalyzed reaction (Yamanaka et al. 2008). At present, pgsC has been found only in the strains that can synthesize γ-PGA, indicating that pgsC plays an important role in γ-PGA synthesis (Vetting et al. 2005; Min et al. 2009).
There are membrane-anchoring regions in pgsA that are responsible for positioning the pgsBCA complex on the membrane (Ashiuchi and Misono 2002b). Homologous sequences of this protein are found in a variety of biological genes, belonging to the cytosolute protein serine/threonine phosphatase with divalent cation binding sites, including Zn2+, Mn2+, Fe2+, and Ca2+. PgsA may be responsible for the extracellular transport of γ-PGA (Nordlund and Eklund 1995; Rusnak and Mertz 2000). In addition, PgsA had high homology with the isomerase MslH, and the strain could synthesize γ-PGA with high l-glutamate ratio after knockout of pgsA gene in B. subtilis. Therefore, it is speculated that PgsA may affect the configuration of glutamic acid monomer during γ-PGA synthesis (Ogasawara et al. 2019; Sawada et al. 2018).
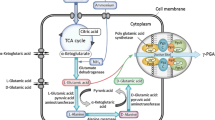
PgsE exists downstream of the pgsBCA gene, but its function remains unclear. The researchers hypothesized that this protein is functionally similar to the CapE protein in B. anthracis. Recent studies by Ashiuchi and Yamashiro et al. have shown that this protein plays an important role in some plasmid-containing γ-PGA-producing bacteria (Ashiuchi and Yamashiro 2009). Recent studies have reported that the expression of PgsE can promote the increase of the molecular weight and yield of γ-PGA. PgsE was similar to the N-terminus of the key protein responsible for the assembly of ubiquitin synthetase. It is speculated that PgsE may stabilize the assembly of the PgsBCA complex, thus promoting the increase of the molecular weight and yield of γ-PGA. In the future, the biological functions and related catalytic mechanisms of PgsE remain to be further explored (Fujita et al. 2021). The catalysis of γ-PGA synthesis by these membrane-bound proteins has not been fully understood. Candela et al. concluded that γ-PGA synthase must perform two functions: polymerization of γ-PGA and transport of γ-PGA. According to the function of each protein, a γ-PGA synthesis model based on PgsB, PgsC, PgsA, and PgsE polymerase systems was also established (Candela and Fouet 2006) (Fig. 2).
Schematic representation of γ-PGA biosynthesis by membrane-bound proteins. An illustration depicts the gamma-PGA biosynthesis. Substrate glutamate isomerized under the action of glutamate racemase (RacE). D-glutamate and L-glutamate plus ATP in the presence of gamma-PGA synthase with the help of PGSA, PGSB, PGSC, PGSE forms a biopolymer gamma-PGA which is secreted into cell culture medium. In this process, there are still gamma-PGA degrading enzyme that can degrade gamma-PGA
3 Fermentation Engineering for γ-PGA Production
3.1 Fermentation Medium for Producing γ-PGA
3.1.1 The Effect of Carbon Source on γ-PGA Production
As one of the nutrients necessary for the growth of microorganisms, carbon source plays an important role in the growth and development of microorganisms. It is a carbon nutrient used by microorganisms to build the carbon skeleton of bacteria and metabolites and to provide energy for life. In the process of microbial fermentation to produce γ-PGA, it commonly used glycerol, fructose, lactose, galactose, glucose, sucrose, maltose, and starch as the carbon sources. Different strains require different carbon sources, and even those using the same carbon source, the amount of the carbon source would be different. Ju et al. found that starch is the best carbon source for the fermentation of B. subtilis MJ80 to produce γ-PGA (Ju et al. 2014). Shi et al. used the response surface method to study the fermentation process of γ-PGA by B. subtilis ZJU-7 and found that using sucrose as a carbon source can maximize the yield of γ-PGA as high as 58.2 g/L (Shi et al. 2006). Jung et al. used B. subtilis RKY3 to produce γ-PGA; glycerol was the best carbon source (Jung et al. 2005). Jiang et al. found that when glucose is used as the carbon source for the fermentation of B. subtilis NX-2 to produce γ-PGA, the maximum yield of γ-PGA can reach 70.9 g/L (Jiang et al. 2016).
3.1.2 The Effect of Nitrogen Source on γ-PGA Production
As an indispensable component in the growth and reproduction process of microorganisms, nitrogen sources can provide nitrogen nutrition for cell life activities. Generally, the available nitrogen sources of microorganisms are divided into organic nitrogen sources and inorganic nitrogen sources. Organic nitrogen sources are beef extract, casein peptone, malt extract, yeast extract, etc., while inorganic nitrogen sources include urea, NH4Cl, NH4NO3, (NH4)2SO4, etc. Different microbial strains have different utilization abilities to these two types of nitrogen sources. Ju et al. found that B. subtilis MJ80 has a strong ability to utilize inorganic nitrogen source urea and can use urea as the best nitrogen source (Ju et al. 2014). Shi et al. used tryptone and cheap corn flour as nitrogen source to produce γ-PGA by B. subtilis ZJU-7 (Shi et al. 2006).
3.1.3 The Effect of Metal Ions on the Yield of γ-PGA
In the process of γ-PGA production by microbial fermentation, K+, Mg2+, Na+, Mn2+, and other metal ions have the effect on γ-PGA yield. Ju et al. found that K2HPO4 and MnSO4 added to the fermentation of Bacillus RKY3 influenced the yield of γ-PGA produced. K2HPO4 can promote the growth of bacterial cells, while Mn2+ can promote the synthesis of γ-PGA. Ashiuchi et al. found that adding 0.5% NaCl to the fermentation system of strain B. subtilis (chungkookjang) can promote the production of γ-PGA per unit volume to reach 13.5 g/L (Ashiuchi et al. 2001a).
3.2 Fermentation Factors for Producing γ-PGA
Temperature, pH, dissolved oxygen, stirring method, and other factors of the fermentation system can all affect the final yield of γ-PGA. Here we mainly discuss dissolved oxygen. The production of γ-PGA by microbial fermentation is generally carried out under aerobic conditions. Oxygen is one of the necessary conditions for the production of γ-PGA. Generally, oxygen can affect the biosynthesis and energy metabolism of microbial cells during fermentation, and the regulation of dissolved oxygen directly affects the yield of γ-PGA.
There are many studies on optimizing dissolved oxygen. Su et al. inserted the vgb (Vitreoscilla hemoglobin gene) into the genome of the γ-PGA-producing strain B. subtilis S18–3. It can increase the ability of B. subtilis S18–3 to carry more oxygen to achieve the purpose of increasing the dissolved oxygen in the fermentation system (Su et al. 2010). Qun et al. adopted batch fermentation method and changed the stirring speed in stages; within 24 h in the fermentation process, the fermenter speed was adjusted to 600 rpm and dropped to 400 rpm after 24 h. Compared with the previous experimental method that did not change the speed (maintained at 400 rpm), the method significantly increased the oxygen content in the fermentation system during the first 24 hours of cell growth (Wu et al. 2006a). Xu et al. developed a method to increase the oxygen mass transfer coefficient by adding oxygen carriers to the fermentation system, which increased the yield of γ-PGA to 39.4 ± 0.19 g/L (Zhang et al. 2012b). Our team designed and used an aerobic plant cellulose bed experiment to increase the amount of dissolved oxygen in the fermentation system and ultimately increase the yield of γ-PGA (Feng et al. 2016).
3.3 Fermentation Method for Producing γ-PGA
At present, the production of γ-PGA by microbial fermentation mainly includes liquid fermentation and solid fermentation. Among them, the most widely used fermentation method is submerged fermentation. Bajaj et al. reported that B. licheniformis ATCC 9945 contained grains (Bajaj and Singhal 2011). The yield of γ-PGA could reach up to 23 g/L in 48 h in the medium that contains acetic acid, glycerol and citric acid. The yield of γ-PGA increased to 35 g/L when glutamic acid was added in the medium (Bajaj and Singhal 2011). When glutamic acid and citric acid were used as the fermentation substrates for B. subtilis IFO 3335 growth, the yield of γ-PGA was only 20 g/L (Bajaj and Singhal 2011).
B. licheniformis CCRC12826, B. licheniformis WBL-3, B. subtilis R23, B. subtilis NX-2, and B. subtilis TAM-4 are also the primary strains used for submerged fermentation production of γ-PGA (Table 1). However, the production of γ-PGA by submerged fermentation is costly and time-consuming. As γ-PGA is a highly viscous, in the later stage of submerged fermentation, the fermentation broth usually becomes extremely viscous, resulting in insufficient dissolved oxygen, which in turn affects the growth and metabolism of the bacteria and ultimately affects the yield of γ-PGA. Therefore, in order to solve the above problems, some researchers have utilized solid-state fermentation method to produce γ-PGA due to its couple of advantages, such as low energy consumption and low pollution. In addition, solid-state fermentation can also use solid waste produced in industry and agriculture as the fermentation substrate. This fermentation method can not only increase the yield of γ-PGA but can also be used for solid waste utilization hence promoting environmental protection (Liu et al. 2013). At present, the strains B. subtilis CCTCC 202048, B. subtilis B6–1, B. subtilis ME714, B. licheniformis NCIM 2324, B. subtilis natto, B. subtilis NX-2, B. subtilis 3–10, and B. licheniformis C1 that can be used to produce γ-PGA by solid-state fermentation have been reported (Table 1) (Junnan et al. 2018).
4 Separation and Purification of γ-PGA
For high-end fields, such as food, cosmetics, and medical materials, high-grade γ-PGA products are often needed to meet the requirements of industry standards. To obtain such high-quality product, separation and purification of γ-PGA from fermentation broth are required. However, most of the studies on the fermentation production of γ-PGA have focused on the screening of strains, the optimization of medium and culture conditions, and the improvement of γ-PGA yields. The extraction and purification of γ-PGA from fermentation broth research is very few.
For most fermentation products, removal of the bacteria from the culture media is the first step for subsequent purification. In general, filtration and centrifugation are the common methods. However, it is not trivial to separate the cells from the fermentation broth containing γ-PGA product. γ-PGA is usually firmly attached to the surfaces of γ-PGA-producing strains as an extracellular capsule in the early stages of growth (Ogunleye et al. 2015). These γ-PGA-coated cells show electronegativity due to the ionization effect under neutral pH conditions, making the cells stable due to the electrostatic repulsion, which in turn makes it difficult to directly achieve the solid-liquid separation through filtration or centrifugation without pre-treatment (Do et al. 2001). Because γ-PGA is a polymer with a relatively high molecular weight, the fermentation broth tends to be very viscous, which slows down the sedimentation rate of centrifugation and therefore requires higher centrifugal force for bacterial separation (Shih and Van 2001).
Do et al. (2001) found that acidification of fermentation broth before centrifugation could significantly improve the process efficiency and energy consumption. By lowering the pH of the fermentation broth to 3, the viscosity and the ξ potential value of the cells of the fermentation broth with 2.5% γ-PGA at 35 °C were reduced to 1/6 and 1/3 of the original value, respectively. After acidification, the bacteria can be removed by centrifugation under 5000 × g centrifugal force for 30 mins, while 22,000 × g for 60 min was required if acidification is not performed. Due to the simple process and low cost, acidification has also become the main step to obtain cell-free clear liquid from γ-PGA fermentation broth in industry. The commonly used acidification reagents include inorganic acids, including trichloroacetic acid (TCA), HCl, and H2SO4. The acidification degree is generally adjusted to about 2–3 pH of fermentation broth.
The next step after obtaining the cell-free broth is to extract the γ-PGA from the solution, which there are three main strategies that are alcohol sedimentation, metal ion-induced sedimentation, and quaternary ammonium salt sedimentation.
Recovery of γ-PGA from cell-free broth by alcohol precipitation is the most commonly used technology (Goto and Kunioka 1992). γ-PGA is heteropeptide in structure, whose molecular status in aqueous solution is similar to that of protein, DNA, polysaccharide, and other water-soluble polymers. Adding intermediate polar organic solvents, such as ethanol, to γ-PGA solution can reduce the activity of water molecules, reduce the dielectric constant of the solution, and repel water molecules from the γ-PGA around. Through the interaction of polar groups, γ-PGA molecules coagulated and precipitated under the action of van der Waals force. The solvents used for γ-PGA precipitation recovery have been reported as ethanol, methanol, and propanol, among which ethanol is the most widely used due to its cheapness, easy availability, and high safety (Do et al. 2001; Goto and Kunioka 1992; Birrer et al. 1994; Kubota et al. 1993). For cell-free broth with 1–2% γ-PGA content, ethanol with a final concentration of 75–80% is usually used. The higher the concentration of γ-PGA, the lesser the amount of ethanol can be used. Therefore, the cost of ethanol can be reduced by concentrating the γ-PGA broth. However, using alcohol as a precipitator has several disadvantages. Firstly, alcohol will lead to the coprecipitation of proteins and nucleic acids in the broth, which would reduce the purity of the product. Secondly, alcohol precipitation method is not enough to recover γ-PGA completely, usually resulting in the loss of 15%. Finally, the use of large quantities of organic solvents may face environmental regulatory issues (Manocha and Margaritis 2010). Therefore, despite the easy operation of alcohol precipitation, many researchers are still searching for more efficient and low-cost γ-PGA recovery processes.
Manocha and Margaritis (2010) proposed a method to recover γ-PGA from cell-free fermentation broth based on metal-ion affinity. Studies have found that γ-PGA side chain is rich of carboxyl groups that can chelate with many metal cations, such as Cu2+, Mn2+, Al3+, Cr3+, Fe3+, etc. When γ-PGA dissolves, its long chain is extended due to the repulsion force that form the negatively charged carboxyl groups. When large metal cations enter the solution, the negative carboxyl groups become neutral due to the chelation, and the long-chain molecules lose the electrostatic repulsion, form flocculates with the bound metal ions, and then settle down. This is also the main mechanism of γ-PGA as a flocculant in the field of water treatment (Salehizadeh and Shojaosadati 2001). Manocha and Margaritis (Manocha and Margaritis 2010) make use of this principle. The cell-free fermentation broth was treated with divalent copper (CuSO4), resulting in γ-PGA precipitation, which was collected as precipitation by centrifugation. The precipitate was then dissolved and dialyzed with deionized water to obtain pure γ-PGA. The study compared the recovery efficiency and selectivity of γ-PGA with ethanol precipitation method. It was found that 85% of the γ-PGA in the broth was recovered by the CuSO4 method and 82% by the ethanol method. In addition, ethanol method detected 48% of the protein in the broth, while CuSO4 method detected only 3% of the protein in the final purified PGA, indicating that later method had better selectivity than former method. Interestingly, the CuSO4 method did not result in the residual Cu in the purified γ-PGA. Therefore, copper-induced precipitation of γ-PGA offers an effective recovery technique that can selectively precipitate γ-PGA from the fermentation broth without the use of expensive organic solvents.
Liu et al. (2018) also proposed a scheme for γ-PGA recovery using Gemini quaternary ammonium salt-induced precipitation. This strategy is similar in principle to the metal ion-induced γ-PGA deposition, which uses quaternary ammonium cation to bind the carboxylic acid group of γ-PGA forming water-insoluble complexes. A Gemini quaternary ammonium salt called DOBP 10 (1,4-bis-[3,3′-(1-decylpyridinium) methyloxy] butane dibromide) was used in the study. 10% DOBP 10 was added to the fourfold diluted cell-free fermentation broth to produce complex precipitation. The precipitate was dissolved in ethanol again, and then 30% NaCl was added to the solution to separate DOBP 10 and precipitate γ-PGA. The recovery rate of γ-PGA was more than 90%. However, compared with alcohol and metal deposition, this process is more complicated.
5 Applications of γ-Polyglutamic Acid
5.1 Agricultural Planting
In recent years, γ-PGA, as a plant biostimulant, has shown great success in the field of agriculture, especially in China. In 1994, Donlar Corporation reported for the first time that γ-PGA could improve the absorption of nitrogen, phosphorus, and potassium by plants and promoted crop growth (Kinnersley et al. 1994). In 2000, Hara (2000) coated the modified γ-PGA on the surface of seeds and spread it on arid sandy land for germination experiments. It was found that seeds could germinate and grow normally, while the control could not. Therefore, γ-PGA is believed to have a good application prospect in barren mountains and desert reconstruction. Wang et al. (Wang et al. 2008) found that γ-PGA had effects on biological control and fertilizer efficiency enhancement. Xu et al. (2014) reported that γ-PGA can improve the nitrogen use efficiency of wheat, rape, and other crops and promote crop yield increase and believed that γ-PGA can regulate the key enzyme activity of plant nitrogen metabolism through Ca2+/CaM signaling pathway, thus improving the absorption and assimilation of nitrogen and thus promoting plant growth. Lei et al. (2015) studied the effects of γ-PGA on the growth, physiological, and biochemical characteristics of rapeseed seedlings under low temperature (4 °C). The results showed that the fresh weight and chlorophyll content of rapeseed seedlings induced by γ-PGA increased by 24.5% and 50.9%, respectively, after 144 h of stress. Similarly, under NaCl stress, γ-PGA significantly increased K+/Na+ value, proline content, and antioxidant enzyme activity of rape seedlings (Lei et al. 2016). The content of malondialdehyde was significantly reduced, indicating that it enhanced the tolerance of rape seedlings to salt stress. Guo et al. (2017) also found the same effect on wheat.
Due to the polycarboxylic acid properties, γ-PGA also has a potential role in mitigating plant heavy metal toxicity. Pang et al. (2018) found that under Pb and Cd stresses, γ-PGA could effectively alleviate the growth inhibition of cucumber seedlings by heavy metals, and with the increase of γ-PGA concentration, the contents of chlorophyll a and b also increased. After γ-PGA treatment, the contents of Pb and Cd in cucumber seedlings decreased by 74.13% and 38.65%, respectively. What is more interesting is that γ-PGA simultaneously mitigates the adverse effects of Cd and Pb on soil microbial community.
Lei and Xu et al. systematically studied the mechanism of γ-PGA in assisting abiotic stress in plant environment (Lei et al. 2017; Xu et al. 2016; Xu et al. 2017a). Fluorescent labeling showed that γ-PGA did not enter the cytoplasm but instead attached to the surface of root protoplasm. Here, it triggered a burst of H2O2 in roots by enhancing the transcription of RbohD and RbohF, and the elicited H2O2 further activated an influx of Ca2+ into root cells. Ca2+ signaling was transmitted via the stem from roots to leaves, where it elicited a fresh burst of H2O2 by Ca2+-binding proteins CBL9, CPK4, and CPK5. The H2O2 signal promoted brassinolide and jasmonic acid biosynthesis by upregulating key genes (dwf4 and lox2, respectively) for synthesizing these compounds. Lastly, brassinolide and jasmonic acid increased H2O2 which promoted proline accumulation and total antioxidant capacity (T-AOC) improvement, resulting in improving the resilience of plants. Unfortunately, some questions remain unanswered. Is there a γ-PGA-specific binding protein on the cell membrane? If so, how does the receptor protein bind to γ-PGA and mediate the production of these signals? These problems will be the last barrier to solve the relationship between γ-PGA and plant stress resistance in the future.
5.2 Food
γ-PGA, also known as natto bacteria gum, was first found in “natto” – fermented beans. Thus, it is food-borne substances with food safety. It has been widely used in food additives, antifreeze agents, dietary supplements, etc.
5.2.1 Food Additives
Food quality is often determined by flavor and shelf life. γ-PGA of high molecular weight can effectively embed the functional components of food, which reduce the damage of harmful factors in food processing and transportation (e.g., temperature, bacteria, etc.). Besides, it has a promoting effect on the stability and improvement of food quality. Previous studies have shown that the addition of γ-PGA to one or more bitter substances (e.g., amino acids, peptides, quinine, caffeine, minerals, etc.) can significantly reduce bitterness (Katsuragi et al. 1998).
γ-PGA can be added in the production of starch foods (mainly baked goods and noodles) to improve quality and extend shelf life. The addition of γ-PGA in the production of wheat bread can improve the water retention ability of bread and significantly improve the rheological properties and thermal properties of wheat dough. After adding γ-PGA, the gelatinization temperature of flour increased to further expand the dough. In the storage process, the addition of PGA makes the wheat bread soft, that is, the bread hardness is reduced, thus effectively delaying the bread aging (Shyu et al. 2008).
γ-PGA can also be used to modify emulsified food and improve its stability. Shyu and Sung (2010) studied the effects of different concentrations of γ-PGA (0.05, 0.1, and 0.5 g/kg w/w) on the viscosity, foam stability, and emulsification properties of sponge cake paste. The viscosity, foam stability, and emulsion stability of the cake were significantly improved when 0.5 g/kg γ-PGA was added. The cross-linking of triglyceride with the polyhydroxyl groups of γ-PGA enhances the stability of the emulsion. During the storage process, γ-PGA reduces the hardness and mastic ability of sponge cake, which maintains the cohesion of sponge cake, and has the effect of preventing cake spoilage.
Further γ-PGA function is its utility as a healthy functional oil reducer in fried food. Lim et al. (Lim et al. 2012) studied the effects of γ-PGA on the oil absorption and water loss of donuts during frying. The results showed that γ-PGA improved the water retention ability of donuts. After frying for 4 min, the oil absorption of the control donut was about 0.7 g/g dough, while the oil absorption of the PGA treated donut was about 0.2 g/g dough. When the PGA concentration was increased from 0.25 g/100 g of dough to 1 g/100 g of dough, the amount of oil absorbed by the doughnut was reduced by a factor of five.
5.2.2 Antifreeze Agents
In addition to being used as a food additive, γ-PGA of low molecular weight is an excellent antifreeze agent. It can effectively inhibit the growth of ice crystals and reduce the amount of water that can be frozen in food, which avoid the destruction of food structure by ice crystals (Jia et al. 2019). The antifreeze activity of γ-PGA in the molecular weight range of less than 20,000 Da is higher than that of glucose, which is called a highly antifreeze substance. Its antifreeze activity tends to decrease with the increase of molecular weight (Mitsuiki et al. 1998). In addition, compared with the commonly used small molecule antifreeze agents, such as glucose and inorganic salts, γ-PGA of low molecular weight has less impact on food quality due to its d-glutamic acid component which has a lighter taste (Park et al. 2005).
The survival ability of probiotics can be also improved by adding γ-PGA in the process of freeze-drying. Bhat et al. (2013) found that the protective effect of 10% γ-PGA on Lactobacillus casei was significantly better than that of 10% sucrose in the freeze-drying process. Compared with trehalose, sorbitol, and NATA (bacterial cellulose produced by Acetobacter xylostae), γ-PGA can provide better protection for lactic acid bacteria during freeze-drying.
5.2.3 Food Nutritions
γ-PGA is also a good dietary fiber, which is usually processed into health products. It can increase gastrointestinal peristalsis, remove the body garbage, as well as maintain the human digestive. Additionally, its final decomposition product is free glutamic acid, which is beneficial to human health (Wang et al. 2016). As γ-PGA contains γ-amide bond, it can reduce the sensitivity of the gastrointestinal enzyme and is often used as a nutritional aid. It can promote the absorption of calcium ions in the human body by increasing calcium solubility and intestinal absorption; thus it can be used for treatment towards osteoporosis. The increase in calcium solubility is attributed to the large number of anions in γ-PGA which inhibit the production of insoluble calcium phosphate. In addition, γ-PGA also promotes intestinal calcium absorption by increasing passive calcium transport in the small intestine (Tanimoto et al. 2001). Tanimoto et al. (2007) found that postmenopausal women who took a single dose of γ-PGA had a better intestinal calcium absorption and individuals with a lower basal absorption capacity than average benefited more from γ-PGA consumption. In recent years, some researchers have added γ-PGA to the animal feed to promote the absorption and utilization of minerals in poultry and improve the quality of meat.
5.3 Daily Chemical Products
γ-PGA is widely used for cosmetics because of its super water absorption, moisture retention, and cell affinity. Since the application process of cosmetics involves the cellular osmotic absorption of molecules, its application effect is closely related to molecular weight. Generally, γ-PGA of low molecular weight can be absorbed by the skin and achieve deep moisturizing effect, and, on the other hand, γ-PGA of high molecular weight can form a membrane structure on the skin surface conducive to protecting skin moisture (Xu et al. 2017b).
γ-PGA, also named natto gum (nattogum) in the cosmetics pharmacopoeia international name, is often added to facial masks, morning and night cream, and other skincare products as highly effective moisturizing ingredients. It plays a significant role in maintaining epidermal moisture and reducing the corneous layer of water loss. When applied in shampooing and hair care products, γ-PGA can lock the moisture of hair surface and hair scales, maintaining hair quality, nourishing scalp, as well as reducing dandruff generation and hair boring, etc.
When used in skincare ointment, γ-PGA can reduce skin allergy, roughness, and skin damage caused by lack of water, restore cell function, and improve skin immunity and metabolism (Wang et al. 2016).
5.3.1 Antioxidant
γ-PGA of low molecular weight often has excellent antioxidant activity. Wang et al. (2020) examined γ-PGA having different molecular weights for removing ultra-oxygen anion free radical (O2·-), hydroxyl radical (·OH), and 1,1-diphenyl-2-trinitrobenzene hydrazine (DPPH). The results showed that all the γ-PGA indicated a certain degree of radical scavenging activity with the clearance of O2·− ·OH, and DPPH reached 31.36%, 97.55%, and 74.64% respectively. In addition, by building a UV-induced mice skin fibroblasts (L929) oxidative damage light aging model, it was observed that γ-PGA with different molecular weights could repair the damage of photoaging cells to a certain extent and reduce the increase of oxygen free radical (ROS) and nitrogen free radical (NO) in the photoaging cells with the γ-PGA having a molecular weight of 3.0 × 105 Da had the best effect.
γ-PGA can also be used as an active component of hyaluronidase inhibitors to maintain skin elasticity and improve skin allergy by inhibiting the permeability of inflammatory cells (Sung et al. 2014). γ-PGA maintains skin moisture and elasticity by inhibiting the activity of hyaluronidase, an enzyme that degrades hyaluronic acid in the dermis. γ-PGA have also been widely used in the production of wet wipes, baby diapers, and other sanitary products (Zhang 2020).
5.3.2 Skin Protection
γ-PGA of high molecular weight has outstanding moisture retention and water absorption, forming a protective film on the skin. It can inhibit the loss of skin moisture and penetrate the deep skin, restoring the skin self-moisturizing system (Liu et al. 2015). Based on γ-PGA as the precursor material of the hydrogel, Wang et al. (2019) constructed a new double-network hydrogel film material (“the second skin “) similar to epidermal skin tissue through covalent cross-linking and molecular self-assembly technology which combined with the polyphenolic structural molecule tannic acid (TA). The hydrogel demonstrated broad-spectrum UV resistance in the range of 275–360 nm, excellent bioadhesion and water resistance (under sweating and dynamic physiological conditions), without invading skin tissues. In addition, when the surface structure of the hydrogel is damaged, it can realize perfect self-repair function within 1 min. On the one hand, the excellent performance is due to the γ-PGA containing many carboxylic acid groups in molecular structure, enhancing the skin cell affinity adaptation ability. On the other hand, the double network formed by the TA molecular assembly with the hydrogel membrane on the skin surface to construct a stable 3D network structure interact with the skin surface to form multiple, strong integration and intelligent self-healing effect.
5.4 Tissue Engineering, Regenerative Medicine, and Drug Delivery
γ-PGA, as a natural polyamino acid produced by microbial fermentation, has great water solubility, biocompatibility, and degradability. Besides, γ-PGA contains many active carboxyl groups in the molecular structure, which are conducive to functional modification. Therefore, γ-PGA has been widely used in tissue engineering scaffolds, regenerative medicine, and drug carriers.
5.4.1 Tissue Engineering and Regenerative Medicine Materials
Because of the excellent water solubility, γ-PGA has been used to develop hydrogel materials in recent years. Studies have shown that wound of human tissue under wet conditions has a faster repair rate and the reduced rate of scar relative to the dry wound. Therefore, the bionic materials prepared with γ-PGA mainly as the wound dressings and hemostatic materials, which are similar with human tissue (such as skin, cartilage, etc.) in their structure and functions, have shown rapid development. Based on γ-PGA as the primary material, Xu et al. constructed a series of wound repair materials by means of enzymatic in situ catalysis (Chen et al. 2017) and chemical cross-linking (Xu et al. 2019b) via molecular modification. Among them, γ-PGA-based injectable hydrogel material had shown excellent function of promoting wound repair and regeneration, wound healing, and hemostasis with cell compatibility. Furthermore, γ-PGA nanofiber materials based on microfluidic spinning technology could effectively promote wound repair and reduce scar generation. Sun et al. (2020) modified the double bond of the γ-PGA and composited with antibacterial polylysine through photopolymerization using visible light to develop the antibacterial and biocompatible hydrogel, achieving the repair and regeneration of skin infections. Hsieh et al. (2005) prepared a γ-PGA/chitosan composite biomaterial and demonstrated that the composite had better hydrophilicity, biocompatibility, and mechanical properties as compared to the conventional chitosan matrix, which was a promising tissue engineering biomaterial. γ-PGA/chitosan composite biomaterial could also be used as wound dressing. Tsao et al. (2011) designed a chitosan/γ-PGA polyelectrolyte complex (PEC) as an easily peeled material to treat wounds. The results showed that chitosan/γ-PGA PECs contain a suitable moisture content and exhibited good mechanical properties, both of which were beneficial for the wound dressing as it can be easily detached from the wound surface without damaging the newly regenerated tissue.
5.4.2 Drug Carrier
γ-PGA is also an ideal drug carrier material, mainly because of the large amount of highly active carboxyl groups in its molecular structure which is suitable for functional modification. Most anticancer drugs (e.g., paclitaxel (TXL), camptothecin (CPT), etc.) are water-insoluble in clinical practice, which greatly limits the clinical application. Moreover, the increase of drug dose not only increases the cost but also increases the drug toxicity. The efficient utilization of controlled/sustained release and targeted delivery of drugs by using water-soluble γ-PGA molecules carrying hydrophobic drugs have attracted extensive attention of drug delivery researchers. Li et al. (1998) developed a water-soluble complex by covalent bonding of paclitaxel drug to γ-PGA (PG-TXL), which demonstrated a more significant antitumor efficiency than TXL. Clinical experiments showed that the uptake of PG-TXL by tumor cells was about five times higher than that of regular TXL at the same dose. The side effects were reduced, with the antitumor activity and the maximum tolerated dose (MTD) were significantly improved in animal models. Besides, PG-TXL has unique tumor-targeting capabilities. The polymer was targeted into the cell and able to decompose to deliver high doses of paclitaxel to the tumor, increasing the antitumor efficiency. Bhatt et al. (2002) formed γ-PGA complex with camptothecin drug (CPT-PGA) by combining the carboxyl group of the γ-PGA with the functional group (20S-hydroxyl) of camptothecin. This way the water solubility and therapeutic effect of the drug can be significantly improved.
γ-PGA can also be used in combination with other anticancer drugs to reduce toxicity. Cisplatin anticancer drugs (CDDP) have been widely used to treat a variety of cancers. However, the inhibition of bone marrow growth, severe nephrotoxicity, and other defects limit the clinical application of CDDP. Zhang et al. (2018) constructed PGA-Asp-maleimide-cisplatin-peptide complex (PAMCP). The results showed that PAMCP reduced the toxicity of CDDP in vitro and in vivo with the increasing antitumor efficiency.
5.5 Environmental Protection
Industrial and agricultural sewage has caused a severe burden to the environment, damaging animal and plant health. At present, the flocculation sedimentation method has been frequently used to achieve efficient sewage treatment. There are many kinds of flocculants, the quality of which directly affects the treatment effect. γ-PGA is a biodegradable polymer material. Compared with traditional flocculants, γ-PGA is environmentally friendly with outstanding water absorption, adhesion, and adsorption bridging effect, which has a broad application prospect.
Flocculation is a commonly used and effective method to remove suspended solids and metal ions in wastewater treatment (Deng et al. 2003). γ-PGA with relatively large molecular weight (more than 1.5 million) can form colloids in water, which has adsorption bridging effect and biodegradability. It can be used as an environment-friendly flocculant to replace the traditional synthetic flocculant in wastewater pollution treatment. Bajaj and Singhal (2009a) studied the flocculation activity of γ-PGA and optimized the flocculation conditions. The results showed that the maximum flocculation activity of the γ-PGA (6.2 × 106 Da) produced by Bacillus subtilis R 23 fermentation was up to 34.7 L /OD with the optimal flocculation pH and coagulant ion Ca2+.
γ-PGA has the excellent chelating ability of metal ions (Ni2+, Cr3+, Cu2+, and Pb2+) due to a good deal of carboxylic anion groups, which can be used for the removal and recovery of heavy metals. Hajdu et al. (2012) combined γ-PGA-Ni complexed nanoparticles with membrane separation technology to remove toxic lead ions from an aqueous solution. More than 99.8% of lead ions in water can be removed by the convenient low-pressure ultrafiltration technology, making the permeate meet the drinking water standard recommended by WHO. Chang et al. (2013) developed γ-PGA-coated superparamagnetic iron oxide NPs (γ-PGA/Fe3O4 NPs) by coprecipitation method for the removal of heavy metal ions. The results showed that γ-PGA/Fe3O4 NPs had a higher specific surface area and removal efficiency for all metal ions, as compared to Fe3O4 NPS or γ-PGA. Specifically, γ-PGA/Fe3O4 NPs showed a better removal activity at the solution pH higher than 6.0, removing more than 99% of Cr3+, Cu2+, Pb2+, and 77% of Ni2+. Sakamoto and Kawase (2016) used both water-insoluble γ-PGA and water-soluble sodium salt form of γ-PGA (γ-PGANa) as a low-cost, safe, and environmentally friendly biological adsorbent to recover cesium from radioactive wastewater. The adsorption principle of γ-PGA and γ-PGANa on cesium in radioactive wastewater is due to the electrostatic interaction between carboxylic acid ions (-COO−) and cesium cation (Cs+) via chemical reaction. The maximum adsorption capacities at equilibrium of γ-PGA and γ-PGANa for Cs were 345 mg-Cs(g-adsorbent)−1 at pH 6.0 and 290 mg-Cs(g-adsorbent)−1 at pH 9.0, respectively.
6 Conclusion and Future Outlook
As a biopolymer, γ-PGA has attracted worldwide attention. At present, remarkable progress has been made in screening and modifying production strains, optimizing fermentation conditions, and purifying γ-PGA. In particular, the industrial applications of γ-PGA have been widely recognized, which has resulted in the in-depth research of its metabolic pathways and essential enzymes.
The biological macromolecular polymer structure regulation and assembling mechanism is a significant problem, even though many studies have explored the key enzymes for γ-PGA synthesis. Understanding the molecular regulatory mechanisms of γ-PGA biosynthesis and control of stereoisomers would undoubtedly prove valuable. However, due to the lack of relevant crystal structure data, the mechanism of γ-PGA polymerization and degradation has not been fully elucidated. As different γ-PGA synthases have differences in stability, activity, and molecular weight of products, future research should analyze its protein structure and regulation mechanism. Such work would result in feasible solutions for the production of γ-PGA of variable structures.
With the increasing trend in using biomass as a carbon source for fermentation processes, much research of γ-PGA bioproduction has aimed at improving the cost-effectiveness and the efficiency of recovery. At present, most of the reported production strains are glutamic acid-dependent, which require large amounts of glutamic acid in the medium. Therefore, some glutamic acid-independent strains, reducing the cost of fermentation by saving the cost of glutamate, have become the new research hotspot for the biosynthesis of γ-PGA. However, their low productivity remains the main bottleneck in industrial application. Further efforts are needed to carry out the production of γ-PGA with the use of cost-effective methods like harnessing the potential of agricultural waste in the production along with the construction of efficient microbial strains which can reduce the cost of fermentative production. Genetic manipulation could also be exploited to develop novel γ-PGA-producing strains, such as thermo- and salt-tolerant bacterial extremophiles. Finally, the establishment of γ-PGA separation processes for different downstream applications could be decisive in improving the cost-effectiveness of production and will help expand the application field of γ-PGA. Therefore, the systematic approach combining synthetic biology, metabolic engineering, and fermentation regulation technology may significantly improve the biosynthetic efficiency and product diversity of γ-PGA, which lay a foundation for further expanding its downstream application.
References
Ashiuchi M, Kamei T, Baek DH et al (2001a) Isolation of Bacillus subtilis (chungkookjang), a poly-γ-glutamate producer with high genetic competence. Appl Micobiol Biotechnol 57:764–769
Ashiuchi M, Nawa C, Kamei T et al (2001b) Physiological and biochemical characteristics of poly-γ-glutamate synthetase complex of Bacillus subtilis. Eur J Biochem 268:5321–5328
Ashiuchi M, Misono H (2002a) Biochemistry and molecular genetics of poly-γ-glutamate synthesis. Appl Microbiol Biotechnol 59(1):9–14
Ashiuchi M, Misono H (2002b) Poly-γ-glutamic acid. In: Fahnestock SR, Steinbüchel A (eds) Biopolymers. Wiley, Weinheim
Ashiuchi M, Shimanouchi K, Horiuchi T et al (2006) Genetically engineered poly-γ-glutamate producer from Bacillus subtilis ISW1214. Biosci Biotechnol Biochem 70:1794–1797
Ashiuchi M, Soda K, Misono H (1999a) A poly-γ-glutamate synthetic system of Bacillus subtilis IFO 3336: gene cloning and biochemical analysis of poly-γ-glutamate produced by Escherichia coli clone cells. Biochem Biophys Res Commun 263:6–12
Ashiuchi M, Soda K, Misono H (1999b) Characterization of yrpC gene product of Bacillus subtilis IFO 3336 as glutamate racemase isozyme. Biosci Biotechnol Biochem 63:792–798
Ashiuchi M, Tani K, Soda K et al (1998) Properties of glutamate racemase from Bacillus subtilis IFO 3336 producing poly-γ-glutamate. J Biochem 123:1156–1163
Ashiuchi M, Yamashiro D. 2009. Moonlighting function of the pgsE gene product, a novel member of the membranous enzyme complex responsible for the synthesis of d-glutamate-containing poly-γ-glutamate. The first international conference of d-amino acid research. Awaji
Bajaj I, Singhal R (2011) Poly (glutamic acid)-an emerging biopolymer of commercial interest. Bioresour Technol 102(10):5551–5561
Bajaj IB, Singhal RS (2009a) Flocculation properties of poly(γ-glutamic acid) produced from Bacillus subtilis isolate. Food Bioprocess Technol 4(5):745–752
Bajaj IB, Singhal RS (2009b) Enhanced production of poly(γ-glutamic acid) from Bacillus licheniformis NCIM 2324 by using metabolic precursors. Appl Biochem Biotechnol 159(1):133–141
Balikό G, Venetianer P (1993) An Escherichia coli gene in search of a function: phenotypic effects of the gene recently identified as murI. J Bacteriol 175:6571–6577
Belitsky BR, Sonenshein AL (1998) Role and regulation of Bacillus subtilis glutamate dehydrogenase genes. J Bacteriol 180:6298–6305
Belitsky BR, Wray L Jr, Fisher SH et al (2000) Role of tnrA in nitrogen source-dependent repression of Bacillus subtilis glutamate synthase gene expression. J Bacteriol 182:5939–5947
Bhat AR, Irorere VU, Bartlett T et al (2013) Bacillus subtilis natto: a non-toxic source of poly-γ-glutamic acid that could be used as a cryoprotectant for probiotic bacteria. AMB Expr 3(1):1–9
Bhatt R, Vries P, Klein J, et al. 2002. Polyglutamic acid-camptothecin conjugates and methods of preparation. WO2001070275A3
Birrer GA, Cromwick AM, Gross RA (1994) Gamma-poly(glutamic acid) formation by Bacillus licheniformis 9945a: physiological and biochemical studies. Int J Biol Macromol 16:265–275
Bovarnick M (1942) The formation of extracellular d(−)-glutamic acid polypeptide by Bacillus subtillis. J Biol Chem 145:415–424
Cai D, Chen Y, He P et al (2018) Enhanced production of poly-γ-glutamic acid by improving ATP supply in metabolically engineered Bacillus licheniformis. Biotechnol Bioeng 115(10):2541–2553
Cai D, He P, Lu X et al (2017) A novel approach to improve poly-γ-glutamic acid production by NADPH regeneration in Bacillus licheniformis WX-02. Sci Rep 7:43404
Candela T, Fouet A (2005) Bacillus anthracis CapD, belonging to the γ-glutamyltranspeptidase family, is required for the covalent anchoring of capsule to peptidoglycan. Mol Microbiol 57(3):717–726
Candela T, Fouet A (2006) Poly-gamma-glutamate in bacteria. Mol Microbiol 60:1091–1098
Candela T, Moya M, Haustant M et al (2009) Fusobacterium nucleatum, the first gram-negative bacterium demonstrated to produce polyglutamate. Can J Microbiol 55:627–632
Cao M, Geng W, Liu L et al (2011) Glutamic acid independent production of poly-γ-glutamic acid by bacillus amyloliquefaciens LL3 and cloning of pgsBCA genes. Bioresour Technol 102(5):4251–4257
Cao M, Geng W, Zhang W et al (2013) Engineering of recombinant Escherichia coli cells co-expressing poly-γ-glutamic acid (γ-PGA) synthetase and glutamate racemase for differential yielding of γ-PGA. Microb Biotechnol 6(6):675–684
Cao M, Song C, Jin Y et al (2010) Synthesis of poly (γ-glutamic acid) and heterologous expression of pgsBCA genes. J Mol Catal B Enzym 67(1–2):111–116
Chang J, Zhong Z, Xu H et al (2013) Fabrication of poly(γ-glutamic acid)-coated Fe3O4 magnetic nanoparticles and their application in heavy metal removal. Chin J Chem Eng 21(11):1244–1250
Chen W, Wang R, Xu T et al (2017) A mussel-inspired poly (γ-glutamic acid) tissue adhesive with high wet strength for wound closure. J Mater Chem B 5(28):5668–5678
Cheng C, Asada Y, Aida T (1989) Production of γ-polyglutamic acid by Bacillus subtilis A35 under denitrifying conditions. Agric Biol Chem 53:2369–2375
Deng S, Bai R, Hu X et al (2003) Characteristics of a bioflocculant produced by bacillus mucilaginosus and its use in starch wastewater treatment. Appl Microbiol Biotechnol 60(5):588–593
Do JH, Chang HN, Lee SY (2001) Efficient recovery of poly (glutamic acid) from highly viscous culture broth. Biotechnol Bioeng 76(3):219–223
Feng J, Gu Y, Quan Y et al (2015) Improved poly-γ-glutamic acid production in bacillus amyloliquefaciens by modular pathway engineering. Metab Eng 32:106–115
Feng J, Gu Y, Quan Y et al (2017) Construction of energy-conserving sucrose utilization pathways for improving poly-γ-glutamic acid production in bacillus amyloliquefaciens. Microb Cell Factories 16:98
Feng X, Tang B, Jiang Y et al (2016) Efficient production of poly-γ-glutamic acid from cane molasses by Bacillus subtilis NX-2 immobilized on chemically modified sugarcane bagasse. J Chem Technol Biotechnol 91(7):2085–2093
Fujita KI, Tomiyama T, Inoi T et al (2021) Effect of pgsE expression on the molecular weight of poly(γ-glutamic acid) in fermentative production. Polym J 53:409–414
Gao W, Liu F, Zhang W et al (2016) Mutations in genes encoding antibiotic substances increase the synthesis of poly-γ-glutamic acid in bacillus amyloliquefaciens LL3. MicrobiologyOpen 6(1):e00398
Goto A, Kunioka M (1992) Biosynthesis and hydrolysis of poly(γ-glutamic acid) from Bacillus subtilis IFO3335. Biosci Biotechnol Biochem 56:1031–1035
Guo Z, Yang N, Zhu C et al (2017) Exogenously applied poly-γ-glutamic acid alleviates salt stress in wheat seedlings by modulating ion balance and the antioxidant system. Environ Sci Pollut Res 24:6592–6598
Hajdu I, Bodnár M, Csikós Z et al (2012) Combined nano-membrane technology for removal of lead ions. J Membr Sci 409-410:44–53
Halmschlag B, Steurer X, Putri SP et al (2019) Tailor-made poly-γ-glutamic acid production. Metab Eng 55:239–248
Hara T (2000) Desert greening: greening by utilization of microbial macromolecules. Kobunshi 49:367–370
Hezayen FF, Rehm BHA, Tindall BJ et al (2001) Transfer of Natrialba asiatica B1T to Natrialba taiwanensis sp. nov. and description of Natrialba aegyptiaca sp. nov., a novel extremely halophilic, aerobic, non-pigmented member of the archaea from Egypt that produces extracellular poly(glutamic acid). Int J Syst Evol Microbiol 51:1133–1142
Hsieh CY, Tsai SP, Wang DM et al (2005) Preparation of gamma-PGA/chitosan composite tissue engineering matrices. Biomaterials 26(28):5617–5623
Ito Y, Tanaka T, Ohmachi T (1996) Glutamic acid independent production of poly(γ-glutamic acid) by Bacillus subtilis TAM-4. Biosci Biotechnol Biochem 60:1239–1242
Ivanovics G, Bruckner V (1937) The chemical nature of the immuno-specific capsule substance of anthrax bacillus. Naturwissenschaften 25:250
Jia WS, Zhi YY, Zhong Y et al (2019) Effect of γ-glutamic acid on the antifreeze protection of frozen dough and noodles. Food Ind Sci Tech 7
Jiang H, Shang L, Yoon SH et al (2006) Optimal production of poly-γ-glutamic acid by metabolically engineered Escherichia coli. Biotechnol Lett 28:1241–1246
Jiang Y, Tang B, Xu Z et al (2016) Improvement of poly-gamma-glutamic acid biosynthesis in a moving bed biofilm reactor by Bacillus subtilis NX-2. Bioresour Technol 218:360–366
Ju WT, Song YS, Jung WJ, Park RD (2014) Enhanced production of poly-gamma-glutamic acid by a newly-isolated Bacillus subtilis. Biotechnol Lett 36(11):2319–2324
Jung DY, Jung S, Yun JS et al (2005) Influences of cultural medium component on the production of poly (γ-glutamic acid) by bacillus sp. RKY3. Biotechnol Bioproc Eng 10(4):289
Junnan F, Juan L, Lishan XU et al (2018) Recent advances in poly-γ-glutamic acid production by microbial fermentation. Chin J Appl Environ Biol 24(5):1041–1049
Katsuragi Y, Sugiura Y, Ono S, et al. 1998. Bitterness-relieving agent. WO2000021390A1
Kimura K, Itoh Y (2003) Characterization of poly-γ-glutamate hydrolase encoded by a bacteriophage genome: possible role in phage infection of Bacillus subtilis encapsulated with poly-γ-glutamate. Appl Environ Microbiol 69(5):2491–2497
Kimura K, Tran LSP, Do TH et al (2009) Expression of the pgsB encoding the poly-gamma-DL-glutamate synthetase of Bacillus subtilis (natto). Biosci Biotechnol Biochem 73:1149–1155
Kimura K, Tran LSP, Uchida I et al (2004a) Characterization of Bacillus subtilis γ-glutamyltransferase and its involvement in the degradation of capsule poly-γ-glutamate. Microbiology 150(12):4115–4123
Kimura K, Tran LSP, Itoh Y (2004b) Roles and regulation of the glutamate racemase isogenes, racE and yrpC, in Bacillus subtilis. Microbiology 150:2911–2920
King EC, Blacker AJ, Bugg TD (2001) Enzymatic breakdown of poly-γ-d-glutamic acid in bacillus licheniformis: identification of a polyglutamyl γ-hydrolase enzyme. Biomacromolecules 1(1):75–83
Kinnersley A M, Koskan L P, Meah A R Y, et al. 1994. Composition and method for enhanced fertilizer uptake by plants. CA2148798A1
Ko HY, Gross AR (1998) Effects of glucose and glycerol on γ-poly(glutamic acid) formation by Bacillus licheniformis ATCC 9945A. Biotechnol Bioeng 57:430–437
Kuboat H, Matsunobu T, Uotani K (1993) Production of poly(γ-glutamic acid) by Bacillus subtilis F-2-01. Biosci Biotech Biochem 57:1212–1213
Kubota H, Matsunobu T, Uotani K et al (1993) Production of poly(γ-glutamic acid) by Bacillus subtilis F-2-01. Biosci Biotech Biochem 57:1212–1213
Kunioka K (1997) Biosynthesis and chemical reactions of poly(amino acid)s from microorganisms. Appl Microbiol Biotechnol 47:469–475
Lei P, Pang X, Feng X et al (2017) The microbe-secreted isopeptide poly-γ-glutamic acid induces stress tolerance in Brassica napus L. seedlings by activating crosstalk between H2O2 and Ca2+. Sci Rep 7:41618
Lei P, Xu Z, Ding Y et al (2015) Effect of poly (γ-glutamic acid) on the physiological responses and calcium signaling of rape seedlings (Brassica napus L.) under cold stress. J Agric Food Chem 63:10399–10406
Lei P, Xu Z, Liang J et al (2016) Poly (γ-glutamic acid) enhanced tolerance to salt stress by promoting proline accumulation in Brassica napus L. Plant Growth Regul 78:233–241
Li C, Yu DF, Newman RA et al (1998) Complete regression of well-established tumors using a novel water-soluble poly(l-glutamic acid)-paclitaxel conjugate. Cancer Res 58(11):2404–2409
Lim SM, Kim J, Shim JY et al (2012) Effect of poly-γ-glutamic acids (PGA) on oil uptake and sensory quality in doughnuts. Food Sci Biotechnol 21(1):247–252
Liu F, Tian W, Li L et al (2013) Optimization of solid-state fermentation conditions for antagonistic Bacillus subtilis SQR9 producing bio-organic fertilizer. Chin J Appl Environ Biol 19(1):90–95
Liu J, Ma X, Wang Y et al (2011) Depressed biofilm production in bacillus amyloliquefaciens C06 causes γ-polyglutamic acid (γ-PGA) overproduction. Curr Microbiol 62:235–241
Liu T, Nobeshima H, Ojima Y, Azuma M (2018) A new method to purify poly-γ-glutamic acid using gemini quaternary ammonium salts and characterization of its ionic complex. J Chem Eng Jpn 51(5):431–437
Liu X, Liu F, Liu SY et al (2015) Moist efficacy and safety evaluation of polyglutamate. Chem Ind Daily Use 45(05):275–278
Manocha B, Margaritis A (2010) A novel method for the selective recovery and purification of γ-polyglutamic acid from Bacillus licheniformis fermentation broth. Biotechnol Prog 26(3):734–742
Min L, Jin Z, Caldovic L et al (2009) Mechanism of allosteric inhibition of N-acetyl-L-glutamate synthase by l-arginine. J Biol Chem 284:4873–4880
Mitsui N, Murasawa H, Sekiguchi J (2011) Disruption of the cell wall lytic enzyme CwlO affects the amount and molecular size of poly-γ-glutamic acid produced by Bacillus subtilis (natto). J Gen Appl Microbiol 57(1):35–43
Mitsuiki M, Mizuno A, Tanimoto H et al (1998) Relationship between the antifreeze activities and the chemical structures of oligo- and poly(glutamic acid). J Agric Food Chem 46(3):891–895
Muro T, Nagamori Y, Okada S et al (1990) Some properties and action of poly(glutamic acid) hydrolase II from Micromonospora melanosporea IFO 12515. J Agric Chem Soc Jpn 54(4):1065–1067
Nordlund P, Eklund H (1995) Di-iron-carboxylate proteins. Curr Opin Struct Biol 5:758–766
Ogasawara Y, Shigematsu M, Sato S et al (2019) Involvement of peptide epimerization in poly-γ-glutamic acid biosynthesis. Org Lett 21(11):3972–3975
Ogawa Y, Yamaguchi F, Yuasa K et al (1997) Efficient production of γ-polyglutamic acid by Bacillus subtilis (natto) in jar fermenters. Biosci Biotech Biochem 61:1684–1687
Ogunleye A, Bhat A, Irorere VU et al (2015) Poly-γ-glutamic acid: production, properties and applications. Microbiology 161(1):1–17
Pang X, Lei P, Feng X et al (2018) Poly-γ-glutamic acid, a bio-chelator, alleviates the toxicity of cd and Pb in the soil and promotes the establishment of healthy Cucumis sativus L. seedling. Environ Sci Pollut Res 25:19975–19988
Park C, Choi JC, Choi YH et al (2005) Synthesis of super-high-molecular-weight poly-γ-glutamic acid by Bacillus subtilis subsp. chungkookjang. J Mol Catal B Enzym 35(4–6):128–133
Peng Y, Jiang B, Zhang T et al (2015) High-level production of poly(γ-glutamic acid) by a newly isolated glutamate-independent strain, bacillus methylotrophicus. Process Biochem 50(3):329–335
Qiu Y, Sha Y, Zhang Y et al (2017) Development of Jerusalem artichoke resource for efficient one-step fermentation of poly-(γ-glutamic acid) using a novel strain bacillus amyloliquefaciens NX-2S. Bioresour Technol 239:197–203
Qiu Y, Zhang Y, Zhu Y, Sha Y, Xu Z, Feng X, Li S, Xu H (2019) Improving poly-(γ-glutamic acid) production from a glutamic acid-independent strain from inulin substrate by consolidated bioprocessing. Bioprocess Biosyst Eng 42(10):1711–1720
Qiu Y, Zhu Y, Sha Y et al (2020) Development of a robust bacillus amyloliquefaciens cell factory for efficient poly(γ-glutamic acid) production from Jerusalem artichoke. ACS Sustain Chem Eng 8(26):9763–9774
Rusnak F, Mertz P (2000) Calcineurin: form and function. Physiol Rev 80:1483–1521
Sakamoto S, Kawase Y (2016) Adsorption capacities of poly-gamma-glutamic acid and its sodium salt for cesium removal from radioactive wastewaters. J Environ Radioact 165:151–158
Salehizadeh H, Shojaosadati SA (2001) Extracellular biopolymeric flocculants: recent trends and biotechnological importance. Biotechnol Adv 19(5):371–385
Sawada K, Araki H, Takimura Y et al (2018) Poly-l-gamma-glutamic acid production by recombinant Bacillus subtilis without pgsA gene. AMB Express 8(1):110
Schneerson R, Kubler-Kielb J et al (2003) Poly(γ-d-glutamic acid) protein conjugates induce IgG antibodies in mice to the capsule of bacillus anthracis: a potential addition to the anthrax vaccine. Proc Natl Acad Sci U S A 100(15):8945–8950
Scoffone V, Dondi D, Biino G et al (2013) Knockout of pgdS and ggt genes improves γ-PGA yield in B. subtilis. Biotechnol Bioeng 110(7):2006–2012
Sha Y, Qiu Y, Zhu Y et al (2020a) CRISPRi-based dynamic regulation of hydrolase for synthesis of poly-γ-glutamic acid with variable molecular weights. ACS Synth Biol 9(9):2450–2459
Sha Y, Huang Y, Zhu Y et al (2020b) Efficient biosynthesis of low-molecular-weight poly-γ-glutamic acid based on stereochemistry regulation in bacillus amyloliquefaciens. ACS Synth Biol 9(6):1395–1405
Sha Y, Sun T, Qiu Y et al (2019a) Investigation of glutamate dependence mechanism for poly-γ-glutamic acid production in Bacillus subtilis based on transcriptome analysis. J Agric Food Chem 67(22):6263–6274
Sha Y, Zhang Y, Qiu Y et al (2019b) Efficient biosynthesis of low-molecular-weight poly-γ-glutamic acid by stable overexpression of PgdS hydrolase in bacillus amyloliquefaciens NB. J Agric Food Chem 67(1):282–290
Sha Y, Tao S, Qiu Y et al (2019c) Investigation of glutamate dependence mechanism for poly-γ-glutamic acid production in Bacillus subtilis on the basis of transcriptome analysis. J Agric Food Chem 67(5):6263–6274
Shi F, Xu Z, Cen P (2006) Optimization of γ-polyglutamic acid production by Bacillus subtilis ZJU-7 using a surface-response methodology. Biotechnol Bioprocess Eng 11(3):251–257
Shih I, Wu P, Shieh C (2005) Microbial production of a poly(γ-glutamic acid) derivative by Bacillus subtilis. Process Biochem 40(8):2827–2832
Shih L, Van YT (2001) The production of poly-(γ-glutamic acid) from microorganisms and its various applications. Bioresour Technol 79(3):207–225
Shyu YS, Hwang JY, Hsu CK (2008) Improving the rheological and thermal properties of wheat dough by the addition of γ-polyglutamic acid. LWT - Food Sci Technol 41(6):982–987
Shyu YS, Sung WC (2010) Improving the emulsion stability of sponge cake by the addition of γ-polyglutamic acid. J Mar Sci Technol 18(6):895–900
Su Y, Li X, Liu Q et al (2010) Improved poly-γ-glutamic acid production by chromosomal integration of the Vitreoscilla hemoglobin gene (vgb) in Bacillus subtilis. Bioresour Technol 101(12):4733–4736
Sun A, He X, Li L et al (2020) An injectable photopolymerized hydrogel with antimicrobial and biocompatible properties for infected skin regeneration. NPG Asia Mater 12(1):1–11
Sung MH, Park C, Choi JC, et al. 2014. Hyaluronidase inhibitor containing poly-gamma-glutamic acid as an effective component. U8916141B2
Suzuki T, Tahara Y (2003) Characterization of the Bacillus subtilis ywtD gene, whose product is involved in γ-polyglutamic acid degradation. J Bacteriol 185(7):2379–2382
Tanaka T, Hiruta O, Futamura T et al (1993) Purification and characterization of poly (γ-glutamic acid) hydrolase from a filamentous fungus, Myrothecium sp. TM-4222. Biosci Biotechnol Biochem 57(12):2148–2153
Tanimoto H, Fox T, Eagles J et al (2007) Acute effect of poly-gamma-glutamic acid on calcium absorption in post-menopausal women. J Am Coll Nutr 26(6):645–649
Tanimoto H, Mori M, Motoki M et al (2001) Natto mucilage containing poly-gamma-glutamic acid increases soluble calcium in the rat small intestine. Biosci Biotechnol Biochem 65(3):516–521
Tomosho JW, Moran RG, Coward JK (2008) Concentration-dependent processivity of multiple glutamate ligations catalyzed by folylpoly-γ-glutamate synthetase. Biochemistry 47:9040–9050
Tsao CT, Chang CH, Lin YY et al (2011) Evaluation of chitosan/γ-poly(glutamic acid) polyelectrolyte complex for wound dressing materials. Carbohydr Polym 84(2):812–819
Vetting MW, De Carvalho LPS, Yu M et al (2005) Structure and functions of the GNAT superfamily of acetyltransferases. Arch Biochem Biophys 433:212–226
Volcani BE, Margalith P (1957) A new species (Flavobacterium polyglutamicum) which hydrolyzes the gamma-L-glutamyl bond in polypeptides. J Bacteriol 74(5):646–655
Wang Q, Chen S, Zhang J et al (2008) Co-producing lipopeptides and poly-γ-glutamic acid by solid-state fermentation of Bacillus subtilis using soybean and sweet potato residues and its biocontrol and fertilizer synergistic effects. Bioresour Technol 99:3318–3323
Wang R, Wang X, Zhan Y et al (2019) A dual network hydrogel sunscreen based on poly-γ-glutamic acid/tannic acid demonstrates excellent anti-UV, self-recovery, and skin-integration capacities. ACS Appl Mater Interf 11(41):37502–37512
Wang WG, Wang W, Zhao YL et al (2016) Research and application progress of γ-polyglutamic acid. J Henan Univ Technol 2
Wang XX, Li S, Ren ZK et al (2020) Antioxidant effect of γ-poly (glutamate) on skin photoaging induced by UV radiation. Bioprocessing 18(05):561–566
Weber J (1989) Poly(γ-glutamic acid)s are the major constituent of nematocysts in hydra (hydrozoa, Cnidaria). J Biol Chem 265(17):9664–9669
Wu Q, Xu H, Shi N et al (2008) Improvement of poly (γ-glutamic acid) biosynthesis and redistribution of metabolic flux with the presence of different additives in Bacillus subtilis CGMCC 0833. Appl Microbiol Biotechnol 79(4):527
Wu Q, Xu H, Zhang L et al (2006a) Production, purifcation and properties of γ-glutamyltranspeptidase from a newly isolated Bacillus subtilis NX-2. J Mol Cat B Enzym 43(1–4):113–117
Wu Q, Xu H, Xu L, Ouyang P (2006b) Biosynthesis of poly(γ-glutamic acid) in Bacillus subtilis NX-2: regulation of stereochemical composition of poly(γ-glutamic acid). Process Biochem 41:1650–1655
Xu G, Zha J, Cheng H et al (2019a) Engineering Corynebacterium glutamicum for the de novo biosynthesis of tailored poly-γ-glutamic acid. Metab Eng 56:39–49
Xu T, Yang R, Ma X et al (2019b) Bionic poly(γ-glutamic acid) electrospun fibrous scaffolds for preventing hypertrophic scars. Adv Healthc Mater 8(13):1900123
Xu Z, Lei P, Feng X et al (2014) Calcium involved in the poly (γ-glutamic acid)-mediated promotion of Chinese cabbage nitrogen metabolism. Plant Physiol Biochem 80:144–152
Xu Z, Lei P, Feng X et al (2016) Analysis of the metabolic pathways affected by poly(γ-glutamic acid) in Arabidopsis thaliana based on genechip microarray. J Agric Food Chem 64:6257–6266
Xu Z, Lei P, Pang X et al (2017a) Exogenous application of poly-γ-glutamic acid enhances stress defense in Brassica napus L. seedlings by inducing cross-talks between Ca2+, H2O2, brassinolide, and jasmonic acid in leaves. Plant Physiol Biochem 118:460–470
Xu H, Feng XH, Xu DL et al (2017b) Development and application prospect of polyamino acid functional polymers. Biotechnology 6
Yamanaka K, Maruyama C, Takagi H et al (2008) ε-Poly-L-lysine dispersity is controlled by a highly unusual nonribosomal peptide synthetase. Nat Chem Biol 4:766–772
Yao J, Jing J, Xu H et al (2009) Investigation on enzymatic degradation of γ-polyglutamic acid from Bacillus subtilis NX-2. J Mol Catal B Enzym 56(2–3):158–164
Yeh CM, Wang JP, Lo SC et al (2010) Chromosomal integration of a synthetic expression control sequence achieves poly-γ-glutamate production in a Bacillus subtilis strain. Biotechnol Prog 26(4):1001–1007
Zeng W, Chen G, Guo Y et al (2017) Production of poly-γ-glutamic acid by a thermotolerant glutamate-independent strain and comparative analysis of the glutamate dependent difference. AMB Expr 7(1):213
Zeng W, Lin Y, Qi Z et al (2013) An integrated high-throughput strategy for rapid screening of poly(γ-glutamic acid)-producing bacteria. Appl Microbiol Biotechnol 97(5):2163–2172
Zhan Y, Zhu C et al (2017) Improvement of glycerol catabolism in Bacillus licheniformis for production of poly-γ-glutamic acid. Appl Microbiol Biotechnol 101(19):7155–7164
Zhang C, Wu DJ, Zheng XJ (2017) Generation of a high γ-polyglutamic acid-producing, high glucose-tolerant Bacillus subtilis strain via genome shuffling. J Biobaased Mater Bioenergy 11(1):73–77
Zhang H, Zhu J, Zhu X et al (2012a) High-level exogenous glutamic acid-independent production of poly-(γ-glutamic acid) with organic acid addition in a new isolated Bacillus subtilis C10. Bioresour Technol 116:241–246
Zhang D, Feng X, Li S et al (2012b) Effects of oxygen vectors on the synthesis and molecular weight of poly (γ-glutamic acid) and the metabolic characterization of Bacillus subtilis NX-2. Process Biochem 47(12):2103–2109
Zhang D, Feng X, Zhou Z et al (2012c) Economical production of poly(γ-glutamic acid) using untreated cane molasses and monosodium glutamate waste liquor by Bacillus subtilis NX-2. Bioresour Technol 114:583–588
Zhang JX (2020) Study on synthesis and application of γ-polyglutamic acid. Green Sci Tech 24:212–215
Zhang L, Zhu X, Wu S et al (2018) Fabrication and evaluation of a γ-PGA-based self-assembly transferrin receptor-targeting anticancer drug carrier. Int J Nanomedicine 13:7873
Zhang W, He Y, Gao W et al (2015) Deletion of genes involved in glutamate metabolism to improve poly-γ-glutamic acid production in B. amyloliquefaciens LL3. J Ind Microbiol Biotechnol 42:297–305
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Li, S., Qiu, Y., Xu, H., Wang, R., Lei, P. (2022). Recent Advances in Poly-(γ-Glutamic Acid) Production by Microbial Fermentation. In: Rehm, B.H.A., Wibowo, D. (eds) Microbial Production of High-Value Products. Microbiology Monographs, vol 37. Springer, Cham. https://doi.org/10.1007/978-3-031-06600-9_9
Download citation
DOI: https://doi.org/10.1007/978-3-031-06600-9_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-06599-6
Online ISBN: 978-3-031-06600-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)