What You Will Learn
-
1.
The issues with conventional fluorescence microscopy techniques that lead to the development of light-sheet fluorescence microscopy
-
2.
The basic principles of light-sheet fluorescence microscopy illumination, detection, and sample imaging
-
3.
The processes involved in sample preparation for light-sheet fluorescence microscopy
-
4.
The limitations surrounding light-sheet fluorescence microscopy, and the innovations that have been developed to compensate for these limitations
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
FormalPara What You Will Learn-
1.
The issues with conventional fluorescence microscopy techniques that lead to the development of light-sheet fluorescence microscopy
-
2.
The basic principles of light-sheet fluorescence microscopy illumination, detection, and sample imaging
-
3.
The processes involved in sample preparation for light-sheet fluorescence microscopy
-
4.
The limitations surrounding light-sheet fluorescence microscopy, and the innovations that have been developed to compensate for these limitations
12.1 Introduction
Organisms are three-dimensional objects (3D). Historically, attempts to understand biological processes within organisms have resorted to the imaging of a single slice of tissues, trying to relate these 2D snapshots to the ultimate processes of life. Recently imaging has seen a push toward understanding this microscopic world in its full 3D version. Steps forward have been carried by a mixture of physical sectioning (e.g., histology) and optical sectioning. Physical sectioning results in very thin slices of a sample being imaged independently, allowing for transmission of light through an otherwise opaque sample. Optical sectioning relies on optical methods selectively imaging only a single plane of a sample while leaving the physical structure of the whole sample untampered. This innovation has most notably been implemented through the widespread use of confocal microscopy. However, these techniques, and confocal microscopy specifically, come with a range of highly problematic side effects that more modern technologies are now trying to resolve and eliminate completely.
12.1.1 Issues with Conventional Fluorescence Microscopy Techniques
Fluorescence imaging relies on the absorption of light at excitation wavelengths by a fluorophore, followed by emission at a longer wavelength, which is then detected. Conventional approaches to fluorescence microscopy use a basic premise of parallel illumination and detection. A beam of light of a defined wavelength (excitation) is shone on a sample. Emission light is then collected through the same aperture, with the light diverted along a different path to end up at a detector using dichroic mirrors and specific filters. This generates important issues, mostly overlooked by inexperienced users. Firstly, even though light may be focused onto the specific plane a user is imaging, the beam of light will have to converge through the preceding tissue and diverge into the subsequent tissue. This means that even if only collecting light from a single plane of a sample, the tissues above and below the plane are still exposed to the light. Exposure to light can eventually make these fluorophores unresponsive to excitation, meaning the more light you expose a fluorophore to, the faster it will stop emitting fluorescence. This process is known as photobleaching (Fig. 12.1). By exposing a whole sample to the excitation light, this process will begin to occur throughout the sample. If you are using microscopy to optically section a sample, as in confocal microscopy, this means that the first layer imaged will be imaged as normal, but the second layer would have already been exposed to one round of excitation light, and the third layer would have been exposed to two rounds before being imaged, and so on for the whole sample. This results in a reduced fluorescent output the more slices you image. This is further compounded if multiple time points are imaged, meaning later slices will appear darker than earlier slices, and later time points will appear darker than earlier time points.
Excessive light exposure can also trigger cellular damage to a sample in two major ways. Firstly, fluorescent excitation always results in the production of free radical oxygen species, which cause intracellular damage to crucial cellular components, including DNA (Fig. 12.1). Free radicals are produced by light interacting with normal oxygen, altering their electron configuration, and turning them into reactive oxygen species. These reactive oxygen species react with intracellular structures and molecules, causing damage. The more fluorescent excitation in a sample, the more free radicals are produced and thus the more damage. Secondly, light transfers energy to a sample in the form of heat, with longer wavelengths of light transferring more heat to a sample. For physiological imaging experiments this poses problems of influencing the environment within a sample, and potential damage to cellular structures sensitive to heat (Fig. 12.1).
Issues of fluorescence imaging. Fluorophore exposure to excitation light will eventually result in decreased fluorescence emission, meaning that over time the brightness of a fluorescent object will decrease the longer it is exposed. Phototoxicity results from excitation light triggering the generation of free radicals within cells, causing damage to intracellular components ultimately leading to cell death. Light exposure over time imparts thermal energy into a sample, also causing disruption and eventually death to a live sample
A compounding issue, particularly with confocal microscopy, is the time necessary for imaging a sample. Confocal microscopy builds up a planar image of a sample layer pixel by pixel. This is achieved by simply counting the number of photons emitted from each pixel within a plane using a specialized photon detector known as a photomultiplier tube. This process is time-consuming, and also requires a high laser intensity for each pixel to excite enough fluorophores to detect the small levels of light emitted. While the spinning disk confocal microscope was developed to reduce this increased imaging time, it is still relatively time-intensive to acquire even a single image. Cameras have developed significantly in the past few decades, with highly sensitive and photodetector-dense sensors capable of capturing up to hundreds of frames per second under the relatively low light conditions associated with microscopy. However, so far point-scanning confocal microscopy is unable to make use of these cameras.
The quest to understand live organisms in 3D has also been hampered by conventional optical slicing techniques due to their limitations on the size of a sample that can be imaged and the field of view that can be acquired at any one time. As described above, confocal microscopy builds up an image pixel by pixel, and so the more pixels needed per image (larger field of view, or more XY areas scanned) the longer it takes to acquire each image.
To deal with these limitations, a new way of optically slicing samples was required—ideally one that flips the geometry and orientation of illumination and detection.
12.1.2 Introducing Light-Sheet Microscopy
Light-sheet microscopy was first published in 1903, when Henry Siedentopf and Richard Zsigmondy utilized a planar sheet or cone of light to image colloids in solution, for which Richard Zsigmondy was later awarded a Nobel Prize. The technique was mainly used in physical chemistry and as a dark field equivalent for bacteria observation but vanished shortly after 1935. In 1993, this technique was revived and applied for the first time to 3D imaging of the mouse cochlea (Voie et al. 1993). Light-sheet microscopy only illuminates a single Z layer of a sample at a time. This is achieved by uncoupling the illumination and detection axes, illuminating a single plane from a 90° angle relative to the detection system. This means that there is no extra light disrupting the rest of the sample during imaging of an optical section, and thus photobleaching and phototoxicity are significantly reduced compared to confocal and wide-field approaches. This allows for a longer duration of imaging before photobleaching and phototoxicity become destructive, meaning more data and information can be extracted about any biological processes. By using high-quality cameras for image acquisition, and by imaging the whole illuminated plane at once, they allow for much larger fields of view and also for faster imaging, meaning biological processes are easier to visualize within the scale of a sample. Light-sheet microscopy also accommodates for the use of much larger samples than confocal microscopy, meaning small animal organs, tissues samples, and even whole embryos can be imaged many times over the imaging duration to observe real-time processes of biology and physiology. A general visual comparison of wide-field, confocal, and light-sheet microscopy techniques can be found in Fig. 12.2.
Comparison of wide-field, confocal, and light-sheet microscopy. Wide-field microscopy illuminates the whole sample indiscriminately, confocal microscopy uses a single point of illumination for detection of each pixel independently, and light-sheet microscopy illuminates a single plane of a sample for each acquisition. From the top view, wide-field and light-sheet microscopy illuminate the whole field of view, while confocal microscopy illuminates a single point at a time, building up each Z plane image pixel by pixel. From the side view panels, you can see the excess illumination light (magenta) from each modality, with wide-field and confocal microscopy illuminating all Z planes of a sample while only imaging one plane, in comparison to light-sheet microscopy. When reconstructing Z planes into a 3D structure, wide-field often lacks the Z resolution desired by microscopists, while confocal and light-sheet microscopy show more accurate results
The ways in which light-sheet microscopy can counter the problems faced with in confocal microscopy relies on the method of illumination, image acquisition, and motion control systems. These solutions are discussed below.
12.2 Principles of Light-Sheet Microscopy
Light-sheet fluorescent microscopy relies mostly on the same physical principles as other fluorescence microscopy techniques. Excitation light can be shone onto a sample where certain molecules containing fluorophores will then absorb the light and reemit it at a longer wavelength which can be selectively imaged. However, the way the sample is illuminated, imaged, and moved during imaging is distinct from other methods of optical sectioning.
12.2.1 Creating and Using a Light Sheet
Light is composed of particles called photons that will travel in a straight line until they enter a medium of a different refractive index. A few things can happen when light rays are at the interface of two media with different refractive indices. (1) The object reflects the light, sending it away from the object at an angle relative to the angle it hit the object at (e.g., mirror) (Fig. 12.3a). (2) The light (or certain wavelengths) is absorbed by the object (e.g., apple, selective absorbance of light wavelengths is why objects have color perceivable to us) (Fig. 12.3b). (3) The light is transmitted through the object, either at the original angle of movement or at a different angle (e.g., glass) (Fig. 12.3c). The general ways in which light can interact with matter are outlined visually in Fig. 12.4.
Light behaves differently when hitting different objects. Photons travel in straight lines until they interact with some sort of matter. As a photon hits matter it can be reflected (as with a mirror, a), it can be absorbed (as with the non-red wavelengths of light hitting an apple, b), or it can be refracted and change direction (as with an optical lens, c). It should be noted that absorption is the method by which photons are used for fluorophore excitation. When light rays enter a lens (d), they are refracted in a different direction. In the case of a convex lens, the rays are refracted to a common point in space known as the focal point. Prior to this point the rays are converging and after this point they are diverging
Light behaves differently when interacting with different matter. (a) When incident light moves into a substrate with a different refractive index (i.e., from air to glass) the light interacts with the interface in several ways. It can be reflected at an angle relative to its angle of incidence, it can be transmitted and move at in the same direction as it was moving beforehand, or it can be refracted whereby it changes direction at the interface between the two refractive indices. (b) As light is traveling through a substrate, individual photons can hit particles within the substrate. These particles can cause the light to alter direction unpredictably, causing scattering of the photons, and diffusing the light. (c) When light interacts with an object, the object may be capable of absorbing certain wavelengths of light. In the case of a red object, all light of wavelengths shorter than red light will be absorbed, while red light will be reflected. As red light is the only light not absorbed by the object, our eyes perceive the object as red
Refractive index refers to the velocity of light traveling through the medium relative to the velocity of light traveling in a vacuum. As light travels through one medium (air, for example) and into a new medium with a different refractive index (e.g., glass), the difference in velocity causes its change in direction (refraction). Lenses can use this property in combination with the shape of the lens to bend light in a specific and predictable manner. We can use a lens to focus light onto a specific point, or to convert the non-parallel rays of light into parallel rays.
When light is passed through a cylindrical lens it curves the light in such a way that a single beam is converted into a planar sheet of light (Fig. 12.3d). This sheet behaves like a beam of light as normal; the rays will travel in straight lines, the plane will reach a point of focus followed by divergence away from the focal point, and at certain wavelengths can trigger fluorescence of specific fluorophores. This conversion of a beam into a sheet is what allows for optical sectioning in light-sheet microscopy. We can pass a sheet of light through a sample and it will only illuminate a section with the thickness of the light sheet. Fluorophores in this plane will be excited by the light and will fluoresce just as in any other fluorescence microscopy system.
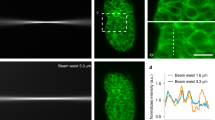
The light sheet will reach a focal point, where the sheet will be optimized to be its thinnest, with thicker regions either side of this point. This means a sample is not necessarily illuminated evenly, and central regions of a sample may be illuminated by a thinner sheet of light than the edges. This is an important consideration when drawing inferences from your imaging data.
If detection of the light were orientated parallel to the illumination, in a similar way to other optical sectioning techniques, this would appear on a camera as a single line of pixels with no detection either side of the light sheet. The acquisition innovation that allows for the detection of the plane of light is the orientation of the camera, using a perpendicular (90°) orientation of the detection to the illumination plane (Fig. 12.5).
Basic light-sheet microscopy system. A beam of excitation light shone through the curved edge of a cylindrical lens will become a light sheet. This light sheet is orientated to be at the exact focal distance from a known objective lens. The light sheet is passed through a fluorescent sample to allow only a single plane of the sample to be illuminated at any one time, allowing the detection of all fluorescence in one plane of the sample. This can be repeated for all planes within the sample, allowing for later 3D reconstruction of the sample
12.2.2 Capturing an Image
As opposed to confocal microscopy, which makes use of a single photodetector and acquires image pixel by pixel, light-sheet microscopy uses a camera to image a whole plane of a sample. This is advantageous for several reasons. The most prominent advantage is that with only a single acquisition required for each plane sample, image acquisition is faster than for confocal microscopy.
Like our eyes, cameras project the light from an object onto a sensor for detection. Unlike our eyes, cameras do not have an adjustable lens that can selectively focus on either an object close to the camera or far from the camera; they have a fixed point of focus. This means that for a camera detection system, the plane of a sample exposed by a light sheet must be kept at an exact focal distance from the camera. This precise distance must be maintained throughout the imaging procedure, and so the illumination and the detection systems must be physically coupled to ensure focus.
The two main types of cameras used in light-sheet microscopy systems are charge-coupled device (CCD) and complementary metal oxide semiconductor (CMOS) cameras. Both are composed of a planar array of sensors, and both rely on a conversion of photons into current, and then into voltage. However, they have different methods of extracting data from the sensor photodetectors. In CCD cameras, each individual pixel is responsible for conversion of photons into electrons to form a current. Each row of pixels then outputs its current to another system, where the current is converted to voltage pixel by pixel. By contrast, in CMOS sensors each pixel is responsible for both the conversion of photons into current and the conversion of current into voltage, which can be performed simultaneously by all pixels on a sensor. Therefore, CMOS cameras are much faster at image acquisition than CCD cameras, and can achieve higher framerates under the same illumination conditions. The disadvantage of CMOS cameras is the increased price associated with the newer and more expensive technology compared to the CCD cameras.
Regardless of the camera type used, the framerate of imaging will be higher than for techniques like confocal microscopy. Increased framerate means that fast imaging of the Z layers of a sample can be achieved, but for this to happen the light sheet must pass through the sample.
12.2.3 Imaging a Whole Sample
One of the major advantages of LSFM is the fast imaging of Z planes of a sample. To achieve this, motion control to move the light sheet through a sample is needed. The light sheet needs to always stay at the same distance from the camera to remain in focus, so there are two options for the motion control system. (1) Move the light sheet and the detection components in tandem to image through a sample. (2) Move the sample through the light sheet. This second option is much more frequently used due to its technical ease over the first option.
For automated scanning of a sample, the Z boundaries (e.g., front and back, or dorsal and ventral edges of the sample) need to be defined by the user. The number of slices between these layers can be set, either by maximizing the number of slices (depending on the light sheet thickness), or performing a lower number of slices for a faster imaging process at the expense of sample Z resolution. The light sheet itself will have a certain thickness throughout the sample, and this information is crucial for determining how far to move the sample between each planar acquisition. If you move too little, then overlap will occur between the images, reducing the Z resolution of your final reconstruction. If you move too far, then planes of the sample will not be imaged, again reducing Z resolution, and potentially missing out on crucial data while interpolating.
12.2.4 Image Properties and Analysis
The scanning process outputs a series of 2D images from different Z levels. If you know the physical distance between each Z plane slice, you can project each 2D slice back that exact distance, adding the Z dimension onto each 2D pixel. This creates a voxel with dimensions X, Y, and Z. Lateral resolution (XY) is often better than axial (Z) resolution, due to several factors, one of which being controlled by the light sheet thickness. This means that the Z dimension of each voxel is often larger than the X or Y dimension (termed anisotropic), and thus, each voxel represents a cuboid as opposed to a cube (isotropic).
In cases of very large samples, it may be necessary to image multiple fields of view sequentially for a single sample (Fig. 12.6). This additional process requires tiling, the process of combining multiple FOVs together by stitching and overlaying their common regions.
Some key considerations in the analysis and interpretation of your imaging data result from the design of the system itself. Issues with light penetration may mean that your sample gets progressively darker the further from the light source. Be careful not to conflate this appearance with any physiological importance, for example if you are performing quantitative fluorescence analysis of protein localization. Additionally, if the motion control system is not working with 100% accuracy, you will generate a 3D drift in Z and so the 3D will not be fully accurate. If movement is larger between planes than requested, your voxels will be too small relative to the actual data you acquired, and vice versa, meaning careful calibration and validation of a system should be performed routinely using samples with known geometries and structural landmarks.
12.3 Preparation of a Sample for Light-Sheet Fluorescence Microscopy
So, we have covered how light-sheet microscopy improves upon other techniques for optical sectioning and shown you how to get images from light-sheet microscopy. But how do you prepare a sample for imaging in the first place?
12.3.1 Dyeing Your Sample
When selecting fluorescent dyes for a sample there are three main considerations which are important to all fluorescent microscopy systems. (1) Method of labeling (i.e., antibody conjugation, secondary antibodies, endogenous fluorophore expression, etc.). (2) Illumination/emission wavelengths and system compatibility. (3) Compatibility with living cells/tissues.
If using an antibody system, there needs to be commercial option in the form of either a bulk manufactured antibody or a company willing to generate a specific antibody for your target molecule. This antibody does not need to be conjugated to a fluorophore itself. Using a “primary” antibody (from an animal that you are not using as a specimen) specific for your molecule (or antigen) of interest can then be followed by using a “secondary” antibody that is just generally specific for the organism you obtained your primary antibody from. If the secondary antibody is conjugated to an active fluorophore, your molecule of interest will now be fluorescent under the right illumination wavelength. Secondary labeling also increases fluorescence compared to direct labeling (Fig. 12.7), as multiple secondary antibodies can bind to each primary antibody, amplifying the signal. Antibody systems can only be applied in fixed tissues, as they require a lethal amount of membrane permeability to allow antibodies to enter cells and bind to their targets.
Secondary antibodies can be used to enhance fluorescent output of a sample. (a) Adding a fluorescently conjugated antibody specific to a target molecule will result in selective fluorescent labeling of a target molecule (b). (c) Through using a primary antibody specific for a target molecule, multiple fluorescently conjugated secondary antibodies can bind each primary antibody, increasing the amount of fluorescence output from a single target molecule
If using an endogenously fluorescent system to image a living organism, for example from transient transfection of fluorescent proteins or from stable expression of fluorescent proteins, the main considerations will be the excitation wavelength used and required filters for excitation/emission. This consideration should be reviewed prior to generating/obtaining the fluorescent construct for the sample. Additionally, commercial fluorescent markers exist for routinely stained features, such as DAPI for nuclei, mitotracker for mitochondria, and others, many of which are compatible for use with live tissue.
The excitation and emission wavelengths of your selected fluorophores or live dyes, regardless of the mechanism of expression, must be selected with your specific optics and hardware capabilities in mind, and should precede any experimental steps.
12.3.2 Clearing Your Sample
Some samples present difficulties when it comes to imaging. As described earlier, different samples permit transmission of different levels of light, with dense samples attenuating the light earlier than less dense samples. This means that some samples, even if relatively small, are incompatible with light-sheet microscopy. To image these samples, it is necessary to remove the problem of optical density from the sample, which is achieved through the process of clearing. Clearing is the process of removing components such as cell membranes and proteins while leaving the cellular structures intact. This attempts to homogenize the refractive index of the sample and prevent light from being attenuated while passing through. These techniques have shown capabilities in creating transparent tissues. Techniques include the use of organic solvents (BABB and iDISCO), hydrogel embedding, high refractive index solutions, or detergent solutions (Fig. 12.8). Each method has its own advantages and disadvantages, as outlined comprehensively elsewhere [2]. The major issue with all these approaches is their incompatibility with live tissue, meaning imaging of a live sample cannot occur.
Sample clearing method using BABB. Large biological samples are fixed overnight in paraformaldehyde (PFA) at 4 °C. Following fixation, samples are dehydrated using increasing concentrations of ethanol, ending with 3 rounds of dehydration in 100% ethanol. Samples are then transferred into a solution of BABB (peroxide-free) for 1–2 days until they become transparent. Protocol obtained from Becker et al. [1]
12.3.3 Mounting Your Sample
Sample preparation for LSFM depends heavily on the type of sample you are using. For imaging of adherent cell culture monolayers, the height of the sample is often too small to make use of the advantages of light-sheet microscopy. To make use of the benefits of LSFM, the monolayer is placed at a 45° angle relative to the light sheet. This means that the light sheet will pass through the specimen diagonally at a 45° angle while maintaining the 90° orientation of light sheet/detection system. The samples themselves are thin enough that light penetration is unlikely to be an issue, but users must take care to adhere their cells to a substrate compatible with light-sheet microscopy imaging.
Larger specimens including organoids, 3D cell cultures, small organs, and embryos need a different approach. One common technique is to embed the object to be images in some kind of material that can be applied as a liquid and then turned solid around the specimen to embed the whole specimen in the middle of a tube of optically transparent solid. Materials like agarose dissolved in water are often used which can be melted to a liquid and solidified by cooling to room temperature. This approach is favored for samples such as whole organs or whole organisms. Thermal considerations must be taken to ensure the embedding material is not excessively hot to the point it will damage a living specimen. A critical consideration when embedding samples is to ensure that the embedding matrix has the same refractive index as whatever fluid you are immersing your sample in. This will prevent detrimental interference with the light sheet when entering the material. Embedding samples is particularly useful in the case of small, live, whole organisms, as you can add nutrients to the embedding matrix to ensure organism survival while also maintaining its physical position and preventing organism movement. This can allow for the long-term imaging of physiological processes, development, and organism functions in stable environmental conditions under relative physiological conditions. This has been applied to the study C. elegans, D. melanogaster, and D. rerio. If a sample is not alive, and thus does not need to be restrained, you may opt for a hooking approach. This technique physically impales the specimen on a sharp hook, allowing it to be suspended within the FOV in air or in solution. The preparation time needed for this approach is obviously less than for embedding; however, it will not work with a specimen that is large, could possibly move, or lacks sufficient integrity to not be torn through by the hook.
12.4 Trade-Offs and Light-Sheet Microscopy: Development of Novel Systems to Meet Demands
The general principle of light-sheet microscopy is not without its own issues. Here, we will discuss some of the biggest issues with light-sheet microscopy and explore the ways in which people have improved upon the design to solve or compensate for them.
12.4.1 Cost and Commercial Systems
The main commercial system available presently is the Zeiss Lightsheet 7. With commercial microscopy systems becoming more specialized and refined, and often becoming more expensive in the process, many groups are left with no financially accessible method of performing large sample 3D imaging using light-sheet microscopy. Without access to a commercial light sheet, labs are left without the necessary technology. Thus, OpenSPIM set to create an open-source and community-driven group for the development and improvement of open-source LSFM. A full parts list, costings, and construction guide is available with step-by-step instructions on how to build the microscope. It is apparently constructible within 1 h by a non-specialist, according to Pavel Tomancak who helped develop the system. The system constructed in full is estimated at $18,000–$40,000, depending on whether you already have a camera available. This is a significant cost saving compared to the commercial system. Independent of a commercial entity, certain aspects gained with a commercial LSFM will be lost, such as warranty and dedicated customer support. However, the community-driven approach has led to a variety of forum-based problem-solving, aiding users in potential issues with their setups. The quality of this open-source system is limited mainly by the quality of the components used and the accuracy of your construction, which may result in a lower quality microscope than a commercial alternative. However, a major benefit of this approach is the adaptability given to the user to alter and improve the design to cater to their specific research goals, not achievable using commercial systems.
12.4.2 Light Penetration
A beam or sheet of light has a finite number of photons capable of interacting with matter. As light progresses through a sample, some photons will hit structures such as lipids, proteins, organelles, or DNA, and will be absorbed or deflected in a different direction. If this light is disrupted before it reaches a fluorophore, it is unable to cause fluorescence as desired. The deeper the light travels into a sample, the more likely it is that a photon collides with disruptive matter. Meaning, the deeper into a sample the fewer photons remain. Eventually, so few photons will remain traveling through the sample that any fluorescence they produce would be of such low intensity that it is undetectable by camera systems. This means that light can only ever penetrate to a certain extent into a sample and still result in usable fluorescence. Where this limit of penetration is depends on the light interaction with during its passage through a sample. A sample with a generally low optical density will allow more photons to travel through it, but light will always become attenuated the further it travels through a sample. Samples that are too large will not have light illuminate the far end of the sample, meaning the whole plane cannot be imaged. Additionally, samples are rarely homogenous in terms of optical density, with some internal structures absorbing more light than others. Opaque or dense structures absorb more light than low-density structures, and thus less light will reach the structures immediately behind the dense ones. This artifact is called shadowing, due to the characteristic appearance of shadows being cast behind optically dense structures. These two problems can be (for the most part) resolved by the addition of a second light sheet at a 180° angle to the original one (Fig. 12.9). This dual illumination means you can image larger samples than with a single light sheet, while also partly solving the problems of shadowing (Fig. 12.10a, b, d). Shadowing is not completely solved with this method, as there may be other optically dense structures from the opposing angle which will cause shadows that overlap with shadows from the original light sheet; however, it is an improvement. An additional method of addressing shadowing artifacts is the use of a scanning light sheet approach, whereby the light sheet is moved quickly laterally to illuminate the sample from multiple angles as opposed to just one (Fig. 12.10c). The problems caused by a sample’s optical density can also be alleviated using clearing agents if possible, but as previously discussed this is not possible when using live samples.
Dual illumination directions can improve illumination uniformity. As light travels through a large sample, it gets attenuated and is thus less able to trigger fluorescence meaning fluorescent objects deeper in the sample show lower fluorescence than those closer to the excitation light source. Addition of a second illumination source from the opposite direction increases uniformity of illumination and reduces this issue. Illumination intensity shown below each image
Removing the impact of shadowing on light-sheet microscopy. (a) A sample with optically dense structures (black circles) will present issues for light-sheet microscopy. (b) Optically dense structures within a sample prevent excitation light from reaching the structures behind them by absorbing the light before it can reach them. (c) By vibrating the excitation light laterally, you allow excitation light to reach behind the dense structures, illuminating their features. (d) By illuminating from opposite directions, you allow for excitation light to reach the spaces behind the optically dense structures
12.4.3 Resolution Limitations
In microscopy, magnification is the degree to which the size of the acquired image is different from the size of the imaged object. Higher magnifications mean that each pixel on your acquired image represents a smaller area of the object (Fig. 12.11). As a smaller area will be imaged, each pixel on the camera sensor is able to detect a higher number of structures in a certain physical area, and thus resolution will increase. Increasing the magnification infinitely will not infinitely increase the resolution of an acquired image, as resolution of a system is limited by two factors: the optics and the detection system. Light itself has a resolution limit itself, based on the wavelength of the light observed. Cameras have a finite number of pixels on their sensor, meaning for any magnification you will only be able to distinguish two objects if they appear on the sensor with one pixel apart.
Field of view vs. resolution. A single sensor has a set number of pixels capable of detecting light. If an object is projected under low magnification onto the sensor, a large number of features will be hidden as multiple features will take up a single pixel space; however, a large field of view will be imaged at this low resolution. If a sample is projected under high magnification onto the sensor, a very small field of view of the sample will be obtained; however, features will be easier to distinguish as they will now be spread out over multiple pixels
There are several ways to improve the resolving power of a microscope: (1) increase the magnification of the optics, (2) improve camera resolution, and (3) apply super-resolution techniques to the imaging process. Increasing magnification involves using high-magnification objective lenses. However, as these focus onto smaller and smaller regions, the amount of light entering the objective will be lower than for lower magnification objectives. This means that camera sensitivity needs to be higher for smaller areas, and the amount of light an objective can take in needs to be increased. To achieve this, higher intensity light can be applied to a sample, and the objectives used can develop higher numerical apertures, often using immersion liquids like oil. Over time, cameras have improved in maximum pixel density by decreasing the size of photodetectors on the camera sensor. However, this only allows for an increased resolution in the X and Y axes, the Z-axis resolution remains unchanged as this is dependent on the thickness and qualities of the light sheet itself. Applications of alternative imaging modalities to LSFM have also led to improvements in resolution. Combination of stimulated emission depletion (STED) microscopy to create a smaller laser beam for thinner light sheet formation has reportedly resulted in a 60% increase in axial resolution [3]. However, pitfalls to this increased resolution are numerous. STED is notorious for causing high levels of photobleaching and photodamage, and thus negatively impacts two major benefits of LSFM for 3D imaging. The equipment and expertise necessary to establish this system is also intense, putting off lower-expertise users, or facility managers not familiar with self-built optical systems. All these issues account for a 60% increase in axial resolution, which very few users would find essential for their research. In terms of resolution as a trade-off, the resolution needed for an experiment need not be excessively high. If performing macrostructure analysis of tissues, it is not necessary to visualize each organelle within each cell, and so excessively high resolution represents an unnecessary amount of data and information.
12.4.4 Sample Size
A significant trade-off in microscopy is that between resolution and field of view. Typically, the higher the resolution, the smaller the FOV, and the larger the FOV the lower the resolution. Light-sheet microscopy users often desire high-resolution imaging of large samples, meaning this trade-off is incompatible with their research goals. A high-tech innovation in this regard is the MesoSPIM, a light-sheet microscopy system that allows for a much larger FOV with relatively high resolution. One pitfall of this technology is the cost and expertise required to build the open-source system from scratch. Thankfully, the community around this technology is open to assisting in a researcher’s goals by offering use of their system, with microscopes available in Switzerland, Germany, and the UK. A significant downside to this equipment is the cost required to build the system, with total cost estimated at between $169,600 and $239,600.
12.4.5 Data Deluge
Light-sheet microscopy has been used to generate full 4D datasets over large time frames of several sample types. This creates a huge amount of data, which is good for getting usable results, but not so good if you need to store or process that data. Using the MesoSPIM, a 3D reconstruction of a full mouse brain has been generated. While the data contained within this reconstruction stands at a modest 12–16 GB for a single time point, time series, multichannel imaging, and numerous equipment users can result in large volumes of data being stored. Datasets of this large size require significant storage space, which can come at a high cost for the user. A dedicated server infrastructure may be necessary if data output is sufficiently high. A further consideration is processing the dataset once imaging has been complete. Even a high-quality computer will struggle to handle the colossal datasets involved in LSFM, so virtual servers may be necessary to even function during normal imaging. Additional problems involve the bandwidth of data able to be transmitted from the device to the storage system. If not high enough, the bottleneck could form in the data transfer, which could abruptly end an imaging run early. All these precautions and additions represent significant financial investment for a group and may not be feasible for a small group with limited funds.
Take-Home Message
Conventional light microscopy systems have major limitations that have led to the development and utilization of LSFM in the life sciences. Light-sheet illumination allows for direct optical sectioning of a sample. Those selected planes can be recorded faster as the technology takes advantage of high-quality cameras with lower illumination power. LSFM can serve as a useful method of optical sectioning of samples for full 3D reconstructive imaging of a wide range of biological sample types, proving its utility in the ever-growing field of LSFM.
References
Becker K, Jährling N, Saghafi S, Weiler R, Dodt H-U. Correction: Chemical clearing and dehydration of GFP expressing mouse brains. PLoS One. 2012;7(8):10.1371/annotation/17e5ee57-fd17-40d7-a52c-fb6f86980def. https://doi.org/10.1371/annotation/17e5ee57-fd17-40d7-a52c-fb6f86980def.
Ariel P. A beginner’s guide to tissue clearing. Int J Biochem Cell Biol. 2017;84:35–9. https://doi.org/10.1016/j.biocel.2016.12.009. Epub 2017 Jan 7. PMID: 28082099; PMCID: PMC5336404.
Friedrich M, Gan Q, Ermolayev V, Harms GS. STED-SPIM: stimulated emission depletion improves sheet illumination microscopy resolution. Biophys J. 2011;100(8):L43–5. https://doi.org/10.1016/j.bpj.2010.12.3748. PMID: 21504720; PMCID: PMC3077687.
Further Reading
History
Cahan D. The Zeiss Werke and the ultramicroscope: the creation of a scientific instrument in context. In: Buchwald JZ, editor. Scientific credibility and technical standards in 19th and early 20th century Germany and Britain. Archimedes (New Studies in the History and Philosophy of Science and Technology), vol. 1. Dordrecht: Springer; 1996. https://doi.org/10.1007/978-94-009-1784-2_3.
Huisken J, Swoger J, Del Bene F, Wittbrodt J, Stelzer EH. Optical sectioning deep inside live embryos by selective plane illumination microscopy. Science. 2004;305:1007–9.
Siedentopf H, Zsigmondy R. Über Sichtbarmachung und Groessenbestimmung ultramikroskopischer Teilchen, mit besonderer Anwendung auf Goldrubinglaesern. Ann Phys. 1903;10:1–39.
Voie AH, Burns DH, Spelman FA. Orthogonal-plane fluorescence optical sectioning: three dimensional imaging of macroscopic biological specimens. J Microsc. 1993;170:229–36.
Reviews
Olarte OE, Andilla J, Gualda EJ, Loza-Alvarez P. Light-sheet microscopy: a tutorial. Adv Opt Photon. 2018;10:111–79.
Power R, Huisken J. A guide to light-sheet fluorescence microscopy for multiscale imaging. Nat Methods. 2017;14:360–73. https://doi.org/10.1038/nmeth.4224.
Reynaud EG, Peychl J, Huisken J, Tomancak P. Guide to light-sheet microscopy for adventurous biologists. Nat Methods. 2015;12(1):30–4. https://doi.org/10.1038/nmeth.3222.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Merces, G.O.T., Reynaud, E.G. (2022). Light-Sheet Fluorescence Microscopy. In: Nechyporuk-Zloy, V. (eds) Principles of Light Microscopy: From Basic to Advanced . Springer, Cham. https://doi.org/10.1007/978-3-031-04477-9_12
Download citation
DOI: https://doi.org/10.1007/978-3-031-04477-9_12
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-04476-2
Online ISBN: 978-3-031-04477-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)