Abstract
The wastewater originating from the industrial and domestic sector with potential hazardous organic and inorganic pollutants when discharged into the water bodies causes undesirable effects on the aquatic environment and human health. It is therefore desirable to treat wastewater prior to its disposal. Recently, the application of nanostructured materials for water remediation has gained attention due to their unique size-dependent properties like large surface area, stability, remarkable reusability and recyclability. Nanoparticles represent a promising new technology for wastewater remediation, not only because of their high treatment efficiency but also for their cost-effectiveness, as they have the flexibility for in-situ and ex-situ applications. Several conventional physical and chemical procedures have been reported for the synthesis of metallic and non-metallic nanoparticles. But the environmental hazards associated with the conventional methods restrict the large-scale production and application in water remediation. It is therefore desirable to synthesize nanoparticles using environmentally safer, rapid and inexpensive biogenic approaches based on microbes. Carbohydrates, proteins, polyphenols, vitamins, polymeric substances and several antioxidants obtained from bacteria, fungi and algae have proven their effectiveness as capping and stabilizing agents during greener synthesis of nanomaterials. Application of microbially synthesized nanomaterials for wastewater treatment is a relatively newer but rapidly escalating area of research. The present book chapter outlines the information on recent advances on the microbial synthesis of different types of metallic and non-metallic nanoparticles, their characterization and role in the removal of different types of organic and inorganic pollutants from wastewater.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Microbial synthesis
- Nanoparticles
- Wastewater
- Remediation
- Recyclability
- Cost-effectiveness
- Non-metallic nanoparticles
5.1 Introduction
The present situation of pollution in aqueous streams has become a major problem for the society (Lu et al. 2016; Moustafa 2017). Water is very essential for the survival of the living beings as well as for the industrial and economic growth of the country. It has been estimated that around 1.1 billion people do not have access to adequate drinking water (Cassivi et al. 2021; Chen et al. 2021; Price et al. 2021). This is due to the increasing cost of potable water, rapidly growing populations and various climatic and environmental concerns. Many harmful chemicals such as pesticides, fertilizers, insecticides, organic compounds, heavy metals, industrial effluents and sewage wastewater are the major environmental contaminants (El-Gendy and Nassar 2021; Mandeep 2020). These contaminants are disposed of in fresh water bodies such as water streams and rivers which leads to water pollution and affects the aquatic ecosystem. The effluent coming out of industrial units are disposed directly in the rivers without treatment due to inefficient wastewater treatment plant. The use of synthetic dyes in excess amount for various industrial activities is also responsible for water as well as soil pollution.
Modern ways of industrialization has increased the tremendous pressure for water usage due to its application in production process. High production resulted in generation of enormous amount of industrial effluents and it requires sustainable and cost-effective technique for achieving sustainable development in case of wastewater treatment. Many electrochemical, valourization techniques and advanced oxidation processes have been utilized to decrease the toxicity of effluents from wastewater and making it fit for drinking and household purpose (Lu et al. 2016). But the techniques mentioned here are not very cost-effective for many industries. Moreover, these conventional methods of wastewater treatment has certain other limitations like energy intensive, excessive cost, requirement of high temperature and pressure, generation of toxic chemicals that are hazardous in nature (Khin et al. 2012; Shankar and Nill 2015). These are uneconomical method because they do not completely purify the wastewater, and it do not give any option to reuse the material. The biological method of wastewater treatment is also applied but they are normally slow, and sometime they cause toxicity to the microorganism due to some hazardous toxic contaminants (Aghalari et al. 2020; Bhat et al. 2020). The physical processes like filtration has tendency to remove the contaminants by changing one phase to another phase but it produces highly concentrated sludge, which is poisonous and it is difficult to dispose in environment.
So, in the aforesaid mentioned context, the wastewater treatment requires more efficient and powerful technology to treat municipal and industrial wastewaters. This can be done either by developing new methods or by improving the techniques that exists. Among several technologies, nanotechnology has emerged as an incredible way for remediating wastewater and certain other environmental related problems. The nanotechnological ways are more effective than their conventional counterparts owing to their small size, excellent chemical properties, high reactivity and large surface area (Aragaw et al. 2021; Kumari et al. 2019; Magalhães-Ghiotto et al. 2021). Synthesizing green nanomaterials from the microorganisms and their extracts have opened the doors towards the environmentally friendly treatment of wastewater pollutants. Nanomaterials possess exceptional surface chemistry in comparison to other conventional methods, due to which they are able to target contaminants with the help of their functional groups for remediation. The properties of nanomaterials such as absorptive capacity, shape, size and chemical components can be altered to enhance the performance of nanomaterial for treatment of contaminants. Application of nanomaterial will help in promoting green technology because there will be less production of a sludge, and it can be the safer option for the remediation of environmental pollutants (Gehrke et al. 2015; Lu et al. 2016; Yaqoob et al. 2020). Iron nanoparticles are the well-known green nanoparticles that are used in remediation of pollutants due to their nontoxic nature, magnetic susceptibility and redox potential. Another type of nanomaterial involved in effective removal of effluent are membrane associated nanomaterial. Nanomaterial are known to improve the permeability of membrane, mechanical and thermal strength for pollutant degradation.
The size of nanoparticles range between 1 and 100 nm. Production of nanoparticles is generally done through top-down and bottom-up methods. In case of top-down method, large structures are converted into small ones by means of physical methods whereas in bottom-up method small atoms, and molecules are utilized to produce nanoparticles by supra-molecular chemistry or self-assembly. The commercial synthesis of nanoparticles is generally carried out by various chemical and physical routes. Among the chemical approaches, the most common methods are sol-gel, coprecipitation, hydrothermal and solvothermal (Ganachari et al. 2017).
The biological method of synthesizing nanoparticle through bottom-up approach has gained popularity as a novel green strategy. The synthesis of nanoparticles biologically is less toxic, biocompatibe, eco-friendly and energy efficient process which produces less sludge. The biological synthesis of nanoparticles is the best alternative method which will overcome the drawback of the abovementioned problems. Among the biological methods, microbial-based synthesis of nanoparticles is of utmost importance due to its cost-effectiveness, ease of synthesis and eco-friendly nature (Singh et al. 2016; Prasad 2016). Microorganisms which are found in contaminated environment have tendency to modify and adapt themselves for degrading xenobiotic compounds and exhibit immense catabolic activity towards polluted environment. Number of studies have been done that shows the efficiency of many newly developed nanomaterials. So, the present chapter focuses upon microbial synthesis of nanomaterials for the application of wastewater treatment.
5.2 Water Pollution: Sources and Environmental Concerns
Water is the most important material for the support of life process in organism. It is the basic need of humans and all the living beings on earth. It is the most precious reserve among all natural resources. Earth is considered as a blue planet as it contains plenty of water on its surface. It is the most valuable asset among all form of natural resources. Freshwater is very important for sustenance as it is needed for household purposes and also for industrial as well as farming purpose. The water used by humans for their day to day activity should be safe i.e. colourless, odourless and free from harmful chemicals and pathogen. The quality of water is a very important issue because decrease in water quality reduces its application for human beings and other living organisms.
Any alteration in the chemical, physical and biological features of water which causes a destructive result on human health and living beings can be called as water pollution. Water plays a vital role in range of human activities like drinking, bathing and other household use. Plenty of water is required for irrigation purpose, and it is a crucial part of agriculture without which the crop production may be affected. Water also plays a major role in biogeochemical reaction that occur below the surface. In the last few decades, scarcity of freshwater has been observed due to rapidly growing population, increased urbanization and farming activities. The industrial revolution has been responsible for rapid industrialization among fast developing countries which has been a major issue as large amount of water is required in industrial processes. Presently, the problem of water scarcity is being observed all over the world among which less developed countries are likely to be more affected (Anderson et al. 2007; Madhura et al. 2019; Shan et al. 2009; Weng 2021).
Many industrial units like thermal power plants, fertilizer, distilleries, oil refineries, cement industries, pulp and paper industries, tanneries, sugar mills, pharmaceuticals, pesticides and textiles along with dye and dye-intermediates generate problem of water pollution through their effluents (Chatterjee et al. 2013; Khan et al. 2005; Sharma et al. 2021; Singh 2001; Solomon 2005). These manufacturing units dispose their effluent containing wastewater into the nearby water bodies or sewage system that create the environmental crisis.
5.2.1 Sources of Water Pollution
Sources of water pollution are broadly classified under two categories:
-
i.
Point source: In point, the harmful substances are directly emitted into the aquatic body e.g. water pollution created by industrial unit discharging its effluent directly into a river. Point source pollution can be monitored and regulated. The strict policies designed and efforts by the government of countries can tackle the problems related to point source water pollution in efficient manner.
-
ii.
Nonpoint source: In nonpoint source, the water pollution occurs through diffuse sources. It negatively affects water bodies. Its example includes runoff from agricultural land draining into a river. Nonpoint source pollution have many sources which makes it difficult to manage. It is very difficult to control nonpoint source water pollution it comes from daily activities of different people, using pesticide, or constructing activity. The major sources of nonpoint source wastewater include nutrient loss from grassland, crop fields, road surfaces and forest. The two major nutrients phosphorus and nitrogen are of great concern. Phosphorus is main part of fertilizers used in agriculture. It is transported to aquatic water bodies through soil erosion. Nitrogen is the other crucial component of fertilizers, and it is a major pollutant of saltwater or brackish estuarine systems where nitrogen is considered as a limiting nutrient. Large amounts of nitrogen causes eutrophication and algal blooms
The major sources of water pollution are described below.
5.2.1.1 Sewage (Wastewater)
Sewage refers to the wastewater generated from domestic and industrial processes. Sewage disposal is a major concern in developing countries because many people in such countries don’t have access to hygienic sanitary conditions and clean water (Kiguchi et al. 2016; Nazeer et al. 2016; Shrivastava et al. 2020). Untreated sewage water can contaminate the environment thus, causing diseases like diarrhoea. Sewage treatment is done in water treatment plants and is often disposed in the sea. Sewage is biodegradable, and it is easily broken down in the environment.
5.2.1.2 Agricultural Pollution
The agriculture sector contribute a major portion of water pollution. Agricultural processes like spreading of manure and slurries, tillage, ploughing on land, pesticide and fertilizers use on land causes water pollution (Evans et al. 2019; Logan 1993).
5.2.1.3 Oil Pollution
Oil spillages severely affects the quality of water in many ways. Oil makes drinking water unsafe for drinking. Large amount of oil released into the oceans and seas can destroy aquatic life and ecosystems that sustain them (Hernández Ruiz et al. 2021; Ukhurebor et al. 2021). Oil spills reduces oxygen in the water environment. The main reason for oil related water pollution are:
-
Leakage from storage facilities
-
Spillage during oil delivery
-
Disposal of waste oil into drainage systems
5.2.1.4 Radioactive Substances
Radioactive waste is also among one of the source of water pollution. Radioactive substances are mostly used in nuclear power plants. They are also used medical, industrial and other scientific processes. These substances are also used in making watches, x-ray machinery and television sets. If the radioactive substance are not properly disposed of, they could result in severe water pollution incidents (Radioactive Pollution 2014).
5.2.1.5 River Dumping
Many people dump their household waste such as discarded bicycles, garden waste and electronic waste into the nearby river. River dumping is not only responsible for water pollution but it also affects wildlife and lead to occurrence of severe floods.
5.2.1.6 Marine Dumping
The Worldwide Fund for Nature (WWF) estimated that substantial amount of waste is dumped into the sea every year. The waste dumped into the sea generally include plastics and other non-degradable materials that is blown or washed from the land. These plastics get fragmented into microplastics and engulfed by the aquatic fauna which affects their metabolism severely.
5.2.2 Concerns of Water Pollution
Infectious diseases of humans are one of the most serious effect of water pollution. Developing countries are much affected by such infectious disease of water pollution because of poor sanitation practices followed by the people. Waterborne diseases affects human beings when parasites or other microorganisms responsible for causing disease are transmitted through contaminated water, mainly the water contaminated by pathogens from excreta (Some et al. 2021). Waterborne diseases include typhoid, intestinal disease, enteric and diarrheal diseases that are caused by various bacteria, viruses, and, parasites. The most severe parasitic diseases are ascariasis, amoebiasis and giardiasis. Water-based diseases are similar to waterborne diseases but they are not directly an effect of water pollution. They occurs from infectious agents that occur in water and naturally spend a part of their life cycle in water. Humans become infected when intake such water or come in contact with it (Abia et al. 2015; Akita et al. 2021; Seurinck et al. 2005; Teklehaimanot et al. 2014). Major water based disease is schistosomiasis, which currently infects many people around the world. Water pollution causes nutrient pollution which is the major, chronic environmental problem of aquatic ecosystem. Nutrients like nitrogen and phosphorus comes from agriculture, waste disposal and fossil fuel use. Once these nutrient reaches into the aquatic water bodies, they stimulates overgrowths of algae, and causes harmful algal bloom, which is very harmful and have direct toxic effects on aquatic flora and fauna. It causes depletion of oxygen in the water body. Zooplankton engulfs the toxic algae and passes these toxins up the food chain, thus, affecting seabirds, marine animals and humans. This results in illness and sometimes proves to be fatal.
Some other sources of pollution like sewage, industrial waste, chemicals, oil spills destroys the marine as well as terrestrial biodiversity. A new emerging threat is hormone-disrupting chemicals. The effects of hormone-disrupting chemicals include thyroid system disorders; reduced immune response; inability to breed and abnormal mating and parenting behaviour. In humans, endocrine disruptors lead to weaken immune function, mental impairment, decreased fertility and increases in some types of cancers.
5.3 Wastewater Remediation: Existing Technologies and Recent Advances
The choice of technology for the treatment of wastewater depends upon its nature. One of the primary reason behind the development of new technology for wastewater treatment is heavy fines which are levied on the industries and factories for the disposal of wastewater that does not meet the standard limits. This has negative impact on the financial aspect of industries that fuelled the need for new and improved wastewater treatment technologies. Some of the commonly used technologies are explained below.
5.3.1 Membrane Technology
Membrane technology (MT) utilizes the scientific as well as engineering approach for the transport of components, through the membranes. This technique is basically adopted for the mechanical separation of gaseous or liquid streams. Membranes are the thin layer barrier for the separation of substances having different size (Santoro et al. 2021). This technology is then combined with chemical and biological treatment processes. In a typical membrane technology, the driving force is a semi-permeable barrier that controls the movement rate of substances by fractional permeation and rejection through pores of various sizes. The permeation and selective rejection depends upon the membrane pore size and chemical affinity that allows for a product stream containing no target components. The major advantages for the usage of membrane technology in wastewater treatment are as follows:
-
1.
It is energy saving and clean technology.
-
2.
It has tendency to replace conventional process of treatment.
-
3.
It has flexible system design
-
4.
It is easier to use
This technology is used in many industries, pharmaceutical process, metallurgical process including treatment of water for domestic use, biological, chemical and in various separation processes.
5.3.2 Microalgal Wastewater Treatment (MWWT)
Phytoremediation is an eco-friendly technique to remove residual pollutants from wastewater and make it potent for re-introduction in the water supply system. The microalgae-based wastewater treatment system has been used for domestic, agricultural, commercial and industrial wastewater. The advantages of microalgae-based wastewater treatment system are as follows:
-
1.
reduces pollutants and pathogens
-
2.
recover nutrients as biomass
-
3.
energy savings
-
4.
mitigate CO2 gas emissions
-
5.
recovery of metabolites
Microalgae-based wastewater treatment technology has many advantages but it also have some challenges. These include requirement of land, environmental and operational condition influence, harvesting of biomass and valourization. Some other limitations are algae biomass separation of algae biomass from water, and less ability of the algae biomass to reduce and remove micropollutant content in wastewater discourages the use of technology at large scale (Abdel-Raouf et al. 2012; Alcántara et al. 2015; Molazadeh et al. 2019).
5.3.3 Microbial Fuel Cells for Wastewater Treatment
Microbial fuel cells are considered as the sustainable way of managing the increasing energy demands for wastewater treatment. It is the promising technique to deal with environmental pollution (Guo et al. 2020). An MFC is basically a device which converts organic matter into electricity by utilizing microbes as biocatalyst. Generally, MFCs consists of three main components: electrodes, electrogens and separator. MFCs have two electrodes, which, can be separated either into one or two chambers. These chambers are known to operate as mixed reactors. Below, each electrode a proton exchange membrane (PEM) or the cation exchange membrane (CEM) is placed. The anode faces towards the chamber that contains the liquid phase, whereas cathode faces towards the chamber that is contained with air. Cation exchange membrane or a salt bridge is used as a separator to keep the chamber. The potential difference which develops between the two chambers make electrons move through the circuit and the microbial degradation of wastewater acts as the substrate to produce bioelectricity. Use of MFCs in treatment of wastewater include a number of advantages like use of renewable resources, long-term sustainability, bio-hydrogen production, degradation of organic and inorganic waste and removal of certain compounds like nitrates (Gude 2016).
5.4 Nanoparticles as a Promising Tool of Remediation of Wastewater
Clean water is a basic need for human and for multi-dimensional development of society and emerging economy. Rise of population, expansion of industries, extensive agriculture practices and urbanization resulted in wastewater generation. This has made water polluted and deadly for drinking purpose. Many people die due to diseases through consumption of contaminated water. Even though several methods are available for wastewater treatment but their use is limited due to number of limitations due to harmful chemicals used, formation of disinfection by-products (DBPs), consumption of time and costly approach (Khin et al. 2012; Shankar and Nill 2015). Nanotechnology is a science of manipulating matter at molecular or atomic level to design new structures and devices that possess excellent electronic, conductive, optical, magnetic and mechanical properties. It is gaining importance as a promising technology, that has shown remarkable results in various areas including wastewater treatment. Nanomaterials possess high surface to volume ratio, a high reactivity and sensitivity, high capacity of adsorption and easy functionalization that makes them appropriate for usage in wastewater treatment (Santoro et al. 2021).
5.5 Nanoparticles: Types, Properties and Synthesis
Nanoparticles are materials which have size ranging from 1 to 100 nm. They are classified into various categories based on their properties, sizes or shapes. The different groups of nanoparticles include fullerenes, metallic nanoparticles, ceramic N nanoparticles and polymeric nanoparticles. Nanoparticles have unique chemical and physical properties due to large surface area and its nanoscale size (Kshtriya et al. 2021; Shukla et al. 2021). The optical properties of nanoparticle are dependent on size, which gives different colour because of absorption in visible region. Their properties like reactivity, toughness are dependent on their size, shape and structure. Due to such character, they are suitable for many commercial and domestic usage, that include catalysis, imaging, energy-based research, medical-based applications and in environmental applications. Heavy metal nanoparticles of lead and mercury have been reported to be very rigid and stable. Their degradation is not easily achieved, and it can lead to several environmental toxicities.
5.5.1 Types of Nanoparticles
Nanoparticles are divided into many categories based on their size, morphology and chemical properties. On the basis of physical and chemical character, some of the popularly known classes of NPs are as follows:
5.5.1.1 Carbon-Based Nanoparticles
Fullerenes and carbon nanotubes are the two main classes of carbon-based NPs. Fullerenes consists of nanomaterial made up of globular hollow cage of allotropic forms of carbon. They are used frequently in various applications due to good electrical conductivity, structure, electron affinity, high strength and versatility (Kokorina et al. 2020; Maiti et al. 2019; Patel et al. 2019). These nanoparticles are elongated and tubular in structure, of 1–2 nm in diameter. They are synthesized by the deposition of carbon precursors mainly the atomic carbons, which are vapourized from graphite by laser or by an electric arc on metal particles. Due to their unique properties, they are not only used in pristine form but also they are used in nanocomposites for number of commercial applications like fillers, as an efficient gas adsorbents for remediation of environment, and as a support medium for many inorganic and organic catalysts.
5.5.1.2 Metal Nanoparticles
Metal nanoparticles are made up of metals precursors. Due to its localized surface plasmon resonance (LSPR) character, such nanoparticles shows unique optoelectrical properties. Nanoparticles of the alkali and noble metals like copper, silver and gold shows broad absorption band in visible zone of electromagnetic solar spectrum. Due to their excellent optical properties, metal nanoparticles have many applications in research areas (Luong et al. 2011; Shnoudeh et al. 2019; Vanden Bout 2002). Gold nanoparticles coating is used for the sampling in scanning electron microscopy (SEM), to increase the electronic stream, that helps in getting high quality SEM images.
5.5.1.3 Ceramics Nanoparticles
Ceramics nanoparticles are the inorganic non-metallic solids, that are synthesized via heat and successive cooling. They are commonly found in polycrystalline, amorphous, dense, hollow forms or porous. These NPs are used frequently in several applications such as catalysis, photodegradation of dyes, photocatalysis and imaging applications (De Guire et al. 2006; Thomas et al. 2015).
5.5.1.4 Semiconductor Nanoparticles
Semiconductor materials shows properties between metals and nonmetals due to which they are used in various applications. Semiconductor nanoparticles have wide bandgaps and upon bandgap tuning they show significant alterations in properties. They are considered as an important materials for photo optics, photocatalysis and electronic devices (Nayak et al. 2017; Uchida and Matsui 2001; Wang et al. 2003). Many semiconductor nanoparticles are efficient in water splitting applications, due to suitable bandgap and band edge positions.
5.5.1.5 Polymeric Nanoparticles
These are organic based nanoparticles. They are nanospheres or nano-capsular shaped. In nanosphere shaped polymeric nanoparticles, matrix particles are generally solid and the other molecules are adsorbed over the outer boundary of the spherical surface (Zielińska et al. 2020). In nano-capsular shaped polymeric nanoparticles, the solid mass is encapsulated completely within the particle. The polymeric nanoparticles are used in numerous applications.
5.5.1.6 Lipid-Based Nanoparticles
These nanoparticles contains lipid moieties. They are used effectively in many biomedical applications. Generally, a lipid nanoparticles is spherical having diameter that range from 10 to 1000 nm. Lipid nanoparticles contains a solid core made up of lipid and a matrix that possess soluble lipophilic molecules (García-Pinel et al. 2019).
5.5.2 Synthesis of Nanoparticles
Various methods have been used for the synthesis of nanoparticles (Fig. 5.1). These methods are classified into two main classes.
-
1.
Bottom-up approach, and
-
2.
Top-down approach
These approaches are divide into various subclasses on the basis of operation, reaction condition and protocols adopted.
5.5.2.1 Top-Down Syntheses
This method utilizes destructive approach. A larger molecule is decomposed into smaller molecules, and then these molecules are converted into nanoparticles. Examples for this method include grinding/milling, physical vapour deposition (PVD) (Aryal et al. 2019; Baig et al. 2021). This approach is also used in synthesizing coconut shell nanoparticles. The colloidal carbon spherical particles with control size are also synthesized from simple top-down approach. The technique of synthesis is based on the continuous adsorption of polyoxometalates (POM) chemically on the carbon interfacial surface. Adsorption transformed the carbon black aggregates into smaller spherical particles. Micrographs revealed that the size of carbon particles reduced with sonication time. Transition-metal dichalcogenide nanodots (TMD-NDs) are also synthesized by top-down techniques using their bulk crystals.
5.5.2.2 Bottom-Up Approach
This approach is in reverse of top-down syntheses as nanoparticles are formed of simpler substances. So, this approach is also known as building up approach. Examples of bottom-up approach are reduction and sedimentation techniques. It includes sol-gel, green synthesis, spinning and biochemical synthesis (Ramanathan et al. 2021). Through this method, TiO2 anatase nanoparticles are synthesized with graphene domains. In TiO2 nanoparticles, titanium isopropoxide and alizarin precursors are used to synthesize the photoactive composite for the degradation of methylene blue photocatalytically. Alizarin is used because it provide strong binding capacity with TiO2 nanoparticles through the axial hydroxyl terminal groups.
5.5.3 Physicochemical Properties of Nanoparticles
Unique properties of nanoparticles such as large surface area, optically active, mechanically strong and chemically reactive make makes it a suitable material for various applications. Some of the important properties of nanoparticles are discussed below.
5.5.3.1 Magnetic Properties
Magnetic property of nanoparticles are of great importance as they are used in number of disciplines. It is used in heterogeneous and homogenous catalysis, magnetic fluids, biomedicine, magnetic resonance imaging (MRI) and in environmental remediation for water decontamination (Akbarzadeh et al. 2012; Issa et al. 2013).
5.5.3.2 Electronic and Optical Properties
The electronic and optical properties of nanoparticles are interdependent. For example, noble metals nanoparticles have optical properties dependent on size and shows a strong UV–visible extinction band which is not found in the spectrum of bulk metal (Asha and Narain 2020; McConnell et al. 2000). This excitation band is seen when incident frequency of photon is constant with the excitation of the conduction electrons and is called as localized surface plasma resonance (LSPR) (Kumbhakar et al. 2014; Zhang 2009). It is well-known that peak wavelength of LSPR depends upon the shape, size, interparticle spacing of nanoparticles, its own dielectric properties and its local environment which includes adsorbates, substrate and solvents. Gold colloidal nanoparticles are responsible for the rusty colours seen in blemished glass door and windows, while silver nanoparticles are yellow. These free electrons over the surface nanoparticles (d electrons in silver and gold) are freely transportable through nanomaterial.
5.5.3.3 Mechanical Properties
Due to distinct mechanical properties nanoparticles, they are used in many important fields like surface engineering, nanomanufacturing and nanofabrication (Reghunadhan et al., 2018; Wu et al. 2020). Different mechanical parameters studied to know the mechanical nature of nanoparticle. Beside such parameters coagulation, surface coating and lubrication are also the mechanical properties of nanoparticle. Nanoparticles show different mechanical properties when compared to microparticles. Good command over the mechanical features of nanoparticles and their interactions with any type of surface are important for enhancing the surface quality and increasing material removal. Fruitful outcomes in these fields generally need a deep insight into the basics of the Some of the mechanical properties of nanoparticles like elastic modulus, hardness, friction and interfacial adhesion, movement law and their size-dependent characteristics (Guo et al. 2014; Wu et al. 2020).
5.5.3.4 Thermal Properties
Metals nanoparticles have thermal conductivities higher than fluids in solid form, for e.g. thermal conductivity of copper is about 700 times higher than that of water at room temperature and about 3000 times higher than that of engine oil (Das et al. 2020; Savage and Rao 2004). Oxides like alumina (Al2O3) have thermal conductivity greater than that of water. Thus, the fluids that contain suspended solid particles display significantly higher thermal conductivities compared to conventional heat transfer fluids. Nanofluids are known to exhibit excellent properties. Nanofluids are formed by dispersing the nanometric solid particles in the liquid like water, oils or ethylene glycol. Recently, it has been examined that the nanofluids containing CuO or Al2O3 nanoparticles in ethylene or water exhibit greater thermal conductivity.
5.6 Microbial Synthesis of Nanoparticle
Number of bacteria, fungi and algae are well-known to produce nanoparticles (Fig 5.2). These nanoparticles of microbial origin are then utilized in number of applications. Some of the important bacteria, fungi and algae for the synthesis of nanoparticles are discussed below.
5.6.1 Synthesis of Nanoparticle by Bacteria
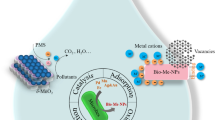
The bacteria are very well-known as for the generation of nanoparticles like gold, platinum, silver, titanium, palladium, magnetite cadmium sulphide and titanium dioxide. Bacteria are the important microbes for synthesis of nanoparticles due to their characteristic feature of getting adapted to adverse environmental conditions. It has been reported in some study that some bacteria are known to reduce or precipitate the soluble inorganic toxic ions into nontoxic metal nanoparticles which are insoluble. Bacteria forms nanoparticles either intracellularly or extracellularly under certain physicochemical conditions such as exposure period, temperature, pH, metal salts and concentration of bacteria. The biomolecules which are present in medium have tendency to reduce the metal ions in extracellular process. But, in intracellular process, the functional groups found on the cell wall attracts the metal, metalloids. Metal ions interacts with proteins that are present inside the cells for producing nanoparticles. Extracellular reduction is more favourable as compared to the intracellular reduction because the extraction procedure is easy and efficiency is high. Fang et al. (2019) revealed that dead bacteria can also be used to synthesize the nanoparticles same as living bacteria. Bacteria can also be used as biocatalyst because they act as biological platform for the mineralization process (Iqtedar et al. 2019). The bacteria mobilizes or immobilizes the metals and reduces or precipitate the metal ions. Bacteria catalyses different reactions due to the presence of various enzymes and produces inorganic nanoparticles. The large amount of nanoparticles can be produced via extracellular secretion of bacterial enzymes in a pure form. The bacterial cells possess metal binding capacity and the presence of S-layer makes them useful for their use in bioremediation. The bacterial cell wall plays important function as metals could percolate in the cytoplasm through cell wall, and it is transferred back to wall for the extracellular secretion. The cell wall of bacteria with metal binding sites could be changed by chemical reactions for some specific groups, like amines and carboxyl groups, that converts positive charge into negative charge. Use of bacteria for production of nanoparticles is profitable process as it do not require any costly and toxic chemicals for the synthesis and stabilization processes.
5.6.2 Synthesis of Nanoparticle by Fungi
The filamentous fungi are used as a potent source for synthesizing nanoparticles. The fungus mycelium has high surface area which is known to secrete large amount of proteins which can participate directly in production of nanoparticles. Nanoparticle production by filamentous fungi is considered to be better due to the capacity of fungi to secrete, enzymes, proteins and metabolites, economic feasibility, simple scaling up and downstream handling, higher surface area due to occurrence of mycelia and low-cost input for production procedures. Some filamentous fungi species are known to grow fast and their maintenance is easy at laboratory conditions (Fouda et al. 2018). The nanoparticles production having nanoscale dimension via fungi shows more monodispersity when compared to those synthesized from bacteria. Fusarium oxysporum produces gold nanoparticles in presence of aqueous AuCl4 − ions with NADH-enzyme-mediated reaction that releases reducing agents in the solution and forms gold nanoparticles. The nanoparticles synthesized show stability for long-term due to protein binding capacity by association of cysteine and lysine residues (Das et al. 2017). The filamentous fungi possess greater regenerative ability for synthesis of metal nanoparticles in good amount with its commercial feasibility. Aureobasidium pullulans, Cladosporium resinae, Aspergillus nigerPenicillium species, Ganoderma lucidum, Funalia trogii, Trametes versicolor and Rhizopus arrhizus have been studied to absorb heavy metals from polluted sites which was the used for production of nanoparticles. Salvadori et al. (2013) have studied the uptake of Cu(II) by Hypocrea lixii dead biomass and then produced copper nanoparticles. The same fungus was also able to produce NiO nanoparticles by extra and intracellularly both. Fusarium oxysporum showed extracellular synthesis of gold and silver nanoparticles when treated with the equimolar mixture of silver nitrate and tetrachloroaurate ion (Senapati et al. 2005). In the presence of hexachloroplatinic acid, it produces platinum nanoparticles (Riddin et al. 2006). Aspergillus flavus produced silver nanoparticles of 9 nm size because it reduces silver ions due to the presence of sil genes in its plasmid (Vigneshwaran et al. 2007). Salvadori et al. (2014) revealed that Aspergillus aculeatus dead biomass produced NiO nanoparticles of 5.89 nm size which were organized in the form of film. Due to the occurrence of metabolites, fungi are known as the better resource for synthesizing nanoparticles as compared to bacteria (Singh et al. 2016). Zhang et al. (2011) found that gold nanoparticles have been biosynthesised in vacuoles of filamentous fungi. They also described the functions of fungal proteins for the capping of gold nanoparticles. Filamentous fungi are the better candidate for the synthesis of metallic nanoparticles due to the occurrence of different enzymes in their cells and simple handling procsess (Khandel and Shahi 2018). Filamentous fungi also shows metal uptake capacities. It can easily be cultured in large amount by using solid substrate fermentation. Verticillium species are known to produce gold nanoparticles intracellularly when it is exposed to chloroauric acid solution. Gericke and Pinches (2006) discovered the synthesis of gold nanoparticles by fungus Verticillium luteoalbum. The age do not show any effect on the shape of gold nanoparticles but the number of nanoparticles decreased significantly when old cells are used. The biomass of Fusarium oxysporum is also used for production of silver nanoparticles (Karbasian et al. 2008). Saxena et al. (2014) studied that genetic modification procedures can be used to increase the properties of nanoparticles.
5.6.3 Synthesis of Nanoparticle by Algae
The algae are the aquatic oxygenic photoautotrophs which are used for production of nanoparticles (Castro et al., 2013). Chlorella vulgaris, Nannochloropsis oculata and Dunaliella salina show tendency to produce silver nanoparticles of size less than 15 nm inside the cells in 48 h (Mohseniazar et al. 2011). The bio reduction character of algae showed significant potential in synthesis of various metal oxide nanoparticles like gold, silver, palladium, platinum, zinc oxide and copper oxide (Momeni and Nabipour 2015). Algae produces complex inorganic nanomaterials by using both intracellular and extracellular process (Sau and Murphy 2004). The gold nanoparticles are synthesized intracellularly in Rhizoclonium fontinale and by extracellular process in Lyngbya majuscula and Spirulina subsalsa (Chakraborty et al. 2009). The metal ions can attach to the cell surface through electrostatic interactions between ions and carboxylate groups. Afterwards, ions are reduced by enzymes which cause nuclei formation and growth with reduction of metal ions (Parial et al., 2012). Accumulation of gold nanoparticles of size 9–20 nm was stated in Chlorella vulgaris dried cell suspension (Hosea et al., 1986). Cyanobacteria are also known to reduce gold (III)–chloride to metallic gold. The intermediates formed are gold (I)–sulphide and Au (I) (Lengke et al. 2006).
5.7 Mechanism of Microbial Synthesis of Nanoparticles
Microorganisms produces nanoparticles by extracellular and intracellular enzymes as defined below.
5.7.1 Extracellular Enzymes
The extracellular microbial enzymes play crucial role in production of metallic nanoparticles and work as a reducing agent in their production. (Subbaiya et al. 2017). The extracellular enzymes like acetyl xylan esterase, glucosidase and cellobiohydrolase D found in fungi play a great role in synthesizing metallic nanoparticles (Ovais et al. 2018). Rhodopseudomonas capsulata have been studied to produce gold nanoparticles through extracellular process by electron transfer to NADH-reliant reductase enzymes from NADH. After acceptance of electrons, gold ions get reduced to produce gold nanoparticles (He et al. 2007). Fusarium oxysporum was also utilized as a reducing agent for synthesizing gold and silver nanoparticles. Fusarium species are known to produce nitrate-reliant reductase and shuttle quinone which are then used for the production of nanoparticles through extracellular process (Senapati et al. 2005). Other two species of Fusarium which are Fusarium solani and Fusarium semitectum also produce silver nanoparticles through extracellular process by using their enzymes (Ingle et al. 2009). Silver nanoparticles are also synthesized through extracellular process by Coriolus versicolor and Cladosporium cladosporioides (Balaji et al., 2009). Aspergillus fumigatus produced silver nanoparticles extracellularly in in a time span of 10 min in comparison to chemical and physical techniques (Bhainsa and D’Souza 2006). Sargassum wightii has been used to reduce Au3+ ions to gold nanoparticles (Singaravelu et al. 2007). Chlorella vulgaris has been used to synthesize gold nanoparticles (Lengke et al. 2006).
5.7.2 Intracellular Enzymes
Actinomycetes like Rhodococcus and Thermomonospora, which are alkalo-thermophilic and alkalo-tolerant, are used for production of gold nanoparticles through intracellular process (Ahmad et al. 2003). Verticillium species produced silver nanoparticles when it is exposed to Ag+ ion solution through intracellular reduction. Same method was used for fabrication of gold nanoparticles using Verticillium as a good source of reducing enzymes (Ovais et al. 2018). Salvadori et al. (2017) suggested a natural method for the synthesis of metal nanoparticles intracellularly using yeasts. Possible mechanism for the production of nanoparticles intracellularly is the electrostatic interaction between amide groups and metal cations which are present in enzymes of yeast cell wall and reduction of ions by the enzymes which then results in metal ions accumulation and nanoparticles formation.
5.8 Characterization of Nanoparticles
Different techniques of characterization have been employed for the analysis of physicochemical properties of nanoparticles. It includes techniques like X-ray diffraction (XRD), infrared (IR), X-ray photoelectron spectroscopy (XPS), Brunauer–Emmett–Teller (BET), Scanning electron microscopy (SEM), transmission electron microscopy (TEM) and particle size analysis.
5.8.1 Morphological Characterizations
The morphological attributes of nanoparticles have always gained great interest as morphology always affects most of the properties of nanoparticles. There are many characterization techniques for the morphological studies, but some microscopic techniques like SEM, polarized optical microscopy (POM), and TEM are the important one. SEM is based on the principle of electron scanning, and it provides all the information available about the nanoparticles at nanoscale level. Numerous literature is available, where researchers have used this instrumentation technique to study the morphology of nanomaterials, as well as the dispersion of nanoparticles in the bulk or matrix. Similar to SEM, TEM is based on the principle of electron transmittance, so it provides information about the bulk material from lesser to higher magnification. The morphologies of gold nanoparticles have been studied through this technique. TEM provides important basic information about the layer of material, like, quadrupolar hollow shell structure of Co3O4 nanoparticle which was observed through TEM. These nanoparticles are exceptionally active as anode in Li-ion batteries (Wang et al. 2013).
5.8.2 Structural Characterizations
The structural features are of great importance in order to study the nature of bonding and composition of nanomaterials. Some of the common techniques which are utilized to study the structural properties of nanoparticles are X-Ray diffraction (XRD), XPS, energy dispersive X-ray (EDX), BET, Raman, IR and Zieta size analyzer. XRD is most important instrumentation technique that reveal the structural properties of nanoparticles. It provides enough information about crystallinity and phase of nanoparticles (Ullah et al. 2017). In case of nanoparticles having size less than hundred of atoms, the correct measurement of structure and may be difficult. Moreover, nanoparticles which have amorphous character different inter atomic lengths can affect the XRD diffractogram. EDX, which is usually attached with field emission scanning electron miscopy (FE-SEM) or TEM instrument, is used widely to know the elemental composition, and it gives a rough idea of weight percent. XPS is also considered as a most sensitive instrumentation technique to determine the exact ratio of element and exact nature of bond of the elements in nanoparticle materials. It is a surface sensitive instrumentation technique and is used for depth profiling studies to determine the overall composition and compositional variation alongwith depth. XPS is another instrumentation technique which is based on basic principles of spectroscopy and a XPS spectrum shows the binding energy (eV) of the electrons on versus X-axis number of electrons on Y-axis plot. FT-IR and Raman spectroscopies give information about the vibrational character of nanoparticles (Dablemont et al. 2008).
5.8.3 Particle Size and Surface Area Characterization
There are many techniques to determine the size of the nanoparticle which include SEM, XRD, AFM, TEM and dynamic light scattering (DLS). SEM, AFM TEM and XRD can give better idea about the particle size (Kestens et al. 2016). Zeta potential size analyser and DLS are used to find the size of nanoparticle at very low level. But, in case of hydrophilicity and agglomeration, DLS may be incapable of correct measurement, so in this case other high-resolution technique like differential centrifugal sedimentation (DCS) should be used (Sikora et al. 2016). Nanoparticle tracking analysis (NTA) is a newer technique, which helpful for dealing with biological systems like proteins, and DNA. In NTA technique, nanoparticles can be visualized and analyzed in liquids media. This technique helps us to find the size distribution profile of nanoparticles with diameter ranging between 10 and 1000 nm in a liquid medium. BET is one of the best technique to estimate the surface area of nanoparticle materials. This instrumentation technique works on adsorption and desorption principle and according to Brunauer–Emme–Teller (BET) theorem.
5.8.4 Optical Characterizations
Optical properties are important in photocatalytic applications and therefore, these characterizations works on the famous beer-lambert law and the basic principles of light. These techniques provide information about the reflectance, absorption, phosphorescence and luminescence properties of nanoparticle. It is very well-known that nanoparticle, especially metallic and semiconductor nanoparticle show different colours and thus, best used for photo-related applications (Kumbhakar et al. 2014; Uchida and Matsui 2001).
5.9 Application of Nanoparticles in Removal of Organic Pollutants from Wastewater
Natural organic matter (NOM) forms various group of hydrophobic and hydrophilic organic compounds. Its contribution is significant towards water contamination (Farrokhi et al., 2013). Number of carbon-based adsorbents are used for the removal of NOM from the wastewater and many factors affect the sorption of NOM (Farrokhi et al. 2013; Ye et al. 2019). Some of the nanomaterials used for the removal of such organic compounds are discussed below.
5.9.1 CNTs
Number of nanomaterial like nano-sorbents like carbon nanotubes (CNTs), polymeric materials, such as dendrimers, and zeolites have shown exceptional properties of adsorption and are used for removal of organic compound from wastewater (Ye et al. 2019; Zhang 2009). CNTs have gained special importance because of their exceptional water treatment capabilities. CNTs have been studied widely. Removal of NOM by using CNTs is higher when compared to carbon-based adsorbents because of large surface areas and some other factors. CNTs have also proved to be effective in removal of polycyclic aromatic organic compounds and atrazine.
5.9.2 TiO2 Nanoparticles
TiO2 and CeO2 are the common nanomaterial of metal oxides that are used as a catalysts for quick and complete degradation of organic contaminant (Farrokhi et al. 2013). TiO2 nanoparticles are used in the treatment of water which is contaminated with organic contaminants like polychlorinated biphenyls (PCBs), chlorinated alkanes and benzenes (Upadhyayula et al. 2009). The addition of TiO2 nanoparticles in the wastewater enhanced the Removal of total organic carbon. TiO2 nanoparticles have also been used in a ‘falling film’ reactor for degrading microcystins in water. Functionalization of multiwalled CNTs with Fe nanoparticles proved to be an effective sorbents for removal of aromatic compounds such as benzene and toluene.
5.9.3 Zero-Valent Iron
Catalysts such as zero-valence metal, bimetallic nanoparticles and semiconductor materials have shown their usage in degradation of environmental pollutants like PCBs, azo dyes and pesticides, due to their large surface area alongwith its shape dependent properties (Kang and Choi 2009; Li et al. 2021; Liu et al. 2021). Magnetic nano-sorbents have also been used effectively in removal of organic contaminants. Nanomaterial of iron oxide showed better capabilities of removal of organic pollutants in comparison to bulk materials. They have also been utilized for removal of coloured humic acids from wastewater.
5.9.4 Other Nanomaterials
Nanostructured ZnO semiconductor films have been used successfully for the degradation of organic contaminants like 4-chlorocatechol (Yu et al. 2015). The nanocatalyst made up of silver and amidoxime fibres have been utilized effectively for the degradation of some organic dyes. Nanocomposite of Pd- Cu/γ-alumina have also been utilized for the reduction of nitrate. Hydrogen and the palladium-based nanoparticles have been investigated to biodegrade traces of halogenated organic compounds. Palladium nanoparticles and bimetallic Pd/Au (gold) nanoparticles have been used for hydrodechlorination of trichloroethylene (TCE) (Arsiya et al. 2017). Mineralization of was accelerated in one study by using films of Hydrogen peroxide and manganese oxide (MnO2) nanoparticles has also been investigated for mineralization of organic dyes.
5.10 Application of Nanoparticles in Removal of Inorganic Pollutants from Wastewater
Nanomaterials of different types have been developed for the heavy metals removal from wastewater. Nano-sorbents like CNTs, dendrimers and zeolites have shown exceptional adsorption properties (Upadhyayula et al. 2009). The CNTs ability to adsorb heavy metals has been studied by several researchers. Composites of CNTs with iron and cerium oxide (CeO2) have been reported for the removal of heavy metal ions (Amin et al. 2014). Nanoparticles of Cerium oxide supported on CNTs have been used in effective manner to adsorb arsenic of CNTs are known to have fast adsorption kinetics due to short intraparticle diffusion distance and highly accessible adsorption sites (Fausey et al. 2019).
The nanomaterials that are metal based showed better removal of heavy metals in comparison to activated carbon. TiO2 nanoparticles and nanosized magnetite have been used for adsorption of arsenic. The usage of TiO2 nanoparticles has been examined to reduce the toxic metal ions in water. Nanocrystalline TiO2 has been effective in removal of different forms of arsenic it has been proven to be more effective photocatalyst as compared to commercially available TiO2 nanoparticles that shows maximum removal of arsenic at neutral pH. Nanocomposite of TiO2 nanoparticles attached on graphene sheet reduce Cr(VI) to Cr(III) in presence of sunlight. Chromium ions were removed using palladium nanoparticles in a study. Removal of arsenic was investigated by utilizing high specific surface area of Fe3O4 nanocrystals. Polymer-grafted Fe2O3 nanocomposite has been used effectively for the removal of divalent heavy metal ions of copper, cobalt and nickel at a pH range of 3 to 7.
For the removal of radioactive metal toxins like uranium dioxide (UO22+) from water, bisphosphonate-modified magnetite nanoparticles has been used efficiently. Zero-valent iron or iron nanoparticles (nZVI or Fe0) have shown to be effective in transformation of heavy metal ions like As(III), As(V), Cu(II), Ni(II), Pb(II) and Cr(VI) (Jézéquel and Chu, 2006).
As(V) and Cr(VI) have been shown to be successfully adsorbed by novel self-assembled 3D flower-like iron oxide nanostructures (Fausey et al., 2019). The 3D nanostructures of CeO2 also proved to be a good adsorbents for arsenic and chromium. The efficacy of NaP1 zeolites was examined for removing heavy metals like (Cr(III), Zn(II), Cu(II), Ni(II), and Cd(II)) from the wastewater stream. Dendritic polymers have also shown their capability for treatment of toxic metal ions. Biopolymers were used for remediating heavy metal from aqueous wastes. Nanoparticles of chitosan for sorption of Pb(II) were also reported. (Arsiya et al. 2017)
Nanofilters has been examined for removal of arsenic and radioactive waste from ground and surface waters (Darab et al. 2007). It has also shown its potential to remove uranium (VI) from the sea water. Novel nanofilter membranes have been prepared by assembling) negative poly (styrene sulfonate) and positive poly (allylamine hydrochloride on porous alumina. They displayed high retention of Ca2+ and Mg2+ions. A dendrimer-UF system showed the removal of Cu2+ ion from water. The addition of iron (hydr)oxide nanoparticles in the porous carbon materials has successfully removed inorganics and organics. Therefore, these filters can be used as point-of-use applications.
5.11 Application of Nanoparticles in Removal of Pathogenic Microbes from Wastewater
Biological contaminants are classified in three categories, such as, natural organic matter (NOM), microorganisms and biological toxins. Microbial contaminants contain free living microbes and human pathogens. Removal of cyanobacterial toxins is a major issue in conventional water treatment methods. Many adsorbents have shown good removal efficiencies but number of factors affects the removal process. Despite several development in disinfection technology, waterborne infections are prevalent in many areas. Therefore, advanced disinfection techniques should be used to eliminate the pathogens. There are several kind of nanomaterials like silver, zinc and titanium which are able to disinfect waterborne disease-causing microorganism. TiO2 photocatalysts and metal oxide nanoparticles are considered among the most effective nanomaterials which showed antimicrobial properties (Azizi-Lalabadi et al. 2019; Darab et al. 2007; Othman et al. 2014). The potential of metal ions for disinfection in water has been investigated by many researchers. Some of the nanoparticle used for removing microbial contamination has been discussed below.
5.11.1 Silver Nanoparticles
Silver is widely used metal because of its microbial inactivation property in water and low toxicity (Pandey et al. 2019). Silver nanoparticles are obtained from its salts like silver chloride and silver nitrate. Although, the antibacterial effect is dependent on size, smaller silver nanoparticles (8 nm) have been most effective, while larger particle size (11–23 nm) showed lower bactericidal activity. Truncated triangular silver nanoplates showed better antibacterial property in comparison to rod-shaped and spherical nanoparticles which indicate their dependency on shape. The mechanisms which is involved during bactericidal effects of silver nanoparticles include, formation of free radicals that damage the bacterial membranes, damaging DNA, alteration in the properties of the membrane by adhering to the cell surface and by damaging enzyme.
5.11.2 TiO2 Nanoparticles
TiO2 nanoparticles are the emerging and promising photocatalysts for purification of water. The basic mechanism of photocatalysts such as low-cost TiO2 that have good photoactivity and less toxicity involves production of highly reactive oxidants, like OH radicals, for disinfecting microorganisms (Desa and Kowshik 2009; Senarathna et al. 2017). TiO2 after simulated solar exposure of 8 hours has been shown to decrease the viability of many waterborne pathogens. Full inactivation of faecal coliforms in the presence of sunlight has been reported, expressing the photocatalytic disinfection potential of TiO2.
5.11.3 CNTs and Others
CNTs have shown their potential in removal of bacterial pathogens. CNTs have received special attention for their ability of removing biological contaminants from the water (Al-Jumaili et al. 2017; Kang and Choi 2009; Liu et al. 2018; Teixeira-Santos et al. 2021). CNTs have shown antimicrobial characteristics against wide range of microbes including bacteria and viruses. Adsorption of cyanobacterial toxins over CNTs is also high when compared to the carbon-based adsorbents due to large specific surface area, large composition of mesoporous volume and external diameter of CNTs.
5.12 Mechanism of Nano-Remediation of Wastewater
Nanotechnology-based pathways, which are being employed for wastewater remediation, are adsorption and biosorption, nanofiltration, photocatalysis, disinfection and pathological control, sensing and monitoring. The mechanism of the nano-remediation of wastewater is discussed below.
5.12.1 Adsorption and Biosorption
Adsorption a surface phenomenon and exothermic process, which involves process of transfer of a phase called adsorbate, on a solid surface called adsorbent to form a monomolecular layer over the surface through physicochemical or the chemical interactions under some specific conditions (Sadegh et al. 2017). Biosorption is an adsorption process in which biological substances like bacteria, fungi and algae act as an adsorbents. Owing to their intrinsic property they bind up heavy metals, from a very dilute aqueous solution or by making use of ATP or through spontaneous physicochemical pathways of uptake. The process of biosorption mainly involves microprecipitation, cell surface and complexation ion exchange.
5.12.2 Nanofiltration
Water filtration is a process of reducing the amount of particulate matter, like suspended particles, microbes and other dangerous biological and chemical pollutant from polluted water to make it safe and clean for drinking, medical applications and pharmaceutical (Abdel-Fatah 2018; Shon et al. 2013). Membrane technology has been used widely and the most important development in membrane technology is the nanofiltration (NF) membrane. NF membranes possess molecular weight cut-off (MWCO) for the uncharged particles in nanometer range. NF membranes are the recent and most preferably used technology for wastewater treatment and drinking water. Nanofilters works on pressure-driven membrane process which lies between ultrafiltration and reverse osmosis, with a pressure in the range of 5–20 bars and pore size between 0.5 and 2.0 nm. NF is widely employed techniques for wastewater treatment due to its exclusive filtration mechanism and availability of several types of membranes. NF is are the suitable technique to filter out all the organic and inorganic contaminants, including harmful microbes from the wastewater.
5.12.3 Photocatalysis
Photocatalysis is a light-induced reaction accelerated by a catalyst. Photocatalysis involves a solid material which is a photocatalyst that absorbs radiation and induces chemical reaction. Photocatalysis is considered as one of the Advanced Oxidation Processes (AOPs) which comprises of in-situ production of potent chemical oxidants with the help of Fenton’s reagent, UV light, hydrogen peroxide (H2O2), ozone or a catalyst. Photocatalysis is basically a surface phenomenon, and its mechanism involves five steps discussed below:
-
Diffusion of pollutants on the surface of photocatalyst
-
Adsorption of pollutants over the surface of photocatalyst
-
Reaction of the adsorbed pollutants
-
Desorption of the products formed, from the surface
-
Removal or diffusion of products from the interface Some of the widely used nanostructured photocatalysts are Fe2O3, TiO2, ZnO, zinc sulphide (ZnS), zirconium dioxide (ZrO2), cadmium sulphide (CdS) and tungsten trioxide (WO3) (Chimupala et al. 2020; Mustapha et al. 2020; Sagir et al. 2020).
5.12.4 Disinfection and Pathological Control
Nanomaterials possess tremendous ability to inactivate pathogens present in water, due to its large surface area and the specific reactivity. The mechanism of inactivation for the nanoparticles involves surface-based electrostatic interaction and the photochemical reactions. It induces the generation of reactive oxygen species (ROS), disruption of the cell walls and the targeted delivery of disinfecting agents. Due to excellent surface properties and reactivity of nanomaterials, pathogens inhibition in water can be done easily. Nanomaterials which are based on CuO, TiO2, silver, polymeric nanoparticles and CNTs have been examined for the disinfection of wastewater (Deshmukh et al. 2019; Teixeira-Santos et al. 2021).
5.12.5 Sensing and Monitoring
Monitoring of quality water, on large scales, is a challenging work because of low concentrations of contaminants, variability and complexity of wastewater matrices. To solve these issues, fast and effective techniques are needed to be developed. In recent past, the researchers have shown their interest towards development of sensors based on nanomaterial to monitor quality of water. Due to their excellent properties, like excellent recognition of trace pollutant, and fast analysis, nanosensors are defined as a device or material that are sensitive towards alterations in surrounding stimuli, like heat, mechanical and chemical stress, changes in volume, gravitational, concentration and the magnetic, as well as electrical forces. They are used to provide physical, chemical and biological information about behaviour and character of nanoparticle from the nanoscale level to macroscopic level (Deshmukh et al. 2019; Lee and El-Sayed 2006; Luo et al. 2019; Segev-Bar and Haick 2013; Willner and Vikesland 2018). Nanosensors contains three main parts specifically, a recognizing component like nanometals, nanowires, nanotubes, nanoparticles, etc. which is connected to a transducer (such as amperometric, spectrophotometric, conductometric) and a display for real time monitoring.
5.13 Constraints in Application of Nanoparticles in Water Remediation
Commercialization of nanomaterials for wastewater and water technology depends on their impact on aquatic ecosystem. Several studies like toxicity tests, technology assessment, life cycle analysis and dispersal of nanomaterial in aquatic water bodies have been done to evaluate the health risks caused by nanomaterials (Ray et al. 2009; Subramaniam et al. 2019). The findings of such studies have given a better understanding of behaviour of nanoparticles like CNTs, silver nanoparticles and TiO2 in aquatic system. Therefore, stakeholders from all fields have supported to form a new laws and regulations or the modification of present ones. Several studies have produced contradictory results, because no general standards for experimental procedures and measurements have been formulated, which slows the necessary process of decision. Nanomaterials that goes into water, do not affect the humans directly, but there is a possibility to uptake nanomaterials through consumption of fish. So the impact of nanoparticles on aquatic organisms should be taken seriously. Nanoparticles are emitted into the environment through various point sources like landfill sites, production units and wastewater treatment plants. Nonpoint sources, for the emission of nanoparticles include washing machines, clothes or other substances that contain nanoparticles, for example, nanosilver nanoparticles are released from clothes after washing. Nanosocks that are commercially available leach 25% of its total silver content at pH 10 within minutes. So, it is necessary to perform the research on the toxicity of nanomaterials in air, water and soil. Moreover, the impact of nanoparticles in the food chain should also be studied thoroughly as it will affect many organism.
5.14 Conclusion and Future Prospectus
The biological resources like microorganisms and their enzymes, when applied successfully, could help in synthesis of nanoparticles biologically which can be termed as a potential strategy used in an eco-friendly and eco-incentive manner. Microbial possess a significant potential for the synthesis of metal nanoparticles because they show less toxic nature alongwith high degradation capacity. It has been found that the mechanism of biologically synthesized nanoparticles are not clear, despite knowing that the stable nanoparticles are generated by selecting appropriate microorganisms and optimizing conditions. Therefore, there is a requirement of choosing appropriate microorganism or microbial consortia for sustainable production of nanoparticles on large-scale or commercial scale. Synthesis of nanoparticles by microorganism can be obtained without the use of pressure, high temperature, stabilizers, energy and toxic chemicals. More research is required to synthesize nanoparticles having wide range of organic functional groups by using microbial enzymes for selective and multi-pollutants removal from the wastewater. The synthesis of nanoparticles through microbes like bacteria, fungi, actinomycetes, yeast and algae has number of benefits like easy production, high efficiency, non-expensive and eco-friendly approach. The nanoparticles obtained from microorganism can be utilized at contaminated sites for the treatment of various pollutants. The residues which are left after degradation of pollutants by nanoparticles produced microbially are biocompatible in nature and can be separated by filtration or by using precipitation technique. The value-added products like construction material can be prepared using left residues by adding biochar, thus, there will be no waste at the end. Synthesis of nanoparticle opens a new channels for number of biotechnological applications using greener path. Bio synthesis of metallic nanoparticles has been done at laboratory scale but its production at industrial scale still needs to be investigated for the mass production. The use of efficient microorganism for nanotechnology can enhance the industrial economy at commercial level but unfortunately only 1% of the nanotechnological material have been commercialized. The cost-incentive and cost-effective production of nanoparticles from microbes is needed to make this procedure economically viable and sustainable. The cost-benefit analysis also needs to be conducted for the commercial exploitation of the usage of microbial nanoparticles because no cost related data is available yet. Utilization of salts, chemicals, stabilizing and reducing agents in chemical synthesis method are costly whereas in microbial process of synthesizing nanoparticles, the usage of metal salts as well as media for growth of microorganism is also expensive. The waste biomass that can be recycled can be prove to be an alternative for producing nanoparticles to reduce the expenditure. Research investigations about biosynthetic pathways of microorganism and research in genetic engineering may pave the way for breakthrough development of nanomaterials for their industrial exploitation as efficient and sustainable strategies in the field of bioremediation. Advanced tools on computation is required to use the omics derived data for good understanding of microbial processes. Thus, green chemistry can be applied successfully for the production of nanoparticles by microorganism and research efforts in this direction can be a giant step for the adoption of green nanotechnology.
References
Abdel-Fatah MA (2018) Nanofiltration systems and applications in wastewater treatment: Review article. Ain Shams Eng J 9:3077–3092. https://doi.org/10.1016/j.asej.2018.08.001
Abdel-Raouf N, Al-Homaidan AA, Ibraheem IBM (2012) Microalgae and wastewater treatment. Saudi J Biol Sci 19:257–275. https://doi.org/10.1016/j.sjbs.2012.04.005
Abia ALK, Ubomba-Jaswa E, du Preez M, Momba MNB (2015) Riverbed sediments in the Apies River, South Africa: recommending the use of both Clostridium perfringens and Escherichia coli as indicators of faecal pollution. J Soils Sed 15:2412–2424. https://doi.org/10.1007/s11368-015-1209-0
Aghalari Z, Dahms H-U, Sillanpää M, Sosa-Hernandez JE, Parra-Saldívar R (2020) Effectiveness of wastewater treatment systems in removing microbial agents: a systematic review. Glob Health 16:13. https://doi.org/10.1186/s12992-020-0546-y
Ahmad A, Senapati S, Kumar R, Ramani R, Srinivas V, Sastry M (2003) Intracellular synthesis of gold nanoparticles by a novel alkalotolerant actinomycetes, Rhodococcus Species. Nanotechnology 14:824
Akbarzadeh A, Davaran SM (2012) Magnetic nanoparticles: preparation, physical properties, and applications in biomedicine. Nanoscale Res Lett 7:144. https://doi.org/10.1186/1556-276X-7-144
Akita LG, Laudien J, Biney C, Akrong MO (2021) A baseline study of spatial variability of bacteria (total coliform, E. coli, and Enterococcus spp.) as biomarkers of pollution in ten tropical Atlantic beaches: concern for environmental and public health. Environ Sci Pollut Res https://doi.org/10.1007/s11356-021-15432-x
Al-Jumaili A, Alancherry S, Bazaka K, Jacob M (2017) Review on the antimicrobial properties of carbon nanostructures. Materials 10:1066. https://doi.org/10.3390/ma10091066
Alcántara C, Posadas E, Guieysse B, Muñoz (2015) Microalgae-based wastewater treatment. In: Handbook of marine microalgae. Elsevier, pp 439–455. https://doi.org/10.1016/B978-0-12-800776-1.00029-7
Amin MT, Alazba AA, Manzoor U (2014) A Review of Removal of pollutants from water/wastewater using different types of nanomaterials. Adv Mater Sci Eng 2014:1–24. https://doi.org/10.1155/2014/825910
Anderson BA, Romani JH, Phillips H, Wentzel M, Tlabela K (2007) Exploring environmental perceptions, behaviors and awareness: waterland water pollution in South Africa. Popul Environ 28:133–161. https://doi.org/10.1007/s11111-007-0038-5
Aragaw TA, Bogale FM, Aragaw BA (2021) Iron-based nanoparticles in wastewater treatment: a review on synthesis methods, applications, and removal mechanisms. J Saudi Chem Soc 25:101280. https://doi.org/10.1016/j.jscs.2021.101280
Arsiya F, Sayadi M, Sobhani S (2017) Arsenic (III) adsorption using palladium nanoparticles from aqueous solution. J Water Environ Nanotechnol 2. https://doi.org/10.22090/jwent.2017.03.004
Aryal S, Park H, Leary JF, Key J (2019) Top-down fabrication-based nano/microparticles for molecular imaging and drug delivery. Int J Nanomed 14:6631–6644. https://doi.org/10.2147/IJN.S212037
Asha, AB, Narain R (2020) Nanomaterials properties. In: Polymer science and nanotechnology. Elsevier, pp 343–359. https://doi.org/10.1016/B978-0-12-816806-6.00015-7
Azizi-Lalabadi M, Ehsani A, Divband B, Alizadeh-Sani M (2019) Antimicrobial activity of Titanium dioxide and Zinc oxide nanoparticles supported in 4A zeolite and evaluation the morphological characteristic. Sci Rep 9:17439. https://doi.org/10.1038/s41598-019-54025-0
Baig N, Kammakakam I, Falath W (2021) Nanomaterials: a review of synthesis methods, properties, recent progress, and challenges. Mater Adv 2:1821–1871. https://doi.org/10.1039/D0MA00807A
Balaji D, Basavaraja S, Deshpande R, Mahesh DB, Prabhakar B, Venkataraman A (2009) Extracellular biosynthesis of functionalized silver nanoparticles by strains of Cladosporium cladosporioides fungus. Colloids Surf B 68:88–92
Bhainsa KC, D’Souza S (2006) Extracellular biosynthesis of silver nanoparticles using the fungus Aspergillus fumigatus. Colloids Surf B 47:160–164
Bhat SA, Cui G, Li W. Wei Y, Li F (2020) Effect of heavy metals on the performance and bacterial profiles of activated sludge in a semi-continuous reactor. Chemosphere 241:125035. https://doi.org/10.1016/j.chemosphere.2019.125035
Cassivi A, Tilley E, Waygood EOD, Dorea C (2021) Evaluating self-reported measures and alternatives to monitor access to drinking water: a case study in Malawi. Sci Total Environ 750:141516. https://doi.org/10.1016/j.scitotenv.2020.141516
Castro L, Blazquez ML, Munoz JA, Gonzalez F, Ballester A (2013) Biological synthesis of metallic nanoparticles using algae. Nanobiotechnol. IET 7:109–116
Chakraborty N, Banerjee A, Lahiri S, Panda A, Ghosh AN, Pal R (2009) Biorecovery of gold using cyanobacteria and an eukaryotic alga with special reference to nanogold formation-a novel phenomenon. J Appl Phycol 21:145–152
Chatterjee S, Mitra A, Datta S, Veer V (2013) Phytoremediation protocols: an overview. In: Gupta DK (ed) Plant-based remediation processes, soil biology. Springer Berlin Heidelberg, Berlin, Heidelberg, pp 1–18. https://doi.org/10.1007/978-3-642-35564-6_1
Chen L, Deng Y, Dong S, Wang H, Li P, Zhang H, Chu W (2021) The occurrence and control of waterborne viruses in drinking water treatment: a review. Chemosphere 281:130728. https://doi.org/10.1016/j.chemosphere.2021.130728
Chimupala Y, Phromma C, Yimklan S, Semakul N, Ruankham P (2020) Dye wastewater treatment enabled by piezo-enhanced photocatalysis of single-component ZnO nanoparticles. RSC Adv 10:28567–28575. https://doi.org/10.1039/D0RA04746E
Dablemont C, Lang P, Mangeney C, Piquemal J-Y, Petkov V, Herbst F, Viau G (2008) FTIR and XPS study of Pt nanoparticle functionalization and interaction with alumina. Langmuir 24:5832–5841
Darab JG, Amonette AB, Burke DSD, Orr RD, Ponder SM, Schrick B, Mallouk TE, Lukens WW, Caulder DL, Shuh DK (2007) Removal of pertechnetate from simulated nuclear waste streams using supported zerovalent iron. Chem Mater 19:5703–5713. https://doi.org/10.1021/cm0607379
Das RK, Pachapur VL, Lonappan L (2017) Biological synthesis of metallic nanoparticles: plants, animals and microbial aspects. Nanotechnol Environ Eng 2:18. https://doi.org/10.1007/s41204-017-0029-4
Das L, Habib K, Saidur R, Aslfattahi N, Yahya SM, Rubbi F (2020) Improved thermophysical properties and energy efficiency of aqueous ionic liquid/MXene nanofluid in a hybrid PV/T solar system. Nanomaterials 10:1372. https://doi.org/10.3390/nano10071372
De Guire MR, Hu MZ, Gogotsi Y, Lu SW (eds) (2006) Ceramic nanomaterials and nanotechnology II: De Guire/Ceramic. Wiley, Hoboken, NJ, USA. https://doi.org/10.1002/9781118406083
Desa VS, Kowshik M (2009) Antimicrobial activity of titanium dioxide nanoparticles synthesized by sol-gel technique. Res J Microbio 4:97–103. https://doi.org/10.3923/jm.2009.97.103
Deshmukh SP, Patil SM Mullani SB, Delekar SD (2019) Silver nanoparticles as an effective disinfectant: a review. Mater Sci Eng C 97:954–965. https://doi.org/10.1016/j.msec.2018.12.102
El-Gendy NSh, Nassar HN (2021) Biosynthesized magnetite nanoparticles as an environmental opulence and sustainable wastewater treatment. Sci Total Environ 774:145610. https://doi.org/10.1016/j.scitotenv.2021.145610
Evans AE, Mateo-Sagasta J, Qadir M, Boelee E, Ippolito A (2019) Agricultural water pollution: key knowledge gaps and research needs. Curr Opin Environ Sustain 36:20–27. https://doi.org/10.1016/j.cosust.2018.10.003
Fang X, Wang Y, Wang Z, Jiang Z, Dong M (2019) Microorganism assisted synthesized nanoparticles for catalytic applications. Energies 12:190
Farrokhi M, Yang J-K, Lee S-M, Shirzad-Siboni M (2013) Effect of organic matter on cyanide removal by illuminated titanium dioxide or zinc oxide nanoparticles. J Environ Health Sci Eng 11:23. https://doi.org/10.1186/2052-336X-11-23
Fausey CL, Zucker I, Shaulsky E, Zimmerman JB, Elimelech M (2019) Removal of arsenic with reduced graphene oxide-TiO2-enabled nanofibrous mats. Chem Eng J 375:122040. https://doi.org/10.1016/j.cej.2019.122040
Fouda A, Saad E, Salem SS, Shaheen TI (2018) In-vitro cytotoxicity, antibacterial, and UV protection properties of the biosynthesized zinc oxide nanoparticles for medical textile applications. Microb Pathog 125:252–261
Ganachari SV, Banapurmath NR, Salimath B, Yaradoddi JS, Shettar AS, Hunashyal AM et al (2017) Synthesis techniques for preparation of nanomaterials. In: Martínez LMT, Kharissova OV, Kharisov BI (eds) Handbook of ecomaterials. Springer, Cham, pp 1–21
García-Pinel B, Porras-Alcalá C, Ortega-Rodríguez A, Sarabia F, Prados J, Melguizo C, López-Romero JM (2019) Lipid-based nanoparticles: application and recent advances in cancer treatment. Nanomaterials 9:638. https://doi.org/10.3390/nano9040638
Gehrke I, Geiser A, Somborn-Schulz A (2015) Innovations in nanotechnology for water treatment. Nanotechnol. Sci Appl l. https://doi.org/10.2147/NSA.S43773
Gude VG (2016) Microbial fuel cells for wastewater treatment and energy generation. In: Microbial electrochemical and fuel cells. Elsevier, pp 247–285. https://doi.org/10.1016/B978-1-78242-375-1.00008-3
Guo D, Xie G, Luo J (2014) Mechanical properties of nanoparticles: basics and applications. J Phys Appl Phys 47:013001. https://doi.org/10.1088/0022-3727/47/1/013001
Guo Y, Wang J, Shinde S, Wang X, Li Y, Dai Y, Ren J, Zhang P, Liu X (2020) Simultaneous wastewater treatment and energy harvesting in microbial fuel cells: an update on the biocatalysts. RSC Adv 10:25874–25887. https://doi.org/10.1039/D0RA05234E
He S, Guo Z, Zhang S, Wang J, Gu N (2007) Bio-synthesis of gold nanoparticles using the bacteria Rhodopseudomonas capsulata. Mater Lett 61:3984–3987
Hernández Ruiz L, Ekumah B, Asiedu DA, Albani G, Acheampong E, Jónasdóttir SH, Koski M, Nielsen TG (2021) Climate change and oil pollution: a dangerous cocktail for tropical zooplankton. Aquat Toxicol 231:105718. https://doi.org/10.1016/j.aquatox.2020.105718
Hosea M, Greene B, McPherson R, Henzl M, Dale Alexander M, Darnall DW (1986) Accumulation of elemental gold on the alga Chlorella vulgaris. Inorg Chim Acta 123:161–165
Ingle A, Rai M, Gade A, Bawaskar M (2009) Fusarium solani: a novel biological agent for the extracellular synthesis of silver nanoparticles. J Nanopart Res 11:2079
Iqtedar M, Aslam M, Akhyar M, Shehzaad A, Abdullah R, Kaleem A (2019) Extracellular biosynthesis, characterization, optimization of silver nanoparticles using Bacillus mojavensis BTCB15 and its antimicrobial activity against multidrug resistant pathogens. Prep Biochem Biotechnol 49:136–142
Issa B, Obaidat I, Albiss B, Haik Y (2013) Magnetic nanoparticles: surface effects and properties related to biomedicine applications. Int J Mol Sci 14:21266–21305. https://doi.org/10.3390/ijms141121266
Jézéquel H, Chu KH (2006) Removal of arsenate from aqueous solution by adsorption onto titanium dioxide nanoparticles. J Environ Sci Health Part A 41:1519–1528. https://doi.org/10.1080/10934520600754201
Kang SH, Choi W (2009) Oxidative degradation of organic compounds using zero-valent iron in the presence of natural organic matter serving as an electron shuttle. Environ Sci Technol 43:878–883. https://doi.org/10.1021/es801705f
Karbasian M, Atyabi S, Siadat S, Momen S, Norouzian D (2008) Optimizing nano-silver formation by Fusarium oxysporum PTCC 5115 employing response surface methodology. Am J Agric Biol Sci 3:433–437
Kestens V, Roebben G, Herrmann J, Jämting Å, Coleman V, Minelli C, Clifford C, De Temmerman P-J, Mast J, Junjie L, Babick F, Cölfen H, Emons H (2016) Challenges in the size analysis of a silica nanoparticle mixture as candidate certified reference material. J Nanopart Res 18:171
Khandel P, Shahi SK (2018) Mycogenic nanoparticles and their bio-prospective applications: current status and future challenges. J Nanostruct Chem 8:369–391
Khan R, Israili SH, Ahmad H, Mohan A (2005) Heavy metal pollution assessment in surface water bodies and its suitability for irrigation around the Neyveli Lignite Mines and associated industrial complex, Tamil Nadu, India. Mine Water Environ 24:155–161. https://doi.org/10.1007/s10230-005-0087-x
Khin MM, Nair AS, Babu VJ, Murugan R, Ramakrishna S (2012) A review on nanomaterials for environmental remediation. Energy Environ Sci 5:8075. https://doi.org/10.1039/c2ee21818f
Kiguchi O, Sato G, Kobayashi T (2016) Source-specific sewage pollution detection in urban river waters using pharmaceuticals and personal care products as molecular indicators. Environ Sci Pollut Res 23:22513–22529. https://doi.org/10.1007/s11356-016-7437-z
Kokorina AA, Ermakov AV, Abramova AM, Goryacheva IYu, Sukhorukov GB (2020) Carbon nanoparticles and materials on their basis. Colloids Interfaces 4:42https://doi.org/10.3390/colloids4040042
Kshtriya V, Koshti B, Gour N (2021) Green synthesized nanoparticles: classification, synthesis, characterization, and applications. In: Comprehensive analytical chemistry. Elsevier, pp 173–222. https://doi.org/10.1016/bs.coac.2020.12.009
Kumari P, Alam M, Siddiqi WA (2019) Usage of nanoparticles as adsorbents for waste water treatment: an emerging trend. Sustain Mater Technol 22:00128. https://doi.org/10.1016/j.susmat.2019.e00128
Kumbhakar P, Ray SS, Stepanov AL (2014) Optical properties of nanoparticles and nanocomposites. J Nanomater 2014:1–2. https://doi.org/10.1155/2014/181365
Lee K-S, El-Sayed MA (2006) Gold and silver nanoparticles in sensing and imaging: sensitivity of plasmon response to size, shape, and metal composition. J. Phys Che B 110:19220–19225. https://doi.org/10.1021/jp062536y
Lengke MF, Fleet ME, Southam G (2006) Synthesis of platinum nanoparticles by reaction of filamentous cyanobacteria with platinum (IV)- chloride complex. Langmuir 22:7318–7323
Li Q, Chen Z, Wang H, Yang H, Wen T, Wang S, Hu B, Wang X (2021) Removal of organic compounds by nanoscale zero-valent iron and its composites. Sci Total Environ 792:148546. https://doi.org/10.1016/j.scitotenv.2021.148546
Liu D, Mao Y, Ding L (2018) Carbon nanotubes as antimicrobial agents for water disinfection and pathogen control. J Water Health 16:171–180. https://doi.org/10.2166/wh.2018.228
Liu J, Zhang L, Lu G, Jiang R, Yan Z, Li Y (2021) Occurrence, toxicity and ecological risk of Bisphenol A analogues in aquatic environment—a review. Ecotoxicol Environ Saf 208:111481. https://doi.org/10.1016/j.ecoenv.2020.111481
Logan TJ (1993) Agricultural best management practices for water pollution control: current issues. In: Agriculture and the environment. Elsevier, pp 223–231. https://doi.org/10.1016/B978-0-444-89800-5.50020-8
Lu H, Wang J, Stoller M, Wang T, Ba Y, Hao H (2016) An Overview of nanomaterials for water and wastewater treatment. Adv Mater Sci Eng 2016:1–10. https://doi.org/10.1155/2016/4964828
Luo Z, Tu Y, Li H, Qiu B, Liu Y, Yang Z (2019) Endocrine-disrupting compounds in the Xiangjiang River of China: spatio-temporal distribution, source apportionment, and risk assessment. Ecotoxicol Environ Saf 167:476–484. https://doi.org/10.1016/j.ecoenv.2018.10.053
Luong NH, Long NN, Vu LV, Hai NH, Nghia PT, Anh NTV (2011) Metallic nanoparticles: synthesis, characterisation and application. Int J Nanotechnol 8:227. https://doi.org/10.1504/IJNT.2011.038201
Madhura L, Singh S, Kanchi S, Sabela M, Bisetty K, Inamuddin (2019) Nanotechnology-based water quality management for wastewater treatment. Environ Chem Lett 17:65–121. https://doi.org/10.1007/s10311-018-0778-8
Magalhães-Ghiotto GAV, de Oliveira AM, Natal JPS, Bergamasco R, Gomes RG (2021) Green nanoparticles in water treatment: a review of research trends, applications, environmental aspects and large-scale production. Environ Nanotechnol Monit. Manag 16:100526. https://doi.org/10.1016/j.enmm.2021.100526
Maiti D, Tong X, Mou X, Yang K (2019) Carbon-based nanomaterials for biomedical applications: a recent study. Front Pharmacol 9:1401. https://doi.org/10.3389/fphar.2018.01401
Mandeep SP (2020) Microbial nanotechnology for bioremediation of industrial wastewater. Front Microbiol 11:590631. https://doi.org/10.3389/fmicb.2020.590631
McConnell WP, Novak JP, Brousseau LC, Fuierer RR, Tenent RC, Feldheim DL (2000) Electronic and optical properties of chemically modified metal nanoparticles and molecularly bridged nanoparticle arrays. J Phys Chem B 104:8925–8930. https://doi.org/10.1021/jp000926t
Mohseniazar M, Barin M, Zarredar H, Alizadeh, Shanehbandi D (2011) Potential of microalgae and Lactobacilli in biosynthesis of silver nanoparticles. Bioimpacts 1:149–152
Molazadeh M, Ahmadzadeh H, Pourianfar HR, Lyon S, Rampelotto PH (2019) The use of microalgae for coupling wastewater treatment with CO2 biofixation. Front Bioeng Biotechnol 7:42. https://doi.org/10.3389/fbioe.2019.00042
Momeni S, Nabipour I (2015) A simple green synthesis of palladium nanoparticles with Sargassum alga and their electrocatalytic activities towards hydrogen peroxide. Appl Biochem Biotechnol 176:1–13
Moustafa MT (2017) Removal of pathogenic bacteria from wastewater using silver nanoparticles synthesized by two fungal species. Water Sci 31:164–176. https://doi.org/10.1016/j.wsj.2017.11.001
Mustapha S, Ndamitso MM, Abdulkareem AS, Tijani JO, Shuaib DT, Ajala AO, Mohammed AK (2020) Application of TiO2 and ZnO nanoparticles immobilized on clay in wastewater treatment: a review. Appl Water Sci 10:49. https://doi.org/10.1007/s13201-019-1138-y
Nayak MK, Singh J, Singh B, Soni S, Pandey VS, Tyagi S (2017) Introduction to semiconductor nanomaterial and its optical and electronics properties. In: Metal semiconductor core-shell nanostructures for energy and environmental applications. Elsevier, pp 1–33. https://doi.org/10.1016/B978-0-323-44922-9.00001-6
Nazeer S, Ali Z, Malik RN (2016) Water quality assessment of River Soan (Pakistan) and source apportionment of pollution sources through receptor modeling. Arch Environ Contam Toxicol 71:97–112. https://doi.org/10.1007/s00244-016-0272-x
Othman SH, Abd Salam NR, Zainal N, Kadir Basha R, Talib RA (2014) Antimicrobial activity of TiO2 nanoparticle-coated film for potential food packaging applications. Int J Photoenergy 2014:1–6.
Ovais M, Khalil AT, Islam NU, Ahmad I, Ayaz M, Saravanan M, et al. (2018) Role of plant phytochemicals and microbial enzymes in biosynthesis of metallic nanoparticles. Appl Microbiol Biotechno 102:6799–6814
Pandey A, Shankar S, Shikha ANK (2019) Amylase-assisted green synthesis of silver nanocubes for antibacterial applications. Bioinspired Biomim Nanobiomater 8:161–170. https://doi.org/10.1680/jbibn.17.00031
Parial D, Patra HK, Dasgupta AKR, Pal R (2012) Screening of different algae for green synthesis of gold nanoparticles. Eur J Phycol 47:22–29
Patel KD, Singh RK, Kim H-W (2019) Carbon-based nanomaterials as an emerging platform for theranostics. Mate Horiz 6:434–469. https://doi.org/10.1039/C8MH00966J
Prasad R (2016) Advances and applications through fungal nanobiotechnology. Springer International Publishing, Cham. ISBN 978-3-319-42989-2. http://www.springer.com/us/book/9783319429892
Price HD, Adams EA, Nkwanda PD, Mkandawire W, Quilliam RS (2021) Daily changes in household water access and quality in urban slums undermine global safe water monitoring programmes. Int J Hyg Environ Health 231:113632. https://doi.org/10.1016/j.ijheh.2020.113632
Radioactive Pollution (2014) Methods of measuring environmental parameters. Wiley, Hoboken, NJ, USA, pp 380–384. https://doi.org/10.1002/9781118914236.ch36
Ramanathan S, Gopinath SCB, Arshad MKM, Poopala P, Perumal V (2021) Nanoparticle synthetic methods: strength and limitations. In: Nanoparticles in analytical and medical devices. Elsevier, pp 31–43. https://doi.org/10.1016/B978-0-12-821163-2.00002-9
Ray PC, Yu H, Fu PP (2009) Toxicity and environmental risks of nanomaterials: challenges and future needs. J Environ Sci Health Part C 27:1–35. https://doi.org/10.1080/10590500802708267
Reghunadhan A, Kalarikkal N, Thomas S (2018) Mechanical property analysis of nanomaterials. In: Characterization of nanomaterials. Elsevier, pp 191–212. https://doi.org/10.1016/B978-0-08-101973-3.00007-9
Riddin T, Gericke M, Whiteley CG (2006) Analysis of the inter and extracellular formation of platinum nanoparticles by Fusarium oxysporum f. sp. lycopersici using response surface methodology. Nanotechnology 17:3482
Sadegh H, Ali GAM, Gupta VK, Makhlouf ASH, Shahryari-ghoshekandi R, Nadagouda MN, Sillanpää M, Megiel E (2017) The role of nanomaterials as effective adsorbents and their applications in wastewater treatment. J Nanostructure Chem 7:1–14. https://doi.org/10.1007/s40097-017-0219-4
Sagir M, Tahir MB, Akram J, Tahir MS, Waheed U (2020) Nanoparticles and significance of photocatalytic nanoparticles in wastewater treatment: a review. Curr Anal Chem 17:38–48. https://doi.org/10.2174/1573411016999200607172553
Salvadori MR, Ando RA, Nascimento CA, Corrêa B (2014) Bioremediation from wastewater and extracellular synthesis of copper nanoparticles by the fungus Trichoderma koningiopsis. J Environ Sci Health A Tox Hazard Subst Environ Eng 49(11):1286–1295
Salvadori MR, Ando RA, Nascimento CAO, Corrêa B (2017) Dead biomass of Amazon yeast: a new insight into bioremediation and recovery of silver by intracellular synthesis of nanoparticles. J Environ Sci Health A Tox Hazard Subst Environ Eng 52:1112–1120
Salvadori MR, Lepre LF, Ando RA, Oller do Nascimento CA, Corrêa B (2013) Biosynthesis and uptake of copper nanoparticles by dead biomass of Hypocrea lixii isolated from the metal mines in the Brazilian Amazon region. PLoS ONE 8:80519
Sau TK, Murphy CJ (2004) Room temperature, high-yield synthesis of multiple shapes of gold nanoparticles in aqueous solution. J Am Chem Soc 126:8648–8649
Santoro S, Estay H, Avci AH, Puglies L, Ruby-Figueroa R, Garcia A, Aquino M, Nasirov S, Straface S, Curcio E (2021) Membrane technology for a sustainable copper mining industry: The Chilean paradigm. Clean Eng Technol 2:100091. https://doi.org/10.1016/j.clet.2021.100091
Savage T, Rao AM (2004) Thermal properties of nanomaterials and nanocomposites. In: Tritt TM (ed) Thermal conductivity, physics of solids and liquids. Springer US, pp 261–284. https://doi.org/10.1007/0-387-26017-X_12
Saxena J, Sharma MM, Gupta S, Singh A (2014) Emerging role of fungi in nanoparticles synthesis and their applications. World J Pharmacy Pharmaceutical Sci 3:1586–1613
Segev-Bar M, Haick H (2013) Flexible sensors based on nanoparticles. ACS Nano 7:8366–8378. https://doi.org/10.1021/nn402728g
Senapati S, Ahmad A, Khan MI, Sastry M, Kumar R (2005) Extracellular biosynthesis of bimetallic Au-Ag alloy nanoparticles. Small 1:517–520
Senarathna ULNH, Fernando SSN, Gunasekara TDCP, Weerasekera MM, Hewageegana HGSP, Arachchi NDH, Siriwardena HD, Jayaweera PM (2017) Enhanced antibacterial activity of TiO2 nanoparticle surface modified with Garcinia zeylanica extract. Chem Cen J 11:7. https://doi.org/10.1186/s13065-017-0236-x
Seurinck S, Verstraete W, Siciliano SD (2005) Microbial source tracking for identification of fecal pollution. Rev Environ Sc Biotechnol 4:19–37. https://doi.org/10.1007/s11157-005-4997-7
Sikora A, Shard AG, Minelli C (2016) Size and f-potential measurement of silica nanoparticles in serum using tunable resistive pulse sensing. Langmuir 32:2216–2224
Singaravelu G, Arockiamary J, Kumar VG, Govindaraju K (2007) A novel extracellular synthesis of monodisperse gold nanoparticles using marine alga, Sargassum wightii Greville. Colloids Surf B 57:97–101
Singh RP (2001) Effect of wastewater disposal and extent of industrial pollution in and around Kanpur, Uttar Pradesh, India. Bull Eng Geol Environ 60:31–35. https://doi.org/10.1007/s100640000079
Singh P, Kim YJ, Zhang D, Yang DC (2016) Biological synthesis of nanoparticles from plants and microorganisms. Trends Biotechnol 34:588–599
Shan G, Surampalli R, Tyagi RD, Zhang TC (2009) Nanomaterials for environmental burden reduction, waste treatment, and nonpoint source pollution control: a review. Front Environ Sci Eng China 3:249–264. https://doi.org/10.1007/s11783-009-0029-0
Shankar S, Nill S (2015) Effect of metal ions and redox mediators on decolorization of synthetic dyes by Crude Laccase from a novel white rot Fungus Peniophora sp. (NFCCI-2131). Appl Biochem Biotechnol 175:635–647. https://doi.org/10.1007/s12010-014-1279-2
Sharma R, Kumar SDK, Sarkar M, Mishra BK, Puri V, Priyadarshini I, Thong PH, Ngo PTT, Nhu V-H (2021) Water pollution examination through quality analysis of different rivers: a case study in India. Environ Dev Sustain. https://doi.org/10.1007/s10668-021-01777-3
Shnoudeh AJ, Hamad I, Abdo RW, Qadumii L, Jaber AY, Surchi HS, Alkelany SZ (2019) Synthesis, characterization, and applications of metal nanoparticles. In: Biomaterials and bionanotechnology. Elsevier, pp 527–612. https://doi.org/10.1016/B978-0-12-814427-5.00015-9
Shon HK, Phuntsho S, Chaudhary DS, Vigneswaran S, Cho J (2013) Nanofiltration for water and wastewater treatment—a mini review. Drink Water Eng Sci 6:47–53. https://doi.org/10.5194/dwes-6-47-2013
Shrivastava KK, Shrivastava RK, Jain SV (2020) Pollution abatement strategies in a stretch of River Kahn at Indore, India. J Inst Eng India Ser A 101:829–834. https://doi.org/10.1007/s40030-020-00479-9
Shukla S, Khan R, Daverey A (2021) Synthesis and characterization of magnetic nanoparticles, and their applications in wastewater treatment: a review. Environ Technol Innov 24:101924. https://doi.org/10.1016/j.eti.2021.101924
Solomon SK (2005) Environmental pollution and its management in sugar industry in India: an appraisal. Sugar Tech 7:77–81. https://doi.org/10.1007/BF02942422
Some S, Mondal R, Mitra D, Jain D, Verma D, Das S (2021) Microbial pollution of water with special reference to coliform bacteria and their nexus with environment. Energy Nexus 1:100008. https://doi.org/10.1016/j.nexus.2021.100008
Subbaiya R, Saravanan M, Priya AR, Shankar KR, Selvam M, Ovais M et al (2017) Biomimetic synthesis of silver nanoparticles from Streptomyces atrovirens and their potential anticancer activity against human breast cancer cells. IET Nanobiotechnol 11:965–972
Subramaniam VD, Prasad SV, Banerjee A, Gopinath M, Murugesan R, Marotta F, Sun X-F, Pathak S (2019) Health hazards of nanoparticles: understanding the toxicity mechanism of nanosized ZnO in cosmetic products. Drug Chem Toxicol 42:84–93. https://doi.org/10.1080/01480545.2018.1491987
Teixeira-Santos R, Gomes M, Gomes LC, Mergulhão FJ (2021) Antimicrobial and anti-adhesive properties of carbon nanotube-based surfaces for medical applications: a systematic review. iScience 24:102001. https://doi.org/10.1016/j.isci.2020.102001
Teklehaimanot GZ, Coetzee MAA, Momba MNB (2014) Faecal pollution loads in the wastewater effluents and receiving water bodies: a potential threat to the health of Sedibeng and Soshanguve communities, South Africa. Environ Sci Pollut Res 21:9589–9603. https://doi.org/10.1007/s11356-014-2980-y
Thomas S, Harshita BSP, Mishra P, Talegaonkar S (2015) Ceramic nanoparticles: fabrication methods and applications in drug delivery. Curr Pharm Des 21:6165–6188. https://doi.org/10.2174/1381612821666151027153246
Uchida Y, Matsui K (2001) Optical properties of ZnS:Mn nanoparticles in sol-gel glasses. In: Studies in surface science and catalysis. Elsevier, pp 331–334. https://doi.org/10.1016/S0167-2991(01)82099-1
Ukhurebor KE, Athar H, Adetunji CO, Aigbe UO, Onyancha RB, Abifarin O (2021) Environmental implications of petroleum spillages in the Niger Delta region of Nigeria: a review. J Environ Manage 293:112872. https://doi.org/10.1016/j.jenvman.2021.112872
Ullah H, Khan I, Yamani ZH, Qurashi A (2017) Sonochemical-driven ultrafast facile synthesis of SnO2 nanoparticles: growth mechanism structural electrical and hydrogen gas sensing properties. Ultrason Sonochem 34:484–490
Upadhyayula VKK, Deng S, Mitchell MC, Smith GB (2009) Application of carbon nanotube technology for removal of contaminants in drinking water: a review. Sci Total Environ 408:1–13. https://doi.org/10.1016/j.scitotenv.2009.09.027
Vanden Bout DA (2002) Metal nanoparticles: synthesis, characterization, and applications (ed: Feldheim DL, Foss, Jr, CA). Marcel Dekker, Inc., New York and Basel. x+ 338 pp $150.00. ISBN: 0-8247-0604-8. J Am Chem So 124:7874–7875. https://doi.org/10.1021/ja015381a
Vigneshwaran N, Ashtaputre NM, Varadarajan PV, Nachane RP, Paralikar KM, Balasubramanya RH (2007) Biological synthesis of silver nanoparticles using the fungus Aspergillus flavus. Mater Lett 61:1413–1418
Wang ZL, Liu Y, Zhang Z (eds) (2003) Semiconductor nanoparticles. In: Handbook of nanophase and nanostructured materials. Springer US, Boston, MA, pp 813–848. https://doi.org/10.1007/0-387-23814-X_23
Wang J, Yang N, Tang H, Dong Z, Jin Q, Yang M, Kisailus D, Zhao H, Tang Z, Wang D (2013) Accurate control of multishelled Co3O4 hollow microspheres as high-performance anode materials in lithium-ion batteries. Angew Chemie Int Ed 52:6417–6420
Weng C-H (2021) Environmental concerns and pollution control in the context of developing countries. Environ Sci Pollut Res 28:46085–46088. https://doi.org/10.1007/s11356-021-15004-z
Willner MR, Vikesland PJ (2018) Nanomaterial enabled sensors for environmental contaminants. J Nanobiotechnol 16:95. https://doi.org/10.1186/s12951-018-0419-1
Wu Q, Miao W, Zhang Y, Gao H, Hui D (2020) Mechanical properties of nanomaterials: a review. Nanotechnol Rev 9:259–273. https://doi.org/10.1515/ntrev-2020-0021
Yaqoob AA, Parveen T, Umar K, Mohamad Ibrahim MN (2020) Role of nanomaterials in the treatment of wastewater: a review. Water 12:495. https://doi.org/10.3390/w12020495
Ye Y, Bruning H, Liu W, Rijnaarts H, Yntema D (2019) Effect of dissolved natural organic matter on the photocatalytic micropollutant removal performance of TiO2 nanotube array. J Photochem Photobiol Chem 371:216–222. https://doi.org/10.1016/j.jphotochem.2018.11.012
Yu X, Qin A, Liao L, Du R, Tian N, Huang S, Wei C (2015) Removal of organic dyes by nanostructure ZnO-bamboo charcoal composites with photocatalysis function. Adv Mater Sci Eng 2015:1–6. https://doi.org/10.1155/2015/252951
Zhang JZ (2009) Optical properties and spectroscopy of nanomaterials. World Sci. https://doi.org/10.1142/7093
Zhang X, He X, Wang K, Yang X (2011) Different active biomolecules involved in biosynthesis of gold nanoparticles by three fungus species. J Biomed Nanotechnol 7:245–254
Zielińska A, Carreiró F, Oliveira AM, Neves A, Pires B, Venkatesh DN, Durazzo A, Lucarini M, Eder P, Silva AM, Santini A, Souto EB (2020) Polymeric nanoparticles: production, characterization. Toxicology and Ecotoxicology. Molecules 25:3731. https://doi.org/10.3390/molecules25163731
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Singh, S., Shankar, S., Shikha (2022). Microbial Synthesis of Nanoparticles for Wastewater Remediation. In: Rai, J.P.N., Saraswat, S. (eds) Nano-biotechnology for Waste Water Treatment. Water Science and Technology Library, vol 111. Springer, Cham. https://doi.org/10.1007/978-3-031-00812-2_5
Download citation
DOI: https://doi.org/10.1007/978-3-031-00812-2_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-00811-5
Online ISBN: 978-3-031-00812-2
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)