Abstract
Accumulating evidence suggests that the cerebellum plays a crucial role not only in the motor and cognitive domains but also in emotions and social behavior. In the present chapter, after a general introduction on the significance of the emotional components of social behavior, we describe recent efforts to understand the contributions of the cerebellum in social cognition focusing on the emotional and affective aspects. Specifically, starting from the description of the cerebello-cortical networks subtending the social-affective domains, we illustrate the most recent findings on the social cerebellum and the possible functional mechanisms by which the cerebellum modulate social-affective behavior. Finally, we discuss the possible consequences of cerebellar dysfunction in the social-affective domain, focusing on those neurological and psychopathological conditions in which emotional and social behavior difficulties have been described as being associated with cerebellar structural or functional alterations.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Cerebellar sequencing
- Social interactions
- Prediction
- Cerebro-cerebellar circuits
- Social-affective alterations
1 General: Emotions in Social Behavior
Emotional processing includes immediate physiological responses and both automatic and intentional behavioral reactions to life events, proving critical to individuals’ survival and adaptive social relationships (Lerner et al. 2015). Humans are intrinsically predisposed to perceive and understand their own and others’ emotions and are constantly engaged in social interactions (Zaki and Ochsner 2011). One of the most primitive and key processes for successful social interactions is the ability to infer others’ emotions from visual and acoustic features, such as facial expressions and vocalizations (Schaller and Rauh 2017). While this ability reflects automatic aspects of emotion recognition, the ability to integrate contextual information with emotional states and to make detailed appraisals based on the interplay between present and past conditions is a reflection of more conceptual aspects of emotion recognition (Siciliano and Clausi 2020). Emotion recognition represents one of the two core components of social cognition, a composite human function defined by Brothers (Brothers and Ring 1990) as “the processing of any information which culminates in the accurate perception of the dispositions and intentions of other individuals.” The other component of this complex function is termed mentalizing or theory of mind (ToM), which consists of the advanced capacity to recognize and attribute mental states, such as emotions, intentions, and beliefs, to others (Premack and Woodruff 1978). This human ability allows us to make abstract social inferences in terms of past, future, or hypothetical events to adaptively predict social-affective behaviors (Van Hoeck et al. 2013). Emotion recognition and ToM are two closely linked processes since the recognition of others’ emotional state and related relevant cues culminate in the recognition of intentions (Brothers and Ring 1990). In this framework, social cognition processes could be viewed along a continuum, ranging from more automatic emotional processing to the recognition of complex emotional and mental states. Along this continuum, the ability to recognize the emotional state of others, based on specific situations and contexts, coincides with the capacity to empathize with them and has been defined as the affective component of ToM (Abu-Akel and Shamay-Tsoory 2011). Whereas affective ToM is thought to involve emotional contagion and empathetic appreciation of others’ emotional states, cognitive ToM is believed to require the understanding that others may have beliefs and intentions that differ from ours (Shamay-Tsoory 2011). Although these two components are separate aspects of ToM, their cooperation is needed to guarantee effective social interactions (Shamay-Tsoory et al. 2009). This model is in line with the one proposed by Coricelli (2005), which described a two-mind reading process: an unconscious/automatic process that allows the decoding of others’ intentions by the recognition of action and emotional contagion and a conscious/voluntary process that is linked to the ability to take the perspective of others and to use anticipatory and comparative mechanisms to make assumptions regarding their mental state (Coricelli 2005).
The multifaceted nature of these functions is supported by extended and dynamic neural networks composed of limbic areas, including the amygdala, hippocampus and insula (Phillips et al. 2003; Kipps et al. 2007; Gu et al. 2012), and cortical and associative areas, such as the anterior cingulate cortex, superior temporal sulcus (STS), temporo-parietal junction (TPJ), medial precuneus, and medial and lateral prefrontal cortex (Kennedy and Adolphs 2012; Van Overwalle et al. 2014). Despite the high specialization of these structures, constant interactions between them are needed to accomplish such complex functions, and overlaps can be found between emotional and social brain (Fossati 2012).
Nevertheless, the large-scale network sustaining all the functions covered under the umbrella term social cognition has yet to be well defined, and the role of the cerebellum is becoming increasingly emphasized (Van Overwalle et al. 2020a). The demonstrated influence of the cerebellar vermis, termed the “limbic cerebellum,” in emotional processing represents a crucial starting point for broadening its role in related affective and social fields (Adamaszek et al. 2015; Leggio and Olivito 2018). Indeed, recent MRI studies have shown cerebellar involvement in different aspects of social-affective behavior (Van Overwalle and Mariën 2016; Van Overwalle et al. 2020b). In particular, it has been highlighted that specific and phylogenetically more recent regions of the cerebellum are activated during complex social cognition tasks and are coupled with brain areas belonging to the mentalizing network (Van Overwalle et al. 2014; Van Overwalle and Mariën 2016). This interesting issue will be described in depth in the following sections.
2 The Role of Cerebro-Cerebellar Circuits in Social-Affective Behavior
Consensus is growing on the starring role of the cerebellum in social-affective behavior. Indeed, although this very interesting field is still at its early stages, many research findings point to a better understanding of the involvement of the cerebellum in social-affective domains. Substantial data come from structural and functional neuroimaging studies, which provide a useful characterization of the cerebellar functional topography for emotional (Stoodley and Schmahmann 2010) and social processing (Van Overwalle et al. 2014, 2015).
The cerebellar vermis has been indicated as the principal target of limbic connections, supporting the modulation of more “primitive” emotions (Schmahmann 1991; Schmahmann 2000) and the processing of stimuli that are critical for the emotional/affective experience (Schmahmann and Sherman 1998). In contrast, more complex emotional processing, such as social mentalizing, recruits specific regions in the posterior cerebellum (Van Overwalle et al. 2014, 2015). Accordingly, in a recent meta-analysis, Van Overwalle et al. (2020b) investigated which areas of the posterior cerebellum are specialized for social mentalizing and found that the Crus II was shown, in approximately 75% of the studies, to support domain-specific social cognition tasks related not only to social mentalizing but also to self-related emotional experience. Interestingly, among the mentalizing tasks, the highest percentages were found in subcategories examining attribution of others’ emotions (27%) and emotional self-experiences (17%). Notably, predominant activity during mentalizing tasks has also been found in the lobule IX of the cerebellum.
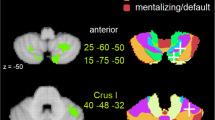
Considering that social cognition is a sophisticate mental processes, neuroimaging studies on brain functional connectivity (FC) have proven very useful in characterizing the complex brain networks involved in this function. In this field, the findings accumulated within the past decade have proven the presence of segregated cerebellar functional organization that allows the cerebellum to play a crucial role in emotional and social processing through its functional interactions with specific emotional and social brain cerebral regions (Leggio and Olivito 2018). Specifically, a study investigating the pattern of resting-state FC among vermal, paravermal, and hemispheric regions of the cerebellum (Sang et al. 2012) suggested that the cerebellum has a third functional subdivision, beyond the motor and cognitive subdivisions, that is devoted to emotional processing. Functional coherence was found between the cerebellum and brain limbic structures typically implicated in emotional regulation, such as the hippocampus and the amygdala (Sacchetti et al. 2009; Milner et al. 1998; Phelps and Le Doux 2005). In particular, the vermal I–VI, Crus II–X, hemispheric I–VI, and Crus II and IX regions are functionally connected to the hippocampus, while the vermal I–V, VIIb, VIIIa,b, and IX and hemispheric I–VI and VIIb regions are more functionally connected to the amygdala (Fig. 15.1a).
Cerebellar functional topography for affective/emotional and social domains. Cerebellar lobules are segregated by affective/emotional and social mentalizing functions and superimposed on the Spatial Unbiased Infratentorial Template. (a) Cerebellar lobules of the limbic/emotional cerebellum having connectivity with limbic brain regions: amygdala (in violet) and hippocampus (in blue); (b) cerebellar lobules of the social cerebellum having connectivity with default/mentalizing brain regions (in green). Verm vermal, Hem hemispheric, TPJ temporo-parietal junction, PRc precuneus, PCC posterior cingulate cortex
Further studies investigating cerebellar contributions to cerebral intrinsic connectivity networks reported patterns of functional coherence between the vermal and hemispheric parts of the lobule VI, the adjacent Crus I, and the dentate nuclei with the salience network (Habas et al. 2009). This network includes the dorsal anterior cingulate cortex and the fronto-insular cortex and is involved in interoception, autonomic regulation, and emotional regulation (Seeley et al. 2007). Lateral hemispheric regions of the posterior cerebellum (especially the Crus I/II), which are part of the “executive cerebellum,” also show connectivity with the salience network (Habas et al. 2009; Stoodley and Schmahmann 2010) and are likely to be recruited when more cognitive aspects of emotional processing are in demand.
Several resting-state fMRI studies also proved the participation of the posterior portions of the cerebellum (Crus I/II) in intrinsic connectivity networks related to social mentalizing (Habas et al. 2009; Buckner et al. 2011). In particular, while motion-related mirroring movement tasks have been shown to recruit “somatomotor” networks in the anterior cerebellum (Buckner et al. 2011), nonmotion-related mentalizing tasks have been shown to recruit the “default/mentalizing” network in the posterior cerebellum (Crus II), specifically when a high level of abstraction is required (Van Overwalle and Mariën 2016).
Connectivity fMRI studies have reported participation of the cerebellum in the default mode network (DMN) (Habas et al. 2009; Buckner et al. 2011), highlighting a crucial role of the cerebellar Crus I/II. The DMN is of particular interest in the context of mentalizing functions since it includes a set of cerebral regions that are particularly relevant for the social understanding of others, such as the TPJ, posterior cingulate cortex, precuneus, lateral parietal/angular gyrus, medial prefrontal cortex, and superior frontal gyrus (Schilbach et al. 2008). The cerebellar Crus I/II has been shown to be functionally coupled to default mode regions, while the anterior Crus I is functionally associated with the cerebral fronto-parietal network (Bernard et al. 2012).
Consistent with these data, altered cerebello-cerebral functional connectivity has been reported in adults with autism spectrum disorder (Olivito et al. 2017a, 2018), a neurodevelopmental disorder typically characterized by an impairment in social mentalizing (Baron-Cohen 1995; Hill and Frith 2003). In particular, altered FC was found between the cerebellar Crus II and cortical regions belonging to the DMN (Olivito et al. 2018). Interestingly, a meta-analysis of connectivity studies has identified a cerebello-cerebral mentalizing network that is particularly involved when a high level of abstraction is required, such as inferring group stereotypes, a person’s traits, or a person’s past (Van Overwalle and Mariën 2016). This network included the right posterior cerebellar region corresponding to the Crus II and bilateral mentalizing regions in the cerebrum, such as the bilateral TPJ, precuneus, and medial prefrontal cortex (Fig. 15.1b). A recent study using dynamic causal modeling showed that bidirectional connectivity exists within this network and that cerebral and cerebellar mentalizing areas are effectively connected via closed loops. In particular, the bidirectional (closed-loop) connectivity between the Crus II and bilateral TPJ has been specifically related to high-level social understanding (Van Overwalle et al. 2019a).
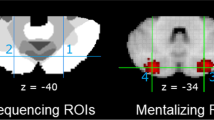
tDCS montage, experimental protocol, stimuli examples and behavioral results. (a) Extra-cephalic montage: the active electrode was placed on the cerebellar vermis and the reference electrode was placed on the right deltoid; (b) tDCS protocol; (c) Stimuli examples of the digital Reading the Mind in the Eyes Test: mental state (M-S) visual perception (V-P) and visual motor (V-M) stimuli are showed; (d) Study results in healthy participants and in one patients affected by cerebellar atrophy. In the healthy participant’s study, each subject was assigned to one of three groups in which anodal, cathodal, or sham stimulations was delivered. Instead, in the cerebellar patient, the anodal, cathodal, and sham stimulations were delivered over the cerebellum in three double-blind sessions (with 1-week interval). Moreover, one occipital lobe stimulation session was performed as further control condition. *p < 0.005
Taken together, these data confirm the existence of cerebellar functional segregation for emotional and social processing and suggest that the cerebellum significantly contributes to emotional processing both in the early stages of emotional perception and recognition and in the modulation of more complex social-affective behaviors.
3 The Role of the Cerebellum in Emotional Component of Social Cognition
3.1 From Emotion Regulation to Mentalizing Abilities: The Advent of the Social Cerebellum
In recent years, different research groups have paid great attention to the contribution of the cerebellum in emotional processing and social-affective behavior, going beyond its well-known role in the motor and cognitive domains. Early research reports regarded cerebellar involvement in emotion regulation and affective disorders, such that impaired modulation of affective behavior has been recognized as a part of “cerebellar cognitive affective syndrome” (CCAS) (Schmahmann and Sherman 1998). Subsequent clinical studies in individuals with cerebellar focal or degenerative damage showed symptomatology that ranged from inappropriate affective reactions to external events, such as pathological laughter and crying (Parvizi et al. 2001, 2007), agitation, impulsivity and irritability, to difficulties in processing negative emotions (fear and anger), mood fluctuations or affective flattening and depressive disorders (Richter et al. 2005; Tavano et al. 2007).
Moreover, clinical evidence has reported that the cerebellum also participates in the ability to consciously define our own affective state. Indeed, patients with cerebellar damage presented with difficulties in feeling conscious emotions of regret subsequent to disadvantageous choices in a gambling task (Clausi et al. 2015) and in explicitly recognizing their bad mood in the presence of clinically relevant depressive disorder (Clausi et al. 2019a).
Most of these alterations have been associated with damage to the vermis, which is part of the limbic cerebellum and is well known for its involvement in emotional processing and regulation (Parvizi et al. 2001; Richter et al. 2005; Schmahmann 2001; Stoodley and Schmahmann 2009). Interestingly, animal studies have shown that potentiation of excitatory and inhibitory synapses, which impinge on Purkinje cells at the level of the vermis, correlated with associative learning of fear, an emotion that is endowed with high adaptive value, and has long-lasting effects (Sacchetti et al. 2009; Zhu et al. 2007; Scelfo et al. 2008). These data allow us to hypothesize that learning-related plasticity at the level of the vermis might be crucial for relaying appropriate emotional and motor behaviors in response to external stimuli and maintaining this information for long periods. Thus, through its connections with the limbic system, the vermis may act as the interface between sensory stimuli, emotional state, and behavioral responses.
Since emotion regulation and affective state awareness are crucial abilities in social interaction and in the adaptation to new social contexts, the cerebellum implication in these functions paved the way to further investigation about the social-affective cerebellum.
Studies have primarily focused on the cerebellar contribution in emotional processing from facial expressions (Adamaszek et al. 2015; Schutter et al. 2009; Ferrucci et al. 2012). Facial expressions are crucial for non-verbal social interactions and are markers of internal states and intentions (Phillips and David 1995; Schupp et al. 2004). Recognizing facial expressions is vital in a complex social world, as it permits one to detect the emotional state of another person and provides cues on how to respond in social situations (Frank and Stennett 2001; Grossmann and Johnson 2007).
The contribution of the cerebellum in this domain is supported by studies using non-invasive neuro-stimulation techniques targeting the human cerebellum, such as transcranial magnetic stimulation (TMS) and transcranial direct current stimulation (tDCS) (van Dun et al. 2017). Specifically, Schutter et al. (2009) found that repetitive TMS (20 Hz) over the cerebellar vermis increased emotional responsiveness to happy facial expressions with no changes in consciously experienced mood. In the same way, tDCS delivered over the cerebellar vermis significantly reduced the time needed to identify negative facial expressions, such as anger and sadness (Ferrucci et al. 2012). More recently, facilitative effects of tDCS over the cerebellar vermis on the ability to recognize mental states of others through the eyes expression was found in both healthy subjects and patients with cerebellar ataxia (personal observations) (Fig. 15.2). These studies used the digital version of Reading the Mind in the Eyes (RME) test, an advanced ToM task that involves more implicit emotional processing and requires recognizing complex facial emotions and mental states from photographs of the eye region (Baron-Cohen et al. 2001).
These preliminary findings are in line with clinical observations in patients affected by neurodegenerative cerebellar pathologies in whom low performance on the RME test, difficulties in attributing negative emotion, and impaired performance on advanced mentalization tasks have been reported (D’Agata et al. 2011; Sokolovsky et al. 2010; Clausi et al. 2019b, 2021). Impairments in visual emotional attention and emotional face recognition have also been reported in subjects affected by cerebellar hemisphere damage (Adamaszek et al. 2013, 2015). In this context, the cerebellum has been characterized as an active interface with large-scale cerebral pathways that are involved in emotionally conscious processes. In line with this, fMRI studies have shown that the cerebellum, in addition to the amygdala and prefrontal cortex, is activated during conscious processing of emotional faces (Fusar-Poli et al. 2009). In fact, specific portions of the cerebellum are part of brain networks, including the amygdala–medial prefrontal circuitry, which contribute to determining the meaning of external stimuli and coherently reacting to them (Ghashghaei et al. 2007). Specifically, the medial prefrontal cortex plays a key role in complex aspects of emotional processing, such as social interactions (Rudebeck et al. 2008). Amygdala activation has also been associated with the perception of emotion (Adolphs 2002) and emotional arousing effects (Le Doux 2007; Wallentin et al. 2011).
The cerebellar involvement in emotions and social-affective behavior is strongly supported by neuroimaging meta-analyses in healthy subjects, which show that different areas of the cerebellum can be linked to specific social-cognitive processes, such as mirroring and mentalizing (Van Overwalle et al. 2014, 2015). In fact, as detailed in Sect. 15.2, although the vermis seems to participate in autonomic and implicit processing of emotions, the posterior portions of the cerebellum (i.e., the Crus I and II) have been proposed to mediate emotional content at higher cognitive levels (Timmann et al. 2010) and social cognition processing (Van Overwalle et al. 2014, 2015). In line with this suggestion, posterior regions of the cerebellum were shown to be activated during conscious feelings and empathy of pain (Singer et al. 2004) and are connected with the temporal, parietal, and prefrontal cortices (Ramnani 2006), which are involved in cognitive elaboration of emotional content (Lane 2008).
Accordingly, the causal role of the posterolateral region of the cerebellum in inferring others’ mental states from observation of their body language has also been demonstrated in neuro-stimulation studies. For example, Ferrari et al. (2018, 2019) showed that TMS over the left posterior cerebellar hemisphere affects the discrimination of emotional facial and body expressions in static pictures of real-life individuals. Overall, the abovementioned data from studies employing non-invasive brain stimulation and neuroimaging techniques converge to the view that the cerebellum acts not only in automatic perceptual processing but also in conscious processing of emotional and mental state information required for adequate social functioning. However, one question remains open: how does the cerebellum participate in processes related to the social-affective domain?
Currently, the most widely accepted hypothesis is that the modulatory actions that the cerebellum exerts on projection areas in the cerebral cortex are crucial not only for motor adaptation to sudden environmental changes but also for the optimization of social interactions and adaptation to the social context in accordance with internal and external emotional changes. This idea has been substantiated by the existence of anatomo-functional circuits (see Sect. 15.2) that allows the cerebellum to exert its function as part of the social-affective brain.
3.2 Cerebellar Modulation of Social-Affective Behavior: Theoretical Hypotheses
As illustrated above, the involvement of the cerebellum in social-affective behavior is becoming an accepted notion in the scientific community (Van Overwalle et al. 2020a). In parallel with the interest in defining the emotional and behavioral aspects by which the cerebellum plays a role and with the need to characterize the cerebello-cortical networks involved in the control of these functions, several groups of researchers in recent years have tried to elaborate specific theoretical hypotheses about the way in which this particular brain area influence the social-affective functioning.
3.2.1 Universal Cerebellar Transform and Dysmetria of Thought Theories in Social Behavior
One of the theories that has been posited to explain cerebellar functioning in emotional processing and social-affective behavior is the “uniform cerebellar transform” (UCT) hypothesis (Schmahmann 1991, 1998, 2000). It states that the cerebellum contributes to different domains by a singular neurological computation due to the repeating cytoarchitecture of its cortex and to the topographical organization of the extensive reciprocal anatomical connections between specific regions in the cerebellum and the sensory-motor and associative cortices (Schmahmann 2001; Schmahmann and Pandya 1997). Although the cerebellar cortex has an essentially uniform, monotonously repetitive architecture, immunohistochemistry has shown that it contains anatomically identifiable parasagittal bands (Hawkes et al. 1993) that appear to have connectional and physiological specificity (Hallem et al. 1999). Thus, the functional specificity of each cerebellar module is determined by the cortical brain region to which it is connected. Indeed, there is anatomical specificity linking each cerebral cortical area with unique patterns of termination in the basilar pons, which in turn is linked with specific regions of the cerebellar cortex. The cerebellar cortico-nuclear projections are then transmitted to specific areas of the thalamus before returning to those cerebral areas from which the projection originated (Kelly and Strick 2003). Based on its characteristic anatomical organization, the authors suggested that the cerebellum performs a universal cerebellar transform on the information to which it has access (Schmahmann 1991, 1998, 2000).
Following this idea, the uniform structure of the cerebellar cortex enables a unique computation that modulates the processing of multiple streams of information not only in sensorimotor and cognitive domains but also in emotional and social-affective domains. The cerebellum may serve as an oscillation damper, smoothing out performance in all domains and modulating behavior (Schmahmann 1998). Specifically, with respect to social-affective behavior, it has been hypothesized that the cerebellum acts to compare the consequences of actions with the intended outcomes, that is, to match reality with perceived reality. This is possible through existing cerebellar anatomical links with systems that control these functions, such that the vermis, fastigial nucleus and flocculo-nodular lobe are linked with the limbic system, whereas the cerebellar posterior areas are linked with paralimbic areas and association cortices concerned with the integration of emotional experiences into the repertoire of perceptions and behaviors required for social interactions (see Sect. 15.2 for further details).
The UCT theory is supported not only by neuroimaging findings on cerebro-cerebellar circuits but also by evidence on the significant relationships between social behavior and other motor and non-motor domains (Schmahmann 2000; Schmahmann and Sherman 1998; Schmahmann et al. 2019). Indeed, a new line of inquiry has examined general organizational principles that are shared between social and other motor and non-motor domains and has provided evidence that cerebellar social neuroanatomy can be contextualized within a larger triple representation principle that is common across numerous non-motor domains in the cerebellar cortex. Specifically, similar to the well-established descriptions of a double motor representation in lobules I–VI and VIII (Snider and Eldred 1952), it has been demonstrated that all non-motor processes in the cerebellar cortex might simultaneously engage some aspects of lobules VI/Crus I (first non-motor representation), lobules Crus II/VIIB (second non-motor representation), and lobules IX/X (third non-motor representation) (Buckner et al. 2011; Guell et al. 2018). In the same way, social processing exhibited a first and contiguous second representation in lobules Crus I/II and a third representation in lobule IX (Guell et al. 2018). This organization allows the parallel processing of complex information such as that required in social-affective behavior.
As a corollary of the UCT hypothesis, the same authors defined the ‘dysmetria of thought’ theory according to which the symptoms consequent to cerebellar damage can reflect universal cerebellar impairment, namely, dysmetria. In line with this, cerebellar malfunctioning can also lead to dysmetria in the cognitive and affective domains, which refers to the concept of dysmetria of the movement typical of patients with ataxia (Schmahmann 1991; Schmahmann and Sherman 1998). Indeed, in the motor domain, it is well known that a cerebellar lesion results in impairments of coordination, precision, and fluidity of motor control; in the same way, in the cognitive and affective domains, a cerebellar lesion leads to impaired coordination, precision, and fluidity of thought and emotion, including social processing (Schmahmann 2000; Schmahmann and Pandya 1997; Schmahmann et al. 2019). Impaired modulation of affect and mismatches between reality and perception of reality are central and defining features of psychoses, including schizophrenia and related disorders, bipolar affective disorders and related illnesses. Therefore, the role of the cerebellum in the pathophysiology of these conditions becomes plausible in the context of the dysmetria of thought hypothesis.
3.2.2 Cerebellar Sequencing and Prediction in Social Interactions
Increasing evidence on the functional diversity of structurally similar cerebellar modules has also enlarged the perspectives for hypothesizing other possible functional processes underpinning cerebellar influence in social-affective behavior. Within this framework, another theory was recently applied to explain the mechanisms by which the cerebellum is involved in non-motor processes, including social-affective domains, and is referred to as the “sequence detection theory” (Van Overwalle et al. 2020a; Leggio et al. 2008, 2011; Leggio and Molinari 2015). Sequencing is defined as the ability to perceive, represent, and execute spatio-temporal relations among events that follow a particular order. It can be considered a supra-modal function encompassing all human activities and crucial to the predictive processing of the brain (Savalia et al. 2016).
The “sequence detection theory” posits that the cerebellum plays a central role in sequencing (Leggio et al. 2008, 2011; Braitenberg et al. 1997). The cerebellum receives patterns of sequential temporal or spatial events via the pontine nuclei and compares them with information conveyed by climbing fibers. This interaction provides data regarding previously encountered sequences and consequently generates internal models useful in making predictions. Because of these mechanisms, the cerebellum can recognize sequential events and identify possible errors in the expected sequence, thereby acting as a feedforward controller that guarantees anticipatory actions (Molinari et al. 2009; Sokolov et al. 2017).
In the traditional view, the cerebellum has been related to motor processes, where internal models are considered to be responsible for the construction, detection, and application of motor sequences (Ito 2008). Through feedback and feedforward control, individuals become capable of predicting and adjusting movements in accordance with sudden environmental changes. More recently, Leggio et al. (2008) and Van Overwalle et al. (2019b) proposed that during human evolution, the cerebellum advanced and began engaging in similar processes for purely mental sequences. The authors argued that the cerebellum is also involved in the construction of internal models of mental processes during social interactions, in which the prediction of sequential events plays a central role (Clausi et al. 2019b; Van Overwalle et al. 2019b). Specifically, in the social-affective domain, as in the sensorimotor domain, the cerebellum may act by matching external information (social inputs) with the internal model of a specific social event linked to previous experiences, contributing to the formation of judgements on the mental state of others and predicting the consequences of social actions based on an individual’s beliefs and the social norms (Koster-Hale and Saxe 2013). Thus, to accomplish these functions in a fluid and automated manner, the cerebellum might modulate higher-order cortical areas by detecting socially predictable sequences (e.g., internal model of a social action) and promoting optimized feedforward control over activity (Sokolov et al. 2017; Van Overwalle et al. 2019b; Middleton and Strick 2000). This allows us to anticipate and understand the consequences of others’ actions and to recognize deviations in the predicted outcomes of social interactions to modify future social expectations. When cerebellar damage occurs, fast and continuous information comparisons between external stimuli and internal model results are affected; thus, subjects fail to recognize deviations/errors in social interactions and to adjust their response according to social expectations, as recently observed in patients with cerebellar neurodegenerative diseases (Clausi et al. 2021; Van Overwalle et al. 2019c).
Strong support for these theoretical hypotheses also comes from recent fMRI studies in healthy subjects (Heleven et al. 2019), in which activation of regions in the posterior cerebellum was observed during the construction of sequences of social actions that required understanding of the mental state of the protagonist (e.g., involving false or true beliefs) or when social predictions were violated (e.g., violations of social norms) (Berthoz et al. 2002).
Overall, the role of the cerebellum in predictive coding and adaptive control could be crucial in the social cognition domain because anticipation, adaptation, and learning are indispensable for successful social interactions and adaptive social behavior. This brain area could assist in learning and understanding social action sequences, supporting optimal predictions about imminent or future social interactions. In line with this theory, impaired sequencing and prediction mechanisms could be considered possible functional substrates of emotional and social-affective alterations in pathologies characterized by cerebello-cerebral dysfunctions.
4 Social-Affective Behavior in the Presence of Cerebellar Structural and Functional Alterations
The characterization of cerebellar involvement in specific aspects of emotional processing and social cognition and deeper knowledge of the mechanisms by which the cerebellum participates in social-affective domains assume strong significance in those pathological conditions that present both maladaptive social-affective behavior, and cerebellar structural and functional alterations. In the present section, we will focus on specific neurodegenerative diseases and psychiatric and neurodevelopmental conditions.
4.1 Social-Affective Behavioral Alterations in Patient with Cerebellar Pathologies
Evidence in the clinical field has shown that difficulties in recognizing basic emotions and difficulties with social behaviors are present in patients with focal and degenerative cerebellar pathologies (Adamaszek et al. 2014, 2015; Sokolovsky et al. 2010). Indeed, since the identification of emotional and affective changes found in patients with focal lesions of the cerebellum leading to the description of the CCAS (Schmahmann and Sherman 1998), the number of studies assessing emotional and social competences in cerebellar patients has exponentially grown. Indeed, difficulties in empathizing and mentalizing abilities have been described in a patient with cerebellar stroke involving the vermis and the posterior regions of the cerebellar hemispheres (Gerschovich et al. 2011). Moreover, clinical studies have evidenced specific patterns of social-affective alterations in patients with different types of cerebellar degenerative pathologies. For example, difficulties in the recognition of emotions were found in individuals with complex cerebello-cerebral degeneration and patients with isolated cerebellar degeneration (Sokolovsky et al. 2010; Hoche et al. 2016), while patients with spinocerebellar ataxia type 1 showed selective difficulties in the attribution of mental states (Sokolovsky et al. 2010).
Interesting findings have come from a recent behavioral and neuroimaging study with patients affected by various forms of cerebellar ataxia (Clausi et al. 2019b). In this study, patients presented with difficulties in basic aspects of social behavior, such as emotional contagion and recognition of the emotions of others, and in its more complex aspects, such as the ability to simulate, anticipate, and predict mental states of others. Intriguingly, cerebellar patients also have gray matter reductions localized in specific portions of the cerebellum (vermis and bilateral Crus I/II), which showed decreased functional connectivity with cerebral areas involved in mirroring and mentalizing processes (Abu-Akel and Shamay-Tsoory 2011; Clausi et al. 2019b).
In a more recent anatomo-functional study in a homogeneous cohort of SCA2 patients (Clausi et al. 2021), alterations in social cognition were found to be associated with structural damage to specific cerebellar lobules and microstructural alterations in the cerebellar peduncles, which are involved in different aspects of social-affective processing. Specifically, the correlational analyses evidenced that impairments in anger attribution were mainly related to the degree of gray matter atrophy in right lobules VIIIB and IX. This finding is consistent with increasing evidence that vermal and paravermal cerebellar areas are involved in the processing of ‘primitive’ emotions (Bauman and Mattingley 2012), such as fear of dangerous stimuli and anger towards aggressors (Schmahmann 1991), and participate in detecting, integrating, and filtering emotional information as a part of a cerebello-cortical-limbic network (Habas et al. 2009). In contrast, patients’ impairments in more complex components of social cognition were associated with atrophy in specific posterior cerebellar regions (right Crus II). As reported above, this cerebellar area is associated with complex brain networks involved in mentalizing abilities (Van Overwalle et al. 2014; Habas et al. 2009; Sokolov et al. 2017).
Moreover, the scores of the patients on the Reading the Mind in the Eyes test were related to the cerebellar peduncle microstructural damage reported in an SCA2 cohort (Olivito et al. 2017b). These data have been explained with consideration of the well-known function of the cerebellum as a predictor (Sokolov et al. 2017; Ito 2008). Indeed, since the abovementioned task requires determination of the meaning of the expression in the eyes and an automatic and rapid inference of another person’s mental state (Baron-Cohen et al. 2001), the cerebellum might act by matching the external information (i.e., expression of the eyes) with the individual’s internal model of eye region expression linked to previous experiences, thus contributing to an immediate judgment about the other’s mental state. Notably, the middle and posterior peduncles are the feedback and feedforward limbs of the cerebello-cortical system, respectively, through which the cerebellum receives information and sends information back to cerebral regions (Ramnani 2006). Therefore, it is reasonable to assume that fiber degeneration within the cerebellar peduncles could interfere with the communication between the cerebellum and the cortical projection areas involved in the more automatic mentalizing processes.
Considering the complexity of information processing in the social cognition domain and the cerebellar interplay with the brain networks involved in these processes, it is reasonable to infer that neuroanatomical damage that occurs in cerebellar patients may account for their behavioral impairments and also alter cerebello-cortical interactions. Accordingly, in a recent study, altered inter-nodal connectivity between the right Crus II and cerebral areas involved in more complex aspects of mentalization, such as the dorsomedial prefrontal cortex and the TPJ, was found in SCA2 patients (Olivito et al. 2020).
In summary, the findings reported above offer a new point of view regarding specific symptomatology in patients with cerebellar ataxia, where the importance of links between social behavior difficulties and cerebellar damage has often been underestimated or neglected, with direct consequences on clinical practice.
4.2 Cerebellar and Social-Affective Alterations in Neurodegenerative Disorders and Psychiatric and Neurodevelopmental Conditions
Several studies have described impairments in social functioning and mentalizing processes not only in patients affected by cerebellar pathology (D’Agata et al. 2011; Sokolovsky et al. 2010; Clausi et al. 2019b) but also in individuals affected by neurodegenerative disorders (Poletti et al. 2012), such as Alzheimer’s and Parkinson’s diseases (Wu and Hallett 2013; Jacobs et al. 2018) and behavioral variants of frontotemporal dementia (Van den Stock et al. 2019), that have often been associated with cerebellar alterations.
A recent study suggested that the impaired cerebellar modulation described in sporadic Alzheimer’s disease may be predictive of the onset of cognitive and neuropsychiatric deficits (Jacobs et al. 2018). With this disease, cerebellar gray matter reductions have been found to progressively evolve, beginning with early involvement in the vermis and the posterior cerebellar lobe and extending to the anterior cerebellar lobe in more advanced stages (Jacobs et al. 2018; Toniolo et al. 2018). As both structural and functional cerebellar modifications progress in parallel with pathological changes in the cerebral cortex, social-affective behavioral alterations evolve as well. Moreover, in people with Parkinson’s disease, cognitive, behavioral, and mentalizing impairments arise after the onset of motor symptoms; this progression of disease parallels the degree of structural and functional changes occurring in both the cerebellum and cortical areas involved in higher-order functions (Wu and Hallett 2013; Camicioli et al. 2009; Nishio et al. 2010). Difficulties in the mentalizing domain have often been reported in patients affected by behavioral variants of frontotemporal dementia (Pardini et al. 2013; Desmarais et al. 2018), who show correlations between task performances and atrophy in posterior regions of the cerebellum (Crus I and II) (Van den Stock et al. 2019; Synn et al. 2018).
A specific role for the cerebellum has been hypothesized in the onset of emotional and mentalizing difficulties described in some psychiatric and neurodevelopmental disorders (Corcoran et al. 1995; Fatemi et al. 2012; Bora et al. 2016; Bora and Berk 2016). Indeed, a number of neuroimaging studies in individuals affected by schizophrenia, depressive and bipolar disorders, and ASD have shown structural and functional alterations in regions of the “social cerebellum” and in cerebello-cerebral mentalizing networks (Adler et al. 2007; Becker and Stoodley 2013; Kim et al. 2014). Specifically, microstructural disruptions in cerebro-cerebellar pathways (Kanaan et al. 2009) and intracerebellar white matter (Kim et al. 2014) were found in schizophrenic individuals, who often present with difficulties in the automatic process of mentalizing and fail to select the appropriate behavioral representation for understanding the actions and intentions of others (Das et al. 2012; Martinez et al. 2019). In these patients, alterations in cerebellar forward modeling have been considered to be the cause of hallucinations because of the inability to distinguish between internal states and external events (Frith et al. 2000; Ford and Mathalon 2012).
Moreover, altered functional connectivity between the posterior cerebellum and mentalizing regions, such as the TPJ, medial prefrontal cortex, and posterior cingulate, has been reported in individuals with a diagnosis of bipolar disorder (Liu et al. 2012; Wang et al. 2015) and in those with depressive states. In particular, reduced volumes and decreased activity in the vermis and posterior cerebellar lobes (Adler et al. 2007; Mills et al. 2005; Narita et al. 2011; Kim et al. 2013; Sani et al. 2016) and reduced functional connectivity between the posterior cerebellum and the amygdala, inferior frontal gyrus (orbital), and striatum have been described in patients with bipolar disorder (Shaffer et al. 2018; Wang et al. 2016; Li et al. 2015). Likewise, decreased functional connectivity between the cerebellum and temporal and parietal regions has been shown in individuals with major depressive disorder (Guo et al. 2013).
In these clinical populations, including individuals in both manic and depressive states (Bora et al. 2016; Bora and Berk 2016) and remitted patients with bipolar disorder (Bora et al. 2005; Bora and Pantelis 2016), alterations in mentalizing are thought to affect social behavior. Accordingly, preliminary studies in patients affected by type 1 or type 2 bipolar disorder (in a euthymic state) showed specific mentalizing problems characterized by difficulties understanding another person’s mental state and considering their beliefs and intentions (personal observations). In a recent single case study, Lupo and colleagues (Lupo et al. 2018) added new insight into the role of the cerebellum in socio-affective processing in the context of specific psychiatric conditions and demonstrated not only that there was an association between the onset of a manic state and the presence of a focal cerebellar lesion but also that the pattern of impaired functional connectivity in the patient overlapped with cerebello-cerebral mentalizing networks.
Finally, the cerebellum seems to be implicated in ASD (Fatemi et al. 2012), a neurodevelopmental condition mainly characterized by difficulties in ToM abilities (Baron-Cohen et al. 2001). Cerebellar dysfunction has recently gained attention as a potential biomarker of this condition (Fatemi et al. 2012). Indeed, early developmental damage in the cerebellum is considered one of the major risk factors for ASD (Wang et al. 2014) and has been associated with deficits in affective and internalizing behaviors and with social withdrawal (Limperopoulos et al. 2007), thus suggesting that atypical cerebellar development as well as dysfunctional cerebello-cerebellar networks could contribute to ASD-related behaviors (Stoodley and Limperopoulos 2016).
Further support for this idea has been derived from resting-state functional connectivity studies in individuals with ASD that reported reduced functional connectivity between specific regions in the posterior cerebellum and regions in the “social brain” relevant for social interaction. Indeed, low resting-state functional connectivity between the Crus II and the TPJ adjacent to the STS (Igelström et al. 2017) and altered functional connectivity between the dentate nucleus and the cortical regions involved in social cognition were reported in adults with ASD (Olivito et al. 2017a, 2018). Accordingly, a recent study in ASD showed that more severe scores on the Autism Diagnostic Observation Schedule were associated with the degree of hypo-connectivity between Crus I/II and lobule IX and brain areas involved in language, emotional and social domains, including the bilateral STS, inferior frontal gyrus, amygdala and specific nodes in the default mode network (Arnold Anteraper et al. 2019).
Taken together, these observations suggest that the study of cerebellar functioning in these pathologies could be crucial for a better comprehension of the neurobiological bases of social behavior impairments in neurodegenerative and psychiatric disorders, holding great promise for a better understanding and treatment of a variety of social impairments.
5 Conclusions and Future Directions
In the present chapter, we have described converging evidence from clinical studies in patients with cerebellar alterations and fMRI and neuro-stimulation studies in healthy individuals that point to recognizing the involvement of the cerebellum in specific aspects in the social-affective domain. The emotional and affective components of social skills are crucial for adaptation to the surrounding environment, especially in the presence of pathological conditions. A better comprehension of the mechanisms by which the cerebellum influences the neural substrates of social-affective behavior could have important clinical implications not only in the presence of a cerebellar pathology but also in specific psychiatric and neurodevelopmental disorders.
Knowledge of these aspects is essential in clinical practice because, on the one hand, behavioral alterations can have an impact on quality of life and compliance with pharmacological and rehabilitative treatments of individuals; on the other hand, knowing the neural basis of these alterations can facilitate the development of rehabilitation protocols to modulate cerebellar excitability, allowing clinicians to influence/improve symptomatology in individuals suffering from social-affective alterations. Among these innovative treatments, non-invasive neuro-stimulation techniques should be mentioned as a useful method for investigating the causal and potential clinical role of the cerebellum in social functioning (van Dun et al. 2017).
References
Abu-Akel A, Shamay-Tsoory S (2011) Neuroanatomical and neurochemical bases of theory of mind. Neuropsychologia 49(11):2971–2984
Adamaszek M, Olbrich S, Kirkby KC et al (2013) Event-related potentials indicating impaired emotional attention in cerebellar stroke—A case study. Neurosci Lett 548:206–211
Adamaszek M, D’Agata F, Kirkby KC et al (2014) Impairment of emotional facial expression and prosody discrimination due to ischemic cerebellar lesions. Cerebellum 13:338–345
Adamaszek M, Kirkby KC, D’Agata F et al (2015) Neural correlates of disturbed emotional face recognition in cerebellar lesions. Brain Res 1613:1–12
Adler CM, DelBello MP, Jarvis K et al (2007) Voxel-based study of structural changes in first-episode patients with bipolar disorder. Biol Psychiatry 61(6):776–781
Adolphs R (2002) Neural systems for recognizing emotion. Curr Opin Neurobiol 12:169–177
Arnold Anteraper S, Guell X, D’Mello A et al (2019) Disrupted cerebrocerebellar intrinsic functional connectivity in young adults with high-functioning autism spectrum disorder: a data-driven, whole-brain, high-temporal resolution functional magnetic resonance imaging study. Brain Connect 9(1):48–59
Baron-Cohen S (1995) Mindblindness: an essay on autism and theory of mind. MIT Press, Cambridge, MA
Baron-Cohen S, Wheelwright S, Skinner R et al (2001) The autism-spectrum quotient (AQ): evidence from Asperger syndrome/high-functioning autism, males and females, scientists and mathematicians. J Autism Dev Disord 31(1):5–17
Bauman O, Mattingley JB (2012) Functional topography of primary emotion processing in the human cerebellum. Neuroimage 61:805–811
Becker EBE, Stoodley CJ (2013) Autism spectrum disorder and the cerebellum. Int Rev Neurobiol 113:1–34
Bernard JA, Seidler RD, Hassevoort KM et al (2012) Resting state cortico-cerebellar functional connectivity networks: a comparison of anatomical and self-organizing map approaches. Front Neuroanat 10:6–31
Berthoz S, Armony JL, Blair RJR et al (2002) An fMRI study of intentional and unintentional (embarrassing) violations of social norms. Brain 125(8):1696–1708
Bora E, Berk M (2016) Theory of mind in major depressive disorder: a meta-analysis. J Affect Disord 191:49–55
Bora E, Pantelis C (2016) Social cognition in schizophrenia in comparison to bipolar disorder: a meta-analysis. Schizophr Res 175(1–3):72–78
Bora E, Vahip S, Gonul AS et al (2005) Evidence for theory of mind deficits in euthymic patients with bipolar disorder. Acta Psychiatr Scand 112(2):110–116
Bora E, Bartholomeusz C, Pantelis C (2016) Meta-analysis of theory of mind (ToM) impairment in bipolar disorder. Psychol Med 46(2):253–264
Braitenberg V, Heck D, Sultan F et al (1997) The detection and generation of sequences as a key to cerebellar function: experiments and theory. Behav Brain Sci 20:229–277
Brothers L, Ring B (1990) A neuroethological framework for the representation of mind. J Cogn Neurosci 4:107–118
Buckner R, Krienen F, Castellanos A et al (2011) The organization of the human cerebellum estimated by intrinsic functional connectivity. J Neurophysiol 106:2322–2345
Camicioli R, Gee M, Bouchard TP (2009) Voxel-based morphometry reveals extra-nigral atrophy patterns associated with dopamine refractory cognitive and motor impairment in parkinsonism. Parkinsonism Relat Disord 15:187–195
Clausi S, Coricelli G, Pisotta I et al (2015) Cerebellar damage impairs the self-rating of regret feeling in a gambling task. Front Behav Neurosci 9:113
Clausi S, Lupo M, Olivito G et al (2019a) Depression disorder in patients with cerebellar damage: awareness of the mood state. J Affect Disord 245:386–393
Clausi S, Olivito G, Lupo M et al (2019b) The cerebellar predictions for social interactions: theory of mind abilities in patients with degenerative cerebellar atrophy. Front Cell Neurosci 12:510
Clausi S, Olivito G, Siciliano L et al (2021) The neurobiological underpinning of the social cognition impairments in patients with spinocerebellar ataxia type 2. Cortex 138:101–112
Corcoran R, Mercer G, Frith CD (1995) Schizophrenia, symptomatology and social inference: investigating “theory of mind” in people with schizophrenia. Schizophr Res 17(1):5–13
Coricelli G (2005) Two-levels of mental states attribution: from automaticity to voluntariness. Neuropsychologia 43:294–300
D’Agata F, Caroppo P, Baudino B et al (2011) The recognition of facial emotions in spinocerebellar ataxia patients. Cerebellum 10:600–610
Das P, Lagopoulos J, Coulston CM et al (2012) Mentalizing impairment in schizophrenia: a functional MRI study. Schizophr Res 134(2–3):158–164
Desmarais P, Lanctot KL, Masellis M et al (2018) Social inappropriateness in neurodegenerative disorders. Int Psychogeriatr 30(2):197–207
Fatemi SH, Aldinger KA, Ashwood P et al (2012) Consensus paper: pathological role of the cerebellum in autism. Cerebellum 11(3):777–807
Ferrari C, Oldrati V, Gallucci M et al (2018) The role of the cerebellum in explicit and incidental processing of facial emotional expressions: a study with transcranial magnetic stimulation. Neuroimage 169:256–264
Ferrari C, Ciricugno A, Urgesi C et al (2019) Cerebellar contribution to emotional body language perception: a TMS study. Soc Cogn Affect Neurosci. https://doi.org/10.1093/scan/nsz074
Ferrucci R, Giannicola G, Rosa M et al (2012) Cerebellum and processing of negative facial emotions: cerebellar transcranial DC stimulation specifically enhances the emotional recognition of facial anger and sadness. Cognit Emot 26(5):786–799
Ford JM, Mathalon DH (2012) Anticipating the future: automatic prediction failures in schizophrenia. Int J Psychophysiol 83(2):232–239
Fossati P (2012) Neural correlates of emotion processing: from emotional to social brain. Eur Neuropsychopharmacol 22(Suppl 3):S487–S491. https://doi.org/10.1016/j.euroneuro.2012.07.008
Frank MG, Stennett J (2001) The forced-choice paradigm and the perception of facial expressions of emotion. J Pers Soc Psychol 80:75–85
Frith CD, Blakemore S, Wolpert DM (2000) Explaining the symptoms of schizophrenia: abnormalities in the awareness of action. Brain Res Brain Res Rev 31(2–3):357–363
Fusar-Poli P, Placentino A, Carletti F et al (2009) Functional atlas of emotional faces processing: a voxel based meta-analysis of 105 functional magnetic resonance imaging studies. J Psychiatry Neurosci 34:418–432
Gerschovich ER, Cerquetti D, Tenca E et al (2011) The impact of bilateral cerebellar damage on theory of mind, empathy and decision making. Neurocase 17(3):270–275
Ghashghaei HT, Hilgetag CC, Barbas H (2007) Sequence of information processing for emotions based on the anatomic dialogue between prefrontal cortex and amygdala. Neuroimage 34:905–923
Grossmann T, Johnson MH (2007) The development of the social brain in human infancy. Eur J Neurosci 25:909–919
Gu X, Gao Z, Wang X et al (2012) Anterior insular cortex is necessary for empathetic pain perception. Brain 135:2726–2735
Guell X, Gabrieli JDE, Schmahmann JD (2018) Triple representation of language, working memory, social and emotion processing in the cerebellum: convergent evidence from task and seed-based resting-state fMRI analyses in a single large cohort. Neuroimage 172:437–449
Guo W, Liu F, Liu J et al (2013) Is there a cerebellar compensatory effort in first-episode, treatment-naïve major depressive disorder at rest? Prog Neuropsychopharmacol Biol Psychiatry 46:13–18
Habas C, Kamdar N, Nguyen D et al (2009) Distinct cerebellar contributions to intrinsic connectivity networks. J Neurosci 29:8586–8594
Hallem JS, Thompson JH, Gundappa-Sulur G et al (1999) Spatial correspondence between tactile projection patterns and the distribution of the antigenic Purkinje cell markers anti-zebrin I and anti-zebrin II in the cerebellar folium crus IIA of the rat. Neuroscience 93:1083–1094
Hawkes R, Blyth S, Chockkan V et al (1993) Structural and molecular compartmentation in the cerebellum. Can J Neurol Sci Suppl 3:S29–S35
Heleven E, van Dun K, Van Overwalle F (2019) The posterior cerebellum is involved in constructing social action sequences: an fMRI study. Sci Rep 9(1):11110
Hill EL, Frith U (2003) Understanding autism: insights from mind and brain. Philos Trans R Soc Lond B Biol Sci 358:281–289
Hoche F, Guell X, Sherman JC et al (2016) Cerebellar contribution to social cognition. Cerebellum 15:732–743
Igelström KM, Webb TW, Graziano MSA (2017) Functional connectivity between the temporoparietal cortex and cerebellum in autism spectrum disorder. Cereb Cortex 27(4):2617–2627
Ito M (2008) Control of mental activities by internal models in the cerebellum. Nat Rev Neurosci 9:304–313
Jacobs HIL, Hopkins DA, Mayrhofer HC et al (2018) The cerebellum in Alzheimer’s disease: evaluating its role in cognitive decline. Brain 141(1):37–47
Kanaan RAA, Borgwardt S, McGuire PK et al (2009) Microstructural organization of cerebellar tracts in schizophrenia. Biol Psychiatry 66(11):1067–1069
Kelly RM, Strick PL (2003) Cerebellar loops with motor cortex and prefrontal cortex of a nonhuman primate. J Neurosci 23(23):8432–8444
Kennedy DP, Adolphs R (2012) Perception of emotions from facial expressions in high-functioning adults with autism. Neuropsychologia 50(14):3313–3319
Kim D, Cho HB, Dager SR et al (2013) Posterior cerebellar vermal deficits in bipolar disorder. J Affect Disord 150(2):499–506
Kim D-J, Kent JS, Bolbecker AR et al (2014) Disrupted modular architecture of cerebellum in schizophrenia: a graph theoretic analysis. Schizophr Bull 40(6):1216–1226
Kipps CM, Duggins AJ, McCusker EA et al (2007) Disgust and happiness recognition correlate with anteroventral insula and amygdala volume respectively in preclinical Huntington’s disease. J Cogn Neurosci 19:1206–1217
Koster-Hale J, Saxe R (2013) Theory of mind: a neural prediction problem. Neuron 79(5):836–848
Lane R (2008) Neural substrates of implicit and explicit emotional processes: a unifying framework for psychosomatic medicine. Psychosom Med 70:214–231
Le Doux J (2007) The amygdala. Curr Biol 17:R868–R874
Leggio M, Molinari M (2015) Cerebellar sequencing: a trick for predicting the future. Cerebellum 14(1):35–38
Leggio M, Olivito G (2018) Topography of the cerebellum in relation to social brain regions and emotions. Handb Clin Neurol 154:71–84
Leggio MG, Tedesco AM, Chiricozzi FR et al (2008) Cognitive sequencing impairment in patients with focal or atrophic cerebellar damage. Brain 131(5):1332–1343
Leggio MG, Chiricozzi FR, Clausi S et al (2011) The neuropsychological profile of cerebellar damage: the sequencing hypothesis. Cortex 47(1):137–144
Lerner JS, Li Y, Valdesolo P et al (2015) Emotion and decision making. Annu Rev Psychol 66:799–823
Li M, Huang C, Deng W et al (2015) Contrasting and convergent patterns of amygdala connectivity in mania and depression: a resting-state study. J Affect Disord 173:53–58
Limperopoulos C, Bassan H, Gauvreau K et al (2007) Does cerebellar injury in premature infants contribute to the high prevalence of long-term cognitive, learning, and behavioral disability in survivors? Pediatrics 120(3):584–593
Liu CH, Li F, Li SF et al (2012) Abnormal baseline brain activity in bipolar depression: a resting state functional magnetic resonance imaging study. Psychiatry Res 203(2–3):175–179
Lupo M, Olivito G, Siciliano L et al (2018) Evidence of cerebellar involvement in the onset of a manic state. Front Neurol 9:774
Martinez G, Mosconi E, Daban-Huard C et al (2019) “A circle and a triangle dancing together”: alteration of social cognition in schizophrenia compared to autism spectrum disorders. Schizophr Res 210:94–100
Middleton FA, Strick PL (2000) Basal ganglia output and cognition: evidence from anatomical, behavioral, and clinical studies. Brain Cogn 42(2):183–200
Mills NP, Del Bello MP, Adler CM et al (2005) MRI analysis of cerebellar vermal abnormalities in bipolar disorder. Am J Psychiatry 162(8):1530–1533
Milner B, Squire LR, Kandel ER (1998) Cognitive neuroscience and the study of memory. Neuron 20:445–468
Molinari M, Restuccia D, Leggio MG (2009) State estimation, response prediction, and cerebellar sensory processing for behavioral control. Cerebellum 8(3):399–402
Narita K, Suda M, Takei Y et al (2011) Volume reduction of ventromedial prefrontal cortex in bipolar II patients with rapid cycling: a voxel-based morphometric study. Prog Neuropsychopharmacol Biol Psychiatry 35(2):439–445
Nishio Y, Hirayama K, Takeda A et al (2010) Corticolimbic gray matter loss in Parkinson’s disease without dementia. Eur J Neurol 17:1090–1097
Olivito G, Clausi S, Laghi F et al (2017a) Resting-state functional connectivity changes between dentate nucleus and cortical social brain regions in autism spectrum disorders. Cerebellum 16(2):283–292
Olivito G, Lupo M, Iacobacci C et al (2017b) Microstructural MRI basis of the cognitive functions in patients with Spinocerebellar Ataxia Type 2. Neuroscience 366:44–53
Olivito G, Lupo M, Laghi F et al (2018) Lobular patterns of cerebellar resting-state connectivity in adults with autism spectrum disorder. Eur J Neurosci 47(6):729–735
Olivito G, Siciliano L, Clausi S et al (2020) Functional changes of mentalizing network in SCA2 patients: novel insights into understanding the social cerebellum. Cerebellum 19(2):235–242
Pardini M, Emberti Gialloreti L, Mascolo M et al (2013) Isolated theory of mind deficits and risk for frontotemporal dementia: a longitudinal pilot study. J Neurol Neurosurg Psychiatry 84(7):818–821
Parvizi J, Anderson SW, Martin CO et al (2001) Pathological laughter and crying: a link to the cerebellum. Brain 124:1708–1719
Parvizi J, Joseph J, Press DZ et al (2007) Pathological laughter and crying in patients with multiple system atrophy-cerebellar type. Mov Disord 22:798–803
Phelps EA, Le Doux JE (2005) Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron 48:175–187
Phillips ML, David AS (1995) Facial processing in schizophrenia and delusional misidentification: cognitive neuropsychiatric approaches. Schizophr Res 17:109–114
Phillips ML, Drevets WC, Rauch SL et al (2003) Neurobiology of emotion perception I: The neural basis of normal emotion perception. Biol Psychiatry 54(5):504–514
Poletti M, Enrici I, Adenzato M (2012) Cognitive and affective theory of mind in neurodegenerative diseases: neuropsychological, neuroanatomical and neurochemical levels. Neurosci Biobehav Rev 36(9):2147–2164
Premack D, Woodruff G (1978) Does the chimpanzee have a ‘theory of mind’? Behav Brain Sci 4:515–526
Ramnani N (2006) The primate cortico-cerebellar system: anatomy and function. Nat Rev Neurosci 7:511–522
Richter S, Schoch B, Kaiser O et al (2005) Behavioral and affective changes in children and adolescents with chronic cerebellar lesions. Neurosci Lett 381:102–107
Rudebeck PH, Bannerman DM, Rushworth MF (2008) The contribution of distinct subregions of the ventromedial frontal cortex to emotion, social behavior, and decision making. Cogn Affect Behav Neurosci 8:485–497
Sacchetti B, Scelfo B, Strata P (2009) Cerebellum and emotional behavior. Neuroscience 162:756–762
Sang L, Qin W, Liu Y et al (2012) Resting-state functional connectivity of the vermal and hemispheric subregions of the cerebellum with both the cerebral cortical networks and subcortical structures. Neuroimage 61:1213–1225
Sani G, Chiapponi C, Piras F et al (2016) Gray and white matter trajectories in patients with bipolar disorder. Bipolar Disord 18(1):52–62
Savalia T, Shukla A, Bapi RS (2016) A unified theoretical framework for cognitive sequencing. Front Psychol 7:1821
Scelfo B, Sacchetti B, Strata P (2008) Learning-related long-term potentiation of inhibitory synapses in the cerebellar cortex. Proc Natl Acad Sci U S A 105:769–774
Schaller MU, Rauh R (2017) What difference does it make? Implicit, explicit and complex social cognition in autism spectrum disorders. J Autism Dev Disord 47:961–979
Schilbach L, Eickhoff SB, Rotarska-Jagiela A et al (2008) Minds at rest? Social cognition as the default mode of cognizing and its putative relationship to the default system of the brain. Conscious Cogn 17:457–467
Schmahmann JD (1991) An emerging concept: the cerebellar contribution to higher function. Arch Neurol 48:1178–1187
Schmahmann JD (1998) Dysmetria of thought. Clinical consequences of cerebellar dysfunction on cognition and affect. Trends Cogn Sci 2:362–370
Schmahmann JD (2000) The role of the cerebellum in affect and psychosis. J Neurolinguistics 13(2–3):189–214
Schmahmann JD (2001) The cerebrocerebellar system: anatomic substrates of the cerebellar contribution to cognition and emotion. Int Rev Psychiatry 13(4):247–260
Schmahmann JD, Pandya DN (1997) The cerebrocerebellar system. Essentials of cerebellum and cerebellar disorders. Int Rev Neurobiol 41:31–60
Schmahmann JD, Sherman JC (1998) The cerebellar cognitive affective syndrome. Brain 141:561–579
Schmahmann JD, Guell X, Stoodley CJ et al (2019) The theory and neuroscience of cerebellar cognition. Annu Rev Neurosci 42(1):337–364
Schupp HT, Ohman A, Junghofer M et al (2004) Facilitated processing of threatening faces: an ERP analysis. Emotion 4:189–200
Schutter DJLG, Enter D, Hoppenbrouwers SS (2009) High-frequency repetitive transcranial magnetic stimulation to the cerebellum and implicit processing of happy facial expressions. J Psychiatry Neurosci 34(1):60–65
Seeley WW, Menon V, Schatzberg AF et al (2007) Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci 27:2349–2356
Shaffer JJ, Johnson CP, Fiedorowicz JG et al (2018) Impaired sensory processing measured by functional MRI in bipolar disorder manic and depressed mood states. Brain Imaging Behav 12(3):837–847
Shamay-Tsoory SG (2011) The neural bases for empathy. Neuroscientist 17:18–24
Shamay-Tsoory SG, Aharon-Peretz J, Perry D (2009) Two systems for empathy: a double dissociation between emotional and cognitive empathy in inferior frontal gyrus versus ventromedial prefrontal lesions. Brain 132:617–627
Siciliano L, Clausi S (2020) Implicit vs. explicit emotion processing in autism spectrum disorders: an opinion on the role of the cerebellum. Front Psychol 11:96. https://doi.org/10.3389/fpsyg.2020.00096
Singer T, Seymour B, O’Doherty J et al (2004) Empathy for pain involves the affective but not sensory components of pain. Science 303:1157–1162
Snider R, Eldred E (1952) Cerebrocerebellar relationships in the monkey. J Neurophysiol 15(1):27–40
Sokolov AA, Miall RC, Ivry RB (2017) The cerebellum: adaptive prediction for movement and cognition. Trends Cogn Sci 21(5):313–332
Sokolovsky N, Cook A, Hunt H et al (2010) A preliminary characterisation of cognition and social cognition in spinocerebellar ataxia types 2, 1, and 7. Behav Neurol 23:17–29
Stoodley CJ, Limperopoulos C (2016) Structure–function relationships in the developing cerebellum: evidence from early-life cerebellar injury and neurodevelopmental disorders. Semin Fetal Neonatal Med 21(5):356–364
Stoodley CJ, Schmahmann JD (2009) Functional topography in the human cerebellum: a meta-analysis of neuroimaging studies. Neuroimage 44(2):489–501
Stoodley CJ, Schmahmann JD (2010) Evidence for topographic organization in the cerebellum of motor control versus cognitive and affective processing. Cortex 46:831–844
Synn A, Mothakunnel A, Kumfor F et al (2018) Mental states in moving shapes: distinct cortical and subcortical contributions to theory of mind impairments in dementia. J Alzheimers Dis 61(2):521–535
Tavano A, Grasso R, Gagliardi C et al (2007) Disorders of cognitive and affective development cerebellar malformations. Brain 130:2646–2660
Timmann D, Drepper J, Frings M et al (2010) The human cerebellum contributes to motor, emotional and cognitive associative learning. A review. Cortex 46:845–857
Toniolo S, Serra L, Olivito G et al (2018) Patterns of cerebellar gray matter atrophy across Alzheimer’s disease progression. Front Cell Neurosci 12:430. https://doi.org/10.3389/fncel.2018.00430
Van den Stock J, De Winter FL, Stam D et al (2019) Reduced tendency to attribute mental states to abstract shapes in behavioral variant frontotemporal dementia links with cerebellar structural integrity. Neuroimage Clin 22:101770. https://doi.org/10.1016/j.nicl.2019.101770
van Dun K, Bodranghien F, Manto M et al (2017) Targeting the cerebellum by non-invasive neurostimulation: a review. Cerebellum 16(3):695–741
Van Hoeck N, Ma N, Ampe L et al (2013) Counterfactual thinking: an fMRI study on changing the past for a better future. Soc Cogn Affect Neurosci 8(5):556–564
Van Overwalle F, Mariën P (2016) Functional connectivity between the cerebrum and cerebellum in social cognition: a multi-study analysis. Neuroimage 124(2016):248–255
Van Overwalle F, Baetens K, Mariën P et al (2014) Social cognition and the cerebellum: a meta-analysis of over 350 fMRI studies. Neuroimage 86(2014):554–572
Van Overwalle F, D’aes T, Mariën P (2015) Social cognition and the cerebellum: a meta-analytic connectivity analysis. Hum Brain Mapp 36(12):5137–5154
Van Overwalle F, Van de Steen F, Mariën P (2019a) Dynamic causal modeling of the effective connectivity between the cerebrum and cerebellum in social mentalizing across five studies. Cogn Affect Behav Neurosci 19(1):211–223
Van Overwalle F, Manto M, Leggio M et al (2019b) The sequencing process generated by the cerebellum crucially contributes to social interactions. Med Hypotheses 128:33–42
Van Overwalle F, De Coninck S, Heleven E et al (2019c) The role of the cerebellum in reconstructing social action sequences: a pilot study. Soc Cogn Affect Neurosci 14(5):549–558
Van Overwalle F, Manto M, Cattaneo Z et al (2020a) Consensus paper: cerebellum and social cognition. Cerebellum 19(6):833–868
Van Overwalle F, Ma Q, Heleven E (2020b) The posterior crus II cerebellum is specialized for social mentalizing and emotional self-experiences: a meta-analysis. Soc Cogn Affect Neurosci 15(9):905–928
Wallentin M, Nielsen AH, Vuust P et al (2011) Amygdala and heart rate variability responses from listening to emotionally intense parts of a story. Neuroimage 58:963–973
Wang SS-H, Kloth AD, Badura A (2014) The cerebellum, sensitive periods, and autism. Neuron 83(3):518–532
Wang Y, Zhong S, Jia Y et al (2015) Interhemispheric resting state functional connectivity abnormalities in unipolar depression and bipolar depression. Bipolar Disord 17(5):486–495
Wang Y, Zhong S, Jia Y et al (2016) Disrupted resting-state functional connectivity in nonmedicated bipolar disorder. Radiology 280(2):529–536
Wu T, Hallett M (2013) The cerebellum in Parkinson’s disease. Brain 136(Pt 3):696–709
Zaki J, Ochsner K (2011) Reintegrating accuracy into the study of social cognition (target article). Psychol Inq 22(3):159–182
Zhu L, Scelfo B, Hartell NA et al (2007) The effects of fear conditioning on cerebellar LTP and LTD. Eur J Neurosci 26:219–227
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Clausi, S., Siciliano, L., Olivito, G., Leggio, M. (2022). Cerebellum and Emotion in Social Behavior. In: Adamaszek, M., Manto, M., Schutter, D.J.L.G. (eds) The Emotional Cerebellum . Advances in Experimental Medicine and Biology, vol 1378. Springer, Cham. https://doi.org/10.1007/978-3-030-99550-8_15
Download citation
DOI: https://doi.org/10.1007/978-3-030-99550-8_15
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-99549-2
Online ISBN: 978-3-030-99550-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)