Abstract
Understanding the chemical and biological mechanisms of peat solidification is vital to protect the environment during geotechnical works. The alteration of peat to increase its strength for the preparation of road construction requires an admixture to be added to the soil. Changes in peat properties affect proximal ecosystems. Peat has a high water content of up to 2000%, is high in fibre and microorganisms and has a low shear strength of around 4–12 kPa. Normal construction work requires around 300 kPa of soil bearing capacity. The addition of admixtures, usually cement, retards bacterial activities, changes the pH from acidic to alkaline and reduces moisture content significantly. Changes in solidified peat may be observed physically by measuring unconfined compressive strength (UCS) followed by scanning electron microscopy (SEM). Chemical reactions are quantified using X-ray diffraction (XRD), X-ray fluorescence (XRF), Fourier transform infrared spectroscopy (FTIR), pH and energy-dispersive X-ray (EDX), while biological reactions may be enumerated by bacterial counts and enzymatic activities. Mathematical modelling may be used to elucidate chemical and biological reactions and understand the kinetics of strength improvement. The Michaelis-Menten equation is applicable as enzymes secreted by bacteria in peat dissolve hydration products in solidified peat. Affirmation of the theory used in determining chemical and biological mechanisms is critical in helping geotechnical engineers to choose the best method of peat solidification with minimal environmental impact.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Geotechnical works in peat areas are always a challenge to engineers. Roads or buildings constructed on peat often encounter settlement issues. Peat treatment has been introduced by researchers to harden the peat prior to engineering works. The chemical reaction that strengthens soil particles due to mixing peat with chemical admixtures has not yet been highlighted by geotechnical engineers. The reaction can be elucidated using mathematical modelling and simulation. In this chapter, the nature of peat and possible strengthening agents are discussed before mathematical modelling and simulation are introduced to clarify the chemical reaction that happens during peat treatment work. Awareness may safeguard our environment before work is done on-site; the use of possible chemical pollutants shoud be reduced, and the problem of dissolved transmissible chemical additives used for peat solidification transferred to neibouring areas should be solved or minimised.
2 Peat and Its Engineering Challenges
Peat is the cumulative product of decayed plants known as soft soil among geotechnical engineers. Peat contains up to 1800% moisture. According to the nature and management of tropical peat soils (Andriesse 1988), soil consisting of more than 65% organic matter is classified as peat soil. ASTM D 2607–69 (ASTM 1990) states that peat is soil with organic content greater than 75%. Overall, peat can be defined as a brownish or black-coloured soil, depending on the location it is formed and climatic conditions. It is formed by accumulation of decomposed organic matter over thousands of years, under anaerobic conditions. Waterlogging promotes formation. A large amount of porous spaces filled with water makes peat easily compressible. These unique characteristics make construction work on it very challenging to engineers. It is suggested that peat should be treated before any construction work is done.
It is estimated that 4.5% of total land areas or 1 billion acres of land in the world consists of peat. A total area of 25,000 km2 peat soil in Malaysia is shown in Table 5.1, ranking the country ninth with the biggest total area of peat soil (Mesri and Ajlouni 2007). The characteristics of peat depend on the original plant material, e.g. moss or fern, and the climate of specific locations. In most European countries, peat originates from bogs and fens. There is variability in microbial communities of peat. In European regions it is normally wet and records constant or similar moisture content throughout the year. Although peats in temperate regions, with tropical climates, are saturated during wet seasons and of low moisture content in dry seasons, peats are easily ignited and are fire hazards (American Coal Ash Association 2007).
Around 2.4 million hectares of Malaysian land area is covered with tropical peat. Sarawak is the state that has the largest peat area, 69.5% of the total peat area in Malaysia (Mutalib et al. 1992). In peninsular Malaysia, the peat area of Pahang, Perak and Selangor states comprises 17.2% of the total land area (Mesri and Ajlouni 2007).
Peat has a low shear strength of 5–17 kPa when tested using vane shear ; it is not a favourable soil for construction work (Hebib and Farell 2003; Wong et al. 2009). One of the reasons for the low strength of peat is its high porosity, affecting all peat types worldwide (Al-Ani et al. 2013). In construction work , there is a minimum strength of soil required before a basement is considered safe; however, no specific guideline has been established for construction in peat areas. The New Zealand Building Code proposed that other types of soil have an ‘allowable bearing’ pressure of 100 kPa before building (New Zealand Building Code Requirements 2011). Building codes of New York City (2008) and New Zealand Building Code (2011) advise a mass stabilisation or vertical piling for construction on peat areas.
As peats are prone to shrinkage and have a very low bearing capacity, they are not suitable for building on (Islam and Hashim 2010). Dramatic peat shrinkage occurs during drier seasons, leaving buildings ‘hanging’ on piling structure or facing settlement issues. The high moisture content of peat effects shrinkage of a few metres beneath the original level during droughts (Holden et al. 2004; Lindsay et al. 2015). When natural organic soils are drained, subsidence caused by consolidation may occur (Van Hardeveld et al. 2017). Perpetually, after the water table is lowered, saturated organic layers are compacted due to a loss of buoyancy (Wösten et al. 1997). When a high negative water pressure occurs due to loss of water, organic materials above water level experience shrinkage and rapid decomposition. Compaction follows decomposition processes when biochemical oxidation takes place (PS Konsultant 1998).
In most countries, peat areas are abandoned as treatment of peat incurs a high cost. However, Brazil, Canada and Denmark take advantage of peat’s natural carbon stock making beneficial products such as peat bricks, peat fuel and supplements for agricultural activity (Peat Society 2015). In Malaysia and in other developed countries such as Sweden, the study of peat treatment has become important. According to Pontian District Municipal Council in a 2014–2018 report, Johor Bahru, Malaysia, (town) has a residential growth of 280% from 2003 to 2013 compared to other peat-based areas in the district with only 10% growth in the same time interval. This highlights the importance of peat treatment to the ministry for the purpose of balanced urbanisation. Knowledge of peat decomposition level is essential as it infers or leads to accurately predict the potential of any physical modification on peat soil (Mohamed et al. 2002). Treatment studies should consider all peat types, to give solutions that suit all peat areas.
Peats originate from organic matter, some of which contains complex aromatic macromolecules known as humic substances that contribute to odour, taste and acidity in water supplies (Fong and Murtedza 2007). The chemical and physical properties of soils involve humic substances, the most chemically active fractions of peat with a high surface area and surface charge (Santagata et al. 2008). Stabilisation may be carried out using the agent ordinary Portland cement (OPC), though hydration processes are interrupted by humic acid, organic matter with a low pH. Stabilisation is disrupted when acid reacts with calcium from cement hydrolysis, resulting in insoluble calcium humic acid, which makes calcium crystallisation a challenging process. Calcium crystallisation products provide the key strengthening of cemented soil (Santagata et al. 2008; Hashim and Islam 2008).
Peat is classified according to its decomposition level. Categories of less decomposed (more than 66% fibre), moderately decomposed (33–66% fibre) and most decomposed (less than 33% fibre) peat are known as fibric, hemic and sapric respectively. The structure of decomposed peat varies in its degree of humification. Physical properties of peat vary with decomposition level. Peat moisture content alone cannot be used to predict decomposition as it changes with location, original vegetation, climate and water table (Rahman and Chan 2013). Abd Rahman (2015) confirms the relationship between peat decomposition level and the solidification process. The amount of fibre in peat determines the amount of filler needed for peat treatment.
The degree of decomposition varies between peat mosses depending on the resistance degree of plants and environmental factors such as the presence of hydrolytic enzymes produced by decomposing microorganisms. Factors affect microbial activity directly and then indirectly on the decomposition process. These include biochemical stability of the peat, water activity, temperature, pH and aeration (Huat 2004). Variations give a wide range of physical properties to peat including colour, texture, density, specific gravity, porosity and pore size, which are all related to the degree of decomposition. Higher decomposition levels of peat lead to a decrease in the particle size of organic matter (Boelter 1968). Microorganisms have more activity with increases in water content and aeration (Wolińska and Stępniewska 2012). Based on the degree of decomposition for each type, fibric peats are rich with organic matter and have a high porosity due to the large pore size, while sapric peats have a small pore size and low organic matter. Figure 5.1 depicts peat layers : The remnants of logs and woody plants can be seen clearly in fibric peat; the remnants of decomposing wood and trunks can also be noted in hemic peat, but both are absent in sapric peat. The decomposition of peat is organised and represents the peat profile, where fibric peat is normally found at the top of the peat soil layer (Abd Rahman 2015).
3 Peat Solidification Theory
Any soil that is not stable, strong and durable is considered weak and unable to bear high load. Soil solidification, stabilisation or modification is the process of improving the physical and engineering properties of a soil to predetermined targets (Eisazadeh et al. 2010). Soil stabilisation is a solution that increases the bearing capacity and strength of the soil. Stabilisation also refers to the selection of the stabiliser in order to achieve target strengths or stiffness values in addition to modification (Makusa 2012).
The challenge in peat stabilisation is finding the best binder, filler and ratio for the admixture. Several studies on binder and filler for soft soil stabilisation have been conducted, which include the use of recycled waste products, rice husks and many more (Huat 2004). According to Makusa (2012), there are two types of soil stabilisation methods: mechanical and chemical stabilisation. Mechanical stabilising methods include induced vibration or compaction, as well as adding other physical properties like barriers and nails. These are best suited for coarse-grained soils or aggregates at optimum or below optimum moisture contents (Dhakal 2012) and are not suitable for peat soils. Chemical stabilisation , often known as soil stabilisation, is based on a chemical reaction between a stabiliser and the minerals in the soil. There are two groups of chemical stabiliser – traditional and non-traditional. Liu et al. (2011) stated that traditional stabilisers such as cement and lime are more common as compared to non-traditional stabilisers including liquid polymers, silicates and lignin derivatives. This may be due to the cheap price of traditional stabilisers especially if the stabilisation needed to cover a large area is easily obtained. According to Dhakal (2012), clayey soil is the most effective for chemical stabilisation. The selection of stabilisation technique depends on the soil type and its condition.
The water content ; the physical, chemical and mineralogical properties of peats; the nature and amount of organic matter included; and the pH of pore water all play a role in peat stabilisation. As different types of peat have their own unique characteristics, no absolute ratio of properties can be used to indicate solidification. The qualities of treated organic soils by binders and fillers are dependent on both the concentration of organic matter and the nature and type of organic matter (Tremblay et al. 2002). The degradation of biodegradable chemicals also affects the strength gained. Fine-grained soils’ engineering behaviour is governed by their specific surface area (Santamarina et al. 2002). A study by Kazemian et al. (2009) found that sapric, hemic and fibric peats have specific surface areas of 93, 69 and 50 m2/g, respectively. On a unit mass or volume basis, as the specific surface of sapric peat increases, more surface area is accessible for reaction. A higher shear strength is obtained in comparison with fibric and hemic peats (Kazemian et al. 2009). In the following subsections, the solidification of peats will be reviewed and evaluated.
3.1 Techniques in Peat Solidification
In many soil solidification studies, cement is used as a binder (Tremblay et al. 2002; Consoli et al. 2000; Rotta et al. 2003; Rao and Shivananda 2005; Ahnberg 2006). Cement is a hydraulic binder. A hydraulic binder is self-curing when in contact with water, while a non-hydraulic binder requires a catalyst to initiate curing. A hydraulic binder will stabilise almost any soil. When a binder interacts with soft soil, it creates a substance with greater engineering qualities than the original soil (Hebib and Farell 2003). The finer the grain size of cement, the more reactive it will be (Kazemian et al. 2009).
Cement is commonly used to alleviate soil acidity, as well as to improve the physical condition of the soil (Rotta et al. 2003). The pozzolanic reaction increases the pH of pore water due to dissolution of hydrated lime. The strong base dissolves both soil silica and alumina from soil minerals (Rao and Shivananda 2005). Care must be taken to ensure homogeneous mixing; unlike lime cement does not diffuse into soil mass (Ahnberg 2006). Thermogravimetric analysis (TGA) is often use to determine the presence of Ca(OH)2 in OPC or solidified soil. Weight loss between 450 and 580 °C was used to determine the Ca(OH)2 composition (El-Jazairi and Illston 1977; Wang et al. 2004). The change of cementitious products can be indicated by the change of Ca(OH)2 by hydration process which produces ettringite (Horpibulsuk 2012).
Beside cement , researchers mix peat with a variety of pozzolan materials to enhance secondary reactions and reduce the cement cost. Pozzolan is a material made up of siliceous or siliceous and aluminous materials with little to no cementitious value. Pozzolan reacts chemically with calcium hydroxide at room temperature in the presence of water to generate cementitious compounds (Mehta 1987). Small amounts of secondary pozzolanic materials are added to admixtures to promote secondary pozzolanic reactions. Fly ash is commonly known as a good pozzolan for soil stabilisation (Wongsa et al. 2016).
Sodium silicate is an admixture used in peat solidification studies (Kazemian et al. 2011a, b, c). According to Karol (2003) and the US Army Corps of Engineers (USACE 1995), when sodium silicate solution and an adequate solution of alkali metal salt (sodium and potassium) are mixed, the reaction generates a gel instantly. As the reaction is fast, solutions do not completely contact with each other; unstable interfaces ensure that enough contact occurs to form a continuous gel network that can be followed through stabilised soil. The study by Kazemian et al. (2009) found that sodium silicates can solidify peat and achieve higher strength; more than 50% of the strength is gained when solidifying using OPC in controlled acidic media. The sodium silicates, however, react more in hemic and sapric peats compared to fibric peat.
The ground granulated blast furnace slag (GGBS), calcium oxide (CaO) and sodium bentonite are among secondary pozzolans that are used in peat. These admixtures are good binders with cement. Most secondary pozzolans cannot achieve significant strength without cement as no hydrolysis takes place; no ettringite is formed to bind the soil particles. Combinations of GGBS and cement lengthen the curing effect as it is reactive for long durations (after years). The mixture was found to be efficient in gaining strength in peat solidification studies (Axelsson et al. 2002; Mass Stabilization Manual 2005). Calcium oxide, on the other hand, works differently. Calcium oxide reacts well with water to produce slake lime. Slake lime is very reactive to carbon dioxide, producing mortar. Mortar is a paste that gradually hardens and cements bricks together. The use of calcium oxide in soil treatment is limited to the soil natural water content; however, in the case of peat, a high water content enables mortar formation when calcium oxide is used.
3.2 Stabilisation of Peat by Cement
Organic soils have been shown to slow or prohibit the hydration of binders such as cement in binder-soil mixes (Hebib and Farell 2003). With a high organic content and less solid particles in peat, cement alone as a chemical admixture is insufficient to provide peat stabilisation. Peat has a significantly lower content of clay particles that enter secondary pozzolanic reactions than clay and silt (Janz and Johansson 2002). As such, the interaction between hydrated lime Ca(OH) and the soil is less effective in secondary pozzolanic reactions. Unless a sufficient amount of cement is put to the soil, peat stabilisation by cement will not lead to a significant increase in strength. A study by Ahnberg et al. (1995) found that peat achieves the lowest shear strength with cement stabilisation compared to other types of soil. Similarly, with the highest water/cement ratio (wcr), cement-stabilised peat demonstrated the lowest shear strength compared to other types of soil (Ahnberg et al. 1995). Evidence from both figures shows that less solid particles and high organic matter make peat soil porous and spongy in nature; the organic matter tends to impede the hydration of cement when it is used to stabilise soil (Wolińska and Stępniewska 2012).
Abd Rahman (2015) discusses the concept of natural water content in peat. As peat is naturally acidic, containing humic acid, the concept of water consumption in peat solidification is varied. Water in the peat increases after neutralisation of humic acid takes place. The neutralisation produces water, which will react with OPC to form ettringite. Neutralisation is a process involving a reaction between acid and alkaline, producing water and salt as shown in Eq. (5.1).
Neutralisation can be detected by pH 7 of a mixture and an increasing amount of water as the result of combination of the aqueous solution from both acid and alkaline. An exact quantity of acid and base (OH− ion) is needed to achieve neutralisation. Exceeding the neutralisation limit by any of the elements causes the material to be dominant by the respective element, either acid or base. The pH of solidified peats was found to be alkaline (Fig. 5.6). OH− ions contained in the alkaline binder dominate the pH mixture. The pH value is determined based on the number of OH− ions present in the solution.
The pH of Pontian peat is around 3–4 and represents the high number of H+ ions in humic acid (Abd Rahman 2015). Humic acid is a weak acid; direct exposure to peat does not give irritation or corrosion. Moisture in peat contains an aqueous humic acid. Since ettringite is formed by the interaction between cement and water, neutralisation does not occur in a mixture of solidified peat. The amount of water present in the mixture depends on the neutralisation process. Studies by Ali et al. (2010), Akol (2012), Wong et al. (2013a, b) and Abd Rahman (2015) show that peat is acidic by nature, while solidified peat is alkaline with a pH range from 8.83 to 12.11 (Fig. 5.2). The alkaline condition is important for the reaction between OPC and water, interlocking the hydration product and soil particles.
The presence of black humic acid and fulvic acid in peat soil makes cementation and hardening of peat-cement admixtures difficult. Wherever calcium is present in solution, black humic acid interacts with it to generate insoluble calcium humic acid (Rahman and Chan 2013). Calcium crystallisation is hard due to the combination of humic acid and calcium ions created during cement hydration; yet, crystallisation increases the strength of the peat soil-cement mixture (Rahman and Chan 2013). Exposure of cement to fulvic acid solutions generates the hydration of cement. The chemical reaction between fulvic acid and cement minerals results in an absorbed layer, which obstructs the hydration process. Furthermore, existing crystals such as calcium aluminate hydrate, calcium sulfate-aluminate hydrate and calcium ferrite-aluminate hydrate are decomposed by fulvic acid, inhibiting the creation of a soil-cement structure (Rahman and Chan 2013). The acids lower the pH of the soil, which has an adverse effect on the binder’s reaction rate, resulting in a slower strength gain in the peat (Axelsson et al. 2002).
Organic acids, when mixed with soil and cement, generate a pH lower than 9 in porous solutions, preventing the creation of cementing products because the pH has become too low to support secondary mineral formation (Liu et al. 2011). The process of soil stabilisation will be slowed unless a large amount of cement is mixed with the soil to neutralise the acids. It is uneconomical to add a big amount of cement to the soil to improve peat ground. As a result, it is obvious that the physico-chemical reactions that occur, such as cement hydration and hardening, as well as interactions between components in the soil and cement hydration products, are responsible for the increased strength of cement-stabilised soil (Abd Rahman 2015).
Excessive organic matter in peat effects a high water retention capacity, and organic particles on the surface of cement and solid soil particles absorb water during cement hydrolysis in the soil. Both the development of cement hydration products and the hydration of solid soil particles and hydration products are hampered by this (Abd Rahman 2015). As a result, only limited increments can be achieved in peat-cement admixture strength. This was evident when Chen and Wang (2006) mentioned that the strength of peat did not reach 300 kPa with deep mixing with a cement ratio of up to 30%, in a foundation reinforcing project on peat undertaken in 1985. A clear understanding on the behaviour of organic matter in the process of stabilisation of peat by suitable chemical admixtures is vital in order to outline effective peat stabilisation.
3.3 Effect of Pozzolan as a Secondary Additive in Peat Stabilisation
To increase the secondary pozzolanic reaction in the stabilised soil, small amounts of pozzolans such as kaolinite, sodium bentonite and fly ash can be added to the cement-stabilised peat (Torkittikul et al. 2017). Under specific conditions, both cement and pozzolan used for peat stabilisation react with water in the soil to generate a high-strength product that binds soil particles together. However, the ratio of lime to silica (Ca(OH)2:SiO2) affects reactivity. The material is more hydraulic if the ratio is higher (Janz and Johansson 2002).
4 Gaps in Peat Improvement Studies
It can be concluded that the effectiveness and dosage of binder type on the stabilisation of peat are site specific since the properties of peat differ geographically. Different types of peat react with different types of binder at certain binder dosage to achieve effective stabilisation. The unconfined compressive strength gain of stabilised soil rose with an increase in binder dosage, filler and curing time, according to a review of numerous experimental examinations of stabilised peat. Abd Rahman (2015) shows that peat strength can be improved. However, two patterns manifest during stabilisation effecting optimum and non-optimum mixtures during the treatment process (Fig. 5.3). The same pattern was detected by Kido et al. (2009), Kalantari et al. (2013) and Huat et al. (2014) where the peat was of less strength after a certain curing period. Most studies stop at this engineering finding. The chemical reaction, other than hydration, is expected to be the main cause of peat solidification. The alteration of the chemical bond in solidified peat may lead to a new product, potentially a more cost-effective and efficient solution.
Strength (qu) versus curing days (Abd Rahman 2015). This infers a potential reaction between microbes and solidified peats. *Red lines indicate optimum mixture
The role of the microbial community in peat decomposition of plants has not been considered. Researchers estimate that microbial activity is frozen during the stabilisation process. However, current research in concrete studies introduces self-healing concrete, where microbes are used to react with chemicals from concrete to self-paste the leaking concrete. The concrete research used CaCO3, the main element found in OPC.
5 Biological Reaction in Peat Solidification
5.1 Microbes in Peat
Microorganisms are responsible for the decomposition of plants in peat. Edaphic communities are varied and comprised of bacteria, actinomycetes, fungi, algae and protozoa. Peat lands contain large microbial populations of a wide metabolic diversity (Williams and Crawford 1983). Studies by Kraigher et al. (2006) and Ausec et al. (2009) explore relationships in microbial activity and structure in two fens and one bog. The result from the studies shows that from the 16S rRNA genes, Proteobacteria and Acidobacteria dominate fen and bog soils (Thrash and Coates 2011). However, bog and fen soils show a clear distinction in their bacterial communities despite sharing dominant phyla. A significant difference can be observed at the level of relative abundance of species affiliated with the phylum Acidobacteria, 23% in the fen and 40% in the bog gene library respectively.
The types of vegetation from which peat originates cause differences in microbial community makeup. Ombrotrophic bogs are made up of slow-decomposing mosses and shrubs that get their nutrients from both dry and wet deposition (Aerts et al. 1999). Minerotrophic fens, on the other hand, are made up of more easily decomposable sedges, plants and shrubs that get their nutrients from groundwater (Bragazza et al. 2007). According to Tamburini et al. (2017), microorganisms using degraded cellulose by a depolymerisation step allow a metabolisation step. Table 5.2 lists the variety of microbes found in different regions of peat.
All microbes have unique characteristics; some are pathogenic and can be harmful to people. The microbes in Pontian peat may be different from peat from Europe countries as studied by Liimatainen et al. (2018), Novak et al. (2018) and Lorenz and Lal (2018).
5.2 Microbes in Solidified Soil
The study of microbes in solidified soil has attracted interest since the last turn of the century. Most studies focus on utilising bacteria in soil treatment (Ivanov and Chu 2008; Kim et al. 2013; Abo-El-Enein 2013; Ismail et al. 2014; Ng et al. 2012). The biocementation concept where microbes and cement are mixed to form a cementation product lessens the void in soil. The bioclogging process was introduced by Ng et al. (2012) where the soil void is filled by microbial-induced biochemical products. In the study, Ng et al. (2012) concentrate used uratic bacteria that are naturally found to produce a cemantitious product. Urase activity was found to increase rapidly in mediums with pH 6–8.
In a study by Ivarson (1977), it was shown that the number of microbes in surface peat (normally fibric peat) was higher compared to those from subsurface (hemic peat) and subsoil (sapric peat). It was recorded that fibric and hemic types have microbe counts of 25 × 105 CFU/g and 5 × 105 CFU/g, respectively. However, when lime was introduced to peat, the number of microbes increased dramatically to 6540 × 105 CFU/g for fibric peat and 2940 × 105 CFU/g for hemic peat. This leads to biocementation where the lime content from cement can be expected to stimulate the microbes in peat.
Ivanov and Chu (2008) investigated the potential of biocementation, which is described as a method that uses microbial activity or products to improve the strength and stiffness properties of soils and rocks. Ivanov and Chu (2008), however, limit their research to clay and sand only. Kucharski et al. (2005) filed a patent application for microbial biocementation, which involves combining a permeable material with a biomass of urease-producing microbes, urea and soluble calcium salts to make a high-strength cement. Microorganisms enable rapid urea hydrolysis, raise pH during urea-to-ammonia hydrolysis and create calcite in soils and rocks. Compressive strength of the cement produced was up to 5 MPa. Conglomerate, breccia, sandstone, siltstone, shale, limestone, gypsum, peat, lignite, sand, soil, clay, sediments and sawdust are among the materials treated by biocementation. Bacillus, Sporosarcina, Sporolactobacillus, Clostridium and Desulfotomaculum are among the urease-producing bacteria used in the study (Ivanov and Chu 2008).
The process of producing urease for the hydrolysis of urea CO (NH2)2 into carbonate (CO32−) and ammonium (NH4+) is as follows (Karthik and Rao 2016):
These reactions increase the pH and form carbonate ions (Ramakrishnan et al. 1998).
5.3 Microbes in Cement Study
Recent trends in concrete or cement studies utilise microbes to develop self-healing concrete. Basically, microbes are known to have unique characteristics which are vulnerable to environmental changes. However, microbes can survive in extreme conditions. The process of self-healing concrete requires adding microbes to the cement before pouring it into the concrete material. In crack-sealing, bacterial concrete is formed via the metabolic conversion of calcium lactate to calcium carbonate (Jonkers et al. 2010; Kim et al. 2013). Whenever cracking occurs in concrete, rainwater fills the crack, stimulating the inserted microbe and reactivating it to produce more carbon dioxide. The carbon dioxide reacts with calcium lactate and produces calcium carbonate which hardens the material (Jonkers 2011). Ismail et al. (2014) and Pradeep Kumar et al. (2015) have confirmed the success of this hypothesis. Small cracking was found to take about 100 days to recover (Joshi et al. 2017). Bacillus spp. work well with cement and are easily obtained in the soil (Ivanov and Chu 2008; Jonkers 2011). Table 5.3 summarises the bacteria that have been used in concrete studies.
Since microbes were reported to be part of the solidification product, microbes present in peat may work the same in solidified peat. However, no successful biocementation field applications have been reported.
6 Chemical Reactions in Peat Solidification
6.1 Chemical Bonding/Sketching for Raw Peat
The chemical bonding of all materials is known as a fingerprint of a substance. Different materials have unique molecular structure. Peat in particular is very dynamic. It consists of several types of plant origin and has different decomposition levels, a wide range of acidity and microbial presence. These parameters have limited the sketching of chemical bonds in peat. A general sketching for peat according to its functional group was introduced by Koch (1982) as seen in Fig. 5.4. In 1993, Yonebayashi et al. (1994a, b) used nuclear magnetic resonance (NMR) and infrared (IR) spectroscopy to locate the functional group of peat. However, Yonebayashi et al. (1994a, b) were unable to sketch the molecular structure of the peat, there was no absolute chain in peat from all three sampling locations and the C/N ratio was unstable. Stevenson (1994) has sketched the molecular bonding of humic acid, a typical acid present in peat soil (Fig. 5.5).
Degradation of plant residues and the formation of humic substances in peat. (Koch 1982)
6.2 Fourier Transform Infrared (FTIR), Nuclear Magnetic Resonance (NMR) and X-Ray Diffraction (XRD) Analysis Explain Chemical Alteration in Peat
FTIR and XRD are used in determining the chemical and mineral elements in samples. FTIR determines the absorbance of infrared rays in a sample. Different functional groups will give different absorbance values and normally read in ranges. XRD works by determining minerals in samples by dispersing X-rays. The combination of information gained by both FTIR and XRD could be utilised to obtain the chemical sketching of specific peat samples. Yonebayashi et al. (1994a, b) examined the occurrence of carboxyl, carbonyl, phenolic hydroxyl and alcoholic hydroxyl groups in humic acids. It has been shown that the amount of lignin and cellulose decreases with increasing humic acid in peat. Yonebayashi et al. (1994a, b) used NMR to classify the chemical in the sample. It was concluded that humic acids from tropical peat have long aliphatic chains due to the origin of their plant source.
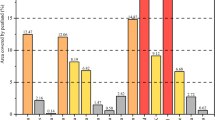
Kyziol (2002) used FTIR to determine functional groups in peat from three different locations. Peat from different sites has the same functional group at wavelength 2900, 2780, 2350, 1480 cm−1 and more minor peaks in the range 800–1300 cm−1 (Fig. 5.6). Table 5.4 summarises the techniques used to consider chemical bonding.
FTIR spectra of selected peats. (Kyziol 2002). W1 Alder Peat Humus, W9b Brushwood Peat Humus, W9c Rush (Reed-Sedge) Peat
6.3 Chemical Reaction Models of Peat Processes
To model chemical reactions that take place in peat solidification, an understanding of the concept of chemical reaction is essential. The kinetics behind chemical reactions are influenced by the rate of reaction, reversible or non-reversible reaction, rate constant, reaction order and reaction mechanism.
The reaction mechanism of materials takes place via adsorption, desorption and surface reaction. These reactions are spurred by temperature, pressure and biological elements. All parameters mentioned must be considered when modelling the chemical reaction of a material. There are chemical reaction models which use conventional equations or mathematical models using software in explaining the reaction that takes place in particular samples.
As peat is derived from plant materials and affected by microbial activity, a biochemical reaction model is suitable for clarifying the reaction inside the solidified peat. The majority of soil biochemical reactions take place in organic dominated layers (McLaren and Peterson 1967). Table 5.5 summarises modelling and simulation of chemical reactions as discussed by Higham (2008).
6.4 Chemical Reactions Behind Soil Improvements
Eisazadeh et al. (2012) used NMR and FTIR techniques to analyse the functional group and local bonding of lime-treated soil. The study treated green bentonite and laterite soil (rich with iron) by using lime in various ratios and curing periods. As bentonite and laterite soils are rich with minerals, changes in clay minerals were expected. Conversely, the silica in quartz was found not to be affected by the presence of lime. The study on the effect of curing period of treated clay found a promising strengthening effect for all types of mixing up to 8 months of curing. However, the NMR and FTIR analysis for all mixing portions and curing periods did not reveal any new element in the mixtures. Comparison between non-treated clay and treated clay differed with a new peak identified as a Ca-OH bond. Ca-OH is a functional group of lime. No new element was found in the treated clay that can explain the strengthening effect gained. According to Eisazadeh et al. (2012), lime treatment did not result in significant changes in functional groups in the soil structure component. The pozzolanic reaction expected happened over the curing period though it was not clearly resolved using FTIR and NMR.
Clay soil is known for its homogeneity in size, pores and retained controllable water. However, peat compounds contain several functional groups, and its chemical structure is hard to be visualised; chemical changes in peat are complex to explore and explain.
7 Case Study in Pontian, Johor, Malaysia
A case study was carried out in Pontian, Johor, Malaysia where three types of peat – fibric, hemic and sapric – were sampled, solidified and tested for strength (qu), bacterial count and crystallite (using XRD) for curing in 7, 14, 28 and 56 days. Two mixing formulations were used following Rahman et al. (2015) for the two patterns of strengthening effects owned by solidified peat. Table 5.7 shows the mixed design for both formulations. This differentiates the chemical and biological reactions that happen when samples steadily increase in strength over the curing period (Mixing 1, M1) and samples that show increase in strength for curing days 7, 14 and 28 while decreasing its strength on day 56 (Mixing 2, M2).
Figures 5.7 and 5.8 show the pattern of bacterial counts and the strength of solidified peat over the curing period for both M1 and M2. In M1 bacteria are depleted but remain present in solidified peat; the depleted colony bears a strong relation with the strength. The stronger the solidified peat, the least bacteria count recorded and vice versa.
The finding from XRD showed that the amount of crystallite formed in solidified peat related with the strength of the sample (Fig. 5.9). The samples were found to be stronger when more crystallite formed and vice versa. The dominant crystal present in all samples was identified as pargasite (NaCa2[Mg4Al](Si6Al2)O22(OH)2) which has similar physical characteristics with ettringite (Ca6Al2[(SO4)3(OH)12]•(24 + 2)H2O) that is normally found in solidified clay as a result of hydration processes.
Pattern of strength and percentage of crystallite formed over curing period for solidified peat using M1 and M2 formulations. (a) Strength and percentage of crystallite formed increase over curing period of solidified peat for Mixing 1. (b) Strength and percentage of crystallite formed increase for D7 and D14 but decrease on D28 and D56 for Mixing 2
The bacteria in this case study were identified as Bacillus sp. and were tested using cellulose agar. This medium allows bacteria secreting the enzyme cellulose to grow. The cellulose was monitored using Congo red, and bacterial activity was recorded, as shown in Table 5.8.
Peat solidification work highlights the formation of crystallite as the product of reaction between OPC, bottom ash (BA), fly ash (FA) and acidic peat. Since bacteria is abundantly present in the peat, the solidification work was not an absolute success as the enzyme secreted by the bacteria was found to dissolve the crystallite formed in M2. This phenomenon may be described using the Michaelis-Menten equation and was used by Cordero et al. (2019) and Sihi et al. (2019) to describe soil enzyme activity.
This finding helps engineers to understand the nature of peat before construction work. It contributes to environmental sustainability by limiting the trial and error during road and basement installation work.
8 Conclusion
It can be concluded that the effectiveness and dosage of binder type on the stabilisation of peat are site specific since the properties of peat differ from site to site. Furthermore, different types of peat react with different types of binder at certain binder dosage to achieve effective stabilisation. The unconfined compressive strength gain of the stabilised soil rose with an increase in binder dosage, filler and curing time, according to a review of numerous experimental examinations of stabilised peat. Most studies stop at this engineering finding with little knowledge exploration of cement hydration theory. The chemical reaction other than hydration theory is expected to be the main reason of peat solidification. The alteration of the chemical bond in solidified peat may lead to a new product finding with more cost-effective and cost-efficient solution.
Although it was previously estimated that microbial activity in peat is frozen during the stabilisation process, current research in concrete studies introduce self-healing concrete where microbes are used to react with chemicals from concrete to self-paste the leaking concrete. The concrete research used CaCO3, the main element also found in OPC. This leads to a reaction between microbes and solidified peat being likely. The Michaelis-Menten equation was found to fit well with peat solidification work. Computational modelling may be developed beyond singular stochastic algorithms. Finally, different types of admixture could be proposed to find the pattern of enzymes reacting with induced chemicals in solidification. The study and nature of microbial activity in peat processes and its optimisation may well prove useful in use and transformation of land resources far into the future.
References
Abd Rahman J (2015) Relationship between decomposition level and induced solidification of peat based on laboratory investigation. Master Degree Thesis. Universiti Tun Hussein Onn Malaysia
Abo-El-Enein SA (2013) Application of microbial biocementation to improve the physico-mechanical properties of cement mortar. Housing and Building National Research Centre
Aerts R, Verhoeven JTA, Whigham DF (1999) Plant-mediated controls on nutrient cycling in temperate fens and bogs. Ecology 80(7):2170–2181. https://doi.org/10.2307/176901
Afifudin H, Hamidah MS, Noor Hana H et al (2011) Microbial participation in the formation of calcium silicate hydrated (CSH) from Bacillus subtilis. Procedia Eng 20:159–165. https://doi.org/10.1016/j.proeng.2011.11.151
Ahnberg H (2006) Consolidation stress effects on the strength of stabilised Swedish soils. Proc Inst Civ Eng Ground Improv 10(1):1–13. https://doi.org/10.1680/grim.2006.10.1.1
Ahnberg H, Ljungkratnz C Holmqvist L (1995) Deep stabilization of different types of soft soils. Proceedings XI ECSMFE, Copenhagen, 7:167–172, 28 May – 1 June 1995
Akol AK (2012) Stabilization of peat soil using lime as a stabilizer. Project Dissertation. Civil Engineering Programme Universiti Teknologi PETRONAS
Al-Ani H, Oh E, Chai G (2013) Engineering properties of peat in estuarine environment. In: 1st International Conference on Foundation and Soft Ground Engineering Challenges in Mekong Delta. 181–191. Thu Dau Mot University, Binh Duong City, Vietnam on June 5–6, 2013
Albert RA, Archambault J, Rossello-Mora R et al (2005) Bacillus acidicola sp. nov., a novel mesophilic, acidophilic species isolated from acidic sphagnum peat bogs in Wisconsin. Int J Syst Evol Microbiol 55(5):2125–2130. https://doi.org/10.1099/ijs.0.02337-0
Ali F, Wong LS, Hashim R (2010) Engineering properties of improved fibrous peat. Sci Res Essay 5(2):154–169
Al-Sadi AM, Al-Zakwani HA, Nasehi A et al (2016) Analysis of bacterial communities associated with potting media. Springerplus 5(1):74. https://doi.org/10.1186/s40064-016-1729-0
Alwi A (2008) Ground improvement of Malaysian peat soils using stabilized peat column techniques. PhD thesis, University of Malaya, Kuala Lumpur, Malaysia
American Coal Ash Association (2007) Coal combustion product (CCP) production and use survey. Available via www.acaa-usa.org. Accessed 13 Feb 2009
Andrew TCS, Syahrizal II, Jamaluddin MY (2012) Effective microorganisms for concrete (EMC) admixture–its effects to the mechanical properties of concrete. In: Awam International Conference on Civil Engineering (AICCE’12) Geohazard Information Zonation (GIZ’12), 28–30 Aug 2012
Andriesse JP (1988) Nature and management of tropical peat soils (No. 59). Food and Agriculture Organization
ASTM International (2005) ASTM Standards: Standards Test Methods and Definitions for Mechanical Testing of Steel Products (A370–02). American Society for Testing and Materials
ASTM (1990) 2607–69. Standard classification on peat, mosses, humus and related products (approved April 25, 1966) 1989 Annual Book of ASTM Standards. American Society for Testing and Materials
Ausec L, Kraigher B, Mandic-Mulec I (2009) Differences in the activity and bacterial community structure of drained grassland and forest peat soils. Soil Biol Biochem 41(9):1874–1881. https://doi.org/10.1016/j.soilbio.2009.06.010
Axelsson K, Johansson SE, Anderson R (2002) Stabilization of organic soils by cement and pozzolanic reaction-feasability study. Swedish Deep Stabilization Research Centre, Report 3, 1–51
Banadaki AD, Ahmad K, Ali N (2013) Influence of natural fillers on shear strength of cement treated peat. Gradevinar 65:633–640
Bang SS, Lippert JJ, Yerra U et al (2010) Microbial calcite, a bio-based smart nanomaterial in concrete remediation. Int J Smart Nano Mater 1(1):28–39. https://doi.org/10.1080/19475411003593451
Billong N, Melo UC, Louvet F et al (2009) Properties of compressed lateritic soil stabilized with a burnt clay-lime binder: effect of mixture components. Constr Build Mater 23:2457–2460. https://doi.org/10.1016/j.conbuildmat.2008.09.017
Boehm MJ, Madden LV, Hoitink HAJ (1993) Effect of organic matter decomposition level on bacterial species diversity and composition in relationship to pythium damping-off severity. Appl Environ Microbiol 59(12):4171–4179
Boelter DH (1968) Important physical properties of peat materials. Proceedings of the Third International Peat Congress. Quebec, Canada 150–156, 18–23 August 1968
Bragazza L, Siffi C, Iacumin P et al (2007) Mass loss and nutrient release during litter decay in peatland: the role of microbial adaptability to litter chemistry. Soil Biol Biochem 39(1):257–267. https://doi.org/10.1016/j.soilbio.2006.07.014
Caldarone MA (2014) High-strength concrete: a practical guide. CRC Press
Chahal N, Siddique R, Rajor A (2012) Influence of bacteria on the compressive strength, water absorption and rapid chloride permeability of fly ash concrete. J Constr Build Mater 28(1):351–356. https://doi.org/10.1016/j.conbuildmat.2011.07.042
Chen H, Wang Q (2006) The behaviour of organic matter in the process of soft soil stabilization using cement. Bull Eng Geo Environ 65(4):445–448. https://doi.org/10.1007/s10064-005-0030-1
Cordero I, Snell H, Bardgett RD (2019) High throughput method for measuring urease activity in soil. Soil Biol Biochem 134:72–77. https://doi.org/10.1016/j.soilbio.2019.03.014
Consoli NC, Zortéa F, De Souza M et al (2011) Studies on the dosage of fiber-reinforced cemented soils. J Mater Civ Eng:1624–1632. https://doi.org/10.1061/(ASCE)MT.1943-5533.0000343
Consoli NC, Rotta GV, Prietto PDM (2000) Influence of curing under stress on the triaxial response of cemented soils. Geotechnique. https://doi.org/10.1680/geot.2000.50.1.99
De Muynck W, Cox K, De Belie N et al (2008) Bacterial carbonate precipitation as an alternative surface treatment for concrete. J Constr Build Mater 22(5):875–885. https://doi.org/10.1016/j.conbuildmat.2006.12.011
Dhakal SK (2012) Stabilization of very weak subgrade soil with cementitious stabilizers. Master Degree Thesis Louisiana State University and Agricultural and Mechanical College, USA
Douglas PR (2004) Properties of self-consolidating concrete containing type F fly ash. Master Degree Thesis. Northwestern University, USA
El-Jazairi B, Illston JM (1977) A simultaneous semi-isothermal method of thermogravimetry and derivative thermogravimetry, and its application to cement plates. Cem Concr Res 7:247–258
Eisazadeh A, Kassim KA, Hadi H (2010) Physicochemical characteristics of phosphoric acid stabilized bentonite. Electron J Geotech Eng 15:327–355
Eisazadeh A, Kassim KA, Nur H (2012) Solid-state NMR and FTIR studies of lime stabilized montmorillonitic and lateritic clays. Appl Clay Sci 67:5–10. https://doi.org/10.1016/j.clay.2012.05.006
Fong SS, Murtedza M (2007) Chemical characterization of humic substances occurring in the peats of Sarawak, Malaysia. Org Geochem 38(6):967–976. https://doi.org/10.1016/j.orggeochem.2006.12.010
Ghosh P, Mandal S, Mandal BD et al (2005) Use of microorganism to improve the strength of cement mortar. J Cem Concr Res 35:1980–1983. https://doi.org/10.1016/j.cemconres.2005.03.005
Ghosh S, Biswas M, Chattopadhyay BD et al (2009) Microbial activity on the microstructure of bacteria modified mortar. J Cem Concr Comp 31(2):93–98. https://doi.org/10.1016/j.cemconcomp.2009.01.001
Green DH, Echavarri-Bravo V, Brennan D et al (2015) Bacterial diversity associated with the coccolithophorid algae Emiliania huxleyi and Coccolithus pelagicus f. braarudii. BioMed Res Int. https://doi.org/10.1155/2015/194540
Guanlin LI, Seongjun KIM, Minji PARK et al (2017) Short-term effects of experimental warming and precipitation manipulation on soil microbial biomass C and N, community substrate utilization patterns and community composition. Pedosphere 27(4):714–724. https://doi.org/10.1016/S1002-0160(17)60408-9
Hartford RA (1993) Smoldering combustion limits in peat as influenced by moisture, mineral content, and organic bulk density. Master Degree Thesis, University of Montana
Hartikainen T, Martikainen PJ, Olkkonen M et al (2002) Peat biofilters in long-term experiments for removing odorous sulphur compounds. Water Air Soil Pollut 133(1–4):335–348. https://doi.org/10.1023/A:1012966705004
Hashim R, Islam S (2008) A Model Study to Determine Engineering Properties of Peat Soil and Effect on Strength after Stabilization. Eur J Sci Res 2:205–215
Hebib S, Farell ER (2003) Some experiences on the stabilisation of Irish peats. Can Geotech J 40:107–120. https://doi.org/10.1139/t02-091
Higham DJ (2008) Modelling and simulating chemical reactions. SIAM Rev 50(2):347–368. https://doi.org/10.1137/060666457
Holden J, Chapman PJ, Labadz JC (2004) Artificial drainage of peatlands: hydrological and hydrochemical process and wetland restoration. Prog Phys Geogr 28(1):95–123. https://doi.org/10.1191/0309133304pp403ra
Horpibulsuk S (2012) Strength and microstructure of cement stabilized clay, scanning electron microscopy. InTech, ISBN: 978-953-51-0092-8
http://ppsj.johor.gov.my/johor-sepintas-lalu/maklumat-asas-negeri-johor (2018) Accessed 12 May 2021
Peat Society (2015). http://www.peatsociety.org. Accessed 12 May 2021
Huat BBK, Kazemian S, Prasad A et al (2011) A study of the engineering behavior of peat stabilized by DMM: lab model and FE analysis. Sci Res Essays J 6(1):196–204
Huat BBK (2004) Organic and peat soil engineering. University Putra Malaysia Press, Serdang
Huat BBK, Prasad A, Asadi A et al (2014) Geotechnics of organic soils and peat. CRC Press, London
Hunter WJ, Kuykendall LD, Manter DK (2007) Rhizobium selenireducens sp. nov.: a selenite-reducing α-proteobacteria isolated from a bioreactor. Curr Microbiol 55(5):455–460. https://doi.org/10.1007/s00284-007-9020-9
Islam MS, Hashim R (2010) Behaviour of stabilised peat: a field study. Sci Res Essays 5(17):2366–2374
Islam S, Hashim R (2008) Stabilization of peat by deep mixing method: a critical review of the state of practice. Electron J Geotech Eng 13:1–9
Ismail N, Mohd Saman H, Kamaruddin K et al (2014) The cement hydration, chemical phases and its microstructural examination of microbed cement based material. In: 2014 IEEE Colloquium on Humanities, Science and Engineering Research (CHUSER 2014), Penang, Malaysia
Istina IN, Widiastuti H, Joy B et al (2015) Phosphate-solubilizing microbe from saprists peat soil and their potency to enhance oil palm growth and P uptake. Procedia Food Sci 3:426–435. https://doi.org/10.1016/j.profoo.2015.01.047
Ivanov V, Chu J (2008) Applications of microorganisms to geotechnical engineering for bioclogging and biocementation of soil in situ. Rev Environ Sci Biotechnol 7(2):139–153. https://doi.org/10.1007/s11157-007-9126-3
Ivarson KC (1977) Changes in decomposition rate, microbial population and carbohydrate content of an acid peat bog after liming and reclamation. Can J Soil Sci 57(2):129–137
Jamaludin M, Ismail YM, Rahman WA et al (2009) Performance of concrete containing effective microorganisms (EM) under various environments. In: Joint Conference of 7th Asia Pacific Structural Engineering and Construction Conference 2009 (APSEC2009) and 2nd European Asian Civil Engineering Forum (EACEF 2009), Malaysia
Janz M, Johansson S (2002) The function of different binding agents in deep stabilization. Report 9, Swedish Deep Stabilization Research Center, Linköping, Sweden. 1-47
Jonkers HM (2011) Bacteria-based self-healing concrete. Heron 56(1/2):1–12
Jonkers HM, Thijssen A, Muyzer G et al (2010) Application of bacteria as self-healing agent for the development of sustainable concrete. Ecol Eng 36:230–235. https://doi.org/10.1016/j.ecoleng.2008.12.036
Joshi S, Goyal S, Mukherjee A et al (2017) Microbial healing of cracks in concrete: a review. J Ind Microbiol Biotech 44(11):1511–1525. https://doi.org/10.1007/s10295-017-1978-0
Kalantari B, Prasad A, Huat BBK (2013) Cement and silica fume treated columns to improve peat ground. Arab J Sci Eng 38:805–816. https://doi.org/10.1007/s13369-012-0369-0
Karim MR, Zain MFM, Jamil M et al (2011) Strength development of mortar and concrete containing fly ash: A review. Int J Phys Sci 6(17):4137–4153
Karol RH (2003) Chemical grouting and soil stabilization, revised and expanded, vol 12. CRC Press
Karthik C, Rao RM (2016) Properties of bacterial-based self-healing concrete- A review. Int J ChemTech Res 9(2):182–188
Kazemian S, Huat BBK, Prasad A et al (2011a) A state of art review of peat: Geotechnical engineering perspective. Int J Phys Sci 6(8):1974–1981
Kazemian S, Huat BK, Prasad A et al (2011b) Effect of peat media on stabilization of peat by traditional binders. Int J Phys Sci 6(3):476–481
Kazemian S, Prasad A, Huat BB et al (2011c) Influence of cement–sodium silicate grout admixed with calcium chloride and kaolinite on sapric peat. J Civ Eng Manag 17(3):309–318. https://doi.org/10.3846/13923730.2011.589209
Kido Y, Nishimoto S, Hayashi H et al (2009) Effects of curing temperatures on the strength of cement-treated peat. International Symposium on Deep Mixing and Admixture Stabilization, Okinawa, 2009
Kim HK, Park SJ, Han JI et al (2013) Microbially mediated calcium carbonate precipitation on normal and lightweight concrete. J Constr Build Mater 38:1073–1082. https://doi.org/10.1016/j.conbuildmat.2012.07.040
Kip N, Van Winden JF, Pan Y et al (2010) Global prevalence of methane oxidation by symbiotic bacteria in peat-moss ecosystems. Nat Geosci 3(9):617–621. https://doi.org/10.1038/ngeo939
Koch W (1982) Findings with operation of biofilters for reduction of odourmtensive emissions. (original in German), Die Fleischmehl-Indusrtie. £, 165–175 (1982) in Domenico M.S. Delicato, Physical-Chemical Properties and Sorption Characteristics of Peat. PhD Thesis, Dublin City University
Kraigher B, Stres B, Hacin J et al (2006) Microbial activity and community structure in two drained fen soils in the Ljubljana Marsh. Soil Biol Biochem 38(9):2762–2771. https://doi.org/10.1016/j.soilbio.2006.04.031
Kucharski ES, Winchester W, Leeming WA et al (2005) Microbial biocementation. Patent Application WO/2006/066326; International Application No.PCT/AU2005/001927
Kyziol J (2002) Effect of physical properties and cation exchange capacity on sorption of heavy metals onto peats. Pol J Environ Stud 11(6):713–718
Liimatainen M, Voigt C, Martikainen PJ et al (2018) Factors controlling nitrous oxide emissions from managed northern peat soils with low carbon to nitrogen ratio. Soil Biol Biochem 122:186–195. https://doi.org/10.1016/j.soilbio.2018.04.006
Lindsay R, Birnie R, Clough J (2015) Impacts of artificial drainage on peatlands. IUCN UK Committee Peatland Programme Briefing Note No. 3. National Committee, United Kingdom
Liu J, Shi B, Jiang H, Huang H et al (2011) Research on the stabilization treatment of clay slope topsoil by organic polymer soil stabilizer. Eng Geol 117(1):114–120. https://doi.org/10.1016/j.enggeo.2010.10.011
Lorenz K, Lal R (2018) Carbon sequestration in wetland soils. In: Carbon sequestration in agricultural ecosystems. Springer, Cham. https://doi.org/10.1007/978-3-319-92318-5_5
Makusa GP (2012) Soil stabilization methods and materials in engineering practice. Lulea University of Technology, Lulea, Sweden
Mandic-Mulec I, Ausec L, Danevčič T et al (2014) Microbial community structure and function in peat soil. Food Technol Biotechnol 52(2):180–187
Mass Stabilization Manual (2005) Idea Chip and Ramboll Finland
McLaren AD, Peterson GH (1967) Introduction to the biochemistry of terrestrial soils. Soil Biochemistry 1:1–15
Mehta PK (1987) Natural pozzolans: Supplementary cementing materials in concrete. CANMET Special Publication 86:1–33
Mesri G, Ajlouni M (2007) Engineering properties of fibrous peats. J Geotech Geoenviron Eng 133:850–866. https://doi.org/10.1061/(ASCE)1090-0241(2007)133:7(850)
Mohamed M, Padmanabhan E, Mei BLH et al (2002) The Peat Soils of Sarawak. Strapeat- status report, University Malaysia Sarawak
Mousavi SE (2018) Utilization of silica fume to maximize the filler and pozzolanic effects of stabilized soil with cement. Geotech Geol Eng 36(1):77–87. https://doi.org/10.1007/s10706-017-0305-x
Muhardi Marto A, Kassim KA, Maktar AM et al (2010) Engineering characteristics of Tanjung Bin coal ash. Electron J Geotech Eng 15:1117–1129
Mutalib AA, Lim JS, Wong MH et al (1992) Characterization, distribution and utilization of peat in Malaysia. In: Aminudin BY (ed) Proceeding of the International Symposium on Tropical Peatland, Serdang: MARDI. 7-16. In: Mohamed M, Padmanabhan E, Mei BLH, Siong WB (2002). The Peat Soils of Sarawak. Strapeat- status report, Universiti Malaysia Sarawak
New York City Building Code (2008) Code Counsel, New York, U.S.A
New Zealand Building Code Requirements (2011) Bearing capacity and geotechnical investigation requirements for buildings. Gisborne District Council, New Zealand
Ng WS, Lee ML, Hii SL (2012) An overview of the factors affecting microbial-induced calcite precipitation and its potential application in soil improvement. World Acad Sci Eng Technol. https://doi.org/10.5281/zenodo.1084674
Novak M, Stepanova M, Buzek F et al (2018) The fate of 15 N tracer in waterlogged peat cores from two central european bogs with different N pollution history. Water Air Soil Pollut 229(3):70. https://doi.org/10.1007/s11270-018-3731-3
Konsultant PS (1998) Detailed Design and Construction Supervision of Flood Protection and Drainage Facilities for Balingian RGC Agricultural Development Project, Sibu Division, Sarawak (Inception Report). Department of Irrigation and Drainage, Kuching
Pradeep Kumar A, Akila D, Anestraj S, Arun S, Santhoshkumar A (2015) An experimental work on concrete by adding Bacillus Subtilis. Int J Emerging Technol Adv Eng 2(4):69–73
Rahman JA, Chan CM (2013) Influence of temperature on the mass losses of tropical peat at different decomposition level. In: Soft Soil Engineering International Conference
Rahman ZA, Lee JY, Rahim SA, Lihan T, Idris WMR (2015) Application of gypsum and fly ash as additives in stabilization of tropical peat soil. J Appl Sci 15(7):1006
Ramakrishnan V, Bang SS, Deo KS (1998) A Novel technique for repairing cracks in high performance concrete using bacteria. In: Proceedings of International conference on high performance high strength concrete. Perth, Australia, pp 597–518
Rao SM, Shivananda P (2005) Compressibility behaviour of lime-stabilized clay. Geotech Geol Eng 23(3):309–319. https://doi.org/10.1007/s10706-004-1608-2
Rashid ZA, Alias AB, Aris MJ et al (2010) Hazardous waste management: current status and future strategies in Malaysia. Int J Environ Eng 2(1):139–158. https://doi.org/10.1504/IJEE.2010.029825
Robert HK, Rober LK (1996) Treatment wetlands. Lewis Publishers, Boca Raton, New York
Romão LP, Lead JR, Rocha JC et al (2007) Structure and properties of Brazilian peat: analysis by spectroscopy and microscopy. J Braz Chem Soc 18(4):714–720. https://doi.org/10.1590/S0103-50532007000400008
Rong H, Qian CX, Li LZ (2012) Study on microstructure and properties of sandstone cemented by microbe cement. J Constr Build Mater 36:687–694. https://doi.org/10.1016/j.conbuildmat.2012.06.063
Rotta GV, Consoli NC, Prietto PDM et al (2003) Isotropic yielding in an artificially cemented soil cured under stress. Geotechnique 53(5):493–501. https://doi.org/10.1680/geot.2003.53.5.493
Rybicki E (1990) The classification of organisms at the edge of life, or problems with virus systematics. S Afr J Sci 86:182–186
Santagata M, Bobet A, Johnston CT et al (2008) One-dimensional compression behavior of a soil with high organic matter content. J Geotech Geoenviron Eng 134(1):1–13
Santamarina JC, Klein KA, Wang YH et al (2002) Specific surface area: determination and relevance. Can Geotech J 39:233–241. https://doi.org/10.1139/t01-077
Sihi D, Inglett PW, Inglett KS (2019) Warming rate drives microbial nutrient demand and enzyme expression during peat decomposition. Geoderma 336:12–21. https://doi.org/10.1016/j.geoderma.2018.08.027
Sposito G (2008) The chemistry of soils, 2nd edn. Oxford University press Inc
Stevenson FJ (1994) Humus chemistry: genesis, composition, reactions. Wiley
Tamburini D, Łucejko JJ, Zborowska M et al (2017) The short-term degradation of cellulosic pulp in lake water and peat soil: a multi-analytical study from the micro to the molecular level. Int Biodeterior Biodegradation 116:243–259. https://doi.org/10.1016/j.ibiod.2016.10.055
Report TBPP (2010) Malakoff Berhad. Johor, Malaysia
The Fly Ash Resource Center (2010) The fly ash resource center. Available via http://www.rmajko.com/flyash.html. Accessed 6 Apr 2010
Thrash JC, Coates JD (2011) Acidobacteriaceae fam. nov. Bergey’s Manual of Systematic Bacteriology 4:728
Torkittikul P, Nochaiya T, Wongkeo W et al (2017) Utilization of coal bottom ash to improve thermal insulation of construction material. J Mater Cycles Waste Manag 19(1):305–317. https://doi.org/10.1007/S10163-015-0419-2
Tremblay H, Duchesne J, Locat J et al (2002) Influence of the nature of organic compounds on fine soil stabilization with cement. Can Geotech J 39(3):535–546. https://doi.org/10.1139/t02-002
USACE (1995) Chemical grouting [R]. US Army Corps of Engineers. Manual No. 1110-1-3500, Washington D C, USA
Van Hardeveld HA, Driessen PPJ, Schot PP et al (2017) An integrated modelling framework to assess long-term impacts of water management strategies steering soil subsidence in peatlands. Environ Impact Assess Rev 66:66–77
Wang KS, Lin KL, Lee TY et al (2004) The hydration characteristics when C2S is present in MSWI fly ash slag. Cem Concr Res 26:323–330. https://doi.org/10.1016/j.jhazmat.2004.03.014
Williams RT, Crawford RL (1983) Microbial diversity of Minnesota peatlands. Microb Ecol 9(3):201–214. https://doi.org/10.1007/BF02097737
Wolińska A, Stępniewska Z (2012) Dehydrogenase activity in the soil environment. In: Dehydrogenases. InTech
Wong LS (2015) Formulation of an optimal mix design of stabilized peat columns with fly ash as a pozzolan. Arab J Sci Eng 40(4):1015–1025. https://doi.org/10.1007/s13369-015-1576-2
Wong LS, Hashim R, Ali F (2008) Engineering behaviour of stabilized peat soil. Eur J Sci Res 21(4):581–591
Wong LS, Hashim R, Ali F (2009) Unconfined compressive strength of cemented peat. Aust J Basic Appl Sci 3(4):3850–3856
Wong LS, Hashim R, Ali F (2013a) Utilization of sodium bentonite to maximize the filler and pozzolanic effects of stabilized peat. Eng Geol 152(1):56–66
Wong LS, Hashim R, Ali F (2013b) Improved strength and reduced permeability of stabilized peat: focus on application of kaolin as a pozzolanic additive. Constr Build Mater 40:783–792. https://doi.org/10.1016/j.conbuildmat.2012.11.065
Wongsa A, Zaetang Y, Sata V et al (2016) Properties of lightweight fly ash geopolymer concrete containing bottom ash as aggregates. Constr Build Mater 111:637–643. https://doi.org/10.1016/j.conbuildmat.2016.02.135
Wösten JHM, Ismail AB, Van Wijk ALM (1997) Peat subsidence and its practical implications: a case study in Malaysia. Geoderma 78(1–2):25–36. https://doi.org/10.1016/S0016-7061(97)00013-X
Yonebayashi K, Okazaki M, Pechayapisit J et al (1994a) Distribution of heavy metals among different bonding forms in tropical peat soils. Soil Sci Plant Nutr 40(3):425–434. https://doi.org/10.1080/00380768.1994.10413320
Yonebayashi K, Pechayapisit J, Vijarnsorn P et al (1994b) Chemical alterations of tropical peat soils determined by Waksman’s proximate analysis and properties of humic acids. Soil Sci Plant Nutr 40(3):435–444. https://doi.org/10.1080/00380768.1994.10413321
Žifčáková L, Větrovský T, Howe A et al (2016) Microbial activity in forest soil reflects the changes in ecosystem properties between summer and winter. Environ Microbiol 18(1):288–301. https://doi.org/10.1111/1462-2920.13026
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Rahman, J.A., Mohamed, R.M.S.R., Durahim, N.H.A., Tajuddin, S.A., Al-Gheethi, A.A.S. (2022). Mathematical Modelling and Simulation of Chemical and Biological Reaction in Peat Solidification Work for Environmental Sustainability. In: Furze, J.N., Eslamian, S., Raafat, S.M., Swing, K. (eds) Earth Systems Protection and Sustainability. Springer, Cham. https://doi.org/10.1007/978-3-030-98584-4_5
Download citation
DOI: https://doi.org/10.1007/978-3-030-98584-4_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-98583-7
Online ISBN: 978-3-030-98584-4
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)