Abstract
Under low nitrogen (N) input into rain-fed peat bogs, Sphagnum moss efficiently filters incoming N, preventing invasion of vascular plants and peat oxygenation. Elevated atmospheric N deposition, in combination with climatic warming, may cause retreat of bryophytes and degradation of peat deposits. There are concerns that higher emissions of greenhouse gases, accompanying peat thinning, will accelerate global warming. Breakthrough of deposited N below living moss has been quantified for two Central European peat bogs dominated by Sphagnum magellanicum. In the 1990s, the northern site, ZL, received three times more atmospheric N (> 40 kg ha−1 year−1) than the southern site, BS. Today, atmospheric N inputs at both sites are comparable (15 and 11 kg ha−1 year−1, respectively). Replicated peat cores were collected from the wet central segments of both study sites, 15N-NO3− tracer was applied on the moss surface, and the peat cores were incubated under water-logged conditions. After 40 weeks, the rate of downcore leaching of the 15N tracer was assessed. The recent history of high N pollution at ZL did not accelerate 15N penetration into deeper peat layers, relative to BS. At both sites, less than 3% of the 15N tracer reached the shallow depth of 9 cm. Analysis of control peat cores, along with a 210Pb chronology, revealed removal of the “excess” N from the ZL peat profiles prior to sampling. Following a decrease of atmospheric N pollution in the past two decades, efficient filtering of atmospheric N by Sphagnum at ZL has been renewed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Since the beginning of the Industrial Revolution, anthropogenic emissions of reactive nitrogen (Nr), mainly nitrate (NO3−) and ammonium (NH4+), have more than doubled atmospheric Nr deposition (Galloway et al. 2004). Today, the biogeochemical cycle of N belongs to the most disturbed cycles among all chemical elements (Johnson and Lindberg 1992). Large amounts of ammonium originate from agricultural practices, whereas nitrate is primarily derived from industrial operations (Dise et al. 2011). Urbanization contributes to the emissions of both reduced and oxidized forms of Nr (Gabor et al. 2017).
Wetlands represent a sizeable store of N, amounting approximately to 16% of global soil N (Wieder and Vitt 2006). There are concerns that global warming, in combination with elevated N deposition, will cause peat degradation and thinning (Gorham 1991, Granath et al. 2014). This will be accompanied by higher biogenic emissions of greenhouse gases (CO2, CH4, N2O) which, in turn, may accelerate global warming (Clymo et al. 1998, Sheppard et al. 2013). While fertilization with elevated N inputs may lead to higher net primary productivity in rain-fed bogs, it will also augment microbial decomposition (Breeuwer et al. 2008, Song et al. 2017). It appears that accelerated litter decomposition under high N deposition is typical only of the early stages of peat formation. By contrast, high N deposition may slow down decomposition of recalcitrant organic matter in later stages of peat formation (Manninen et al. 2016).
Most high-latitude peatlands are severely N-limited (Sorrell et al. 2011). Lamers et al. (2000) interpreted the response of Sphagnum mosses to increasing N deposition as a three-step process with different underlying biogeochemical controls. Under low N depositions (< 12 kg N ha−1 year−1), increasing N input has no effect on N concentrations in Sphagnum. Incoming N is rapidly taken up by the moss because N availability limits the growth. Nitrogen concentration is determined by productivity and not by N deposition rates. Increased C uptake leads to a relatively constant N concentration. Under medium N depositions (12–18 kg N ha−1 year−1), there is a positive relationship; higher N inputs lead to higher N concentration in the moss. Nitrogen is no longer a growth-limiting nutrient and it accumulates in the tissue. Under high N depositions (18–60 kg N ha−1 year−1), there is again no positive response. Atmospheric N inputs are no longer bound to the moss layer; the ecosystem reaches N saturation. Nitrogen is leached into deeper bog layers, where it becomes available to the roots of vascular plants. An invasion of vascular plants is detrimental to the preservation of C and N pools in wetland soil. The roots of vascular plants introduce oxygen to deeper peat layers and augment decomposition of organic matter (Heijmans et al. 2002). Litter of vascular plants per se is more easily degradable than litter of Sphagnum, also decreasing C and N accumulation (Jirousek et al. 2011). The scenario by Lamers et al. (2000) has been modified by Bragazza et al. (2005, 2006) and Harmens et al. (2014).
The mechanisms responsible for the mass transfer of atmospheric N into Sphagnum (the top ca. 5 cm) and peat (deeper than 5 cm) are still poorly understood (Xing et al. 2011). Downward translocation of atmospheric N could be dominated by particulate organic N (Blodau et al. 2006) or microbial immobilization following the transport of dissolved Nr along a concentration gradient (Pinsonneault et al. 2016).
In recent years, Lamers’s threshold for N breakthrough and leaching (ca. 18 kg N ha−1 year−1) has been tested in several N polluted bogs (e.g., Xing et al. 2011, Chiwa et al. 2016, Zając and Blodau, 2016). These data have shown that under N-rich conditions (over 18 kg N ha−1 year−1) Sphagnum still can effectively filter inorganic N and immobilize it in the thin topmost layer.
Nitrogen has two stable isotopes, 14N (natural abundance of 99.635%) and 15N (natural abundance of 0.365%). The heavier stable isotope 15N has been used as an efficient tracer of translocations and transformations of N in natural systems. An artificial mixture containing 98% of 15N was applied in several field and laboratory studies to investigate the dispersion of atmospheric N in the wetland (Li and Vitt 1997; Franzes and Loiseau 1999; Williams et al. 1999, Nordbakken et al. 2003; Blodau et al. 2006; Xing et al. 2011; Fritz et al. 2014; Zając and Blodau, 2016). These studies typically combined high and low water table periods, relative to the capitula of living Sphagnum. As a rule, inclusion of a (natural or simulated) drought resulted in a higher downward mobility of atmospheric N in peat. The 15N tracer reached the unsaturated Sphagnum and substrate surfaces above the low water table almost immediately following its application (Williams et al. 1999). Vice versa, water saturation resulted in slower penetration of the tracer into deeper substrate layers (Blodau et al. 2006). In the long term, such experimental designs gave realistic results on the overall vertical mobility of N in peat, but the maximum depth reached by the tracer often reflected the relative duration of high and low water table conditions; the longer the drought, the deeper the tracer distribution. In our previous studies (Novak et al. 1994, 2003, Vile et al. 2000, Bohdalkova et al. 2013), we observed that large segments of mountain-top ombrotrophic peat bogs in Central Europe (usually those situated close to a bog water pool) remained water-saturated throughout the year. Given that the hydraulic conductivity in saturated peat rapidly decreases with an increasing depth, the question arises of how the generally wet conditions in Central European peat bogs affect the vertical N mobility in peat.
Here, we present a 15N tracer study using replicated peat cores from two Sphagnum-dominated sites with contrasting N pollution histories. Until recently, the industrial northeast of the Czech Republic received three times more atmospheric N than the semi-natural ecosystems in the southwest (Fottova 2003). Today, both regions receive similar, relatively small amounts of atmospheric N. Our objective was to evaluate the robustness of N retention in waterlogged Sphagnum peat at a northeastern and a southwestern site by comparing the rates of penetrating of atmospheric Nr into deeper substrate layers. We hypothesized that at the northeastern site N saturation at a time of peak air pollution 20–30 years ago could have led to N leaching and removal from the peat profiles. Present-day N filtering capacity of living Sphagnum at the northeastern site could be re-established, becoming as high as the N filtering capacity at the southwestern site.
2 Materials and Methods
2.1 Study Sites
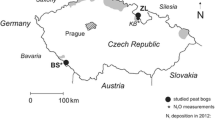
The two study sites are ombrotrophic, Sphagnum-dominated peat bogs situated in spruce forested mountains near the state borders of the Czech Republic, Central Europe (Fig. 1, Table 1). Since the beginning of the Holocene (11.5 thousand years BP), both sites have accumulated at least 7 m of freshwater peat (Dohnal et al. 1965). The less polluted study site, Blatenska stat (BS), is part of the Sumava Mts. National Park in southwestern Bohemia. The area near BS is dominated by forestry and agriculture. Torfowisko Pod Zielencem (ZL) is located in Silesia, Poland, 260 km northeast of BS, 3 km from the Czech border. ZL and the nearby Eagle Mts. are situated in the vicinity of a highly industrialized area with numerous open-pit soft coal mines, coal-fired power stations, base-metal smelters, and heavy industry operations (Erbanova et al. 2008, Bohdalkova et al. 2014a). The mountain range near ZL was affected by spruce die-back resulting from SOx and NOx emissions during the period of central planning (Oulehle et al. 2017). Air pollution peaked in 1987 in the Czech Republic and around 1990 in Poland (Kopacek and Vesely 2005). Atmospheric S and N deposition decreased following the return of market economy to both countries during the 1990s. The central segments of both peat bogs are unforested and relatively wet. At both sites, bog water pH is between 3.9 and 5.3, and slightly increases downcore. Stagnating water column with little vertical mixing is typical throughout the year. Following major precipitation events, 95% of horizontal water flow occurs in the topmost several centimeters of the Sphagnum cover. No groundwater inputs were detected. Nitrogen pools and fluxes at both sites were studied using natural abundance N isotope ratios by Novak et al. (2016).
2.2 Sampling
At each study site, six peat cores, 10-cm in diameter, 15-cm long, were taken in a lawn, avoiding hummocks and hollows. The distance between the two adjacent peat cores was 20 to 40 m. Sampling took place in late April, i.e., 1 month after the end of the snowmelt period. All 12 peat cores were 100% covered with Sphagnum magellanicum Brid., and the water table level was 1 to 2 cm below surface. During sampling, care was taken not to drain the porewater; the cores were kept vertically, transported to the laboratory, and placed in a growth chamber (15 °C day, 9 °C night, daylight duration of 14 h per day) for a 2-week acclimation.
2.3 Experimental Design
The design of the peat incubation experiment is summarized in Table 2. Six replicate peat cores per site were randomly divided in a group of three cores that were subjected to the 15N treatment and a group of three control cores. The surface of each treatment core received 0.087 mg 15N in the form of water-soluble Na15NO3 in one dose at the onset of the incubation experiment. The control cores were incubated with no added 15N. The peat cores, placed vertically in a growth chamber, were allowed to drain gravitationally through a mesh into a beaker; however, no pore water was recovered at the bottom of the peat cores throughout the 40-week experiment. Bog water from each site was used to top up the polyethylene cylinders with peat so that the water table level was kept 2 cm below Sphagnum capitula throughout the experiment. The day/night and temperature regime during the experiment (Table 2) was identical to the acclimation period. After 40 weeks, the peat cores were cut into 3-cm segments, dried at 40 °C to a constant mass, and homogenized.
2.4 Analysis
Bog water and peat from BS and ZL were analyzed for major cations and anions using conventional methods (Oulehle et al. 2017). For NO3− analysis, HPLC Knauer 1000 was used with a detection limit of 0.3 mg L−1; for NH4+ analysis, a Perkin-Elmer Lambda 25 spectrophotometer was used with a detection limit of 0.02 mg L−1. The detection limits for K and P in peat were 50 ppm. For the N concentration and isotope analysis, two separate 10-mg aliquots of peat were placed in a tin (Sn) capsule and combusted at 1040 °C with copper (Cu) grains and oxides in a Fisons 1108 elemental analyzer. Nitrogen concentrations in peat were determined with a reproducibility of ± 1.5%. The N isotope compositions were determined on a Delta V mass spectrometer coupled with the Fisons 1108 elemental analyzer. Natural abundance N isotope ratios were expressed in the δ15N notation as a ‰ deviation of the 15N/14N composition of the sample from that of a standard (atmospheric N2). IAEA isotope standards were analyzed every 1-day session (N1 with a δ15N = 0.5 ‰ and N2 with a δ15N = 20.3 ‰). Additionally, in-house standards [(NH4)2SO4 with a δ15N = − 1.7‰ and glycine with a δ15N = 4.0‰] were analyzed following every six samples during each analytical run. The reproducibility of the δ15N values of peat was ± 0.35‰.
The results of the 15N tracer experiment were expressed as the content of 15N atoms in total N atoms. 15N excess values (in atomic %) were obtained by subtracting the background 15N of Sphagnum or peat from the labeled samples (see Xing et al. 2011 and Zając and Blodau, 2016 for details).
Statistical analysis was performed using the R software, version 3.4.2 (R Core Team 2017) and the nlme package (Pinheiro et al. 2016). The two study sites were compared by the mean of generalized least squares (Pinheiro and Bates 2000), assuming possibly different variability of the outcomes at BS and ZL. The comparison was adjusted for a possible effect of depth. In the following sections, means and standard errors are given.
3 Results
3.1 Chemistry of the Bog Water
Bog water from each site was used as the moisturizing solution during peat incubation. Its chemistry is given in Table 3. Conductivity of the bog water from the historically more polluted site ZL was higher by a factor of three, compared to the less polluted site BS. The ZL bog water was more acidic, compared to the BS bog water (pH of 4.2 vs. 5.3). Concentrations of NO3− and NH4+ in the bog water were nearly twice higher at ZL than at BS (Table 3). Concentration of SO42− in ZL bog water was more than twice higher, compared to BS.
3.2 Peat Density and Ash Content
Statistically, peat bulk density did not differ between the two sites (p > 0.05). The mean peat bulk density at BS (0.049 ± 0.004 g cm−3) was slightly higher than the mean peat bulk density at ZL (0.045 ± 0.002 g cm−3). At both sites, the peat bulk density increased with an increasing depth (Fig. 2a). Cumulative peat mass increased smoothly downcore and was indistinguishable at both sites (p > 0.05; Fig. 2b). Ash content at both sites was below 5 wt%, which is the customary upper limit for ombrotrophic peatlands. Significantly more ash was found at ZL (2.06 ± 0.22 wt%) than at BS (1.29 ± 0.08 wt%; p < 0.05).
3.3 Carbon and Nutrients in Peat
Carbon (C) concentration in peat was significantly higher at BS (42.3 ± 0.3 wt.%) than at ZL (40.7 ± 0.4 wt.%; p < 0.05; Table S1 in the Electronic Annex). Nitrogen concentration was statistically indistinguishable at the two sites (0.91 ± 0.04 wt% at BS and 0.84 ± 0.04 wt% at ZL; p > 0.05; Table S1 in the Electronic Annex). With an increasing depth, N concentration in peat generally increased (Fig. 3a). C:N ratios were similar at both sites and decreased with an increasing peat depth (Fig. 4a). Phosphorus (P) concentration was significantly higher at ZL (377 ± 29 ppm) than at BS (286 ± 12 ppm; p < 0.05; Table S1 in the Electronic Annex). N:P ratios were between 20 (ZL, deeper than 3 cm) and 40 (BS, deeper than 10 cm), with no clear-cut depth gradient. One exception was a sharp downcore decrease in N:P between the two topmost peat samples (Fig. 4b). Potassium (K) concentration was statistically indistinguishable between the two sites (p > 0.05). The mean K concentration at BS was 1040 ± 240 ppm, the mean K concentration at ZL was 1180 ± 210 ppm (Table S1 in the Electronic Annex). N:K ratios increased with an increasing peat depth at both sites and were either similar at both sites or higher at BS (Fig. 4c).
3.4 Natural Abundance N Isotope Ratios in Peat
All 30 measured δ15N values of bulk peat were negative (δ15N of the standard corresponds to N isotope composition of atmospheric N2; δ15Nstd = 0 ‰). The δ15N values of bulk peat were significantly less negative at BS (− 2.9 ± 0.2‰) than at ZL (− 3.4 ± 0.1‰; p < 0.05; Table S1 in the Electronic Annex). In the shallowest depth interval, δ15N values of bulk peat increased more at BS than at ZL. In deeper peat sections, only a very small downcore increase in δ15N values was observed (Fig. 3b).
3.5 Downcore Leaching of the 15N-NO3 − Tracer in Peat
Total 15N recovery was calculated at the end of the 40-week peat incubation experiment by summing up 15N yields in five 3-cm thick peat segments (depths of 0 to 15 cm below peat surface). As mentioned already in the “Materials and Methods” section, no 15N was leached below the depth of 15 cm. Relative to the amount of 85 μg of 15N applied on the surface of each core at time t = 0, the 15N recovery in peat at the end of the experiment was 99.6 ± 0.2%.
The result of the 15N tracer experiment is given in Fig. 3c. In incubated peat from both sites, the amount of excess 15N decreased with an increasing depth. This decrease was just slightly faster at BS, compared to ZL (see the second shallowest peat segment, 3–6 cm below surface in Fig. 3c). However, the maximum depth reached by the 15N tracer was the same in peat cores from both sites (p > 0.05). Only 2.6% of the 15N tracer was detected deeper than 9 cm below peat surface.
4 Discussion
4.1 Contrasting N Pollution History of BS and ZL
Regional differences in atmospheric N deposition in the Czech Republic have been discussed by Fottova (1995, 2003), Fottova and Skorepova (1998), Fisak et al. (2002), Oulehle et al. (2011, 2016, 2017), Hunova et al. (2014), and Novak et al. (2014, 2015a,b, 2016). Historical reconstructions of atmospheric N loads in the Czech Republic were performed by Kopacek et al. (2001), Kopacek and Vesely (2005), and Kopacek and Posch (2011). Table 4 summarizes the chemistry of open-area deposition (bulk precipitation) and spruce canopy throughfall in the hydrological year 1995, i.e., at the beginning of hydrogeochemical monitoring in the GEOMON system of small headwater catchments (Fottova and Skorepova 1998). The two sites selected for Table 4 are located close to the currently studied peat bogs; LIZ is located near BS in the southwest, while UDL is located near ZL in the northeast (Fig. 1). In the water year 1995, NO3− deposition in clearings at UDL was by a factor of 2.7 higher than that at LIZ. NH4+ deposition in clearings at UDL was by a factor 2.2 higher than that at LIZ. In the same year, throughfall at UDL contained six times more NO3− and seven times more NH4+ than throughfall at LIZ. Open-area deposition is a better measure of the relative N pollution levels in the two regions, since part of the N in throughfall is leached from the canopy, and some atmospheric N is intercepted by the canopy. Novak et al. (2015a) have shown that horizontal deposition in N-polluted peat bogs adds approximately 30% of N to the vertical deposition in an open area.
Nitrogen deposition in 2013, i.e., in the year of peat sampling for the 15N tracer experiment, was measured directly at BS and ZL (Novak et al. 2016). Figure 5 compares annual N depositions in 1995 and 2013 for both nitrate and ammonium. Whereas total N deposition into BS was generally lower and did not change over the 18-year period, total N deposition into ZL decreased 2.8 times. In 2013, N deposition into the Sphagnum cover was 11.4 kg ha−1 year−1 at BS and 15.1 kg ha−1 year−1 at ZL (Fig. 5). The contribution of NO3− to total N deposition was about 40% at BS and 60% at ZL (Fig. 5).
Atmospheric deposition of reactive nitrogen in 1995 and 2013. For site location see Fig. 1
As we have seen in Table 3, N concentrations in surface bog water reflect higher pollution at ZL, relative to BS. In 2013, NO3− and NH4+ concentrations in bog water were less than twice higher at ZL than at BS. This agrees well with the less than twice higher atmospheric deposition at ZL, compared to BS, in 2013 (Fig. 5). A similar pattern was reported by Limpens et al. (2003) and Bragazza and Limpens (2004), who found higher N concentrations in interstitial bog water under higher atmospheric N inputs.
According to the classification by Lamers et al. (2000), present-day BS with 11.4 kg N ha−1 year−1 is a site with low N pollution but close to the lower limit of medium-polluted sites (12 kg N ha−1 year−1), and ZL with 15.1 kg N ha−1 year−1 is a medium-polluted site (i.e., falls within the range of 12–18 kg N ha−1 year−1). In the mid-1990s, ZL was a highly N polluted site (42 kg ha−1 year−1; Fig. 5), while BS was characterized by a low N pollution level (10 kg ha−1 year−1; Fig. 5).
4.2 δ15N Trends in Bulk Peat
Shallow peat cores often exhibit an increase in δ15N values with an increasing depth (Nadelhoffer et al. 1996, Kuhry and Vitt 1996, Högberg 1997, Novak et al. 1999, 2014, Kalbitz and Geyer 2002, Blodau et al. 2006, Esmeijer-Liu et al. 2012). As seen in Fig. 3b, a downcore increase in δ15N values of bulk peat was more pronounced at BS than at ZL. Previous literature ascribed this N isotope trend to preferential release of isotopically light (14N-enriched) nitrogen during the degradation of organic molecules, typical of early stages of peat diagenesis. The BS trend in δ15N in peat is similar to previously published trends in δ13C and δ34S values along vertical peat profiles (Novak et al. 1994, 1999, 2010, Bohdalkova et al. 2014b). Indeed, isotopically heavier C, N, and S in deeper horizons are also typical of aerated forest soils (Nadelhoffer and Fry 1988, Novak et al. 1996). The proposed controlling mechanism is always the same: preferential removal of isotopically light C, N, and S during decomposition of organic matter, with isotopically heavier residual C, N, and S, observed in older/deeper soil layers. We suggest that peat maturation and removal of mobile, low-δ15N nitrogen were responsible for the BS trend in Fig. 3b. Recently, several studies have shown that temporal changes in environmental parameters also affect δ15N trends in ageing peat. For example, peat decomposition was faster during periods of higher ash inputs (Broder et al. 2012). Multiple microbial recycling of atmospheric N within the peat profile, availability of other nutrients, such as P, shifts in plant community structure, and land use changes also affect the downcore δ15N profiles (Kohzu et al. 2003, Limpens et al., 2004, Inglett et al. 2007, Jones et al. 2010, Tfaily et al. 2014, Biester et al. 2014, Kruger et al. 2015). Generally, higher δ15N values in BS peat at most peat depths, compared to ZL peat (Fig. 3b), may be related to the fact that microbial N2 fixation could have played a more important role at BS as the less N polluted site. BS may have experienced a convergence of δ15N values of bulk peat toward the N isotope signature of atmospheric N2 (0‰) as a result of N2 fixation. Microbial N2 fixation, a thermodynamically costly process, would be less likely at ZL as the more N polluted site. For more detailed discussion of N2 fixation by Sphagnum-dominated wetlands see Larmola et al.(2014), Vile et al. (2014), Novak et al. (2016), and Van den Elzen et al. (2017).
4.3 Nitrogen Mobility in Peat and Downcore Leaching of the 15N Tracer
As we have mentioned in the “Introduction,” peak tropospheric N pollution in the study region dates to the late 1980s and early 1990s. Our 15N incubation experiment took place in 2013, which is 20 to 30 years after the maximum atmospheric N input onto the Sphagnum surface. Lead-210 dating at Czech sites repeatedly shows that 20- to -30-year-old substrate is found in rather shallow layers (5–15 cm; Mihaljevic et al. 2006, 2008, Novak et al. 1994, 2008). Such shallow depths coincide with the depth of penetration of 15N in previous tracer studies in highly polluted wetlands (e.g., Heijmans et al. 2002, Nordbakken et al. 2003, Blodau et al. 2006). We hypothesized that shallow peat layers at ZL may contain as little N as analogical shallow peat layers at BS because of the past N saturation at ZL. Indeed, the N concentration in bulk peat at both study sites was indistinguishable (Fig. 3a). Statistically, no peak in N concentration in bulk peat was detected (p > 0.05). We conclude that the “excess” N deposited on the Sphagnum surface at ZL around 1990, with respect to today’s deposition (Fig. 5), was leached to peat layers deeper than the studied interval of 0–15 cm below surface and/or removed laterally via surface runoff. Some of the excess N could have been emitted as N2O or N2 resulting from denitrification (see Novak et al. 2015a,b for data from a peat bog close to ZL). This conclusion is corroborated by Fig. 6 in which we converted peat depth into an age scale. For the purpose of Fig. 6, we interpolated 210Pb ages from 2-cm peat segments (Novak et al. 2017, Table S2 in the Electronic Annex) to 3-cm peat segments. Figure 6a depicts the atmospheric N emissions, dominated by the industrial north of the Czech Republic according to Kopacek and Vesely (2005). Figure 6b plots N concentrations in peat from BS and ZL in a temporal perspective, and Fig. 6c gives N accumulation rates in individual peat segments, based on N concentrations, peat bulk density, and 210Pb dates. Neither Fig. 6b nor Fig. 6c exhibit the 1990 maximum in atmospheric N emissions seen in Fig. 6a.
Historical N emission rates in the Czech Republic, dominated by the industrial north according to Kopacek and Vesely (2005) (a), plot of N concentration in peat vs. age (b), and plot of N accumulation rates in peat vs. age (c)
In light of the nearly identical vertical N concentration and accumulation patterns in peat from BS and ZL, it may not be surprising that the maximum depth of penetration of the 15N tracer in our incubation experiment was the same at both sites (Fig. 3c). No 15N tracer was detected deeper than 12 cm below surface, and only less than 3% of the tracer were found deeper than 9 cm below surface. Neither of the study sites currently belongs to the “highly N polluted” sites (> 18 kg N ha−1 year−1) sensu Lamers et al. (2000). At both sites, green Sphagnum plants were present to a depth of 5 cm below surface. Forty weeks after tracer application, most tracer remained in the Sphagnum cover (Fig. 3c, two topmost samples).
4.4 Comparison with Previous 15N Tracer Experiments in Freshwater Wetlands
Our experiment differs from previously published 15N tracer studies in that the vertical peat profiles were kept water-saturated. None of the previous studies reported more efficient interception of a 15N tracer in the topmost peat segments, compared to Fig. 3c. Zając and Blodau (2016) studied the fate of 15N nitrate in mesocosms from five peatlands differing in N deposition rates and found that the decline in N retention above atmospheric input of 15 kg N ha−1 year−1 was more modest than previously thought. After 24 weeks and multiple 15N applications, Sphagnum (including Sp. magellanicum Brid.) intercepted about one half of the 15N tracer, and 78–98% of the 15N tracer pool in peat were recovered in the top 20 cm of the profile. Because of its lower density, Sphagnum continued to be a more efficient filter for the 15N tracer than the underlying peat, even at high atmospheric N depositions of more than 50 kg ha−1 year−1. The water table level was 28 and 8 cm below the moss layer. Dissolved inorganic N (DIN) contributed less than 1% to the 15N retention, regardless of the N pollution level (Zając and Blodau, 2016). Xing et al. (2011) reported the results of a15NO3− tracer experiment in two ombrotrophic peat bogs in Canada. The highest atmospheric N deposition found then in Canada was 15 kg ha−1 year−1, which is close to the N input at ZL in 2013, the year of our incubation experiment. At the Canadian sites, rapid immobilization of inorganic 15N by microorganisms and mycorrhizal assimilation was observed. A sharp decrease of the 15N signal was seen below the depth of 10 cm after 4 weeks. This depth was similar to that in our 40-week-long experiment. 15N content in the microbial biomass in the Canadian study was lower compared to that in Sphagnum and the underlying peat, and cycling of the tracer by microorganisms was fast. The mean residence time of 15N in microbial biomass was mere 7 days. Xing et al. (2011) also showed that the relative amount of the tracer found in the microbial biomass decreased with an increasing atmospheric N deposition.
Blodau et al. (2006) studied peat receiving 6 and 15 kg N ha−1 year−1. In the experiment, a NH415NO3 addition increased N input to 15 and 47 kg ha−1 year−1, while the water table was kept 1 and 32 cm below Sphagnum capitula. After 14 weeks, substantial 15N mobility was ascribed to the water table drawdown. Up to 11% of the tracer was found 8 to 12 cm below surface, with up to 99% of the tracer retained in the unsaturated upper zone. At the end of the experiment, only 1% of the 15N tracer was found in DIN and DON, indicating relative stability of the organic N forms. Blodau et al. (2006) suggested that most downcore N mobility in peat is controlled by particulate organic matter transport. This mechanism is unlikely in our experimental design because of the low hydraulic conductivity in waterlogged peat (Fraser et al. 2001). An important conclusion of the Blodau et al. (2006) study was that even an extremely high N input of 47 kg ha−1 year−1 did not compromise the filtering function of the topmost layers of the peatland.
Li and Vitt (1997) working in relatively unpolluted parts of Canada reported that most 15N was immediately sequestered by the moss layer. However, due to an extremely low water table, some tracer was recovered as deep as 40 cm below surface. Nordbakken et al. (2003) found that nearly all N entering the bog was incorporated into the organic matter even when inorganic N input was increased to 40 kg ha−1 year−1 for a period of 3 years. The 15N label was recovered mainly from the peat depths of 5 and 10 cm below surface, similar to our results (Fig. 3).
Chiwa et al. (2016) suggested that the Sphagnum N filter is more compromised when NH4+ largely dominates the elevated atmospheric N deposition, compared to NO3−. In our experiments (Fig. 3c), we did not study NH4+mobility in peat, however, ammonium did not largely dominate atmospheric N deposition at either study site. Slightly more ammonium N, compared to nitrate N, was deposited at the southwestern less polluted site BS (Fig. 5). It remains to be seen whether this small difference in N speciation in the atmospheric input affects the overall N leaching at BS and ZL. Heijmans et al. (2002) used 15N-labeled ammonium in a peat core experiment with the water table fluctuating between 5 and 20 cm below surface. The 15N tracer applied as ammonium did reach deeper peat layers than in our experiment with 15N labeled nitrate (15 cm vs. < 9 cm), but this greater N mobility in the Heijmans et al. (2002) experiment was likely a result of more aerated peat, compared to our experiment.
Fritz et al. (2014) conducted a combined 15NH4+/15NO3− study in one pristine and one polluted wetland confirming the conclusion by Nordbakken et al. (2003) that 15N binding at adsorption sites was negligible. The NH4+-N uptake rates by Sphagnum moss were initially higher than those of NO3−-N, but over time the uptake rates of both N species became similar. Fritz et al. (2014) proposed that long exposure to high N inputs significantly decreases N uptake rates by Sphagnum and the underlying peat at all doses. This conclusion is consistent with our interpretation of Fig. 3a and Fig. 6b, c, specifically with the missing accumulation of excess industrial N in 20- to 30-year-old peat layers at ZL. Leaching of N may result from saturation of temporal N storage pools in the vacuoles and cell walls. The concept proposed by Fritz et al. (2014) broadens the scenarios of Lamers et al. (2000) which specified the bog’s response to changing N deposition. There may be no fixed upper threshold of N uptake by Sphagnum related to N saturation. Instead, N uptake may decrease over time, regardless of N concentrations in Sphagnum, microbes and topmost peat layers.
4.5 Nutritional Constraints in Sphagnum Under Different N Inputs
Clearly, the N filter function of Sphagnum is influenced by an interaction with other nutrients. Bragazza et al. (2004) argued that at N depositions higher than 10 kg ha−1 year−1 Sphagnum in rain-fed bogs changes from being N limited to P and/or K limited. Specifically, the P limitation occurs under N:P > 30, and N:K > 3. Accordingly, Sphagnum at both BS and ZL is currently P limited (Fig. 4b). Older peat layers record N:P ratios greater than 30 only at BS, indicating that P may have not been a limiting nutrient. However, caution must be exercised because diagenesis alters elemental ratios in peat profiles. The N:K ratio in living Sphagnum was greater than 3 only at BS (Fig. 3c), indicating K limitation. Historically, N:K ratios were much greater than 3 in both peat profiles but, again, may have been modified by diagenesis. We note that Jirousek et al. (2011) found generally low N:P ratios across numerous Sphagnum-dominated sites in Central and Eastern Europe, suggesting that relatively high P availability rather than low N availability prevented P limitation. In general, differences in P and K availability at BS and ZL did not cause nutrient imbalances strong enough to affect downcore 15N leaching in our peat incubation experiment.
5 Conclusions
A 40-week laboratory incubation experiment using replicated peat cores from two Central European peat bogs confirmed our hypothesis that contrasting N pollution history would not affect downcore leaching of a 15NO3− trace, as long as today’s atmospheric N inputs are similar. The maximum depth of the tracer recovery was the same at both sites. At the end of the experiment, less than 3% of the tracer was found deeper than 9 cm below surface. The low rate of 15N leaching downcore at both sites was a result not only of relatively low present-day atmospheric N inputs (11 and 15 kg ha−1 year−1 at BS and ZL, respectively) but also of the complete water saturation of incubated peat cores (the water table was kept at 2 cm below the capitula of Sp. magellanicum Brid.). By contrast, most previous field and laboratory studies included a period of water table drawdown which facilitated vertical penetration of the 15N tracer to deeper layers of the acrotelm. Importantly, N contents and accumulation rates in shallow peat layers at ZL, corresponding to the peak pollution period (> 40 kg N ha−1 year−1), were as low as those at the significantly less polluted BS. The high atmospheric Nr inputs at ZL 20–30 years ago temporarily compromised the filtering function of Sphagnum, and the excess N was leached from the peat profile. Following a decrease to atmospheric N pollution levels comparable to BS, efficient filtering of atmospheric N by Sphagnum at ZL has been renewed.
References
Biester, H., Knorr, K. H., Schellekens, J., Basler, A., & Hermanns, Y. M. (2014). Comparison of different methods to determine the degree of peat decomposition in peat bogs. Biogeosciences, 11(10), 2691–2707.
Blodau, C., Basiliko, N., Mayer, B., & Moore, T. R. (2006). The fate of experimentally deposited nitrogen in mesocosms from two Canadian peatlands. Science of the Total Environment, 364, 215–228.
Bohdalkova, L., Curik, J., Kubena, A. A., & Buzek, F. (2013). Dynamics of methane fluxes from two peat bogs in the Ore Mountains, Czech Republic. Plant Soil Environment, 1, 14–21.
Bohdalkova, L., Novak, M., Stepanova, M., Fottova, D., Chrastny, V., Mikova, J., & Kubena, A. A. (2014a). The fate of atmospherically derived Pb in Central European catchments: insights from spatial and temporal pollution gradients and Pb isotope ratios. Environmental Science & Technology, 48(8), 4336–4343.
Bohdalkova, L., Novak, M., Buzek, F., Kreisinger, J., Bindler, R., Pazderu, K., & Pacherova, P. (2014b). The response of a mid- and high latitude peat bog to predicted climate change: methane production in a 12-month peat incubation. Mitigation and Adaptation Strategies for Global Change, 19(7), 997–1010.
Bragazza, L., & Limpens, J. (2004). Dissolved organic nitrogen dominates in European bogs under increasing atmospheric N deposition. Global Biogeochemical Cycles, 18(4), GB4018.
Bragazza, L., Tahvanainen, T., Kutnar, L., Rydin, H., Limpens, J., Hajek, M., et al. (2004). Nutritional constraints in ombrotrophic Sphagnum plants under increasing atmospheric nitrogen deposition in Europe. New Phytologist, 163(3), 609–616.
Bragazza, L., Limpens, J., Gerdol, R., Grosvernier, P., Hajek, M., Hajek, T., et al. (2005). Nitrogen concentration and δ15N signature of ombrotrophic Sphagnum mosses at different N deposition levels in Europe. Global Change Biology, 11(1), 106–114.
Bragazza, L., Freeman, C., Jones, T., Rydin, H., Limpens, J., Fenner, N., et al. (2006). Atmospheric nitrogen deposition promotes carbon loss from peat bogs. Proceedings of the National Academy of Sciences of the United States of America, 103(51), 19386–19389.
Breeuwer, A., Heijmans, M., Robroek, B. J. M., Limpens, J., & Berendse, F. (2008). The effect of increased temperature and nitrogen deposition on decomposition in bogs. Oikos, 117(8), 1258–1268.
Broder, T., Blodau, C., Biester, H., & Knorr, K. H. (2012). Peat decomposition records in three pristine ombrotrophic bogs in southern Patagonia. Biogeosciences, 9(4), 1479–1491.
Core Team, R. (2017). R: a language and environment for statistical computing. Vienna, Austria: R Foundation for statistical computing http://www.R-projetc.org.
Chiwa, M., Sheppard, L. J., Leith, I. D., Leeson, S. R., Tang, Y. S., & Cape, J. N. (2016). Sphagnum can ‘filter’ N deposition, but effects on the plant and pore water depend on the N form. Science of the Total Environment, 559, 113–120.
Clymo, R. S., Turunen, J., & Tolonen, K. (1998). Carbon accumulation in peatland. Oikos, 81, 368–388.
Dise, N. B., Ashmore, M., Belyazid, S., Bleeker, A., Bobbink, R., de Vries, W., et al. (2011). Nitrogen as threat to European terrestrial biodiversity. In M. A. Sutton, C. M. Howard, J. W. Erisman, G. Billen, A. Bleeker, P. Grennfelt, H. van Grinsven, & B. Grizetti (Eds.), The European Nitrogen Assessment (pp. 463–494). Cambridge: Cambridge University Press.
Dohnal, Z., Kunst, M., Mejstrik, V., Raucina, S., & Vydra, V. (1965). Czechoslovak Peatlands. Czechoslovakia: Czechoslovak Academy of Sciences.
Erbanova, L., Novak, M., Fottova, D., & Dousova, B. (2008). Export of arsenic from forested catchments under easing atmospheric pollution. Environmental Science & Technology, 42(19), 7187–7192.
Esmeijer-Liu, A. J., Kurschner, W. M., Lotter, A. F., Verhoeven, J. T. A., & Goslar, T. (2012). Stable carbon and nitrogen isotopes in a peat profile are influenced by early stage diagenesis and changes in atmospheric CO2 and N deposition. Water Air and Soil Pollution, 223(5), 2007–2022.
Fisak, J., Tesar, M., Rezacova, D., Elias, V., Weignerova, V., & Fottova, D. (2002). Pollutant concentrations in fog and low cloud water at selected sites of the Czech Republic. Atmospheric Research, 64(1–4), 75–87.
Fottova, D. (1995). Regional evaluation of mass element fluxes - GEOMON network of small catchments. Environmental Monitoring and Assessment, 34(2), 215–221.
Fottova, D. (2003). Trends in sulphur and nitrogen deposition fluxes in the GEOMON network, Czech Republic, between 1994 and 2000. Water Air and Soil Pollution, 150(1–4), 73–87.
Fottova, D., & Skorepova, I. (1998). Changes in mass element fluxes and their importance for critical loads: GEOMON network, Czech Republic. Water Air and Soil Pollution, 105(1–2), 365–376.
Franzes, A. J., & Loiseau, P. (1999). The fate of mineral nitrogen in a fen with Sphagnum fallax klinggr. and Carex rostrata stokes (Massif central, France). Canadian Journal of Botany, 77, 1136–1143.
Fraser, C. J. D., Roulet, N. T., & Lafleur, M. (2001). Groundwater flow patterns in a large peatland. Journal of Hydrology, 246(1–4), 142–154.
Fritz, C., Lamers, L. P. M., Riaz, M., van den Berg, L. J. L., & Elzenga, T. J. T. M. (2014). Sphagnum mosses - masters of efficient N-uptake while avoiding intoxication. PLoS One, 9(1), 1–11.
Gabor, R. S., Hall, S. J., Eiriksson, D. P., Jameel, Y., Millington, M., Stout, T., et al. (2017). Persistent urban influence on surface water quality via impacted groundwater. Environmental Science & Technology, 51(17), 9477–9487.
Galloway, J. N., Dentener, F. J., Capone, D. G., Boyer, E. W., Howarth, R. W., Sitzinger, S. P., et al. (2004). Nitrogen cycles: past, present, future. Biogeochemistry, 70, 153–226.
Gorham, E. (1991). Northern peatlands: role in the carbon cycle and probable responses to climatic warming. Ecological Applications, 1, 182–195.
Granath, G., Limpens, J., Posch, M., Mücher, S., & de Vries, W. (2014). Spatio-temporal trends of nitrogen deposition and climate effects on Sphagnum productivity and European peatlands. Environmental Pollution, 187, 73–80.
Harmens, H., Schnyder, E., Thoni, L., Cooper, D. M., Mills, G., Leblond, S., et al. (2014). Relationship between site-specific nitrogen concentrations in mosses and measured wet bulk atmospheric nitrogen deposition across Europe. Environmental Pollution, 194, 50–59.
Heijmans, M. M. P. D., Klees, H., de Visser, W., & Berendse, F. (2002). Effects of increased nitrogen deposition on the distribution of 15N-labeled nitrogen between Sphagnum and vascular plants. Ecosystems, 5, 500–508.
Högberg, M. (1997). Tansley review no. 95. 15N abundance in soil-plant system. New Phytologist, 137, 179–203.
Hunova, I., Maznova, J., & Kurfurst, P. (2014). Trends in atmospheric deposition fluxes of sulphur and nitrogen in Czech forests. Environmental Pollution, 184, 668–675.
Inglett, P. W., Reddy, K. R., Newman, S., & Lorenzen, B. (2007). Increased soil stable nitrogen isotopic ratio following phosphorus enrichment: historical patterns and tests of two hypotheses in a phosphorus-limited wetland. Oecologia, 153(1), 99–109.
Jirousek, M., Hajek, M., & Bragazza, L. (2011). Nutrient stoichiometry in Sphagnum along a nitrogen deposition gradient in highly polluted region of Central-East Europe. Environmental Pollution, 159(2), 585–590.
Johnson, D. W., & Lindberg, S. E. (Eds.). (1992). Atmospheric deposition and forest nutrient cycling. Berlin Heidelberg New York: Springer.
Jones, M. C., Peteet, D. M., & Sambrotto, R. (2010). Late-glacial and Holocene δ15N and δ13C variation from a Kenai Peninsula, Alaska peatland. Palaeogeography Palaeoclimatology Palaeoecology, 293(1–2), 132–143.
Kalbitz, K., & Geyer, S. (2002). Different effects of peat degradation on dissolved organic carbon and nitrogen. Organic Geochemistry, 33(3), 319–326.
Kohzu, A., Matsui, K., Yamada, T., & Sugimoto, A. (2003). Significance of rooting depth in mire plants: evidence from natural 15N abundance. Ecological Research, 18(3), 257–266.
Kopacek, J., & Posch, M. (2011). Anthropogenic nitrogen emissions during the Holocene and their possible effect on remote ecosystems. Global Biogeochemical Cycles, 25(GB2017), 1–16.
Kopacek, J., & Vesely, J. (2005). Sulfur and nitrogen emissions in the Czech Republic and Slovakia from 1850 till 2000. Atmospheric Environment, 39(12), 2179–2188.
Kopacek, J., Vesely, J., & Stuchlik, E. (2001). Sulphur and nitrogen fluxes and budgets in the Bohemian Forest and Tatra Mountains during the Industrial Revolution (1850–2000). Hydrology and Earth System Sciences, 5(3), 391–405.
Kruger, J. P., Leifeld, J., Glatzel, S., Szidat, S., & Alewell, C. (2015). Biogeochemical indicators of peatland degradation—a case study of a temperate bog in northern Germany. Biogeosciences, 12(10), 2861–2871.
Kuhry, P., & Vitt, D. H. (1996). Fossil carbon/nitrogen ratios as a measure of peat decomposition. Ecology, 77, 271–275.
Lamers, L. P. M., Bobbing, R., & Roelofs, J. G. M. (2000). Natural nitrogen filter fails in polluted raised bogs. Global Change Biology, 6, 583–586.
Larmola, T., Leppanen, S. M., Tuitila, E.-S., Aarva, M., Merila, P., Fritze, H., & Tiirola, M. (2014). Methanotrophy induces nitrogen fixation during peatland development. Proceedings of the National Academy of Sciences of the United States of America, 112(2), 734–739.
Li, Y. H., & Vitt, D. H. (1997). Patterns of retention and utilization of aerially deposited nitrogen in boreal peatlands. Ecoscience, 4, 106–116.
Limpens, J., Berendse, F., & Klees, H. (2003). N deposition affects N availability in interstitial water, growth of Sphagnum and invasion of vascular plants in bog vegetation. New Phytologist, 157, 339–347.
Limpens, J., Berendse, F., & Klees, H. (2004). How P affects the impact of N deposition on Sphagnum and vascular plants in bogs. Ecosystems, 7, 793–804.
Manninen, S., Kivimäki, S., Leith, I. D., Leeson, S. R., & Sheppard, L. J. (2016). Nitrogen deposition does not enhance Sphagnum decomposition. Science of the Total Environment, 571, 314–322.
Mihaljevic, M., Zuna, M., Ettler, V., Chrastny, V., Sebek, O., Strnad, L., & Kyncl, T. (2008). A comparison of tree rings and peat deposit geochemical archives in the vicinity of a lead smelter. Water Air and Soil Pollution, 188(1–4), 311–321.
Mihaljevic, M., Zuna, M., Ettler, V., Sebek, O., Strnad, L., & Golias, V. (2006). Lead fluxes, isotopic and concentration profiles in a peat deposit near a lead smelter (Pribram, Czech Republic). Science of the Total Environment, 372(1), 334–344.
Nadelhoffer, K., & Fry, B. (1988). Controls on natural nitrogen-15 and carbon-13 abundances in forest soil organic matter. Soil Science Society of America Journal, 52, 1633–1640.
Nadelhoffer, K., Shaver, G., Fry, B., Giblin, A., Johnson, L., & McKane, R. (1996). N-15 natural abundances and N use by tundra plants. Oecologia, 107(3), 386–394.
Nordbakken, J. F., Ohlson, M., & Högberg, P. (2003). Boreal bog plants: Nitrogen sources and uptake of recently deposited nitrogen. Environmental Pollution, 126, 191–200.
Novak, M., Bottrell, S. H., Fottova, D., Buzek, F., Groscheova, H., & Zak, K. (1996). Sulfur isotope signals in forest soils of Central Europe along an air-pollution gradient. Environmental Science & Technology, 30(12), 3473–3476.
Novak, M., Buzek, F., & Adamova, M. (1999). Vertical trends in δ13C, δ15N and δ34S ratios in bulk Sphagnum peat. Soil Biology & Biogeochemistry, 31(9), 1343–1346.
Novak, M., Buzek, F., Jackova, I., Stepanova, M., & Prechova, E. (2017). Isotope constraints on microbial N2-fixation in ombrotrophic peat bogs. Annual Report, Czech Geological Survey, Prague. Grant project no. 16-18079S (Czech Science Foundation).
Novak, M., Emmanuel, S., Vile, M. A., Erel, Y., Veron, A., Paces, T., et al. (2003). Origin of lead in eight Central European peat bogs determined from isotope ratios, strengths and operation times of regional pollution sources. Environmental Science & Technology, 37, 437–445.
Novak, M., Erel, Y., Zemanova, L., Bottrell, S. H., & Adamova, M. (2008). A comparison of lead pollution record in Sphagnum peat with known historical Pb emission rates in the British Isles and the Czech Republic. Atmospheric Environment, 42, 8997–9006.
Novak, M., Gebauer, G., Thoma, M., Curik, J., Stepanova, M., Jackova, I., et al. (2015b). Denitrification at two nitrogen-polluted, ombrotrophic Sphagnum bogs in Central Europe: insights from porewater N2O-isotope profiles. Soil Biology & Biogeochemistry, 81, 48–57.
Novak, M., Jackova, I., Curik, J., Stepanova, M., Veselovsky, F., Buzek, F., et al. (2016). Contrasting δ15N values of atmospheric deposition and Sphagnum peat bogs: N fixation as a possible cause. Ecosystems, 19, 1037–1050.
Novak, M., Stepanova, M., Jackova, I., Vile, M. A., Wieder, R. K., Buzek, F., et al. (2014). Isotopic evidence for nitrogen mobility in peat bogs. Geochimica et Cosmochimica Acta, 133, 351–361.
Novak, M., Veselovsky, F., Curik, J., Stepanova, M., Fottova, D., Prechova, E., & Myska, O. (2015a). Nitrogen input into Sphagnum bogs via horizontal deposition: an estimate for N-polluted high-elevation sites. Biogeochemistry, 123(1–2), 307–312.
Novak, M., Wieder, R. K., & Schell, W. R. (1994). Sulfur during early diagenesis in Sphagnum peat: insights from δ34S ratio profiles in 210Pb-dated peat cores. Limnology and Oceanography, 39(5), 1172–1185.
Novak, M., Zemanova, L., Buzek, F., Jackova, I., Adamova, M., Komarek, A., et al. (2010). The effect of a reciprocal peat transplant between two contrasting Central European sites on C cycling and C isotope ratios. Biogeosciences, 7(3), 921–932.
Oulehle, F., Chuman, T., Hruska, J., Kram, P., McDowell, W. H., Myska, O., et al. (2017). Recovery from acidification alters concentrations and fluxes of solutes from Czech catchments. Biogeochemistry, 132(3), 251–272.
Oulehle, F., Evans, C. D., Hofmeister, J., Krejci, R., Tahovska, K., Persson, T., et al. (2011). Major changes in forest carbon and nitrogen cycling caused by declining sulphur deposition. Global Change Biology, 17(10), 3115–3129.
Oulehle, F., Kopacek, J., Chuman, T., Cernohous, V., Hunova, I., Hruska, J., et al. (2016). Predicting sulphur and nitrogen deposition using a simple statistical method. Atmospheric Environment, 140, 456–468.
Pinheiro, J. C., & Bates, D. (2000). Mixed-effects models in S and S-Plus. New York: Springer-Verlag.
Pinheiro, J. C., Bates, D., DebRoy, S., Sarkar, D., & R Core Team. (2016). Nlme: linear and nonlinear mixed effect models. R package version, 3, 1–128 http://CRAN.R-project.org/package=nlme.
Pinsonneault, A. J., Moore, T. R., & Roulet, N. T. (2016). Effects of long-term fertilization on peat stoichiometry and associated microbial enzyme activity in an ombrotrophic bog. Biogeochemistry, 129(1–2), 149–164.
Sheppard, L. J., Leith, I. D., Leeson, S. R., van Dijk, N., Field, C., & Levy, P. (2013). Fate of N in a peatland, Whim bog: immobilization in the vegetation and peat, leakage into pore water and losses as N2 depend on the form of N. Biogeosciences, 10, 149–160.
Song, Y., Song, C., Meng, H., Swarzenski, C. M., Wang, X., & Tan, W. (2017). Nitrogen additions affect litter quality and soil biochemical properties in a peatland of Northeast China. Ecological Engineering, 100, 175–185.
Sorrell, B. K., Chague-Goff, C., Basher, L. M., & Partridge, T. R. (2011). N:P ratios, δ15N fractionation and nutrient resorption along a nitrogen to phosphorus limitation gradient in an oligotrophic wetland complex. Aquatic Botany, 94(2), 93–101.
Tfaily, M. M., Cooper, W. T., Kostka, J. E., Chanton, P. R., Schadt, C. W., Hanson, P. J., et al. (2014). Organic matter transformations in the peat column at Marcell Experimental Forest: humification and vertical stratification. Journal of Geophysical Research – Biogeosciences, 119(4), 661–675.
Van den Elzen, E., Kox, M. A. R., Harpenslager, S. F., Hensgens, G., Fritz, C., Jetten, M. S. M., et al. (2017). Symbiosis revisited: phosphorus and acid buffering stimulate N2 fixation but not Sphagnum growth. Biogeosciences, 14(5), 1111–1122.
Vile, M. A., Wieder, R. K., & Novak, M. (2000). 200 years of Pb deposition throughout the Czech Republic: patterns and sources. Environmental Science & Technology, 34, 12–21.
Vile, M. A., Wieder, R. K., Zivkovic, T., Scott, K. D., Vitt, D. H., Hartsock, J. A., et al. (2014). N2-fixation by methanotrophs sustains carbon and nitrogen accumulation in pristine peatlands. Biogeochemistry, 121(2), 317–328.
Wieder, R. K., & Vitt, D. H. (Eds.). (2006). Boreal peatland ecosystems. Berlin Heidelberg New York: Springer.
Williams, B. L., Silcock, D. J., & Young, M. (1999). Seasonal dynamics of N in two Sphagnum moss species and the underlying peat treated with 15NH4 15NO3. Biogeochemistry, 45, 285–302.
Xing, Y., Bubier, J., Moore, T. R., Murphy, M., Basiliko, N., Wendel, S., & Blodau, C. (2011). The fate of 15N-nitrate in a northern peatland impacted by long term experimental nitrogen, phosphorus and potassium fertilization. Biogeochemistry, 103, 281–296.
Zając, K., & Blodau, C. (2016). The fate of 15N-nitrate in mesocosms from five European peatlands differing in long-term nitrogen deposition rate. Biogeosciences, 13, 707–722.
Acknowledgements
We thank Dr. Andrea Kucerova of the Botanical Institute of the Czech Academy of Sciences, Trebon, for a consultation on phosphorus cycling.
Funding
This work was funded by the Czech Science Foundation, Project No. 16-18079S to MN.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Novak, M., Stepanova, M., Buzek, F. et al. The Fate of 15N Tracer in Waterlogged Peat Cores from Two Central European Bogs with Different N Pollution History. Water Air Soil Pollut 229, 70 (2018). https://doi.org/10.1007/s11270-018-3731-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-018-3731-3