Abstract
Biocompatible artificial or synthetic materials have wide application in chest surgery and surgery in general today. In the field of chest surgery, they are being used to reconstruct chest wall defects after extensive resection due to primary or secondary neoplasm, congenital anomalies, infections, radiation injuries and trauma. After an extensive wall resection, large defects of soft tissue and bone structures (sternum and ribs) can remain, which require carefully planned and extensive reconstruction of such defects. Reconstruction of the chest wall after such extensive resections is a procedure aimed at stabilizing the bone wall and reconstructing the soft tissues. When planning resection of the chest wall due to neoplasm, a particular attention must be paid to the general condition of the patient as well as to the size and the localization of the tumor, since the defects extending from 5 to 7 cm in the largest diameter rarely require stabilization, while larger defects mostly require some type of stabilization procedures in order to preserve the normal lung function. Also, localization of resection is very important. For example, the resection covering the area of the scapula, or the area of the large chest muscles rarely requires stabilization of the chest wall, while on the other hand, those involving the lateral or the lower part of anterior portion of the thoracic wall should always be subjected to the stabilization procedure.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
1 Introduction
Today, numerous biological and synthetic materials are used for the reconstruction of the chest wall. Biological materials may be autologous, such as: fascia lata, bone grafts (ribs, tibia, fibula, iliac crest, dura mater, pericardium) or heterologous (dura mater, pericardium, fascia). The expanded use of synthetic materials for the reconstruction of the chest wall encourages their diversity, availability, inert nature, and ease of use. It should be kept in mind that no material is absolutely inert and that the human body reacts to the presence of a foreign body by inflammation and the formation of pseudocapsules. Synthetic materials are rigid and fragile and can migrate into tissue, which can sometimes lead to serious injury to internal organs (i.e., lungs). Most of the synthetic materials used for implantation are produced in the form of plates, supporters or meshes. The plates can be made of metal or fiberglass, while meshes are fabricated from Teflon (polytetrafluoroethylene), nylon, polypropylene, prolene or vicryl. Also, today there are synthetic prostheses such as those consisted of acrylic derivatives or Teflon, as well as those made of composite materials. Nowadays, composite protective products made of methacrylate and marble meshes are increasingly being applied. In difference to synthetic woven meshes, the composite protective products made of methacrylate and marble meshes are increasingly being used due to their ability to fully cover massive chest wall defects, preclude paradoxical respirations, and avoid the development of chest malformations (Tamburini et al. 2019). As part of the preparation of the surgical intervention, the size of the chest wall defect that remains after radical resection is being assessed, and after that, a methyl acrylate implant is being created by 3D printing to be finally incorporated into the defect site between the two layers of the Marlex mesh. In addition to their hardness, these protheses maintain the physiological curvature of the chest wall, they are easily fixed to the chest wall, and, above all, they are fairly inexpensive (Goldsmith et al. 2020). In the process of their production, experts of various specialties (surgeons, bioengineers, immunologists, etc.) are involved, which in the future can lead to the application of biodegradable scaffolds with implantation of stem cells in order to gain the artificial bones to be applied in various areas of surgery such as orthopedics, maxillofacial, thoracic surgery and neurosurgery.
2 Historical Aspects of Implantable Materials for Chest Wall Reconstruction
The first relevant attempt to reconstruct the chest wall dates to the early twentieth century when Tansini used latissimus dorsi myocutaneous flap for closuring the soft-tissue defect after radical mastectomy (Maxwell 1980). About 60 years later, chest wall reconstruction techniques were first introduced. In the 1940s Watson and James described the use of fascia lata grafts for closure of chest wall defects (Watson and James 1947). Bisgard and Swenson were the first to use rib grafts as horizontal struts for reconstruction after sternal resection (Bisgard and Swenson 1948). The main drawback associated with these methods of autotransplantation refers to the lack of available tissues and harm of place from which the donor tissue was taken, especially after an extensive resection had been performed. Therefore, although Gangolphe initially published the insertion of a metal prosthesis about 110 years ago (Gangolphe 1909).
In the fifties of the last century began the implantation of artificial prosthetic materials sporadically, with the intensive development of surgical techniques and materials in the coming years. Initially, woven, and soft synthetic types of prosthetics were being used, such as meshes made of polypropylene or polyester or polytetrafluoroethylene, but their inability to provide the full protection of inner thoracic organs from the effects of external factors led to subsequent introduction of rigid materials, e.g., sandwich-like composite material made of methyl methacrylate and polypropylene mesh. The introduction of the latter improved the stability and protection of the thoracic wall, but also reduced its flexibility during breathing movements, which could lead to potential impairment of respiratory rhythm and plasticity in such patients. Besides, albeit to a significantly lesser extent, this procedure could also result to abrasion of attached subjacent anatomical structures, implant rejections and infections.
Several years ago, based on the previous positive experience in other surgical branches, reinforced prostheses with titanium plates or meshes were proposed as the best possible artificial material for the reconstruction of the chest wall because it provides adequate rigidity of the thoracic cage, as well as prevents injuries and infections of the internal thoracic organs to a significant extent most likely due to the high degree of biological inertia and biocompatibility, which in turn leads to better tolerance of patients compared to the above mentioned synthetic meshes. Another potentially fascinating approach involves the transplantation of cryopreserved bone allografts, bearing in mind its superiority in relation to autotransplantations and implantation of synthetic materials in terms of relatively easier, safer, and cheaper procurement of implantable materials, as well as unlimited number of bones collected in the bone banks. Although the first bone bank was established more than 70 years ago, the first allograft transplantation of chest wall bone was awaited until 1993, when Cara et al. reported on reconstruction of sternum using iliac bone and musculocutaneous flaps (Cara et al. 1993). Subsequently, the other authors are also reported on successful combination of iliac bone allografts with other biological or synthetic materials to cover massive thoracic defects, highlighting another advantage of bone allograft versus systemic reflected in the greater ability to fully incorporate into the host organism and become its essential component over time. In accordance with the current achievements in the process of improving the production and quality of synthetic implantable materials, in the future it is much expected from the application of biodegradable scaffolds with implantation of stem cells in order to get the artificial bones for the complete closure of the large defects in various areas of surgery (i.e., orthopedics, maxillofacial, chest and neurosurgery, etc.), which would be an important step forward when it comes to all types of implant procedures. Finally, it is important to emphasize that until now the selection of the most suitable artificial material for the reconstruction of bone structures of the chest has not yet been clearly established, unlike biological, especially autogenous grafts that are widely being used to restore soft tissue defects.
3 Applications of Biodegradable Synthetic Materials in Thoracic Surgery
Biocompatible artificial or synthetic materials have wide application in chest surgery and surgery in general today. In the field of chest surgery, they are being used to reconstruct chest wall defects after extensive resection due to primary or secondary neoplasm, congenital anomalies, infections, radiation injuries and trauma. After extensive wall resections, large defects of soft tissue and bone structures (sternum and ribs) can remain and requires carefully planned and extensive reconstruction of such defects. Reconstruction of the chest wall after such extensive resections is a procedure aimed at stabilizing the bone wall and reconstructing the soft tissues. When planning resection of the chest wall due to neoplasm, a particular attention must be paid to the general condition of the patient as well as to the size and the localization of the tumor, since the defects extending from 5 to 7 cm in the largest diameter rarely require stabilization, while larger defects mostly require some type of stabilization procedures in order to preserve the normal lung function. Also, localization of resection is very important. For example, the resection covering the area of the scapula, or the area of the large chest muscles, rarely requires stabilization of the chest wall, while on the other hand, those involving the lateral or the lower part of anterior portion of the thoracic wall should always be subjected to the stabilization procedure (Sanna et al. 2017).
The chest wall has both a structural and a functional role. The structural role is reflected in the fact that it protects the internal vital organs (heart, lungs, liver, etc.). It also provides a flexible skeletal frame to stabilize shoulder and arm function. The inspiratory and expiratory muscles of the chest wall work in a precisely coordinated movement to execute a functional breath (Clemens et al. 2011).
Careful preoperative evaluation, meticulous surgical technique, and active postoperative treatment are important for any chest wall reconstruction. The choice of reconstruction is individual and is based on the nature, size and location of the defect, as well as on the general health condition of the patient and the prognosis. The goals of reconstruction are to provide the structural and functional role of the chest (Tukiainen 2013).
As described earlier, different types of synthetic, biological and metallic materials are available for the reconstruction of chest wall defects. Each prosthetic material has its advantages and disadvantages, and none has proven to be ideal (Weyant et al. 2006).
The main potential disadvantages of this prosthesis seem to be its negligible permeability to fluid that can lead to exacerbation of preexisting painful condition and enhanced rigidity of the chest wall. Rarely, the occurrence of methacrylate fractures usually followed by an infection, when the implant must be removed immediately. Owing to its outstanding inertness, low density, and resistance to weakening by rusting and stretching, the plates or meshes with titanium provide a certain advantage over other synthetic materials. Besides, these are materials repelled by a magnetic field thus they can be used without any restriction when magnetic resonance imaging scan is being performed. Although insertion of titanium implants is rarely associated with early and delayed respiratory and infective complications only a couple of them were noticed, such as fracture and/or dislocation of system’s components as well as severe chest pain. Isolated implantation of titanium plates is particularly useful for reconstructing the chest wall after resection due to traumatic events (e.g., reconstruction of sternum after partial or total sternectomy needed to maintain thoracic wall stable), while titanium meshes are usually used in conjunction with plates and some other biological materials to cover large, fully thickened defects after radical, mutilating surgery due to locally advanced tumors (Thomas and Brouchet 2010).
In cases of a full-thickness chest wall defect, many rigid implants may be used. Modern titanium devices are rigid, corrosion-free, chemically inert implants that are quickly and precisely adaptable to the shape of the thoracic wall. Moreover, titanium can safely be imaged with both computer tomography (CT) and magnetic resonance imaging, and therefore it does not affect the follow-up. Traditionally, most of the available chest wall prosthesis materials are evolved from implanted devices used in other fields such as abdominal repair. A common feature of these materials is their biological “inertness,” nonreactivity, and durability. According to these principles, several meshes, such as Prolene and Marlex meshes, have gained acceptance. However, the use of these materials may lead to an infection. Chronic and persistent pain, erosion, bleeding, hematoma, and pulmonary restrictive disease may occur due to inadequate incorporation, mesh shrinkage, and migration. In such circumstances, most of the prostheses must be removed. In cases of the sternal, chest wall, and diaphragm reconstruction, Composix Mesh™, titanium mesh, and Marlex mesh as well as methyl methacrylate were also successfully used. Although resection of tumors in this region with sufficient margins may lead to a large chest wall defect, primary closure of these defects can be satisfactorily achieved in most cases (Khalil et al. 2010). As far as our knowledge is concerned, this seems to be the very first attempt to produce anatomical design of sternal defect. The ideal prosthetic material characteristics should be:
-
1.
strong enough to withstand physiologic stresses over a long period.
-
2.
conform to the chest wall.
-
3.
promote strong host tissue ingrowth, which mimics normal tissue healing and ensuring wall incorporation.
-
4.
resist erosions into surrounding tissue and visceral structures.
-
5.
not induce allergic or adverse foreign body reactions.
-
6.
resist infection.
-
7.
be easy to use.
Successful reconstruction of the chest wall after extensive surgical resections plays a key role in the proper functioning of vital thoracic organs as well as the whole organism. As a matter of fact, the loss of completeness of the full thickness of thoracic wall layers inevitably leads to respiratory failure, which significantly increases the risk of death in such patients. In recent decades many and various biodegradable materials are being used in thoracic surgery in order to repair either acquired or congenital large chest wall defects and ensure the stability of thoracic cage. The choice of an adequate materials and surgical techniques used for such indispensable procedure should be always based on multidisciplinary approach, with inclusion of different clinicians (e.g., thoracic surgeons, plastic surgeons, neurosurgeons, orthopedists, radiologists, oncologists, pediatricians), but also the other experts, such as biomedical engineers, chemists, physicists, microbiologists, immunologists, pharmacologists, etc. Materials used to reconstruct chest wall can be of biological (xeno-, allo- and auto-grafts), synthetic and metallic origin, whereby the implantation of any of them has certain benefits and drawbacks, without proven dominance over others. Such pros and cons should be taken into account when deciding to apply each of them rationally in a particular situation according to the needs of an individual patient. In fact, to achieve a successful clinical outcome all potential benefits to a patient must be carefully balanced over the possibility of early and delayed surgical and non-surgical complications after the insertion of these prosthetic materials, on an individual basis. Today, the implantation of modern biomaterials, such as particularly hybrid human tissue-engineered products containing proteins, stem cells and/or other biological ingredients, implies a rather promising approach to optimize risk/benefit ratio (e.g., the emergence of infections versus favorable effects referring to structural and functional stability), wherever possible.
The expanded use of various synthetic materials for the reconstruction of the chest wall is supported by their diversity, availability, relatively inert nature, and apparent simplicity of use. However, considering no material is absolutely inert, the human body usually responds to insertion of prostheses by inflammation (displaying both types of immune response) and subsequent irregular formation of fibrous tissue containing numerous immune and foreign body giant cells that surround such an implant. Hence, since the organism can have relevant biological and physical-chemical influence on the implanted material in terms of its absorption, degradation, calcification, abrasion, oxidation, etc., on the other hand the implant can also demonstrate local and systemic effects to entire organism that are clinically manifested with occurrence of infection, toxic and allergic reactions, carcinogenesis, etc. In this way, due to the variable bidirectional interactions between implants and tissues in a particular environment the materials used in different tissues are expected also to cause the different responses, which is essential for their development and usage. Besides, synthetic materials are rigid and fragile enough, and also have the possibility to migrate deep into tissues, which can sometimes lead to serious injury to internal organs (i.e., heart, lungs, blood vessels, etc.) (Hayashi et al. 2019).
Regardless of its origin, the characteristics of implantable materials used to reconstruct the chest are more or less approaching the optimal material in the sense that they provide sufficient stiffness of the chest wall necessary to preclude paradoxical movements, while enabling the proper adaptation of the chest wall during breathing; in addition, they are also sufficiently inert to harmonize the growth of fibrous tissue around the implants and the risk of developing the infection, while at the same time, the monitoring and detection of potential local tumor recurrences are facilitated when these materials are used since they are often shown as radiolucent (hypodense) regions on radiographic imaging procedures making an anatomic landmark of separation (Weyant et al. 2006).
4 Chest Wall Resection and Reconstruction with Methacrylate Implant
The human musculoskeletal system is prone to injuries and various diseases such as tumors. In addition to the division into primary and secondary, benign, and malignant, all chest wall tumors are divided into soft tissue tumors and tumors of the bone structures of the chest wall. Surgery with resection has shown to be the best option for primary tumors and a selection of secondary tumors of the chest wall and may even be curative. In the surgical treatment of tumors, it is necessary to consider the localization, size and depth of the tumor tissue, the condition of the local tissue, the general condition of the patient, the expected survival and prognosis. Planning and preparation of operations are a key factor for the success of a surgical intervention. Chest wall reconstruction depends primarily on the size and localization of the tumor. The implant that will replace the bone or part of the bone must match the shape and function of the replaced bone, and the manufacturing technology used should save time and costs.
Chest wall sarcomas is usually formed in the cartilage, soft tissues, and bones of the thoracic cavity, including chondrosarcomas, osteosarcomas, rhabdomyosarcomas, plasmacytomas, malignant fibrous histiocytomas, and Ewing sarcomas.
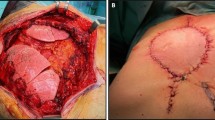
The chondrosarcoma is the most common primary malignant tumor of the chest wall and accounts for about 95% of all malignant tumors of the bone structures of the chest wall. It occurs as a slow-growing mass on the costochondral joints and most often occurs in the third and fourth decades of life. It is more common in men than in women. The chondrosarcoma is treated exclusively surgically because it is resistant to chemo and radiotherapy. Adequate surgical procedure with removal to a healthy length of about 5 cm is a surgical position that should always be followed (Bajaj and Aboeed 2021). Chondrosarcoma of the sternum is extremely rare and often untreatable. Removal of the sternum due to a malignant tumor leads to large defects of the bones and soft tissue, which leads to deformities and paradoxical movements of the chest wall and makes the recontruction of the chest wall very important (Fig. 8.1). If possible, it is necessary to preserve the manubrium of the sternum in order to maintain the stability of the thorax (Haraguchi et al. 2006).
CT scans of the affected bones and images of healthy bones are used for geometric analysis of bones and further processed in a 3D medical image processing software package for three-dimensional design and modeling (Stojkovic et al. 2010).
Based on the 3D model created in Dassault Catia software, the export in STL format results in a model suitable for 3D printing. Due to the specifics of subsequent processing, another additional model is made, which in addition to the main geometry includes a partial surface (which also has a thickness of about 2 mm), in order of forming mold easy. These models are then read in the dedicated ZPrint software specialized for 3D printing by ZCorporation ZPrinter 310 System. The loaded model and the device on which the output is performed are shown in Figs. 8.2 and 8.3.
The core prototype (core cavity of the mold) was used for the fabrication of the casting mold for the sternum implant manufacturing, while the sternum implant prototype was used for visualization in preoperative treatment planning. Before starting of development, it is necessary to determine the printing parameters. This specifically refers to the choice of working material and binder (in this case the powder was ZP130, and the binder ZB58), as well as the layer thickness that affects the surface quality. Since the printed part is the original molds for production, it is of the great importance that the quality of the surface is as good as possible, for the purpose of easier making and separation of the mold. Printing time depends on these parameters, so if a thicker layer is selected for fabrication, more time is required. In addition, the models are positioned in the working volume in a way that maximizes the surface quality and strength of the finished parts. The production process and the printed original are shown in Fig. 8.4.
The printing process itself takes about 2 h, and then the parts are left to harden for another 45 min before being removed from the powder. After the parts are removed from the powder, the excess powder is removed with compressed air. In order to achieve the best possible mechanical characteristics and shorten the drying time, the parts are placed in an oven at 100 °C for 90 min. Only after this operation the sternum implant prototypes for making the mold are obtained (Fig. 8.5).
Since the original mold are not safe for direct use for prosthetic purposes, it is necessary to make a mold which would have implants from medically tested prosthetic material. A special type of polyurethane plastic is used to make the mold, which has the hardness required for casting acrylates, which make up the largest percentage of the mentioned material (75% methyl methacrylate–styrene-copolymer and 15% polymethylmethacrylate). First, the lower part of the mold is poured into the box, using an auxiliary model with a “cloak”, and then the upper part of the mold is made using the original. The auxiliary model is destroyed in the process of making the mold, while the original remains undamaged, and can be reused if the need arises. The result of this procedure is given in Fig. 8.6, as well as the procedure of filling the mold with prosthetic material.
The prepared prosthetic material is applied to the cavities of the mold and, after the expansion, it completely fills the mold. After the time allowed for the prosthetic material to harden, the implant is removed from the mold, as shown in Fig. 8.7.
This is followed by mechanical processing of the material, cutting and grinding, and finally the implant is sterilized.
In the case of chondrosarcoma of the sternum shown above and the intraoperative finding shown in Fig. 8.8, after the preparation of an adequate, patient- adapted implant, a specified surgical method is performed in accordance with the preoperative plan. The resection of the sternum in the length of 100 mm and the cartilages of the second and third ribs on both sides including cuneiform resection of the anterior segment of right superior lobe was done (Fig. 8.9). The implant of sternum is fixed to the proximal fragment of the manubrium and distal part of the body of sternum with K-wires. In addition, artificial costal cartilages of the implant are matched and connected to the patient’s second and third thoracic rib with K-wires. Mesh wire is placed above the implant which is sutured to the chest wall using PROLEN 0 (Fig. 8.10). After that, the chest wall was closed (Milisavljevic et al. 2013). A control CT of the chest was performed with a recontruction that shows the correct position of the sternum implant. (Fig. 8.11).
The pleomorphic Sarcoma of Bone, formerly known as Malignant Fibrous Histiocytoma, are rare malignant histiocytic lesions of bone. Treatment is the same as conventional osteosarcoma, including pre- and postoperative chemotherapy and surgical resection (Malik et al. 2020).
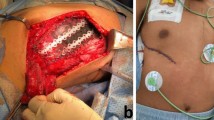
Below is an example of Malignant Fibrous Histiocytoma localized at the posterior end of the 8th rib on the left side (Fig. 8.12). The 8th rib on the left side was resected and replaced with a methyl acrylate implant created by 3D printing, and the reconstruction of the chest wall was supplemented with mesh wire in order to improve the stability of the chest wall (Fig. 8.13).
Not only the tumors of the chest wall require surgical resection and reconstruction of the chest wall, but also the rarely tumors originating from the breast can also lead to infiltration of the chest wall. An extreme example of a phylloid breast tumor with infiltration of all layers of the chest wall, including bone structures, i.e., ribs is present on (Fig. 8.14).
The phylloid breast tumor was excised and the chest wall was resected, including three ribs (the 4, 5 and 6th ribs on the left side) (Fig. 8.15). The implant is fixed with K-wires and mesh wire is placed above the implant. Chest wall was then closed. The defect was additionally reconstructed with omentoplasty and skin flap (Fig. 8.16).
5 Conclusion
After an extensive wall resection, complex chest wall defects, which includes large defects of the soft tissue and bone structures, can be some of the most challenging problems that thoracic surgeons must face. Successful reconstruction after extensive surgical resections of the chest wall plays a key role in the proper functioning of vital thoracic organs as well as the whole organism. The length of postoperative recovery is related to the size of the defect and the choice of appropriate materials and surgical techniques. Each case requires an individual plan, approach, selection of appropriate material and surgical techniques. The choice of an adequate materials and surgical techniques should be always based on multidisciplinary approach. The use of synthetic materials for chest reconstruction is supported by their diversity, availability, relatively inert nature and ease of use. Chest reconstruction with biocompatible artificial or synthetic materials is safe and effective in most defects. It also expands the indications for resection and reconstruction of the chest wall and reduces overall costs. It is important to emphasize that until now, the selection of the most suitable artificial material for the reconstruction of structures of the chest wall has not yet been clearly established. The implantation of the modern biomaterials (particularly hybrid human tissue-engineered products containing proteins, stem cells and/or other biological ingredients) implies a rather promising approach to optimize risk/benefit ratio.
References
Bajaj T, Aboeed A (2021) Chest wall tumors. In: StatPearls (Internet). Treasure Island (FL). StatPearls Publishing
Bisgard JD, Swenson SA Jr (1948) Tumors of the sternum; report of a case with special operative technic. Arch Surg 56(5):570–578
Cara JA, Laclériga AF, Cañadell J (1993) Iliac allograft used for sternal reconstruction after resection of a chondrosarcoma. Int Orthop 17(5):297–299
Clemens MW, Evans KK, Mardini S, Arnold PG (2011) Introduction to chest wall reconstruction: anatomy and physiology of the chest and indications for chest wall reconstruction. Semin Plast Surg 25(1):5–15
Gangolphe L (1909) Enorme enchondrome de la fourchette sternale. Lyon Chir 2:112
Goldsmith I, Evans PL, Goodrum H, Warbrick-Smith J, Bragg T (2020) Chest wall reconstruction with an anatomically designed 3-D printed titanium ribs and hemi-sternum implant. 3D Print Med 6(1):26
Haraguchi S, Hioki M, Hisayoshi T, Yamashita K, Yamashita Y, Kawamura J, Hirata T, Yamagishi S, Koizumi K, Shimizu K (2006) Resection of sternal tumors and reconstruction of the thorax: a review of 15 patients. Surg Today 36(3):225–229
Hayashi T, Sakakura N, Ishimura D, Kozawa E, Yoshida M, Sakao Y, Yamada H, Tsuku-shi S (2019) Surgical complication and postoperative pulmonary function in patients undergoing tumor surgery with thoracic wall resection. Oncol Lett 17(3):3446–3456
Khalil el-SA, El-Zohairy MA, Bukhari M (2010) Reconstruction of large full thickness chest wall defects following resection of malignant tumors. J Egypt Natl Canc Inst 22(1):19-27
Malik AT, Baek J, Alexander JH, Voskuil RT, Khan SN, Scharschmidt TJ (2020) Malignant fibrous histiocytoma of bone: a survival analysis from the National Cancer Database. J Surg Oncol 121(7):1097–1103
Maxwell GP (1980) Iginio Tansini and the origin of the latissimus dorsi musculocutaneous flap. Plast Reconstr Surg 65(5):686–692
Milisavljevic S, Grujovic NN, Mrvic S, Stojkovic D, Arsenijevic M, Jeremic B (2013) Sternum resection and chest wall reconstruction with metaacrilate implant in tuberculosis. Indian J Surg 75(Suppl 1):257–260
Sanna S, Brandolini J, Pardolesi A, Argnani D, Mengozzi M, Dell’Amore A, Solli P (2017) Materials and techniques in chest wall reconstruction: a review. J Vis Surg 3:95
Stojkovic M, Milovanovic J, Vitkovic N, Trajanovic M, Grujovic N, Milivojevic V, Milisavljevic S, Mrvic S (2010) Reverse modeling and solid free-form fabrication of sternum implant. Australas Phys Eng Sci Med 33(3):243–250
Tamburini N, Grossi W, Sanna S, Campisi A, Londero F, Maniscalco P, Dolci G, Quaran-totto F, Daddi N, Morelli A, Cavallesco G, Dell’Amore A (2019) Chest wall reconstruction using a new titanium mesh: a multicenters experience. J Thorac Dis 11(8):3459–3466
Thomas PA, Brouchet L (2010) Prosthetic reconstruction of the chest wall. Thorac Surg Clin 20(4):551–558
Tukiainen E (2013) Chest wall reconstruction after oncological resections. Scand J Surg SJS Official Organ Finnish Surg Soc Scand Surg Soc 102(1):9–13
Watson WL, James AG (1947) Fascia lata grafts for chest wall defects. J Thorac Surg 16(4):399–406
Weyant MJ, Bains MS, Venkatraman E, Downey RJ, Park BJ, Flores RM, Rizk N, Rusch VW (2006) Results of chest wall resection and reconstruction with and without rigid prosthesis. Ann Thorac Surg 81(1):279–285
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Milisavljevic, S., Lukovic, A. (2022). Application of Artificial Materials in Thoracic Surgery. In: Canciglieri Junior, O., Trajanovic, M.D. (eds) Personalized Orthopedics. Springer, Cham. https://doi.org/10.1007/978-3-030-98279-9_8
Download citation
DOI: https://doi.org/10.1007/978-3-030-98279-9_8
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-98278-2
Online ISBN: 978-3-030-98279-9
eBook Packages: EngineeringEngineering (R0)