Abstract
Reversible cerebral vasoconstriction syndrome (RCVS) and central nervous system (CNS) vasculitis both present with focal or diffuse vasculopathy and intracranial stenosis. Distinguishing features include signs and symptoms, gender predilection, etiology, MRI findings, treatment, and disease course. Daily transcranial Doppler (TCD) is well validated in the subarachnoid hemorrhage (SAH) population to assess proximal vessel vasospasm. In particular, MCA mean flow velocity (MFV) > 200 cm/s and basilar MFV > 100 cm/s represent severe spasm. Pulsatility index (PI) levels >1.19 may also signify distal stenosis. Thus, TCD represents a cost-effective, mobile, and non-invasive tool for repeated assessment of intracranial vasculopathy. Here we describe TCD application to RCVS and vasculitis: for diagnosis, monitoring during therapy, and ultimately for prognostication.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Reversible cerebral vasoconstriction syndrome
- CNS vasculitis
- Primary angiitis of the CNS
- Transcranial Doppler
Introduction
Reversible cerebral vasoconstriction syndrome (RCVS) and central nervous system (CNS) vasculitis both present with focal or diffuse vasculopathy and intracranial stenosis. Distinguishing features include signs and symptoms, gender predilection, etiology, MRI findings, treatment, and disease course. Daily transcranial Doppler (TCD) is well validated in the subarachnoid hemorrhage (SAH) population to assess proximal vessel vasospasm. In particular, MCA mean flow velocity (MFV) > 200 cm/s and basilar MFV > 100 cm/s represent severe spasm. Pulsatility index (PI) levels >1.19 may also signify distal stenosis. Thus, TCD represents a cost-effective, mobile, and non-invasive tool for repeated assessment of intracranial vasculopathy. Here we describe TCD application to RCVS and vasculitis: for diagnosis, monitoring during therapy, and ultimately for prognostication.
Literature Review
TCD to Detect Stenosis and Occlusion
Anterior Circulation
TCD can reliably detect stenosis in the anterior circulation including the middle cerebral artery M1 segment and the internal carotid siphon in both the stroke and SAH populations (Table 1). Compared to angiography, TCD has an approximate sensitivity in the MCA of 80% to 90%, specificity of 90% to 95%, positive predictive value of 85%, and negative predictive value of 98% [1, 2]. It is less reliable for the anterior cerebral arteries because of collateral flow patterns and shunting to the ACA contralateral to the spastic segment [3]. In the ACAs, sensitivity is approximately 13% to 18% and specificity is 100%.
Posterior Circulation
As compared to angiography, several studies have shown that TCD can detect stenosis and occlusion in the posterior circulation including the intracranial vertebral, and proximal basilar with an approximate sensitivity of 80% and specificity of 75% to 80% [1, 2]. The BA/VA ratio may improve the sensitivity for basilar vasospasm [4]. Given collateral flow, the sensitivity for PCA territories is poor with close to 100% specificity [3].
Vasospasm and SAH
TCD is much more sensitive in detecting proximal versus distal vasospasm [5]. Proximal vasospasm results in segmental or diffuse elevations in mean flow velocities without flow velocity increase in the extracranial vessels.
Daily TCD examinations help identify SAH patients at risk of delayed cerebral ischemia. Literature in the SAH population has shown that mean MCA flow velocity values greater than 120 cm/s or greater than 200 cm/s reliably predict the presence of clinically significant vasospasm [1, 5,6,7]. For the basilar artery, mean flow velocity >85 cm/s or >100 cm/s predicts severe vasospasm. These values are affected by factors such as age, intracranial pressure (ICP), mean arterial blood pressure, hematocrit, arterial C02 content, and collateral flow patterns. As above, TCD is most reliable for detecting vasospasm in the MCA and ICA territories, and less so for the ACA territories and vertically oriented branches of the intracranial arteries distal to the basal cisterns. TCD is well validated for the detection of cerebral vasospasm (class II, level of evidence B) [1]. Transcranial color-coded sonography is another non-invasive tool that may be utilized as well [8,9,10]. It combines pulsed wave Doppler ultrasound with a cross sectional view, allowing identification of arteries, velocities, and direction of flow.
As discussed in Chapter “Transcranial Doppler in Subarachnoid Hemorrhage”, the ratio between the intracranial MCA and the extracranial carotid or the Lindegaard ratio (Vintracranial MCA/VeICA) helps to avoid reading hyperemia as vasospasm and is well validated when compared to angiography [11]. Hyperemia results in flow elevations in MCA and ICA with LR < 3 while vasospasm results in elevated MCA over ICA flow with LR > 6. Increased pulsatility index indicates increased resistance distal to the site of insonation, and is a surrogate marker of distal vasospasm. A similar ratio (Vbasilar artery/Vextracranial vertebral artery) exists for basilar vasospasm [4].
Gosling’s pulsatility index ((PSV-EDV)/MFV) may reflect vascular resistance, with proximal stenosis lowering the PI due to distal arterial dilatation, and distal stenosis increasing the PI (normal range 0.5–1.19) [12]. Notably, the PI is not a direct reflection of vascular resistance, but reflects the interplay between CPP, arterial pulse amplitude, compliance and other factors [13].
Additionally, diagnosis of >50% stenosis using TCD is based on the following criteria: (1) increased velocity through the stenotic segment; (2) decrease in velocity distally (post-stenotic dilation); (3) left versus right side differences in velocity; and (4) disturbances in flow such as turbulence and murmurs [14, 15]. Absent flow at a normal position and depth, and increased flow in collateral vessels may signal vessel occlusion. Examples of collateral flow include flow reversal in ACA or MCA, flow reversal in the ophthalmic artery, and prominent Acomm or Pcomm flow.
The Stroke Prevention Trial in Sickle Cell Disease (STOP) trial utilized TCD to detect intracranial stenosis and stroke risk in children with SCD [16]. Children with Vmean >200 mc/s in the ICA or MCA territory were randomly assigned to standard care or periodic blood transfusion, with a 92% stroke risk reduction in the transfusion group. This study validated the use of TCD for SCD-related intracranial stenosis in children.
TCD is well-validated in vasospasm secondary to subarachnoid hemorrhage [6]. In this population, serial TCD is utilized to diagnose vasospasm, guide need for endovascular intervention, and monitor response to therapy.
Hemodynamic Effects in Vasospasm
In the SAH population, vasospasm induces a decrease in vessel lumen diameter, which causes an increase in flow resistance. Mild narrowing may not cause a sufficient change to influence flow, and cerebral autoregulation may induce elevated arterial blood pressure to compensate. In moderate narrowing, the flow velocity will increase inversely to the change in lumen area with good correlation to angiography. In severe narrowing, this relationship is more complicated [3]. Figure 1 shows a reliable relationship between diameter and velocity in mild or moderate spasm (I, ‘forward side’). II shows the ‘plateau’ where flow is reduced while velocity remains relatively independent of diameter. III, the ‘backside’ shows lower velocities with additional diameter reduction with loss of cerebral autoregulation with severe narrowing. Given these changes, TCD velocities need to be correlated to angiography and clinical findings.
Cerebral autoregulation is the ability to maintain cerebral blood flow over a range of mean arterial pressures. Cerebral autoregulation may be static or dynamic depending on the variability in blood pressure.
As discussed in other chapters, the pulsatility index may be a surrogate marker for cerebral autoregulation. It is also related to ICP, with a correlation coefficient of 0.938 (p < 0.0001) [17]. PI = (MCA peak systolic velocity – MCA end diastolic velocity)/ MCA mean flow velocity.
RCVS
RCVS is characterized by reversible cerebral vasoconstriction of acute onset, often with severe “thunderclap” headache [18,19,20,21]. It has a female predilection with a ratio of 2-3 to 1. RCVS is often associated with vasoactive medications including sympathomimetic and serotonergic drugs, and illicit substances such as cocaine and methamphetamine. Endocrine and hormonal factors may play a role as well. Treatment is often calcium channel blockers such as nimodipine or verapamil.
Cortical SAH, ICH, seizures, and reversible posterior leukoencephalopathy often occur within the first week with ischemic complications (stroke and TIA) within the second week [22]. By definition, stenosis improves within 4–12 weeks (Fig. 2).
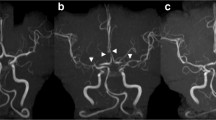
(a) Catheter angiography at disease onset: multiple narrowing and dilatations, (b) Control catheter angiography at 3 months, (c) MRA at disease onset and (d) Control MRA [22]
Cerebral angiography may show single or multi-vessel narrowing and dilation (“string and beads”) with improvement following intra-arterial calcium channel blockers. As compared to vasculitis, RCVS has a relatively normal CSF profile. While the gold standard for diagnosis is angiography, TCD has been used successfully to show improvement in the blood flow velocities over time, but may underestimate changes in vessel diameter in the acute phase [18, 23,24,25].
Ducros et all looked at TCD in 64 patients with RCVS with 44 (69%) having increased arterial velocities with a mean of 163 ± 27 cm/s in middle cerebral arteries and 148 ± 20 cm/s in carotid siphons. Angiography confirmed multifocal segmental arterial constriction. Twelve patients had serial TCD with a moderate increase in velocities on first TCD (mean 6.8 days) but marked on the second TCD (mean 22.5 days), long after the headache had subsided [22]. Levin et al. also found that TCD flow velocities in the MCA (VMCA) reached a mean peak three to four weeks after headache onset [26]. Another case series reported bimodal peaks with increased velocities at days 15 and 17 [27]. Marsh et al. demonstrated improvement in velocities in seven patients with RCVS, which correlated to initiation of a calcium channel blocker [24].
Chen et al. utilized transcranial color-coded sonography (TCSS) to investigate hemodynamic changes in patients with RCVS [28]. They analyzed the mean flow velocity of MCA (VMCA), Lindegaard Index (LI), and maximum values every 10 days over the first month and every 20 days thereafter (average 3.9 studies per patient). They utilized vasospasm criteria for SAH patients with mild vasospasm as VMCA >120 cm/sec; and moderate-to-severe with VMCA > 200 cm/sec. Patients with VMCA > 120 cm/sec and LI > 3, had a greater risk of posterior leukoencephalopathy and ischemic strokes.
Topcuoglu et al. described impaired vasomotor reactivity in seven of eight patients with RCVS, which highlights the impaired autoregulation through vasodilation in this disease [29]. Vasomotor reactivity may be considered as an ancillary study, but the significance of abnormal findings remains unknown.
Thus, TCD remains a cost-effective, mobile, and noninvasive technology with utility for both diagnosis and therapeutic monitoring in RCVS. We still recommend the gold standard for initial diagnosis (cerebral angiography, CTA, MRA), and to determine disease extension and disease progression, particularly in small vessel vasculitides [10].
TCD offers real-time monitoring of blood flow in an intracranial artery. Therefore, it represents an important, non-invasive and non-nephrotoxic tool to monitor intracranial stenosis associated with RCVS and vasculitis over extended periods. Additionally, assessment of stenosis over time can assist with prognostication.
RCVS v Vasculitis
As compared to RCVS, CNS vasculitis (aka primary angiitis of the central nervous system [PACNS]) does not have a gender predilection, is often subacute, and CSF profile is typically abnormal [18]. MRI is abnormal in 90%, and often shows ischemic infarcts in multiple vascular territories of varying ages. Cerebral angiography may be similar but does not show reversibility with administration of intra-arterial calcium channel blockers. Other authors have reported angiographic findings of “sausage on a string” in RCVS versus “irregular notched” appearance in vasculitis [30]. Findings do not typically reverse spontaneously, and treatment includes high-dose steroids and immunotherapy. Clinical outcomes are typically better in RCVS. Singhal et al. described a 90% discharge mRS of 0-3 in patients with RCVS as compared to 75% with vasculitis [30]. A significantly greater number of RVCS patients had mRS 0-1.
Krasnianski et al. described three patients with RCVS (migraine, eclampsia, toxic encephalopathy), and three patients with vasculitis (2 with PACNS and 1 with Crohn’s disease associated vasculitis). They found that TCD (elevated velocities) correlated to MRA and cerebral angiography and clinical disease course [31].
Vasculitis
CNS vasculitis (primary angiitis of the CNS) refers to inflammation of the blood vessels of the brain, spinal cord and meninges [32]. Secondary causes of CNS vasculitis include infectious causes such as varicella zoster, large, medium and small vessel vasculitides, malignancy, and autoimmune diseases such as systemic lupus erythematosus and sarcoidosis. It is a rare disease, predominantly affecting males around 50 years old, and presenting in an indolent or subacute way. MRI findings include ischemic and hemorrhagic strokes, often in multiple vascular territories and of varying age, subarachnoid hemorrhage, and leptomeningeal enhancement.
Calabrese et al. proposed diagnostic criteria for primary CNS vasculitis in 1988 including: 1) new neurological or psychiatric deficit; 2) angiographic or histopathological features of CNS arteriopathy; and (3) no evidence of systemic vasculitis or other mimics [33]. Angiography reveals “beading” and multifocal vascular stenosis. The gold standard in diagnosis is pathology.
Lowe et al. performed serial TCD in two children with PACNS and one child with West Nile vasculitis [34]. All three girls had MCA infarcts, and abnormal findings on TCD (elevated peak systolic velocities over multiple examinations) which correlated with abnormalities in both MRA and cerebral angiography.
TCD Findings in Vasculitis
Several studies have validated use of TCD in cerebral vasculitis (particularly for proximal cerebral vessels) for both diagnosis and disease course as compared to cerebral angiography [10, 35,36,37].
Gonzalez-Suarez et al. reported TCD findings in inactive Antineutrophil Cytoplasmic Antibody (ANCA)-associated vasculitis. They found that lower mean flow velocity and lower middle cerebral artery pulsatility index was related to altered SPECT perfusion, lower Montreal cognitive assessment test scores, and younger age [38].
Cantu et al. evaluated 21 patients with Takayasu arteritis, a large-vessel arteriopathy [36]. They compared MRA, color Doppler flow imaging, and TCD with angiography. For TCD, temporal windows were used to evaluate the MCA, ACA, and PCA. The transorbital approach was used for the intracranial ICA, and the transoccipital approach was used for the basilar artery and distal vertebral arteries. Extracranial obstruction was suggested by dampened or blunted waveforms, slow acceleration, and decreased pulsatility in the MCA and basilar artery. They found that non-invasive techniques showed at least one abnormality in 20 (95%) patients. In addition, MRA and color Doppler flow imaging highly correlated to angiography for detection of vessel occlusion. Interestingly, high resolution ultrasound detected common carotid wall thickening in vessels that were normal on other imaging studies.
Ameriso et al. also found that the systolic/diastolic ratios and pulsatility indexes were extremely low in a patient with Takayasu arteritis [37]. They also noted damping in every flow waveform recorded, consistent with pulseless flow in the intracranial circulation.
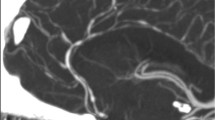
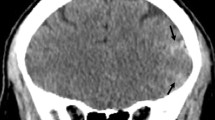
Others have validated use of ultrasound in vasculitis as compared to cerebral angiography and MRA (Figs. 3 and 4) [10, 39, 40]. Ultrasound may show inflammatory wall thickening. In the case of temporal arteritis, color Doppler ultrasound shows a concentric, hypoechoic dark wall swelling secondary to edema; increased flow velocity due to stenosis (particularly with increased velocities proximally, turbulent waveforms with several colors present, and reduced velocities behind the area of stenosis [14]. They noted that these findings improve with corticosteroids, which highlights the benefit of non-invasive techniques such as ultrasound or transcranial Doppler for real-time monitoring of treatment effect. Ultimately, improvement of stenosis with treatment over time can assist in prognostication.
Imaging studies of a 37-year-old man with newly diagnosed and untreated active Wegener’s granulomatosis with involvement of the carotid bifurcation and the internal carotid artery. (a) Angiography of the left carotid artery shows narrowing of the proximal internal carotid artery. Kinking and coiling of the artery occurs further distally. (b) The T1-weighted magnetic resonance image of the left carotid bifurcation shows a perivascular infiltrate. The ultrasound image in a longitudinal (c) and transverse view (d) delineates narrowing of the artery, hyperechoic (bright) wall thickening, and a perivascular infiltrate [10]
Ultrasound images of vasculitis. The arrows indicate inflammatory wall thickening. The scans are longitudinal to the course of the vessels. (a) Left parietal ramus in acute temporal arteritis. (b) Left subclavian artery in acute large-vessel giant cell arteritis. (c) Left common carotid artery in Takayasu’s arteritis. The right side of the image delineates a thickened artery wall. The distal common carotid artery (left side) is normal. (d) Isolated abdominal aortitis
Suggested Protocol
Obtain a complete standard TCD, including extracranial ICA flow velocities to calculate the Lindegaard ratio, to establish a baseline after diagnosis (see Chapter “Transcranial Doppler in Subarachnoid Hemorrhage”: SAH and vasospasm). Repeat TCD studies daily, following additional treatment, or with a change in clinical examination.
Technical Considerations
The examination is often done in the neurocritical care unit under less than optimal conditions. Audible signals from narrowed vessels are weak and a good quality headset is recommended. All studies should be conducted with the patients at rest with steady state ventilator settings (arterial CO2 content causing large artery dilation and arteriolar constriction), ICP, hematocrit, temperature, blood pressure, and mental or motor activity (neurovascular coupling).
The evaluation of RCVS or vasculitis should be similar to the routine TCD performed for vasospasm, utilizing classic acoustic windows with a 2 MHz frequency ultrasound probe at previously recommended depths.
We recommend the highest possible PRF setting to reduce the risk of PRFs aliasing [3]. Musical murmurs and bruits in distal segments can guide the sample volume toward the highest velocities.
Distal vessels such as M2 should be scanned for elevated velocities. Given the fluctuation in flow velocities, we recommend at least daily assessment over a sufficient amount of time.
Obtain extracranial Doppler for a reference velocity from the internal carotid artery (eICA) as close as possible to the base of the skull. Aim the probe inferiorly or slightly posteriorly to the angle of the jaw, with the depth set to 40 to 50 mm.
References
Babikian VL, Feldmann E, Wechsler LR, Newell DW, Gomez CR, Bogdahn U, Caplan LR, Spencer MP, Tegeler C, Ringelstein EB, Alexandrov AV. Transcranial Doppler ultrasonography: year 2000 update. J Neuroimaging. 2000;10(2):101–15.
Zanette EM, Fieschi C, Bozzao L, Roberti C, Toni D, Argentino C, Lenzi GL. Comparison of cerebral angiography and transcranial Doppler sonography in acute stroke. Stroke. 1989;20(7):899–903.
Aaslid R. Transcranial Doppler assessment of cerebral vasospasm. Eur J Ultrasound. 2002;16(1-2):3–10.
Sviri GE, Ghodke B, Britz GW, Douville CM, Haynor DR, Mesiwala AH, Lam AM, Newell DW. Transcranial Doppler grading criteria for basilar artery vasospasm. Neurosurgery. 2006;59(2):360–6.
Lysakowski C, Walder B, Costanza MC, Tramèr MR. Transcranial Doppler versus angiography in patients with vasospasm due to a ruptured cerebral aneurysm: a systematic review. Stroke. 2001;32(10):2292–8.
Sloan MA, Haley EC, Kassell NF, Henry ML, Stewart SR, Beskin RR, Sevilla EA, Tomer JC. Sensitivity and specificity of transcranial Doppler ultrasonography in the diagnosis of vasospasm following subarachnoid hemorrhage. Neurology. 1989;39(11):1514.
Harders AG, Gilsbach JM. Time course of blood velocity changes related to vasospasm in the circle of Willis measured by transcranial Doppler ultrasound. J Neurosurg. 1987;66(5):718–28.
Proust F, Callonec F, Clavier E, Lestrat JP, Hannequin D, Thiebot J, Freger P. Usefulness of transcranial color-coded sonography in the diagnosis of cerebral vasospasm. Stroke. 1999;30(5):1091–8.
Kubo S, Nakata H, Tatsumi T, Yoshimine T. Headache associated with postpartum cerebral angiopathy: monitoring with transcranial color‐coded sonography. Headache J Head Face Pain. 2002;42(4):297–300.
Schmidt WA. Use of imaging studies in the diagnosis of vasculitis. Curr Rheumatol Rep. 2004;6(3):203–11.
Lindegaard KF, Nornes H, Bakke SJ, Sorteberg W, Nakstad P. Cerebral vasospasm diagnosis by means of angiography and blood velocity measurements. Acta Neurochir. 1989;100(1):12–24.
Gosling RG, King DH. The role of measurement in peripheral vascular surgery: arterial assessment by Doppler-shift ultrasound.
de Riva N, Budohoski KP, Smielewski P, Kasprowicz M, Zweifel C, Steiner LA, Reinhard M, Fábregas N, Pickard JD, Czosnyka M. Transcranial Doppler pulsatility index: what it is and what it isn’t. Neurocrit Care. 2012;17(1):58–66.
Schmidt WA, Gromnica‐Ihle E. Incidence of temporal arteritis in patients with polymyalgia rheumatica: a prospective study using colour Doppler ultrasonography of the temporal arteries. Rheumatology. 2002;41(1):46–52.
Rasulo FA, De Peri E, Lavinio A. Transcranial Doppler ultrasonography in intensive care. Eur J Anaesthesiol (EJA). 2008;25:167–73.
Fullerton HJ, Adams RJ, Zhao S, Johnston SC. Declining stroke rates in Californian children with sickle cell disease. Blood. 2004;104(2):336–9.
Bellner J, Romner B, Reinstrup P, Kristiansson KA, Ryding E, Brandt L. Transcranial Doppler sonography pulsatility index (PI) reflects intracranial pressure (ICP). Surg Neurol. 2004;62(1):45–51.
Calabrese LH, Dodick DW, Schwedt TJ, Singhal AB. Narrative review: reversible cerebral vasoconstriction syndromes. Ann Intern Med. 2007;146(1):34–44.
Call GK, Fleming MC, Sealfon S, Levine H, Kistler JP, Fisher CM. Reversible cerebral segmental vasoconstriction. Stroke. 1988;19(9):1159–70.
Singhal AB, Hajj-Ali RA, Topcuoglu MA, Fok J, Bena J, Yang D, Calabrese LH. Reversible cerebral vasoconstriction syndromes: analysis of 139 cases. Arch Neurol. 2011;68(8):1005–12.
Rocha EA, Topcuoglu MA, Silva GS, Singhal AB. RCVS2 score and diagnostic approach for reversible cerebral vasoconstriction syndrome. Neurology. 2019;92(7):e639–47.
Ducros A, Boukobza M, Porcher R, Sarov M, Valade D, Bousser MG. The clinical and radiological spectrum of reversible cerebral vasoconstriction syndrome. A prospective series of 67 patients. Brain. 2007;130(12):3091–101.
Bogousslavsky J, Despland PA, Regli F, Dubuis PY. Postpartum cerebral angiopathy: reversible vasoconstriction assessed by transcranial Doppler ultrasounds. Eur Neurol. 1989;29(2):102–5.
Marsh EB, Ziai WC, Llinas RH. The need for a rational approach to vasoconstrictive syndromes: transcranial doppler and calcium channel blockade in reversible cerebral vasoconstriction syndrome. Case Rep Neurol. 2016;8(2):161–71.
Razumovsky AY, Wityk RJ, Geocadin RG, Bhardwaj A, Ulatowski JA. Cerebral vasculitis: diagnosis and follow‐up with transcranial Doppler ultrasonography. J Neuroimaging. 2001;11(3):333–5.
Levin JH, Benavides J, Caddick C, Laurie K, Wilterdink J, Yaghi S, Silver B, Khan M. Transcranial Doppler ultrasonography as a non-invasive tool for diagnosis and monitoring of reversible cerebral vasoconstriction syndrome. R I Med J. 2016;99(9):38.
Terasawa Y, Arai A, Sakai K, Mitsumura H, Iguchi Y. Transcranial color-coded sonography findings of patients with reversible cerebral vasoconstriction syndromes. J Clin Neurosci. 2019;61:290–2.
Chen SP, Fuh JL, Chang FC, Lirng JF, Shia BC, Wang SJ. Transcranial color doppler study for reversible cerebral vasoconstriction syndromes. Ann Neurol. 2008;63(6):751–7.
Topcuoglu MA, Chan ST, Silva GS, Smith EE, Kwong KK, Singhal AB. Cerebral vasomotor reactivity in reversible cerebral vasoconstriction syndrome. Cephalalgia. 2017;37(6):541–7.
Singhal AB, Topcuoglu MA, Fok JW, Kursun O, Nogueira RG, Frosch MP, Caviness VS Jr. Reversible cerebral vasoconstriction syndromes and primary angiitis of the central nervous system: clinical, imaging, and angiographic comparison. Ann Neurol. 2016;79(6):882–94.
Krasnianski M, Schluter A, Neudecker S, Spielmann RP, Stock K. Serial magnet resonance angiography in patients with vasculitis and vasculitis-like angiopathy of the central nervous system. Eur J Med Res. 2004;9:247–55.
Hajj-Ali RA, Calabrese LH. Diagnosis and classification of central nervous system vasculitis. J Autoimmun. 2014;48:149–52.
Calabrese LH, Mallek JA. Primary angiitis of the central nervous system. Report of 8 new cases, review of the literature, and proposal for diagnostic criteria. Medicine. 1988;67(1):20–39.
Lowe LH, Morello FP, Jackson MA, Lasky A. Application of transcranial Doppler sonography in children with acute neurologic events due to primary cerebral and West Nile vasculitis. Am J Neuroradiol. 2005;26(7):1698–701.
Morgenlander JC, McCallum RM, Devlin T, Moore MS, Gray L, Alberts MJ. Transcranial doppler sonography to monitor cerebral vasculitis. J Rheumatol. 1996;23(3):561–3.
Cantú C, Pineda C, Barinagarrementeria F, Salgado P, Gurza A, de Pablo P, Espinosa R, Martínez-Lavín M. Noninvasive cerebrovascular assessment of Takayasu arteritis. Stroke. 2000;31(9):2197–202.
Ameriso S, Bernard JT, Fisher M, Weaver F. “Pulseless” transcranial Doppler findings in Takayasu’s arteritis. J Clin Ultrasound. 1990;18(7):592–6.
González-Suárez I, Arpa J, Ríos-Blanco JJ. Brain microvasculature involvement in ANCA positive vasculitis. Cerebrovasc Dis. 2016;41(5-6):313–21.
Ritter MA, Dziewas R, Papke K, Lüdemann P. Follow-up examinations by transcranial Doppler ultrasound in primary angiitis of the central nervous system. Cerebrovasc Dis. 2002;14(2):139–42.
Duna GF, Calabrese LH. Limitations of invasive modalities in the diagnosis of primary angiitis of the central nervous system. J Rheumatol. 1995;22(4):662–7.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Strohm, T. (2022). Reversible Cerebral Vasoconstriction Syndrome (RCVS) and Vasculitis. In: Ziai, W.C., Cornwell, C.L. (eds) Neurovascular Sonography . Springer, Cham. https://doi.org/10.1007/978-3-030-96893-9_6
Download citation
DOI: https://doi.org/10.1007/978-3-030-96893-9_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-96892-2
Online ISBN: 978-3-030-96893-9
eBook Packages: MedicineMedicine (R0)