Abstract
Subarachnoid hemorrhage (SAH) is a frequently encountered neurological emergency in patients hospitalized in the neurocritical care units. SAH originating from aneurysmal rupture (aSAH) carries significant morbidity and mortality in these patients. Transcranial Doppler (TCD) is an important technology for the assessment and management of patients with aSAH in the neurocritical care units. In particular, surveillance TCD examination has been used a screening tool for the development of cerebral vasospasm, an important complication associated with outcomes of these patients. Monitoring of cerebral blood flow velocities and Lindegaard ratio are considered the most frequently used markers for the detection of cerebral vasospasm. In addition, TCD offers a non-invasive technology for assessment and monitoring of additional parameters that can be utilized in the management of patients with aSAH such as the cerebral autoregulation and microembolic signals. Those will need to be further assessed in future clinical studies.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction to Subarachnoid Hemorrhage
Subarachnoid hemorrhage (SAH) is a life-threatening emergency that occurs due to bleeding into the subarachnoid space between the arachnoid membrane and the pia matter [1]. The annual incidence of SAH in the United States is between 10–14 cases per 100,000 [2]. Clinically, SAH presents with acute onset of severe headache that is often described as the “worst headache of life”, nausea, vomiting, photophobia, neck pain and loss of consciousness [3]. Risk factors for SAH can be divided into modifiable factors such as hypertension, smoking, heavy alcohol use and sympathomimetic drug use like cocaine and non-modifiable factors including advanced age, female sex, specific ethnicities, prior personal or familial history of SAH, type IV Ehlers-Danlos syndrome or enlarged aneurysmal diameter [2, 3]. While women have overall higher rates of aSAH than men, one study showed this may start to occur in women at the age of late thirties [4]. The overall outcomes are similar between men and women [5].
The etiology of SAH include aneurysmal rupture, arteriovenous malformation, trauma and secondary to other etiologies such as anticoagulation or other types of brain injuries [3]. Common forms of subarachnoid hemorrhage include spontaneous aneurysmal rupture that has a prevalence of 85% of the cases while perimesencephalic SAH constitutes about 5% of the cases. Trauma contributes to variable rates of SAH ranging between 25–53% of traumatic brain injury patients [6,7,8]. The location of brain aneurysm in cases of aneurysmal SAH (aSAH) are typically found in the anterior circulation ranging between 80–90% of the cases. The anterior communicating artery (ACOM) constitutes the most common site (35%), followed by internal carotid artery (ICA) in 30% (main ICA, posterior communicating artery (PCOM) and the ophthalmic artery), and middle cerebral artery (MCA) in 22% of the cases [9, 10]. The posterior circulation accounts for only 10–20% of cerebral aneurysms location with the most common site being the tip of the basilar artery [9, 10]. Cerebral angiogram remains the gold standard test to detect aneurysms in aSAH [11]. In cases of SAH that are suggestive of aneurysmal etiology, repeating cerebral angiography is recommended if the initial test was normal or equivocal for the presence of aneurysm which reduces the overall negative rate to 15.6% [12].
Aneurysmal subarachnoid hemorrhage constitutes the most severe form of SAH. Typical complications include re-bleeding, hydrocephalus, delayed cerebral ischemia (DCI), cerebral vasospasm, seizures, chemical meningitis and bacterial meningitis in addition to other medical complications [9]. The morbidity and mortality of aSAH are related to these neurological complications as well as other medical complications such as pneumonia [13]. Several scales have been created to grade the severity of aSAH which reflects mortality or poor functional outcome. In particular, the Hunt & Hess score is a clinical score that ranges between 1–5 based on the severity of the clinical symptoms at presentation where grade 1 is considered asymptomatic or with minimal headache while patients with grade 5 are in deep coma [14]. The modified fisher scale is another scale for predicting the risk of cerebral vasospasm and severity of aSAH based on the amount and distribution blood in the subarachnoid space and the ventricles [15]. We will discuss in the following section cerebral vasospasm in further details since it is the main neurological complication following aSAH in addition to the significant role of TCD in the management of it.
Cerebral Vasospasm in SAH
The complications after subarachnoid hemorrhage can be divided into three phases based on the timing of their occurrence after SAH including the acute phase (day 0 – day 3), subacute phase (day 3 – day 30) and late phase (after day 30) [16]. Cerebral vasospasm is defined as a narrowing of the large and medium-sized intracranial arteries that typically occurs in the subacute phase (Day 3 to day 30) with a peak incidence between (7–10) days from aSAH [16, 17]. It is considered a serious complication after aSAH that is associated with regional cerebral hypoperfusion and delayed cerebral ischemia (DCI). The exact underlying pathophysiological mechanisms of cerebral vasospasm and DCI remain unknown. However, endothelial dysfunction with reduced production of endothelial nitric oxide and activation of endothelin-1 receptor on vascular smooth muscles resulting in vasoconstrictions have been implicated. In addition, oxidative stress from activation of inflammatory cascades with subsequent release of oxygen free radicals will add further injury to the vascular smooth muscle cells with subsequent vasoconstriction. The breakdown of erythrocytes in the subarachnoid space could be implicated in the initiation of these pathophysiological cascades [17, 18]. While cerebral vasospasm is typically seen following aSAH, it still can be seen following traumatic SAH and the incidence varies between 19% to 68% [19]. Interestingly, the incidence of vasospasm detected by TCD was higher in cases of traumatic epidural and subdural hematomas than intracerebral or intraventricular hemorrhage [20].
Detection of Cerebral Vasospasm
Several imaging modalities have been utilized in the detection of cerebral vasospasm and associated delayed cerebral ischemia in patients with aSAH in the neurocritical care units with variable degrees of success. Some modalities focus on the vessel directly such as TCD, CT angiography (CTA) and digital subtraction angiography (DSA). Others utilize changes in the brain function or perfusion as indirect surrogates for this phenomenon such as brain tissue oxygenation monitoring (BTO), microdialysis, CT perfusion (CTP), continuous electroencephalogram (cEEG), thermal diffusion monitoring, jugular bulb oximetry and near infrared spectroscopy (NIRS) [21]. The ideal diagnostic modality would provide an early recognition of cerebral vasospasm before the clinical symptoms occur in order to prevent the consequences of vasospasm. Additional considerations include the non-invasive nature of the technology and cost effectiveness. Digital subtraction angiography (DSA) remains the gold standard test to diagnose cerebral vasospasm. However, it is an invasive procedure that may carry risk of arterial dissection or induce thrombosis [11]. Transcranial Doppler (TCD) technology is frequently used in the neurocritical care setting as a non-invasive modality for bedside surveillance and early detection of cerebral vasospasm.
The Role of TCD in Cerebral Vasospasm Detection and Management Following SAH
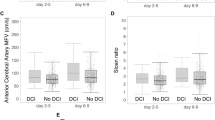
Transcranial Doppler measures cerebral blood flow velocity (CBFV) in the arteries. In case of arterial diameter reduction, as seen in cerebral vasospasm, CBFV will be increased. The magnitude of increase in CBFV will serve as an indirect indicator of the severity of cerebral vasospasm [22]. Indeed, in a meta-analysis by Kumar et al., cerebral vasospasm detection by TCD accurately predicted DCI with high sensitivity, high negative predictive value and fair specificity [23]. However, evidence for the impact of TCD in cases of SAH on mortality and functional outcome remains lacking [23]. It is important to note though that there are several factors that may influence CBFV in addition to cerebral vasospasm (Table 1). Hence, understanding of these factors is critical for accurate TCD interpretation [24]. These factors include:
-
1.
Age:
Previous studies have shown a decline in the total cerebral blood flow with aging which has been estimated to be around 2.6 mL/min per year [25]. In association, CBFV demonstrates a decrease with aging, in particular in people older than 60 years of age. This has clinical significance as significant clinical vasospasm can be seen at lower velocities compared to younger individuals. Table 2 below lists normal reference values for CBFV across age.
-
2.
Sex and pregnancy status:
Women are known to have higher CBF values compared to men [26]. This is attributed to several hormonal factors involving the lower blood viscosity in women [27], effect on estrogen on the brain leading to higher cerebral glucose metabolism [28], the lower brain weight in women [28] and the higher systemic blood flow in females due to higher cardiac index and lower peripheral vascular resistance [29]. In addition, CBF tends to increase further throughout pregnancy reaching a maximum increase of 20% above the non-pregnant value along with decreased cerebrovascular resistance [30].
-
3.
Fever:
Cerebral blood flow changes in response to changes in the cerebral metabolism due to temperature variations [31]. Hyperthermia increases metabolic rate and cerebral blood flow, whereas hypothermia does the opposite leading to decrease in intracerebral volume and intracranial pressure [32, 33]. Patients with SAH and cerebral vasospasm are often febrile and fluctuations of CBFV in these patients could be related to body temperature variations.
-
4.
Intravascular volume and hemodynamic factors:
Cerebral blood flow is augmented by increasing the intravascular volume as in the cases of severe anemia and insufficient delivery of oxygen to the brain in these patients [34]. Additional medications that are used in the treatment of cerebral vasospasm in the setting of SAH may further complicate the interpretation of TCD in these cases. For example, vasodilators and their intra-arterial administration such as milrinone, verapamil, nicardipine or nimodipine are all used in the treatment of cerebral vasospasm. They do not only increase CBF but also improve mean transient time (MTT) in ischemic regions in patients with aSAH induced vasospasm [35]. Additionally, vasoactive medications are administered for the majority of SAH patients with cerebral vasospasm to augment the cerebral perfusion and that may further increase CBFV on TCDs by inducing further vasoconstriction of the brain vessels. These therapeutic interventions in patients with cerebral vasospasm may further complicate the interpretation of the TCD findings [36].
Timing of TCD surveillance in patients with aSAH :
Studies have shown that TCD examination in the first 4 to 10 days following aSAH can detect rapid increases in CBFV which helps in identifying patients at risk for developing delayed cerebral ischemia and neurological deficits [37]. Earlier application of TCD in the first 2–5 days following SAH can also help in detecting radiographic cerebral vasospasm before it becomes clinically apparent which may help inform the treating physicians to predict the occurrence of clinically relevant cerebral vasospasm [37, 38]. The utilization of TCD examination in the next two days following SAH, (5–7) days, can also help in monitoring the progression of cerebral vasospasm towards the development of delayed cerebral ischemia which can be utilized in planning therapeutic and interventional studies [39]. Sloan et al. revealed that the maximum sensitivity of TCD in detecting cerebral vasospasm is at 8 days following aSAH [38]. TCD monitoring after day 12 of SAH can reveal information about the resolution of cerebral vasospasm as well as detecting late or rebound cerebral vasospasm (late 2nd or mid 3rd week following SAH) [40]. However, it is not necessary in the majority of cases.
Determining the severity of cerebral vasospasm by TCD :
The severity of cerebral vasospasm is classically graded into mild, moderate and severe based on the combination of several TCD measurements including the mean CBFV (cm/s), Lindegaard ratio (LR) and Sviri ratio.
-
1.
Lindegaard Ratio (LR) :
In 1976, Lindegaard et al. investigated 76 patients with known aSAH by comparing cerebral angiogram findings to TCD results for the diagnosis of cerebral vasospasm [41]. They identified elevation in the middle cerebral artery (MCA) CBFV compared to the CBFV in distal extracranial ICA on TCD when there was angiographic spasm in the MCA [41]. The term “Lindegaard index” was coined which can be calculated from the TCD by dividing mean CBFV of the MCA by the ipsilateral extracranial ICA CBFV to give information on the severity of MCA spasm [41, 42]. The LR shows correlation with elevation of CBFV in the anterior circulation. We include in Table 3 a commonly used grading scale of SAH severity based on the mean CBFV and LR values. Figures 1, 2 and 3 show examples of normal waveforms at baseline and at time of cerebral vasospasm in aSAH.
-
2.
Sviri ratio:
Sviri ratio can be calculated from TCD by dividing the mean flow velocity of the basilar artery (BA) by the extracranial vertebral artery (VA) velocity [43]. In 2006, Sviri conducted a study of one hundred twenty-three patients with aSAH using TCD and cerebral arteriograms. He found the BA/VA ratio to improve the sensitivity and specificity of TCD detection of BA vasospasm. The grading of cerebral vasospasm severity according to the mean CBFV in the posterior circulation and Sviri ratio is shown Table 3.
The reliability of TCD in detecting cerebral vasospasm:
TCD technology has been extensively utilized in the screening and detection of cerebral vasospasm. However, questions have been raised regarding the specificity of this study, in particular, predicting the conversion of radiographic vasospasm to clinically relevant vasospasm. In this section, we will present the reliability of TCD in diagnosing cerebral vasospasm according to the most commonly assessed intracranial vessels.
-
1.
Anterior circulation :
In a meta-analysis of five studies involving 198 patients and 317 TCD examination comparing TCD findings to angiographic cerebral vasospasm as a screening tool for MCA vasospasm, Lysakowski et al. found TCD sensitivity to be 67% (95% CI 48% to 87%), specificity of 99% (98% to 100%), positive predictive value (PPV) of 97% (95% to 98%), and negative predictive value (NPV) of 78% (65% to 91%) [44]. These data also suggested that most patients who were predicted to have vasospasm on TCD did actually have vasospasm on cerebral angiogram (high PPV) [44]. However, this study did not take into consideration the severity of the cerebral vasospasm on the TCD or its clinical correlates. In summary, TCD has high predictability for MCA spasm in patients who have a suspicion for it.
In anterior cerebral artery vasospasm, a similar meta-analysis of 3 studies including 108 patients and 171 tests identified sensitivity of 42% (11% to 72%), specificity of 76% (53% to 100%), PPV of 56% (27% to 84%), and NPV of 69% (43% to 95%) for TCD in comparison to diagnostic angiogram [44]. This suggests that TCD has lower sensitivity and specificity in detecting cerebral vasospasm in the ACA compared to the MCA.
-
2.
Posterior circulation :
In a study of 47 patients that included 84 TCD examinations of the PCAs during the cerebral vasospasm risk period in comparison to cerebral angiography within 24 hours of the test, TCD had sensitivity of 48%, specificity of 69%, PPV of 37%, and NPV of 78% [45]. The main false positive findings included occlusion of the vessel which was attributed to either anatomical factors or operator error. In another study that evaluated the reliability of TCD in the vertebrobasilary system for the detection of cerebral vasospasm in comparison to cerebral angiogram, TCD had sensitivity of 76.9%, specificity of 79%, PPV of 63%, and NPV of 88% [46]. They also found sensitivity of 43.8%, specificity of 88%, PPV of 54%, and NPV of 82% for detection of vertebral artery vasospasm by TCD [46]. This study was conducted in 64 vertebral arteries and 42 basilar arteries.
TCD and Cerebral Autoregulation in SAH
Cerebral autoregulation has been known since 1959 as the tendency of cerebral blood flow (CBF) to remain approximately constant in response to changes of mean arterial pressure (MAP) [47]. The CBF range through which autoregulation operates is typically between a MAP of 60 to 150 mmHg [48]. The assessment of cerebral autoregulation is made by measuring the relative blood flow changes in response to slow changes in blood pressure which is known as static autoregulation and by measuring the rapid cerebral flow blood flow changes with blood pressure which is known as dynamic autoregulation [49]. While there are currently several tools that are utilized for determining cerebral autoregulation, TCD is considered an excellent non-invasive tool for this aim. There are several parameters that can be derived from TCD signals which reflect the function of cerebrovascular reactivity and autoregulation. These include analysis of CBFV waveforms, their characteristics and relative changes in comparison to changes of the systemic blood pressure [49, 50]. Additional maneuvers can be applied for measuring the cerebrovascular reactivity such as CO2 challenge [51], or the early transient hyperemic response test through brief compression of the carotid and measuring the subsequent hyperemic response of the unilateral CBFV [52]. The full extent of cerebral autoregulation assessment by TCD is beyond the scope of this chapter. We refer to previous excellent papers in this area [49, 53]. The significance of assessment of cerebral autoregulation by TCD in subarachnoid hemorrhage has been found in recent studies. For example, impaired early transient hyperemic response measurement in aSAH predicted poor outcome in one study [52]. Another study showed that impaired cerebral autoregulation early in aSAH predicts the occurrence of delayed cerebral ischemia [54]. The role of autoregulation assessment by TCD in SAH is an area of future research for the utility of TCD and advanced intracranial monitoring in the neurocritical care unit.
A simple commonly used measure by TCD which reflects CBF waveform characteristics is the pulsatility index (PI). This can also be an indirect measure of the impairment of autoregulation and it is defined as: (MCA peak systolic velocity – MCA end diastolic velocity)/MCA mean CBFV [49]. Therefore, the greater decrease in diastolic velocities relative to systolic ones, the more increase in PI [49]. The principle reason for the increase in PI is due to an increase in CBFV pulsation just before complete loss of autoregulation. A strong correlation is found between PI and intracranial pressure (ICP). In a study of 81 patients with a variety of intracranial disorders that required intraventricular catheter placement (46 SAH, 21 closed head injury, 14 with other neurological disorders), a total of 658 TCD measurements were made in parallel with ICP registrations. The study identified a strong correlation between ICP and PI of 93.8% (correlation coefficient). In addition, a negative moderate correlation between cerebral perfusion pressure (CPP) and PI was also identified (correlation coefficient of −0.493) [55]. In SAH, these could constitute signs of decreased cerebral compliance, increased cerebral edema or development of hydrocephalus and the need for placement of intraventricular catheter for cerebrospinal fluid placement.
TCD and Emboli Detection
TCD is a considered as a sensitive technique to detect microembolic signals (MESs) which help to identify patients at high risk for cerebrovascular ischemic events. These signals are characterized by unidirectional high intensity increase, short duration, random occurrence, and a “whistling” sound on TCD [56]. Microembolic signals have been detected in a variety of cerebrovascular diseases that are associated with ischemic events including carotid artery stenosis, aortic arch plaques, atrial fibrillation, myocardial infarction, prosthetic heart valves, patent foramen ovale (PFO), valvular stenosis, during invasive procedures (angioplasty, percutaneous transluminal angioplasty) and surgery (carotid, cardiopulmonary bypass) [56]. The preferred time duration of monitoring for these MESs using the TCD varies based on the clinical scenario. For example, monitoring for 30 minutes in patients with implanted artificial heart valves in whom MESs can be detected, in the vast majority of patients is enough. Extended monitoring for patients with atrial fibrillation or carotid arterial stenosis for more than an hour is required given the low frequency of embolic signals [57]. A few small studies have evaluated MESs in patients with SAH [58,59,60]. In these studies, MESs were detected in up to 70% of SAH patients. These studies did not associate the presence of MESs with the development of cerebral vasospasm. However, in one of these studies, MESs were independently associated with the development of ischemic symptoms in SAH [58]. It is important to note, however, that these previous studies had small sample sizes and future larger studies are needed to confirm the relevance of MEs in patients with SAH.
Conclusions
TCD offers a non-invasive methodology for assessment of patients with SAH. It has been particularly useful in the treatment of aSAH for the assessment of development and management of cerebral vasospasm and it is currently considered an important neurocritical care unit management tool in the United States. TCD offers an additional promising technology for advanced intracranial monitoring of cerebral autoregulation in patients with SAH that will need to be further assessed in future clinical studies.
References
Abraham MK, Chang WW. Subarachnoid hemorrhage. Emerg Med Clin North Am. 2016;34(4):901–16.
Ziu E, Mesfin FB. Subarachnoid hemorrhage. Treasure Island (FL): StatPearls; 2021.
Muehlschlegel S. Subarachnoid hemorrhage. Continuum (Minneap Minn). 2018;24(6):1623–57.
Wang YX, He J, Zhang L, Li Y, Zhao L, Liu H, et al. A higher aneurysmal subarachnoid hemorrhage incidence in women prior to menopause: a retrospective analysis of 4,895 cases from eight hospitals in China. Quant Imaging Med Surg. 2016;6(2):151–6.
Hamdan A, Barnes J, Mitchell P. Subarachnoid hemorrhage and the female sex: analysis of risk factors, aneurysm characteristics, and outcomes. J Neurosurg. 2014;121(6):1367–73.
Kundra S, Mahendru V, Gupta V, Choudhary AK. Principles of neuroanesthesia in aneurysmal subarachnoid hemorrhage. J Anaesthesiol Clin Pharmacol. 2014;30(3):328–37.
Hartings JA, Vidgeon S, Strong AJ, Zacko C, Vagal A, Andaluz N, et al. Surgical management of traumatic brain injury: a comparative-effectiveness study of 2 centers. J Neurosurg. 2014;120(2):434–46.
Flaherty ML, Haverbusch M, Kissela B, Kleindorfer D, Schneider A, Sekar P, et al. Perimesencephalic subarachnoid hemorrhage: incidence, risk factors, and outcome. J Stroke Cerebrovasc Dis. 2005;14(6):267–71.
Petridis AK, Kamp MA, Cornelius JF, Beez T, Beseoglu K, Turowski B, et al. Aneurysmal subarachnoid hemorrhage. Dtsch Arztebl Int. 2017;114(13):226–36.
Keedy A. An overview of intracranial aneurysms. Mcgill J Med. 2006;9(2):141–6.
Mascia L, Del Sorbo L. Diagnosis and management of vasospasm. F1000 Med Rep. 2009;1:33.
Pathirana N, Refsum SE, McKinstry CS, Bell KE. The value of repeat cerebral angiography in subarachnoid haemorrhage. Br J Neurosurg. 1994;8(2):141–6.
Rosengart AJ, Schultheiss KE, Tolentino J, Macdonald RL. Prognostic factors for outcome in patients with aneurysmal subarachnoid hemorrhage. Stroke. 2007;38(8):2315–21.
Hunt WE, Hess RM. Surgical risk as related to time of intervention in the repair of intracranial aneurysms. J Neurosurg. 1968;28(1):14–20.
Frontera JA, Claassen J, Schmidt JM, Wartenberg KE, Temes R, Connolly ES Jr, et al. Prediction of symptomatic vasospasm after subarachnoid hemorrhage: the modified fisher scale. Neurosurgery. 2006;59(1):21-7; discussion -7.
Daniere F, Gascou G, Menjot de Champfleur N, Machi P, Leboucq N, Riquelme C, et al. Complications and follow up of subarachnoid hemorrhages. Diagn Interv Imaging. 2015;96(7–8):677–86.
Daou BJ, Koduri S, Thompson BG, Chaudhary N, Pandey AS. Clinical and experimental aspects of aneurysmal subarachnoid hemorrhage. CNS Neurosci Ther. 2019;25(10):1096–112.
Ciurea AV, Palade C, Voinescu D, Nica DA. Subarachnoid hemorrhage and cerebral vasospasm - literature review. J Med Life. 2013;6(2):120–5.
Modi NJ, Agrawal M, Sinha VD. Post-traumatic subarachnoid hemorrhage: a review. Neurol India. 2016;64(Suppl):S8–S13.
Zubkov AY, Lewis AI, Raila FA, Zhang J, Parent AD. Risk factors for the development of post-traumatic cerebral vasospasm. Surg Neurol. 2000;53(2):126–30.
Kistka H, Dewan MC, Mocco J. Evidence-based cerebral vasospasm surveillance. Neurol Res Int. 2013;2013:256713.
Nicoletto HA, Burkman MH. Transcranial Doppler series part II: performing a transcranial Doppler. Am J Electroneurodiagnostic Technol. 2009;49(1):14–27.
Kumar G, Shahripour RB, Harrigan MR. Vasospasm on transcranial Doppler is predictive of delayed cerebral ischemia in aneurysmal subarachnoid hemorrhage: a systematic review and meta-analysis. J Neurosurg. 2016;124(5):1257–64.
D’Andrea A, Conte M, Scarafile R, Riegler L, Cocchia R, Pezzullo E, et al. Transcranial Doppler ultrasound: physical principles and principal applications in neurocritical care unit. J Cardiovasc Echogr. 2016;26(2):28–41.
Amin-Hanjani S, Du X, Pandey DK, Thulborn KR, Charbel FT. Effect of age and vascular anatomy on blood flow in major cerebral vessels. J Cereb Blood Flow Metab. 2015;35(2):312–8.
Rodriguez G, Warkentin S, Risberg J, Rosadini G. Sex differences in regional cerebral blood flow. J Cereb Blood Flow Metab. 1988;8(6):783–9.
Shaw TG, Mortel KF, Meyer JS, Rogers RL, Hardenberg J, Cutaia MM. Cerebral blood flow changes in benign aging and cerebrovascular disease. Neurology. 1984;34(7):855–62.
Baxter LR Jr, Mazziotta JC, Phelps ME, Selin CE, Guze BH, Fairbanks L. Cerebral glucose metabolic rates in normal human females versus normal males. Psychiatry Res. 1987;21(3):237–45.
Messerli FH, Garavaglia GE, Schmieder RE, Sundgaard-Riise K, Nunez BD, Amodeo C. Disparate cardiovascular findings in men and women with essential hypertension. Ann Intern Med. 1987;107(2):158–61.
Nevo O, Soustiel JF, Thaler I. Maternal cerebral blood flow during normal pregnancy: a cross-sectional study. Am J Obstet Gynecol. 2010;203(5):475 e1–6.
Sahuquillo J, Vilalta A. Cooling the injured brain: how does moderate hypothermia influence the pathophysiology of traumatic brain injury. Curr Pharm Des. 2007;13(22):2310–22.
Bisschops LL, Hoedemaekers CW, Simons KS, van der Hoeven JG. Preserved metabolic coupling and cerebrovascular reactivity during mild hypothermia after cardiac arrest. Crit Care Med. 2010;38(7):1542–7.
Clifton GL, Miller ER, Choi SC, Levin HS, McCauley S, Smith KR Jr, et al. Lack of effect of induction of hypothermia after acute brain injury. N Engl J Med. 2001;344(8):556–63.
Kuwabara Y, Sasaki M, Hirakata H, Koga H, Nakagawa M, Chen T, et al. Cerebral blood flow and vasodilatory capacity in anemia secondary to chronic renal failure. Kidney Int. 2002;61(2):564–9.
Nogueira RG, Lev MH, Roccatagliata L, Hirsch JA, Gonzalez RG, Ogilvy CS, et al. Intra-arterial nicardipine infusion improves CT perfusion-measured cerebral blood flow in patients with subarachnoid hemorrhage-induced vasospasm. AJNR Am J Neuroradiol. 2009;30(1):160–4.
Manno EM, Gress DR, Schwamm LH, Diringer MN, Ogilvy CS. Effects of induced hypertension on transcranial Doppler ultrasound velocities in patients after subarachnoid hemorrhage. Stroke. 1998;29(2):422–8.
Babikian VL, Feldmann E, Wechsler LR, Newell DW, Gomez CR, Bogdahn U, et al. Transcranial Doppler ultrasonography: year 2000 update. J Neuroimaging. 2000;10(2):101–15.
Sloan MA, Alexandrov AV, Tegeler CH, Spencer MP, Caplan LR, Feldmann E, et al. Assessment: transcranial Doppler ultrasonography: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2004;62(9):1468–81.
Alexandrov AV, Sloan MA, Tegeler CH, Newell DN, Lumsden A, Garami Z, et al. Practice standards for transcranial Doppler (TCD) ultrasound. Part II. Clinical indications and expected outcomes. J Neuroimaging. 2012;22(3):215–24.
Samagh N, Bhagat H, Jangra K. Monitoring cerebral vasospasm: how much can we rely on transcranial Doppler. J Anaesthesiol Clin Pharmacol. 2019;35(1):12–8.
Lindegaard KF, Nornes H, Bakke SJ, Sorteberg W, Nakstad P. Cerebral vasospasm diagnosis by means of angiography and blood velocity measurements. Acta Neurochir. 1989;100(1–2):12–24.
Aaslid R, Huber P, Nornes H. Evaluation of cerebrovascular spasm with transcranial Doppler ultrasound. J Neurosurg. 1984;60(1):37–41.
Sviri GE, Ghodke B, Britz GW, Douville CM, Haynor DR, Mesiwala AH, et al. Transcranial Doppler grading criteria for basilar artery vasospasm. Neurosurgery. 2006;59(2):360–6; discussion -6.
Lysakowski C, Walder B, Costanza MC, Tramer MR. Transcranial Doppler versus angiography in patients with vasospasm due to a ruptured cerebral aneurysm: a systematic review. Stroke. 2001;32(10):2292–8.
Wozniak MA, Sloan MA, Rothman MI, Burch CM, Rigamonti D, Permutt T, et al. Detection of vasospasm by transcranial Doppler sonography. The challenges of the anterior and posterior cerebral arteries. J Neuroimaging. 1996;6(2):87–93.
Sloan MA, Burch CM, Wozniak MA, Rothman MI, Rigamonti D, Permutt T, et al. Transcranial Doppler detection of vertebrobasilar vasospasm following subarachnoid hemorrhage. Stroke. 1994;25(11):2187–97.
Claassen JA, Meel-van den Abeelen AS, Simpson DM, Panerai RB. International Cerebral Autoregulation Research N. Transfer function analysis of dynamic cerebral autoregulation: a white paper from the International Cerebral Autoregulation Research Network. J Cereb Blood Flow Metab. 2016;36(4):665–80.
Paulson OB, Strandgaard S, Edvinsson L. Cerebral autoregulation. Cerebrovasc Brain Metab Rev. 1990;2(2):161–92.
Bellapart J, Fraser JF. Transcranial Doppler assessment of cerebral autoregulation. Ultrasound Med Biol. 2009;35(6):883–93.
Zeiler FA, Smielewski P, Stevens A, Czosnyka M, Menon DK, Ercole A. Non-invasive pressure reactivity index using Doppler systolic flow parameters: a pilot analysis. J Neurotrauma. 2019;36(5):713–20.
Fierstra J, Sobczyk O, Battisti-Charbonney A, Mandell DM, Poublanc J, Crawley AP, et al. Measuring cerebrovascular reactivity: what stimulus to use? J Physiol. 2013;591(23):5809–21.
Rynkowski CB, de Oliveira Manoel AL, Dos Reis MM, Puppo C, Worm PV, Zambonin D, et al. Early transcranial Doppler evaluation of cerebral autoregulation independently predicts functional outcome after aneurysmal subarachnoid hemorrhage. Neurocrit Care. 2019;31(2):253–62.
Panerai RB. Transcranial Doppler for evaluation of cerebral autoregulation. Clin Auton Res. 2009;19(4):197–211.
Budohoski KP, Czosnyka M, Smielewski P, Kasprowicz M, Helmy A, Bulters D, et al. Impairment of cerebral autoregulation predicts delayed cerebral ischemia after subarachnoid hemorrhage: a prospective observational study. Stroke. 2012;43(12):3230–7.
Bellner J, Romner B, Reinstrup P, Kristiansson KA, Ryding E, Brandt L. Transcranial Doppler sonography pulsatility index (PI) reflects intracranial pressure (ICP). Surg Neurol. 2004;62(1):45–51. discussion
Vukovic-Cvetkovic V. Microembolus detection by transcranial Doppler sonography: review of the literature. Stroke Res Treat. 2012;2012:382361.
Hudorovic N. Clinical significance of microembolus detection by transcranial Doppler sonography in cardiovascular clinical conditions. Int J Surg. 2006;4(4):232–41.
Romano JG, Rabinstein AA, Arheart KL, Nathan S, Campo-Bustillo I, Koch S, et al. Microemboli in aneurysmal subarachnoid hemorrhage. J Neuroimaging. 2008;18(4):396–401.
Azarpazhooh MR, Velayati A, Chambers BR, Nejad HM, Nejad PS. Microembolic signals in subarachnoid hemorrhage. J Clin Neurosci. 2009;16(3):390–3.
Romano JG, Forteza AM, Concha M, Koch S, Heros RC, Morcos JJ, et al. Detection of microemboli by transcranial Doppler ultrasonography in aneurysmal subarachnoid hemorrhage. Neurosurgery. 2002;50(5):1026–30. discussion 30-1
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Bittar, J., Hannawi, Y. (2022). Transcranial Doppler in Subarachnoid Hemorrhage. In: Ziai, W.C., Cornwell, C.L. (eds) Neurovascular Sonography . Springer, Cham. https://doi.org/10.1007/978-3-030-96893-9_5
Download citation
DOI: https://doi.org/10.1007/978-3-030-96893-9_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-96892-2
Online ISBN: 978-3-030-96893-9
eBook Packages: MedicineMedicine (R0)