Abstract
Growing volume of environmental plastic waste has generated global concerns because of its persistence in the environment. Plastic is one of the main environmental problems because of its slow degradation rate or organic matter’s non-biodegradability in natural conditions. Plastic contamination and global warming are caused not only by increasing the issue of waste disposal and landfilling but also by burning CO2 and dioxins. Not only the environment is contaminated, but plastic also poses health risks to wildlife. Plastic waste burning produces poisonous gases that pose a health hazard after inhalation causing cancer and lung diseases. Biodegradation of plastics by microbes is one possible solution to this problem. Plastic waste has not been much treated so far by means of combination of biological along with the physicochemical methods; this chapter explores the potential of cohesive methods for remediation of plastic waste. Main benefits of plastic are lightweight, inertness, toughness, strength, and low cost, but it also has the disadvantages such as recalcitrant biodegradation and difficult to naturally degrade. This chapter highlights the effect of cohesive methods on the plastic degradation by potential microbes. PET type of plastic was treated biologically by various microbes including microalgae, lichens, fungi, and bacteria subjected to either pretreatment or not. It is concluded from the different review studies that pretreatment had marked effect on the cracking and alteration of plastic polymer which helped to grow microbial species on the cracked surface.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
14.1 Introduction
Plastic consumption is increasing day by day, and plastic pollution has amplified manifold (Haward 2018). About 100 million tons of plastics are manufactured around the world every year (Payne et al. 2015). The use of the synthetic polymers is part of our daily life; polythene and plastics are widely used as packaging, food, medicine, clothing, shelter, transport, industry, and agriculture in not only rural but also urban areas since the last few decades (Cragg et al. 2015). This causes waste disposal and contamination due to extensive use of polythene and plastics. The best (Eichinger et al. 2005) way to degrade water treatment and remove pollutants from the environment is through biodegradation (Sanchez et al. 2015). Biodegradation is the degradation of the natural and artificial polymers by different microorganisms (fungi, bacteria) (Sanchez et al. 2015).
Plastic pollution is increasing at such an alarming rate that plastic disposal seems very difficult due to limited dumping sites (Restrepo-Flórez et al. 2014). In all economic sectors, plastic polymer has been widely used for manufacturing various useful products. Because of being versatile, elastic, durable, and recalcitrant, it has brought quality to life as it acts as an outstanding barrier to external stress (Singh and Sharma 2008; Alimi et al. 2018). Moreover, it has unique mechanical and thermal properties: cost-effective, low-weight indestructible, and highly moldable, and is widely used at industrial and household levels. Plastic polymer like polyethylene is a man-made polymer which has high molecular weight and greatly hydrophobic in nature (Ahmed et al. 2014). It causes adverse impact to the environment because of its recalcitrant nature and extensive use in the production of grocery bags, conveyance material, and beverage bottles and in making of electrical and medical appliances (Krupp and Jewell 1992).
Polythene is sustainable and will require up to 1000 years for natural environmental degradation (Sangale et al. 2012). Plastic is one of the main environmental problems because of its slow degradation rate or organic matter’s non-biodegradability in natural conditions. Ali et al. have clarified in 2009 that improper disposal of waste and landfilling are not the only cause of plastic contamination and global warming, but other activities like burning carbon dioxide as well as carcinogenic hydrocarbons called dioxins are also contributing to it. Burning of plastic waste produces poisonous gases that pose a health hazard after inhalation causing cancer and lung diseases (Pramila and Ramesh 2011). The accumulation of synthetic polymers in the environment is mostly because of the unavailability of effective methods of disposing these polymers and thus increasing the environmental hazards to plants and animals (Barnes et al. 2009).
The main characteristics of plastic are lightweight, inertness, toughness, strength, and low cost, but it also has some drawbacks, like it is intractable to degradation and very hard to decay naturally (Leja and Lewandowicz 2010). The best (Eichinger et al. 2005) way to degrade water treatment and remove pollutants from the environment is by biodegradation (Sanchez et al. 2015). Biodegradation is the method of degrading natural polymers like cellulose, lignin, and artificial polymers like polystyrene and polyethylene by the enzymatic activity of microbes such as fungi and bacteria into metabolites such as H2O, CO2, CH4, biomass, etc. (Sheel and Pant 2018).
14.2 Plastic as Source of Pollution
The amount of plastic deposition in the environment is evident that environmental contamination is caused by plastic. Around 10% of global waste disposal is reported to be plastic (Lebreton and Andrady 2019). According to a general estimate, the annual production of plastic has reached to approximately 52 million tons and is increasing day by day and contributes to almost 60% of ocean debris, and it is estimated that total accumulation of plastic will be reached to 250 million tons till 2025. Plastic contamination has been described as a global concern in freshwater and marine environment. Plastic waste is estimated to account for 60–80% of marine debris, exceeding 90–95% in some areas (Kibria 2017). Eighty percent of the total plastic waste is from sources that are land based, while the remaining 20% has ocean origin, which includes different sources like ropes and nets used for fishing (Van Sebille et al. 2016). Plastic debris is an increasing pollutant which does not degrade easily, but remains for long periods in the aquatic ecosystem. An estimated 5 trillion fragments of plastic float in the oceans of the planet (Tunçer et al. 2019).
The plastics are manufactured at a large scale because it is used in textile industry, food synthesis, construction activities, and for the synthesis of carrying and conveyance material. The accumulation of plastic in the water bodies is considered a major problem and may undergo mechanical and chemical breakdown, causing water pollution and threat to aquatic life (Eriksen et al. 2014). Its direct contact to the ultraviolet rays from sunlight and disintegration by the ocean waves breaks plastic into micro and nano forms which more toxic and easily become the part of the food chain. The studies suggest that the plastic present in the oceans is difficult to degrade due to pertaining environmental conditions (i.e., exposure to UV radiations and its conversion into micro and nano form) (Cole et al. 2011). The high-density polyethylene usually sinks down into water, undergoing torpid degradation and changing into microplastics, causing huge threat to marine biota (Andrady 2017), while the low-density polyethylene and polypropylene floats on water surface (Alimi et al. 2018). Dangerous waste has been exposed to the natural environment. In many areas, the local environment has been destroyed by major disasters including oil spills every year, and the unnecessary use of plastics has degraded soil and water. Million tons of polythene are produced worldwide. It is used in the development of plastic films to make containers and other products like cups. Polythene waste also causes damage to the health of fish, birds, goats, deer, and other animals (Lora and Andrade 2009). Plastic pollution emerges as a threat to global ecology, as it is resilience to degradation (Saxena et al. 2009). The continuous plastic pollutant flow is sustained by two means: deliberately by unsafe domestic and industrial waste disposal and by improperly contained static and transported waste directed by the weather; land-based plastic waste migrates to the oceans, where it is further introduced by the dumping or destruction of underwater ships and offshore oil platforms. Such pollution has a number of deleterious consequences. Plastic waste in the marine environment is the cause of numerous dangerous and environmentally harmful effects. Plastic remains pose a direct threat to wildlife, with many and varied species recognized as having an undesirable impact on plastic objects (Mussgnug et al. 2010).
The one main source of environmental pollution is LDPE (Romero Saez et al. 2017). High-density polyethylene generally sinks into water undergoing degradation and converting into microplastics (Andrady 2011), and the low-density polyethylene and polypropylene floats on the surface of water (Alimi et al. 2018).
The key risks coupled with plastic objects to animals are the entanglement and ingestion of such objects (McKendry 2002). Not only the environment is contaminated, but also plastic poses health risks to wildlife. The improperly disposed and utilized plastic bags don’t allow air and water to enter the earth which leads to exhaustion of fresh water and is also an ultimatum to a creature’s life. Direct contact to ultraviolet rays from sunlight degrades plastic into small toxic parts, which can be easily entered into the food chain by ingestion of especially aquatic animals (Denuncio et al. 2011). A wide range of humans have been noted to have negative impacts on plastic wreckage, ocean birds, sea turtles, cetaceans, filter feeders, fur seals, and sharks (Srirangan et al. 2012). Marine birds are particularly prone to plastic items through ingestion, which they mistake for food (Sims et al. 2006). Furthermore, plastic contamination annually records higher amounts of marine mammal death approximately 1 lakh and seabirds deaths up to 1 million (Kedzierski et al. 2018).
Plastic eaten or taken up by these animals does not digest and contribute to decrease stimulation of the diet and blocking of gastro intestine, reduce secretion of gastric enzymes, and lower the steroid hormone level and raising reproductive problems (Horton et al. 2017). Plastic particulates in the ocean were shown to retain very high concentrations of organic toxins. Harmful chemicals like polychlorinated biphenyls (PCBs), nonylphenols (NPs), organic pesticides including dichlorodiphenyltrichloroethane (DDT), polycyclic aromatic hydrocarbons (PAHs), polybrominated diphenyl ethers (PBDEs), as well as bisphenol A (BPAs) have been consistently found across oceanic plastic wreckage (Sharma et al. 2019).
The existence of these substances even further raises the risk involved with the consumption of plastic debris by wild animals (Kurian et al. 2013).
14.3 Nature and Kinds of Plastics
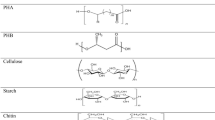
Plastics are synthetic long-chain polymeric particles and are considered very resilient toward environmental changes (Kumar et al. 2017). Plastics can be defined as long-chain polymers, which can occur naturally or synthetically (Ghosh et al. 2013). Natural plastics consist of polymers that include chitin, lignin, starch, etc., whereas synthetic plastics are generally derived from petrochemicals such as polyethylene, polystyrene, silicone, nylon, etc. (Narwal and Gupta 2017). Polymers are mainly petrochemical in nature and have semisynthetic organic compounds and high molecular weight which can be molded into different shapes like bags, household items, wires, packaging and wrapping material (40% consumption), agriculture mulch, pipes, and electronics (Accinelli et al. 2012). Monomers of ethylene form the polyethylene, which is an organic polymer, nonpolar, porous, high molecular weight hydrocarbons formed primarily by cracking ethane and propane, naphtha, and gas oil (Khoddami et al. 2013). High-density polyethylene (HDPE) and low-density polyethylene (LDPE) are the two most common types of polyethylene. Polyethylene is completely linear and available from 0.91 to 0.97 g/cm3 with elastic thickness range. Furthermore, high-density polyethylene is additionally linear with marginal splitting resulting in high packing density (Kigozi et al. 2013).
Basically, polyethylene is an organic polymer which is made up of long-chain monomers known as ethylene (C2H4) molecules. Polythene is produced by ethane degradation or cracking propane and gas oil (Sangale et al. 2012). The general formula of polyethylene is CnH2n, and the n is the number of carbon. Polythene compounds have two types that are most common which include HDPE and LDPE (Romero Saez et al. 2017). Polyethylene having little density is completely linear and accessible from 0.91 to 0.97 g/cm3 with elastic thickness range. High-density polyethylene is additionally linear with marginal splitting resulting in high packing density (Kigozi et al. 2013). Polyethylene (HDPE, LDPE, LLDPE, and MDPE), polystyrene (PS), polypropylene (PP), and polyvinyl chloride (PVC) have been classified as commonly used plastics (Muhonja et al. 2018).
Plastic is composed of different elements like oxygen, nitrogen carbon, hydrogen chloride, and silicon. Coal and hydrocarbons are used in extracting the fundamental plastic material (Ali et al. 2016). Therefore plastics are classify into two various types, that is, thermoset and thermoplastics. Thermoplastics are classified as nonbiodegradable, and these are generally allowed for their hardening and softening to cool and heat repetitively. On the other hand, thermoset plastics have a strong cross-linked structure, and they are considered linear solids (Bardají et al. 2020).
14.4 Uses of Different Plastic Types
Food, cosmetics, pharmaceutical chemicals, and detergents are packed mostly by using synthetic plastics. Thirty percent of plastics have their usage in packaging worldwide. Plastic’s usage is increasing at a rapid level of 12% annually (Sabir 2004). The most commonly used plastics in packaging are polyethylene (HDPE , LDPE, LLDPE, and MDPE), polyethylene terephthalate (PET), polystyrene (PS), polypropylene (PP), polyvinyl chloride (PVC), polyurethane (PUR), polybutylene terephthalate (PBT), and nylons which are shown in Table 14.1 below. The major use of plastics is not restricted to their mechanical and thermal characteristics but depends on the durability and stability of plastics.
14.5 Plastic Waste Disposal
Plastic disposal is a global issue. Around the world, the most widely plastic disposal methods are recycling, landfilling, and incineration (Silva et al., 2020).
14.5.1 Combustion of Plastic
Combustion of plastic products also produces gases as by-product, and these released gases are hazardous as their inhalation by living organisms especially humans can lead to several respiratory disorders and in some cases can also cause cancer (Pramila and Ramesh 2011). The plastic waste is mainly burned in landfills, this threatens Natural environment due to emission of toxics gases from it causing air pollution and ozone layer depletion, in addition to this dioxins release that have potential of causing various anthropogenic problems like hormonal abnormalities (Raziyafathima et al. 2016). The aromatic rings, chains, and bonds make the plastic polymer resistant to microbial degradation, thus persisting for centuries and affecting the natural environment, causing serious air, water, and soil pollution (Eriksen et al. 2014).
14.5.2 Landfilling
Around 60% of plastic waste is discarded in a landfill, which is known to be one of the best methods of disposing of solid waste material. It’s a widely used method for disposal of plastic, but the main drawback of this approach is that on the open land, the dumped plastic is not quickly degraded (Qasaimeh et al. 2016). Plastic litter, which is dumped openly or in sanitary landfills, produces harmful leachate. In addition, disposing plastic in landfills results in land degradation and also increases the chance of plastic ingestion by animals. Ultimately, the release of plastics into aquatic environments leads to their intake by aquatic animals which causes death of such organisms and creates a high risk of biomagnifications and bioamplification of harmful plastic substances (Denuncio et al. 2011).
14.5.3 Recycling
It is essential to have proper treatment as well as disposal options for plastic waste treatment. Recycling plastic is a better disposal option, but this method has its drawbacks since it can no longer be recycled at the end of the life of products. The production and use of biodegradable plastics is another method (Al-Salem et al. 2009).
14.6 The Assessment of Biodegradable Nature
The assessment of the biodegradable nature of plastic can be done by measuring the structural changes under the microscope or by evaluating the growth of microorganisms after the biological and enzymatic action along with the evolution of carbon dioxide (Kissi et al. 2001; Ahmed et al., 2018). Plastics are of numerous kinds such as polystyrene (PS), polyethylene (PE), polyurethane (PU), polypropylene (PP), poly(vinyl chloride) (PVC), and poly(ethylene terephthalate) (PET). Plastic polymers basically comprise of hydrocarbon monomers created by natural and synthetic organic and inorganic raw material. Generally, there are two main kinds of plastic, that is, thermoplastics and thermoset plastics. Thermoplastics are long linear carbon chains, having different molecules which are joined end to end. Among different kinds of plastics, the microplastic is more dangerous (Cole et al. 2011) as it is difficult to identify and tracked (Kutten, 2019) and is accumulating inside the body of different organisms causing severe anomalies resulting visible decline in abundance and diversity of fish and coastal birds.
14.6.1 Factors Affecting Biodegradation of Plastics
The effects of the biodegradation process are also depending on some main factors like characteristics of the polymers and the condition of the environment. The characteristics of the polymer include functional group, morphology, molecular weight, stability, crystalline nature, additives, cross-link, and copolymers. Low molecular weight polymers are favorable for biodegradation (Varjani and Upasani 2017), and environmental conditions like temperature, ultraviolet radiations, pH, hydrophobicity, salinity, presence or absence of O2, etc. also affect the biodegradation of plastics (Gu 2003). In the formation of polymers, stabilizers and antioxidant are used which drop off biodegradation time, and also it can be harmful for microbes (Kale et al. 2015). In addition to all listed above, the structural factors (linearity and polymer branching; bonding type, such as C-C, amide, and ester), the polymers’ molecular composition, and physical structure such as powder, films, pellets, and fibers can also affect the polymer’s biodegradability. The rate at which the polymer degrades eventually depends upon the procedure of decaying and speeding of process.
14.6.2 Different Steps of Biodegradation Mechanisms
Biodegradation is a process of degrading complex organic polymers into simpler forms by using microbes (Restrepo-Flórez et al. 2014). Processes causing alterations in polymer functions or properties due to chemical, physical, or biological reactions ultimately result in bond breakage. Chemical alterations have been categorized as polymer degradation (Ghosh et al. 2013). Optical mechanical or electrical properties of a substance or material change due to degradation through cracking, crazing, erosion, discoloration, or phase separation. The changes comprise bond breakage and chemical transformation, and new functional groups are also formed (Nigam 2013).
Degraded particles are again scattered in the environment, and those particles are generally nontoxic. For biodegradation, microbes form the catalytic enzymes in nature (Hadad et al. 2005). Through various enzymatic activities and cleavage of bonds, the degradation process is achieved by microbes (Pathak and Navneet 2017). The degradation process occurs in sequential phase’s bio-deterioration (changing the the physicochemical characteristics of the polymer), bio-fragmentation (breakdown of polymers into simpler form through enzymatic cleavage), assimilation (microbes uptake the molecules), and mineralization (after the degradation, oxidized metabolites such as CO2, H2O, and CH4 are produced, which are shown in Fig. 14.1).
14.6.3 Aerobic and Anaerobic Degradation of Polymers
Degradation of polymers depends on polymers’ physicochemical properties. The weight and crystalline nature are the main properties of polymers which affect the efficiency of microbial degradation. The polymer degradation enzymes are divided into two types, that is, extracellular depolymerase and intracellular depolymerase (Gu 2003). In the degradation of complex polymers into simple units such as monomers and dimers, exoenzymes are generally involved. They are also used as carbon and energy sources by microbes. During or at the end of processes, polymer degradation (mineralization) produces new products, for example, CO2, H2O, or CH4 (Gu 2003). On the availability of oxygen, this degradation process is dependent. In both aerobic and anaerobic cases, mineralization of polymers takes place. Polymers are converted into simple form by using microorganisms, and mineralization produces CO2 and H2O in the presence of oxygen, while in anaerobic conditions, it produces organic acids like CH4 and H2O which are shown in Fig. 14.2. Biodegradation is an efficient way to clean up the waste of plastic with the help of microbes (Kumar et al. 2011). Enzymes of microbial species are used for pollution control and contribute to the creation of an environment that is friendly (Gu 2003). Various types of microbes are known to be used through the process of mineralization.
14.7 Major Properties Affecting Plastic Biodegradation
14.7.1 Characteristics of Plastics
Plastic products take hundreds of years to degrade naturally, and the material only partially degrades after that. It is important to know the structure and characteristics of polymers that change with external environmental factors according to the life cycle of the material. Weight reduction is an important parameter that helps to measure the degradation rates. This section describes some of the important properties that assist in indicating the rate of degradation (Artham and Doble 2008).
14.7.2 Weight of Plastic Films
Plastic film is preferred as it is Light weight, durable nature, and low cost. Therefore, due to these properties of plastic it is used in the world for many applications and mostly in packaging (Kumar et al. 2013). Plastic is not easily degraded because they are durable and made up of such a strong C-H bond. One way for the degradation of plastic is to degrade plastic biologically, which is commonly known as biodegradation (Moharir and Kumar 2019). Some researchers have placed the films of plastic in the soil to analyze the rate of biodegradation of plastics (Chiellini et al. 2003). The loss of plastic film rate before and after the study is used as a degradation rate parameter with some other relevant parameters. Weight loss of plastic film is the indicating parameter of degradation. Therefore the essential parameter to be considered is the weight of plastic films before and after the process of degradation (Hadad et al. 2005).
14.7.3 Thickness of Plastic Films
Any plastic material strength is related to the thickness of material similarly thickness of plastic shows its strength, thicker film will be more strength and vice-versa. In plastic film degradation studies, it is one of the most significant parameters that must be taken into account. The degradation rate of thicker plastic films is slow, and a thinner plastic film will degrade quickly. The thickness of plastic depends on the application in which they are used (Moharir and Kumar 2019).
14.7.4 Density of Plastic Films
Types of plastic are categorized by their densities. With the range of thickness, every plastic has its density. It is an important parameter to count the viability of a film, and it plays an important role in which the transparency of the film is a key factor needed for certain applications (Gulmine et al. 2003).
14.7.5 Mechanical Properties of Plastics
Plastic’s mechanical and elastic properties are useful in identifying the plastic strength that would be useful for any further research to be carried out and used in particular applications (Gerald 2000). Stress increases as these polymers distort under pressure, and it relies on the polymers’ structure and mechanical characters, and some of the main polymer mechanical properties are as follows.
14.7.6 Tensile Strength of Plastic Films
The Tensile strength is a significant mechanical character that shows the durability, strength, and rigidity of the polymers (Ferreira et al. 2005). In many applications, various thickness levels of polymers are tested in for tensile strength to know the effectiveness in resisting external loading. The tensile test is performed to control tension till the failure stage to understand the strength and capability of the polymer.
14.7.7 Plastic Film Elongation
Elongation is another important character. The breakpoint of polymers can be identified by performing this test that will help to understand the efficiency of material breakage at a particular length (Pedroso and Rosa 2005). Some external factors such as temperature, sunlight, and UV radiation can affect the elongation of the polymer.
14.7.8 Young’s Modulus of Plastics
Now the term Young’s modulus is replaced by the elastic modulus. For determining any solid, material stiffness is measured by Young’s modulus. The linear elasticity of materials basically defines the solid material stiffness. With different temperatures, Young’s modulus varies. Young’s modulus of polymers is measured to analyze its elastic behavior. The stress-strain relationship is defined by this mechanical property (Pedroso and Rosa 2005).
14.8 Potential Degradation Procedures
A process which causes the breakage or splitting of complex or larger fragments into simple and small size particles is known as degradation; degradation of these polymers into monomers can be done as a result of any physical and chemical changes; in the degradation process, a lot of procedures are involved. The plastic degradation includes all processes (either natural or synthetic) accounting for changing plastic properties (Yousif and Haddad 2013). Depending upon composition and type, the degradation time of plastics varies like plastic bottles require 400–500 years and grocery bags require 10–1000 years to degrade (Eriksen et al. 2014). Plastics can be degraded by a number of physical, chemical, and biological methods. Physical degradation includes polymer recrystallization or denaturation of protein structures and exposure to ultraviolet rays which refer to the polymer breakdown by photooxidation, releasing different chemical compounds and radicals, decreasing molecular weight leading to polymer destruction, and converting it into a more hazardous form. Several procedures like photochemical and thermal degradation can be used for the biodegradation of polythene (Lu et al. 2009). Plastic accumulation in our environment is a very serious concern, and its accumulation in the environment is causing long-term problem to living organisms and their habitats. It is actually destroying the natural habitat of flora and fauna, especially those in the aquatic environment (Restrepo-Flórez et al. 2014). Degradation of plastic mainly occurs by three processes which are physical, chemical, and biological degradation process (Yousif and Haddad 2013). The biodegradation of plastics occurs actively, depending on their properties, under different conditions, and degrading microorganism varies from one another and has its own optimum soil and water growth conditions. Ecological factors such as humidity, pH, temperature, salinity, aerobic and anaerobic conditions, solar light, water, pressure, as well as plant environment not only affect the degradation of polymers but also have a critical impact on the microbial community and enzymatic activity (Nigam 2013).
Plastic biodegradation requires adherence of the microbes to the polymer layer, microorganism growth by using the polymer as the origin of carbon source, and polythene degradation (Gunatillake et al., 2006). The biological decomposition of plastic, extracellular depolymerization and intracellular metabolism, involves at least two forms of enzymes (Tilman 1977). From the previous work, it is observed that UV due to the synergistic effect of nitric acid and microbial action encouraged an oxidation reaction that improves and increases the rate of LDPE biodegradation (Egli 1995).
14.9 Properties and Applications
PET is semicrystalline thermoplastic polyester (Harrison and Wren 1976). It is produced by different companies under separate trade names. PET is strong and durable, stable both chemically and thermally with low gas permeability, and easy to handle and manage (Ingram et al. 1999). This combined effect of properties makes PET a valuable product for a variety of applications and a considerable component of global plastic usage (Chen et al. 2011). More than 50% of the synthetic fabrics generated globally consist of PET, and global usage of PET has been reported to exceed $17 billion per year (Chen et al. 2011). The Sheets, films, fabrics, food and beverage packaging (especially soft drinks and water bottles), appliances, auto parts, home appliances, lighting products power tools, sports goods, photography devices, X-ray sheets and textiles are products based on the actual use and the desired properties of PET. By controlling the polymerization conditions, PET can be manufactured to specifications (Nigam 2013).
14.10 Pretreatments for Plastic Degradation
It was anticipated that physical treatment may enhance the biodegradation rate (Arkatkar et al. 2009). Physicochemical treatments of the polymer by microbes like fungus lead to oxidation and further breakdown. Thus physicochemical pretreatment of polymers helps in easy assimilation by the microbes (Arkatkar et al. 2009). The oxidized polymer helps in adhesion of microorganisms and easily adheres to the oxidized polymers surface, which become less hydrophobic and it the prerequisite biodegradation.
14.10.1 Physical Methods
The physical treatment strategies involve decreasing the plastic waste through physical methods like crushing (Al-Salem et al. 2009), incineration, and pulverizing. Some of the other treatments of physical mode include thermal treatment, treatment with ultraviolet rays, photooxidative degradation, etc. In addition, these types are responsible for altering the polymer’s nature, shape, and structure to some extent (Mohan et al. 2016). Plastic modifications are identified by surface splitting, cracking, polymer disintegration, degradation, decreased polymer weight, discoloration, and gaps on the polymer surface, also referred to as aging.
Mechanical stress is provided by giving high speed or stirring and powdering, splitting, mixing, chopping, crushing, etc.; this mechanical strength breaks the polymer and decreases its molecular bulk (Ahmed et al. 2018). Plastic polymers is complex compounds and microorganisms cannot ability to utilized it directly. The oxidation process reduces the long chain of plastic polymers and make availability to microorganism by reducing its hydrophilic level . It also included UV exposure and provides temperature.
14.10.2 Photodegradation
In order to degrade the larger and complex molecules into simple, smaller particles, high-intensity photon particles are used like ultraviolet radiations to react with the photo reactive groups, so the chains of polymers cleave in a proficient way. Photodegradation is a polymer degradation process that includes placement of films under the sunlight or ultraviolet rays and photodegrade them with time (Moharir and Kumar 2019). Strong bond of polymers is hard to disrupt, but this kind of pretreatment will increase the rate of biodegradation.
14.10.3 Thermal Degradation
Thermal degradation is similar to the photodegradation (Ray and Cooney 2018). Photodegradation includes chemical reactions occurring on the outer surface, while thermal degradation occurs on the greater surface of the polymer (Ray and Cooney 2018). Thermal degradation occurs with heat treatments such as incineration, pyrolysis, and gasification, but treatments such as incineration emit toxic gases, like dioxins and furans which are known to be the main hazards to humans and to the environment as well (Erceg et al. 2018).
14.10.4 Chemical Degradation
Chemical treatment methods depend on different chemicals usage that have capability of splitting plastic polymer’s chain and converting it into forms which are nontoxic in nature (Lin and Yang 2009). These methods cannot be used on large scale due to the problem of disposing these chemicals that are used in these methods. Treating plastic polymer with nitric acid enhances the rate of biodegradation (Leja and Lewandowicz 2010). Many previous investigations also reported that providing pretreatment enhanced the rate of biodegradation (Ahmed et al. 2018). Pretreatment may enhance the breaking of polymer bond due to oxidation, and it also improved the hydrophilic level and converted plastics into a more available form which can be easily assimilated by microorganisms (Vimala and Mathew 2016). UV , nitric acid, and temperature exposure are the best ways for physicochemical degradation as reported by Ray and Cooney (2018).
14.10.5 Biological Degradation
Biodegradation is another method for the disposal of plastic waste, it is an environmental friendly technique, and it is more suitable than physical and chemical degradation (Shah et al. 2008). Latest developments have advocated that many organisms (bacterial and fungal species) have the ability to degrade plastics, and in this process, by-products are produced that are nontoxic in nature (Restrepo-Flórez et al. 2014). As no secondary contaminants are produced like landfilling and incineration, this method of treatment is cheap, efficient, and profitable that can be applied on large scale in reactor operation for organic waste treatment (Michaud et al. 2007). Ethanol and biofuels are useful end products of microbial degradation of pollutants (Iranzo et al. 2001). But this technique is not practically applied at commercial scale (Shah et al. 2008).
The natural process of organic and inorganic degradation by microbes into nutrients is known as biodegradation. Degradation is conducted by different organisms (bacteria, algae), insects, and those organisms which eat dead matter and recycle it into new forms. All naturally produce plastic like cellulose, chitin, and PHAs which can be completely broken down by microorganisms. Under natural conditions, polyethylene degrades slowly, or it is nonbiodegradable, and this causes major environmental problems. Nondegradable solid waste such as polyethylene is most commonly used, and increasing the amount of waste in the environment has been a threat to the world (Arkatkar et al. 2009). Decaying of polymer or plastic means any type of physicochemical changes in the plastic construction and nature results from the ecological factors, like light, heat, humidity, biological activities, or any chemical condition (Nigam 2013). Polymeric substances are the potential carbon and energy sources for heterotrophic microorganisms, including bacteria and fungi, in many respects (Ghosh et al. 2013). Biodegradation is defined as the decaying or dissolution of pollutant molecules by microbial actions and secretion of enzyme. Microorganisms biodegrade the polymers which are natural or synthetic. The making alterations mostly deteriorate the sturucture/function of the polymers like bond breakage having biological, physical and chemical reactions and are categorized as polymer degradation processes . (Ghosh et al. 2013). These degradation processes bring changes in the optical mechanical or electrical characteristics, crazing, cracking, discoloration, erosion, or phase separation. The changes comprise bond scission, chemical transformation, and the formation of new functional groups (Nigam 2013).
Biodegradation refers to the degradation or deterioration of complex organic polymer into simpler components by the help of microbes (Restrepo-Flórez et al. 2014). Biochemical or microbial degradation is a novel concept which involves living microbes in the degradation process by expending the enzyme produced by these organisms. In the complete process, these microbes utilize these polymers as their carbon and energy source (Nigam 2013).
Biodegradation happens by enzyme activity and chemical degradation by living organisms. Initially, the breakdown of polymers into smaller molecules, that is, monomers, is through abiotic reactions such as oxidation (Patel and Bhaskaran 2016). Photo degradation also known as hydrolysis, or biotic reactions such as microorganism degradation during this many microbes are active in the presence of oxygen or in the absence of oxygen (Zheng et al. 2005). Environmental factors such as light, temperature, humidity, and chemical conditions also help in plastic degradation. Breakdown of polymers by means of microbes, that is, algae, fungi, and bacteria, is actually chemical degradation material, that is, polymer degradation, so it is a type of degradation involving biological activity (Patel and Bhaskaran 2016). It generally denotes the degradation and accommodation of polymers by living microorganisms to produce degradation products. Biodegradable polymer is then degraded, and carbon dioxide, methane, and biomass are the end products (Zheng et al. 2005).
This is comparatively more suitable method than physical and chemical degradation as it has less or no hazardous impacts and has relatively fast and efficient degradation potential and is an environmentally friendly technique, but yet not practically applied at commercial scale (Shah et al. 2008). Polymer conversion is done by microbes by mineralization, and under aerobic conditions mineralization produces carbon dioxide and water, while in the absence of oxygen, it forms methane and carbon dioxide (Singh and Sharma 2008). In our natural environment, the biodegradation process is performed by different microbes, but their polymer consumption rate is slow (Shah et al. 2008). It is evident that some microbial strains have the potential to degrade the plastic; thus such strains can be employed in the degradation of polymer by providing appropriate controlled environment (Zheng et al. 2005). Both natural and artificial plastic can be utilized effectively by different microbial strains. Degradation is a very complex and slow process. It doesn’t start by direct action of microorganisms but is highly influenced by ecological factors, that is, pH, temperature, and UV. The biological degradation is accompanied by solubilization, dissolution, charge formation, enzyme-catalyzed degradation, and hydrolysis (Singh and Sharma 2008).
Microbes are present in all kind of environments where life exists. Different organisms mainly fungi bacteria produce variety of enzymes they also differs between the similar species, and these enzymes also help in degradation of polymer (Zheng et al. 2005). Microbes have special plan for the utilization of plastic as it acts as a carbon and energy source for them. Degradation of plastic waste by means of microbes is the most expedient method of degradation. It is generally based on 2 steps, in the first step adhering of enzyme substrate(polyethylene) occurs and causes hydrolytic cleavage, fungus and bacteria that produces intra and extracellular enzymes and further cause the degradation of polymer (Singh and Sharma 2008).
The most prominent microbes that have the capability of polymer degradation are fungi and bacteria, but both have quite different mechanisms of degradation, and both require different conditions for their growth (Anthony 2016). The assessment of biodegradable nature of plastic can be done by performing the measurement of structural changes by microscope observation or by evaluating the growth of microorganisms after the biological and enzymatic action along with the evolution of carbon dioxide (Anthony 2016). Many microorganisms (bacteria, fungus, and algae) have been isolated with the ability to grow on polyethylene. The effects of these microbes have been described on the physiochemical properties of these polymers, including changes in crystalline, molecular weight, sample topography, and functional groups found on the polythene layer. While several scientists have demonstrated the biodegradation of polyethylene, the enzymes involved and the mechanisms associated with these phenomena remain unclear (Restrepo-Flórez et al. 2014).
Biodegradation is influenced by various factors, including the features of plastic, the type of pretreatment, and the type of organisms. The characteristics of polymers like mobility, tacticity, crystalline nature, molecular weight, the form of functional groups and substituents present in their structure, and the added plasticizers or additives to the polymer all play an important role in their degradation (Hu et al. 2019). Microbes are present in every environment where life exists; different organisms mainly fungi and bacteria produce a variety of enzymes, and they also differ between similar species, and these ten enzymes also help in the degradation of polymer (Zheng et al. 2005). The most prominent microbes that have the capability to degrade polymers are fungi and bacteria, but both have quite different mechanisms of degradation, and both require different conditions for their growth.
14.11 Enzymatic Degradation by Microbial Agents
Different microbial species excrete different enzymes for LDP degradation, such as bacterial enzymes which include laccases, peroxidases, lipases, hydrolases, and glycosidase and fungal enzymes which include catalases, proteases, ureases, hydrolases, laccases, peroxidases, and lipases (Bhardwaj et al. 2013). Different enzyme like Lignin that is a degrading enzyme produced by fungi and manganese peroxidase, that is partially purified from the strain of Phanerochaete chrysosporium also aids in the degradation of high molecular weight polyethylene under carbon limited and nitrogen-limited conditions (Restrepo-Flórez et al. 2014).
14.12 Mechanism of Enzymatic Degradation
14.12.1 Lignin-Degrading Enzymes
A number of enzymes are involved in hydrolysis of polymers. These enzymes are laccase, manganese peroxidase, glycol oxidase, lignin peroxidase, sugar oxidase, alcohol oxidase, and quinone oxidoreductase (Martínez et al. 2005). Lignin-degrading fungi release more than 100 laccases to date and can range in size from 60 to 70 kDa (kilo Dalton) (Baldrian 2006). Laccases are copper-containing oxidases that catalyze the electron oxidation of primarily phenolic and lower-redox potential compounds, although they can oxidize non-phenolic compounds in the presence of mediators, such as 2,2’-azino-bis (3-ethylbenzothiazoline-6-sulphonic acid) (Martínez et al. 2005). Glycol oxidase, sugar oxidase, and alcohol oxidase are a diverse group of enzymes that also take part in lignin degradation. These enzymes produce H2O2, a vital product in ligninase activity through the oxidation of ligninase enzymes (Martínez et al. 2005). Lignin is a plant woody biomass organic aromatic polymer and intransigent to nature degradation. White rot fungi have been widely studied for plastic degradation as the most effective lignin degrading microbes. Polyethylene membrane degradation by lignin-degrading fungus. Under various nutrient conditions, Phanerochaetechrysosporium, Trametesversicolor, showed that manganese peroxidase (MnP) is the primary enzyme in polymer degradation.
In the presence of Tween 80, Mn (II) and Mn (III) chelators, partially distilled MnP used for polyethylene surface treatment led to major degradation (Iiyoshi et al. 1998). Four groups, CHO, NHCHO, CH3, and CONH2 were formed through nuclear magnetic resonance (NMR) examination of bio-degraded nylon-66 with fungus IZU-154 that decay lignin also showed that the nylon66 was decayed oxidatively (Deguchi et al. 1997).
Lignin-degrading fungi primarily secrete laccases , where the oxidation of various polyaromatic compounds is catalyzed. Laccase is also known for nonaromatic substrate degradation (Mayer and Staple 2002). Laccase can also perform oxidation of polyethylene’s hydrocarbon backbone. There will be reduction of 20% and 15%, respectively, of the average molecular weight and molecular number of polyethylene when we incubate cell-free lacquers with polyethylene (Bhardwaj et al. 2013). The degradable polymer (comprising pro Oxidant and 6% starch) was allowed to treat with Phanerochaete chrysosporium, Streptomyces viridosporus T7A, S. badius 252, S. Setonii75 Vi2 among them Streptomyces viridosporus T7A treated plastic showed a 50% decline in tensile strength.
The ability of Pleurotus ostreatus to break down oxo-biodegradable plastics without prior physical intervention, such as UV or thermal heating, racks, and small holes was formed on the surface of plastic from the formation of OH groups and C-O bonds after the incubation period of 45 days. In fact, the dye deterioration in such bags has been noted. La and MnP enzymes secreted by polyethylene can be degraded through Chaetomium globosum fungus (Sowmya et al. 2015). Various biological mechanisms such as chemical, thermal, photo, and biodegradation play their role in polyethylene deterioration (Shah et al. 2008). Microbes as part of secondary metabolism have a natural capability to convert or absorb mass of substances, including hydrocarbons (PAHs), pharmaceutical products, and metals. Plastic polymer build functional groups by solubilizing through enzymes to enhance hydrophilicity, and the main polymer chains are weakened, which results in bad mechanical properties and in low molecular weight polymers which makes them more available for further microbial incorporation (Shah et al. 2009).
Biodegradation of plastics often contributes to the breakdown of polymers by certain enzyme systems into oligomers and monomers or further transformation into organic intermediates (such as acids, alcohols, and ketones) (Arkatkar et al. 2009). Microbial cells absorb these water-soluble cleaved products where they are metabolized. After aerobic metabolism, CO2 and water formed, while anaerobic metabolism produced carbon dioxide, water, and methane as end products. The polymers mechanically breakdown through dome physical variables like pressure, temperature, and humidity by which the process is stimulated by biological agents such as enzymes as well as other metabolites.
Plastic biodegradation due to difficulties in penetrating extracellular enzymes into the plastic polymer is usually called surface degradation, therefore acting only on the surface of the plastic polymer. Plastic degradation is when the pro-oxidants catalyze the formation of free radicals in polyethylene that react to the polyethylene matrix with molecular oxygen. The enzymatic hydrolysis degradation of plastic polymers is a two-step process: Initially, the enzyme attaches to the substratum of the polymer and then catalyzes a hydrolytic cleavage. Intracellular deterioration is the hydrolysis of an endogenous carbon supply by the growing microbe itself, whereas extracellular degradation does not necessarily require the use of an exogenous carbon source. La and MnP enzymes secreted by Chaetomium globosum fungus were responsible for the degradation of plastic polymer (Sowmya et al. 2014).
14.12.2 Laccases
Laccase enzyme is a benzene diol, a multi-copper enzyme and one of the three main ligninases. Laccase catalyze the oxidation of wide range of phenolic substrates including diphenols, polyphenols, substituted phenols, diamines, and aromatic amines, with the reduction of molecular oxygen to water. Laccase is widely present in the number of bacterial fungal and plant species as well. Laccase is involved in the degradation of lignocellulose substances, plant pathogenesis, and pigment production; they also act as oxidizing agent for variety of inorganic and organic compounds like aromatic amines, substituted phenols, diphenols, polyphenols, and diamines with associated reduction of molecular oxygen to water; in recent studies laccases are also being used in industrial applications like biopolymer modification, bioremediation, bleaching agent in textile industry, detoxification of effluent, and pulp lignification. For industrial application discovery of novel laccases with variety of substrate specifications is very important that makes them highly useful biocatalysts for various biotechnological applications .
14.13 Plastic Biodegradation Analysis Techniques
Biodegradability of polyethylene can be described through tracking the modification of CO2, the intake of O2, improvements in the attribution of polymers (physicochemical), and the maturing of species. Several experiments could be carried out to assess plastic degradation for the mentioned purposes (Mohan and Shrivastava 2010). Loss of weight may be caused by chemical leaching, including plasticizers. The degradation of polymers having low molecular weight fraction without degrading long chains can result in the development of carbon dioxide. Very minor changes in the chemical composition or skipping any additives of plastics affect the quality of plastic. To check the level as well as nature of decaying, there are a number of techniques that are available.
14.13.1 Mechanical Properties
The Tensile strength-Elongation at fail and modulus of the plastic polymer is mostly examined by dynamic mechanical Analysis (DMR).
14.13.2 Physical Properties
Morphology , that is, micro cracks, are analyzed by scanning electron microscope (SEM).
14.13.3 Chemical Properties
Chemical properties are determined by Fourier transform infrared spectroscopy (FTIR).
14.13.4 Molecular Weight
The thin layer Chromatography (TLC), Chemilluminesence, Gas Chromatography-Mass Spectrometry (GC-MS), Gas Chromatography (GC), Nuclear Magnetic Resonance (NMR), Matrix Assisted Laser Desorption Ionization-Time of Flight.
14.14 Mechanism of Plastic Biodegradation
Different microorganisms like algae, fungi, and bacteria produce different chemicals like mucilaginous substances by algae, while bacteria and fungi produce laccases, hydrolases, PETases, peroxidases, and lipases which help in cleaving the polymer structure into simpler and available form for microbes as reported by Bhardwaj et al. (2013). Lignin-degrading fungi secrete laccase enzyme having capability to break the complex structures like aromatic and polyaromatic compounds. Meanwhile, laccases are also involved in degradation of nonaromatic compounds (Restrepo-Flórez et al. 2014).
Different microorganisms produce a variety of enzymes, and they also differ between the similar species, and these enzymes also help in the degradation of polymers like plastics (Zheng et al. 2005). Microbes have a special plan for the utilization of plastic as it acts as a carbon and energy source for them. Degradation of plastic waste by means of microbe is the most expedient method of degradation. It is based generally on two steps; in the first step adhering of enzyme to substrate (polyethylene) occurs, and the second involves the hydrolytic cleavage as fungus and bacteria produce intra- and extracellular enzymes that further cause the degradation of polymer (Singh and Sharma 2008). Recently, it has been reported (Kumar et al. 2017) that the most dominant microalgae were Scenedesmus dimorphus (green microalga), Anabaena spiroides (blue-green alga), and Navicula pupula (diatom). It was shown that polyethylene sheet showed the proliferation of microalgae in both outer and inner sides, and the erosion cum degradation was obvious.
14.14.1 Microbial Growth and Plastic Degradation
It was evident that the microbial species produced biofilms on the surface of PET plastic during the current study and the growth of microbes on plastic films was efficient when provided with pretreatment. The growth of various microbial species like bacteria, fungi, microalgae, and lichens on plastic was regarded as a milestone in plastic biodegradation. It was evident from compound microscopy and scanning electron microscope (SEM) analyses that microbes were able to grow on cracks and fishers created during pretreatment. Very few literature is published on the PET biological degradation or its utilization to support microbial growth. Rare examples are members of the filamentous fungi Fusarium oxysporum and F. solani, which have been shown to grow on a mineral medium with PET yarn (although growth rates have not been given). Once identified, microorganisms with the enzymatic machinery needed to break down PET could serve as an environmental remediation strategy as well as a breakdown and/or fermentation platform for the biological recycling of PET waste products . The use of PCL as a selective isolation substrate deserves a comment. Cutinase, a serine esterase secreted by many phytopathogenic fungi, including F. solani f. sp. pisi (Murphy et al. 1996), may have a low substrate specificity. Certain cutinases hydrolyze cutin and can also degrade PET (Lin and Kolattukudy 1978). Cutinase is induced by cutin or suberin (Murphy et al. 1996) and can be repressed by glucose (Lin and Kolattukudy 1978). On this general basis, PCL hydrolysis has been used as an initial screen to study fungal isolates that produce enzymes that may be active on aromatic synthetic polyesters such as PET. Esterases and cutinases from various fungi and bacteria can hydrolyze ester bonds in PET (Muller et al. 2001; Nimchua et al. 2007).
Regarding PET degradation by microalgae , a recent report showed that diatoms (a group of microalgae) have the ability to degrade PET through the production of the enzyme PETase under eosinophilic marine conditions (Moog et al. 2019). Another aspect of the biodegradation of plastic is the development of efficient closed or open loop recycling strategies for TPA (and EG) in order to synthesize new PET from its own degradation products through to further metabolism.
Engineering microalgae metabolism to create cells that can fully metabolize PET and use it as a carbon source (Moog et al. 2019). The same authors suggested that physically treated PET can be efficiently biodegraded by enzymes such as PETase, which are produced by microalgae such as diatoms. Once an enzyme such as PETase or laccase has started to break down PET, it is speculated that its by-products may be further affected by some other enzymes produced by other microbial consortia in the same environment. down PET, it is speculated that its by-products may be further affected by some other enzymes produced by other microbial consortia in the same environment.
14.14.2 Products of Microbial Degraded or Treated Plastics
Previous research has demonstrated that carbon dioxide is the prime product released through polythene biodegradation. The production of aldehydes, ketones, and carboxylic acids was reported in LDPE film extrusion smoke in the extrusion coating. Rhodococcus rubber (C208) generated polysaccharides and proteins using polythene as a carbon source in another study. Rhodococcus rhodochrous ATCC29672 (bacterium) and Clados produced polysaccharides and proteins. Rhodococcus rubber (C208) formed polysaccharides and proteins using polythene as a carbon source (Sivan et al. 2006). In one more study, Rhodococcus rhodochrous ATCC29672 (Bacterium) and Cladosporium cladosporioides ATCC 20251 (fungus) utilized polythene to generate polysaccharides and proteins, while Nocardia asteroides GK911 (bacterium) generated proteins only.
14.15 Conclusion
Different pretreatment and cohesive methods with the most effective species are very helpful in the degradation of plastic. The use of different efficient species on different types of plastic and examine which plastic type can be more easily degraded. In order to understand the complete process of biodegradable plastics over a longer period of time, prolonged biodegradation studies on plastics using selected microorganisms should be carried out.
References
Anthony P (2016) High molecular weight polylactic acid synthesized by applying different binary catalyst. Int J Chem Pharm Sci.
Arkatkar A, Arutchelvi J, Bhaduri S, Uppara PV, Doble M (2009) Degradation of unpretreated and thermally pretreated polypropylene by soil consortia. International Biodeterioration & Biodegradation 63(1): 106–111.
Accinelli C, Saccà ML, Mencarelli M, Vicari A (2012) Deterioration of bioplastic carrier bags in the environment and assessment of a new recycling alternative. Chemosphere. 1;89(2):136–43.
Ahmed S, Islam MT, Karim MA, Karim NM (2014) Exploitation of renewable energy for sustainable development and overcoming power crisis in Bangladesh. Renewable Energy, 72, 223–235.
Ahmed T, Shahid M, Azeem F, Rasul I, Shah AA, Noman M, Muhammad S (2018) Biodegradation of plastics: current scenario and future prospects for environmental safety. Environmental Science and Pollution Research 25(8):7287–7298.
Al-Salem SM, Lettieri P, Baeyens J. 2009 Recycling and recovery routes of plastic solid waste (PSW): A review. Waste management 29(10):2625–43.
Ali MI, Perveen Q, Ahmad B, Javed I, Razi-Ul-Hussnain R, Andleeb S, Atique N, Ghumro PB, Ahmed S, Hameed A (2009) Studies on biodegradation of cellulose blended polyvinyl chloride films. Int J Agric Biol. 11(5):577–80.
Ali SS, Qazi IA, Arshad M, Khan Z, Voice TC, Mehmood CT(2016) Photocatalytic degradation of low density polyethylene (LDPE) films using titania nanotubes. Environmental nanotechnology, monitoring & management 5:44–53.
Alimi OS, Farner Budarz J, Hernandez LM, Tufenkji N (2018) Microplastics and nanoplastics in aquatic environments: aggregation, deposition, and enhanced contaminant transport. Environmental science & technology 52(4):1704–24.
Andrady AL (2011) Microplastics in the marine environment. Marine pollution bulletin 62(8):1596–605.
Andrady AL (2017) The plastic in microplastics: A review. Marine pollution bulletin 119(1):12–22.
Artham T, Doble M (2008) Biodegradation of aliphatic and aromatic polycarbonates. Macromol Biosci 8(1):14–24.
Baldrian P (2006) Fungal laccases: occurrence and properties. FEMS Microbial Rev 30:215–242
Barnes DK, Galgani F, Thompson RC, Barlaz M (2009) Accumulation and fragmentation of plastic debris in global environments. Philosophical transactions of the royal society B: biological sciences 364(1526):1985–98.
Bardají DKR, Moretto JAS, Furlan JPR, Stehling EG (2020) A mini-review: current advances in polyethylene biodegradation. World J Microbiol Biotechnol 36:32.
Bhardwaj H, Gupta R, Tiwari A (2013) Communities of microbial enzymes associated with biodegradation of plastics. Journal of Polymers and the Environment 1;21(2):575–9.
Cragg SM, Beckham GT, Bruce NC, Bugg TD, Distel DL, Dupree P, Etxabe AG, Goodell BS, Jellison J, McGeehan JE, McQueen-Mason SJ (2015) Lignocellulose degradation mechanisms across the Tree of Life. Current opinion in chemical biology 29:108–19.
Chiellini E, Corti A, Swift G (2003) Biodegradation of thermally oxidized, fragmented low density polyethylene, Polymer Degradation and Stability 81:341–351.
Cole M, Lindeque P, Halsband C, Galloway TS (2011) Microplastics as contaminants in the marine environment: a review Mar Pollut Bull 62:2588–2597.
Eichinger L, Pachebat JA, Glöckner G, Rajandream MA, Sucgang R, Berriman M, Song J, Olsen R, Szafranski K, Xu Q, Tunggal B (2005) The genome of the social amoeba Dictyostelium discoideum. Nature 435(7038):43–57.
Egli T (1995) The ecological and physiological significance of the growth of heterotrophic microorganisms with mixtures of substrates. Advances in microbial ecology 305–86.
Deguchi T, Kakezawa M, Nishida T (1997) Nylon biodegradation by lignin-degrading fungi. Appl Environ Microbiol 63:329–331.
Denuncio P, Bastida R, Dassis M, Giardino G, Gerpe M, Rodríguez D (2011) Plastic ingestion in Franciscana dolphins, Pontoporia blainvillei (Gervais and d’Orbigny, 1844), from Argentina. Marine pollution bulletin 62(8):1836–41.
Erceg M, Krešić I, Vrandečić NS, Jakić M (2018) Different approaches to the kinetic analysis of thermal degradation of poly (ethylene oxide). Journal of Thermal Analysis and Calorimetry 31(1):325–34.
Eriksen M, Lebreton LC, Carson HS, Thiel M, Moore CJ, Borerro JC, Galgani F, Ryan PG, Reisser J (2014) Plastic pollution in the world’s oceans: more than 5 trillion plastic pieces weighing over 250,000 tons afloat at sea. PloS one 9(12):e111913.
Ferreira LM, Falcao AN, Gil MH (2005) Modification of LDPE molecular structure by gamma irradiation for bioapplications. Nuclear Instruments and Methods in Physics Research Section B: Beam Interactions with Materials and Atoms 236(1–4):513–20.
Gerald S (2000) Green polymers. Polymer Degradation and Stability 68(1):1–7.
Gu JD (2003) Microbiological deterioration and degradation of synthetic polymeric materials: recent research advances. International biodeterioration & biodegradation 52(2):69–91.
Gulmine JV, Janissek PR, Heise HM, Akcelrud L(2003) Degradation profile of polyethylene after artificial accelerated weathering. Polymer degradation and stability 79(3):385–97.
Ghosh SK, Pal S, Ray S (2013) Study of microbes having potentiality for biodegradation of plastics. Environmental Science and Pollution Research 20(7):4339–55.
Gunatillake P, Mayadunne R, Adhikari R (2006) Recent developments in biodegradable synthetic polymers. Biotechnol Annu Rev 12:301–347.
Harrison DE, Wren SJ (1976) Mixed microbial cultures as a basis for future fermentation processes. Process Biochemistry 11(8):30–2.
Hadad D, Geresh S, Sivan A (2005) Biodegradation of polyethylene by the thermophilic bacterium Brevibacillus borstelensis. Journal of applied microbiology 98(5):1093–100.
Haward M (2018) Plastic pollution of the world’s seas and oceans as a contemporary challenge in ocean governance. Nature communications 9(1):1–3.
Horton AA, Walton A, Spurgeon DJ, Lahive E, Svendsen C (2017) Microplastics in freshwater and terrestrial environments: evaluating the current understanding to identify the knowledge gaps and future research priorities. Science of the total environment. 586:127–41.
Hu H, Zhang R, Ying WB, Shi L, Yao C, Kong Z, Wang K, Wang J, Zhu J (2019) Sustainable and rapidly degradable poly (butylene carbonate-co-cyclohexanedicarboxylate): influence of composition on its crystallization, mechanical and barrier properties. Polymer Chemistry 10(14):1812–22.
Iiyoshi Y, Tsutsumi Y, Nishida T (1998) Polyethylene degradation by lignin-degrading fungi and manganese peroxidase. J Wood Sci, 44:222–229.
Ingram LO, Aldrich HC, Borges AC, Causey TB, Martinez A, Morales F, Saleh A, Underwood SA, Yomano LP, York SW, Zaldivar J (1999) Enteric bacterial catalysts for fuel ethanol production. Biotechnology progress 15(5):855–66.
Iranzo M, Sainz-Pardo I, Boluda R, Sanchez J, Mormeneo S (2001) The use of microorganisms in environmental remediation. Annals of microbiology 51(2):135–44.
Kale SK, Deshmukh AG, Dudhare MS, Patil VB (2015) Microbial degradation of plastic: a review. Journal of Biochemical Technology 6(2):952–61.
Kedzierski M, d’Almeida M, Magueresse A, Le Grand A, Duval H, César G, Sire O, Bruzaud S, Le Tilly V (2018) Threat of plastic ageing in marine environment. Adsorption/desorption of micropollutants. Marine pollution bulletin 127:684–94.
Kibria, G (2017) Plastic waste, plastic pollution-a threat to all nations, Technical Report 2017. https://doi.org/10.13140/RG.2.2.11169.51048.
Kurian JK, Nair GR, Hussain A, Raghavan GV (2013) Feedstocks, logistics and pre-treatment processes for sustainable lignocellulosic biorefineries: A comprehensive review. Renewable and Sustainable Energy Reviews 25:205–19.
Khoddami A, Wilkes MA, Roberts TH (2013) Techniques for analysis of plant phenolic compounds. Molecules 18(2):2328–75.
Kigozi R, Aboyade A, Muzenda E (2013) Biogas production using the organic fraction of municipal solid waste as feedstock. World 5:6.
Kissi M, Mountadar M, Assobhei O, Gargiulo E, Palmieri G, Giardina P, Sannia G (2001) Roles of two white-rot basidiomycete fungi in decolorisation and detoxification of olive mill waste water. Applied microbiology and biotechnology 57(1):221–6.
Krupp LR, Jewell WJ (1992) Biodegradability of modified plastic films in controlled biological environments. Environmental science & technology 26(1):193–8.
Kumar AA, Karthick K, Arumugam KP (2011) Biodegradable polymers and its applications. International Journal of Bioscience, Biochemistry and Bioinformatics 1(3):173.
Kumar RV, Kanna GR, Elumalai S (2017) Biodegradation of polyethylene by green photosynthetic microalgae. J Bioremediat Biodegrad 8(381):2.
Kumar S, Das MP, Rebecca LJ, Sharmila S (2013) Isolation and identification of LDPE degrading fungi from municipal solid waste. Journal of Chemical and Pharmaceutical Research 5(3):78–81.
Kutten K (2019) The Potential of Africa’s Plastic Market and Its Impact on Plastic Waste Management. Master’s Thesis, Lappeenranta University of Technology, Lappeenranta, Finland,
Lebreton L, Andrady A (2019) Future scenarios of global plastic waste generation and disposal. Palgrave Communications 5(1):1–1.
Leja K, Lewandowicz G (2010) Polymer biodegradation and biodegradable polymers-a review. Polish Journal of Environmental Studies 19(2).
Lora ES, Andrade RV (2009) Biomass as energy source in Brazil. Renewable and sustainable energy reviews 13(4):777–88.
Lu DR, Xiao CM, Xu SJ (2009) Starch-based completely biodegradable polymer materials. Express polymer letters 3(6):366–75.
Lin TS, Kolattukudy PE (1978) Induction of a biopolyester hydrolase (cutinase) by low levels of cutin monomers in Fusarium solani i. sp. pisi. J. Bacteriol. 133, 9.
Lin YH, Yang MH (2009) Tertiary recycling of commingled polymer waste over commercial FCC equilibrium catalysts for producing hydrocarbons. Polymer Degradation and Stability 94(1):25–33.
Martínez ÁT, Mariela S, Francisco J, Ruiz-Dueñas PF, Susana C, Francisco G, Martínez MJ, Gutiérrez A, del Río JC (2005) Biodegradation of lignocellulosics: microbial, chemical, and enzymatic aspects of the fungal attack of lignin. International Microbiology 8:195–204.
Mayer AM, Staples RC (2002) Laccase: new functions for an old enzyme. Phytochemistry 60:551–565.
Murphy CA, Cameron JA, Huang SJ, Vinopal RT (1996) Fusarium polycaprolactone depolymerase is cutinase. Appl Environ Microbiol 62:456–460
Mussgnug JH, Klassen V, Schlüter A, Kruse O (2010) Microalgae as substrates for fermentative biogas production in a combined biorefinery concept. Journal of biotechnology 150(1):51–6.
McKendry P(2002) Energy production from biomass (part 1): overview of biomass. Bioresource technology 83(1):37–46.
Moog D, Schmitt J, Senger J, Zarzycki J, Rexer KH, Linne U, Erb T, Maier UG (2019) Using a marine microalga as a chassis for polyethylene terephthalate (PET) degradation. Microbial cell factories 18(1):1–5.
Michaud L, Di Marco G, Bruni V, Giudice AL (2007) Biodegradative potential and characterization of psychrotolerant polychlorinated biphenyl-degrading marine bacteria isolated from a coastal station in the Terra Nova Bay (Ross Sea, Antarctica). Marine pollution bulletin 54(11):1754–61.
Mohan AJ, Sekhar VC, Bhaskar T, Nampoothiri KM (2016) Microbial assisted high impact polystyrene (HIPS) degradation. Bioresource technology 213:204–7.
Mohan KS, Srivastava T (2010) Microbial deterioration and degradation of polymeric materials. J Biochem Tech 2(4):210–215.
Moharir RV, Kumar S (2019) Challenges associated with plastic waste disposal and allied microbial routes for its effective degradation: a comprehensive review. Journal of Cleaner Production 208:65–76.
Muhonja CN, Makonde H, Magoma G, Imbuga M (2018) Biodegradability of polyethylene by bacteria and fungi from Dandora dumpsite Nairobi-Kenya. PloS one 3(7):e0198446.
Muller RJ, Kleeberg I, Deckwer WD (2001) Biodegradation of polyesters containing aromatic constituents. J Biotechnol 86:87–95
Nimchua T, Punnapayak H, Zimmermann W (2007) Comparisonof the hydrolysis of polyethylene terephthalate Wbers by a hydrolase from Fusarium oxysporum LCH I and Fusarium solani. Biotechnology 2:361–364
Narwal S K, R Gupta (2017) Biodegradation of xenobiotic compounds: An Overview. Handbook of research on inventive bioremediation techniques, IGI Global: 186–212.
Nigam PS (2013) “Microbial enzymes with special characteristics for biotechnological applications.” Biomolecules 3(3): 597–611.
Payne CM, Knott BC, Mayes HB, Hansson H, Himmel ME, Sandgren M, Beckham GT (2015) Fungal cellulases. Chemical reviews, 115(3), 1308–1448.
Patel RJ, Bhaskaran Lakshmi (2016) Screening of novel ascomycetes for the production of laccase enzyme using different lignin model compounds. International Journal of Pharmacological Biological Science 7(4), 452–458.
Pathak VM, Navneet (2017) Review on the current status of polymer degradation: a microbial approach. Bioresour, Bioprocess 4(15),
Pedroso AG, DdS Rosa (2005) Mechanical, thermal and morphological characterization of recycled LDPE/corn starch blends. Carbohydrate Polymers 59(1): 1–9.
Pramila R, KV Ramesh (2011) Biodegradation of low density polyethylene (LDPE) by fungi isolated from marine water a SEM analysis. African Journal of Microbiology Research 5(28): 5013–5018.
Qasaimeh A, Abdallah-Qasaimeh MR, Hani FB (2016) A review on biogas interception processes in municipal landfill. Journal of Environmental Science and Technology 9(1):1–25.
Ray S, Cooney RP (2018) Thermal degradation of polymer and polymer composites. InHandbook of environmental degradation of materials (pp. 185–206). William Andrew Publishing.
Raziyafathima M, Praseetha PK, Rimal Isaac RS (2016) Microbial degradation of plastic waste: a review. J Pharm Chem Biol Sci 4(2):231–42.
Restrepo-Flórez JM, Bassi A, Thompson MR (2014) Microbial degradation and deterioration of polyethylene–A review. International Biodeterioration & Biodegradation 88:83–90.
Romero Saez M, Jaramillo LY, Saravanan R, Benito N, Pabón E, Mosquera E, Gracia Caroca F. (2017) Notable photocatalytic activity of TiO2-polyethylene nanocomposites for visible light degradation of organic pollutants.
Sabir I (2004) Plastic industry in Pakistan. 6(7):2017.
Sanchez DL, Nelson JH, Johnston J, Mileva A, Kammen DM (2015) Biomass enables the transition to a carbon-negative power system across western North America. Nature Climate Change 5(3), 230.
Srirangan K, Akawi L, Moo-Young M, Chou CP (2012) Towards sustainable production of clean energy carriers from biomass resources. Applied Energy 100, 172–186.
Sims RE, Hastings A, Schlamadinger B, Taylor G, Smith P (2006) Energy crops: current status and future prospects. Global Change Biology 12(11), 2054–2076.
Sharma HK, Xu C, Qin W (2019) Biological pretreatment of lignocellulosic biomass for biofuels and bioproducts: an overview. Waste and Biomass Valorization 10(2), 235–251.
Sangale MK, Shahnawaz M, Ade AB (2012) A review on biodegradation of polythene: the microbial approach. J Bioremed Biodeg 3(10): 1–9.
Saxena RC, Adhikari DK, Goyal HB (2009) Biomass-based energy fuel through biochemical routes: a review. Renewable and Sustainable Energy reviews 13(1), 167–178.
Shah AA, Hasan F, Akhter JI, Hameed A, Ahmed S (2008) Degradation of polyurethane by novel bacterial consortium isolated from soil. Annals of microbiology 58(3): 381.
Shah AA, Hasan F, Hameed A, Ahmed S (2009) Biological degradation of plastics: a comprehensive review. Biotechnology advances 26(3): 246–265.
Silva PAL, Prata JC, Walker TR, Campos D, Duarte AC, Soares AMVM, Barcelò D, Rocha-Santos T (2020) Rethinking and optimising plastic waste management under COVID-19 pandemic: Policy solutions based on redesign and reduction of single-use plastics and personal protective equipment. Sci Total Environ, 742, 140565.
Sheel A, D Pant (2018) Microbial Depolymerization. Waste Bioremediation, Springer 61–103.
Singh B, N Sharma (2008) Mechanistic implications of plastic degradation. Polymer Degradation and Stability 93(3): 561–584.
Sivan A, Szanto M, Pavlov V (2006) Biofilm development of the polyethylene-degrading bacterium Rhodococcus ruber. Applied microbiology and biotechnology 72(2): 346–352.
Sowmya HV, Krishnappa M, Thippeswamy B (2014) Degradation of polyethylene by Trichoderma harzianum—SEM, FTIR, and NMR analyses. Environmental monitoring and assessment 186(10): 6577–6586.
Sowmya HV, Ramalingappa, Krishnappa R, Thippeswamy B (2015) Degradation of polyethylene by Penicillium simplicissimum isolated from local dumpsite of Shivamogga district. Environment, Development and Sustainability 17(4): 731–745.
Tilman D (1977) Resource competition between planktonic algae: an experimental and theoretical approach. Ecology 58: 338–348.
Tunçer S, Gündoğdu S, Çevik C, Zilifli A (2019) Belone belone (Linnaeus, 1760) and Spicara smaris (Linnaeus, 1758) Entangled in Plastic Collars in the Dardanelles Strait, Turkey. Annales: Series Historia Naturalis, Scientific and Research Center of the Republic of Slovenia.
Van Sebille, E Spathi C, Gilbert A (2016) The ocean plastic pollution challenge: towards solutions in the UK. Grant. Brief. Pap, 19, 1–16.
Varjani SJ, VN Upasani (2017) A new look on factors affecting microbial degradation of petroleum hydrocarbon pollutants. International Biodeterioration & Biodegradation 120: 71–83.
Vimala P, L Mathew (2016) Biodegradation of Polyethylene using Bacillus subtilis. Procedia Technology 24:232–239.
Yousif E, Haddad R (2013) Photodegradation and photostabilization of polymers, especially polystyrene: review. SpringerPlus 2, 398
Zheng Y, Yanful EK, Bassi AS (2005) A review of plastic waste biodegradation. Critical reviews in biotechnology, 25(4), 243–250.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Zeb, B.S., Mahmood, Q., Abbasi, H.Z., Zeb, T. (2022). Remediation of Plastic Waste Through Cohesive Approaches. In: Ahmed, T., Hashmi, M.Z. (eds) Hazardous Environmental Micro-pollutants, Health Impacts and Allied Treatment Technologies. Emerging Contaminants and Associated Treatment Technologies. Springer, Cham. https://doi.org/10.1007/978-3-030-96523-5_14
Download citation
DOI: https://doi.org/10.1007/978-3-030-96523-5_14
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-96522-8
Online ISBN: 978-3-030-96523-5
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)