Abstract
Cadmium (Cd) pollution in agricultural soils has become a great concern for global food security and the environment. Cd is a nonessential heavy metal and a group-I carcinogen. Excessive uses of phosphate fertilizers, dispersal of municipal waste, sewage sludge disposal and atmospheric deposition have polluted agricultural soils with cadmium. Accumulation of Cd in crops may cause severe damages to plant growth and agricultural productivity. Human beings get exposed to cadmium toxicity through the food chain. In recent times, plant growth-promoting rhizobacteria (PGPR)-mediated Cd detoxification in plants emerged as an excellent alternative to physicochemical approaches as it is economical and environmentally sustainable. Generally, PGPR enhances plant growth by nitrogen fixation, producing phytohormones, ACC deaminase (ACCD), siderophores, and solubilizing inorganic or organic phosphates. PGPR enhance Cd bioremediation through different mechanisms, such as biosorption, complexation, chelation, sequestration and biotransformation. The application of Cd resistant PGPR to alleviate Cd stress in plants has an exciting prospect, and early findings look promising for boosting food security, especially in contaminated soil, for the increasing global population.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- ACC deaminase
- Bioremediation
- Cadmium
- Detoxification
- Immobilization
- Plant growth promoting rhizobacteria (PGPR)
- Siderophore
1 Introduction
Agricultural soil health deteriorated considerably in the last few decades due to heavy metal contamination in soil. In general, heavy metals are found in the earth’s crust; however, heavy metal contamination is mainly the consequence of increased industrial activities, combustion of coal and petroleum products, mining, smelting, use of agrochemicals (e.g. fertilizers) and disputed agricultural practices, such as the release of industrial effluents, municipal wastes and sewage sludge in agricultural soils. Atmospheric deposition, geogenic activities such as weathering, leaching and volcanism have also contributed to heavy metal pollution to a great extent (Kubier et al. 2019; Singh et al. 2021). Heavy metals are non-biodegradable; most show toxicity even at a low concentration and accumulate in the soil. Consequently, they adversely impact the functions of all the living entities present there and eventually invade the food chain via edible crops and pose a major threat to human well-being and food safety (Kumar 2012; Sharma and Archana 2016).
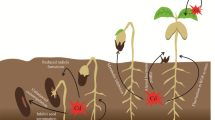
Heavy metals may be classified as a group of metals that have a high atomic weight and high density (>5 g cm−3) (Nies 1999). They may be essential in trace amounts (e.g. Mn, Cu, Fe and Zn) or nonessential with no known physiological role (e.g. Cd, Hg, As and Pb) (Shanmugaraj et al. 2019). Cadmium (Cd) is highly mobile in soils and is the most toxic nonessential metal with a long biologic half-life. Cadmium is a trace element in the earth’s crust (0.2 mg/kg) and generally occurs as oxides, sulfides, and carbonates in zinc, lead, and copper ores. In the past, cadmium was used mainly in metal electroplating, pigments and stabilizers for plastics. In recent decades, the use of cadmium has been growing up for its application in cadmium-nickel batteries, the modern electronics and communication industry, and the power industry. Cadmium is also released into the environment, naturally through volcanic activity and weathering, but mostly it is released into the environment through municipal waste incineration, fossil fuel combustion and smelting. Use of phosphate fertilizers and pesticides, irrigation with municipal waste, and sewage sludge are mainly liable for agricultural soil contamination with cadmium (Fig. 1). Cadmium content in agricultural soils ranges between 0.01 and 1 mg kg−1, averaging 0.36 mg kg−1 worldwide (WHO 2000; Kubier et al. 2019). The World Health Organization (WHO) has recommended a guideline value of 3 µg/L for cadmium in drinking water (WHO 2010).
Chronic Cd exposure causes kidney damage, respiratory disorders, cardiovascular disorder, hypertension, cerebral infarction, disorders in glucose, calcium and vitamin-D metabolism, bone lesions, osteoporosis, and diabetes in humans. Cadmium also has embryotoxic, teratogenic and carcinogenic effects. It has been reported in several studies that the lung, kidney, breast and prostate are the primary target organs for Cd carcinogenicity. The first documented chronic cadmium poisoning incidence was the Itai-Itai disease that occurred in Japan in the 1950s. Cadmium is toxic to living beings even at a low level, and has been classified as a Group-I carcinogen to humans by International Agency for Research on Cancer (IARC) in 1993 (WHO 2000; Hu et al. 2016; Khan et al. 2017b). Cadmium entry into the human body takes place mainly through the dietary intake (e.g. cereals, vegetables) and bio-accumulates in different organs as it is non-degradable, persistent, and has a biological half-life of 10–35 years. In comparison to other cereals, the accumulation of cadmium in rice is much higher and, therefore, increases the health risk in manifold of the rice-consuming population of the world (Hu et al. 2016; Kubier et al. 2019).
Besides animal toxicity, cadmium exhibits phytotoxicity even at a low concentration. Cadmium is easily absorbed by roots of crop plants, especially rice and other cereals and leafy vegetables, during cultivation in Cd-polluted soil and can be translocated to the aerial parts. Cadmium accumulation in plant parts severely damages crop productivity (Bolan et al. 2014). Cadmium toxicity triggers diverse morphological, physio-biochemical, and molecular disturbances in plants, such as stunting overall plant growth, wilting, senescence, reduced photosynthesis, reduced pigment synthesis, leaf chlorosis, inhibition of seed germination, interference in nutrient uptake and disruption in the electron transport chain. Cadmium stimulated oxidative stress may also injure plasma membranes and a variety of different biological molecules, such as nucleic acids and proteins, by generating an excessive amount of reactive oxygen species (ROS) (Gallego et al. 2012; Roy et al. 2016; Moradi et al. 2019; El Rasafi et al. 2020).
Many traditional methods, such as soil dressing, soil removal, chemical washing, soil liming, electrochemical treatment, reverse osmosis, biochar amendment, bio-slurries and other agronomic approaches, are used for cadmium-contaminated soil remediation. These conventional physicochemical Cd remediation methods are usually expensive, require high maintenance and skilled labour, and typically cause harm to the soil in the long run by the resultant secondary toxic products (Volesky 2001; Vinod and Sashidhar 2011; Singh and Gadi 2012). Phytoremediation through hyperaccumulator plants is a possible alternative technique for the bioremediation of contaminated sites. However, they have little practical value in the heavy metal toxicity alleviation from the soil due to their slow growth rate and small biomass (Blaylock et al. 1997; Kayser et al. 2000).
Bioremediation involving microorganisms has attracted increasing interest in recent years (Dixit et al. 2015). The free-living rhizospheric bacteria that assist in plant growth and development are generally regarded as plant growth-promoting rhizobacteria (PGPR). The approach of using PGPR to alleviate heavy metal stress, including cadmium, is environment-friendly and inexpensive. PGPR also promote plant growth by producing growth promoters (Kloepper et al. 1980; Pramanik et al. 2017; Abbas et al. 2018). Microbial remediation of heavy metal toxicity involves bioadsorption, bioaccumulation, complexation, precipitation and biotransformation. PGPR like Bacillus subtilis, Burkholderia gladioli, Citrobacter spp., Enterobacter aerogenes and Pseudomonas spp., have been found effective in mitigating Cd toxicity in plants (Kumar 2012; Pramanik et al. 2018; Khanna et al. 2019a; Halim et al. 2020). In this chapter, we summarized Cd uptake, its toxicity and plant response to cadmium stress. Furthermore, we have discussed Cd tolerance strategies found in PGPR and the different PGPR mechanisms involved in Cd detoxification in plants.
2 Cadmium Uptake and Transport in Plants
Cadmium is readily taken into the inside of plant root and then translocated to the aerial plant parts. The uptake of Cd in the higher plants is regulated by diverse aspects of soil and plant characteristics, such as soil type, soil pH, presence of organic matter, Cd availability, plant species, and their genotypes, plant age and growth stage, presence of organic matter, mineral elements, and nutrients. The adsorption and complexation of Cd with soil minerals regulate its mobilization and bioavailability in soil. An increase in soil pH and organic matter stimulates Cd immobilization in soil mainly through precipitation and chelation. Cd ions could be absorbed by root cell transmembrane carriers, meant for uptake of essential micronutrients, such as Ca2+, Fe2+, Mg2+, Cu2+ and Zn2+ (Dalcorso et al. 2008; El Rasafi et al. 2020; Halim et al. 2020). The presence of Zn in ample amount in soil decreases Cd uptake by plants as both of them use the same route to gain entry into the root cell. It is worth noting that modulation of soil conditions through soil management approaches can significantly change the bioavailability of Cd (Hu et al. 2016). The quotient of Cd concentration in the plant to that in the soil defines the Cd transfer factor (TF), which ranges between 0.01 and 0.3 (Smolders 2001). Plant root cell walls can transport cadmium to the xylem through passive transport (diffusion) (Redjala et al. 2011). Cd can be transported symplastically in root cortical cells through membrane transporters, such as zinc transporter [ZIP], iron transporter [IRT]) and metals pumping ATPase (Gallego et al. 2012; Wu et al. 2015; Yamaguchi et al. 2011; Sebastian and Prasad 2018). Also, natural resistance-associated macrophage protein (NRAMP) family, cation/proton exchangers (CAX), P-type ATPase, lysosomal cystine transporter (LCT) family and ATP-binding cassette (ABC) transporters distribute Cd in different plants parts (Gallego et al. 2012; Song et al. 2017; El Rasafi et al. 2020). Cd ions are chelated to organic molecules and distributed to different parts of the plant body through xylem and phloem translocation after xylem loading via apoplast or symplast route (Dalcorso et al. 2008).
3 Phytotoxicity of Cadmium
Due to the toxic effects of Cd, plants and other living beings have no use for it. However, a few diatoms present in seawater utilize Cd in the enzyme Cd-carbonic anhydrase (Lane and Morel 2000). In plants, the bioaccumulation of Cd causes severe toxicity symptoms, such as reduced photosynthesis, chlorosis, wilting, altered enzyme activities, altered membrane functioning, stunted growth and development, and finally, plant death. However, the severity of Cd toxicity depends on plant species and their genotypes (Shanmugaraj et al. 2019). Cd binding with sulfhydryl groups in proteins, due to its high affinity for it, interferes with protein configuration, inhibits enzymatic activities and their regulation (Hall and Brown 2002). Also, Cd2+ ions can displace chemically identical cations, such as Cu2+, Ca2+, Zn2+ and Fe2+, from catalytic sites of enzymes. The released free ions increase the oxidative stress and could cause damage by the Fenton reaction triggered by free Fe/Cu ions (Roy et al. 2016).
3.1 Effect on Plant Root
Roots accumulate more Cd, like other heavy metals, than above-ground parts and show initial symptoms of Cd toxicity (Singh and Shah 2015). Cd interferes with the micronutrient (Ca, Mg, Zn, K, P and Fe) uptake by the roots and thus, disturbs the plant-water balance. Cd+2 ions mainly bind with the negatively charged components of the cell walls of the root. Exposure to Cd inhibits root growth and lateral root formation but stimulates root hair formation (Benavides et al. 2005; Daud et al. 2009). Cd could disrupt the growth and elongation of the root in a dose-dependent manner, as seen in soybean (Sahile et al. 2021). The reduction of root length, decline in root surface area and swelling of root diameter affect the nutrient uptake capacity of roots. Cd forms a callus-like structure in the root through enlargement of parenchyma cells and unorganized cell differentiation (Halim et al. 2020). Cd stress changes the appearance of the root system, and the roots become rigid, necrotic, decomposing, twisted, and mucilaginous. Browning of the root is commonly associated with cd stress (Rascio and Navari-Izzo 2011; Abbas et al. 2017). In tomato plants, roots become thick and sturdy under Cd stress (Chaffei et al. 2004). Cd stress injures the DNA and the nucleoli in the root-cap and root tip cells (Seth et al. 2008). Also, prolonged exposure to Cd could increase the nucleus number in the differentiated root cells and disrupt the mitotic index, induce chromosomal anomaly, irregular mitotic behaviour, and affect micronucleus formation when exposed to Cd (Fusconi et al. 2006; Shanmugaraj et al. 2019).
3.2 Effect on Photosynthetic Apparatus
When a plant counters Cd contamination in its vicinity, it affects photosynthetic growth parameters, such as total chlorophyll and carotenoid contents, photochemical efficacy, and intensity of photosynthesis. Cd exposure causes leaf roll, damages chlorophyll content in old leaves and inhibits biosynthesis of chlorophyll in newer ones to cause leaf chlorosis (He et al. 2008; Xue et al. 2013). In several economically important crops, such as Pisum sativum, Zea mays, Hordeum vulgare, Brassica juncea, Triticum and Oryza sativa, inhibition of photosynthesis due to a short and long period of Cd exposure was well documented (Ci et al. 2010; Popova et al. 2012; Irfan et al. 2014; Pramanik et al. 2018; Almuwayhi 2021). Cd toxicity also triggers stomatal closing and, subsequently, a reduction in photosynthetic activity in higher plants. Cd strongly binds with several proteins involved in photosystems I (PSI) and II (PSII). Cd toxicity also injures the light-harvesting complex (Küpper et al. 2007; Haider et al. 2021). Ribulose-1, 5-bisphosphate carboxylase (RuBisCo), and phosphoenolpyruvate carboxylase (PEPCase) are essential enzymes for CO2 fixation during photosynthesis. Cd replaces cofactor Mg+2, needed for the carboxylation step of Calvin cycle, of enzyme RuBisCo and inhibits its activity. It also decreases the activity of PEPCase (Siedlecka et al. 1998; Tran and Popova 2013). Cd toxicity also reduces the e− flow from QA to QB by altering the QB binding site. Cd ions can bind competitively at Ca-binding sites and replace Ca+2 ions in Ca/Mn clusters of the water-splitting complex of PSII (Sigfridsson et al. 2004; Faller et al. 2005). Cd exposure induces striking changes in chloroplast number and ultrastructure, resulting in distortion of shape and size of thylakoids (Najeeb et al. 2011). Cd stress also deforms thylakoid discs and grana, decreases stored starch, and plastoglobuli deposit, as reported in Picris divarticata, Hordeum vulgare, Oryza sativa L.) and Brassica (Ying et al. 2010; Wang et al. 2011; Elhiti et al. 2012; Parmar et al. 2013).
3.3 Effect on Plant Growth and Biomass
Cadmium toxicity negatively affects general growth, induces growth deformities in many species of plants (Haider et al. 2021). A substantial decrease in the leaf growth and development was reported in Capsicum annuum L. and Brassica oleracea L. under Cd exposure (León et al. 2002; Jinadasa et al. 2016). Also, Cd toxicity decreased shoot and root growth of Solanum tuberosum L. at 60 mg/kg of Cd in pot trials, shoot dry matter of cucumber at 0.05 mM of Cd concentration, and the development of root, stem, and leaves of pepper at 2 mM and 10 mM of Cd, respectively, in the hydroponic system (Xin et al. 2014; Hassan et al. 2016). The long-term effect of Cd stress exhibits a rapid and significant decline in crop yields, especially in cereal production, due to disruption of nutrient uptake and photosynthesis in plants (Rizwan et al. 2016). Plant growth inhibition under Cd stress is well reported in many species, such as rice (Oryza sativa), rape plant (Brassica napus L.), mungbean (Vigna mungo), chickpea (Cicer arietinum L.), tomato (Lycopersicon esculentum L.), sorghum (Sorghum bicolour), lentil (Lens culinaris L.), durum wheat (Triticum turgidum) and soybean ((Glycine max L.) (Rizwan et al. 2012; Mondal et al. 2013; Roy et al. 2016; Dutta et al. 2018; Pramanik et al. 2018; Pal and Sengupta 2019; Zhao et al. 2019; Zhi et al. 2020; Bansal et al. 2021).
3.4 Effect on Seed Germination
Cadmium toxicity to plants diminishes water content in seedlings and delays the breaking of seed dormancy, and ultimately, the seed fails to germinate. The failure of seed germination severely hampers crop productivity. The inhibitory effect of reduced water content for embryos resulting from Cd stress was reported in seedling and seed germination of Arabidopsis sp., and cowpea (Vigna unguiculata L.) (Li et al. 2005; Vijayaragavan et al. 2011). Water deficiency, endospermic starch immobilization, and a decrease in sugar transport to the embryo resulted in the failure of seed germination (Kuriakose and Prasad 2008). Under Cd exposure, low activity of hydrolyzing enzymes, such as α-amylase, has resulted in slow transport of stored foods (Kalai et al. 2016; Haider et al. 2021). Under Cd stress, seeds were failed to germinate in sunflower (Helianthus Annuus) by >50% after being treated with 40 and 50 mg kg−1 Cd, wheat by 31% at 0.03–4.8 mM of Cd, soybean by 8.0% at 5 mg/L, lettuce by 19% at 5 mg/L, sugarbeet by 18% at 5 mg/L and rice by 100% at 1.0 mM of Cd (Ahsan et al. 2007; Jadia and Fulekar 2008; Li et al. 2013; de Souza Guilherme et al. 2015). However, a little increase in germination at low Cd concentration was reported due to the limiting effect of metal on free oxygen radicals and nitric oxide, which regulate oxidative stress (Shanying et al. 2017). Moreover, Cd has a strong affinity for the Ca-calmodulin binding sites. The binding of Cd to calmodulin greatly affects metabolic activity and seed germination (Huybrechts et al. 2019).
3.5 Oxidative Stress
Cadmium toxicity in plants is mainly caused due to reactive oxygen species (ROS) generation and change in the antioxidant system, which increases oxidative stress. However, Cd is redox-inactive and cannot transfer single electrons to generate reactive oxygen species (ROS). Cd toxicity may generate ROS indirectly through the alternation of the electron transfer chain by disrupting chloroplasts and also by damaging antioxidant defence (Gallego et al. 2012). ROS examples include superoxide (O2¯), hydrogen peroxide (H2O2), and hydroxyl radicals (OH¯). In plants, Cd-induced oxidative damage results in lipid and protein peroxidation, and consequently, disrupts lipid-rich plasma membrane, as well as DNA (Younis et al. 2016; Shanmugaraj et al. 2019). Plants have evolved an advanced antioxidant system to manage oxidative stress that primarily involves enzymatic, such as glutathione reductase (GR), peroxidase (POX), superoxide dismutase (SOD), glutathione peroxidase (GPX), ascorbate peroxidase (APX), catalase (CAT), monodehydroascorbate reductase (MDHAR), dehydroascorbate reductase (DHAR) and non-enzymatic antioxidants, such as α-tocopherols, non-protein amino acids, alkaloids, phenolic compounds, carotenoids, ascorbic acid (ASA) and reduced glutathione (GSH). Cd stress alters the activity of antioxidative enzymes and non-enzymatic antioxidants (El Rasafi et al. 2020). Under the exposure to Cd, the activity of GR and APX increases in wheat. GR activity also increases in rapeseed (Brassica juncea L.), cotton, and mungbean (Vigna mungo L.) (Gill and Tuteja 2010; Tran and Popova 2013). However, the scavenging activities of POX in rapeseed, SOD, and CAT in sunflower, common bean, and pea, decrease under Cd stress (Sandalio et al. 2001; Markovska et al. 2009; Haider et al. 2021).
4 Cadmium Detoxification Mechanisms in Plant
To manage Cd toxicity, plants can employ either or both tolerance and avoidance approaches. To avoid Cd toxicity, plants minimize the uptake of Cd through the roots by immobilization. In the tolerance approach, plants store and accumulate Cd in vacuoles, bind it to cell walls, phytochelatins (PCs), peptides, amino acids and proteins. Stress signaling pathways and signaling molecules, such as jasmonic acid, salicylic acid, ethylene and nitric oxide, take part in key pathways to reduce toxic effects of Cd in plants (Tran and Popova 2013; Haider et al. 2021). Plants have several strategies to minimize Cd stress, such as immobilization, dissemination, expulsion, chelation, vacuolar sequestration and compartmentalization, synthesis of stress-signaling molecules and proteins. Plants can immobilize Cd in the rhizosphere by secreting root exudates which contain several low- and high molecular weight organic compounds, including proteins, polysaccharides and phenolic compounds. In the root cell wall, pectins, having egg-box structures, and hemicelluloses are the primary site for cd binding and retention. The plasma membrane can exclude Cd ions from entering the cytosol and help in efflux from the cell. Under Cd stress, plants activate the synthesis of phytochelatin, small metal-binding peptides linked to sulfur metabolism. Phytochelatins with thiolic (–SH) groups of Cys chelates Cd to form complex structures, and as a result, prevent dissemination of free Cd+2 ions inside the cytosol. Synthesis of metallothioneins also helps in the chelation of Cd in the cytosol. Plant vacuoles play a very significant role in Cd detoxification by sequestrating it with the help of different ions and metabolites inside the vacuoles. Vacuolar sequestration checks the distribution of free Cd ions inside the cell. Vacuoles have ATPases, NRAMP family transporters, Ca2+ ion transporters, and ATP-binding cassette (ABC) type C transporters in their wall, which controls Cd detoxification in the cell vacuole. In Arabidopsis, heavy metal ATPase3 (HMA3) in roots regulates Cd concentration in leaves by accumulating Cd in the roots (Di Toppi and Gabbrielli 1999; Halim et al. 2020). Plant antioxidant defence mechanisms can also minimize oxidative damages caused by Cd toxicity (Wang et al. 2008).
5 Mechanisms of Cadmium Tolerance in Plant Growth-Promoting Rhizobacteria
Plant-associated non-symbiotic rhizospheric bacterial strains that assist in plant growth, directly or indirectly, are regarded as Plant growth promoting rhizobacteria (PGPR) (Glick 1995). PGPR plays a significant part in increasing agricultural yield through plant–microbe interaction even in contaminated soil. They are also utilized for the remediation of heavy metals, including Cd, polluted sites. Cd-tolerant PGPR, which helps in phytoextraction to remove Cd from the soil, improve Cd mobilization and bioavailability, increase root surface area for Cd uptake, and elevate translocation of Cd from root to aerial parts to boost Cd accumulation in plants. However, many PGPR strains help in plant growth promotion without raising Cd levels in edible crops that grow in contaminated soils. Several mechanisms have evolved in Cd-tolerant PGPR to cope with the heavy metal toxicity, and as a result, reduce Cd stress in plants. These include efflux, extracellular complexation, biosorption, precipitation, biotransformation and sequestration (Sharma and Archana 2016).
After entry into the cell, Cd must be rapidly and effectively removed from the cell or transformed into a non-or-less toxic form. The energy-dependent cadA efflux transporter protein, encoded by cadA gene of plasmid pI258 in Staphlococcus aureus, is involved in the removal of cadmium from the cell (Ganesan 2008). The gene CadB located on the same plasmid also confers Cd resistance by changing the binding site (Wheaton et al. 2015). The Cad system was also reported in Ralstonia sp. CH34. The cadA gene codes for cadmium resistance. The cadB gene expression is possible only when there is no cadA gene. Alcaligenes eutrophus confers Cd resistance due to the presence of the Czc system which effluxes cadmium and other heavy metals (zinc and cobalt) (Nies 2003; Hynninen 2010). The efflux system for Cd resistance, consisting of czcB and smtAB gene, is also present in the E. coli P4 strain (Khan et al. 2015). P-type ATPases, cation diffusion facilitator (CDF) family, CBA (Capsule biogenesis/assembly) family, and chemiosmotic family of transporters help in the efflux of Cd ions and Cd resistance (Nies 2003).
Biosorption plays a significant role in minimizing Cd toxicity to the bacterial cell under Cd exposure. The biosorption of Cd ions depends on metal adsorption, complexation, and bioaccumulation and makes it non-available to other organisms (Coelho et al. 2015). Metallothioneins in bacteria are cysteine-rich low molecular weight cytoplasmic proteins that help in positively charged metal (Cd) binding (Naik and Dubey 2017). Many bacteria with negatively charged cell walls or envelop can bind with dissolved Cd+2 cations. Bacterial exopolysaccharides (EPSs) with their anionic groups play a significant part in absorbing Cd ions from their vicinity. Metal biotransformations through oxido-reduction reactions, methylation and demethylation confer resistance against heavy metals in bacteria (Silver and Phung 2005).
6 Cadmium Resistant PGPR in Cadmium Detoxification in Plants
Cd bioavailability in the rhizospheric region has been the major reason for Cd toxicity in plants. The use of PGPR strains for plant growth and minimization of Cd uptake in edible crops provides an efficient, ecologically sustainable alternative strategy for bioremediation and maintaining food safety. However, in non-hyperaccumulator plants, Cd-tolerant PGPR could lower the uptake and distribution of Cd into the above-ground plant parts; whereas in hyperaccumulator plants, it may facilitate the Cd uptake and bioaccumulation in the plant. Cd resistant PGPR, such as Bacillus sp., Pseudomonas spp., Burkholderia sp., Ochrobactrum, Chryseobacterium sp., Enterobacter sp., Serratia sp., Klebsiella sp., reduce a significant amount of the Cd content in edible crops (Table 1). PGPR can alleviate cadmium toxicity through several mechanisms, ensuing plant growth. PGPR characters, such as the production of plant growth regulators including IAA, 1-aminocyclopropane-1-carboxylate deaminase (ACCD) production, siderophore production, organic acid secretion, and phosphate solubilization (Fig. 2), help in plant growth enhancement and minimization of Cd toxicity in Cd-polluted soil (Table 2) (Pramanik et al. 2018).
6.1 Cadmium Immobilization in Soil
PGPR-induced Cd stabilization in soil has great importance for diminishing Cd bioaccumulation in crops and simultaneously enhancing agricultural productivity and crop quality. PGPR can reduce the mobilization and phytoavailability of Cd by acting directly as biosorbents or as bioaccumulators (Voleskya and Holant 1995). Due to the high area-to-volume ratio of the bacterial cell and many metal attachment sites, PGP bacteria can act as excellent biosorbents (Gadd 1990). Cd binding extracellular polymers, such as exopolysaccharides and proteins, are produced by PGPR strains and could bind a substantial quantity of harmful heavy metals including Cd to immobilize them by precipitating as insoluble sulfides and oxides. Cd ions bind to the polyphosphate bodies, phytochelatins (PCs), metallothioneins (MTs) and other proteins to form various types of metal complexes, as reported in Pseudomonas putida. Chelator-Cd complexes are then transported to the vacuole for sequestration (Rayner and Sadler 1989; Dong et al. 2007). The release of organic molecules and slimes outside the bacterial cell wall increase Cd biosorption and sequestration in the root (Madhaiyan et al. 2007).
6.2 Cadmium Precipitation
PGP bacteria have anions, such as sulfides and phosphates on their cell walls. Cd2+ ions could bind with these negatively charged surfaces. The binding and subsequent precipitation of Cd2+ reduces its phytoavailability (Lamelas et al. 2006). For example, sulfate-reducing bacteria carried out sulfate reduction in presence of organic substances or H2, and as a by-product, precipitate less soluble Cd sulfides (CdS) (Violante et al. 2010; Menon and Voordouw 2018). Also, PGPR under Cd and other heavy metals exposure produce H2S that reacts with free Cd+2 extracellularly to precipitate, as CdS.
6.3 Plant Growth-Promoting Activities to Counter Cadmium Toxicity
6.3.1 Nitrogen Fixation
Nitrogen (N) is by far the most vital micronutrient for plant growth enhancement and agricultural productivity. It also enhances Cd tolerance in plants, with the production of nitrogen metabolites, such as GSH and phytochelatins, which play a significant part in defence against Cd toxicity. The presence of nitrogen in agricultural soil increases RuBisCo activity and photosynthetic yield, along with Cd tolerance (Jalloh et al. 2009). PGPR can fix free atmospheric nitrogen, act as a biofertilizer and remove N limitation in soil for plants. It was reported in a study that N2-fixing Cd-tolerant Klebsiella mobilis promotes grain production in barley and reduces Cd concentration under Cd stress (Pishchik et al. 2002).
6.3.2 Phosphorus Solubilization
Phosphorus (P) also plays a significant function in overall plant growth and crop productivity. Complexation and biosorption of Cd with the phosphate groups present in the cell wall play significant roles in regulating Cd uptake and distribution in plant parts. P amendment in Cd-polluted soil enhances the quantity of chlorophyll and, as a result, improves photosynthetic yield in Zea mays (Jiang et al. 2007). ( Many bacteria are capable of organic and inorganic phosphate complexes solubilization in soils, resulting in enhancement of P bioavailability. Application of phosphate solubilizers in Cd-polluted soils stimulates Cd immobilization as a result of the precipitation of Cd-phosphate complexes (Park et al. 2010). Similarly, many zinc solubilizing PGPR increases Zn phytoavailability (Saravanan et al. 2011). It is believed that Zn solubilization in the soil is an efficient strategy to promote crop productivity by limiting Cd bioavailability to plants and diminishing Cd uptake through roots.
6.3.3 Secretion of Organic Acid
Excretion of organic acids, such as gluconic acid, succinic acid, salicylic acid, oxalic acid and citric acid by PGPR, are well reported. These organic acids help in Cd detoxification in plants by chelating with free Cd+2 ions. The release of organic acids by PGPR is a well-known mechanism that affects the mobility of Cd ions in rhizospheric soil by altering soil pH, organic matter contents and ionic strength (Halim et al. 2020). For instance, gluconic acid production by glucose dehydrogenase enzyme was studied in many PGPR. It was reported that gluconic acid produced by Enterobacter asburiae enhances growth in Vigna radiata under Cd exposure. Organic acids also upregulate antioxidant defence systems, such as SOD and POX under Cd stress (Goldstein 1995; Kavita et al. 2008), and help in phosphate solubilization in soil.
6.3.4 Siderophore Production
Siderophores play an important role in improving the iron status of the plant. It also binds with heavy metals to restrict metal mobility and increase accumulation (Rajkumar et al. 2010). These are low molecular weight compounds released by rhizospheric bacteria that bind to iron (Fe+3) ions with great affinity. Siderophores, with their iron-binding ability, improve iron bioavailability which would result in plant growth. Also, the increase in iron level, in return, would affect the uptake of Cd, thus imparting Cd resistance. In Pseudomonas sp., synthesis of green pigmented siderophore, i.e. pyoverdine, has been reported under Cd stress (Dao et al. 1999). It enhances plant growth and reduces Cd intake in Vigna mungo (Tripathi et al. 2005). Siderophore producing P. aeruginosa also enhances iron intake in Brassica sp. under Cd stress (Sinha and Mukherjee 2008).
6.3.5 ACC Deaminase Production
Ethylene, a stress-signaling molecule, is produced from L-methionine through the intermediate products, S-adenosyl-1-methionine (SAM) and 1-aminocyclopropane-1-carboxylic acid (ACC). Ethylene triggers the production of SOD, APX and ROS, which ultimately results in senescence in plants. ACC deaminase (ACCD) cleaves the immediate ethylene precursor, ACC, to produce α-ketoglutarate and ammonia, and resultantly, reduce ethylene formation. The production of ACCD plays a significant role in Cd resistance mechanisms in plants (Glick 2005; Saleem et al. 2007). ACCD activity stimulates seed germination, root formation in tomato and plant growth in mustard and rape plants under Cd stress (Grichko et al. 2000; Belimov et al. 2001).
6.3.6 IAA Production
PGP traits, such as root hair formation and root elongation, shoot elongation, are immensely controlled by the productions of phytohormones, e.g. IAA, gibberellins and cytokinins. IAA production is regarded as one of the widely accepted plant growth-promoting traits for PGPR. IAA produced by PGPR strains enhances root elongation in Brassica napus (Sheng and Xia 2006). Plant growth promotion and alleviation of Cd toxicity by IAA producing Enterobacter aerogenes MCC 3092 and Pseudomonas sp. SNA5 in rice and wheat, respectively, was reported when exposed to Cd (Verma et al. 2015; Pramanik et al. 2018).
7 Conclusions and Future Prospects
PGPR has been enhancing crop productivity and crop quality in stressed soil through different plant growth-promoting mechanisms. With the recent interesting progress, bioremediation of cadmium stress in plants through PGPR has emerged as a promising technique. However, the use of PGPR on a commercial scale will require much deliberation regarding the preservation of the quality and efficacy of the PGPR product and delivery mechanisms. Future studies will also look into the bacterial genes responsible for PGP traits. It might help in developing and designing bacteria with many PGP traits. The use of genetically engineered PGPR will be more effective in reducing Cd toxicity and plant growth promotion with their multifunctional PGP traits (Glick 2012). Rapid improvement and application of modern tools and nanotechnology open the door for the production of PGPR-mediated bionanohybrids, nano-fertilizers and biosensors. These bionanohybrids will play a vital role in Cd immobilization and maintaining macro and micronutrient balance in the rhizospheric soil. Future improvement and advancement of PGPR-based new technology in Cd detoxification in soil and plants will guide and bring in agricultural prosperity in the coming decades.
In recent decades, rapid accretion in anthropogenic activities led to cadmium contamination in the environment. The increase of cadmium pollution in the agricultural soil has led many scientists to focus on developing rapid, low-cost and efficient Cd detoxification technologies for plants. Further research on how plant growth-promoting rhizobacteria interact with Cd ions and plants in response to cadmium stress would allow us to comprehend the knowledge of the phytoavailability of cadmium in rhizospheric soil effectively. The knowledge about these processes provides insight into the strategies employed by bacteria for Cd detoxification in plants. It would also aid in the prediction of the plant response in a stressed environment. This chapter summarizes the current understanding of natural and anthropogenic sources of cadmium contamination, the intricate interaction between rhizospheric growth-promoting bacteria, soil and plant under Cd stress. Here, PGPR acts as a mediator that regulates bioavailable Cd level and their detoxification in plant cells in a sustainable manner. The knowledge about these processes offers valuable insights into the strategies for developing PGPR-based bioremediation technologies to mitigate the growing risk of Cd toxicity for worldwide agricultural yield and productivity.
Abbreviations
- ABC:
-
ATP-binding cassette
- ACC:
-
1-Aminocyclopropane-1-carboxylic acid
- ACCD:
-
1-Aminocyclopropane-1-carboxylic acid
- APX:
-
Ascorbate peroxidase
- ASA:
-
Ascorbic acid
- CAT:
-
Catalase
- CAX:
-
Cation/proton exchangers
- CDF:
-
Cation Diffusion Facilitator
- CBA:
-
Capsule Biogenesis/Assembly
- Cd:
-
Cadmium
- Czc:
-
Cobalt/zinc/cadmium
- DHAR:
-
Dehydroascorbate reductase
- EPS:
-
Exopolysaccharide
- GPX:
-
Glutathione peroxidise
- GR:
-
Glutathione reductase
- GSH:
-
Glutathione (reduced)
- H2O2:
-
Hydrogen peroxide
- IAA:
-
Indole Acetic Acid
- IARC:
-
International Agency for Research on Cancer
- IRT:
-
Iron-Regulated Transporter
- LCT:
-
Lysosomal Cystine Transporter
- MDHAR:
-
Monodehydroascorbate reductase
- MT:
-
Metallothionein
- NRAMP:
-
Natural Resistance-Associated Macrophage Protein
- PC:
-
Phytochelatin
- PEPCase:
-
Phosphoenolpyruvate carboxylase
- PGPR:
-
Plant Growth Promoting Rhizobacteria
- POX:
-
Peroxidase
- QA:
-
A bound primary plastoquinone
- QB:
-
A secondary plastoquinone
- RuBisCo:
-
Ribulose-1, 5-bisphosphate carboxylase
- ROS:
-
Reactive Oxygen Species
- SAM:
-
S-Adenosyl-1-methionine
- SOD:
-
Superoxide dismutase
- TF:
-
Transfer Factor
- WHO:
-
World Health Organization
- ZIP:
-
ZRT-IRT-like Proteins
References
Abbas T, Rizwan M, Ali S et al (2017) Effect of biochar on cadmium bioavailability and uptake in wheat (Triticum aestivum L.) grown in a soil with aged contamination. Ecotoxicol Environ Saf 140:37–47
Abbas SZ, Rafatullah M, Hossain K et al (2018) A review on mechanism and future perspectives of cadmium-resistant bacteria. Int J Environ Sci Technol 15(1):243–262
Adhikari A, Lee KE, Khan MA et al (2020) Effect of silicate and phosphate solubilizing Rhizobacterium Enterobacter ludwigii GAK2 on Oryza sativa L. under cadmium stress. J Microbiol Biotechnol 30(1):118–126
Ahmad I, Akhtar MJ, Asghar HN et al (2016) Differential effects of plant growth-promoting rhizobacteria on maize growth and cadmium uptake. J Plant Growth Regul 35(2):303–315
Ahsan N, Lee SH, Lee DG, Lee H et al (2007) Physiological and protein profiles alternation of germinating rice seedlings exposed to acute cadmium toxicity. C R Biologies 330(10):735–746
Almuwayhi MA (2021) Effect of cadmium on the molecular and morpho-physiological traits of Pisum sativum L. Biotechnol Biotechnol Equip 35(1):1374–1384
Asif M, Pervez A, Irshad U et al (2020) Melatonin and plant growth-promoting rhizobacteria alleviate the cadmium and arsenic stresses and increase the growth of Spinacia oleracea L. Plant Soil Environ 66(5):234–241
Bansal R, Priya S, Dikshit HK et al (2021) Growth and antioxidant responses in iron-biofortified lentil under cadmium stress. Toxics 9(8):182
Belimov AA, Safronova VI, Sergeyeva TA et al (2001) Characterization of plant growth promoting rhizobacteria isolated from polluted soils and containing 1-aminocyclopropane-1-carboxylate deaminase. Can J Microbiol 47(7):642–652
Benavides MP, Gallego SM, Tomaro ML (2005) Cadmium toxicity in plants. Braz J Plant Physiol 17:21–34
Bhattacharyya PN, Jha DK (2012) Plant growth-promoting rhizobacteria (PGPR): emergence in agriculture. World J Microbiol Biotechnol 28(4):1327–1350
Blaylock MJ, Salt DE, Dushenkov S et al (1997) Enhanced accumulation of Pb in Indian mustard by soil-applied chelating agents. Environ Sci Technol 31(3):860–865
Bolan N, Kunhikrishnan A, Thangarajan R et al (2014) Remediation of heavy metal(loid)s contaminated soils–to mobilize or to immobilize? J Hazard Mater 266:141–166
Ci D, Jiang D, Wollenweber B, Dai T et al (2010) Cadmium stress in wheat seedlings: growth, cadmium accumulation and photosynthesis. Acta Physiol Plant 32(2):365–373
Chaffei C, Pageau K, Suzuki A et al (2004) Cadmium toxicity induced changes in nitrogen management in Lycopersicon esculentum leading to a metabolic safeguard through an amino acid storage strategy. Plant Cell Physiol 45(11):1681–1693
Chen Y, Chao Y, Li Y et al (2016) Survival strategies of the plant-associated bacterium Enterobacter sp. strain EG16 under cadmium stress. Appl Environ Microbiol 82(6):1734–1744
Chmielowska-Bąk J, Gzyl J, Rucińska-Sobkowiak R et al (2014) The new insights into cadmium sensing. Front Plant Sci 5:245
Coelho LM, Rezende HC, Coelho LM et al (2015) Bioremediation of polluted waters using microorganisms. In: Shiomi N (ed) Advances in bioremediation of wastewater and polluted soil, vol 10, p 60770. https://doi.org/10.5772/60770
DalCorso G, Farinati S, Maistri S et al (2008) How plants cope with cadmium: staking all on metabolism and gene expression. J Integr Plant Biol 50(10):1268–1280
Dao KHT, Hamer KE, Clark CL et al (1999) Pyoverdine production by Pseudomonas aeruginosa exposed to metals or an oxidative stress agent. Ecol Appl 9(2):441–448
Daud MK, Sun Y, Dawood M (2009) Cadmium-induced functional and ultrastructural alterations in roots of two transgenic cotton cultivars. J Hazard Mater 161(1):463–473
de Souza Guilherme MDF, de Oliveira HM, da Silva E (2015) Cadmium toxicity on seed germination and seedling growth of wheat Triticum aestivum. Acta Sci Biol Sci 37(4):499–504
Dixit R, Malaviya D, Pandiyan K et al (2015) Bioremediation of heavy metals from soil and aquatic environment: an overview of principles and criteria of fundamental processes. Sustainability 7(2):2189–2212
Di Toppi LS, Gabbrielli R (1999) Response to cadmium in higher plants. Environ Exp Bot 41(2):105–130
Dong J, Mao WH, Zhang GP et al (2007) Root excretion and plant tolerance to cadmium toxicity-a review. Plant Soil Environ 53(5):193
Dutta P, Karmakar A, Majumdar S, et al (2018) Klebsiella pneumoniae (HR1) assisted alleviation of Cd (II) toxicity in Vigna mungo: a case study of biosorption of heavy metal by an endophytic bacterium coupled with plant growth promotion Euro-Mediterr J Environ Integr 3(1):1–10
Elhiti M, Yang C, Chan A et al (2012) Altered seed oil and glucosinolate levels in transgenic plants overexpressing the Brassica napus SHOOTMERISTEMLESS gene. J Exp Bot 63(12):4447–4461
El Rasafi T, Oukarroum A, Haddioui A et al (2020) Cadmium stress in plants: A critical review of the effects, mechanisms, and tolerance strategies. Crit Rev Environ Sci Technol 1–52
Fahsi N, Mahdi I, Mesfioui A et al (2021) Plant Growth-Promoting Rhizobacteria isolated from the Jujube (Ziziphus lotus) plant enhance wheat growth, Zn uptake, and heavy metal tolerance. Agriculture 11(4):316
Faller P, Kienzler K, Krieger-Liszkay A (2005) Mechanism of Cd2+ toxicity: Cd2+ inhibits photoactivation of Photosystem II by competitive binding to the essential Ca2+ site Biochim Biophys Acta Bioenerg 1706(1–2):158–164
Fernández-Llamosas H, Ibero J, Thijs S (2020) Enhancing the rice seedlings growth promotion abilities of Azoarcus sp. CIB by heterologous expression of ACC deaminase to improve performance of plants exposed to cadmium stress. Microorganisms 8(9):1453
Fusconi A, Repetto O, Bona E (2006) Effects of cadmium on meristem activity and nucleus ploidy in roots of Pisum sativum L. cv. Frisson seedlings. Environ Exp Bot 58(1–3):253–260
Gadd GM (1990) Heavy metal accumulation by bacteria and other microorganisms. Experientia 46(8):834–840
Gallego SM, Pena LB, Barcia RA et al (2012) Unravelling cadmium toxicity and tolerance in plants: insight into regulatory mechanisms. Environ Exp Bot 83:33–46
Ganesan V (2008) Rhizoremediation of cadmium soil using a cadmium-resistant plant growth-promoting rhizopseudomonad. Curr Microbiol 56(4):403–407
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48(12):909–930
Glick BR (1995) The enhancement of plant growth by free-living bacteria. Can J Microbiol 41(2):109–117
Glick BR (2005) Modulation of plant ethylene levels by the bacterial enzyme ACC deaminase. FEMS Microbiol Lett 251(1):1–7
Glick BR (2012) Plant growth-promoting bacteria: mechanisms and applications. Scientifica 2012
Goldstein AH (1995) Recent progress in understanding the molecular genetics and biochemistry of calcium phosphate solubilization by gram negative bacteria. Biol Agric Hortic 12(2):185–193
Govindasamy V, Senthilkumar M, Annapurna K (2015) Effect of mustard rhizobacteria on wheat growth promotion under cadmium stress: characterization of acd S gene coding ACC deaminase. Ann Microbiol 65(3):1679–1687
Grichko VP, Glick BR FB (2000) Increased ability of transgenic plants expressing the bacterial enzyme ACC deaminase to accumulate Cd Co, Cu, Ni, Pb, and Zn. J Biotechnol 81(1):45–53
Guo J, Chi J (2014) Effect of Cd-tolerant plant growth-promoting rhizobium on plant growth and Cd uptake by Lolium multiflorum Lam. and Glycine max (L.) Merr. in Cd-contaminated soil. Plant soil 375(1):205–214
Hall MJ, Brown MT (2002) Copper and manganese influence the uptake of cadmium in marine macroalgae. Bull Environ Contam Toxicol 68(1):49–55
Haider FU, Liqun C, Coulter JA et al (2021) Cadmium toxicity in plants: impacts and remediation strategies. Ecotoxicol Environ Saf 211:111887
Halim MA, Rahman MM, Megharaj M et al (2020) Cadmium immobilization in the rhizosphere and plant cellular detoxification: role of plant-growth-promoting rhizobacteria as a sustainable solution. J Agric Food Chem 68(47):13497–13529
Hassan W, Bano R, Bashir S et al (2016) Cadmium toxicity and soil biological index under potato (Solanum tuberosum L.) cultivation. Soil Res 54(4):460–468
He JY, Ren YF, Cheng ZHU et al (2008) Effects of cadmium stress on seed germination, seedling growth and seed amylase activities in rice (Oryza sativa). Rice Sci 15(4):319–325
Hu Y, Cheng H, Tao S (2016) The challenges and solutions for cadmium-contaminated rice in China: a critical review. Environ Int 92:515–532
Huybrechts M, Cuypers A, Deckers J et al (2019) Cadmium and plant development: an agony from seed to seed. Int J Mol Sci 20(16):3971
Hynninen A (2010) Zinc, cadmium and lead resistance mechanisms in bacteria and their contribution to biosensing. Doctoral dissertation, University of Helsinki
Irfan M, Ahmad A, Hayat S (2014) Effect of cadmium on the growth and antioxidant enzymes in two varieties of Brassica juncea. Saudi J Biol Sci 21(2):125–131
Jadia CD, Fulekar MH (2008) Phytoremediation: the application of vermicompost to remove zinc, cadmium, copper, nickel and lead by sunflower plant. Environ Eng Manag J 7(5)
Jalloh MA, Chen J, Zhen F et al (2009) Effect of different N fertilizer forms on antioxidant capacity and grain yield of rice growing under Cd stress. J Hazard Mater 162(2–3):1081–1085
Jiang HM, Yang JC, Zhang JF (2007) Effects of external phosphorus on the cell ultrastructure and the chlorophyll content of maize under cadmium and zinc stress. Environ Pollut 147(3):750–756
Jinadasa N, Collins D, Holford P et al (2016) Reactions to cadmium stress in a cadmium-tolerant variety of cabbage (Brassica oleracea L.): is cadmium tolerance necessarily desirable in food crops?. Environ Sci Pollut Res 23(6):5296–5306
Kalai T, Bouthour D, Manai J et al (2016) Salicylic acid alleviates the toxicity of cadmium on seedling growth, amylases and phosphatases activity in germinating barley seeds. Arch Agron Soil Sci 62(6):892–904
Kavita B, Shukla S, Kumar GN et al (2008) Amelioration of phytotoxic effects of Cd on mung bean seedlings by gluconic acid secreting rhizobacterium Enterobacter asburiae PSI3 and implication of role of organic acid. World J Microbiol Biotechnol 24(12):2965–2972
Kayser A, Wenger K, Keller A et al (2000) Enhancement of phytoextraction of Zn, Cd, and Cu from calcareous soil: the use of NTA and sulfur amendments. Environ Sci Technol 34(9):1778–1783
Khan Z, Nisar MA, Hussain SZ et al (2015) Cadmium resistance mechanism in Escherichia coli P4 and its potential use to bioremediate environmental cadmium. Appl Microbiol Biotechnol 99(24):10745–10757
Khan AR, Park GS, Asaf S et al (2017a) Complete genome analysis of Serratia marcescens RSC-14: a plant growth-promoting bacterium that alleviates cadmium stress in host plants. PloS one 12(2):e0171534
Khan MA, Khan S, Khan A et al (2017b) Soil contamination with cadmium, consequences and remediation using organic amendments. Sci Total Environ 601:1591–1605
Khanna K, Jamwal VL, Gandhi SG et al (2019a) Metal resistant PGPR lowered Cd uptake and expression of metal transporter genes with improved growth and photosynthetic pigments in Lycopersicon esculentum under metal toxicity. Sci Rep 9(1):1–14
Khanna K, Jamwal VL, Sharma A et al (2019b) Supplementation with plant growth promoting rhizobacteria (PGPR) alleviates cadmium toxicity in Solanum lycopersicum by modulating the expression of secondary metabolites. Chemosphere 230:628–639
Kloepper JW, Schroth MN, Miller TD (1980) Effects of rhizosphere colonization by plant growth-promoting rhizobacteria on potato plant development and yield. Phytopathology 70(11):1078–1082
Kubier A, Wilkin RT, Pichler T (2019) Cadmium in soils and groundwater: a review. Appl Geochem 108:104388
Kumar A (2012) Role of plant-growth-promoting rhizobacteria in the management of cadmium-contaminated soil. In: Zaidi A, Wani P, Khan M (eds) Toxicity of heavy metals to legumes and bioremediation. Springer, Vienna, pp 163–178
Kumar A, Dewangan S, Lawate P et al (2019) Zinc-solubilizing bacteria: a boon for sustainable agriculture. In: Sayyed R, Arora N, Reddy M (eds) Plant growth promoting rhizobacteria for sustainable stress management. Microorganisms for sustainability, vol 12. Springer, Singapore, pp 139–155
Kumari M, Thakur IS (2018) Biochemical and proteomic characterization of Paenibacillus sp. ISTP10 for its role in plant growth promotion and in rhizostabilization of cadmium. Bioresour Technol Rep 3:59–66
Küpper H, Parameswaran A, Leitenmaier B et al (2007) Cadmium-induced inhibition of photosynthesis and long-term acclimation to cadmium stress in the hyperaccumulator Thlaspi caerulescens. New Phytol 175(4):655–674
Kuriakose SV, Prasad MNV (2008) Cadmium stress affects seed germination and seedling growth in Sorghum bicolor (L.) Moench by changing the activities of hydrolyzing enzymes. Plant Growth Regul 54(2):143–156
Lamelas C, Benedetti M, Wilkinson KJ et al (2006) Characterization of H+ and Cd2+ binding properties of the bacterial exopolysaccharides. Chemosphere 65(8):1362–1370
Lane TW, Morel FM (2000) A biological function for cadmium in marine diatoms. Proc Natl Acad Sci 97(9):4627–4631
León AM, Palma JM, Corpas FJ et al (2002) Antioxidative enzymes in cultivars of pepper plants with different sensitivity to cadmium. Plant Physiol Biochem 40(10):813–820
Li Y, Dhankher OP, Carreira L et al (2005) Arsenic and mercury tolerance and cadmium sensitivity in Arabidopsis plants expressing bacterial γ-glutamylcysteine synthetase. Environ Toxicol Chem 24(6):1376–1386
Li Q, Lu Y, Shi Y et al (2013) Combined effects of cadmium and fluoranthene on germination, growth and photosynthesis of soybean seedlings. J Environ Sci 25(9):1936–1946
Li Y, Zeng J, Wang S et al (2020) Effects of cadmium-resistant plant growth-promoting rhizobacteria and Funneliformis mosseae on the cadmium tolerance of tomato (Lycopersicon esculentum L.). Int J Phytoremediat 22(5): 451–458
Madhaiyan M, Poonguzhali S, Sa T (2007) Metal tolerating methylotrophic bacteria reduces nickel and cadmium toxicity and promotes plant growth of tomato (Lycopersicon esculentum L.). Chemosphere 69(2):220–228
Majewska M, Kurek E (2011) Effect of Cd concentration in growth media on Secale cereale roots and Cd interaction with rhizosphere microorganisms originating from different parts of the grain. Eur J Soil Biol 47(2):95–101
Markovska YK, Gorinova NI, Nedkovska MP et al (2009) Cadmium-induced oxidative damage and antioxidant responses in Brassica juncea plants. Biol Plant 53(1):151–154
Menon P, Voordouw G (2018) Impact of light oil toxicity on sulfide production by acetate-oxidizing, sulfate-reducing bacteria. Int Biodeterior Biodegrad 126:208–215
Mitra S, Pramanik K, Sarkar A et al (2018a) Bioaccumulation of cadmium by Enterobacter sp. and enhancement of rice seedling growth under cadmium stress. Ecotoxicol Environ Saf 156:183–196
Mitra S, Pramanik K, Ghosh PK et al (2018b) Characterization of Cd-resistant Klebsiella michiganensis MCC3089 and its potential for rice seedling growth promotion under Cd stress. Microbiol Res 210:12–25
Mitra S, Purkait T, Pramanik K et al (2019) Three-dimensional graphene for electrochemical detection of Cadmium in Klebsiella michiganensis to study the influence of Cadmium uptake in rice plant. Mater Sci Eng C 103:109802
Mondal NK, Chittaranjan D, Satinath R et al (2013) Effect of varying cadmium stress on chickpea (Cicer arietinum L) seedlings: an ultrastructural study. Ann Environ Sci 7:59–70
Moradi R, Pourghasemian N, Naghizadeh M (2019) Effect of beeswax waste biochar on growth, physiology and cadmium uptake in saffron. J Clean Prod 229:1251–1261
Naik MM, Dubey SK (2017) Lead-and mercury-resistant marine bacteria and their application in lead and mercury bioremediation. In: Naik M, Dubey S (eds) Marine pollution and microbial remediation. Springer, Singapore, pp 29–40
Najeeb U, Jilani G, Ali S et al (2011) Insights into cadmium induced physiological and ultra-structural disorders in Juncus effusus L. and its remediation through exogenous citric acid. J Hazard Mater 186(1):565–574
Nies DH (1999) Microbial heavy-metal resistance. Appl Microbiol Biotechnol 51(6):730–750
Nies DH (2003) Efflux-mediated heavy metal resistance in prokaryotes. FEMS Microbiol Rev 27(2–3):313–339
Pal AK, Sengupta C (2019) Isolation of cadmium and lead tolerant plant growth promoting rhizobacteria: Lysinibacillus varians and Pseudomonas putida from Indian Agricultural Soil. Soil Sediment Contam 28(7):601–629
Park J, Bolan N, Megharaj M et al (2010) Isolation of phosphate-solubilizing bacteria and characterization of their effects on lead immobilization. In: International symposium: challenges to soil degradation towards sustaining life and environment. Tokyo Metropolitan University Symposium Series No. 2, 2009. Pedologist 53(3):67–75
Parmar P, Kumari N, Sharma V (2013) Structural and functional alterations in photosynthetic apparatus of plants under cadmium stress. Bot Stud 54(1):1–6
Pishchik VN, Vorobyev NI, Chernyaeva II et al (2002) Experimental and mathematical simulation of plant growth promoting rhizobacteria and plant interaction under cadmium stress. Plant Soil 243(2):173–186
Popova LP, Maslenkova LT, Ivanova A et al (2012) Role of salicylic acid in alleviating heavy metal stress. In: Ahmad P, Prasad M (eds) Environmental adaptations and stress tolerance of plants in the era of climate change. Springer, New York, NY, pp 447–466
Pramanik K, Mitra S, Sarkar A et al (2017) Characterization of cadmium-resistant Klebsiella pneumoniae MCC 3091 promoted rice seedling growth by alleviating phytotoxicity of cadmium. Environ Sci Pollut Res 24(31):24419–24437
Pramanik K, Mitra S, Sarkar A et al (2018) Alleviation of phytotoxic effects of cadmium on rice seedlings by cadmium resistant PGPR strain Enterobacter aerogenes MCC 3092. J Hazard Mater 351:317–329
Pramanik K, Mandal S, Banerjee S et al (2021) Unraveling the heavy metal resistance and biocontrol potential of Pseudomonas sp. K32 strain facilitating rice seedling growth under Cd stress. Chemosphere 274:129819
Rajkumar M, Ae N, Prasad MNV et al (2010) Potential of siderophore-producing bacteria for improving heavy metal phytoextraction. Trends Biotechnol 28(3):142–149
Rascio N, Navari-Izzo F (2011) Heavy metal hyperaccumulating plants: how and why do they do it? And what makes them so interesting? Plant Sci 180(2):169–181
Rayner MH, Sadler PJ (1989) Cadmium accumulation and resistance mechanisms in bacteria. In: Poole RK, Gadd GM (eds) Metal-Microbe interactions. Society for General Microbiology, IRL Press/Oxford University Press, New York, pp 39–47
Redjala T, Zelko I, Sterckeman T et al (2011) Relationship between root structure and root cadmium uptake in maize. Environ Exp Bot 71(2):241–248
Rizwan M, Meunier JD, Miche H et al (2012) Effect of silicon on reducing cadmium toxicity in durum wheat (Triticum turgidum L. cv. Claudio W.) grown in a soil with aged contamination. J Hazard Mater 209:326–334
Rizwan M, Ali S, Abbas T et al (2016) Cadmium minimization in wheat: a critical review. Ecotoxicol Environ Saf 130:43–53
Roy SK, Cho SW, Kwon SJ et al (2016) Morpho-physiological and proteome level responses to cadmium stress in sorghum. PLoS One 11(2): p.e0150431
Sahile AA, Khan MA, Hamayun M et al (2021) Novel Bacillus cereus strain, ALT1, enhance growth and strengthens the antioxidant system of soybean under cadmium stress. Agronomy 11(2):404
Saleem M, Arshad M, Hussain S et al (2007) Perspective of plant growth promoting rhizobacteria (PGPR) containing ACC deaminase in stress agriculture. J Ind Microbiol Biotechnol 34(10):635–648
Sandalio LM, Dalurzo HC, Gomez M et al (2001) Cadmium-induced changes in the growth and oxidative metabolism of pea plants. J Exp Bot 52(364):2115–2126
Saravanan VS, Kumar MR, Sa TM (2011) Microbial zinc solubilization and their role on plants. In: Maheshwari D (ed) Bacteria in agrobiology: plant nutrient management. Springer, Berlin, Heidelberg, pp 47–63
Sebastian A, Prasad MNV (2018) Exogenous citrate and malate alleviate cadmium stress in Oryza sativa L.: probing role of cadmium localization and iron nutrition. Ecotoxicol Environ Saf 166:215–222
Seth CS, Misra V, Chauhan LKS et al (2008) Genotoxicity of cadmium on root meristem cells of Allium cepa: cytogenetic and Comet assay approach. Ecotoxicol Environ Saf 71(3):711–716
Shabayev VP, Bocharnikova EA, Ostroumov VE (2020) Remediation of cadmium-polluted soil using plant growth-promoting rhizobacteria and natural zeolite. Eurasian Soil Sci 53(6):809–819
Shahid M, Javed MT, Masood S et al (2019) Serratia sp. CP‐13 augments the growth of cadmium (Cd)‐stressed Linum usitatissimum L. by limited Cd uptake, enhanced nutrient acquisition and antioxidative potential. J Appl Microbiol 126(6):1708–1721
Shanying HE, Xiaoe YANG, Zhenli HE et al (2017) Morphological and physiological responses of plants to cadmium toxicity: a review. Pedosphere 27(3):421–438
Siedlecka A, Samuelsson G, Gardeström P et al (1998) The “activatory model” of plant response to moderate cadmium stress-relationship between carbonic anhydrase and Rubisco. In: Garab G (ed) Photosynthesis: mechanisms and effects. Springer, Dordrecht, pp 2677–2680
Sigfridsson KG, Bernát G, Mamedov F et al (2004) Molecular interference of Cd2+ with photosystem II. Biochim Biophys Acta Bioenergy 1659(1):19–31
Silver S, Phung LT (2005) A bacterial view of the periodic table: genes and proteins for toxic inorganic ions. J Ind Microbiol Biotechnol 32(11–12):587–605
Singh N, Gadi R (2012) Bioremediation of Ni (II) and Cu (II) from wastewater by the nonliving biomass of Brevundimonas vesicularis. J Environ Chem Ecotoxicol 4(8):137–142
Singh I, Shah K (2015) Evidences for suppression of cadmium induced oxidative stress in presence of sulphosalicylic acid in rice seedlings. Plant Growth Regul 76(1):99–110
Singh N, Ghosh PK, Chakraborty S et al (2021) Decoding the pathways of arsenic biotransformation in bacteria. Environ Sustain 1–23
Shanmugaraj BM, Malla A, Ramalingam S (2019) Cadmium stress and toxicity in plants: an overview. In: Hasanuzzaman M, Prasad MNV, Fujita M (eds) Cadmium toxicity and tolerance in plants. Academic Press, Cambridge, MA, USA, pp 1–17
Sharma RK, Archana G (2016) Cadmium minimization in food crops by cadmium resistant plant growth promoting rhizobacteria. Appl Soil Ecol 107:66–78
Sheng XF, Xia JJ (2006) Improvement of rape (Brassica napus) plant growth and cadmium uptake by cadmium-resistant bacteria. Chemosphere 64(6):1036–1042
Sinha S, Mukherjee SK (2008) Cadmium–induced siderophore production by a high Cd-resistant bacterial strain relieved Cd toxicity in plants through root colonization. Curr Microbiol 56(1):55–60
Smolders E (2001) Cadmium uptake by plants. Int J Occup Med Environ Health 14(2):177–183
Song Y, Jin L, Wang X (2017) Cadmium absorption and transportation pathways in plants. Int J Phytoremediation 19(2):133–141
Tran TA, Popova LP (2013) Functions and toxicity of cadmium in plants: recent advances and future prospects. Turk J Botany 37(1):1–13
Tripathi M, Munot HP, Shouche Y et al (2005) Isolation and functional characterization of siderophore-producing lead-and cadmium-resistant Pseudomonas putida KNP9. Curr Microbiol 50(5):233–237
Verma C, Singh P, Kumar R (2015) Isolation and characterization of heavy metal resistant PGPR and their role in enhancement of growth of wheat plant under metal (cadmium) stress condition. Arch Appl Sci Res 7(7):37–43
Vijayaragavan M, Prabhahar C, Sureshkumar J et al (2011) Toxic effect of cadmium on seed germination, growth and biochemical contents of cowpea (Vigna unguiculata L.) plants. Int Multidiscip Res J 1(5)
Vinod VTP, Sashidhar RB (2011) Bioremediation of industrial toxic metals with gum kondagogu (Cochlospermum gossypium): a natural carbohydrate biopolymer. Indian J Biotechnol 10(1):113–120
Violante A, Cozzolino V, Perelomov L et al (2010) Mobility and bioavailability of heavy metals and metalloids in soil environments. J Soil Sci Plant Nutr 10(3):268–292
Volesky B (2001) Detoxification of metal-bearing effluents: biosorption for the next century. Hydrometallurgy 59(2–3):203–216
Volesky B, Holan ZR (1995) Biosorption of heavy metals. Biotechnol Prog 11(3):235–250
Wang Z, Zhang Y, Huang Z et al (2008) Antioxidative response of metal-accumulator and non-accumulator plants under cadmium stress. Plant Soil 310(1):137–149
Wang MY, Chen AK, Wong MH et al (2011) Cadmium accumulation in and tolerance of rice (Oryza sativa L.) varieties with different rates of radial oxygen loss. Environ Pollut 159(6):1730–1736
Wang C, Liu Z, Huang Y et al (2019) Cadmium-resistant rhizobacterium Bacillus cereus M4 promotes the growth and reduces cadmium accumulation in rice (Oryza sativa L.). Environ Toxicol Pharmacol 72:103265
Wheaton G, Counts J, Mukherjee A (2015) The confluence of heavy metal biooxidation and heavy metal resistance: implications for bioleaching by extreme thermoacidophiles. Minerals 5(3):397–451
World Health Organization (2000) The world health report 2000: health systems: improving performance. World Health Organization, Geneva, Switzerland
World Health Organization (2010) Exposure to cadmium: a major public health concern. World Health Organization, Geneva, Switzerland
Wu Z, Zhao X, Sun X et al (2015) Xylem transport and gene expression play decisive roles in cadmium accumulation in shoots of two oilseed rape cultivars (Brassica napus). Chemosphere 119:1217–1223
Xin J, Huang B, Dai H et al (2014) Characterization of cadmium uptake, translocation, and distribution in young seedlings of two hot pepper cultivars that differ in fruit cadmium concentration. Environ Sci Pollut Res 21(12):7449–7456
Xue ZC, Gao HY, Zhang LT (2013) Effects of cadmium on growth, photosynthetic rate and chlorophyll content in leaves of soybean seedlings. Biol Plant 57(3):587–590
Yamaguchi N, Mori S, Baba K et al (2011) Cadmium distribution in the root tissues of solanaceous plants with contrasting root-to-shoot Cd translocation efficiencies. Environ Exp Bot 71(2):198–206
Ying RR, Qiu RL, Tang YT et al (2010) Cadmium tolerance of carbon assimilation enzymes and chloroplast in Zn/Cd hyperaccumulator Picris divaricata. J Plant Physiol 167(2):81–87
Younis U, Malik SA, Rizwan M et al (2016) Biochar enhances the cadmium tolerance in spinach (Spinacia oleracea) through modification of Cd uptake and physiological and biochemical attributes. Environ Sci Pollut Res 23(21):21385–21394
Zhao Y, Hu C, Wu Z et al (2019) Selenium reduces cadmium accumulation in seed by increasing cadmium retention in root of oilseed rape (Brassica napus L.). Environ Exp Bot 158:161–170
Zhi Y, Sun T, Zhou Q et al (2020) Screening of safe soybean cultivars for cadmium contaminated fields. Sci Rep 10(1):1–12
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Ghosh, P.K., Majumdar, S. (2022). Cadmium Stress Management in Plants: Prospects of Plant Growth-Promoting Rhizobacteria. In: Roy, S., Mathur, P., Chakraborty, A.P., Saha, S.P. (eds) Plant Stress: Challenges and Management in the New Decade. Advances in Science, Technology & Innovation. Springer, Cham. https://doi.org/10.1007/978-3-030-95365-2_15
Download citation
DOI: https://doi.org/10.1007/978-3-030-95365-2_15
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-95364-5
Online ISBN: 978-3-030-95365-2
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)