Abstract
In recent years, engineered nanoparticles have been the focus of intensive scientific and technological development in different applications, including agriculture and food production/security. Copper-based nanoparticles have interesting features, such as low production cost and potent antimicrobial actions at concentrations considered safe to humans and to the environment, making them good candidates for agricultural applications. Moreover, copper-based nanomaterials can be prepared not only by traditional chemical and physical methods but also by green routes involving biogenic methods in a sustainable manner. Copper is involved in plant growth, metabolism, and defense, and it has been used in agriculture as a key player in fungicides in the combat of plant diseases. Recently, the design of copper-based nanoparticles has opened new avenues to protect and defend crops, with superior results and lower toxic effects compared with bulk copper (massive copper). In this scenario, the current chapter presents and discusses recent progress in the design and applications of copper-based nanoparticles with potent antimicrobial applications for agricultural pest management, green routes to synthesize the nanoparticles, and recent progress in the applications of copper-based nanoparticles as pesticides, as well as their phytotoxic activity. We hope that this chapter opens new avenues in this important topic involving nanotechnology and agriculture.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Copper-based nanoparticles
- Copper oxide nanoparticles
- Metallic nanoparticles
- Pesticides
- Pest control
- Agriculture
1 Introduction: Importance of Copper in Agriculture
The biological role of copper (Cu) arose during the evolution of photosynthetic organisms, which changed the Earth’s atmosphere from anaerobic to aerobic due to the progressive accumulation of oxygen (Burkhead et al., 2009). Under physiological conditions, Cu exists in two forms: the reduced state (Cu+) and the oxidized state (Cu2+), and it can bind to different substrates depending on its state. Cu has a significant influence on plant metabolism due to its presence in several biomolecules and its participation in numerous metabolic routes in the plant, as a metal cofactor in certain metalloproteins involved in electron transport and oxidative stress response. In chloroplasts, Cu is a constituent of plastocyanin (Pc), the most abundant Cu protein in plant chloroplasts, which acts as an electron carrier in primary photosynthetic reactions. Cu is also a constituent of stromal Cu/Zn superoxide dismutase (Cu/Zn-SOD), which protects against reactive oxygen species (ROS) generated during the oxygenic photosynthetic reactions (Yruela, 2013).
In addition to being essential for plant metabolism, Cu has been used in agricultural practice for years as an active ingredient of fungicides to enhance crop production by controlling plant diseases. The most common Cu-based fungicide formulations contain Cu sulfate, Cu hydroxide, Cu oxychloride, or Cu carbonate (Husak, 2015). The Bordeaux mixture (a complex of Cu sulfate pentahydrate and lime) has been used in viticulture as a plant protection product against the stated fungal diseases since the eighteenth century, being the first fungicide to be used on a worldwide scale. Nowadays, a Cu hydroxide- and Cu sulfate-based fungicide is the only product allowed under organic standards, which is effective against Plasmopara viticola (Vitanovic, 2012).
Since the Bordeaux mixture, there has been rapid growth in the development and use of Cu-based fungicides, revolutionizing plant protection in the twentieth century. Among the advantages conferred to the use of Cu in agriculture, we can highlight the low cost, relatively high toxicity to plant pathogens, chemical stability, and long residual periods (Lamichhane et al., 2018). Cu is used as an active ingredient strictly for its protective function, as it has no curative or systemic activity and, once applied, Cu particles may adhere to leaf surfaces to provide a protective film. This film is a reservoir that, when in contact with water and low pH, releases Cu ions, which act on the pathogen cells (Lamichhane et al., 2018). In other words, as Cu-based fungicides do not penetrate and translocate well in plants, coverage of the target is achieved through the application of large amounts of the product.
In this scenario, the frequent and extensive use of Cu-based fungicides, coupled with the limited Cu mobility in the soil, results in the accumulation of this metal in the upper soil layers as a consequence of direct application, drift, or dripping from leaf surfaces (Fan et al., 2011; Brunetto et al., 2016; Amlal et al., 2020). The long-term foliar application of Cu-based fungicides can easily increase the concentration of this metal to levels close to 200 mg kg−1, contrasting with Cu concentration in noncontaminated agricultural soils that usually varies from 5 to 30 mg kg−1 (Adrees et al., 2015).
The heavy metals that act as micronutrients (e.g., Cu, iron, manganese, nickel, and zinc), when present in soils in concentrations above the optimum level, compromise plant growth and development due to changes in physicochemical properties of soil. In addition, they trigger adverse effects in various physiological processes of plants (Tiwari & Lata, 2018).
These metals cannot be degraded or destroyed, although their chemical forms can change. Once dispersed in water, soil, and air, they can accumulate in plant tissues (Cheng et al., 2017), posing a severe threat to human health through contamination of the food chain (Nuapia et al., 2018). Despite the environmental problems caused by the continuous use of heavy metal-based protective fungicides, there are additional problems related to synthetic pesticides in general.
The conventional application of synthetic pesticides coupled with a lack of proper rules and regulations causes serious environmental problems, releasing toxic compounds that contaminate the surrounding medium through leaching or rainfall runoff, reaching water bodies and even groundwater (Pradhan & Mailapalli, 2020). Moreover, only a minimal quantity of the applied pesticides (less than 1%) reaches the target species, while the remainder affects nontarget organisms, promoting resistance in weeds, insects, and pathogens, in addition to having an environmental impact (Usman et al., 2020).
In this context, nanotechnology has been studied in agriculture as a tool to increase the effectiveness of different agrochemicals as fertilizers and pesticides, helping to reduce the amount released into the environment (Kumaraswamy et al., 2018). Nanomaterials can be used to synthesize nanofertilizers (nano-sized nutrients, nano-coated fertilizers, or engineered metal-oxide/carbon-based nanomaterials) and nanopesticides (inorganic nanomaterials or nanoencapsulated active ingredients) to provide targeted/controlled release of nutrients and agrochemicals. Thus, they can deliver precisely the recommended dosage for plants, improving the biological efficacy and with less environmental damage (Iavicoli et al., 2017; Bhan et al., 2018).
Some studies have recently combined different nanotechnological approaches with Cu bioactivity, showing promising effects on plants. As examples, we can cite Cu nanoparticles (Cu NPs) (Hafeez et al., 2015), polymeric (chitosan) nanoparticles containing copper ions (Cu2+) (Choudhary et al., 2017a, b), nanocomposites of chitosan/alginate loaded with Cu oxide (Leonardi et al., 2021), Cu3(PO4)2 and CuO nanosheets, and copper oxide nanoparticles (CuO NPs) (Ma et al., 2020) developed as nanofertilizers to improve the efficiency of micronutrient use, aiming to enhance plant growth and development.
However, the association between nanotechnology and Cu bioactivity has been mainly used for the development of nanopesticides against plant pathogens (Giannousi et al., 2013; Kanhed et al., 2014; Saharan et al., 2015; Vanathi et al., 2016; Choudhary et al., 2017b; Sathiyabama & Manikandan, 2018; Pariona et al., 2019; Ma et al., 2020). In addition, this combination has been applied for the control of storage pests (El-Saadony et al., 2020), for antibacterial composite food packaging (Longano et al., 2012), and to extend the shelf-life of stored tomatoes (Solanum lycopersicum L.) (Meena et al., 2020).
Here, we review recent progress in the design and use of Cu-based nanomaterials in agriculture, highlighting their potent actions as an antimicrobial agent in pest management.
2 Nanotechnology: Definition and Applications in Agriculture
Notably, the field that addresses nanotechnology (also known as “nanoscience”) has received significant attention in recent years from scientific research (Arya et al., 2018; Camacho-Flores et al., 2015). As a form of technology and scientific study, nanotechnology addresses the study of materials developed at the nanoscale (Arya et al., 2018; Mohanpuria et al., 2008). Commonly, nanoparticles are classified as particles with a size on the scale of 1–100 nanometers (nm); however, some recent works address these same materials—also known as nanostructured materials—in a size range of 1–1000 nm, taking into account the composition and formation of these types of material, their properties, and applications in relation to their mass macrostructure (Arya et al., 2018; Camacho-Flores et al., 2015; Jeevanandam et al., 2018).
Several different kinds of nanoparticles (metallic, metal oxide, and hybrid nanoparticles) have attracted considerable attention due to their physical, biological, chemical, catalytic, optical, and, in some cases, magnetic characteristics, with promising applications in several fields, including, more recently, agriculture (Burdusel et al., 2018; Jeevanandam et al., 2018; Giannousi et al., 2017). Hybrid nanoparticles represent an example of versatile nanomaterials with superior advantages compared to monofunctional nanoparticles, allowing the design of nanostructures with different combinations in a unique stable nanostructure, which enables improvement in their application, including in agriculture and food storage (Burdusel et al., 2018; Kumar et al., 2018; Tavaf et al., 2017).
The considerable increase in agricultural production in recent years together with growing concern about environmental issues has accompanied innovation in the area of nanotechnology and nanobiotechnology, where science seeks the development and improvement of materials such as metallic nanoparticles, cationic polymers, and antimicrobial agents (Giannousi et al., 2017; Ahamed et al., 2014). Cu-based nanoparticles have been used as a priming agent post-harvest and in food storage, in addition to enabling some aspects of the harvest, such as an increase in productivity and a reduction in the impacts of abiotic and biotic stress factors, including pest control (Kasana et al., 2017; Ahamed et al., 2014).
2.1 Copper Nanoparticles (Cu NPs) and Copper Oxide Nanoparticles (CuO NPs)
Cu NPs particularly are a type of material with a low cost of production (Gawande et al., 2016; Shobha et al., 2014; Evano et al., 2008). Despite the extensive history of applications and large-scale uses of Cu in various fields, one must always consider the instability that Cu0 presents under an ambient atmosphere, causing its oxidation (Gawande et al., 2016; Shobha et al., 2014; Hafeez et al., 2015). In this way, methods are being explored for the development of more stable Cu NPs to avoid or minimize the oxidation of this type of nanomaterial, aiming at the development of structurally more complex Cu-based materials, leading to the formation of “core–shell” nanomaterials (Gawande et al., 2016; Giannousi et al., 2017; Hafeez et al., 2015).
Nanotechnology can provide advantages for the agricultural sector to develop more sustainable activities (Hafeez et al., 2015; Gawande et al., 2016). Crop yield is controlled by different and complex characteristics that can be explained by biotic and abiotic factors linked to the genetic issues of each species (Hafeez et al., 2015). According to some studies, the contamination of soil or water caused by various microorganisms can cause disturbances to agricultural health as well as to human health (Ahamed et al., 2014). As such, Cu NPs or CuO NPs find their places in agriculture as part of mitigating actions in irrigation and management, breeding, protection, fertilization, pest control, and production of numerous crops of wheat (Triticum aestivum L.), cotton (Gossypium hirsutum L.), and lettuce (Lactuca sativa L.), among others (Hafeez et al., 2015; Kasana et al., 2017; Pelegrino et al., 2020; Pereira et al., 2021).
Cu itself is an important micronutrient, playing an essential role in plant nutrition and health. Cu NPs and CuO NPs can promote soil remediation, protection against pathogens, and plant growth (Seabra et al., 2014; Rajput et al., 2017; Pelegrino et al., 2020). Some desirable advantages in the application of these nanomaterials are demonstrated by their potential effects on the decrease in post-harvest plant sensitivity, reducing the potential adverse effects observed during the storage, transport, and exposure of the final product (Managa et al., 2018). In this way, Cu-based nanoparticles can improve not only crop production, but also health and food safety when applied in agriculture as fertilizers, herbicides, and antimicrobial agents (Pelegrino et al., 2020; Wang et al., 2019; Kumar et al., 2015).
2.2 Chemical and Biological Routes to Prepare cu NPs and CuO NPs
There are several routes to synthesize Cu-based nanoparticles (Gawande et al., 2016). Metallic and metal oxide nanoparticles can be prepared using physical, chemical, or biological methods (Pereira et al., 2021). Each synthetic route demonstrates advantages and disadvantages, including parameters to control nanoparticle features, such as particle size, degree of agglomeration, surface charge, and morphology (Gawande et al., 2016; Umer et al., 2012; Mijatovic et al., 2005).
Cu NPs and CuO NPs can be synthesized by chemical routes, such as condensation, chemical reduction, and oxidation (Gawande et al., 2016; Ahamed et al., 2014). Basically, the synthesis of Cu NPs is based on the reduction of Cu2+. Commonly, the chemical routes for obtaining nanoparticles are performed under a controlled experimental setting, leading to nanomaterials with controllable size, aggregation state, stability, and morphology (Gawande et al., 2016). However, in some cases, chemical routes might involve high energy input and the presence of toxic chemicals.
In contrast, biological routes to synthesize nanoparticles are considered a low-cost, clean, nontoxic, and eco-friendly approach (Salvadori et al., 2013; Thakkar et al., 2010). Our group has reported the plant-mediated synthesis of CuO NPs for agricultural approaches (Pelegrino et al., 2020; Kohatsu et al., 2021). Green tea-synthesized CuO NPs were applied on lettuce seedlings, in the range of 0.2 and 300 μg mL−1. As expected, low nanoparticle concentrations (up to 40 μg mL−1) enhanced seed germination, whereas higher concentrations (higher than 40 μg mL−1) inhibited seed germination. Moreover, CuO NPs increased the levels of nitrite and nitric oxide, molecules involved in plant growth and defense (Pelegrino et al., 2020). In a further study, green tea CuO NPs were applied (either by foliar application or soil irrigation) on lettuce under greenhouse conditions. Foliar administration of CuO NPs (20 mg per plant) improved lettuce dry weight, number of leaves, CO2 assimilation, and macronutrient content, enhancing the nutritional value of the lettuce (Kohatsu et al., 2021).
Biogenic synthesis of nanoparticles is based on biological entities that act as reducing agents, leading to the formation of the nanoparticles while promoting their coating, which diminishes nanoparticle oxidation and degradation. Thus, nanoparticles can be biologically synthesized by plants, fungi, some yeasts, and bacteria (Krumov et al., 2009; Rahman et al., 2009; Honary et al., 2012). For instance, Cu NPs were biologically synthesized by various plant extracts, such as gotu kola (Centella asiatica L.), flowers (Aloe vera), latex (Calotropis procera (Aiton) W.T Aiton), brown algae (Bifurcaria bifurcata R. Ross), and coffee (Coffea Arabica L.) powder extract (Shobha et al., 2014). The Cu source employed can be copper nitrate, acetate, or sulfate, leading to Cu NPs with different sizes and antimicrobial activity (Kasana et al., 2017; Shobha et al., 2014; Lee et al., 2008; Mohanpuria et al., 2008). Overall, biological routes are cost-effective and eco-friendly methods to synthesize Cu-based nanoparticles, and these green routes demonstrate advantages over traditional chemical routes (Hafeez et al., 2015; Shobha et al., 2014; Salvadori et al., 2013).
2.3 Copper-Based Nanocomposites in Agriculture
In addition to the use of Cu NPs and CuO NPs in agriculture, other kinds of nanomaterials, such as silver (Ag NPs), selenium (Se NPs), silica (SiO NPs), zinc (Zn NPs), and gold (AuNPs) nanoparticles can be used as fertilizers, increasing seed germination and crop growth, in addition to acting as natural pesticides and antimicrobial agents (Pestovsky & Martínez-Antonio, 2017).
Nowadays, versatile nanomaterials can be prepared by using a combination of different kinds of nanoparticles, and thus the synthesis of hybrid nanoparticles consists of the combination of nanomaterials with specific properties to compose a single nanomaterial (Tung et al., 2016). Core–shell nanoparticles might present advantages over simple nanoparticles, enhancing the nanomaterial biocompatibility, stability, and dispersion in the environment in which they are inserted (Iravani, 2020). Some types of nanoparticles that additionally have a layer of another type of nanomaterial or a non-toxic agent end up not only improving the property of the hybrid nanomaterial but also protecting their core against oxidation, degradation, and incompatibility (Wakaskar, 2018; Iravani, 2020; Pestovsky & Martínez-Antonio, 2017).
In this direction, the antimicrobial actions of Cu NPs covered with silica were reported in tomato plants (Carvalho et al., 2019). In a similar approach, Cu silica gel coated with ZnO NPs was effective in bacterial control in plants, proving to be more effective than commercially available Cu-based bactericides (Iravani, 2020; Carvalho et al., 2019). Likewise, iron nanoparticles and Cu NPs increased the antioxidant activity in wheat seeds, inducing resistance against abiotic stress (Pereira et al., 2021). Although each of these nanoparticles, in isolated form, demonstrates a specific type of antimicrobial activity on crops, turning these nanomaterials into hybrid nanosystems might enhance their advantages for agricultural applications by increasing their antimicrobial activities. Thus, the use of Cu-hybrid NPs in pest control is a promising topic to be further explored.
3 Applications of Cu-Based Nanoparticles as Nanopesticides
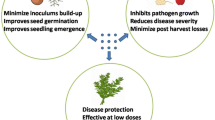
Currently, more than 30% of crop production is lost due to various plant diseases caused by bacteria, fungi, viruses, and insects (Rai et al., 2018). Cu-based compounds have been used since early times for pest control, as they are able to damage biomolecules such as DNA, lipids, and proteins (Borkow & Gabbay, 2005). Among various forms of Cu, copper sulfate (CuSO4), copper oxide (CuO/Cu2O), and copper hydroxide (Cu(OH)2) are the most commonly employed as pesticides, although they present potential risks such as soil damage and environmental hazard (Wilbois et al., 2009). In this field, nanoscaled pesticides demonstrate promising improvement compared to conventional bulk pesticides, promoting better penetration and higher efficiency of Cu (Parisi et al., 2014). Therefore, the evaluation of Cu-based NPs on crops, both as a micronutrient and pesticide, has increased in the last decade. Figure 1 illustrates possible applications of Cu-based nanoparticles in crops, enabling their translocation and action as a micronutrient and/or pesticide.
It should be noted that Cu might positively or negatively affect plants, mainly depending on its concentration. In this direction, the administration of Cu-based nanomaterials in crops might allow sustained and controlled Cu release, avoiding undesired effects. Among different Cu-based nanomaterials, nanostructured Cu(OH)2 has been one of the most studied as a nanopesticide. The increasing number of scientific articles employing nanostructured Cu(OH)2 mainly results from the commercialization of a formulation containing 20-nm needles of Cu(OH)2, Kocide® 3000 (Li et al., 2019). In this sense, Kocide® 3000 has boosted the agricultural market regarding the use of nano-formulations and the research field regarding the evaluation of the benefits and impacts of Kocide® 3000, as well as comparisons with other Cu-based nanoparticles. For example, the beneficial effects of Kocide® 3000 on crops were compared with bulk copper chloride (CuCl2) and CuO and with nanoparticulated CuO and Cu NPs in sugar cane (Saccharum officinarum L.) (Tamez et al., 2020). For nanoparticulated formulations, including Kocide® 3000, significant changes were observed in root Cu levels, while the translocation of Cu in the leaves was consistent with all forms of analyzed copper. Moreover, the accumulation of Cu in sugar juice and alteration in the activity of antioxidant enzymes were also observed in the highest evaluated concentration (60 mg kg−1).
Regarding the application of Cu-based nanomaterials as nanopesticides, the long-term effects of Cu(OH)2 NPs were monitored over one year in both soil microorganisms and plants (Simonin et al., 2018). Even after three sequential applications of Kocide® 3000 (6.68 mg L−1), no negative side effects were observed in plants and in the microbiota. Positive effects were verified in plants treated with the Cu(OH)2 product, evidenced by an increase of 27% in the biomass. In contrast, there were no significant modifications in nontarget soil microbiota, corroborating previous publications (Hong et al., 2015; Zhao et al., 2016; Zhao et al., 2017).
Although presenting promising potential, it has been revealed that Cu(OH)2 treatment using Kocide® 3000 was not efficient for reducing bacterial disease (Qushim et al., 2018). Bacterial spot disease was favored by humid weather in tomato plants, which were treated with various commercial products, including Kocide® 3000. Results indicated that Cu(OH)2 nano-needles present in the formulation did not reduce bacterial spot disease severity (Qushim et al., 2018). Furthermore, in a study with tobacco (Nicotiana tabacum L.) hornworm (Manduca sexta)-infected tomato leaves treated with either Kocide® 3000 or laboratory-synthesized Cu(OH)2 nanowires, it was evidenced that the life-stage of the pest is a key point for the application of Cu(OH)2 nanopesticides, as significant results were observed in the first-instar larvae, but not in the second-instar larvae for both treatments (Li et al., 2019). Interestingly, the growth retardation of tobacco hornworm was higher for Kocide® 3000 than for the laboratory-synthesized Cu(OH)2 nanoparticles. This tendency was associated with the dissolution percentage of Cu ions (five times higher for Kocide® 3000), indicating that the release of the Cu ions is an important aspect for pest control.
Besides Cu(OH)2 nanoparticles, other Cu-based nanoparticulated forms have been used as nanopesticides, such as Cu NPs (Cumplido-Nájera et al., 2019), CuO NPs (Giannousi et al., 2013; Ma et al., 2020; Vanathi et al., 2016), CuS NPs (Shang et al., 2020), Cu-chitosan NPs (Vanti et al., 2020), and Cu-SiO2 NPs (Xu et al., 2020). Cumplido-Nájera et al. (2019) evaluated the combination of Cu NPs and potassium silicate in the control of Clavibacter michiganensis in tomato plants (Cumplido-Nájera et al., 2019). Cu NPs presented spherical morphology, with a size of 42 nm. At both evaluated concentrations (50 and 250 mg L−1), Cu NPs were effective in reducing the plant contamination, inducing the activity of the enzymes superoxide dismutase (SOD), phenylalanine ammonia-lyase (PAL), glutathione peroxidase (GPX), and ascorbate peroxidase (APX). Besides changing levels of key defense compounds in tomato plants, Cu NPs promoted a reduction of 16.1% in yield loss (Cumplido-Nájera et al., 2019).
A similar pattern was observed using Cu NPs against Alternaria solani infesting tomato plants (Quiterio-Gutiérrez et al., 2019). The contamination was significantly reduced by Cu NPs, while the activity of antioxidant enzymes increased in the leaves, and GPX activity also increased in the fruit. Moreover, Cu NPs increased the content of nonenzymatic antioxidant compounds, such as vitamin C, chlorophyll, phenols, and flavonoids.
In vitro studies have also evidenced the potential of Cu NPs as nanopesticides (Banik & Pérez-de-Luque, 2017; El-Saadony et al., 2020). Biosynthesized Cu NPs presented a spherical shape and a diameter ranging from 10 to 70 nm, coated with characteristic biomolecules, such as phenols, amines, and alcohol (El-Saadony et al., 2020). When evaluated against Tribolium castaneum at six different concentrations (from 50 to 300 μg mL−1), it was observed that Cu NPs were able to promote 100% mortality after 5 days. Moreover, better results were obtained for biosynthesized Cu NPs when compared to chemically synthesized Cu NPs, which might be attributed to the characteristic surface coating. A similar pattern was observed for commercial Cu NPs tested against various pathogenic microorganisms, employing concentrations from 100 to 400 mg L−1 (Banik & Pérez-de-Luque, 2017).
CuS NPs are less commonly employed in crops compared to Cu(OH)2 NPs, Cu NPs, or CuO NPs, although CuS NPs have demonstrated promising potential and advantages depending on the targeted application (Shang et al., 2020). CuS NPs demonstrated the highest antimicrobial activity in vitro compared to both control and CuO NPs. In a greenhouse study, rice seedlings (Oryza sativa L.) were infected with Gibberella fujikuroi and treated with CuS NPs, CuO NPs, and Kocide® 3000. Both forms of Cu nanoparticles effectively inhibited the infection, highlighting the highest efficacy of CuS NPs. In contrast, Kocide® 3000 demonstrated no effect against G. fujikuroi infection in rice seedlings. In foliar application, CuS and CuO NPs (50 mg L−1) reduced the infection by 30%, while Kocide® 3000 achieved only 15%.
Cu NPs may also be allied to other molecules and/or nanoparticles. For instance, a nanocomposite based on Cu NPs and chitosan demonstrated 98% inhibition of phytopathogens Rhizoctonia solani and Pythium aphanidermatum, allied with beneficial effects on chilli (Capsicum annuum L.), cowpea (Vigna unguiculata (L.) Walp), and tomato plants (Vanti et al., 2020).
4 Phytotoxic Effects of Cu-Based Nanopesticides
Nanopesticides have been developed as an efficient alternative to reduce the impacts of agricultural practices on the environment and on nontarget organisms, creating better crop protection management. However, the effects of these agrochemicals on plants have not been fully characterized, and more research is essential to distinguish the benefits and risks they confer to the agrosystem (Carley et al., 2020).
Different studies in the literature have discussed the dual effect of nanoparticles on crops, which can exhibit both negative and positive impacts. The effects triggered on the plant are dependent on factors such as plant species, size, structure, shape, concentration, stability, and other chemical properties of nanoparticles (Gabal et al., 2018). The toxicity of metal-based nanoparticles to plants may involve at least three different mechanisms: i) released ions from nanoparticles may be toxic to exposed plants, ii) nanoparticle interactions with environmental media may produce chemical radicals able to generate oxidative stress on plants, and iii) nanoparticles interact directly with plants, leading to toxic effects on metabolism (Chen, 2018). Although engineered nanomaterials can suppress crop diseases by directly acting on pathogens through ROS generation (Adisa et al., 2019), the same mechanism, when excessively induced, causes phytotoxicity, leading to plant oxidative damage (Ahmed et al., 2019).
Considering the diversity of studies over the years on Cu-based nanomaterials applied as nanopesticides, a summary of applications and potential phytotoxic effects on plants is presented in Table 1. Some of these are discussed in more detail in the text below.
The application of Cu-based NPs of different compositions and sizes against Phytophthora infestans was tested in tomato plants (Lycopersicon esculentum var. Belladona) in comparison to the performance of the registered commercially used Cu-based products (Giannousi et al., 2013). Cu2O NP was the most efficient formulation against P. infestans (73.53%) in comparison to all products ten days after application. In general, all Cu-based NPs were found to be effective, while the applied dose of the products was reduced significantly without affecting their efficacy. In addition, phytotoxicity symptoms such as small necrotic spots and some chlorotic spots on the leaves were observed in plants treated with the Cu2O NPs and Cu/Cu2O composite nanoparticles, 3 and 7 days after application, which disappeared 10 days after application. However, no phytotoxicity symptoms were found in fruits and flowers. Cu/Cu2O composite NPs exhibited the highest phytotoxicity (3.75%) compared to the other formulations. This behavior can be attributed to the presence of the metallic core in the NPs, which can be considered more bioreactive than the oxides. Although Cu/Cu2O composite NPs demonstrate excellent efficiency in suppressing the pathogen growth, their application approaches the limit between plant protection and phytotoxicity.
Young and Santra (2014) reported that a composite material of sol–gel silica host matrix loaded with mixed-valence Cu could be an alternative to conventional biocides against Xanthomonas alfalfa strain F1 ATCC 49120. Phytotoxicity studies were performed using Vinca sp. and Hamlin orange (Citrus sinensis (L.) Osb) under greenhouse conditions to observe potential plant tissue damage. Formulations were sprayed at concentrations of 90, 450, and 900 ppm of metallic Cu, and observations were taken at 24, 48, and 72 h after spray application. Except for CuCl2 and Kocide® 3000 (commercial product), all other treatments containing Cu at 900 ppm induced mild phytotoxic symptoms in Vinca sp. 24 h after application. In addition, Vinca sp. exhibited moderate to high levels of plant tissue damage 48 h after application of CuSiNG (water-soluble composite copper (II) loaded silica nanogels) and MV-CuSiNG (composite mixed-valence copper loaded silica nanogel), which remained after 72 h. On the other hand, Hamlin orange exhibited strong tolerance to Cu-induced phytotoxicity even at the highest Cu concentration (900 ppm), regardless of the formulation.
Saharan et al. (2015) synthesized chitosan NPs loaded with Cu ions and evaluated their growth promotion and antifungal efficacy in tomato seedlings (Solanum lycopersicum Mill cv. Navodhya) under laboratory conditions. Seeds treated with Cu–chitosan NPs (0.08% and 0.10%) showed improved seed germination and seedling growth compared to all other treatments. On the other hand, at the highest NP concentration (0.12%), slight decreases in seedling length, vigor index, and biomass were observed compared to 0.08% and 0.10%, but not when compared to the control (water), chitosan (dissolved in 0.1% acetic acid), and CuSO4 0.1% (dissolved in water) treatments. Furthermore, the 0.12% concentration was the most effective treatment in disease control during the experiment.
As can be observed in studies from the last eight years that used Cu-based nanoparticles as nanopesticides, there is a lack of information about the possible phytotoxicity conferred by the application of these nanoformulations. A few studies have performed specific analyses or more careful monitoring to detect possible phytotoxic symptoms. As previously described, some symptoms appear some hours after application and may disappear or intensify during the following days, depending on the plant species, nanoformulation type, and concentration (Li et al., 2020; Ma et al., 2020; Sathiyabama et al., 2020; Cumplido-Nájera et al., 2019; Quiterio-Gutiérrez et al., 2019). In addition to the complete characterization of antifungal activity in vitro and in vivo, careful monitoring of plants (visible symptoms, morphophysiological, and/or metabolic alterations) after nanopesticide application is of utmost importance for better characterization of the effects of Cu-based nanopesticides, highlighting the pros and cons of their use for plant protection.
Because the evaluations of effectiveness and potential uses are directly related to the effects on plant growth, some studies in which Cu-based nanomaterials were applied as nanofertilizers reported relevant information about phytotoxicity.
Lee et al. (2008) evaluated in vitro the growth of beans (Phaseolus radiates L.) and wheat seedlings, as well as the bioaccumulation of Cu NPs applied at concentrations of 0, 200, 400, 600, 800, and 1,000 mg L−1 with an exposure period of 48 h. A decrease in seedling length was observed for both species, reaching the lowest values at the highest concentration (1,000 mg L−1). Beans were more sensitive than wheat to Cu NPs, with the induction of root necrosis. The no-observed-adverse-effect concentrations for wheat root and shoot exposed to Cu NPs were less than 200 and 800 mg L−1, respectively. In addition, bioaccumulation increased with increasing concentrations of Cu NPs. The cupric ions released from Cu nanoparticles had negligible effects in the concentration ranges used in this study, which suggests that the apparent toxicity resulted from Cu NPs.
Hafeez et al. (2015) carried out a study to determine the potential of Cu NPs to enhance the growth and yield of wheat cultivar Millat-2011. Although germination was not affected by Cu NP concentrations up to 0.8 ppm, it decreased significantly with nanoparticle application in concentrations equal to or higher than 1 ppm, using a medium composed of three layers of sterilized filter paper in Petri dishes. Cu NP concentrations higher than 2 ppm were deleterious to wheat plants in solution culture, whereas lower concentrations (0.2, 0.4, 0.6, 0.8, and 1.0 ppm) enhanced seedling growth. When applied to the soil, Cu NPs (10, 20, 30, 40, and 50 ppm) significantly increased the growth and yield of wheat compared with control. The results showed that Cu NPs can enhance the growth and yield of wheat, but their effects are dependent on the concentration and the growth medium.
Zuverza-Mena et al. (2015) evaluated the impact of Cu-based formulations on agronomic and physiological parameters of cilantro (Coriandrum sativum L.) plants. The treatments (Cu(OH)2; Cu NPs; Cu μPs (micro-Cu); CuO NPs; CuO μPs (micro-Cu oxide) or CuCl2) were applied at 20 or 80 mg Cu per kg of commercial substrate. Cu NPs, CuO NPs, CuO μPs, and CuCl2 reduced seed germination at both concentrations, while only CuO μPs decreased shoot growth. All Cu-based treatments impaired nutrient accumulation in shoots, except Fe and Ni. The results showed that, even at a low concentration (20 mg kg−1), the Cu-based nanoparticles or compounds might affect plant nutritional quality.
Yang et al. (2015) evaluated the roles of dissolved metal ions in the CuO NP phytotoxicity against maize (Zea mays L.) and rice. Root elongation was significantly inhibited by CuO NPs in both species in a concentration-dependent manner (25 to 2000 mg L−1), which was not related to Cu2+ release.
The data discussed here show that there is a narrow concentration range between the protective and the phytotoxic effects induced by engineered Cu-based nanomaterials applied to plants as nanofertilizers and/or nanopesticides. Moreover, factors such as nanomaterial concentration, plant species, and exposure route are determinants for the intensity of each effect. Studies need to describe all the conditions involved in the application of nanomaterials and provide as much information as possible about their effects on plants to allow the continuous development of nanostructures aimed at improving agricultural practices.
5 Final Remarks
In recent years, nanotechnology and agriculture have been areas of intensive interest from the scientific, technological, and commercial fields. In general, engineered nanoparticles can be used to promote plant growth and defense against pathogens while increasing crop resistance under biotic stress. Cu is an important micronutrient in plants, participating in several endogenous activities, acting in the metabolism of carbohydrates and proteins as well as being directly involved in the role of chlorophyll synthesis in photosynthesis. However, it is known that the use of Cu at high concentrations can have negative effects on plants.
Cu-based nanoparticles are nanomaterials with potent antimicrobial effects that can be used as pesticides in agriculture. The use of nanomaterials has several advantages over massive (bulk) materials, including higher efficacy and less toxicity. Recently, greener routes to synthesize Cu-based nanoparticles have been widely investigated. These nanoparticles can be prepared using several approaches, their surface can be coated or functionalized with active polymers or other metallic nanoparticles, or they can be incorporated into inorganic or organic materials leading to the formation of hybrid nanoparticles. These strategies can minimize nanoparticle toxicity and maximize their biological effects and biocompatibility. Moreover, Cu-based nanoparticles might have superior effects to commercially used fertilizers, pesticides, and herbicides, which do not contain nanomaterials.
Considering the last few years, several signs of progress have been achieved in using Cu-based nanoparticles as pesticides in agriculture. However, further studies are still required to better understand the phytotoxicity of these nanoparticles. It is essential to highlight that the safe and conscious use of nanomaterials in different crops could minimize ecological impacts, such as pollution and ecotoxicity. Thus, recent efforts have been focused on understanding and improving nanomaterials to mitigate unwanted effects on plants and the environment. The use of Cu-based nanoparticles as active agents in pesticides is a promising and realistic approach in agriculture.
References
Abd-Elsalam, K. A., Vasil’kov, A. Y., Said-Galiev, E. E., Rubina, M. S., Khokhlov, A. R., Naumkin, A. V., Shtykova, E. V., & Alghuthaymi, M. A. (2018). Bimetallic blends and chitosan nanocomposites: Novel antifungal agents against cotton seedling damping-off. European Journal of Plant Pathology, 151, 57–72. https://doi.org/10.1007/s10658-017-1349-8
Adisa, I. O., Pullagurala, V. L. R., Peralta-Videa, J. R., Dimkpa, C. O., Elmer, W. H., Gardea-Torresdey, & White, J. C. (2019). Recent advances in nano-enabled fertilizers and pesticides: A critical review of mechanisms of action. Environmental Science. Nano, 6, 2002–2030. https://doi.org/10.1039/C9EN00265K
Adrees, M., Ali, S., Rizwan, M., Ibrahim, M., Abbas, F., Farid, M., Zia-ur-Rehman, M., Irshad, M. K., & Bharwana, S. A. (2015). The effect of excess copper on growth and physiology of important food crops: A review. Environmental Science and Pollution Research, 22, 8148–8162. https://doi.org/10.1007/s11356-015-4496-5
Ahamed, M., Alhadlaq, H. A., Majeed Khan, M. A., Karuppiah, P., & Al-Dhabi, N. A. (2014). Synthesis, characterization, and antimicrobial activity of copper oxide nanoparticles. Journal of Nanomaterials, 2014, 637858. https://doi.org/10.1155/2014/637858
Ahmed, B., Rizvi, A., Zaidi, A., Khan, M. S., & Musarrat, J. (2019). Understanding the phyto-interaction of heavy metal oxide bulk and nanoparticles: Evaluation of seed germination, growth, bioaccumulation, and metallothionein production. RSC Advances, 9, 4210–4225. https://doi.org/10.1039/C8RA09305A
Al-Dhabaan, F. A., Shoala, T., Ali, A. A. M., Alaa, M., & Abd-Elsalam, K. A. (2017). Chemically-produced copper, zinc nanoparticles and chitosan–bimetallic nanocomposites and their antifungal activity against three phytopathogenic fungi. IJAT, 13, 753–769.
Amlal, F., Drissi, S., Makroum, K., Dhassi, K., Er-rezza, H., & Houssa, A. A. (2020). Influence of soil characteristics and leaching rate on copper migration: Column test. Heliyon, 6, e03375. https://doi.org/10.1016/j.heliyon.2020.e03375
Arya, A., Gupta, K., Chundawat, T. S., & Vaya, D. (2018). Biogenic synthesis of copper and silver nanoparticles using green alga botryococcus braunii and its antimicrobial activity. Bioinorganic Chemistry and Applications, 2018, 7879403. https://doi.org/10.1155/2018/7879403
Banik, S., & Pérez-de-Luque, A. (2017). In vitro effects of copper nanoparticles on plant pathogens, beneficial microbes and crop plants. Spanish Journal of Agricultural Research, 15, e1005. https://doi.org/10.5424/sjar/2017152-10305
Bhan, S., Lalit, M., & Srivastava, C. N. (2018). Nanopesticides: A recent novel ecofriendly approach in insect pest management. Journal of Entomological Research, 42, 263–270. https://doi.org/10.5958/0974-4576.2018.00044.0
Borkow, G., & Gabbay, J. (2005). Copper as a biocidal tool. Current Medicinal Chemistry, 12, 2163–2175. https://doi.org/10.2174/0929867054637617
Bouson, S., Krittayavathananon, A., Phattharasupakun, N., Siwayaprahm, P., & Sawangphruk, M. (2017). Antifungal activity of water-stable copper-containing metal-organic frameworks. Royal Society Open Science, 4, 170654. https://doi.org/10.1098/rsos.170654
Brunel, F., Gueddari, N. E. E., & Moerschbacher, B. M. (2013). Complexation of copper(II) with chitosan nanogels: Toward control of microbial growth. Carbohydrate Polymers, 92, 1348–1356. https://doi.org/10.1016/j.carbpol.2012.10.025
Brunetto, G., Melo, G. W. B., Terzano, R., Buono, D. D., Astolfi, S., Tomasi, N., Pii, Y., Mimmo, T., & Cesco, S. (2016). Copper accumulation in vineyard soils: Rhizosphere processes and agronomic practices to limit its toxicity. Chemosphere, 162, 293–307. https://doi.org/10.1016/j.chemosphere.2016.07.104
Burdusel, A. C., Gherasim, O., Grumezescu, A. M., Mogoantă, L., Ficai, A., & Andronescu, E. (2018). Biomedical applications of silver nanoparticles:Aan up-to-date overview. Nanomaterials, 8, 681. https://doi.org/10.3390/nano8090681
Burkhead, J. L., Gogolin Reynolds, K. A., Abdel-Ghany, S. E., Cohu, C. M., & Pilon, M. (2009). Copper homeostasis. The New Phytologist, 182, 799–816. https://doi.org/10.1111/j.1469-8137.2009.02846.x
Camacho-Flores, B. A., Martínez-Álvarez, O., Arenas-Arrocena, M. C., Garcia-Contreras, R., Argueta-Figueroa, L., de la Fuente-Hernández, J., & Acosta-Torres, L. S. (2015). Copper: Synthesis techniques in nanoscale and powerful application as an antimicrobial agent. Journal of Nanomaterials, 2015, 415238. https://doi.org/10.1155/2015/415238
Carley, L. N., Panchagavi, R., Song, X., Davenport, S., Bergemann, C. M., McCumber, A. W., Gunsch, C. K., & Simonin, M. (2020). Long-term effects of copper nanopesticides on soil and sediment community diversity in two outdoor mesocosm experiments. Environmental Science & Technology, 54, 8878–8889. https://doi.org/10.1021/acs.est.0c00510
Carvalho, R., Duman, K., Jones, J. B., & Paret, M. L. (2019). Bactericidal activity of copper-zinc hybrid nanoparticles on copper-tolerant Xanthomonas perforans. Scientific Reports, 9, 20124. https://doi.org/10.1038/s41598-019-56419-6
Chen, H. (2018). Metal based nanoparticles in agricultural system: Behavior, transport, and interaction with plants. Chemical Speciation & Bioavailability, 30, 123–134. https://doi.org/10.1080/09542299.2018.1520050
Cheng, J., Zhang, X., Tang, Z., Yang, Y., Nie, Z., & Huang, Q. (2017). Concentrations and human health implications of heavy metals in market foods from a Chinese coal-mining city. Environmental Toxicology and Pharmacology, 50, 37–44. https://doi.org/10.1016/j.etap.2017.01.011
Choudhary, R. C., Joshi, A., Kumari, S., Kumaraswamy, R. V., & Saharan, V. (2017a). Preparation of Cu-chitosan nanoparticle and its effect on growth and enzyme activity during seed germination in maize. Journal of Pharmacognosy Phytochemistry, 6, 669–673.
Choudhary, R. C., Kumaraswamy, R. V., Kumari, S., Sharma, S. S., Pal, A., Raliya, R., Biswas, P., & Saharan, V. (2017b). Cu-chitosan nanoparticle boost defense responses and plant growth in maize (Zea mays L.). Scientific Reports, 7, 9754. https://doi.org/10.1038/s41598-017-08571-0
Cumplido-Nájera, C. F., González-Morales, S., Ortega-Ortíz, H., Cadenas-Pliego, G., Benavides-Mendoza, A., & Juárez-Maldonado, A. (2019). The application of copper nanoparticles and potassium silicate stimulate the tolerance to Clavibacter michiganensis in tomato plants. Scientia Horticulturae, 245, 82–89. https://doi.org/10.1016/j.scienta.2018.10.007
El-Saadony, M. T., El-Hack, M. E. A., Taha, A. E., Fouda, M. M. G., Ajarem, J. S., Maodaa, S. N., Allam, A. A., & Elshaer, N. (2020). Ecofriendly synthesis and insecticidal application of copper nanoparticles against the storage pest tribolium castaneum. Nanomaterials, 10, 587. https://doi.org/10.3390/nano10030587
Evano, G., Blanchard, N., & Toumi, M. (2008). Copper-mediated coupling reactions and their applications in natural products and designed biomolecules synthesis. Chemical Reviews, 108, 3054–3131. https://doi.org/10.1021/cr8002505
Fan, J., He, Z., Ma, L. Q., & Stoffella, P. J. (2011). Accumulation and availability of copper in citrus grove soils as affected by fungicide application. Journal of Soils and Sediments, 11, 639–648. https://doi.org/10.1007/s11368-011-0349-0
Gabal, E., Ramadan, M. M., Amal-Asran, A. M. A., & Abd-Elsalam, K. A. (2018). Copper nanostructures applications in plant protection. In K. A. Abd-Elsalam & R. Prasad (Eds.), Nanobiotechnology applications in plant protection: Nanotechnology in the life sciences (Vol. 26, pp. 63–86). Springer.
Gawande, M. B., Goswami, A., Felpin, F. X., Asefa, T., Huang, X., Silva, R., Zou, X., Zboril, R., & Varma, R. S. (2016). Cu and Cu-based nanoparticles: Synthesis and applications in catalysis. Chemical Reviews, 116, 3722–3811. https://doi.org/10.1021/acs.chemrev.5b00482
Giannousi, K., Avramidis, I., & Dendrinou-Samara, C. (2013). Synthesis, characterization and evaluation of copper based nanoparticles as agrochemicals against Phytophthora infestans. RSC Advances, 3, 21743. https://doi.org/10.1039/c3ra42118j
Giannousi, K., Pantazaki, A., & Dendrinou-Samara, C. (2017). Copper-based nanoparticles as antimicrobials. In A. Ficai & A. M. Grumezescu (Eds.), Nanostructures for antimicrobial therapy (1st ed., pp. 515–529). Elsevier. https://doi.org/10.1016/B978-0-323-46152-8.00023-8
Hafeez, A., Razzaq, A., Mahmood, T., & Jhanzab, H. M. (2015). Potential of copper nanoparticles to increase growth and yield of wheat. JNAT, 1, 6–11. https://doi.org/10.24218/jnat.2015.02
Honary, S., Barabadi, H., Gharaeifathabad, E., & Baghibi, F. (2012). Green synthesis of copper oxide nanoparticles using penicillium aurantiogriseum, penicillium citrinum and penicillium waksmanii. Digest Journal of Nanomaterials and Biostructures, 7, 999–1005.
Hong, J., Rico, C. M., Zhao, L., Adeleye, A. S., Keller, A. A., Peralta-Videa, J. R., & Gardea-Torresdey, J. L. (2015). Toxic effects of copper-based nanoparticles or compounds to lettuce (Lactuca sativa) and alfalfa (Medicago sativa). Environmental Science. Processes & Impacts, 17, 177–185. https://doi.org/10.1039/C4EM00551A
Husak, V. (2015). Copper and copper-containing pesticides: Metabolism, toxicity and oxidative stress. JPNU, 2, 38–50. https://doi.org/10.15330/jpnu.2.1.38-50
Iavicoli, I., Leso, V., Beezhold, D. H., & Shvedova, A. A. (2017). Nanotechnology in agriculture: Opportunities, toxicological implications, and occupational risks. Toxicology and Applied Pharmacology, 329, 96–111. https://doi.org/10.1016/j.taap.2017.05.025
Iravani, S. (2020). Core-shell hybrid nanoparticles: Production and application in agriculture and the environment. In K. A. Abd-Elsalam (Ed.), Multifunctional hybrid nanomaterials for sustainable agri-food and ecosystems (1st ed., pp. 21–32). Elsevier. https://doi.org/10.1016/B978-0-12-821354-4.00002-9
Jeevanandam, J., Barhoum, A., Chan, Y. S., Dufresne, A., & Danquah, M. K. (2018). Review on nanoparticles and nanostructured materials: History, sources, toxicity and regulations. Beilstein Journal of Nanotechnology, 9, 1050–1074. https://doi.org/10.3762/bjnano.9.98
Kanhed, P., Birla, S., Gaikwad, S., Gade, A., Seabra, A. B., Rubilar, O., Duran, N., & Rai, M. (2014). In vitro antifungal efficacy of copper nanoparticles against selected crop pathogenic fungi. Materials Letters, 115, 13–17. https://doi.org/10.1016/j.matlet.2013.10.011
Kasana, R. C., Panwar, N. R., Kaul, R. K., & Kumar, P. (2017). Biosynthesis and effects of copper nanoparticles on plants. Environmental Chemistry Letters, 15, 233–240. https://doi.org/10.1007/s10311-017-0615-5
Kaur, P., Duhan, J. S., & Thakur, R. (2018). Comparative pot studies of chitosan and chitosan- metal nanocomposites as nano-agrochemicals against fusarium wilt of chickpea (Cicer arietinum L.). Biocatalysis and Agricultural Biotechnology, 14, 466–471. https://doi.org/10.1016/j.bcab.2018.04.014
Khamis, Y., Hashim, A. F., Margarita, R., Alghuthaymi, M. A., & Abd-Elsalam, K. A. (2017). Fungicidal efficacy of chemically-produced copper nanoparticles against penicillium digitatum and fusarium solani on citrus fruit. Philippine Agricultural Scientist, 100, 69–78.
Kohatsu, M. Y., Pelegrino, M. T., Monteiro, L. R., Freire, B. M., Pereira, R. M., Fincheira, P., Rubilar, O., Tortella, G., Batista, B. L., de Jesus, T. A., Seabra, A. B., & Lange, C. N. (2021). Comparison of foliar spray and soil irrigation of biogenic CuO nanoparticles (NPs) on elemental uptake and accumulation in lettuce. Environmental Science and Pollution Research International. https://doi.org/10.1007/s11356-020-12169-x
Krumov, N., Perner-Nochta, I., Oder, S., Gotcheva, V., Angelov, A., & Posten, C. (2009). Production of inorganic nanoparticles by microorganisms. Chemical Engineering and Technology, 32, 1026–1035. https://doi.org/10.1002/ceat.200900046
Kumar, P. P. N. V., Shameem, U., Kollu, P., Kalyani, R. L., & Pammi, S. V. N. (2015). Green synthesis of copper oxide nanoparticles using aloe vera leaf extract and its antibacterial activity against fish bacterial pathogens. BioNanoScience, 5, 135–139. https://doi.org/10.1007/s12668-015-0171-z
Kumar, S., Shuklaa, A., Baul, P. P., Mitrab, A., & Halder, D. (2018). Biodegradable hybrid nanocomposites of chitosan/gelatin and silver nanoparticles for active food packaging applications. Food Packaging and Shelf Life, 16, 178–184. https://doi.org/10.1016/j.fpsl.2018.03.008
Kumaraswamy, R. V., Kumari, S., Choudhary, R. C., Pal, A., Raliya, R., Biswas, P., & Saharan, V. (2018). Engineered chitosan-based nanomaterials: Bioactivities, mechanisms and perspectives in plant protection and growth. International Journal of Biological Macromolecules, 113, 494–506. https://doi.org/10.1016/j.ijbiomac.2018.02.130
Lamichhane, J. R., Osdaghi, E., Behlau, F., Köhl, J., Jones, J. B., & Aubertot, J. N. (2018). Thirteen decades of antimicrobial copper compounds applied in agriculture. A review. Agronomy for Sustainable Development, 38, 28. https://doi.org/10.1007/s13593-018-0503-9
Lee, W. M., An, Y. J., Yoon, H., & Kweon, H. S. (2008). Toxicity and bioavailability of copper nanoparticles to the terrestrial plants mung bean (Phaseolus radiatus) and wheat (Triticum aestivum): Plant agar test for water-insoluble nanoparticles. Environmental Toxicology and Chemistry, 27, 1915–1921. https://doi.org/10.1897/07-481.1
Leonardi, M., Caruso, G. M., Carroccio, S. C., Boninelli, S., Curcuruto, G., Zimbone, M., Allegra, M., Torrisi, B., Ferlito, F., & Miritello, M. (2021). Smart nanocomposites of chitosan/alginate nanoparticles loaded with copper oxide as alternative nanofertilizers. Environmental Science. Nano, 8, 174. https://doi.org/10.1039/d0en00797h
Li, J., Rodrigues, S., Tsyusko, O. V., & Unrine, J. M. (2019). Comparing plant–insect trophic transfer of cu from lab-synthesised nano-cu(OH)2 with a commercial nano-cu(OH)2 fungicide formulation. Environment and Chemistry, 16, 411–418. https://doi.org/10.1071/EN19011
Li, Y., Liu, Y., Yang, D., Jin, Q., Wu, C., & Cui, J. (2020). Multifunctional molybdenum disulfide-copper nanocomposites that enhance the antibacterial activity, promotes rice growth and induces rice resistance. Journal of Hazardous Materials, 394, 122551. https://doi.org/10.1016/j.jhazmat.2020.122551
Li, Y., Yang, D., & Cui, J. (2017). Graphene oxide loaded with copper oxide nanoparticles as an antibacterial agent against Pseudomonas syringae pv. tomato. RSC Advances, 7, 38853. https://doi.org/10.1039/c7ra05520j
Longano, D., Ditaranto, N., Cioffi, N., Di Niso, F., Sibillano, T., Ancona, A., Conte, A., Del Nobile, M. A., Sabbatini, L., & Torsi, L. (2012). Analytical characterization of laser-generated copper nanoparticles for antibacterial composite food packaging. Analytical and Bioanalytical Chemistry, 403, 1179–1186. https://doi.org/10.1007/s00216-011-5689-5
Ma, C., Borgatta, J., Hudson, B. G., Tamijani, A. A., Torre-Roche, R. D. L., Zuverza-Mena, N., Shen, Y., Elmer, W., Xing, B., Mason, S. E., Hamers, R. J., & White, J. C. (2020). Advanced material modulation of nutritional and phytohormone status alleviates damage from soybean sudden death syndrome. Nature Nanotechnology, 15, 1033–1042. https://doi.org/10.1038/s41565-020-00776-1
Malandrakis, A. A., Kavroulakis, N., & Chrysikopoulos, C. V. (2019). Use of copper, silver and zinc nanoparticles against foliar and soil-borne plant pathogens. Science of the Total Environment, 670, 292–299. https://doi.org/10.1016/j.scitotenv.2019.03.210
Managa, M. G., Tinyani, P. P., Senyolo, G. M., Soundy, P., Sultanbawa, Y., & Sivakumar, D. (2018). Impact of transportation, storage, and retail shelf conditions on lettuce quality and phytonutrients losses in the supply chain. Food Science & Nutrition, 6, 1527–1536. https://doi.org/10.1002/fsn3.685
Meena, M., Pilania, S., Pal, A., Mandhania, S., Bhushan, B., Kumar, S., Gohari, G., & Saharan, V. (2020). Cu-chitosan nano-net improves keeping quality of tomato by modulating physio-biochemical responses. Scientific Reports, 10, 21914. https://doi.org/10.1038/s41598-020-78924-9
Mijatovic, D., Eijkel, J. C. T., & Den Berg, V. (2005). A technologies for nanofluidic systems: Top-down vs bottom-up-a review. Lab on a Chip, 5, 492–500. https://doi.org/10.1039/b416951d
Mohamed, E. A., Gaber, M. H., & Elsharabasy, S. F. (2018). Evaluating the in vivo efficacy of copperchitosan nanocomposition for treating vascular wilt disease in date palm. International Journal Environment, Agriculture and Biotechnology, 3, 447–454. https://doi.org/10.22161/ijeab/3.2.17
Mohanpuria, P., Rana, N. K., & Yadav, S. K. (2008). Biosynthesis of nanoparticles: Technological concepts and future applications. Journal of Nanoparticle Research, 10, 507–517. https://doi.org/10.1007/s11051-007-9275-x
Mondal, K. K., & Mani, C. (2012). Investigation of the antibacterial properties of nanocopper against Xanthomonas axonopodis pv. punicae, the incitant of pomegranate bacterial blight. Annales de Microbiologie, 62, 889–893. https://doi.org/10.1007/s13213-011-0382-7
Nuapia, Y., Chimuka, L., & Cukrowska, E. (2018). Assessment of heavy metals in raw food samples from open markets in two African cities. Chemosphere, 196, 339–346. https://doi.org/10.1016/j.chemosphere.2017.12.134
Pariona, N., Mtz-Enriquez, A. I., Sánchez-Rangel, D., Carrión, G., Paraguay-Delgado, & Rosas-Saito, G. (2019). Green-synthesized copper nanoparticles as a potential antifungal against plant pathogens. RSC Advances, 9, 18835. https://doi.org/10.1039/c9ra03110c
Parisi, C., Vigani, M. and Rodriguez-Cerezo, E. (2014). In proceeding of a workshop on nanotechnology for the agriculture sector: from research to field, JRC Scientific and Policy reports, European Commission, 1.
Pelegrino, M. T., Kohatsu, M. Y., Seabra, A. B., Monteiro, L. R., Gomes, D. G., Oliveira, H. C., Rolim, W. R., de Jesus, T. A., Batista, B. L., & Lange, C. N. (2020). Effects of copper oxide nanoparticles on growth of lettuce (Lactuca sativa L.) seedlings and possible implications of nitric oxide in their antioxidative defense. Environmental Monitoring and Assessment, 192, 232. https://doi.org/10.1007/s10661-020-8188-3
Pereira, A. E. S., Oliveira, H. C., Fraceto, L. F., & Santaella, C. (2021). Nanotechnology potential in seed priming for sustainable agriculture. Nanomaterials, 11, 267. https://doi.org/10.3390/nano1102026
Pestovsky, Y., & Martínez-Antonio, A. (2017). The use of nanoparticles and nanoformulations in agriculture. Journal of Nanoscience and Nanotechnology, 17, 8699–8730. https://doi.org/10.1166/jnn.2017.15041
Ponmurugan, P., Manjukarunambika, K., Elango, V., & Gnanamangai, B. M. (2016). Antifungal activity of biosynthesised copper nanoparticles evaluated against red root-rot disease in tea plants. Journal of Experimental Nanoscience, 11, 1019–1031. https://doi.org/10.1080/17458080.2016.1184766
Pradhan, S., & Mailapalli, D. R. (2020). Nanopesticides for pest control. In E. Lichtfouse (Ed.), Sustainable agriculture reviews 40 (Vol. 40, pp. 43–74). Springer.
Quiterio-Gutiérrez, T., Ortega-Ortiz, H., Cadenas-Pliego, G., Hernández-Fuentes, A. D., Sandoval-Rangel, A., Benavides-Mendoza, A., Cabrera-de la Fuente, M., & Juárez-Maldonado, A. (2019). The application of selenium and copper nanoparticles modifies the biochemical responses of tomato plants under stress by alternaria solani. International Journal of Molecular Sciences, 20, 1950. https://doi.org/10.3390/ijms20081950
Qushim, B., Wu, F., Guan, Z. and Vallad, G. (2018). Optimal decisions under risk in pest management: The case of tomato disease management. Annual Meeting, August 5–7, Washington, D.C. 274362, Agricultural and Applied Economics Association.
Rahman, A., Ismail, A., Jumbianti, D., Magdalena, S., & Sudrajat, H. (2009). Synthesis of copper oxide nano particles by using Phormidium cyanobacterium. Indonesian Journal of Chemistry, 9, 355–360. https://doi.org/10.22146/ijc.21498
Rai, M., Ingle, A. P., Pandit, R., Paralikar, P., Shende, S., Gupta, I., Biswas, J. K., & da Silva, S. S. (2018). Copper and copper nanoparticles: Role in management of insect-pests and pathogenic microbes. Nanotechnology Reviews, 7, 303–315. https://doi.org/10.1515/ntrev-2018-0031
Rajput, V. D., Minkina, T., Suskova, S., Mandzhieva, S., Tsitsuashvili, V., Chapligin, V., & Fedorenko, A. (2017). Effects of copper nanoparticles (cuo nps) on crop plants: A mini review. BioNanoScience, 8, 36–42. https://doi.org/10.1007/s12668-017-0466-3
Rubina, M. S., Vasil’kov, A. Y., Naumkin, A. V., Shtykova, E. V., Abramchuk, S. S., Alghuthaymi, M. A., & Abd-Elsalam, K. A. (2017). Synthesis and characterization of chitosan–copper nanocomposites and their fungicidal activity against two sclerotia-forming plant pathogenic fungi. Journal of Nanostructures Chemistry, 7, 249–258. https://doi.org/10.1007/s40097-017-0235-4
Safaei, M., Taran, M., & Imani, M. M. (2019). Preparation, structural characterization, thermal properties and antifungal activity of alginate-CuO bionanocomposite. Materials Science and Engineering: C, 101, 323–329. https://doi.org/10.1016/j.msec.2019.03.108
Saharan, V., Mehrotra, A., Khatik, R., Rawal, P., Sharma, S. S., & Pal, A. (2013). Synthesis of chitosan based nanoparticles and their in vitro evaluation against phytopathogenic fungi. International Journal of Biological Macromolecules, 62, 677–683. https://doi.org/10.1016/j.ijbiomac.2013.10.012
Saharan, V., Sharma, G., Yadav, M., Choudhary, M. K., Sharma, S. S., Pal, A., Raliya, R., & Biswas, P. (2015). Synthesis and in vitro antifungal efficacy of Cu–chitosan nanoparticles against pathogenic fungi of tomato. Macromolecules, 75, 346–353. https://doi.org/10.1016/j.ijbiomac.2015.01.027
Salvadori, M. R., Lepre, L. F., Ando, R. A., do Nascimentos, C. A. O., & Correa, B. (2013). Biosynthesis and uptake of copper nanoparticles by dead biomass of Hypocrea lixii isolated from the metal mine in the brazilian amazon region. PLoS One, 8, e80519. https://doi.org/10.1371/journal.pone.0080519
Sathiyabama, M., & Manikandan, A. (2018). Application of copper-chitosan nanoparticles stimulate growth and induce resistance in finger millet (eleusine coracana gaertn.) plants against blast disease. Journal of Agricultural and Food Chemistry, 66, 1784–1790. https://doi.org/10.1021/acs.jafc.7b05921
Sathiyabama, M., Indhumathi, M., & Amutha, T. (2020). Preparation and characterization of curcumin functionalized copper nanoparticles and their application enhances disease resistance in chickpea against wilt pathogen. Biocatalysis and Agricultural Biotechnology, 29, 101823. https://doi.org/10.1016/j.bcab.2020.101823
Seabra, A. B., Rai, M., & Durán, N. (2014). Nano carriers for nitric oxide delivery and its potential applications in plant physiological process: A mini review. Journal of Plant Biochemistry and Biotechnology, 23, 1–10. https://doi.org/10.1007/s13562-013-0204-z
Shang, H., Ma, C., Li, C., White, J., Polubesova, T., Chefetz, B., & Xing, B. (2020). Copper sulfide nanoparticles suppress Gibberella fujikuroi infection in rice (Oryza sativa L.) by multiple mechanisms: Contact-mortality, nutritional modulation and phytohormone regulation. Environmental Science. Nano, 7, 2632–2643. https://doi.org/10.1039/D0EN00535E
Shobha, G., Vinutha, M., & Ananda, S. (2014). Biological synthesis of copper nanoparticles and its impact: A review. International Journal of Pharmaceutical Science Invention, 3, 28–38.
Simonin, M., Colman, B. P., Tang, W., Judy, J. D., Anderson, S. M., Bergemann, C. M., Rocca, J. D., Unrine, J. M., Cassar, N., & Bernhardt, E. S. (2018). Plant and microbial responses to repeated Cu(OH)2 nanopesticide exposures under different fertilization levels in an agro-ecosystem. Frontiers in Microbiology, 9, 1769. https://doi.org/10.3389/fmicb.2018.01769
Tamez, C., Molina-Hernandez, M., Medina-Velo, I. A., Cota-Ruiz, K., Hernandez-Viezcas, J. A., & Gardea-Torresdey, J. (2020). Long-term assessment of nano and bulk copper compound exposure in sugarcane (Saccharum officinarum). Science of the Total Environment, 718, 137318. https://doi.org/10.1016/j.scitotenv.2020.137318
Tavaf, Z., Tabatabaei, M., Khalafi-Nezhad, A., & Panahi, F. (2017). Evaluation of antibacterial, antibofilm and antioxidant activities of synthesized silver nanoparticles (AgNPs) and casein peptide fragments against Streptococcus mutans. European Journal of Integrative Medicine, 12, 163–171. https://doi.org/10.1016/j.eujim.2017.05.011
Thakkar, K. N., Mhatre, S. S., & Parikh, R. Y. (2010). Biological synthesis of metallic nanoparticles. Nanomedicine, 6, 257–262. https://doi.org/10.1016/j.nano.2009.07.002
Tiwari, S., & Lata, C. (2018). Heavy metal stress, signaling, and tolerance due to plant-associated microbes: An overview. Frontiers in Plant Science, 9, 452. https://doi.org/10.3389/fpls.2018.00452
Tung, L. M., Cong, N. X., Huy, L. T., Lan, N. T., Phan, V. N., Hoa, N. Q., Vinh, L. K., Thinh, N. V., Tai, L. T., Ngo, D. T., Mølhave, K., Huy, T. Q., & Le, A. T. (2016). Synthesis, characterizations of superparamagnetic fe3o4–ag hybrid nanoparticles and their application for highly effective bacteria inactivation. Journal of Nanoscience and Nanotechnology, 16, 5902–5912. https://doi.org/10.1166/jnn.2016.11029
Umer, A., Naveed, S., Ramzan, N., & Rafique, M. S. (2012). Selection of a suitable method for the synthesis of copper nanoparticles. Nano, 07, 1230005. https://doi.org/10.1142/s1793292012300058
Usman, M., Farooq, M., Wakeel, A., Nawaz, A., Cheema, S. A., Rehman, H. U., Ashraf, I., & Sanaullah, M. (2020). Nanotechnology in agriculture: Current status, challenges and future opportunities. Science of the Total Environment, 721, 137778. https://doi.org/10.1016/j.scitotenv.2020.137778
Vanathi, P., Rajiv, P., & Sivaraj, R. (2016). Synthesis and characterization of Eichhornia-mediated copper oxide nanoparticles and assessing their antifungal activity against plant pathogens. Bulletin of Materials Science, 39, 1165–1170. https://doi.org/10.1007/s12034-016-1276-x
Vanti, G. L., Masaphy, S., Kurjogi, M., Chakrasali, S., & Nargund, V. B. (2020). Synthesis and application of chitosan-copper nanoparticles on damping off causing plant pathogenic fungi. International Journal of Biological Macromolecules, 156, 1387–1395. https://doi.org/10.1016/j.ijbiomac.2019.11.179
Viet, P. V., Nguyen, H. T., Cao, T. M., & Hieu, L. V. (2016). Fusarium antifungal activities of copper nanoparticles synthesized by a chemical reduction method. Journal of Nanomaterials, 2016, 1957612. https://doi.org/10.1155/2016/1957612
Vitanovic, E. (2012). Use of Cu fungicides in vineyards and olive groves. In D. Dhanasekaran, N. Thajuddin, & P. S. Annamalai (Eds.), Fungicides for plant and animal diseases (1st ed., pp. 279–298). IntechOpen.
Wakaskar, R. R. (2018). General overview of lipid–polymer hybrid nanoparticles, dendrimers, micelles, liposomes, spongosomes and cubosomes. Journal of Drug Targeting, 26, 311–318. https://doi.org/10.1080/1061186X.2017.1367006
Wang, Y., Lin, Y., Xu, Y., Yin, Y., Guo, H., & Du, W. (2019). Divergence in response of lettuce (var. ramosa Hort.) to copper oxide nanoparticles/microparticles as potential agricultural fertilizer. Environmental Pollution Bioavailability, 31, 80–84. https://doi.org/10.1080/26395940.2019.1578187
Wilbois, K. P., Kauer, R., Fader, B., Kienzle, J., Haug, P., Fritzsche-Martin, A., Drescher, N., Reiners, E., & Röhrig, F. (2009). Copper as a plant protection product with special regards to organic farming. Journal of Kulturpflanzen, 61, 140–152. https://doi.org/10.12924/of2017.03010066
Xu, C., Shan, Y., Bilal, M., Xu, B., Cao, L., & Huang, Q. (2020). Copper ion chelated mesoporous silica nanoparticles via dopamine chemistry for controlled pesticide release regulated by coordination bonding. Chemical Engineering Journal, 395, 125093. https://doi.org/10.1016/j.cej.2020.125093
Yang, Z., Chen, J., Dou, R., Gao, X., Mao, C., & Wang, L. (2015). Assessment of the phytotoxicity of metal oxide nanoparticles on two crop plants, maize (Zea mays L.) and Rice (Oryza sativa L.). International Journal of Environmental Research and Public Health, 12, 15100–15109. https://doi.org/10.3390/ijerph121214963
Young, M. I., & Santra. (2014). Copper (Cu)-silica nanocomposite containing valence-engineered Cu: A new strategy for improving antimicrobial efficacy of Cu biocides. Journal of Agricultural and Food Chemistry, 62, 6043–6052. https://doi.org/10.1021/jf502350w
Yruela, I. (2013). Transition metals in plant photosynthesis. Metallomics, 5, 1090–1109. https://doi.org/10.1039/C3MT00086A
Zhao, L., Hu, Q., Huang, Y., & Keller, A. A. (2017). Response at genetic, metabolic, and physiological levels of maize (Zea mays) exposed to a Cu(OH)2 nanopesticide. ACS Sustainable Chemistry & Engineering, 5, 8294–8301. https://doi.org/10.1021/acssuschemeng.7b01968
Zhao, L., Huang, Y., Hannah-Bick, C., Fulton, A. N., & Keller, A. A. (2016). Application of metabolomics to assess the impact of cu (OH)2 nanopesticide on the nutritional value of lettuce (Lactuca sativa): Enhanced cu intake and reduced antioxidants. NanoImpact, 3, 58–66. https://doi.org/10.1016/j.impact.2016.08.005
Zuverza-Mena, N., Medina-Velo, I. A., Barrios, A. C., Tan, W., Peralta-Videa, J. R., & Gardea-Torresdey, J. L. (2015). Copper nanoparticles/compounds impact agronomic and physiological parameters in cilantro (Coriandrum sativum). Environmental Science. Processes & Impacts, 17, 1783–1793. https://doi.org/10.1039/c5em00329f
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Gomes, D.G., Pieretti, J.C., Lourenço, I.M., Oliveira, H.C., Seabra, A.B. (2022). Copper-Based Nanoparticles for Pesticide Effects. In: Fernandes Fraceto, L., Pereira de Carvalho, H.W., de Lima, R., Ghoshal, S., Santaella, C. (eds) Inorganic Nanopesticides and Nanofertilizers. Springer, Cham. https://doi.org/10.1007/978-3-030-94155-0_6
Download citation
DOI: https://doi.org/10.1007/978-3-030-94155-0_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-94154-3
Online ISBN: 978-3-030-94155-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)