Abstract
Due to the physicochemical characteristics derived from having at least one dimension <100 nm, nanomaterials are very reactive from a biological perspective. Concentration, surface free energy, charges, roughness, porosity, and functional groups of the coating or corona, among other properties, determine the nanomaterial’s impact on organisms. The impact is variable, from biostimulation to toxicity, depending on the plant species and the route of application or entry of the nanomaterial into the plant. This chapter presents an overview of knowledge about the physiology and molecular biology of plants in response to synthetic nanomaterials. It begins with an introduction that indicates the framework and objectives and then continues by briefly presenting the pathways of entry of nanomaterials to ecosystems due to contamination or intentional application. Subsequently, the nanomaterial’s interactions in the plant interfaces (root, leaves, stems, fruits in the epidermis, stomata, etc.) are reviewed. Next, the entry mechanisms to the apoplast and the cytoplasm, as well as cell compartmentalization and transport, are discussed. In each of the previous sections, the plant’s physiological and molecular responses are described.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Plant nanotechnology
- Plant biostimulants
- Nanobiostimulants
- Plant nanotoxicology
- Nanomaterial metabolism
- Plant stress
- Biostimulation and stress
1 Introduction
Food production represents a significant source of environmental impact. The growing human population, the greater life expectancy, and, in general, the higher standard of living of the population translates each year into growing needs for products obtained from agriculture, livestock, and forestry. The population projection of 9.6–12.3 × 109 people for the year 2100 (Gerland et al., 2014), in the complicated context of climate change that is expected to modify the distribution of precipitation, atmospheric humidity, and temperatures, represents a monumental multifaceted challenge (Mukherjee et al., 2018; Arora et al., 2018; Tong & Ebi, 2019). Until now, the growing need for food, fiber, and metabolites such as pigments and biofuels has been solved with the tools generated during the Green Revolution, which prevented potential famine in the second half of the twentieth century (Evans & Lawson, 2020). These tools, such as improved varieties, fertilizers, pesticides, and intense mechanization, seem to be reaching a limit of efficiency, in the sense that their extensive application for a larger world population represents an environmental impact that reaches unacceptable levels of contamination and degradation of soil, water, and natural ecosystems (Arora et al., 2018). The solution to the above issues requires social, cultural, ecological, economic, and technical considerations that must be applied integrally since none can function effectively on its own.
Solving the above challenge, in addition to the forced adjustments in the lifestyle and diet of the human population (Hurni et al., 2015), requires urgent advances and the application of techniques that, on the one hand, increase the efficiency in the use of inputs and energy used in agricultural, livestock, and forestry activities and that, on the other hand, substantially reduce the ecological impact of said activities (Evans & Lawson, 2020). An example of the above would be those techniques that increase agricultural or forestry productivity without changing the use of a larger surface area of land or applying fewer amounts of water, fertilizers, or pesticides.
Among the set of techniques that can be applied to improve the efficiency of inputs and energy use in agricultural, livestock, and forestry activities are those related to nanotechnology. Nanotechnology is the set of techniques to hold the advantage of the physicochemical characteristics of materials, called nanomaterials (NMs), which arise when they have dimensions in the range of 0.4–100 nm. This 0.4–100-nm range is simply an arbitrary formal agreement to delineate boundaries between materials since the characteristic properties of NMs can be observed as a continuum in dimensions (d) between NMs and micrometric materials 1 nm < d < 1000 nm (Miernicki et al., 2019).
The NMs useful for food production are multiple and varied; they are derived from metals and their oxides, from semimetals such as nanosilicon and nanoselenium, from inorganic materials such as nanoclays, from carbon materials such as graphene, and organic compounds such as nanochitosan, among others. The applications refer to their use as nanofertilizers, nanocarriers of fertilizers, nanopesticides, nanobiostimulants, nanocarriers of pesticides, regulators and other biomolecules, and nanosensors (Vázquez-Núñez et al., 2018; He et al., 2019; Medina-Pérez et al., 2019).
This chapter refers to the use of NMs as biostimulant compounds. The ability of NMs to act as biostimulants is related to several physicochemical properties of the NM. Still, it depends significantly on the NM’s concentration in the medium where the cells are found. The response to concentration is adjusted to a biphasic or hormesis response (Agathokleous et al., 2019).
The biostimulant capacity of NMs results from a large amount of surface free energy (and a consequential reactivity) as an outcome of their high surface:volume ratio (Juárez-Maldonado et al., 2019). But it is also the result of other properties such as shape, aspect ratio, hydrophobicity and hydrophilicity, and the composition of the core of the material itself and the composition of the corona (Nel et al., 2009; Chowdhury et al., 2020).
Biostimulation of plant cells is thought to be the result of a two-phase process. The first phase occurs through interfacial interactions between NMs and their corona with cell walls and membranes. These interactions depend on the surface free energy, the interactions between surface charges, and the hydrophobic and hydrophilic interactions between the surfaces. The second phase results from the chemical properties of the corona and the NM core and occurs both in the apoplast and inside the cell when the functional groups of the corona or the core of NM, or the ions released from the NM’s core induce modifications in the behavior or functionality in the integral proteins of the cell wall and membrane, or the internal membrane systems or the organelles (Juárez-Maldonado et al., 2021).
2 Nanomaterials in Ecosystems
In natural systems, the existence of NMs is a common reality. NMs represent a form of matter in a certain dimensional range defined arbitrarily from 0.4 to 100 nm, which presents characteristic properties that differ from those observed in other dimensional ranges smaller or larger than that spectrum of magnitudes. The occurrence of nano-dimensional structures in abiotic and biotic systems has been well documented; examples are viruses, ferritins, exosomes, and magnetosomes (Stanley, 2014). In the same way, many natural phenomena such as volcanism, fires, weathering, and various mechanical and chemical interactions can transform materials of lower-dimensional magnitudes (such as ions) or larger (such as micrometric materials) into nanostructures (Akaighe et al., 2011; Tepe & Bau, 2014; Hochella et al., 2019). Therefore, the presence of NMs in nature is not a novelty. In fact, they are considered dynamic and important actors at various scales (from atomic to planetary) of terrestrial evolution. However, in addition to the complex series of transformations related to NMs in nature (aggregation, corona formation, chemical alteration, biological assimilation, dissolution, evaporation, shape change, migration between ecosystem’s phases), human activities have significantly modified the presence of NMs both in the amount that is released year after year in nature and in the diversity of NMs that reach ecosystems (Hochella et al., 2019).
In recent decades, the scale of manufacturing NMs with industrial applications has grown substantially. To note some examples, although precise data are not available, it is estimated that each year about 5500 tons of SiO2, 3000 tons of TiO2, 550 tons of ZnO, 300 tons of carbon nanotubes, and 55 tons of NMs of Ag, FeOx, AlOx, and CeOx are produced (Piccinno et al., 2012). Other sources indicate the production of 55 to 1,500,000 tons per year of SiO2 NMs, 5.5 to 100,000 tons per year of CeO2, and 5.5 to 550 tons per year of Ag NMs (Giese et al., 2018). These NMs, used in the biomedical, chemical, manufacturing, and food industries, among others, can be released into the atmosphere, water, or soil through emissions from industries; another alternative is through garbage or by-products that reach the soil or water directly, or are recycled, incinerated or used in biosolids for use in landfills or soil amending material once the useful life of the product containing the NMs ends (Lead et al., 2018). Until now, there is no precise information about the volume of NMs discharged to the atmosphere, water and soils, and sediments. Based on the results of their mathematical model (Giese et al., 2018), the discharge of about 17 tons per year of Ag NMs, 1090 tons per year of CeO2, and 58,000 tons per year SiO2 is estimated. TiO2, ZnO, and Ag are probably the NMs most likely to enter soils in large quantities because of the application of biosolids (Lead et al., 2018).
Another type of NMs, those used in agricultural and livestock activities, can be incorporated as pollutants into ecosystems due to the degradation or disuse of the material that contains them or when used in the treatment of water or recovery of contaminated soils. This type of unintended contamination is analogous to that which occurs with the NMs for industrial use described above. One example of this type of contamination is that which occurs when agroplastics are degraded by abiotic weathering or by the activity of the soil or water microbiome, generating micro and nanoplastics that move between the different components of ecosystems, including through the trophic chain (Fig. 1) (Guo et al., 2020).
Mobilization of NMs between different compartments of ecosystems from industrial, agricultural, livestock, and natural emissions. Figure from Morales-Díaz et al. (2017)
Another way NMs designed for agriculture or livestock can be incorporated into ecosystems or agroecosystems is by mobilization after they are intentionally used as nanofertilizers or nanopesticides applied to soils, substrates, irrigation water, and plants (González-Morales et al., 2020). Other NMs with potential agricultural and livestock use, such as nanosensors, molecular vehicles for the transport of DNA or RNA and other biomolecules, and materials with nanobionic application to increase the metabolic capacities of plants, do not seem to be an important source of contamination taking into account that its use involves very localized applications and in minimal quantities (Omar et al., 2019).
Whether the incorporation of NMs is intentional or not, the result is the contact and interaction of NMs with biotic and abiotic components of ecosystems, including natural toxins and synthetic pollutants such as pesticides and hydrocarbons with which they can interact synergistically. The interaction of NMs with the various media in which they can move (water, soil, living organisms) causes changes in the composition and identity of NM’s core and the NM’s corona (Uddin et al., 2020). These NMs’ corona changes have an unpredictable impact on their stability, mobilization capacity, and bioavailability. Depending on the environmental context and the type of NM in question, exposure to the environment may increase the NM’s potential toxicity or decrease it (Nasser et al., 2020). Examples of the interaction of NMs with soil colloids and with dissolved organic matter illustrate this last point (Fig. 2) (González-Morales et al., 2020).
Scheme of the modifications and interactions of NMs with different abiotic and biotic environmental components, with the consequent increase or decrease in toxicity. Figure from González-Morales et al. (2020)
In addition to the corona changes, another situation that makes the prediction of the trajectory and environmental impact of NMs complicated is the interaction with environmental toxins or synthetic pollutants. The interactions between NMs and pollutants seem to be mainly physicochemical, with the adsorption process predominating, modifying both the original properties of NM and the pollutant molecule. Among the most studied types of interaction are those referring to heavy metals and metalloids such as Pb, Cd, Cu, and As, as well as organic molecules such as diuron, pyrene, atrazine, and polychlorinated biphenyls, among others, finding cases of toxicity increased or decreased by synergy, antagonism, or additive behavior (Liu et al., 2018).
One process that has received much attention is the trophic transfer of NMs. Trophic transfer causes the presence of NMs in organisms that, without being in direct contact with these materials, ingest them through the consumption of other organisms that have directly absorbed or ingested the NMs (Lead et al., 2018). During the trophic transfer, NMs can also carry other molecules such as toxins and contaminants, modifying the trophic transfer process and biomagnification of contaminants (Lu et al., 2021). Theoretically, the trophic transfer can span several trophic levels; however, it is not a proven fact at the ecosystem scale, and several studies indicate limited transfer rates to the superior trophic levels (Lammel et al., 2020; Shi et al., 2020). In human consumers, it has not been shown to occur, but it is not considered an impossibility (Parsai & Kumar, 2020). Historical examples of other contaminants such as heavy metals, pesticides, and radioactive material indicate that it is highly likely (Uddin et al., 2020). It is unknown the long-term consequences of the exposure of the human body to synthetic NMs to which it could potentially be exposed by trophic transfer (Morales-Díaz et al., 2017).
The previous data indicate that NMs will be present in ecosystems in increasing frequency and quantity. An example of this type of contamination is the case of microplastics and nanoplastics, which are present in the water and soils of practically all the planet, being found in the same way inside living organisms (Huang et al., 2020a). The preceding allows us to conclude that, although the use of NMs can result in great productive and economic advantages for agricultural and livestock activities (Medina-Pérez et al., 2019), their application must be based on the appropriate level of knowledge about the dynamics and impact on ecosystems. An adequate level of knowledge implies having information about the behavior of NMs in ecologically relevant times (years), in ecologically relevant concentrations (even in very low concentrations) to take into account the biomagnification phenomena (Uddin et al., 2020) and responses to the chronic exposure (González-Morales et al., 2020).
Another direct ecological impact of NMs on plants occurs through the soil microbiome and the rhizosphere microbiome. Under natural conditions, both the internal media, the epidermis, and the rhizosphere and soil volume near the plant’s roots contain a complex community of microorganisms called the microbiome. The abiotic environmental variables and the microbiome’s physiological and biochemical action on plant cells are key determinants to modeling plants’ phenotype (Bahram et al., 2018). The microbiome is a dynamic soil–plant constituent that induces biostimulation and tolerance to stress. Therefore, any factor that modifies the biodiversity, profile of microorganism species, or their relative abundance will change the plant’s biostimulation response to the microbiome (Berg et al., 2014).
The soil microbiome’s exposition to NMs alters the species composition and relative abundance of microorganisms, mainly soil bacteria and protozoans. The above was demonstrated in several classes of NMs, including those contained in biosolids or subjected to environmental weathering (Asadishad et al., 2018). The concentrations of NMs capable of impacting the microbiome metabolism, enzymatic activities, abundance, or biodiversity were 5–50 mg kg−1 soil in the case of C60 fullerenes (Johansen et al., 2008), 1.2 kg TiO2 NPs ha−1 (Simonin et al., 2016), and 1 mg Ag NPs kg−1 soil (Grün et al., 2018).
As with plants, the effect of NMs on the microbiome is dose-dependent, with positive effects on some variables when concentrations are low (e.g., <1 mg kg−1 soil) in the soil (Rahmatpour et al., 2017). Even though, in general, microorganisms are more tolerant than plants to abiotic stresses, in the case of NMs, the sensitivity of microorganisms seems to be much higher compared to those of plants (Juárez-Maldonado et al., 2021).
In the long-time range, the modifications in the soil microbiome could also modify the composition of the communities of protozoa and mesofauna and maybe plants, with a potential change in the structure of the ecological communities. Until now, there is not enough knowledge about how the distinct microbiomes can regulate and mold the properties of soil, groundwater, and plant and animal communities (González-Morales et al., 2020).
Considering the above, it can be affirmed that the use of NMs as biostimulants can be a form of application of NMs in agriculture with a potentially low environmental impact. The application of NMs as biostimulants, as seed priming (López-Vargas et al., 2020), seedling priming, or an inductor of tolerance or fertilizer or nanofertilizer vehicles in adult plants (Chhipa, 2017; Abdel-Aziz et al., 2019), involves the use of these compounds in low concentrations. The foregoing is the result of the ability of NMs to induce biostimulation and defense responses in plant cells even at low concentrations (Juárez-Maldonado et al., 2021).
3 Impact of Nanomaterials on Cellular Surfaces and Apoplast
As previously mentioned, biostimulation of plants occurs in two phases: the first one occurs through interfacial physicochemical processes, with an impact on the activity of proton pumps, receptors, channels, and transporters of cell walls and membranes; the above modifies the transmembrane potential and consequently the transport of ions and metabolites, cell signaling, energy metabolism, and gene expression. The second phase of biostimulation occurs through a mixture of physicochemical and biochemical processes in response to the internment of NMs, the contact of the corona and core components with cellular metabolites, and the subsequent release of chemical components (ions, functional groups, and low-molecular-weight metabolites) that compose the NMs and their corona (Juárez-Maldonado et al., 2019).
This section of the chapter deals with the first phase of biostimulation with NMs, which has been proposed to depend on the interaction between the NMs’ surface charges and the cell surface charges.
3.1 The Cell Surface Charges
The surface charges of structural components, integral proteins, and functional groups of cell walls and membranes allow chemical interactions at the cell apoplast interface. Examples of these interactions are ionization of functional groups, acid/base dissociations, adsorption of ions and other chemical species, and the partial dissolution of some structural components of cell membranes (Wang et al., 2014). The density of surface charges (quantity by surface area) of the cell wall or membranes modifies the cellular interactions with the ions and other chemical species located in the apoplast. The surface charge density modifies the electrical potential of the surface of the membranes (ψ0) as well as the transmembrane potential that sustains the ion channels and other integral proteins functional (Kinraide & Wang, 2010). Any change in ψ0 and in the transmembrane potential implies an event of biostimulation and the consequent modification of cell metabolism. This is because the surface electrical potentials have an impact on the activity of channels, transporters, receptors, or in the importation via exosomes of ions (e.g., silicon and phosphorus), carbohydrates, lipids, lipoproteins, hormones, and other growth regulators (Haak et al., 2017).
The intensity and the final balance of the chemical interactions between the apoplast and the cell surfaces depend mainly on the ionic strength, pH, oxidation–reduction potential, and other extracellular medium properties. The cell surface maintains an equilibrium with the external fluids, where the interface acquires a net negative charge because the number of positive charges is less than the negative charges. The movement of ions in the apoplast, through attraction and repulsion, results in an electrical double layer (EDL) on the surface of the cell membrane (Fig. 3). The charge density, equivalent to the number of charges per unit area, determines the electrical potential of membrane surfaces and the transmembrane potential that supports the functionality and structure of integral proteins (Perry et al., 2016).
Schematic illustration of a charged cell wall or membrane or nanomaterial (NM) forming an electric double layer (EDL) when exposed to the apoplast. EDL indicates two parallel layers of charges on the surface. The Stern layer includes ions adsorbed via chemical interactions and has a positive net charge. The diffuse layer includes ions associated with the Stern layer via the Coulomb force and has a negative net charge. The diffuse layer contains free ions under the influence of thermal motion and electric attraction. The Debye length is the thickness of EDL with mobile ions and denotes the distance under the influence of the surface’s electric potential. The zeta potential is the electrical potential at the slipping plane. The volume included under the slipping plane shows tangential molecular motion about the surface. In plants, the Debye length is within 1–2 nm. As a consequence that the transmembrane domains of integral proteins can protrude from 2 to 7 nm, the receptors and the functional groups of proteins with positive and negative charges are located outside the EDL, which favor interfacial interactions with the EDL of NMs. Figure from Juárez-Maldonado et al. (2019)
3.2 The Surface Charges of NMs
The characteristics of NMs, such as size, charge, roughness, shape, and hydrophobicity, among others (Barkataki & Singh, 2019), induce different cell responses when they meet with plant surfaces. However, it is believed that the surface charges of NMs produce the first metabolic changes and in cellular gene expression (Pérez-Labrada et al., 2020). NMs have a greater surface area vs volume compared to conventional materials, which results in a large amount of surface free energy and high reactivity (Pacheco & Buzea, 2018).
NMs do not appear in a pristine form in environments such as water, soil, biological fluids, or plant surfaces. Inorganic and organic compounds and biomolecules are joined by adsorption to the core of NM, forming a single layer or several layers of molecules, which constitute a structure called corona. The physicochemical characteristics and the biological reactivity of the corona depend on the profile of the adsorbed molecules. In biological fluids, it is common for the NM’s corona to be constituted by proteins (Francia et al., 2019).
The formation of the NM’s corona occurs spontaneously as a means of decreasing the free energy of the system containing the dispersed NMs. In a contaminant-free system, such as in a laboratory, pristine NMs have the same tendency to decrease free energy, but in this case, they do so through the agglomeration of the NM’s particles. In both cases, the process is guided spontaneously toward a decrease in enthalpy (or an increase in entropy). The surface charge of the diffuse layer of the EDL of pristine NMs is commonly negative, while in biological fluids with pH <7 the diffuse layer of the EDL of the NM with corona has a net positive charge (Simon et al., 2018) (Fig. 4). This net positive charge on the corona surface facilitates interaction with the plant cell’s EDL with a net negative charge.
Representation of formation of NM’s corona in natural media. On the left side of (a), the net charge of the protein’s surface is positive at pH < 7 (with a negatively charged Stern layer). On the right side of (a), the pristine nanoparticle (NP) shows a surface negative charge (with a positively charged Stern layer). In (b), due to the opposite charges of the protein’s and NP’s diffuse layers, the electrostatic interactions that give rise to the corona occur. Figure from Juárez-Maldonado et al. (2019)
The NMs’ EDL acquires different characteristics depending on the coating used for their functionalization (Simon et al., 2018). For example, Li et al. (2019a) studied CeO2 nanoparticles (NPs) with three different coatings (diethylaminoethyl dextran, dextran, and carboxymethyl dextran), observing that the three NMs showed different Zeta potential (+13, −3, and −15 mV, respectively). In another study, Li et al. (2016) observed that the tomato and rice’s uptake of Au NPs of nearly identical size (8–12 nm) coated with cysteamine, cysteine, and thioglycolic acid was dependent on the surface charge of the functionalized NPs and related to the species of ligand used for the coating. The negatively charged Au NPs capped with cysteine were more efficiently absorbed in roots and transferred to stems and leaves than the NPs capped with cysteamine and thioglycolic acid.
As described, the surface free energy and the surface charges of NMs are key determinants in interfacial interactions. The final biological identity of the NM (that is, the impact it exerts on cell behavior) depends substantially on the asymmetric spatial distribution of surface charges, which in turn is the result of the aggregation/agglomeration of NMs, from the components and identity of the corona, and of the inorganic compounds present in the medium, such as Na+, K+, and Li+. Therefore, the same NM placed in different environments or media will have a different impact on biological organisms (González-Morales et al., 2020).
3.3 Corona and Cell Surface Interactions
The positive net surface charge of the NM’s corona can interact with the wall’s or cell membranes’ negatively charged surfaces. It can also interact with the negative or positive charges of the peripheral and integral proteins. The above activity can proceed without the intervention of specific cellular receptors (Fig. 5). The bonding process between the NM’s and cell’s surfaces also depend on the particles’ hydrophobicity and particles’ surface energy as aggregation factors to increase the entropy in NMs (Juárez-Maldonado et al., 2019).
Graphical representation of the interaction of charges on the surface of the proteins of corona, cell wall, or membrane. Figure from Juárez-Maldonado et al. (2019)
The interaction between the surfaces of NMs and cells causes changes in the membrane potential and the activity of the cell walls and membranes’ receptors and channels, causing metabolic adjustments (such as changes in ion fluxes) and energy metabolism and gene expression modifications (Hossain et al., 2016).
Interfacial interactions produce changes in the plant phenotype, from positive effects (biostimulation) to negative effects (toxicity), depending on the concentration and the physicochemical characteristics of the NMs, as well as the identity of the corona and the NM’s core composition (Table 1).
The functionalization of NMs influences the surface charge, also changing the biological impact. Spielman-Sun et al. (2019) studied the interfacial interactions of CeO2 NPs with different surface charges using corn, rice, tomato, and lettuce plants. The positively charged NPs showed greater adsorption in the root cells; meanwhile, the negatively charged and neutral particles showed greater translocation from the root to the stems. Translocation was more effective in tomato and lettuce plants compared to corn and rice plants. The functionalization of engineered NMs allows obtaining surfaces with specific characteristics and biological impact. Still, the characteristics and the biological impact can be modified once the materials are released in the environment or biological fluids and acquire a corona that modifies the surface functionalization (Goswami et al., 2017).
Depending on the type and concentration of NM, and on the characteristics of the corona or the coating chemicals used for surface functionalization, the physiological, biochemical, and genetic impacts are different in organisms. The first interactions of NMs with the epidermis of the root or leaves can cause modifications in the cell structure. For example, NMs of CeO2 caused lesions in tomato root hairs, necrosis, and malformations (Li et al., 2019a). A similar effect was reported in rice roots when exposed to Ag NPs, causing damage to the root cells (Huang et al., 2020b). Similarly, the first contact of some NMs with cell membranes can cause lipid peroxidation, evidenced by the increase in malondialdehyde (MDA) observed in maize plants using Y2O3 NPs (Gong et al., 2019). The same effect of increasing MDA was observed in rice seedlings when subjected toY2O3 NPs (Zhao et al., 2020). Even NMs made with essential elements for plants are toxic when they exceed adequate concentrations, as in the case of ZnO NPs applied at a concentration of 100 mg L−1 and which induced oxidative stress and alterations in the cell walls of the root epidermis of Brassica napus and Brassica juncea (Molnár et al., 2020).
Positive effects of NMs are also reported, manifested as modifications in cell surfaces. An example is the application of SiO2 NPs in rice plants, which was associated with an increase in the cell wall thickness, restricting the flow of arsenic (As) to the cells (Cui et al., 2020). In this same study, the SiO2 NPs induced changes in the cell wall’s electrochemical potential (from −35 to −10 mV) in the presence of 40 μmol of As3+ in the medium. These adjustments did not occur in the absence of the SiO2 NPs. As is known, changes in the surface potentials of the cell wall or membrane are the prelude to physiological adjustments and changes in gene expression (Juárez-Maldonado et al., 2019).
The above-mentioned interfacial interactions, upon the first contact of NMs with plant cells, produce biochemical signals (such as ABA, salicylates, or other hormones and metabolites) from root or leaf cells. These signals move through the vascular structures toward the rest of the plant, resulting in plant biostimulation in the form of adjustments in metabolism and gene expression and greater tolerance to biotic and abiotic stresses (Pérez-Labrada et al., 2020) (Fig. 6). An example is the impact of SiO2 NPs in reducing the expression of the PgSWEET gene, responsible for regulating the flow of sugars in the apoplast, which favors the resistance to certain pathogens in Panax ginseng (Abbai et al., 2019).
A proposed general process of nanoparticles interaction with plants. The mechanism designated as an “unknown mechanism” is what this chapter calls the two-phase biostimulation process. Figure from Rastogi et al. (2017)
The biological impact of NMs, either as biostimulation or toxicity, is also manifested in plant gene expression. In different studies, the physiological and biochemical response has been verified in parallel with gene expression changes. An example is that of multiwalled carbon nanotubes (MWCNTs) that enter protoplasts and can increase the expression of the aquaporin genes PIP1s and PIP2s in broccoli root. The result was a change in water permeability in the cells (Martinez-Ballesta et al., 2020). In maize plants exposed to different concentrations of La2O3 NPs, the content of abscisic acid increased, and water absorption was reduced by accelerating the development of apoplastic barriers in the roots, which caused growth inhibition in the plants. Also, the expression of some genes related to lignin biosynthesis was changed: some, such as ZmPAL, ZmCCR2, and ZmCAD6, were overexpressed, while the ZmF5H gene was repressed (Yue et al., 2019).
Hossain et al. (2016) studied the proteomic response associated with the phytotoxicity of the Al2O3, ZnO, and Ag NPs. A high oxidative burst was evidenced in the treatments with ZnO-NP and Ag-NP. The proteomic analysis of the roots revealed modifications in the amount of 104 proteins in the treatments with NPs; the proteins were associated with secondary metabolism, cell organization, and hormonal metabolism. Besides, Al2O3 NPs increased the expression of genes related to oxidation–reduction metabolism in roots, while the opposite occurred with the ZnO and Ag NPs. In the study of Xun et al. (2017), the maize plants with exposure to ZnO NPs modified the transcriptomic profile of the roots, showing an increase in the N metabolism pathways and synthesis of cellular components, while the processes related to metabolic rate were reduced.
Studies of transcriptomes have shown that the number of genes that modify their gene expression by exposition to NMs is significant, reporting that NMs of TiO2 and ZnO induced the differential expression of 509 genes in leaves and 3666 genes in lettuce roots (Wang et al., 2017b); the genes were associated with different metabolic pathways such as photosynthesis, N metabolism, antioxidant metabolism, and carbohydrate metabolism. In another study, Zhang et al. (2019) found that Ag NPs modified the expression of 626 genes in Arabidopsis; in this case, the genes were associated with photosynthesis, antioxidant metabolism, response to ethylene, and responsivity to other metabolites and environmental challenge.
The changes that occur in transcriptomes and proteomes after exposure of plants to different NMs are extensive. Therefore, it is unlikely that the impact of NMs occurs through a single mechanism; rather, it is expected that a set of mechanisms involving multiple signaling pathways and their crosstalk participate. This situation explains the difficulty of predicting the global and long-term impact of NMs on plant organisms. Additionally, NMs can act synergistically or antagonistically depending on the environmental context, making the prediction and explanation of the mechanisms of action more difficult. An example of this is the synergism between the TiO2 NPs and the high concentration of CO2 in rice plants, while each factor separately did not influence the plants used in the experiment (Xu et al., 2019). However, as with other biostimulants whose mechanism of action is still not well understood (González-Morales et al., 2021), NMs used in low concentrations and by the most appropriate application routes (for example, as seed priming or by foliar spraying with preference over the application to the soil/substrate or the nutrient solution) can surely constitute a valuable alternative within the alternatives available to carry out biostimulation of crops (Juárez-Maldonado et al., 2021).
4 Cellular Internalization and Compartmentalization of Nanomaterials
During the second phase of the biostimulation process by NMs, the internalization and compartmentalization of NMs occur in plant cells. Like the first interactions of NMs with cells, internment depends on the material’s characteristics, such as size, functional groups of the corona, or the compounds used for NM functionalization, shape, surface charge, hydrophobicity, and roughness, among others (Liu et al., 2020).
The following are the main pathways in which NMs can access plant cells (Fig. 7):
-
1.
Through pores in cell walls and membranes. It can occur through pre-existing pores, or indeed the surface free energy of NM can enlarge the cell wall pores or create new pores in the membrane and allow access to the cellular environment (Yan & Chen, 2019; Barkataki & Singh, 2019; Singh & Kumar, 2020), maybe a main access route for NMs smaller than 100 nm.
-
2.
Clathrin-mediated endocytosis is the main endocytic mechanism in plants, maybe a main access route for NMs 120–200 nm (Santiago et al., 2020).
-
3.
Membrane microdomain-associated endocytosis. Membrane microdomains are nanodomains at the plasma membrane (PM) that are enriched in sterol and sphingolipids (Fan et al., 2015).
Active and passive cell uptake of particles and NMs in animal and plant cells: (a) phagocytosis, (b) caveolin-mediated endocytosis, (c) clathrin–caveolin-independent endocytosis, (d) clathrin-mediated endocytosis, (e) macro-pinocytosis, (f) ion pumps, (g) exocytosis, (h) facilitated diffusion, and (i) simple diffusion. Figure from Sabourian et al. (2020)
There are not many studies regarding the cellular internment of NMs in plant cells. However, the forms of access appear to be similar in plant and animal cells. Table 2 shows the main cellular access pathways for NMs, depending on size, charge, and particle shape.
NMs constitute a point and reactive source that provides nutrients and other elements for cells. On the other hand, the ions of the different essential, beneficial, and toxic elements constitute a diffuse source with less reactivity whose cellular internment occurs through channels and transporter proteins that effectively regulate the entry and compartmentalization of these ions. In the case of NMs, as previously stated, there are several access pathways, several of them dependent on the surface free energy of the NM, which facilitates internment into the cytoplasm and organelles without showing the regulation that occurs for ions (Juárez-Maldonado et al., 2019).
The above possibly partially explain the differences observed in the impact of conventional fertilizers versus nanofertilizers on plants. Conventional fertilizer contributes ions that dissolve in the apoplast and from there are interned into the cell by mechanisms subject to strong regulation. In contrast, nanofertilizers provide NMs that initially induce biostimulation by the interaction of surfaces and later allow the entry of NMs through pores, membranes, and endocytosis. After entering the cells, the second phase of biostimulation occurs, followed by the release of the nanofertilizer ions that originate the well-known nutritional responses described for this category of elements. Together, the biostimulation and the nutrients provided by the nanofertilizers translate into a substantial improvement in the metabolism and growth of the crop, also increasing tolerance to environmental stress (Dimkpa & Bindraban, 2018; El-Desouky et al., 2021; Neto et al., 2021; Ahmadian et al., 2021). Additionally, the stability and bioavailability of nanofertilizers in the soil or substrate are greater than conventional fertilizers (Ojeda-Barrios et al., 2020).
After entering the plant cells, the NMs, according to the identity of the corona, will accumulate in certain organelles, cell compartments, or the cell membrane, or they will react with the different metabolites of the cell environment, releasing the components of the corona or the components of the NM’s core (Banerjee et al., 2019). An example is the release of Ag+ and Cu+ ions from Ag and Cu NPs. If the Cu+ concentration is adequate, it will function as a nutrient (cofactor), and this positive effect will be added to the biostimulation created by the Cu NPs. But beyond a certain concentration threshold, the Cu+ will cause toxicity. In the case of Ag+, there is no known function as cofactors in living organisms, and rather they compete with Cu+ as a cofactor of some proteins. Therefore, for Ag NPs, an impact is expected to occur as a biostimulant when it is in low concentration or as toxic when it exceeds a certain threshold. The toxicity threshold (20–100 mg L−1) will depend on the plant species and the environmental context (Yan & Chen, 2019).
The second phase of biostimulation by NMs begins with the wide range of interactions that occur between NMs internalized to cells and the cell components: membranes, proteins, nucleic acids, regulation and signaling complexes, and diverse metabolites. The result is a series of modifications in metabolism, which originate biochemical and physiological changes and adjustments in gene expression that change cellular proteomes and metabolomes and the plant’s phenotype (Zuverza-Mena et al., 2017; Anjum et al., 2019).
Seed priming is an example of the biostimulation process induced by NMs. NMs in contact with the seed coat can pass through this structure through the intercellular spaces in the parenchyma or through the creation of pores in the cell walls. In both cases, the presence of NMs causes the induction of enzymes that initiate germination events and the expression of genes associated with aquaporins. This effect of acceleration of germination and greater capacity of the seed to absorb water is explained as a response to eustress or biostimulation. It has been described for several NMs and is exemplified by the positive impact of carbon nanotubes on germination (Miralles et al., 2012). The biostimulant impact of carbon NMs is not limited to germination events but can modify plants’ antioxidant status in later stages of development (López-Vargas et al., 2020).
The changes associated with the second phase of biostimulation were exemplified by Yan et al. (2020) in maize plants grown in soil with Fe3O4 NPs (0, 50, 500 mg kg−1). The maize plants did not show impact on plant biomass or photosynthesis, but root length significantly increased, with decreased malondialdehyde (MDA) level, higher accumulation of Fe in root tissues, and a reprogramming of root metabolome with a decrease in pathways related to nitrogen metabolism, antioxidant metabolism, and defense. Another example of metabolic adjustments elicited by NMs was described by Anjum et al. (2019). It refers to the use of NMs (Ag, Cu, Au, Co, Zn) as biostimulants to induce the accumulation of specialized metabolites with pharmacological or nutraceutical applications in distinct plant species under different culture systems such as cell culture, organ culture, or growing seedlings. The concentration of NMs depended on the plant species and the cultivation system and was between 0.3 and 900 mg L−1 for metallic and metal oxide NMs and 2 and 500 mg L−1 for carbon NMs. In fact, this biostimulant potential of NMs can be widely applied in the agricultural practice for the nutraceutical improvement of harvested products (Juárez-Maldonado et al., 2018).
The compartmentalization of NMs can have positive or negative effects on plants, depending mainly on the NM concentration. If the levels of NMs are not high, those that are made up of essential elements for plants, such as Ca, Mg, Zn, and Fe, are expected to induce a dual effect of biostimulation and nutrition. Biostimulation occurs by the interaction of NMs with internal membrane systems and protein complexes or RNAs that regulate gene expression or post-translational modification of proteins; nutrition by the release of ions in the internal cell environment and their use as cofactors or by interaction with other ions present in the cell environment or the apoplast. On the other hand, the NMs of elements such as Ti, Ce, and Cd will cause biostimulation or toxicity depending mainly on the concentration and location of the NMs in the different cell compartments (Juárez-Maldonado et al., 2021).
In the case of NMs formed by essential elements and those formed by other elements, when a certain concentration threshold is exceeded, toxicity will occur. The threshold is highly variable, as it depends on the type of NM, the composition of the corona or capping material, the plant species and the stage of development of the plants, and the environmental context, e.g., temperature, the composition of the medium, and the presence of compounds that can antagonize or synergize with NMs (Juárez-Maldonado et al., 2021). Phytotoxicity can be manifested as inhibition in seed germination, root growth, biomass, and leaf area. At the physiological level is associated with oxidative stress and lipid peroxidation, alteration in fluidity and permeability of the cell’s membranes, alteration of cell structure and cell division, hormonal balance changes, and a decline in chlorophyll, nutrient uptake, and transpiration rate (Yan & Chen, 2019).
When NMs reach high concentrations, vacuoles seem to play an important role in regulating the concentration of the released materials that result from the reaction of NMs with cellular metabolites such as organic acids, chelating agents, and redox metabolites (Ma et al., 2018). On the other hand, mobilization of NMs toward the vacuoles through endosomes also appears to occur, as reported for CeO2 NPs (Li et al., 2019a) and CuO NPs (Dai et al., 2018). The compartmentalization also depends on the cellular structure of the plant species. Spielman-Sun et al. (2019) reported that CeO2 NPs were accumulated in mesophyll cells to a greater extent in dicotyledonous plants (lettuce and tomato) than in monocotyledons (rice and maize), an effect attributed to the greater volume of intercellular spaces in the mesophyll of dicotyledons.
The two-phase biostimulation process, or the toxicity when NPs’ concentration is high, occurs immediately (<24 h) in the cells adjacent to the NMs’ entry sites or in cell cultures (Dai et al., 2018). In terrestrial plants, the entry sites can be the root epidermis, the epidermis of stems and leaves, the stomatal pores and lenticels, and the epidermis of flowers and fruits. In all cases, exposure to NMs induces changes in the cellular phenotypes of the different tissues (Zuverza-Mena et al., 2017). The phenotypic modification associated with biostimulation or toxicity is followed by metabolic, biochemical, and genetic adjustments followed by signaling toward other cells not directly exposed to NMs, which also modifies their phenotypes. The above mechanism is analogous to that proposed for other biostimulants and factors inducing biotic and abiotic stress (Fig. 8), mainly through induction of ROS synthesis, followed by an oxidative burst that unchains Ca2+ fluxes, and the subsequent action of ion channels (e.g., K+ and Cl−), hormones, and other regulatory metabolites, and non-coding RNAs. The regulator substances (e.g., salicylic acid and ABA) can be extruded to the apoplast or transported by plasmodesmata. Finally, the signaling spreads all the plant organs through the signaling agents’ long-distance transport by the vasculature (Yan & Chen, 2019; Pérez-Labrada et al., 2020).
The proposed mechanism to explain the biostimulation and elicitation capacity of NMs in plant cells. Figure from Anjum et al. (2019)
In addition to the signaling process of the second phase of biostimulation, dependent on hormones and other metabolites, the migration of NMs can also occur from the site where they entered toward other plant structures and organs. The process is described in the next section.
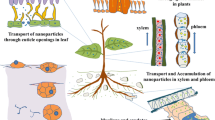
5 Transportation of Nanomaterials between the Organs of the Plant
The transport of NMs can be visualized as a phenomenon that in plants can occur in several dimensions: (1) from the initial point of entry to other plant organs; (2) from the different organs of the plant toward other organisms at different trophic levels (e.g., the direct transference of NMs to herbivores or impact through changes in the nutritional or nutraceutical quality); (3) from one generation to another through transgenerational modifications (e.g., epigenomic changes) or even by direct transfer. Topic (1) is the one that will be described in this section.
As already mentioned, NMs that come into contact with plants do so initially with the surfaces of the roots, stems, leaves, flowers, and fruits. NMs that enter the plant’s internal volume move from the apoplast into the cytoplasm and cell organelles after interactions between the surfaces of the NMs and the walls and membranes occur. Subsequently, NMs can be subjected to chemical transformations or compartmentalization or migrate from one cell to another through symplastic transport. The above can be an important mechanism for the radial transport of NMs from the epidermis of the root or aerial structures toward the different organs’ internal volume (Miralles et al., 2012).
Radial transport allows NMs to reach the cortex’s internal tissues, the xylem and phloem tissues, and the pith. NMs enter the vascular structures and are mobilized by axial transport to the rest of the plant (Miralles et al., 2012). When the initial point of entry is via the root, the main transport route is believed to be via the xylem. On the other hand, when the entry of NMs occurs through the epidermis of leaves, stems, and fruits, the initial internment that seems to occur by simple diffusion is through the stomatal pores and lenticels, which can represent about 5% of the surface of the epidermis. Once the NMs reach the substomatal cavity or the intercellular spaces of the lenticels’ complementary cells, it is believed that the phloem carries out the subsequent transport to the rest of the plant. It is not excluded that some NMs passively enter through the cuticle that covers the epidermis of leaves and stems, which presents pores <5 nm (Su et al., 2019). On the other hand, there is a possibility that the entry of NMs >5 nm through the cuticle could occur as a result of the lipophilicity of some NMs or the interaction of the surface free energy of NMs with the hydrocarbon molecules that build up the cuticle (Juárez-Maldonado et al., 2019).
Axial transport of NMs from the initial entry points to more distant organs triggers other biostimulation or toxicity events, depending on the concentrations, types of material, and the environmental context. These new events are different from those initially triggered since the target organs have different phenotypes and consequently respond differently to NMs. For example, if TiO2 NPs are applied in the substrate of a plant in low concentration (e.g., 1–5 mg L−1), these would enter through the epidermis of the root and promote biostimulation events in the root (with physiological impacts on the whole plant derived from the root’s signaling with hormones and other metabolites). When the xylem transports the TiO2 NPs to other plant organs, they will cause new biostimulation events, but now in the cells of the tissues of the stems or leaves, which would present different response profiles to those of the cells of the root tissues (Fig. 9).
Graphic representation of NMs’ effects on plants. Positive impacts are depicted in green, negative ones in brown. More controversial topics as the trophic transfer and the transgenerational impacts are followed by question marks. Figure from Coman et al. (2019)
The amount of the NM that moves radially or axially from the initial entry point to the rest of the plant is highly variable. It initially depends on the lifetime of the NM in the cell environment, in other words, on whether it is rapidly subject to chemical transformations that release elemental components, e.g., when Cu NPs are transformed into Cu2+. The mobilization depends secondly on the characteristics of the NM and the plant species, the stage of development, growth rate, and its environmental context.
The different plant taxa present substantial anatomical and physiological differences; these intrinsic differences constitute another factor that significantly modifies the response, transport, and fate of NMs in the plant. As an example, there is a difference in the root structure between monocots (fibrous root) and dicots (taproot), which suggests that monocots may be more sensitive to NMs (Su et al., 2019). Analogous reasoning suggests that the differences in the root structure between crops in soil and crops in substrates different from the soil (e.g., peat moss, perlite) or in hydroponics would make the responses to NMs different in each environmental situation.
Photosynthesis appears to be a metabolic pathway sensitive to the presence of NMs in plant cells (Tighe-Neira et al., 2018); for that reason, like germination and increase in biomass, it is widely used in studies on toxicity and biostimulation. Whether the application of NMs in plants occurs via the roots or by foliar spraying, the impact of NMs on photosynthetic activity depends on the axial transport (presumably through the xylem) of NMs from the epidermis of the root, or radial and then axial transport (presumably through the phloem) from the stomatal pores toward the mesophyll of the leaves (Su et al., 2019).
Different variables associated with photosynthesis have been used to describe the impact of different NMs on plants. From Tighe-Neira et al. (2018), the following can be mentioned:
-
CO2 assimilation rate and stomatal conductance. With negative impacts of 1 mg L−1 CuO NPs, 0.2% w/v TiO2 NPs, 200 mg L−1 CeO2 NPs, 300 mg L−1 ZnO NPs, 800 mg kg−1 ZnO NPs.
-
The concentration of photosynthetic pigments. With negative impacts of 1–400 mg L−1 CuO NPs, 5–10 mg L−1 Ag NPs, 25 mg kg−1 ZnS NPs, with a positive effect of 250 mg kg−1 CeO2 NPs in tomato and negative effect of 250 mg kg−1 CeO2 NPs in beans, and 400 mg kg−1 CeO2 NPs in maize.
-
Efficiency in the transport of electrons. With negative impacts of 32 mg L−1 CuO NPs, 5–300 mg L−1 Ag NPs, 200 mg L−1 CeO2 NPs, 1–100 mg L−1 ZnO NPs, and with positive effects of 0.25% w/v TiO2 NPs.
A significant amount of the above results pointed to negative impacts on photosynthesis variables. It is possible that these results, in many cases, were dependent on the use of high concentrations of NMs (e.g., >75 mg L−1) (Juárez-Maldonado et al., 2021).
Many crop plant studies indicate positive impacts of NMs on antioxidant activity, biomass, and yield (Zuverza-Mena et al., 2017). It is not easy to think that these results are obtained without a positive effect on photosynthetic activity or other related activities such as respiration or photorespiration. However, as far as we know, there are no studies where the effect of NMs on plant metabolism is considered comprehensively (e.g., photosynthesis, photorespiration, respiration, biomass allocation; from physiological, biochemical, and molecular points of view). Considering that the biostimulant impact of NMs occurs through multiple signaling cascades and different metabolic pathways, studies aimed at understanding the impact of NMs should consider a more comprehensive view of plant responses.
6 Perspective of Crops Biostimulation with Nanomaterials
Biostimulation is a complex biological phenomenon that has been described for many physical processes, materials, substances, and organisms. NMs constitute a part of the universe of possibilities for the development of biostimulants. What is presented in this chapter indicates that there is a large amount of information about the positive impact of NMs in plants, not necessarily presented with the biostimulation label, but showing the characteristics of the phenomenon.
As with other biostimulants such as humic acids, chitosan, and growth-promoting fungi and bacteria, the responses of plants are not described by a simple model or limited to a few physiological, biochemical, transcriptomic, or proteomic responses. To reach a complete understanding of the biostimulation phenomenon of plants with NMs, great efforts will be necessary to integrate the existing information, e.g., in the form of meta-analysis or other kinds of models that integrate huge amounts of information, or comprehensive experiments that include a large number of response variables in plants, using series of response variables whose causal relationships are reasonably understood, located in different ambits of complexity, from the molecular level to the levels of populations and plant communities.
It is manifest that there are still many unresolved issues regarding the commercial-scale applications of NMs; the main topics still under discussion refer to ecological, economic, and innocuity issues. The possible assortment of interactions between NMs, plant species, soil types and substrates, climatic regimes, and agronomic management practices are numerous. It is quite a challenge to establish the first definition of a few selected NMs to be applied to certain crops under certain environmental conditions. This initial definition is possibly an important first step in advancing the commercial application of NMs as biostimulants in agriculture. The information obtained from the above-mentioned comprehensive studies would be useful for defining a selected group of NMs that could constitute the first wave of new materials for agriculture whose use would increase yield, mitigating the environmental impact of current agronomic practices, with the final objective of promoting the sustainable crop production.
References
Abbai, R., Kim, Y.-J., Mohanan, P., El-Agamy Farh, M., Mathiyalagan, R., Yang, D.-U., Rangaraj, S., Venkatachalam, R., Kim, Y.-J., & Yang, D.-C. (2019). Silicon confers protective effect against ginseng root rot by regulating sugar efflux into apoplast. Scientific Reports, 9, 18259. https://doi.org/10.1038/s41598-019-54678-x
Abdel-Aziz, H. M. M., Hasaneen, M. N. A., & Omer, A. M. (2019). Impact of engineered nanomaterials either alone or loaded with NPK on growth and productivity of French bean plants: Seed priming vs foliar application. South African Journal of Botany, 125, 102–108. https://doi.org/10.1016/j.sajb.2019.07.005
Agathokleous, E., Feng, Z., Iavicoli, I., & Calabrese, E. J. (2019). The two faces of nanomaterials: A quantification of hormesis in algae and plants. Environment International, 131, 105044. https://doi.org/10.1016/j.envint.2019.105044
Ahmadian, K., Jalilian, J., & Pirzad, A. (2021). Nano-fertilizers improved drought tolerance in wheat under deficit irrigation. Agricultural Water Management, 244, 106544. https://doi.org/10.1016/j.agwat.2020.106544
Ahmed, H., Gaafar, R., Elsherif, D., Haider, A., & Shanshory, A. (2020). Effect of silver nanoparticles and Chlorella sp. suspension on vegetative growth of Eruca sativa. Egyptian Journal of Experimental Biology (Botany), 16, 27. https://doi.org/10.5455/egyjebb.20200222113502
Akaighe, N., MacCuspie, R. I., Navarro, D. A., Aga, D. S., Banerjee, S., Sohn, M., & Sharma, V. K. (2011). Humic acid-induced silver nanoparticle formation under environmentally relevant conditions. Environmental Science & Technology, 45, 3895–3901. https://doi.org/10.1021/es103946g
Anjum, S., Anjum, I., Hano, C., & Kousar, S. (2019). Advances in nanomaterials as novel elicitors of pharmacologically active plant specialized metabolites: Current status and future outlooks. RSC Advances, 9, 40404–40423. https://doi.org/10.1039/C9RA08457F
Arora, N. K., Fatima, T., Mishra, I., Verma, M., Mishra, J., & Mishra, V. (2018). Environmental sustainability: Challenges and viable solutions. Environmental Sustainability, 1, 309–340. https://doi.org/10.1007/s42398-018-00038-w
Asadishad, B., Chahal, S., Akbari, A., Cianciarelli, V., Azodi, M., Ghoshal, S., & Tufenkji, N. (2018). Amendment of agricultural soil with metal nanoparticles: Effects on soil enzyme activity and microbial community composition. Environmental Science & Technology, 52, 1908–1918. https://doi.org/10.1021/acs.est.7b05389
Bahram, M., Hildebrand, F., Forslund, S. K., Anderson, J. L., Soudzilovskaia, N. A., Bodegom, P. M., Bengtsson-Palme, J., Anslan, S., Coelho, L. P., Harend, H., Huerta-Cepas, J., Medema, M. H., Maltz, M. R., Mundra, S., Olsson, P. A., Pent, M., Põlme, S., Sunagawa, S., Ryberg, M., … Bork, P. (2018). Structure and function of the global topsoil microbiome. Nature, 560, 233–237. https://doi.org/10.1038/s41586-018-0386-6
Banerjee, K., Pramanik, P., Maity, A., Joshi, D. C., Wani, S. H., & Krishnan, P. (2019). Chapter 4—Methods of using nanomaterials to plant systems and their delivery to plants (mode of entry, uptake, translocation, accumulation, biotransformation and barriers). In M. Ghorbanpour & S. H. Wani (Eds.), Advances in Phytonanotechnology (pp. 123–152). Academic Press.
Barkataki, M. P., & Singh, T. (2019). Chapter two—Plant-nanoparticle interactions: Mechanisms, effects, and approaches. In S. K. Verma & A. K. Das (Eds.), Comprehensive analytical chemistry (pp. 55–83). Elsevier.
Berg, G., Grube, M., Schloter, M., & Smalla, K. (2014). Unraveling the plant microbiome: Looking back and future perspectives. Frontiers in Microbiology, 5. https://doi.org/10.3389/fmicb.2014.00148
Chhipa, H. (2017). Nanofertilizers and nanopesticides for agriculture. Environmental Chemistry Letters, 15, 15–22. https://doi.org/10.1007/s10311-016-0600-4
Chowdhury, M. A., Shuvho, M. B. A., Hossain, M. I., Ali, M. O., Kchaou, M., Rahman, A., Yeasmin, N., Khan, A. S., Rahman, M. A., & Mofijur, M. (2020). Multiphysical analysis of nanoparticles and their effects on plants. Biotechnology and Applied Biochemistry. https://doi.org/10.1002/bab.2049
Coman, V., Oprea, I., Leopold, L. F., Vodnar, D. C., & Coman, C. (2019). Soybean interaction with engineered nanomaterials: A literature review of recent data. Nanomaterials, 9, 1248. https://doi.org/10.3390/nano9091248
Cui, J., Li, Y., Jin, Q., & Li, F. (2020). Silica nanoparticles inhibit arsenic uptake into rice suspension cells via improving pectin synthesis and the mechanical force of the cell wall. Environmental Science: Nano, 7, 162–171. https://doi.org/10.1039/C9EN01035A
Cui, X., Wan, B., Yang, Y., Ren, X., & Guo, L.-H. (2017). Length effects on the dynamic process of cellular uptake and exocytosis of single-walled carbon nanotubes in murine macrophage cells. Scientific Reports, 7, 1518. https://doi.org/10.1038/s41598-017-01746-9
Dai, Y., Wang, Z., Zhao, J., Xu, L., Xu, L., Yu, X., Wei, Y., & Xing, B. (2018). Interaction of CuO nanoparticles with plant cells: Internalization, oxidative stress, electron transport chain disruption, and toxicogenomic responses. Environmental Science: Nano, 5, 2269–2281. https://doi.org/10.1039/C8EN00222C
Dimkpa, C. O., & Bindraban, P. S. (2018). Nanofertilizers: New products for the industry? Journal of Agricultural and Food Chemistry, 66, 6462–6473. https://doi.org/10.1021/acs.jafc.7b02150
Ding, L., Yao, C., Yin, X., Li, C., Huang, Y., Wu, M., Wang, B., Guo, X., Wang, Y., & Wu, M. (2018). Size, shape, and protein Corona determine cellular uptake and removal mechanisms of gold nanoparticles. Small, 14, 1801451. https://doi.org/10.1002/smll.201801451
El-Desouky, H. S., Islam, K. R., Bergefurd, B., Gao, G., Harker, T., Abd-El-Dayem, H., Ismail, F., Mady, M., & Zewail, R. M. Y. (2021). Nano iron fertilization significantly increases tomato yield by increasing plants’ vegetable growth and photosynthetic efficiency. Journal of Plant Nutrition, 0, 1–15. https://doi.org/10.1080/01904167.2021.1871749
Evans, J. R., & Lawson, T. (2020). From green to gold: Agricultural revolution for food security. Journal of Experimental Botany, 71, 2211–2215. https://doi.org/10.1093/jxb/eraa110
Fan, L., Li, R., Pan, J., Ding, Z., & Lin, J. (2015). Endocytosis and its regulation in plants. Trends in Plant Science, 20, 388–397. https://doi.org/10.1016/j.tplants.2015.03.014
Francia, V., Yang, K., Deville, S., Reker-Smit, C., Nelissen, I., & Salvati, A. (2019). Corona composition can affect the mechanisms cells use to internalize nanoparticles. ACS Nano, 13, 11107–11121. https://doi.org/10.1021/acsnano.9b03824
Gerland, P., Raftery, A. E., Ševčíková, H., Li, N., Gu, D., Spoorenberg, T., Alkema, L., Fosdick, B. K., Chunn, J., Lalic, N., Bay, G., Buettner, T., Heilig, G. K., & Wilmoth, J. (2014). World population stabilization unlikely this century. Science, 346, 234–237. https://doi.org/10.1126/science.1257469
Giese, B., Klaessig, F., Park, B., Kaegi, R., Steinfeldt, M., Wigger, H., von Gleich, A., & Gottschalk, F. (2018). Risks, release and concentrations of engineered nanomaterial in the environment. Scientific Reports, 8, 1565. https://doi.org/10.1038/s41598-018-19275-4
Gong, C., Wang, L., Li, X., Wang, H., Jiang, Y., & Wang, W. (2019). Responses of seed germination and shoot metabolic profiles of maize ( Zea mays L.) to Y 2 O 3 nanoparticle stress. RSC Advances, 9, 27720–27731. https://doi.org/10.1039/C9RA04672K
González-Morales, S., Parera, C. A., Juárez-Maldonado, A., la Fuente, M. C. D., & Benavides-Mendoza, A. (2020). Chapter 13—The ecology of nanomaterials in agroecosystems. In A. Husen & M. Jawaid (Eds.), Nanomaterials for agriculture and forestry applications (pp. 313–355). Elsevier.
González-Morales, S., Solís-Gaona, S., Valdés-Caballero, M. V., Juárez-Maldonado, A., Loredo-Treviño, A., & Benavides-Mendoza, A. (2021). Transcriptomics of biostimulation of plants under abiotic stress. Frontiers in Genetics, 12. https://doi.org/10.3389/fgene.2021.583888
Goswami, L., Kim, K.-H., Deep, A., Das, P., Bhattacharya, S. S., Kumar, S., & Adelodun, A. A. (2017). Engineered nano particles: Nature, behavior, and effect on the environment. Journal of Environmental Management, 196, 297–315. https://doi.org/10.1016/j.jenvman.2017.01.011
Grün, A.-L., Straskraba, S., Schulz, S., Schloter, M., & Emmerling, C. (2018). Long-term effects of environmentally relevant concentrations of silver nanoparticles on microbial biomass, enzyme activity, and functional genes involved in the nitrogen cycle of loamy soil. Journal of Environmental Sciences, 69, 12–22. https://doi.org/10.1016/j.jes.2018.04.013
Guo, J.-J., Huang, X.-P., Xiang, L., Wang, Y.-Z., Li, Y.-W., Li, H., Cai, Q.-Y., Mo, C.-H., & Wong, M.-H. (2020). Source, migration and toxicology of microplastics in soil. Environment International, 137, 105263. https://doi.org/10.1016/j.envint.2019.105263
Haak, D. C., Fukao, T., Grene, R., Hua, Z., Ivanov, R., Perrella, G., & Li, S. (2017). Multilevel regulation of abiotic stress responses in plants. Frontiers in Plant Science, 8. https://doi.org/10.3389/fpls.2017.01564
He, X., Deng, H., & Hwang, H. (2019). The current application of nanotechnology in food and agriculture. Journal of Food and Drug Analysis, 27, 1–21. https://doi.org/10.1016/j.jfda.2018.12.002
Hochella, M. F., Mogk, D. W., Ranville, J., Allen, I. C., Luther, G. W., Marr, L. C., McGrail, B. P., Murayama, M., Qafoku, N. P., Rosso, K. M., Sahai, N., Schroeder, P. A., Vikesland, P., Westerhoff, P., & Yang, Y. (2019). Natural, incidental, and engineered nanomaterials and their impacts on the earth system. Science, 363. https://doi.org/10.1126/science.aau8299
Hossain, Z., Mustafa, G., Sakata, K., & Komatsu, S. (2016). Insights into the proteomic response of soybean towards Al2O3, ZnO, and Ag nanoparticles stress. Journal of Hazardous Materials, 304, 291–305. https://doi.org/10.1016/j.jhazmat.2015.10.071
Huang, D., Tao, J., Cheng, M., Deng, R., Chen, S., Yin, L., & Li, R. (2020a). Microplastics and nanoplastics in the environment: Macroscopic transport and effects on creatures. Journal of Hazardous Materials, 124399. https://doi.org/10.1016/j.jhazmat.2020.124399
Huang, X., Li, Y., Chen, K., Chen, H., Wang, F., Han, X., Zhou, B., Chen, H., & Yuan, R. (2020b). NOM mitigates the phytotoxicity of AgNPs by regulating rice physiology, root cell wall components and root morphology. Environmental Pollution, 260, 113942. https://doi.org/10.1016/j.envpol.2020.113942
Hurni, H., Giger, M., Liniger, H., Mekdaschi Studer, R., Messerli, P., Portner, B., Schwilch, G., Wolfgramm, B., & Breu, T. (2015). Soils, agriculture and food security: The interplay between ecosystem functioning and human well-being. Current Opinion in Environmental Sustainability, 15, 25–34. https://doi.org/10.1016/j.cosust.2015.07.009
Johansen, A., Pedersen, A. L., Jensen, K. A., Karlson, U., Hansen, B. M., Scott-Fordsmand, J. J., & Winding, A. (2008). Effects of C60 fullerene nanoparticles on soil bacteria and protozoans. Environmental Toxicology and Chemistry, 27, 1895–1903. https://doi.org/10.1897/07-375.1
Joshi, S. M., De Britto, S., & Jogaiah, S. (2020). Myco-engineered selenium nanoparticles elicit resistance against tomato late blight disease by regulating differential expression of cellular, biochemical and defense responsive genes. Journal of Biotechnology. https://doi.org/10.1016/j.jbiotec.2020.10.023
Juárez-Maldonado, A., González-Morales, S., Cabrera-De la Fuente, M., Medrano-Macías, J., & Benavides-Mendoza, A. (2018). Nanometals as promoters of nutraceutical quality in crop plants. In A. M. Grumezescu & A. M. Holban (Eds.), Impact of nanoscience in the food industry (pp. 277–310). Academic Press.
Juárez-Maldonado, A., Ortega-Ortíz, H., Morales-Díaz, A. B., González-Morales, S., Morelos-Moreno, Á., Cabrera-De la Fuente, M., Sandoval-Rangel, A., Cadenas-Pliego, G., & Benavides-Mendoza, A. (2019). Nanoparticles and nanomaterials as plant biostimulants. International Journal of Molecular Sciences, 20, 162. https://doi.org/10.3390/ijms20010162
Juárez-Maldonado, A., Tortella, G., Rubilar, O., Fincheira, P., & Benavides-Mendoza, A. (2021). Biostimulation and toxicity: The magnitude of the impact of nanomaterials in microorganisms and plants. Journal of Advanced Research. https://doi.org/10.1016/j.jare.2020.12.011
Kang, B., Chang, S., Dai, Y., Yu, D., & Chen, D. (2010). Cell response to carbon nanotubes: Size-dependent intracellular uptake mechanism and subcellular fate. Small, 6, 2362–2366. https://doi.org/10.1002/smll.201001260
Kinraide, T. B., & Wang, P. (2010). The surface charge density of plant cell membranes (σ): An attempt to resolve conflicting values for intrinsic σ. Journal of Experimental Botany, 61, 2507–2518. https://doi.org/10.1093/jxb/erq082
Lammel, T., Thit, A., Cui, X., Mouneyrac, C., Baun, A., Valsami-Jones, E., Sturve, J., & Selck, H. (2020). Trophic transfer of CuO NPs from sediment to worms ( Tubifex tubifex ) to fish ( Gasterosteus aculeatus ): A comparative study of dissolved Cu and NPs enriched with a stable isotope tracer ( 65 Cu). Environmental Science: Nano, 7, 2360–2372. https://doi.org/10.1039/D0EN00227E
Lead, J. R., Batley, G. E., Alvarez, P. J. J., Croteau, M.-N., Handy, R. D., McLaughlin, M. J., Judy, J. D., & Schirmer, K. (2018). Nanomaterials in the environment: Behavior, fate, bioavailability, and effects—An updated review. Environmental Toxicology and Chemistry, 37, 2029–2063. https://doi.org/10.1002/etc.4147
Li, H., Ye, X., Guo, X., Geng, Z., & Wang, G. (2016). Effects of surface ligands on the uptake and transport of gold nanoparticles in rice and tomato. Journal of Hazardous Materials, 314, 188–196. https://doi.org/10.1016/j.jhazmat.2016.04.043
Li, J., Tappero, R. V., Acerbo, A. S., Yan, H., Chu, Y., Lowry, G. V., & Unrine, J. M. (2019a). Effect of CeO 2 nanomaterial surface functional groups on tissue and subcellular distribution of Ce in tomato ( Solanum lycopersicum ). Environmental Science: Nano, 6, 273–285. https://doi.org/10.1039/C8EN01287C
Li, Y., Li, W., Zhang, H., Dong, R., Li, D., Liu, Y., Huang, L., & Lei, B. (2019b). Biomimetic preparation of silicon quantum dots and their phytophysiology effect on cucumber seedlings. Journal of Materials Chemistry B, 7, 1107–1115. https://doi.org/10.1039/C8TB02981D
Lichtenberg, S. S., Tsyusko, O. V., Palli, S. R., & Unrine, J. M. (2019). Uptake and bioactivity of chitosan/double-stranded RNA Polyplex nanoparticles in Caenorhabditis elegans. Environmental Science & Technology, 53, 3832–3840. https://doi.org/10.1021/acs.est.8b06560
Liu, N., Tang, M., & Ding, J. (2020). The interaction between nanoparticles-protein corona complex and cells and its toxic effect on cells. Chemosphere, 245, 125624. https://doi.org/10.1016/j.chemosphere.2019.125624
Liu, Y., Nie, Y., Wang, J., Wang, J., Wang, X., Chen, S., Zhao, G., Wu, L., & Xu, A. (2018). Mechanisms involved in the impact of engineered nanomaterials on the joint toxicity with environmental pollutants. Ecotoxicology and Environmental Safety, 162, 92–102. https://doi.org/10.1016/j.ecoenv.2018.06.079
López-Vargas, E. R., González-García, Y., Pérez-Álvarez, M., Cadenas-Pliego, G., González-Morales, S., Benavides-Mendoza, A., Cabrera, R. I., & Juárez-Maldonado, A. (2020). Seed priming with carbon nanomaterials to modify the germination, growth, and antioxidant status of tomato seedlings. Agronomy, 10, 639. https://doi.org/10.3390/agronomy10050639
Lu, J., Wang, P., Tian, S., Qian, W., Huang, Y., Wang, Z., Zhu, X., & Cai, Z. (2021). TiO2 nanoparticles enhanced bioaccumulation and toxic performance of PAHs via trophic transfer. Journal of Hazardous Materials, 407, 124834. https://doi.org/10.1016/j.jhazmat.2020.124834
Ma, C., White, J. C., Zhao, J., Zhao, Q., & Xing, B. (2018). Uptake of engineered nanoparticles by food crops: Characterization, mechanisms, and implications. Annual Review of Food Science and Technology, 9, 129–153. https://doi.org/10.1146/annurev-food-030117-012657
Martinez-Ballesta, M. C., Chelbi, N., Lopez-Zaplana, A., & Carvajal, M. (2020). Discerning the mechanism of the multiwalled carbon nanotubes effect on root cell water and nutrient transport. Plant Physiology and Biochemistry, 146, 23–30. https://doi.org/10.1016/j.plaphy.2019.11.008
Medina-Pérez, G., Fernández-Luqueño, F., Campos-Montiel, R. G., Sánchez-López, K. B., Afanador-Barajas, L. N., & Prince, L. (2019). Chapter 2—Nanotechnology in crop protection: Status and future trends. In O. Koul (Ed.), Nano-biopesticides today and future perspectives (pp. 17–45). Academic Press.
Miernicki, M., Hofmann, T., Eisenberger, I., von der Kammer, F., & Praetorius, A. (2019). Legal and practical challenges in classifying nanomaterials according to regulatory definitions. Nature Nanotechnology, 14, 208–216. https://doi.org/10.1038/s41565-019-0396-z
Miralles, P., Church, T. L., & Harris, A. T. (2012). Toxicity, uptake, and translocation of engineered nanomaterials in vascular plants. Environmental Science & Technology, 46, 9224–9239. https://doi.org/10.1021/es202995d
Molnár, Á., Rónavári, A., Bélteky, P., Szőllősi, R., Valyon, E., Oláh, D., Rázga, Z., Ördög, A., Kónya, Z., & Kolbert, Z. (2020). ZnO nanoparticles induce cell wall remodeling and modify ROS/ RNS signalling in roots of brassica seedlings. Ecotoxicology and Environmental Safety, 206, 111158. https://doi.org/10.1016/j.ecoenv.2020.111158
Morales-Díaz, A. B., Ortega-Ortíz, H., Juárez-Maldonado, A., Cadenas-Pliego, G., González-Morales, S., & Benavides-Mendoza, A. (2017). Application of nanoelements in plant nutrition and its impact in ecosystems. Advances in Natural Sciences: Nanoscience and Nanotechnology, 8, 013001. https://doi.org/10.1088/2043-6254/8/1/013001
Mukherjee, S., Mishra, A., & Trenberth, K. E. (2018). Climate change and drought: A perspective on drought indices. Current Climate Change Reports, 4, 145–163. https://doi.org/10.1007/s40641-018-0098-x
Nasser, F., Constantinou, J., & Lynch, I. (2020). Nanomaterials in the environment acquire an “eco-Corona” impacting their toxicity to daphnia magna—A call for updating toxicity testing policies. Proteomics, 20, 1800412. https://doi.org/10.1002/pmic.201800412
Nel, A. E., Mädler, L., Velegol, D., Xia, T., Hoek, E. M. V., Somasundaran, P., Klaessig, F., Castranova, V., & Thompson, M. (2009). Understanding biophysicochemical interactions at the nano–bio interface. Nature Materials, 8, 543–557. https://doi.org/10.1038/nmat2442
Neto, M. E., Britt, D. W., Jackson, K. A., Coneglian, C. F., Cordioli, V. R., Braccini, A. L., Inoue, T. T., & Batista, M. A. (2021). Assessments in early growth of corn seedlings after hausmanite (Mn3O4) nanoscale seed priming. Journal of Plant Nutrition, 1–10. https://doi.org/10.1080/01904167.2021.1871745
Neto, M. E., Britt, D. W., Lara, L. M., Cartwright, A., dos Santos, R. F., Inoue, T. T., & Batista, M. A. (2020). Initial development of corn seedlings after seed priming with nanoscale synthetic zinc oxide. Agronomy, 10, 307. https://doi.org/10.3390/agronomy10020307
Ojeda-Barrios, D. L., Morales, I., Juárez-Maldonado, A., Sandoval-Rangel, A., Fuentes-Lara, L. O., & Benavides-Mendoza, A. (2020). Importance of nanofertilizers in fruit nutrition. In A. K. Srivastava & C. Hu (Eds.), Fruit Crops (pp. 497–508). Elsevier.
Omar, R. A., Afreen, S., Talreja, N., Chauhan, D., & Ashfaq, M. (2019). Impact of nanomaterials in plant systems. In R. Prasad (Ed.), Plant nanobionics: Volume 1, Advances in the understanding of nanomaterials research and applications (pp. 117–140). Springer International Publishing.
Pacheco, I., & Buzea, C. (2018). Nanoparticle uptake by plants: Beneficial or detrimental? In M. Faisal, Q. Saquib, A. A. Alatar, & A. A. Al-Khedhairy (Eds.), Phytotoxicity of nanoparticles (pp. 1–61). Springer International Publishing.
Parsai, T., & Kumar, A. (2020). Tradeoff between risks through ingestion of nanoparticle contaminated water or fish: Human health perspective. Science of the Total Environment, 740, 140140. https://doi.org/10.1016/j.scitotenv.2020.140140
Pérez-Labrada, F., Hernández-Hernández, H., López-Pérez, M. C., González-Morales, S., Benavides-Mendoza, A., & Juárez-Maldonado, A. (2020). Chapter 13—Nanoparticles in plants: Morphophysiological, biochemical, and molecular responses. In D. K. Tripathi, V. Pratap Singh, D. K. Chauhan, S. Sharma, S. M. Prasad, N. K. Dubey, & N. Ramawat (Eds.), Plant life under changing environment (pp. 289–322). Academic Press.
Perry, D., Paulose Nadappuram, B., Momotenko, D., Voyias, P. D., Page, A., Tripathi, G., Frenguelli, B. G., & Unwin, P. R. (2016). Surface charge visualization at viable living cells. Journal of the American Chemical Society, 138, 3152–3160. https://doi.org/10.1021/jacs.5b13153
Piccinno, F., Gottschalk, F., Seeger, S., & Nowack, B. (2012). Industrial production quantities and uses of ten engineered nanomaterials in Europe and the world. Journal of Nanoparticle Research, 14, 1109. https://doi.org/10.1007/s11051-012-1109-9
Rahmatpour, S., Shirvani, M., Mosaddeghi, M. R., Nourbakhsh, F., & Bazarganipour, M. (2017). Dose–response effects of silver nanoparticles and silver nitrate on microbial and enzyme activities in calcareous soils. Geoderma, 285, 313–322. https://doi.org/10.1016/j.geoderma.2016.10.006
Rastogi, A., Zivcak, M., Sytar, O., Kalaji, H. M., He, X., Mbarki, S., & Brestic, M. (2017). Impact of metal and metal oxide nanoparticles on plant: A critical review. Frontiers in Chemistry, 5. https://doi.org/10.3389/fchem.2017.00078
Sabourian, P., Yazdani, G., Ashraf, S. S., Frounchi, M., Mashayekhan, S., Kiani, S., & Kakkar, A. (2020). Effect of physico-chemical properties of nanoparticles on their intracellular uptake. International Journal of Molecular Sciences, 21, 8019. https://doi.org/10.3390/ijms21218019
Santiago EF, Pontes MS, Arruda GJ, Caires ARL, Colbeck I, Maldonado-Rodriguez R, Grillo R (2020) Understanding the interaction of nanopesticides with plants. In: Fraceto LFSS, de Castro VL, Grillo R, Ávila D, Caixeta Oliveira H, Lima R (eds) Nanopesticides: From research and development to mechanisms of action and sustainable use in agriculture. Springer International Publishing, , 69–109.
Saulite, L., Dapkute, D., Pleiko, K., Popena, I., Steponkiene, S., Rotomskis, R., & Riekstina, U. (2017). Nano-engineered skin mesenchymal stem cells: Potential vehicles for tumour-targeted quantum-dot delivery. Beilstein Journal of Nanotechnology, 8, 1218–1230. https://doi.org/10.3762/bjnano.8.123
Shi, Q., Wang, C. L., Zhang, H., Chen, C., Zhang, X., & Chang, X.-L. (2020). Trophic transfer and biomagnification of fullerenol nanoparticles in an aquatic food chain. Environmental Science: Nano, 7, 1240–1251. https://doi.org/10.1039/C9EN01277J
Simon, J., Müller, L. K., Kokkinopoulou, M., Lieberwirth, I., Morsbach, S., Landfester, K., & Mailänder, V. (2018). Exploiting the biomolecular corona: Pre-coating of nanoparticles enables controlled cellular interactions. Nanoscale, 10, 10731–10739. https://doi.org/10.1039/C8NR03331E
Simonin, M., Richaume, A., Guyonnet, J. P., Dubost, A., Martins, J. M. F., & Pommier, T. (2016). Titanium dioxide nanoparticles strongly impact soil microbial function by affecting archaeal nitrifiers. Scientific Reports, 6, 33643. https://doi.org/10.1038/srep33643
Singh, D., & Kumar, A. (2020). Understanding the effect of the interaction of nanoparticles with roots on the uptake in plants. In N. Dasgupta, S. Ranjan, & E. Lichtfouse (Eds.), Environmental nanotechnology (Vol. 3, pp. 277–304). Springer International Publishing.
Song, U., & Kim, J. (2020). Zinc oxide nanoparticles: A potential micronutrient fertilizer for horticultural crops with little toxicity. Horticulture, Environment and Biotechnology, 61, 625–631. https://doi.org/10.1007/s13580-020-00244-8
Spielman-Sun, E., Avellan, A., Bland, G. D., Tappero, R. V., Acerbo, A. S., Unrine, J. M., Giraldo, J. P., & Lowry, G. V. (2019). Nanoparticle surface charge influences translocation and leaf distribution in vascular plants with contrasting anatomy. Environmental Science: Nano, 6, 2508–2519. https://doi.org/10.1039/C9EN00626E
Stanley, S. (2014). Biological nanoparticles and their influence on organisms. Current Opinion in Biotechnology, 28, 69–74. https://doi.org/10.1016/j.copbio.2013.11.014
Su, Y., Ashworth, V., Kim, C., Adeleye, A. S., Rolshausen, P., Roper, C., White, J., & Jassby, D. (2019). Delivery, uptake, fate, and transport of engineered nanoparticles in plants: A critical review and data analysis. Environmental Science: Nano, 6, 2311–2331. https://doi.org/10.1039/C9EN00461K
Tepe, N., & Bau, M. (2014). Importance of nanoparticles and colloids from volcanic ash for riverine transport of trace elements to the ocean: Evidence from glacial-fed rivers after the 2010 eruption of Eyjafjallajökull Volcano, Iceland. Science of the Total Environment, 488–489, 243–251. https://doi.org/10.1016/j.scitotenv.2014.04.083
Tighe-Neira, R., Carmora, E., Recio, G., Nunes-Nesi, A., Reyes-Diaz, M., Alberdi, M., Rengel, Z., & Inostroza-Blancheteau, C. (2018). Metallic nanoparticles influence the structure and function of the photosynthetic apparatus in plants. Plant Physiology and Biochemistry, 130, 408–417. https://doi.org/10.1016/j.plaphy.2018.07.024
Tong, S., & Ebi, K. (2019). Preventing and mitigating health risks of climate change. Environmental Research, 174, 9–13. https://doi.org/10.1016/j.envres.2019.04.012
Uddin, M. N., Desai, F., & Asmatulu, E. (2020). Engineered nanomaterials in the environment: Bioaccumulation, biomagnification and biotransformation. Environmental Chemistry Letters, 18, 1073–1083. https://doi.org/10.1007/s10311-019-00947-0
Vázquez-Núñez, E., López-Moreno, M. L., de la Rosa Álvarez, G., & Fernández-Luqueño, F. (2018). Incorporation of nanoparticles into plant nutrients: The real benefits. In F. López-Valdez & F. Fernández-Luqueño (Eds.), Agricultural nanobiotechnology: Modern agriculture for a sustainable future (pp. 49–76). Springer International Publishing.
Wang, T., Wang, L., Li, X., Hu, X., Han, Y., Luo, Y., Wang, Z., Li, Q., Aldalbahi, A., Wang, L., Song, S., Fan, C., Zhao, Y., Wang, M., & Chen, N. (2017a). Size-dependent regulation of intracellular trafficking of polystyrene nanoparticle-based drug-delivery systems. ACS Applied Materials & Interfaces, 9, 18619–18625. https://doi.org/10.1021/acsami.7b05383
Wang, Y., Chen, R., Hao, Y., Liu, H., Song, S., & Sun, G. (2017b). Transcriptome analysis reveals differentially expressed genes (DEGs) related to lettuce (Lactuca sativa) treated by TiO2/ZnO nanoparticles. Plant Growth Regulation, 83, 13–25. https://doi.org/10.1007/s10725-017-0280-5
Wang, Y., Deng, C., Cota-Ruiz, K., Peralta-Videa, J. R., Sun, Y., Rawat, S., Tan, W., Reyes, A., Hernandez-Viezcas, J. A., Niu, G., Li, C., & Gardea-Torresdey, J. L. (2020). Improvement of nutrient elements and allicin content in green onion (Allium fistulosum) plants exposed to CuO nanoparticles. Science of the Total Environment, 725, 138387. https://doi.org/10.1016/j.scitotenv.2020.138387
Wang, Y.-M., Kinraide, T. B., Wang, P., Hao, X.-Z., & Zhou, D.-M. (2014). Surface electrical potentials of root cell plasma membranes: Implications for ion interactions, rhizotoxicity, and uptake. International Journal of Molecular Sciences, 15, 22661–22677. https://doi.org/10.3390/ijms151222661
Xu, M.-L., Zhu, Y.-G., Gu, K.-H., Zhu, J.-G., Yin, Y., Ji, R., Du, W.-C., & Guo, H.-Y. (2019). Transcriptome reveals the rice response to elevated free air CO2 concentration and TiO2 nanoparticles. Environmental Science & Technology, 53, 11714–11724. https://doi.org/10.1021/acs.est.9b02182
Xun, H., Ma, X., Chen, J., Yang, Z., Liu, B., Gao, X., Li, G., Yu, J., Wang, L., & Pang, J. (2017). Zinc oxide nanoparticle exposure triggers different gene expression patterns in maize shoots and roots. Environmental Pollution, 229, 479–488. https://doi.org/10.1016/j.envpol.2017.05.066
Yan, A., & Chen, Z. (2019). Impacts of silver nanoparticles on plants: A focus on the phytotoxicity and underlying mechanism. International Journal of Molecular Sciences, 20, 1003. https://doi.org/10.3390/ijms20051003
Yan, L., Li, P., Zhao, X., Ji, R., & Zhao, L. (2020). Physiological and metabolic responses of maize (Zea mays) plants to Fe3O4 nanoparticles. Science of the Total Environment, 718, 137400. https://doi.org/10.1016/j.scitotenv.2020.137400
Younes, N. A., Hassan, H. S., Elkady, M. F., Hamed, A. M., & Dawood, M. F. A. (2020). Impact of synthesized metal oxide nanomaterials on seedlings production of three Solanaceae crops. Heliyon, 6, e03188. https://doi.org/10.1016/j.heliyon.2020.e03188
Yue, L., Chen, F., Yu, K., Xiao, Z., Yu, X., Wang, Z., & Xing, B. (2019). Early development of apoplastic barriers and molecular mechanisms in juvenile maize roots in response to La2O3 nanoparticles. Science of the Total Environment, 653, 675–683. https://doi.org/10.1016/j.scitotenv.2018.10.320
Yusefi-Tanha, E., Fallah, S., Rostamnejadi, A., & Pokhrel, L. R. (2020). Zinc oxide nanoparticles (ZnONPs) as a novel nanofertilizer: Influence on seed yield and antioxidant defense system in soil grown soybean (Glycine max cv. Kowsar). Science of the Total Environment, 738, 140240. https://doi.org/10.1016/j.scitotenv.2020.140240
Zhang, C. L., Jiang, H. S., Gu, S. P., Zhou, X. H., Lu, Z. W., Kang, X. H., Yin, L., & Huang, J. (2019). Combination analysis of the physiology and transcriptome provides insights into the mechanism of silver nanoparticles phytotoxicity. Environmental Pollution, 252, 1539–1549. https://doi.org/10.1016/j.envpol.2019.06.032
Zhao, X., Zhang, W., He, Y., Wang, L., Li, W., Yang, L., & Xing, G. (2020). Phytotoxicity of Y2O3 nanoparticles and Y3+ ions on rice seedlings under hydroponic culture. Chemosphere, 127943. https://doi.org/10.1016/j.chemosphere.2020.127943
Zuverza-Mena, N., Martínez-Fernández, D., Du, W., Hernandez-Viezcas, J. A., Bonilla-Bird, N., López-Moreno, M. L., Komárek, M., Peralta-Videa, J. R., & Gardea-Torresdey, J. L. (2017). Exposure of engineered nanomaterials to plants: Insights into the physiological and biochemical responses—a review. Plant Physiology and Biochemistry, 110, 236–264. https://doi.org/10.1016/j.plaphy.2016.05.037
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
1 Electronic Supplementary Material
Dynamic representation of the movement of the ions in the electric double layer (EDL) on the surface of the cell wall (https://antonionarro-my.sharepoint.com/:v:/g/personal/adalberto_benavides_uaaan_edu_mx/ET1Y5QCoqx1IrUF_0esl-IoBVqtNn-X-EodyEi9La2-Msw?e=ntvyVL) (MP4 6502 kb)
Dynamic representation of the formation of a corona of proteins on the NPs’ surface through electrostatic interactions (https://antonionarro-my.sharepoint.com/:v:/g/personal/adalberto_benavides_uaaan_edu_mx/ESobKlPXCAxOhwNDAQcgaGcBV94Z56KBVx8PEBDNBxU1tQ?e=jOGTms) (MP4 6174 kb)
Movement of NPs across the cell surface facilitated by the charges on the corona and cell’s integral proteins (https://antonionarro-my.sharepoint.com/:v:/g/personal/adalberto_benavides_uaaan_edu_mx/EbMf9u5ucLBHs2BEepL5gosB5is76hmwtlkbgDoFA7p5zg?e=s1nLYr) (MP4 7271 kb)
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
González-Morales, S., Cárdenas-Atayde, P.A., Garza-Alonso, C.A., Robledo-Olivo, A., Benavides-Mendoza, A. (2022). Plant Biostimulation with Nanomaterials: A Physiological and Molecular Standpoint. In: Fernandes Fraceto, L., Pereira de Carvalho, H.W., de Lima, R., Ghoshal, S., Santaella, C. (eds) Inorganic Nanopesticides and Nanofertilizers. Springer, Cham. https://doi.org/10.1007/978-3-030-94155-0_5
Download citation
DOI: https://doi.org/10.1007/978-3-030-94155-0_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-94154-3
Online ISBN: 978-3-030-94155-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)