Abstract
Oxygen is probably the most often used drug in medicine in general, especially in critical care. The earth’s atmosphere with 21% oxygen is sufficient in healthy human beings to attain adequate oxygenation, but critically ill patients frequently need extra oxygen administration. The oxygenation status can continuously and non-invasively be monitored as oxygen saturation by pulse oximetry, while oxygen pressure is measured discontinuously and invasively. However, pressure is the driving force of oxygen diffusion and in the high saturation range (97 to 100%), oxygen saturation is an unreliable predictor of pressure. Hyperoxemia may go undetected if only oxygen saturation is measured. When breathing air, the ambient oxygen pressure is reduced via the oxygen cascade to 0.5–3 kPa in the mitochondria were oxygen is used in the tricarboxylic acid cycle to produce energy. Hypoxemia will hamper aerobic glycolysis, causing energy depletion in the cells. Hyperoxia has direct toxic effects on the airways and lungs. Hyperoxemia in the cells and the mitochondria stimulates production of reactive oxygen species, which can cause cellular and organ injury. The results of studies on the effects of liberal or conservative oxygen administration in critically ill patients are not uniform. However, it seems prudent to avoid far-conservative and far-liberal oxygenation values. It is presently unclear if there are specific subgroups within the critically ill population that could benefit from oxygenation targets outside the normoxemia ranges. Two currently ongoing large randomized controlled trials (Mega-ROX and UK-ROX) might answer the question of benefit of different oxygenation targets in specific subgroups.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Oxygen is crucial for the critically ill. These patients have a high risk of hypoxemic harm, which can lead to compensatory hyperoxemia by superfluous oxygen administration. Hyperoxia can cause direct toxic effects on the lungs, whereas hyperoxemia can exert vasoconstrictive effects on the circulation, and lead to cellular and organ injury by increased production of reactive oxygen species (ROS). In the last 5 years, an increasing number of large randomized, controlled trials (RCTs) have been performed to determine optimal oxygenation targets, but the discussion is ongoing. In this chapter, we will elaborate on the physiological and pathophysiological background of oxygen, and subsequently discuss the current status of clinical evidence.
2 Origin of Oxygen in the Earth’s Atmosphere
Over a period of approximately 4.5 billion years, the oxygen fraction (FO2) of the atmosphere of the earth changed from 0 to 0.21. A complex interaction between biological evolution and geology led to the present FO2 and the diversity of life forms on earth [1, 2]. Based on the distance to the sun and the position in the solar system, the earth is within the ‘habitable zone’. Oxygen is an extremely reactive element produced by photosynthesis in cyanobacteria or plants’ chloroplasts. Contemporary plants still use symbiosis with cyanobacteria for photosynthesis. Before the appearance of cyanobacteria, anaerobic microbes used sulfate instead of oxygen for their energetic needs. Approximately 2.45 billion years ago, cyanobacteria took over from anaerobes, producing the extremely reactive element oxygen in the period known as the Great Oxidation Event. After another billion years, during which there were very few new developments (the boring billion), the O2 concentration became high enough for the development and further evolution of animal life. During the history of the earth’s atmosphere, a maximum FO2 of approximately 0.30 has been reached, now stabilizing at 0.21. Its high electronegativity and abundance made oxygen uniquely suitable as the final electron acceptor in a series of transfers from high-energy to low-energy molecular states: the electron transport chain. In this way, the increasing oxygen levels were a prerequisite for the development of many new organisms, including human beings. However, the same levels were toxic for others. Oxygen’s propensity to acquire electrons meant that organisms had to develop antioxidant defenses to prevent inadvertent molecular oxidation and dysfunction. As Paracelsus stated “the dose makes the poison”.
3 Measurements and Estimations of Oxygenation
To estimate the oxygenation status of critically ill patients, several different methods and parameters can be used: oxygen saturation of the blood (SO2) by pulse oximetry (SpO2) or by arterial blood gas analysis (SaO2), oxygen pressure (PO2) by arterial blood gas analysis, oxygen extraction by adding central or mixed venous blood gas analysis (ScvO2 or SvO2), lactate concentration and oxygen delivery (DO2). A pulse oximeter measures SpO2 with use of two near infrared wavelengths combined with the circulatory pulsations. The two wavelengths are differently absorbed by oxygenated (O2-Hb) versus de-oxygenated (H-Hb) hemoglobin. Carbon monoxide bonded to hemoglobin (CO-Hb) is not differentiated from O2-Hb by the two wavelength pulse oximeter and will thus cause a falsely high SpO2 reading. The main advantages of SpO2 measurement are that it can be measured easily, continuously, and non-invasively. Disadvantages are the relative unreliable results in patients with dark skin color [3] and the impossibility to detect hyperoxemia. The method using near infrared wavelengths can also be used directly on blood and with up to four different wavelengths instead of the two used in pulse oximetry, enabling determination of concentrations of methemoglobin and CO-Hb [4]. PO2 can be measured in blood samples with a polarographic electrode that has an electrical resistance varying with PO2, and tissue PO2 can be measured by small polarographic electrodes on the skin or on organs. The major limitation of this application is the restricted depth of the measurements. The advantage of PO2 measurements in arterial blood (PaO2) is its accuracy. In addition, pressure is the driving force of O2 diffusion, making pressure a more relevant parameter than saturation, which is not directly related to O2 diffusion. Disadvantages are the discontinuous and invasive nature of this method. There are equations to calculate PO2 from SO2, and vice versa, for oxygen levels within normal ranges [5, 6]. However, these equations do not completely take into account the effects of temperature, 2,3-diphosphoglycerate, pH and PCO2 (the Bohr effect) on the lateral position (right or left shift) of the oxyhemoglobin dissociation curve and are thus only of limited clinical value. Furthermore, in the high oxygen range with high arterial O2 saturations (SaO2 > 97%) large changes of PO2 are related to extremely small changes of SO2 that cannot be accurately measured. Thus in the SpO2 range > 97% hyperoxemia can go undetected unless PO2 is simultaneously measured. Adequate oxygenation can be estimated by calculating oxygen extraction between arterial and venous blood or by measuring lactate production [7]. Extraction of oxygen and production of lactate depends on severity of hypoxemia, but also on the conservation of tissue perfusion and adequate supply of glucose or other metabolic substrates. Calculating oxygen extraction of the whole body can be done by simultaneous sampling of arterial and central or preferably mixed venous blood. ScvO2 can be sampled relative easily and is a well-documented parameter for assessing the circulation of patients in shock. The problem of SvO2 measurement is the requirement of a pulmonary artery catheter. Lactate is a simple measurement, but its level can be affected by many other variables [8]. Tissue DO2 can be calculated using Hb, SaO2, and PaO2 [(1.34 x Hb x SpO2 x 0.01) + (0.023 x PaO2)] and cardiac output and is about 1000 ml of O2 per minute under normal conditions. DO2 is related to oxygen extraction and to oxygen uptake (VO2), but dependent on many circulatory and metabolic variables.
4 Definition of Hypoxemia, Normoxemia, and Hyperoxemia
Administration of extra oxygen can lead to hyperoxia, which is generally defined as any FO2 >0.21. Supranormal FO2 in normal physiological conditions will lead to hyperoxemia or higher than normal PaO2. The normal range of PaO2 is 10–13.3 kPa. Thus any PaO2 >13.3 kPa can be considered as hyperoxemia and any PaO2 value <10 kPa as hypoxemia. Many different definitions and cut-off values for hypoxemia, normoxemia, and (mild, moderate, or severe) hyperoxemia are used in the O2-literature, which makes comparison of study results very difficult. Due to the shape of the oxyhemoglobin dissociation curve and the weak correlation, especially at the high extreme of the SO2 range, between PaO2 and SO2, the measurement of SO2 is an unreliable method to distinguish mild, moderate, and severe hyperoxemia.
5 Physiology of Oxygen in Humans
The transport and the levels of oxygen (expressed as PO2) from the inspired gas (air and/or O2) to the mitochondria are described in the oxygen cascade (Fig. 6.1). Oxygen transport along the cascade is facilitated by ventilation and circulation on the one hand and by diffusion on the other. The PO2 of dry atmospheric air is 21.1 kPa. In the airways, the air is saturated with H2O at 37 ° C and the inspiratory PO2 (PIO2) drops from 21.1 kPa to 19.9 kPa. The next decrease is caused by the exhalation of carbon dioxide (CO2) in the alveoli. The alveolar carbon dioxide pressure (PACO2) is determined by the alveolar ventilation. Using a simplified alveolar air equation and the respiratory exchange ratio (RQ), the alveolar PAO2 can be calculated to be approximately 13 kPa under normal conditions and when breathing air. Hypoventilation when breathing air may cause a significant decrease in PAO2; hyperventilation on the contrary causes only a relatively small increase of PAO2. This can also be explained by using the alveolar air equation and the PIO2 (19.9 kPa) in H2O saturated air in the airways. The highest PAO2 will be (19.9-PACO2) kPa when breathing air.
Before the circulation takes over the oxygen transport, diffusion of oxygen from the alveoli to the arterial blood is necessary. In normal subjects the alveolar/arterial PO2 gradient (ΔPA-aO2) is limited to less than 2 kPa and is mainly caused by resistance to diffusion. Venous admixture, decreased ventilation/perfusion ratios within the lung, and decreased PAO2 are the most common causes of an increased ΔPA-aO2. The normal arterial O2 pressure (PaO2) is therefore slightly higher than 11 kPa. This is the pressure available for diffusion and is directly related to the small amount of oxygen dissolved in blood (0.0232 ml O2 per 100 ml blood per kPa PO2).
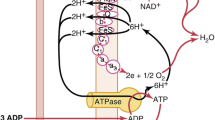
Most oxygen carried by the blood is bound to hemoglobin (maximum 1.39 ml oxygen per gram of hemoglobin at an SO2 of 100%). There is a direct equilibrium between dissolved oxygen in blood and oxygen bound to hemoglobin, determined by the oxyhemoglobin dissociation curve and its lateral position. The next stop in the oxygen cascade is the capillary. Over its length in the tissues, more and more O2 is extracted from the blood by diffusion along a pressure gradient to the tissues. The mean value of PO2 in capillary blood is slightly >5 kPa. Further steps in the cascade are from capillary to tissue and within the cell. Finally, oxygen reaches the mitochondria. Physiological mitochondrial PO2 is estimated to be between 3 and 0.5 kPa. In the cells and the mitochondria, O2 is used by enzymes (oxidases), such as the cytochrome c oxidase system and cytochrome P450. In the mitochondria, O2 is utilized for aerobic metabolism by oxidation of mainly carbohydrates (glucose). The first step of this process is anaerobic glycolysis taking place in the cells and converting glucose into pyruvate while producing only two ATP per glucose molecule. In anaerobic conditions, the next step will be the conversion of pyruvate to lactate. Lactate levels in metabolic acidosis can be used to estimate the severity of disease and to a lesser extent of hypoxia [8, 9]. In aerobic circumstances, pyruvate will enter the tricarboxylic acid (TCA) cycle in the mitochondria allowing oxidative phosphorylation, thus producing as much as 36 ATP for each glucose molecule. Further products of this metabolic pathway are CO2, NADH, FADH2 and H2O.
6 Pathophysiology of Oxygen in Humans: Hypoxemia and Effects of Lack of O2
When critically ill patients become hypoxemic, aerobic glycolysis will be hampered, causing energy depletion, cellular dysfunction, and progressive metabolic lactic acidosis. Longer lasting (chronic) hypoxemia activates intracellular hypoxia-inducible factor, which can activate gene transcription leading to pathophysiological effects opposing hypoxia. These effects include: increased production of hemoglobin through erythropoietin, an increase in vascular growth factors improving tissue perfusion, and sympathetic activation.
7 Pathophysiology of Oxygen in Humans: Hyperoxia, Hyperoxemia, and O2 Toxicity
At a normal atmospheric pressure of 101 kPa, changing FO2 from 0.21 to 1.0 has negligible effects on SaO2 and thus on O2 content (C-O2) of the blood. However, PaO2 will largely increase with an increase in FO2. Hyperoxia therefore will increase the risks of O2 toxicity without significantly increasing tissue DO2. Increasing FO2 is used in clinical medicine to improve oxygenation in cases of hypoventilation, venous admixture or pulmonary pathology with a diffusion disorder. In anemic patients, the factor limiting C-O2 is hemoglobin and administration of red blood cells (RBCs) is far more efficient than an increase in FO2; in patients in shock, tissue DO2 can best be improved by optimizing the circulation. The increase in FO2 results in hyperoxia, which has a direct effect on the airways and lungs [10]. Signs and symptoms of toxicity are tracheobronchial inflammation, retrosternal pain, cough, resorption atelectasis, and finally hyperoxic acute lung injury (HALI) at the alveolar level. Alveolar damage is related to nitric oxide (NO) production increased by inflammation.
Hyperoxemia leads to higher than normal levels of O2 in the cells and mitochondria. At these high levels, mitochondrial O2 can be transformed to ROS, which have unpaired electrons in the outer shell. In vitro experiments showed a strong, exponential correlation between increasing oxygen levels and ROS production in porcine lung mitochondria [11] and capillary endothelial cells from rat lungs [12]. In a reperfusion/re-oxygenation model of cultured hepatocytes, increasing oxygen from 0 to 2% led to a sharp increase in ROS production, whereas further increase in the oxygen content up to 95% induced a steady rise in the formation of ROS [13].
ROS are highly reactive and harmful because they react with intracellular molecules damaging proteins and DNA [14,15,16]. Thus, unopposed ROS react with fatty acids of lipid membranes causing damage to these membranes and lipid peroxidase reactions leading to further cell damage. The effects of ROS may be increased by systemic inflammation, frequently present in critically ill patients. ROS are physiologically inactivated by superoxide dismutase and catalase, enzymes which support the conversion of ROS into H2O and normal O2. ROS can also be inactivated by for example vitamin C, vitamin E, and glutathione in the cells. Antioxidant drugs are pharmacological options to influence ROS formation and deformation. Hyperoxemia not only increases serum ROS but also inflammatory cytokines and is linked to progressive inflammation and organ dysfunction. Oxidative stress in hyperoxemia lowers NO levels and causes vasoconstriction in the microcirculation. Depending on circulatory pathology (the type of shock), vasoconstriction may have different effects on the circulation.
8 Effects of Normoxia, Hypoxia and Hyperoxia in Critically Ill Patients
Based on the effects of hypoxemia and hyperoxemia described above, large numbers of observational and interventional studies have been performed over the last decade in critically ill patients. In 2008, a retrospective observational landmark study described the oxygenation targets in 36,307 ICU patients in 50 ICUs in the Netherlands and the relationships between used FiO2 or achieved PaO2 and clinical outcome [17]. A U-shaped relationship between PaO2 and mortality (corrected for several variables) was found, with the lowest mortality in the PaO2 range from 8.9 to 10.6 kPa. Recently [18], a higher nadir for the relationship between PaO2 and hospital mortality (13.2 kPa) and ICU mortality (13.5 kPa) was reported in ARDS patients. Since 2008, several RCTs have studied the effects of lower versus higher FiO2, PaO2, SaO2 and/or SpO2 in critically ill patients [19,20,21,22,23,24,25]. These RCTs differed in patient selection, target oxygenation in the low and high oxygen groups, and in primary and secondary endpoints. Table 6.1 gives an overview of the characteristics of the RCTs and of two ongoing RCTs.
The first study [19] was a small feasibility study with 103 participants. There were significant differences between PaO2, SaO2, and SpO2 in the experimental and control groups. The authors concluded that conservative oxygenation targets were feasible even though there were significantly more hypoxemic (SpO2 < 88%) and hyperoxemic (SpO2 > 98%) saturations found in the conservative versus the liberal oxygenation groups, respectively. The secondary endpoints (new organ dysfunction, mortality in the ICU and at 90 days) were not significantly different in the two groups in this small study.
The Oxygen-ICU study [20] planned to include 660 patients randomized to normoxic PaO2 and SpO2 targets versus ‘standard practice’ of allowing hyperoxic PaO2 and SpO2 values. The study was stopped prematurely after inclusion of 480 patients of whom 434 were analyzed. The primary endpoint (ICU mortality) was significantly lower in the conservative group (12% vs. 20%). Among the secondary endpoints, new shock, new liver failure, and bloodstream infections occurred less frequently in the conservative group. The authors considered their conclusions to be preliminary due to the smaller than planned size of the analyzed groups.
The HYPERS2S study [21] combined a study of oxygenation targets (hyperoxia at FiO2 of 1.00 compared to SO2 of 88 to 95%) with a study of isotonic versus hypertonic saline infusion in a two-by-two factorial, multicenter RCT in mechanically ventilated patients with septic shock. The primary endpoint of 28-day mortality was not significantly different in the two oxygenation groups. However, the trial was stopped prematurely due to a high number of serious adverse events (SAEs) in the hyperoxia group. Atelectasis occurred significantly more frequent with an FiO2 of 1.00 and there was a potentially important but statistically not significant difference in the incidence of ICU-acquired weakness. The authors concluded that in patients with septic shock an FiO2 of 1.00 might increase the risk of mortality, although at the moment of discontinuation, mortality was not significantly higher in the hyperoxic group.
The ICU-ROX trial [22] included 1000 patients with at least 1 day of mechanical ventilation. Hypoxemia was prevented by setting the lower limit of SpO2 to 90%. In the conservative group, the upper limit of SpO2 was 97% and the FiO2 was lowered to a minimal value of 0.21 as long as SpO2 was >90%. In the usual (conventional) oxygen group, there were no limits to FiO2 or SpO2. The primary endpoint (ventilator-free days at day 28) was not different between the usual and experimental oxygenation groups. Mortality up to 180 days after inclusion was also not different.
In the LOCO2 trial [23], patients with acute respiratory distress syndrome (ARDS) were exposed to either conservative oxygenation targets (PaO2 55–80 mmHg and SpO2 88–92%) or liberal oxygenation targets (PaO2 90 to 105 mmHg and SpO2 ≥96%) for 7 days. Mechanical ventilation strategies were identical in both groups. The primary endpoint of mortality at 28 days was not significantly different between the groups. However, the trial was stopped prematurely because five cases of mesenteric ischemia occurred in the conservative oxygenation group versus none in the liberal oxygenation group and because the mortality at 90 days was higher (44 vs. 30%) in the conservative oxygenation group.
The HOT-ICU [24] is the largest RCT of lower-oxygenation versus higher-oxygenation in critically ill patients up to now, in which 2888 patients admitted to the ICU for hypoxemic respiratory failure were randomized for target PaO2 of either 60 mmHg or 90 mmHg. The primary outcome of mortality after 90 days was not significantly different, at 42.9% and 42.2% in the lower-oxygenation and the higher oxygenation groups, respectively. Secondary endpoints were similar in the two groups.
Recently we published results from an RCT in 400 patients with at least two positive systemic inflammatory response syndrome (SIRS) criteria [25]. Target oxygenation was chosen within the range of clinical practice for both the control and the experimental groups. The control group (high-normal) was targeted for a PaO2 of 14 to 18 kPa and the risk of hyperoxic pulmonary damage was limited by restricting the FiO2 to a maximum of 0.60 in this group as much as clinically possible. The experimental group (low-normal) had a target PaO2 of 8 to 12 kPa. A novel substitute endpoint for organ failure (ranked sequential organ failure assessment [SOFARANK]) was developed based on a previous study [26]. SOFARANK is a ranked outcome of SOFA scores over the first 14 days after randomization and excluding the respiratory component of the SOFA score since that is being influenced by the targeted oxygenation. Organ failure ranking in the two groups was not significantly different (p = 0.06), but tended to favor the high-normal oxygenation target (i.e., the confidence interval was not consistent with clinically important harm from high-normal oxygen target). Mild hypoxemia occurred more often in the low-normal oxygenation group, but severe hypoxemia (PaO2 <5 kPa) was similar in both the oxygenation groups. Other secondary endpoints (duration of mechanical ventilation and mortality) were similar in the two groups.
In a post hoc subgroup analysis of ventilated patients with sepsis from the ICU-ROX study [27], no differences were found between conservative and usual oxygen therapy for the primary endpoint (mortality at day 90) or for any of the secondary endpoints. Point estimates of treatment effects favored usual (conventional) oxygen therapy, in line with our recent results [25].
Since 2014, a number of systematic reviews and meta-analyses [28,29,30,31] including the above mentioned RCTs and other studies in critically ill patients have been published.
In 2014, a meta-analysis was published [28] including 17 observational studies and 1 prospective before-after study. Only four of the included studies specifically addressed critical care patients. Due to the heterogeneity of these four studies, data could not be pooled. Mortality was increased in the hyperoxic groups in the pooled studies of post-cardiac arrest, stroke, and traumatic brain injury patients.
In the IOTA systematic review and meta-analysis [29], 27 RCTs with 16,037 acutely ill patients from several subgroups were included. In-hospital, 30 day, and longest follow-up mortality were significantly increased in the overall liberal oxygen versus the overall conservative oxygenation groups.
In 2021, a meta-analysis including seven RCTs with 5265 patients was published [30]. Longest follow-up mortality was identical in the conservative and conventional oxygenation groups overall. However, in a subgroup analysis of three studies that only included patients with mild to moderate hypoxemia (PaO2/FiO2 >100 mmHg), mortality was significantly lower when using conservative oxygen therapy.
Also in 2021, a network meta-analysis of eight RCTs in mechanically ventilated critically ill patients was performed [31]. Surface under the cumulative ranking (SUCRA) scores and survival curves suggested superiority of the moderate (90–150 mmHg) PaO2 target in the trinary and quadruple classification and also the conservative (70–90 mmHg) PaO2 in the quadruple classification when compared to liberal (>150 mmHg) and far-conservative targets (55–70 mmHg).
9 Future Studies
Two large ongoing RCTs comparing conservative versus conventional oxygenation targets in ICU patients have been registered and are currently recruiting [32, 33]. The Mega-ROX trial [32] aims to include 40,000 adults admitted to the ICU for invasive mechanical ventilation or starting invasive mechanical ventilation after admission. For the control group (liberal oxygen or usual care) the only condition is FiO2 ≥0.30. In the treatment group (conservative oxygen therapy), FiO2 will be decreased to 0.21 if possible, but limited to a minimum acceptable SpO2 of 90% and aiming for a maximum SpO2 of 94 or 95%. The primary endpoint is in-hospital mortality up to 90 days after randomization. Using a response-adaptive randomization procedure, more subjects will be assigned to the group with the lowest mortality during the ongoing period of recruitment, stratified by subgroup. Power calculations were made based on data from the IOTA systematic review and meta-analysis [29] and from the ICU-ROX trial [22]. Heterogeneity of treatment effect in diagnostic subgroups (hypoxic ischemic encephalopathy, acute brain injury, and sepsis) of critically ill patients will be explored.
The UK-ROX trial [33] aims to include 16,500 adult patients admitted to the critical care unit for mechanical ventilation or receiving mechanical ventilation after being admitted for another reason. For the control group (usual oxygen therapy), there are no conditions other than usual care as per local practice. For the intervention group (conservative oxygen therapy), SpO2 values of 88 to 92% are the target. The primary endpoints are mortality at day 90 and economic outcome (incremental costs, quality-adjusted life year (QALY) and net monetary benefit at 90 days). Economic outcome has not been studied previously in this setting.
10 Conclusion
In heterogeneous critically ill patients, the potentially deleterious effects of hyperoxemia found in preclinical research and large observational studies have not been confirmed in well-sized RCTs. In view of the current body of literature, it seems reasonable and prudent to avoid far-conservative and far-liberal oxygenation values. It is presently unknown whether there are specific subgroups or conditions within the critically ill population that might benefit from oxygenation targets outside the normoxemia range. As far as oxygen is concerned half a millennium after the Paracelsus statement ‘‘the dose makes the poison”, it is still not completely clear at what dose the transition from useful drug to dangerous poison takes place.
References
Canfield DE. Oxygen: a four billion year history. Princeton: Princeton University Press; 2014.
Kump LR. The rise of atmospheric oxygen. Nature. 2008;451:277–8.
Sjoding MW, Dickson RP, Iwashyna TJ, Gay SE, Valley TS. Racial bias in pulse oximetry measurement. N Engl J Med. 2020;383:2477–8.
Zijlstra WG, Buursma A, Meeuwsen-van der Roest WP. Absorption spectra of human fetal and adult oxyhemoglobin, de-oxyhemoglobin, carboxyhemoglobin, and methemoglobin. Clin Chem. 1991;37:1633–8.
Kelman GR. Digital computer subroutine for the conversion of oxygen tension into saturation. J Appl Physiol. 1966;21:1375–6.
Sauthier M, Tuli G, Jouvet PA, Brownstein JS, Randolph AG. Estimated Pao2: a continuous and noninvasive method to estimate Pao2 and oxygenation index. Crit Care Explor. 2021;3:e0546.
Vincent JL. Monitoring tissue perfusion. Can J Anaesth. 1996;43:R55–60.
Bakker J, Postelnicu R, Mukherjee V. Lactate: where are we now? Crit Care Clin. 2020;36:115–24.
Kushimoto S, Akaishi S, Sato T, et al. Lactate, a useful marker for disease mortality and severity but an unreliable marker of tissue hypoxia/hypoperfusion in critically ill patients. Acute Med Surg. 2016;3:293–7.
Kallet RH, Matthay MA. Hyperoxic acute lung injury. Respir Care. 2013;58:123–41.
Turrens JF. Mitochondrial formation of reactive oxygen species. J Physiol. 2003;552:335–44.
Brueckl C, Kaestle S, Kerem A, et al. Hyperoxia-induced reactive oxygen species formation in pulmonary capillary endothelial cells in situ. Am J Respir Cell Mol Biol. 2006;34:453–63.
Littauer A, de Groot H. Release of reactive oxygen by hepatocytes on reoxygenation: three phases and role of mitochondria. Am J Phys. 1992;262:G1015–20.
Nakane M. Biological effects of the oxygen molecule in critically ill patients. J Intensive Care. 2020;8:95.
Kulkarni AC, Kuppusamy P, Parinandi N. Oxygen, the lead actor in the pathophysiologic drama: enactment of the trinity of normoxia, hypoxia, and hyperoxia in disease and therapy. Antioxid Redox Signal. 2007;9:1717–30.
Chandel NS, Budinger GR. The cellular basis for diverse responses to oxygen. Free Radic Biol Med. 2007;42:165–74.
de Jonge E, Peelen L, Keijzers PJ, et al. Association between administered oxygen, arterial partial oxygen pressure and mortality in mechanically ventilated intensive care unit patients. Crit Care. 2008;12:R156.
Boyle AJ, Holmes DN, Hackett J, et al. Hyperoxaemia and hypoxaemia are associated with harm in patients with ARDS. BMC Pulm Med. 2021;21:285.
Panwar R, Hardie M, Bellomo R, et al. Conservative versus liberal oxygenation targets for mechanically ventilated patients. A pilot multicenter randomized controlled trial. Am J Respir Crit Care Med. 2016;193:43–51.
Girardis M, Busani S, Damiani E, et al. Effect of conservative vs conventional oxygen therapy on mortality among patients in an intensive care unit: the oxygen-ICU randomized clinical trial. JAMA. 2016;316:1583–9.
Asfar P, Schortgen F, Boisrame-Helms J, et al. Hyperoxia and hypertonic saline in patients with septic shock (HYPERS2S): a two-by-two factorial, multicentre, randomised, clinical trial. Lancet Respir Med. 2017;5:180–90.
Mackle D, Bellomo R, Bailey M, et al. Conservative oxygen therapy during mechanical ventilation in the ICU. N Engl J Med. 2020;382:989–98.
Barrot L, Asfar P, Mauny F, et al. Liberal or conservative oxygen therapy for acute respiratory distress syndrome. N Engl J Med. 2020;382:999–1008.
Schjorring OL, Klitgaard TL, Perner A, et al. Lower or higher oxygenation targets for acute hypoxemic respiratory failure. N Engl J Med. 2021;384:1301–11.
Gelissen H, de Grooth HJ, Smulders Y, et al. Effect of low-normal vs high-normal oxygenation targets on organ dysfunction in critically ill patients: a randomized clinical trial. JAMA. 2021;326:940–8.
de Grooth HJ, Geenen IL, Girbes AR, Vincent JL, Parienti JJ, Oudemans-van Straaten HM. SOFA and mortality endpoints in randomized controlled trials: a systematic review and meta-regression analysis. Crit Care. 2017;21:38.
Young P, Mackle D, Bellomo R, et al. Conservative oxygen therapy for mechanically ventilated adults with sepsis: a post hoc analysis of data from the intensive care unit randomized trial comparing two approaches to oxygen therapy (ICU-ROX). Intensive Care Med. 2020;46:17–26.
Damiani E, Adrario E, Girardis M, et al. Arterial hyperoxia and mortality in critically ill patients: a systematic review and meta-analysis. Crit Care. 2014;18:711.
Chu DK, Kim LH, Young PJ, et al. Mortality and morbidity in acutely ill adults treated with liberal versus conservative oxygen therapy (IOTA): a systematic review and meta-analysis. Lancet. 2018;391:1693–705.
Chen XL, Zhang BL, Meng C, Huang HB, Du B. Conservative oxygen therapy for critically ill patients: a meta-analysis of randomized controlled trials. J Intensive Care. 2021;9:47.
Zhao X, Xiao H, Dai F, Brodie D, Meng L. Classification and effectiveness of different oxygenation goals in mechanically ventilated critically ill patients: network meta-analysis of randomised controlled trials. Eur Respir J. 2021;58:2002928.
The Mega Randomised Registry Trial Comparing Conservative vs. Liberal OXygenation Targets (Mega-ROX). Available at: https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=379432&isReview=true. Accessed 23 Oct 2021.
Intensive care unit randomised trial comparing two approaches to oxygen therapy (UK-ROX). Available at: https://www.isrctn.com/ISRCTN13384956. Accessed 23 Oct 2021.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Gelissen, H.P.M.M., de Grooth, H.J., de Man, A.M.E. (2022). Oxygen: Origin, Physiology, Pathophysiology, and Use in the Critically Ill. In: Vincent, JL. (eds) Annual Update in Intensive Care and Emergency Medicine 2022. Annual Update in Intensive Care and Emergency Medicine. Springer, Cham. https://doi.org/10.1007/978-3-030-93433-0_6
Download citation
DOI: https://doi.org/10.1007/978-3-030-93433-0_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-93432-3
Online ISBN: 978-3-030-93433-0
eBook Packages: MedicineMedicine (R0)