Abstract
Background
Hypertrophic cardiomyopathy (H- CM) is hereditary cardiomyopathy that is characterized by left ventricular hypertrophy (LVH). LVH can develop in response to some factors, such as hypertension, aortic stenosis, or diffuse myocardial fibrosis (DMF). Cardiovascular magnetic resonance (CMR) has been widely used to quantify DMF by calculating the extracellular volume fraction (ECV) of the myocardium. Our aim was to investigate whether the severity of ventricular hypertrophy is associated with the degree of DMF in patients with HCM.
Materials and methods
Patients with HCM (n = 125, age 58 ± 16 yrs) and Brugada syndrome (Brs) which was considered as the relative normal heart control (n = 58, age 51 ± 14 yrs) were retrospectively enrolled in this study. Pre- and postcontrast T1 maps and Cine images were acquired at 1.5 T for the assessment of ECV and wall thickness (WT). ECV and WT measurements at 4 different regions, including the anterior, septal, posterior, and lateral wall regions, were measured, and all groups were compared. Regional ECV was correlated with the corresponding regional WT. Group comparison between two groups in ECV and WT were analyzed with the Mann–Whitney U test. Linear regression was used to obtain a correlation coefficient between regional ECV and WT. The statistical tests were two-tailed, and statistical significance was defined as p < 0.05.
Results
For the group comparison, HCM patients showed a significantly higher WT compared to that in controls (11.3 ± 5.1 mm vs. 6.0 ± 2.2 mm, p < 0.001). In addition, HCM patients also revealed significantly higher ECV compared to that in controls (27.5 ± 4.9% vs. 25.0 ± 2.3%, p < 0.001). The regional ECV was significantly correlated with the corresponding regional WT in patients with HCM (r = 0.311, p < 0.001). In contrast, no significant correlation (r = 0.139, p = 0.297) was found between regional ECV and WT in controls.
Conclusion
In this study, we demonstrated that HCM pa tients with higher WT compared with controls. This finding supports that HCM patients have significant LVH. The increased ECV was positively associated with the corresponding regional wall thickness. This finding suggests that the severity of LVH might be associated with the degree of DMF in patients with HCM.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Hypertrophic cardiomyopathy (HCM) is a hereditary heart disease [1, 2]. The main cause is abnormal muscle alignment of the heart muscle [3,4,5], which leads to poor contraction of the heart and compensatory hypertrophy of the myocardium, resulting in thickening of the myocardium and the appearance of ventricular hypertrophy [6, 7]. This hypertrophy and lack of elasticity may lead to difficulty in ventricular relaxation and heart failure [1, 8, 9]. Left ventricular hypertrophy can develop due to factors such as hypertension, aortic stenosis or diffuse myocardial fibrosis (DMF) etc. [10,11,12]. Myocardial fibrosis refers to tissue damage and scarring caused by ischemia, inflammation and hypertension of the myocardium. Previous studies have demonstrated that HCM presents the DMF, which correlate with estimated LV filling pressure, suggesting a mechanistic link between DMF and abnormal diastolic function. However, it remains unknown whether the degree of DMF is associated with LVH in patients with HCM.
Cardiovascular magnetic resonance (CMR) has been widely used to quantify DMF by calculating the extracellular volume fraction (ECV) of the myocardium [13, 14]. In this study, our aim was to investigate whether the severity of LVH is associated with the degree of DMF in patients with HCM.
2 Material and Methods
-
A.
Patient selection
This study is a retrospective analysis and the CMR was performed at the National Taiwan University Hospital. A total of 125 patients who had a clinical evidence of HCM and 58 patients with Brugada syndrome (Brs) which was considered as the relative normal heart control were enrolled in this study. Patient with Brs had abnormal electrical potential activity in the heart, resulting in arrhythmias, but showed no significant different in the structure and function with healthy subjects were treated as control group. All study participants provided written informed consent.
-
B.
CMR sequences
We performed CMR using a clinical 1.5-T scanner (Area 1.5 T, Siemens, Erlangen, Germany). Cine CMR was performed using a segmented balanced steady-state gradient echo pulse sequence with a retrospective electrocardiography (ECG)-R-wave trigger. The scanning parameters were as follows: repetition time (TR), 3.0 ms; echo time (TE), 1.5 ms; matrix size, 256 × 208; and spatial resolution, 1.21 mm. Multiple short-axis (SA) slices were prescribed in the left ventricle (LV) from the mitral orifice to apex with slice thickness of 8 mm and gap of 2 mm. The true temporal resolution was 63 ms, and 30 cardiac phases were reconstructed retrospectively for each slice level.
Myocardial T1 mapping was performed with an electrocardiography (ECG)-triggered modified Look-Locker inversion recovery (MOLLI) sequence before and 10 min after 0.15 mmol/kg intravenous administration of the gadolinium-based contrast agent (Dotarem, Guerbet, France). The MOLLI protocol used 2 Look-Locker cycles to acquire 7 images over 11 heartbeats, and the scanning parameters were as follows: TR/TE, 1.9 ms/1.0 ms; minimum inversion time, 110 ms; inversion time increment, 80 ms; matrix size, 256 × 192; slice thickness, 6 mm; spatial resolution, 1.28 mm; number of inversions, 2; images acquired after first inversion, 5; pause, 4 heartbeats; and images acquired after second inversion, 2 [15]. Three evenly spaced SA slices were acquired from the LV base to the apex. After postcontrast T1 acquisition, late gadolinium enhancement (LGE) images were acquired using an ECG-triggered phase-sensitive inversion recovery prepared segmented fast gradient-echo pulse sequence to identify the focal fibrosis or scarring.
-
C.
Image analysis
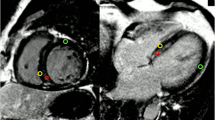
Cine images were used to quantify the LV function and mass by determination of the endocardial and epicardial borders for each diastolic and systolic frame on all SA slices and then normalized by body surface area (BSA). Quantitative analysis of ECV and regional wall thickness (WT) were performed on T1 maps. The ECV was calculated from pre- and postcontrast T1 maps using a region-based method and was then calibrated with the last available hematocrit (Hct) data. Four different regions, including the anterior, septal, posterior and lateral wall regions of the LV, and the area in the central area of the LV cavity were drawn on the T1 map at the mid-ventricular slice. The average T1 values of the segmented regions of interest were then computed. After obtaining the pre- and postcontrast T1 values, ECV values were calculated by using the following formula [16]:
The WTs of the same regions were measured at the same level of the T1 mapping. Group comparisons in LV function, ECV and WT were compared between two group. The correlation between ECV and WT, including averaged and each segment, were assess for each group. In addition, the regional ECV was excluded if the regional myocardium showed hyper-enhancement on the LGE image. Image analysis was performed using software developed in-house provided by MATLAB 7.9 (MathWorks, Inc., Natick, Massachusetts) and Image J (NIH, USA).
-
D.
Statistical analysis
Data are expressed as mean ± standard deviation (SD). Group comparisons in the myocardial ECV, WT and LV functional indexes were tested by the nonparametric Mann–Whitney U test. Linear regression was used to obtain a correlation coefficient between regional ECV and WT for each group. The statistical tests were two-tailed, and statistical significance was defined as p < 0.05. Statistical analyses were performed using SPSS software package version 20.0 (SPSS Inc., Chicago, IL, USA).
3 Results
-
A.
Patient characteristics and Group comparisons
The demographics of the study population are summarized in Table 1. The HCM group was older but body surface area was similar as compared with control group. There were significant differences in LV function and mass between the patient and control groups (Table 1). For the group comparison, HCM group had a significant higher averaged ECV compared to that in control group (27.5 ± 4.9% vs. 25.0 ± 2.3%, p < 0.001). This finding was similar in averaged WT between HCM and control groups (11.3 ± 5.1 mm vs. 6.0 ± 2.3 mm, p < 0.001).
-
B.
Extracellular Volumes and Wall thickness
The regional ECV and regional WT measurements from the four different regions in each group are listed in Table 2. For regional comparisons, the septal region had the highest ECV compared with the other three regions in all the groups. To further investigate the correlation between the myocardial ECV and WT, we performed linear correlation analysis for each group. Our results demonstrated that the regional ECV significantly correlated with the regional WT in HCM group (r = 0.381, p = 0.0001) (Fig. 1). In contrast, no significant correlation (r = 0.139, p = 0.297) was found between regional ECV and WT in control group.
4 Discussion
In this study, we demonstrated that CMR-derived ECV was significantly correlated with the myocardial wall thickness. This finding suggests that LVH is significant associated with the severity of DMF in patients with HCM. Myocardial fibrosis is defined as a significant increase in the collagen content in the myocardium. HCM are known to develop diffuse, as well as regional myocardial fibrosis. Once fibrosis develops, it increases myocardial stiffness and eventually deteriorates diastolic and systolic functions [17, 18].
Our results also showed that ECV varies with the myocardial region, suggested that regional ECV may lead to clinically significant errors if ECV was assessed and compared in different myocardial regions. Therefore, caution must be exercised in estimating the ECV in patients with significant ventricular hypertrophy.
5 Study Limitations
First, our study was done with a single T1 pulse sequence (MOLLI). Different sequences of T1 mapping have been reported to yield different absolute ECV values. Whether our findings are identical in different pulse sequences is unknown and needs further investigation. Second, regional variation of ECV was only performed in one representative mid-ventricular slice, future studies should consider apical and basal slices for more comprehensive assessments. Third, although all subjects were carefully controlled in data acquisition and analysis, our results do not have histological evidence to support the suggestion that this interregional variation of ECV reflects a pathophysiological difference rather than a technical difference.
6 Conclusions
In conclusion, our study demonstrated that CMR-derived ECVs vary with myocardial region in patient with HCM. This interregional variation is associated with the severity of ventricular hypertrophy. Regional ECV is feasible for differentiating myocardial abnormalities and the position of the ROI is essential for comparing ROIs within the same region.
References
Maron BJ (2002) Hypertrophic cardiomyopathya systematic review. JAMA 287(10):1308–1320. https://doi.org/10.1001/jama.287.10.1308
Maron BJ et al (2003) American college of cardiology/European society of cardiology clinical expert consensus document on hypertrophic cardiomyopathy: a report of the American college of cardiology foundation task force on clinical expert consensus documents and the European S. J Am Coll Cardiol 42(9):1687–1713. https://doi.org/10.1016/S0735-1097(03)00941-0
Alcalai R, Seidman JG, Seidman CE (2008) Genetic basis of hypertrophic cardiomyopathy: From bench to the clinics. J Cardiovasc Electrophysiol 19(1):104–110. https://doi.org/10.1111/j.1540-8167.2007.00965.x
Seidman CE, Seidman JG (2011) Identifying sarcomere gene mutations in HCM: a personal history. Breast Dis 108(6):1–15. https://doi.org/10.1161/CIRCRESAHA.110.223834.Identifying
Richard P et al (2003) Hypertrophic cardiomyopathy distribution of disease genes, spectrum of mutations, and implications for a molecular diagnosis strategy. https://doi.org/10.1161/01.CIR.0000066323.15244.54
M. S.Maron et al., “Hypertrophic Cardiomyopathy Phenotype Revisited After 50 Years With Cardiovascular Magnetic Resonance,” J. Am. Coll. Cardiol., vol. 54, no. 3, pp. 220–228, 2009, doi: https://doi.org/10.1016/j.jacc.2009.05.006.
Klues HG, Schiffers A, Maron BJ (1995) Phenotypic spectrum and patterns of left ventricular hypertrophy in hypertrophic cardiomyopathy: morphologic observations and significance as assessed by two-dimensional echocardiography in 600 patients. J Am Coll Cardiol 26(7):1699–1708. https://doi.org/10.1016/0735-1097(95)00390-8
Maron BJ, Rowin EJ, Udelson JE, Maron MS (2018) Clinical spectrum and management of heart failure in hypertrophic cardiomyopathy. JACC: Heart Failure 6(5). Elsevier Inc., pp. 353–363. https://doi.org/10.1016/j.jchf.2017.09.011
Ellims AH, Iles LM, Ling LH, Hare JL, Kaye DM, Taylor AJ (2012) Diffuse myocardial fibrosis in hypertrophic cardiomyopathy can be identified by cardiovascular magnetic resonance, and is associated with left ventricular diastolic dysfunction. J Cardiovasc Magn Reson 14(1):1. https://doi.org/10.1186/1532-429X-14-76
Moon JC (2017) Left ventricular hypertrophy revisited cell and matrix expansion have disease-specific relationship. Circulation 136:2519–2521. https://doi.org/10.1161/CIRCULATIONAHA.117.029895
Lazzeroni D, Rimoldi O, Camici PG (2016) From left ventricular hypertrophy to dysfunction and failure focus reviews on heart failure. Circ J 80. https://doi.org/10.1253/circj.CJ-16-0062
Lovic D et al (2017) Left ventricular hypertrophy in athletes and hypertensive patients. J Clin Hypertens 19:413–417. https://doi.org/10.1111/jch.12977
Mewton N, Liu CY, Croisille P, Bluemke D, Lima JAC (2011) Assessment of myocardial fibrosis with cardiovascular magnetic resonance. J Am Coll Cardiol 57(8):891–903. https://doi.org/10.1016/j.jacc.2010.11.013
Kellman P, Hansen MS (2014) T1-mapping in the heart: accuracy and precision. https://doi.org/10.1186/1532-429X-16-2
Messroghli DR, Greiser A, Fröhlich M, Dietz R, Schulz-Menger J (2007) Optimization and validation of a fully-integrated pulse sequence for modified look-locker inversion-recovery (MOLLI) T1 mapping of the heart. J Magn Reson Imaging 26(4):1081–1086. https://doi.org/10.1002/jmri.21119
Wong TC et al (2012) Imaging association between extracellular matrix expansion quantified by cardiovascular magnetic resonance and short-term mortality. https://doi.org/10.1161/CIRCULATIONAHA.111.089409
RMA (1998) Pathologic fibrosis and connective tissue matrix in left ventricular hypertrophy due to chronic arterial hypertension in humans. J Hypertens Jul, 6(7):1031–1041. https://doi.org/10.1097/00004872-199816070-00018
Su MYM et al (2014) CMR-verified diffuse myocardial fibrosis is associated with diastolic dysfunction in HFpEF. JACC Card Imag 7(10):991–997. https://doi.org/10.1016/j.jcmg.2014.04.022
Brouwer WP et al (2014) In-vivo T1 cardiovascular magnetic resonance study of diffuse myocardial fibrosis in hypertrophic cardiomyopathy. J Cardiovasc Magn Reson 16(1):1–7. https://doi.org/10.1186/1532-429X-16-28
Acknowledgements
I'm very grateful to my teacher, Mr. Mao-Yuan Su. He taught me a lot about MRI. He also helped me with this research project. I would like to thank Mr. Chiu for his constant encouragement and guidance.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this paper
Cite this paper
Yang, SC., Chiu, HW., Su, MY. (2022). CMR-Derived Extracellular Volume Fraction is Associated with the Ventricular Wall Thickness in Patients with Hypertrophic Cardiomyopathy. In: Lin, KP., Liu, RS., Yang, BH. (eds) Future Trends and Challenges of Molecular Imaging and AI Innovation. FASMI 2020. Springer Proceedings in Physics, vol 272. Springer, Cham. https://doi.org/10.1007/978-3-030-92786-8_4
Download citation
DOI: https://doi.org/10.1007/978-3-030-92786-8_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-92785-1
Online ISBN: 978-3-030-92786-8
eBook Packages: Physics and AstronomyPhysics and Astronomy (R0)