Abstract
We assessed whether cardiac MRI (CMR) and echocardiography (echo) have significant differences measuring left ventricular (LV) wall thickness (WT) in hypertrophic cardiomyopathy (HCM) as performed in the clinical routine. Retrospectively identified, clinically diagnosed HCM patients with interventricular-septal (IVS) pattern hypertrophy who underwent CMR and echo within the same day were included. Left Ventricular WT was measured by CMR in two planes and compared to both echo and contrast echo (cecho). 72 subjects, mean age 50.7 ± 16.2 years, 68 % males. Interventricular septal WT by echo and CMR planes showed good to excellent correlation. However, measurements of the postero-lateral wall showed poor correlation. Bland–Altman plots showed greater maximal IVS WT by echo compared to CMR measurement [SAX = 1.7 mm (−5.8, 9.3); LVOT = 1.1 mm (−5.6, 7.8)]. Differences were smaller between cecho and CMR [SAX = 0.8 mm (−9.2, 10.8); LVOT = −0.2 mm (−10.0, 9.6)]. Severity of WT by quartiles showed greater differences between echo and SAX CMR WT compared to cecho. Echocardiography typically measures greater WT than CMR, with the largest differences in moderate to severe hypertrophy. Contrast echocardiography more closely approximates CMR measurements of WT. These findings have potential clinical implications for risk stratification of subjects with HCM.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Hypertrophic cardiomyopathy (HCM) is the most common genetic cardiac disorder in adults, with a prevalence of 1 in 500 persons in general population [1]. HCM is known to be the most common cause of sudden death in young adults, with annual mortality rate between 1 and 5 % [2].
Measurements of myocardial wall thickness have a critical role for diagnosis, prognosis, and treatment decisions in patients with HCM. Morphologic diagnosis is based on the presence of LV myocardial thickness of ≥15 mm in adults or a septum to posterior wall ratio of >1.5 by echo [3, 4]. Wall thickness has prognostic value, with the magnitude of wall thickness strongly linked to increased risk of sudden cardiac death [5]. The presence of massive hypertrophy >30 mm is an important clinical high-risk marker that is used for ICD placement decision-making. However, in some patients echocardiography is inconclusive due to poor acoustic windows and limited myocardial visualization [6]. In this setting, contrast echocardiography may be used to improve wall definition or patients may be referred to CMR for definitive evaluation. Given its high spatial and contrast resolution and lack of operator dependence, CMR is considered the gold standard for morphologic evaluation in HCM. Previous studies have shown that in general, CMR measurements of maximal wall thickness in HCM are similar, although in some patients MRI was able to detect hypertrophy in the basilar anterolateral wall missed by echo [7].
However, measurements of wall thickness by echo and CMR are not necessarily interchangeable. CMR generally provides better visualization of the endocardial and epicardial borders of the LV which facilitates wall thickness measurements. In our clinical experience, however, we have encountered several cases in which echo significantly overestimated the magnitude of septal hypertrophy compared to CMR, which resulted in a change from high risk to low risk categories and impacted the decision for ICD placement. However, risk stratification guidelines were formulated on the basis of echocardiographic studies that have not yet been translated to CMR. Additionally, in borderline cases with hypertrophy <15 mm, a septal to posterior wall ratio of greater than 1.5 by echocardiography is considered diagnostic of HCM. However, whether this criterion is also true for CMR is uncertain. Further adding to the potential for discrepancy is the fact that LV wall thickness is often measured by different planes on CMR and echo. At our institution and others, maximal septal wall thickness is measured by echo on the parasternal long axis view [8], whereas short-axis measurements are considered the gold standard by CMR. A complete understanding of the effect of these factors on potential measurement differences between CMR and echo is lacking.
Therefore, the purpose of our study was to compare standard echo measurements of estimated LV mass and maximal septal and posterior wall thickness to CMR measurements in both left ventricular outflow tract plane (LVOT) and short-axis planes (SAX) in order to determine inter-modality differences and implications for clinical decision making in HCM. We hypothesize that CMR, given its increased contrast resolution compared to echocardiography and better definition of the septal wall, will demonstrate consistently lower measurements of maximal septal wall thickness compared to echo and that these differences will be greater in the patients with the most extreme wall thickness. In addition, we hypothesize that contrast echocardiography will permit more accurate definition of the wall and have maximal thickness values more closely approximated to CMR.
Materials and methods
This retrospective study was approved by our internal Institutional Review Board (IRB) and was Health Insurance Portability and Accountability Act compliant. Patients referred to the Johns Hopkins HCM Clinic for suspicion of HCM underwent echocardiography and CMR for clinical indications between July 2005 and July 2012. Those who fulfilled the standard diagnostic criteria for HCM were asked to enroll in the Johns Hopkins HCM Registry. The diagnosis of HCM was based on the presence of unexplained LV hypertrophy ≥15 mm wall thickness in the absence of other conditions capable of producing a similar degree of hypertrophy (e.g. moderate-to-severe valvular disease, hypertension). We excluded patient’s <18 years old, those with prior septal myectomy or alcohol septal ablation, and patients with other than septal patterns of HCM including apical HCM cases.
Ninety-one consecutive patients with the diagnosis of basal or mid asymmetric septal hypertrophic cardiomyopathy were enrolled into the HCM registry. Nineteen patients were excluded who did not have CMR and echo on the same day. Therefore, a total of seventy-two patients were analyzed. Imaging analysis was blinded to clinical data. In addition, echo images and CMR images were independently analyzed from each other.
Echocardiography imaging
All echo images were acquired with a commercially available Vivid 7 cardiac ultrasound machine (GE Healthcare, Milwaukee, Wisconsin) with a 3.5-MHz transducer. Patients underwent echo contrast imaging (Definity® Lantheus Medical Imaging, N. Billerica MA or Optison® GE Healthcare, Milwaukee WI) in 65 % of cases to provide improved visualization of the apex. For 25 patients, contrast echocardiography was not performed as imaging quality for the apex was considered adequate.
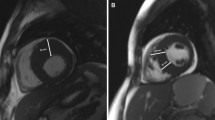
Maximal end-diastolic WT by conventional echo and contrast echo (cecho) were measured on parasternal long axis plane at the basilar and mid antero-septal wall and basilar and mid posterior wall (Fig. 1).
Maximal wall thickness of the basal and mid cavity septum and lateral wall as measured by CMR a left outflow tract view (LVOT), b, c cine short-axis view (SAX), d echocardiography (ECHO), and e contrast echocardiography (cECHO) in end-diastolic phase. Measurements CMR LVOT Base—anterolateral 17.8 mm, posterolateral 11.4 mm; Mid-anterolateral 19.8 mm, posterolateral 12.8 mm. CMR SAX Base—anterolateral 17.2 mm, posterolateral 11.8 mm; Mid-anterolateral 15.2 mm, posterolateral 11.8 mm. ECHO Base—anterolateral 17 mm, posterolateral 11 mm; Mid-anterolateral 18 mm, posterolateral 15 mm. cECHO Base—anterolateral 17 mm, posterolateral 12 mm; Mid-anterolateral 18 mm, posterolateral 12 mm
Cardiac magnetic resonance imaging
All CMR imaging for this study was performed on a 1.5-T MRI (MAGNETOM Avanto, Siemens Healthcare, Erlangen, Germany) system. A standard multi-sequence CMR protocol included breath-hold cine images at 1.5-T using a combined 6-channel torso coil and six channel embedded spine coil. Retrospective, ECG-gated steady state free precession cine images were acquired in the short axis and LVOT planes. Nine to thirteen consecutive MR image sections in the short axis were obtained from the apex to the mitral valve plane covering the whole LV (TR/TE 2.4/1.2 ms; slice thickness 8 mm; flip angle 80°, typical in-plane resolution 1.2 × 1.2 mm). Thirty cardiac phases per slice location were reconstructed with temporal resolution of 30–40 ms.
CMR images were analyzed using Qmass® version 7.4 (Medis, Netherlands) by consensus by 2 experienced investigators, blinded to the echo results and clinical data
Quantification of maximal IVS and PW thickness were measured on LVOT and SAX planes at the base and mid LV cavity for the septum and posterior wall manually in consensus by two readers (CPCV and SLZ). The SAX view was selected because it is widely considered the gold standard for wall thickness measurement. The LVOT plane was selected because it is most similar to the parasternal long axis echocardiographic view (Fig. 1). Short-axis plane measurements of maximal end-diastolic wall thickness were performed manually in segment 2 (basal antero-septal), segment 8 (mid antero-septal), segment 5 (basal infero-lateral) and segment 11 (mid infero-lateral) according to the AHA 17 segment model [9] (Fig. 1).
Statistical analysis
Comparisons between echo, cecho, and CMR for LV wall thickness were performed. For all statistical analysis a p value of <0.05 was considered significant. Continuous variables were expressed by mean ± standard deviation. Two-tailed paired Student t test was used for comparisons. Linear correlation between methods was determined by Pearson correlation (r); <0.3, 0.3–0.5, 0.5–0.7, 0.7–0.9 and ≥0.9 were considered to represent negligible (poor), low positive (poor to fair), moderate positive (fair to good), high positive (excellent) and very high positive (almost perfect) agreement, respectively [10]. Bland–Altman models were used to determine measurement agreement. Statistical analysis was performed with STATA/IC® software (version 12.0) and MedCalc® (Version 12.7.7.0).
Results
Seventy-two subjects were analyzed by CMR and by conventional echo, and 52 subjects were analyzed by contrast echo. Mean age for our cohort was 50.7 ± 16.2 years (range 18–88 years), with 68 % males (n = 49), 53 % Caucasians (n = 38) Table 1.
LV wall thickness measurements
Interventricular septum
Mean septal and posterior WT by echo, LVOT CMR, and SAX CMR are described in Table 2. Overall, mean maximal IVS WT was significantly greater when measured by echo compared to CMR (1.1 ± 3.4 mm for LVOT plane and 1.7 ± 3.8 mm for SAX plane; both p < 0.01). On a per segment basis, mean differences were greatest in the mid septum (2.8 ± 4.5 mm for LVOT and 2.9 ± 4.6 mm for SAX planes; both p < 0.0001), whereas the basilar septal measurements were not significantly different. There was good to excellent linear correlation between echo and either LVOT or SAX CMR for both maximal IVS WT and per-segment measurements (r = 0.52–0.76; all p < 0.001), Table 3. Correlation was consistently better between LVOT views and echo than SAX views for either maximal IVS measurements or segmental IVS WT measures. Bland–Altman plots comparing echo and CMR methods for measurement of maximal IVS WT are shown in Fig. 2. The smallest bias was between echo and LVOT CMR measurements (−1.1 mm; −5.6, 7.8) although SAX bias and limits were of similar magnitude.
A total of 47 patients (65 %) underwent contrast echo measurements. Unlike standard echo, there were no significant differences in mean maximal IVS wall thickness between cecho and either LVOT or SAX CMR measurements (p > 0.05), Table 4. On a per segment basis there were small but significant differences in IVS wall thickness for the basal septum between cecho and CMR in the LVOT plane and for the mid septum in both LVOT and SAX planes (all p < 0.01) Table 4. Similar to conventional echo, there was good to excellent linear correlation between cecho and LVOT CMR for the basilar and mid LV antero-septal wall thickness (r = 0.64, 95 % CI 0.45–0.79; p < 0.0001; r = 0.75, 95 % CI 0.61–0.86; p < 0.0001, respectively) and for SAX CMR (r = 0.63, 95 % CI 0.44–0.77; p < 0.0001; r = 0.74, 95 % CI 0.59–0.85; p < 0.0001, respectively) Table 3. Unlike conventional echo, both LVOT and SAX plane CMR measurements had similar agreement with cecho measurements (Table 3). Bland–Altman plots comparing cecho and both LVOT and SAX planes for CMR measurement of maximal wall thickness are shown in Fig. 2. Compared to conventional echo, cecho shows smaller bias (−0.2 mm for LVOT and 0.8 mm for SAX CMR), however, wider limits of agreement when compared to CMR measurements.
Posterior wall
Unlike septal measurements, there were no significant differences in mean maximal PW WT between conventional echo and either LVOT or SAX CMR measures. On a per segment basis, there were small (< 1 mm) but significant differences for both the basal and mid postero-lateral wall between echo and SAX plane CMR measurements, but not for LVOT plane measurements. The mean difference for these segments was small, both less than 1 mm. Unlike septal measurements, correlation between conventional echo and CMR segmental or overall WT measurements in either plane were either poor or poor to fair (Table 3). For the PW, there was slight negative bias for CMR by LVOT demonstrated on Fig. 2. Bland–Altman plots, however, showed relatively small bias between echo and CMR posterior wall measurements obtained in either LVOT or SAX CMR planes (range −0.5 to 0.1) with limits of agreement of approximately 7 mm in magnitude, similar to septal measurements (Fig. 2).
As with conventional echo, there were no significant differences between cecho measurements of the posterior wall and CMR measures on an overall or segmental basis. Correlation between cecho and CMR measures of the PW were also either poor or poor to fair. Bland–Altman plots, however, showed only small bias between cecho and overall CMR maximal posterior WT measurements in either plane (range −0.2 to 0.4) with similar limits of agreement.
Measurement differences between echo and CMR
We identified four groups for which differences between echo and CMR that could impact diagnosis or affect management. (1) Echo WT measures <15 mm but CMR measures are >15 mm. We did not have any such patients in our cohort due to our entrance criteria (all patients with >15 mm WT by echo). (2) Echo WT measurements are >15 mm but CMR WT is <15 mm. This population may not be diagnosed as HCM if patients undergo a CMR rather than echo at the time of diagnosis. In our cohort 7 cases were discordant, classified as >15 mm by echo but <15 mm by CMR (range 11.0–14.7 mm). (3) Echo WT measurements are <30 mm but CMR WT is >30 mm. This results could impact risk stratification. In our cohort there was 1 case in this group: the WT by echo was 27 mm versus 30 mm by CMR. (4) Echo WT measurements are >30 mm but CMR WT is <30 mm In our group there was one case where WT by echo was 30.0 mm compared to 22.9 by CMR.
Measurement differences according to severity of wall thickness
We divided our cohort by quartiles to determine if there were differences between the most extreme IVS wall thickness measurements as described in Table 5 for conventional echo and Table 6 for cecho. Subjects with in the highest wall thickness quartile (25–32 mm) had the greatest differences between echo and either LVOT (2.6 ± 3.6 mm) or SAX (4.7 ± 3.6 mm) CMR measurements of maximal IVS wall thickness. For cecho there were differences in both extremes, lowest quartile CMR SAX (−3.1 ± 3.1 mm) and LVOT (−1.9 ± 4.5 mm) and highest quartile SAX (3.4 ± 5.5 mm) and LVOT (0.8 ± 5.1 mm) for the IVS wall (Table 6).
Discussion
Identification of patients at higher risk of sudden cardiac death is important for prognosis and treatment in the HCM population. Therefore, accurate assessment of the magnitude of LV hypertrophy with echocardiography and cardiac magnetic resonance imaging is necessary. Although CMR is considered the gold-standard method for LV quantification of volumes and mass [11], echocardiography is the non-invasive test of choice in most centers for HCM diagnosis due to widespread availability and low cost. In the current study, we compared echo-derived estimates of septal wall thickness in patients with HCM to cardiac MRI. Our primary findings were (1) Echocardiographic measurements of myocardial wall thickness are generally higher than CMR measures, especially in patients with moderate to severe hypertrophy, (2) CMR measurements obtained in the LVOT plane are more closely correlated with echocardiographic measurements than SAX plane measurements, and (3) wall thickness differences are smaller for contrast echo compared to CMR, although with greater variability.
Echocardiography has well known technical limitations, such lack of optimal acoustic window in some patients that may limit correct delineation of the endo- and epicardial borders. Septomarginal trabeculae can be mistakenly measured as part of the wall, resulting in artificially increased IVS wall diameter. Finally, obliquity may result in difficulty assessing the epicardial border of the left ventricular free wall [12]. Comparisons of left ventricular wall thickness and left ventricular mass measurements obtained by echocardiography and CMR in HCM have been published [6, 13–18]. However, previous studies have compared echocardiographic vs. CMR images without reference to the views or anatomical landmarks [15, 16] or even though they standardized views for comparison the study was based on a small sample size or old MRI techniques [7, 13, 19, 20].
Our results showed that echo and cecho measurements for the maximal IVS wall thickness are consistently higher than CMR. The differences were smaller with cecho, likely due to better ability to resolve the endocardium. Limits of agreement were greater with cecho, but this may be attributable to a smaller sample size in the current study (47 subjects vs. 72 in the conventional echo group). As expected, CMR measurements obtained in the LVOT plane, similar to the standard echo parasternal long axis view, showed closer correlation with echo measurements. Interestingly, posterior wall measurements were very poorly correlated between echo and CMR.
These results are important to consider for clinical management, given that LV wall thickness is used as a risk factor. The overall magnitude of difference between MRI and echo is small for the cohort (1.7 ± 3.8 mm if SAX measurements are used versus 1.1 ± 3.4 mm if LVOT measurements are used). However, we found that when stratified into quartiles of wall thickness, there were larger differences in patients with extreme wall thickness groups. In the group with the highest wall thickness (>25 mm), echo measurements were 4.7 ± 3.6 mm thicker than SAX CMR measurements and 2.6 ± 3.6 mm thicker than LVOT CMR measurements. This could be sufficient to change a patient from a high risk (>3 cm) to a low risk category, depending on which modality is used for measurements. Indeed, we found 1 case for which CMR WT meaurements would have placed patients in the lower risk category (<30 mm vs. >30 mm on echo) and another patient for which the opposite was true. Since current risk stratification data is based on large outcome studies using echocardiographic data, CMR based wall thickness measurements should be used with caution for the purposes of risk stratification as they will likely underestimate risk given that echocardiographic studies were used to create current guidelines. Similar confusion can be generated when a patient is diagnosed with HCM based on WT by echo and then CMR is performed and WT is <15 mm, not an infrequent clinical scenario that was found in 7 patients from our cohort. There is no current consensus on which study should take diagnostic precedence. Practically, we would consider patients to meet criteria for HCM if they have WT >15 mm on either modality.
Our study comes with some limitations. One of the limitations of our study is that we choose HCM subjects with basilar and mid septal hypertrophy given that is the most frequent phenotype. However, HCM is presented as diverse patterns of wall thickening involving also the anterolateral wall, the posterior wall and the apex. We suspect the same differences would apply for those other segments, but further research will be needed for confirmation We did not include measurements of normal controls, therefore we do not know if these wall thickness differences are specific to HCM or are consistent across different groups. Not all of our patients had both cecho and standard echo, resulting in a smaller sample size in the cecho group.
Conclusion
Echocardiography measures higher wall thickness than CMR in HCM patients with septal hypertrophy, especially those with moderate to severe disease. For these reasons, we suggest that echocardiographically based risk stratification metrics should not be applied to CMR.
Abbreviations
- Echo:
-
Echocardiography
- Cecho:
-
Contrast echocardiography
- LV:
-
Left ventricle
- LVED:
-
LV end-diastolic
- WT:
-
Wall thickness
- HCM:
-
Hypertrophic cardiomyopathy
- IVS:
-
Interventricular-septal
- CMR:
-
Cardiac magnetic resonance imaging
- LVOT:
-
LV outflow tract plane
- SAX:
-
Short axis planes
- LVIDd:
-
LV internal diastolic dimension
- IVSd:
-
Inter-ventricular septum dimension
- PWd:
-
Posterior wall dimension
References
Maron BJ, Maron MS (2013) Hypertrophic cardiomyopathy. Lancet 381:242–255. doi:10.1016/S0140-6736(12)60397-3
Cannan CR, Reeder GS, Bailey KR, Melton LJ 3rd, Gersh BJ (1995) Natural history of hypertrophic cardiomyopathy. A population-based study, 1976 through 1990. Circulation 92:2488–2495
Gersh BJ, Maron BJ, Bonow RO et al (2011) 2011 ACCF/AHA guideline for the diagnosis and treatment of hypertrophic cardiomyopathy: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines developed in collaboration with the American Association for Thoracic Surgery, American Society of Echocardiography, American Society of Nuclear Cardiology, heart failure Society of America, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol 58:e212–e260. doi:10.1016/j.jacc.2011.06.011
Cardim N, Galderisi M, Edvardsen T et al (2015) Role of multimodality cardiac imaging in the management of patients with hypertrophic cardiomyopathy: an expert consensus of the European Association of Cardiovascular Imaging endorsed by the Saudi Heart Association. Eur Heart J Cardiovasc Imaging 16:280. doi:10.1093/ehjci/jeu291
Spirito P, Bellone P, Harris KM, Bernabo P, Bruzzi P, Maron BJ (2000) Magnitude of left ventricular hypertrophy and risk of sudden death in hypertrophic cardiomyopathy. N Engl J Med 342:1778–1785. doi:10.1056/NEJM200006153422403
Fananapazir L, Epstein ND (1995) Prevalence of hypertrophic cardiomyopathy and limitations of screening methods. Circulation 92:700–704
Rickers C, Wilke NM, Jerosch-Herold M et al (2005) Utility of cardiac magnetic resonance imaging in the diagnosis of hypertrophic cardiomyopathy. Circulation 112:855–861. doi:10.1161/CIRCULATIONAHA.104.507723
Lang RM, Badano LP, Mor-Avi V et al (2015) Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 16:233–270. doi:10.1093/ehjci/jev014
Cerqueira MD, Weissman NJ, Dilsizian V et al (2002) Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the cardiac imaging committee of the council on clinical cardiology of the American Heart Association. Circulation 105:539–542
Mukaka MM (2012) Statistics corner: a guide to appropriate use of correlation coefficient in medical research. Malawi Med J 24:69–71
Di Cesare E (2001) MRI of the cardiomyopathies. Eur J Radiol 38:179–184
Maron BJ, Gottdiener JS, Bonow RO, Epstein SE (1981) Hypertrophic cardiomyopathy with unusual locations of left ventricular hypertrophy undetectable by M-mode echocardiography. Identification by wide-angle two-dimensional echocardiography. Circulation 63:409–418
Devlin AM, Moore NR, Ostman-Smith I (1999) A comparison of MRI and echocardiography in hypertrophic cardiomyopathy. Br J Radiol 72:258–264
Katz J, Milliken MC, Stray-Gundersen J et al (1988) Estimation of human myocardial mass with MR imaging. Radiology 169:495–498. doi:10.1148/radiology.169.2.2971985
Park JH, Kim YM, Chung JW, Park YB, Han JK, Han MC (1992) MR imaging of hypertrophic cardiomyopathy. Radiology 185:441–446. doi:10.1148/radiology.185.2.1410351
Pons-Llado G, Carreras F, Borras X, Palmer J, Llauger J, Bayes de Luna A (1997) Comparison of morphologic assessment of hypertrophic cardiomyopathy by magnetic resonance versus echocardiographic imaging. Am J Cardiol 79:1651–1656
Olivotto I, Maron MS, Autore C et al (2008) Assessment and significance of left ventricular mass by cardiovascular magnetic resonance in hypertrophic cardiomyopathy. J Am Coll Cardiol 52:559–566. doi:10.1016/j.jacc.2008.04.047
Rangel I, Goncalves A, de Sousa C et al (2014) Spirito–Maron echocardiographic score: a marker for morphological and physiological assessment of patients with hypertrophic cardiomyopathy. Echocardiography. doi:10.1111/echo.12471
Posma JL, Blanksma PK, van der Wall EE, Hamer HP, Mooyaart EL, Lie KI (1996) Assessment of quantitative hypertrophy scores in hypertrophic cardiomyopathy: magnetic resonance imaging versus echocardiography. Am Heart J 132:1020–1027
Allison JD, Flickinger FW, Wright JC et al (1993) Measurement of left ventricular mass in hypertrophic cardiomyopathy using MRI: comparison with echocardiography. Magn Reson Imaging 11:329–334
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standards
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Rights and permissions
About this article
Cite this article
Corona-Villalobos, C.P., Sorensen, L.L., Pozios, I. et al. Left ventricular wall thickness in patients with hypertrophic cardiomyopathy: a comparison between cardiac magnetic resonance imaging and echocardiography. Int J Cardiovasc Imaging 32, 945–954 (2016). https://doi.org/10.1007/s10554-016-0858-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-016-0858-4