Abstract
The integration of water treatment and disinfection processes is beneficial for reducing energy requirements. The photo-Fenton-like process using UVA light-emitting diode (365 nm) and peroxydisulfate (PDS) was investigated in terms of light fluence (dose) requirements for concurrent degradation of 20 μM emerging organic contaminant (bisphenol A) and inactivation of 105 CFU/mL bacteria (E. coli and E. faecalis) in aqueous solution. The photo-Fenton-like oxidation system {UVA/PDS/Fe2+} was the most efficient for degrading bisphenol A without co-existing bacteria and inactivation of E. faecalis in the absence of bisphenol A. The highest UV fluences of up to 5.7 J/cm2 were obtained for 90% degradation of bisphenol A in the presence of bacteria. These fluences concurrently inactivated 100% co-existing bacterial cells due to their lower fluence requirements for total inactivation (up to 2.1 J/cm2). The lowest fluences were needed for total inactivation of E. coli regardless of the presence of bisphenol A (~1 J/cm2). The obtained fluences are comparable with the literature values for separate degradation or inactivation by other photo-induced advanced oxidation processes at 365 nm. Results show the feasibility and energy-efficiency of the integrated processes for efficient elimination of both chemical and biological contaminants from water.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Pollution of aquatic ecosystems with emerging organic contaminants and pathogenic microflora is of a global environmental concern. To date, advanced oxidation processes (AOPs) are regarded as one of the most efficient means of removing them from wastewater effluents and reducing their level in water bodies. Specifically, the homogenous photo-Fenton-like processes have attracted a significant research attention for water treatment and disinfection. Peroxydisulfate (S2O82−, PDS) and ferrous (Fe2+) ions are efficient and environmentally friendly reagents for producing hydroxyl radicals (•OH) and sulfate radical anions (SO4•–) in water through Fenton-like reactions (1–5):
Ultraviolet (UV) light is employed for activating peroxydisulfate and enhancing the production of SO4•– (6):
The radicals can be used for concurrent water treatment and disinfection due to their dual ability of degrading organic contaminants and inactivating microbial cells. The integration of degradation and inactivation processes is beneficial for reducing the total energy consumption. Over the last few years, there has been a growing research interest in concurrent removal of organic contaminants and pathogenic microorganisms from water and wastewater by different AOPs. Most works focused on the solar-assisted photo-Fenton processes using hydrogen peroxide [1,2,3,4,5]. Regarding photo-Fenton-like processes, recent research showed that the addition of Fe2+, PDS or peroxymonosulfate significantly enhanced the solar degradation of organic contaminants and inactivation of microorganisms in drinking water under mild conditions [6, 7]. It should be pointed out that the target organic pollutants and microorganisms were not treated in a mixture in the above investigations. However, under optimized operating conditions, the photo-Fenton-like treatment with low-pressure mercury lamp (254 nm) provided the concurrent removal of bacteria and organic contaminants from urban wastewater [8].
Meanwhile, switching to energy-efficient and mercury-free light sources, such as UVA/visible light-emitting diodes (LED), is crucial to saving energy and supporting the Minamata Convention on Mercury (2013). To date, UVA diodes (315–400nm) have become rather cost-effective and have a relatively high wall-plug-efficiency [9]. Recently, it was found that the photo-Fenton process with UVA LED was efficient for elimination of propranolol and bacteria from agricultural effluents [10]. However, the photo-Fenton-like systems using PDS towards concurrent degradation and inactivation remain less investigated. Moreover, the energy requirements in terms of light fluences (doses) have been rarely reported in the related literature.
This study aimed at calculating and comparing the UV fluences for degradation of endocrine disrupting compound bisphenol A (BPA) and inactivation of bacteria Escherichia coli and Enterococcus faecalis in the photo-Fenton-like system {UVA/PDS/Fe2+} using UVA LED (365 nm).
2 Materials and Methods
Bisphenol A (99%, Sigma-Aldrich), potassium peroxydisulfate (Vekton, Russia), iron (II) sulfate and sulfuric acid (Khimreaktivsnab, Russia) were used as received. All stock and working solutions were prepared in MilliQ-water (Simplicity®UV system, Millipore). The solvents acetic acid and acetonitrile for HPLC were supplied by Khimreaktivsnab and Cryochrom (Russia), respectively.
The stock suspensions of E. coli K-12 and E. faecalis B 4053 strains (SRIGSIM, Russia) were prepared from the overnight cultures after centrifugation, double washing out and resuspension in phosphate buffered saline (Gibco® Life technologies, UK). The overnight cultures were grown by aerobic incubation in nutrient broth (SRCAMB, Russia) and tryptic soy broth (Merck), respectively.
Experiments were conducted in a thermostabilized glass photoreactor under magnetic stirring. A UVA LED array (Yonton, 100 W, China) was positioned above the open reactor and also thermostated by a circulating water. The LED emission spectrum with a maximum at 365 nm is shown in Fig. 1.
The incident irradiance (at the surface of the sample), measured with a polychromatic ferrioxalate actinometry [11,12,13,14], was 2.46 mW/cm2 over the 300–400 nm range. The model aqueous solution, contaminated with 20 μM BPA and/or 105 CFU/mL E. coli (E. faecalis), was irradiated by UVA LED in the photo-Fenton-like system at pH 5.0 in the presence of PDS and Fe2+ ions. Control experiments were performed under sole UVA irradiation, UVA/PDS and PDS/Fe2+ (dark) treatments. To monitor the degradation and inactivation kinetics, the samples were taken at selected times and analyzed by HPLC (BPA) and plating technique (bacteria) for residual concentrations and counts of survived cells, respectively. The BPA analysis details were described previously [15].
The absorbance spectra for fluence calculations were measured using a Shimadzu UV-1800 spectrophotometer. The fluences were calculated as the product of the average irradiance throughout the water volume (mW/cm2) and the corresponding treatment times (s). In turn, the calculation of average irradiance accounted for the water absorbance and the relative emission spectrum of the LED across the UVA spectrum [16].
3 Results and Discussion
Initially, the efficient Fe2+ dosage was selected in the photo-Fenton-like system {UVA/PDS/Fe2+} by varying the molar ratios at a fixed PDS concentration of 84.5 mg/L (312.5 μM) [17]. The obtained plots of BPA degradation showed that the measurable kinetics was observed at the lowest tested Fe2+ concentration of 1 mg/L (17.4 μM) (Fig. 2). Therefore, a molar ratio of 18:1 (312.5 μM PDS: 17.4 μM Fe2+) was used in further experiments.
Further on, degradation experiments were conducted in the oxidation systems, namely, {UVA} (direct photolysis), {PDS/Fe2+} (dark Fenton-like), {UVA/PDS} and {UVA/PDS/Fe2+}, and the obtained plots are shown in Fig. 3. BPA with two absorption spectrum peaks at 225 and 276 nm was not photolyzed by sole UVA irradiation. Addition of peroxydisulfate lead to generation of SO4•– and complete BPA degradation in 30 min (kBPA, SO4•− = 1.37 × 109 [18]). The highest degradation rate was observed in the UVA/PDS/Fe2+ system, which was 3.7 times higher than that found in the UVA/PDS system. Dark treatment (PDS/Fe2+) resulted in removing approximately 30% by the Fenton-like reaction.
The experiments on BPA degradation in the presence of bacteria were conducted in the most efficient UVA/PDS/Fe2+ system. The pseudo-first-order fluence-based rate constants, which were obtained from the corresponding fluence-based linear plots (not shown), are given in Table 1. Evidently, the degradation rates significantly decreased after adding bacteria. This is consistent with the literature data on inhibition effect of co-existing microorganisms on the degradation of organic contaminants at relatively high initial concentrations at mg/L level [19,20,21,22,23]. These organic substrates are competed with each other for •OH and SO4•–, thereby decreasing the radical levels and exposures.
Additionally, the sorption of compound molecules onto bacterial cells also contributes to the inhibition. This was supported by control experiment (BPA + bacteria), which showed 8–12% BPA decay in 20 min stirring without any exposure.
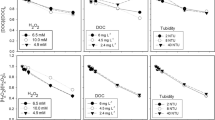
The UV fluences required for 90% degradation were determined from the corresponding fluence-based constants. As shown in Fig. 4, the fluences for BPA removal in the presence of E. coli or E. faecalis are expectedly higher than those obtained without bacteria, increasing to 5.7 J/cm2.
Mostly, the obtained UV fluences were within the range of the previously reported values for degrading organic contaminants by UV365-induced AOPs. Specifically, the fluences for degrading BPA (along with other emerging contaminants) by LED/NO2− and crotamiton by UVA/FeIII-NTA/Oxone were 3.66 [24] and 3.9 J/cm2 [25], respectively. LED/Chlorine treatment required 1.9 J/cm2 for removing 96% acetaminophen [26]. Other literature fluences, which were estimated by multiplying the given irradiance by irradiation time, reached 9.4 J/cm2 for degrading acetamiprid by LED/Fenton [27] and varied in the range of 30.6–122.4 (60–240 min) for decomposing p-hydroxybenzoic acid by LED/TiO2 process [28]. Another estimated fluence of 2.36 J/cm2 (0.15 kJ) was also reported for photocatalytic elimination of sulfamethoxazole, oxytetracyline and 17-α-Ethynylestradiol [29].
Unlike degradation, the plots of E. coli and E. faecalis inactivation did not follow the pseudo-first-order kinetics and some exhibited the lag time, during which no measurable inactivation was observed. Comparing the inactivation rates by sole UVA, UVA/PDS, PDS/Fe2+ and target UVA/PDS/Fe2+ processes, the latter was the fastest for total inactivation of E. faecalis (10 min). Its efficiency for E. coli was similar to the UVA/PDS treatment, achieving the total inactivation in 7 min by both processes. This suggests the predominant contribution of high-intensity UV radiation to inactivation of gram-negative E. coli, which is more sensitive to UV light than gram-positive E. faecalis. Indeed, E. coli was inactivated faster than E. faecalis under sole UVA irradiation (10 versus 20 min). Dark treatment (PDS/Fe2+) did not cause a measurable inactivation of both species.
When BPA was present in the solution, the inactivation rates also decreased that support the statement of competition for radicals between co-existing chemical compounds and microorganisms (Fig. 5). Notably, the inhibition effect of BPA was more pronounced during E. faecalis inactivation.
The UV fluences required for total (100%) bacterial inactivation are depicted in Fig. 6.
Overall, the UV fluences for E. faecalis were higher and reached 2.1 J/cm2. The concurrent presence of organic contaminant prolonged the total inactivation and increased the final fluence by ~30%. Since BPA did not cause the significant inhibition of E. coli inactivation, the corresponding fluence was the same regardless of co-existing contaminant (~1 J/cm2, 7 min). The obtained fluences are substantially lower that those reported in the literature with UVA LED alone. However, only a few studies reported the UV fluences at 365 nm for microbial inactivation by photo-based AOPs. Specifically, the fluences of 0.688–0.870 and 0.105 J/cm2 were needed to attain 3-log [30] and 0.4-log reductions of E. coli by UVA/TiO2 treatment [31]. The reported values are comparable with the obtained fluences for E. coli. Overall, the UV fluences for total inactivation of E. coli and E. faecalis are considerably lower than those found for BPA degradation.
4 Conclusions
The homogenous photo-Fenton-like process using UVA LED and peroxydisulfate is efficient towards the concurrent degradation of BPA and inactivation of E. coli and E. faecalis in model aqueous solution. Although the degradation rates substantially decreased in the presence of bacteria, the required fluences (doses) are comparable with the previously reported values for degradation of organic contaminants by other UV365-based advanced oxidation processes without any co-existing microorganisms. The UV fluences required for degradation of 90% BPA in the presence of bacterial cells provide their concurrent inactivation. In this regard, degradation and inactivation in a single treatment step appear to be a promising energy-effective process for further up-scaling.
References
Gutiérrez-Zapata HM, Sanabria J, Rengifo-Herrera JA (2017) Addition of hydrogen peroxide enhances abiotic sunlight-induced processes to simultaneous emerging pollutants and bacteria abatement in simulated groundwater using CPC solar reactors. Sol Energy 148:110–116. https://doi.org/10.1016/j.solener.2017.03.068
Villegas-Guzman P, Giannakis S, Rtimi S et al (2017) A green solar photo-Fenton process for the elimination of bacteria and micropollutants in municipal wastewater treatment using mineral iron and natural organic acids. Appl Catal B 219:538–549. https://doi.org/10.1016/j.apcatb.2017.07.066
Alvear-Daza JJ, Sanabria J, Rengifo Herrera JA et al (2018) Simultaneous abatement of organics (2,4-dichlorophenoxyacetic acid) and inactivation of resistant wild and laboratory bacteria strains by photoinduced processes in natural groundwater samples. Sol Energy 171:761–768. https://doi.org/10.1016/j.solener.2018.07.026
Ioannou-Ttofa L, Raj S, Prakash H et al (2019) Solar photo-Fenton oxidation for the removal of ampicillin, total cultivable and resistant E. coli and ecotoxicity from secondary-treated wastewater effluents. Chem Eng J 355:91–102. https://doi.org/10.1016/j.cej.2018.08.057
de la Obra Jiménez I, Giannakis S, Grandjean D et al (2020) Unfolding the action mode of light and homogeneous vs. heterogeneous photo-Fenton in bacteria disinfection and concurrent elimination of micropollutants in urban wastewater, mediated by iron oxides in Raceway Pond Reactors. Appl Catal B 263:118158. https://doi.org/10.1016/j.apcatb.2019.118158
Rodríguez-Chueca J, Giannakis S, Marjanovic M et al (2019) Solar-assisted bacterial disinfection and removal of contaminants of emerging concern by Fe2+-activated HSO5- vs. S2O82- in drinking water. Appl Catal B 248:62–72. https://doi.org/10.1016/j.apcatb.2019.02.018
Marjanovic M, Giannakis S, Grandjean D et al (2018) Effect of μM Fe addition, mild heat and solar UV on sulfate radical-mediated inactivation of bacteria, viruses, and micropollutant degradation in water. Water Res 140:220–231. https://doi.org/10.1016/j.watres.2018.04.054
Rodríguez-Chueca J, García-Cañibano C, Lepistö RJ et al (2019) Intensification of UV-C tertiary treatment: Disinfection and removal of micropollutants by sulfate radical based advanced oxidation processes. J Hazard Mater 372:94–102. https://doi.org/10.1016/j.jhazmat.2018.04.044
Matafonova G, Batoev V (2018) Recent advances in application of UV light-emitting diodes for degrading organic pollutants in water through advanced oxidation processes: a review. Water Res 132:177–189. https://doi.org/10.1016/j.watres.2017.12.079
López-Vinent N, Cruz-Alcalde A, Malvestiti JA et al (2020) Organic fertilizer as a chelating agent in photo-Fenton at neutral pH with LEDs for agricultural wastewater reuse: micropollutant abatement and bacterial inactivation. Chem Eng J 388:124246. https://doi.org/10.1016/j.cej.2020.124246
Calvert JG, Pitts JN Jr (1966) Photochemistry. Wiley, New York
Jin S, Mofidi AA, Linden KG (2006) Polychromatic UV fluence measurement using chemical actinometry, biodosimetry, and mathematical techniques. J Environ Eng 132:831–841. https://doi.org/10.1061/(ASCE)0733-9372(2006)132:8(831)
Kheyrandish A, Taghipour F, Mohseni M (2018) UV-LED radiation modeling and its applications in UV dose determination for water treatment. J Photochem Photobiol A 352:113–121. https://doi.org/10.1016/j.jphotochem.2017.10.047
Keshavarzfathy M, Malayeri AH, Mohseni M et al (2020) UV-LED fluence determination by numerical method for microbial inactivation studies. J Photochem Photobiol A 392:112406. https://doi.org/10.1016/j.jphotochem.2020.112406
Popova SA, Matafonova GG, Batoev VB (2019) Removal of organic micropollutants from water by sonophotolytic activated persulfate process. IOP Conf Ser: Mater Sci Eng 687:066051. https://doi.org/10.1088/1757-899X/687/6/066051
Beck SE, Ryu H, Boczek LA et al (2017) Evaluating UV-C LED disinfection performance and investigating potential dual-wavelength synergy. Water Res 109:207–216. https://doi.org/10.1016/j.watres.2016.11.024
Popova S, Matafonova G, Batoev V (2019) Simultaneous atrazine degradation and E. coli inactivation by UV/S2O82-/Fe2+ process under KrCl excilamp (222 nm) irradiation. Ecotoxicol Environ Saf 169:169–177. https://doi.org/10.1016/j.ecoenv.2018.11.014
Sánchez-Polo M, Abdel daiem MM, Ocampo-Pérez R et al (2013) Comparative study of the photodegradation of bisphenol A by HO(•), SO4(•-) and CO3(•-)/HCO3 radicals in aqueous phase. Sci Tot Environ 463-464:423–431. https://doi.org/10.1016/j.scitotenv.2013.06.012
Moncayo-Lasso A, Mora-Arismendi LE, Rengifo-Herrera JA et al (2012) The detrimental influence of bacteria (E. coli, Shigella and Salmonella) on the degradation of organic compounds (and vice versa) in TiO2 photocatalysis and near-neutral photo-Fenton processes under simulated solar light. Photochem Photobiol Sci 11:821–827. https://doi.org/10.1039/c2pp05290c
Timchak E, Gitis V (2012) A combined degradation of dyes and inactivation of viruses by UV and UV/H2O2. Chem Eng J 192:164–170. https://doi.org/10.1016/j.cej.2012.03.054
Barrera M, Mehrab M, Gilbride KA et al (2012) Photolytic treatment of organic constituents and bacterial pathogens in secondary effluent of synthetic slaughterhouse wastewater. Chem Eng Res Des 90:1335–1350. https://doi.org/10.1016/j.cherd.2011.11.018
Subramanian G, Parakh P, Prakash H (2013) Photodegradation of methyl orange and photoinactivation of bacteria by visible light activation of persulfate using a tris(2,2’-bipyridyl)ruthenium (II) complex. Photochem Photobiol Sci 12:456–466. https://doi.org/10.1039/C2PP25316J
He J, Zeng X, Lan S et al (2019) Reusable magnetic Ag/Fe, N-TiO2/Fe3O4@SiO2 composite for simultaneous photocatalytic disinfection of E. coli and degradation of bisphenol a in sewage under visible light. Chemosphere (2019) 217:869–878. https://doi.org/10.1016/j.chemosphere.2018.11.072
Zhou S, Li L, Wu Y et al (2020) UV365 induced elimination of contaminants of emerging concern in the presence of residual nitrite: roles of reactive nitrogen species. Water Res 178:115829. https://doi.org/10.1016/j.watres.2020.115829
Wang Z, Zhu K, Chen J et al (2021) Oxone activation by UVA-irradiated FeIII-NTA complex: Efficacy, radicals formation and mechanism on crotamiton degradation. Chem Eng J 408:127324. https://doi.org/10.1016/j.cej.2020.127324
Li B, Ma X, Li Q et al (2020) Factor affecting the role of radicals contribution at different wavelengths, degradation pathways and toxicity during UV-LED/chlorine process. Chem Eng J 392:124552. https://doi.org/10.1016/j.cej.2020.124552
de la Obra I, Esteban García B, García Sánchez JL et al (2017) Low cost UVA-LED as a radiation source for the photo-Fenton process: a new approach for micropollutant removal from urban wastewater. Photochem Photobiol Sci 16:72–78. https://doi.org/10.1039/c6pp00245e
Ferreira LC, Fernandes JR, Rodríguez-Chueca J et al (2020) Photocatalytic degradation of an agro-industrial wastewater model compound using a UV LEDs system: kinetic study. J Environ Manage 269:110740. https://doi.org/10.1016/j.jenvman.2020.110740
Malkhasian AYS, Izadifard M, Achari G (2014) Photocatalytic degradation of agricultural antibiotics using a UV-LED light source. J Environ Sci Health B 49:35–40. https://doi.org/10.1080/03601234.2013.836871
Xiong P, Hu J (2013) Inactivation/reactivation of antibiotic-resistant bacteria by a novel UVA/LED/TiO2 system. Water Res 47:4547–4555. https://doi.org/10.1016/j.watres.2013.04.056
Montenegro-Ayo R, Barrios AC, Mondal I et al (2020) Portable point-of-use photoelectrocatalytic device provides rapid water disinfection. Sci Tot Environ 737:140044. https://doi.org/10.1016/j.scitotenv.2020.140044
Acknowledgements
The authors thank Dr. Sara Beck for methodological help and fruitful discussions. This research was conducted within the frame of the Basic Research Program of Baikal Institute of Nature Management SB RAS (Project No. 0273-2021-0006) using the equipment of CCU BINM SB RAS (Ulan-Ude, Russia).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this paper
Cite this paper
Matafonova, G., Popova, S., Tsenter, I., Garkusheva, N., Batoev, V. (2022). Comparison of UV Fluences (365 nm) for Water Treatment by Photo-Fenton-Like Process. In: Radionov, A.A., Ulrikh, D.V., Timofeeva, S.S., Alekhin, V.N., Gasiyarov, V.R. (eds) Proceedings of the 5th International Conference on Construction, Architecture and Technosphere Safety. Lecture Notes in Civil Engineering, vol 168. Springer, Cham. https://doi.org/10.1007/978-3-030-91145-4_44
Download citation
DOI: https://doi.org/10.1007/978-3-030-91145-4_44
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-91144-7
Online ISBN: 978-3-030-91145-4
eBook Packages: EngineeringEngineering (R0)