Abstract
During the last three decades, Copahue volcano has been one of the most studied volcanoes in Argentina and Chile. Extensive research has been devoted to studying the geochemistry of rocks and fluids of the Copahue-Caviahue volcanic complex, paying particular attention to the geochemical behavior of the water system. In this study, 275 published analyses of water geochemistry were compiled, in order to describe and revise the processes that control it. Thus, different processes that were previously described in the main volcanic-hydrological system were reanalyzed, such as ions and elements dilution along the Agrio River; alunite, jarosite and barite precipitation in the headwaters; schwertmannite and basaluminite precipitation in the Caviahue Lake and the lower Agrio River. Also, processes such as the incorporation of some elements (As, Tl and Pb) into waters through magmatic gases, and the absortion/adsortion of As, V, Cr and rare earth elements in the hydroxysulfates precipitates are described for the first time.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
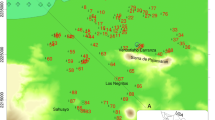
Hydrological systems related to active volcanoes around the world usually present characteristics that reflect the contribution of deep magmatic input (Giggenbach 1992; Rowe 1994; Deely and Sheppard 1996; Kusakabe et al. 2000; D’Alessandro et al. 2008; Varekamp 2015; Tassi et al. 2016). Particularly in the Argentinian Patagonia, the Copahue-Caviahue Volcanic Complex (CCVC) hosts a very uncommon hydrological system, conformed by an acidic river and lake: the Agrio River and the Caviahue Lake (Fig. 1). Copahue volcano (37°51′08′′ S—71°10′03′′ W), one of the most active volcanoes in the country, located inside the Agrio caldera, is constantly emitting volcanic gases. Also, during eruptive events it emits ashes from the eruptive center, affecting the main hydrological system in the area (Naranjo and Polanco 2004; Agusto et al. 2012, 2017). This volcano presented several recent eruptions in the last 30 years (Delpino and Bermúdez 1993; Naranjo and Polanco 2004; Petrinovic et al. 2014; Caselli et al. 2016; Agusto and Velez 2017), being this the reason why Copahue is one of the most studied volcanoes in Argentina (Mas et al. 1996; Linares et al. 1999; Folguera and Ramos 2000; Melnick et al. 2006; Varekamp et al. 2006; Ibáñez et al. 2008; Velez et al. 2011; Agusto et al. 2013; Chiodini et al. 2015; Tamburello et al. 2015; Balbis et al. 2016; Daga et al. 2016; Roulleau et al. 2016, 2017; Tassi et al. 2017; Albite et al. 2019; Barcelona et al. 2019; Lamberti et al. 2019; Báez et al. 2020; Cabrera et al. 2020). In the CCVC, acidic volcanic gases are constantly emitted from the deep magmatic chamber, reaching the surface and interacting with superficial meteoric waters. These gases heat and acidify the waters, developing an acidic shallow hydrothermal system which affects the surface water composition, that was widely studied for over three decades (Mas et al. 1996; Panarello 2002; Agusto et al. 2012, 2017; Varekamp 2008; Varekamp et al. 2009; Pedrozo et al. 2010; Farnfield et al. 2012; Cabrera et al. 2016, 2020; Gaviria Reyes et al. 2016; Temporetti et al. 2019; Candela-Becerra et al. 2020).
In this work, all the available chemical analysis from different authors (Gammons et al. 2005; Parker et al. 2008; Chiacchiarini et al. 2010; Alexander 2014; Agusto and Varekamp 2016; Llano 2016; Rodriguez et al. 2016; Szentiványi 2018; Llano et al. 2020) were compiled, in order to revise the characteristics and processes previously described in the literature. Besides, interpretations of processes that have not been described yet in the system are provided here: such as the incorporation of some trace elements to waters through the gas emission, or the adsorption and absorption processes in the hydroxysulfates which precipitate in the Agrio River and Caviahue Lake.
2 Study Area
2.1 Geological Setting
The CCVC is comprised in the South Volcanic Zone (33° S—46° S) of the Andean range and it is located almost 30 km to the east of the actual volcanic arc front (Folguera et al. 2002; Melnick et al. 2006). The Agrio caldera is part of the CCVC, with Copahue volcano as the most distinctive geographical feature (Fig. 1). This is an active andesitic to basaltic stratovolcano of 1.2 Ma (Linares et al. 1999). It has 9 craters oriented N40°E. Nowadays, the active crater is the one located easternmost. Phreatomagmatic and phreatic eruptions have been constant during the last 250 years (Naranjo and Polanco 2004). The last eruptive cycles occurred in 1992, 1995, 2000 and from 2012-to nowadays (Delpino and Bermúdez 1993, 2002; Petrinovic et al. 2014; Agusto et al. 2017; OAVV 2021). The CCVC water geochemistry is modified by the gas emissions from the magmatic chamber (Agusto 2011; Agusto et al. 2012; Agusto and Varekamp 2016), evidenced by lower pH, higher conductivities and higher ion concentrations during eruptive periods.
Inside the CVCC there is a geothermal zone located in the northeast of the volcano (Fig. 1), that consist of five areas with fluid emissions of boiling, bubbling and mud pools with temperatures up to 96 °C and fumaroles with temperatures up to 160 °C (Agusto et al. 2013; Agusto and Velez 2017).
2.2 The Hydrological System of the Caviahue-Copahue Volcanic Complex
The water bodies inside the Agrio caldera have different geochemical characteristics. Agusto (2011) classified them into three distinct groups: waters from the volcanic hydrological system, steam heated waters of the geothermal zone and snow melted waters. The first group corresponds to the headwaters at the volcano summit and the hydrological system that is originated at this site, being characterized by low pH (0.5–2.5) and high conductivity. High ionic concentrations are also distinctive features of this group, which are controlled by the interactions between the meteoric waters, the magmatic gases and host rock dissolution. The main acid magmatic gases (CO2, SO2, HCl, HF) interact with the aquifer producing the principal anions (SO42−, Cl−, F−), not being in solution HCO3− due to the high water acidity (Agusto and Varekamp 2016). The major cations (Na+, K+, Ca2+, Mg2+), minor cations (Fe, Al), trace elements and rare earth elements (REE) are readily released from the host rock to solution. This process is favored by the low pH and the high temperatures of waters. The snow melted waters group is characterized by meteoric compositions with typical neutral pH and low conductivity values.
The main acidic waters constitute a hydrological system formed in the upper part of the Copahue volcano (Fig. 1). In addition, depending on the volcano activity, a high temperature and low pH volcanic crater lake is developed (Fig. 2a; Agusto and Velez 2017). During the volcano quiescent periods, the active crater usually presents a lake with diameters between 200 and 250 m and 35 m deep (Varekamp 2008). However, its appearance is highly variable depending on seasonal changes and volcanic state of activity (Agusto and Varekamp 2016; Llano et al. 2020). Its usual aspect is gray to green with permanent vapors emission and floating yellow sulfur (Varekamp 2008; Agusto 2011). The hot and acidic aquifer, a shallow volcanic-hydrothermal system developed below the top of the volcanic edifice, feds two springs (pH 1–2) which emerge from the eastern flank (Fig. 2b) and merge downstream to form the Upper Agrio River (Fig. 2c). This river flows from the eastern flank of the volcano for 18 km before reaching the Caviahue Lake (Fig. 2e), which has seasonally controlled temperature and pH values of 2–3. This lake has a horseshoe shape formed by glacial canyons or tectonovolcanic faults, with a maximum depth of approximately 90 m deep (Melnick et al. 2006; Varekamp 2008). It is stratified during summer season with a termocline around 35 m deep, while during winter season is fully mixed. Nevertheless, the lake does not present strong compositional stratification (Varekamp 2008). The only lake effluent is located in the northern arm, and it is called the Lower Agrio River, which merges with other rivers several kilometers outside the Agrio caldera. Along the Agrio River and the Caviahue Lake, the inflow of melted waters is constant (Fig. 2d). This contribution comes from permanent tributaries, such as the Pucón Mahuida stream, Dulce River, Trolope River, Cajón Chico stream and ephemeral tributaries developed during the spring and summer seasons.
3 Methodology
Many authors have studied the hydrological system related to Copahue volcano, providing a large amount of chemical analysis that we have compiled in this work. In this way, 275 water chemical analysis of the CVCC that have been published in the last twenty years (Gammons et al. 2005; Parker et al. 2008; Chiacchiarini et al. 2010; Alexander 2014; Agusto and Varekamp 2016; Llano 2016; Rodriguez et al. 2016; Szentiványi 2018; Llano et al. 2020) were used to describe the system and revise the processes that control water geochemistry. Of the total samples, 241 correspond to the volcanic hydrological system waters and 34 to melted waters. It is important to bear in mind that not all the chemical analyses are complete: most of them do not present trace elements and REE analyses, and there are many gaps in the physico-chemical parameters data.
For a better comparison between the different analyzed elements, a rock sample NV-30 from Las Mellizas formation (Roulleau et al. 2018) was used as reference for the host rock composition. Also, Cl− and Na+ were used as reference for all the binary diagrams, due to their condition of conservative elements (Agusto 2011).
4 Results
Although many of the cited authors have described the system chemistry, only a few have classified the waters using a ternary or a Langelier-Ludwing diagram (Agusto 2011; Agusto and Varekamp 2016; Szentiványi 2018; Llano et al. 2020). Figure 3 corresponds to the ternary diagram where major ionic composition is represented.
Ternary diagrams for the different samples used in this study. Purple: Crater Lake; green: Springs; blue: Upper Agrio River; brown: Caviahue Lake; gray: Lower Agrio River; light blue: Melted waters; black circle: NV-30 rock sample (Roulleau et al. 2018)
Waters from the main volcanic hydrological system are SO42−–Cl−, where samples from the upstream sector of the system, including the crater lake, springs and the Upper Agrio River, have SO42−/Cl− ratios that vary from 0.7 to 10, whereas the Caviahue Lake and the Lower Agrio River exhibit SO42−/Cl− ratios which are more homogeneous, closer to the sulfate corner, with an average of 5.3 (Fig. 3). This may be due to the significant input of melted waters to the lower part of the hydrological system, as this group has an average SO42−/Cl− ratio of 3.8. The reason why melted waters exhibit these ratio values is its null interaction with magmatic gases. However, the interaction between some waters with volcanic ash might be a noteworthy process (Naranjo and Polanco 2004). An example of this is provided by the Pucón Mahuida stream (Fig. 1) where pH is usually below 6 and the F− concentrations are above 2 ppm (Daga et al. 2016; Rodriguez et al. 2016; Szentiványi 2018; Llano et al. 2020). Nevertheless, melted waters composition varies from SO42− to SO42−–HCO3− and to strictly HCO3−.
The cations ternary diagram shows that almost all the samples are depleted on Ca2+ as compared to the NV-30 sample (Fig. 3). On the other hand, some samples show a Mg2+ enrichment, due to the input and dissolution of fresh magmatic material during eruptive events (Varekamp 2008; Agusto et al. 2012; Agusto and Varekamp 2016). All sampling sites show a spread plot, with no preferential cation ratios.
A common process in CCVC waters is the dilution of acidic waters all along the Agrio River due to the constant inflow of melted waters. This phenomenon can be inferred from the diagrams shown in Fig. 4, where a continuous decrease in the conductivity values together with the Cl− concentrations is observed (Fig. 4a), as it happens with the SO42− (Fig. 4b) and the F− (Fig. 4c). Both, Cl− and F− have a conservative behavior, while SO42− participates in the precipitation of different solids, such as alunite or anhydrite in the headwaters (Varekamp 2015; Agusto and Varekamp 2016). Nevertheless, no significant change in SO42− concentrations is noticed, apart from the dilution along the system. Particularly, the pH (Fig. 4d) shows a continuous but more heterogeneous increase along the Agrio River watercourse, with a break around pH 3, particularly in the Lower Agrio River samples.
a Electrical Conductivity, b SO42−, c F− and d pH versus Cl−. The samples tendency is presented with the dilution process. In d its also recognized the change in the pH, breaking the buffer state. Purple: Crater Lake; green: springs; blue: Upper Agrio River; brown: Caviahue Lake; gray: Lower Agrio River; light blue: Melted waters
5 Discussion
5.1 Major Cations
The dilution process affects not only the physico-chemical parameters and the major ions, but also the contents of trace elements and REE. In Fig. 5, major cation concentrations versus Na+ concentrations in the hydrological system are plotted.
a K+, b Ca2+, c Mg2+, d Fe and e Al versus Na+. It is presented the samples tendency with the dilution process. In d and e it is also recognized the hydroxysulfates precipitation due to pH increase. Purple: Crater Lake; green: Springs; blue: Upper Agrio River; brown: Caviahue Lake; gray: Lower Agrio River; light blue: Melted waters; black line: NV-30 rock sample ratio (Roulleau et al. 2018)
Potassium concentrations plot on the line defined by the host rock K-Na+ ratios (Fig. 5a), showing that the incorporation of this element to waters is mainly through the dissolution of the host rock. Nevertheless, some samples of the crater lake and streams show an enrichment and also depletion in this element. This might be due to the precipitation and possibly redissolution of alunite at the hot and acidic headwaters (Gammons et al. 2005; Varekamp 2015; Agusto and Varekamp 2016). Downstream, K+ is only affected by dilution along the hydrological system. Also at the headwaters, the precipitation of anhydrite or gypsum directly affects Ca2+ concentrations (Fig. 5b; Gammons et al. 2005; Varekamp 2008, 2015; Agusto and Varekamp 2016), but in this case the redissolution of these minerals does not occur. For this reason, all waters from the CCVC hydrological system show a depletion in the Ca2+ concentrations compared to the line defined by the host rock Ca-Na ratios. Mg2+ concentrations show a very similar trend to the ratios of NV-30 sample (Fig. 5c), although an enrichment of this element in waters previously and after a volcanic eruption was recognized, through the dissolution of fresh material incorporated to the system (Gammons et al. 2005; Varekamp 2008). Both Ca2+ and Mg2+ concentrations along the main system are affected by the dilution caused by melted waters input.
Regarding Fe concentrations (Fig. 5d), very similar values to the line defined by host rock ratios can be recognized, although precipitation of jarosite is described at the headwaters system (Gammons et al. 2005). Dilution is recognized along the main course of the Agrio River, until Na+ concentrations of nearly 50 ppm are reached. At this point, in some samples from Caviahue Lake and Lower Agrio River, Fe concentrations reach values below 1 ppm, showing a slope break (Fig. 5d). This drop in Fe concentrations is directly related to the precipitation of schwertmannite, which occurs at approximately pH 3 in the study system (Caraballo et al. 2013; Alexander 2014; Agusto and Varekamp 2016; Llano 2016; Rodriguez et al. 2016; Llano et al. 2020). As the volcanic-hydrological system is very dynamic, the geographical position where the system reaches this pH continuously changes, therefore the schwertmannite precipitation occurs at different places through time. This occurs for two main reasons: the eruptive activity of Copahue volcano, which causes a decrease of pH during high activity periods (Agusto et al. 2012; Alexander 2014; Agusto and Varekamp 2016; Szentiványi 2018); and seasonal melted water inflows.
The Al presents a similar behavior to Fe. Along most of the system, Al-Na+ ratios are close to the host rock ratios (Fig. 5e). Particularly, at the headwaters some samples show enriched values due to the redissolution of alunite (Varekamp 2015; Agusto and Varekamp 2016). When the system reaches concentrations near 10 ppm of Na+, a break in the Al-Na+ ratios slope is recognized showing samples with Al concentrations below 1 ppm. In this case, the Al hydroxysulfate precipitation occurs at pH between 4 and 5 (Bigham and Nordstrom 2000; Sánchez-España et al. 2011).
5.2 Trace and Rare Earth Elements
According to their behavior, trace elements in the CCVC hydrological system can be categorized into four different groups: (a) mobile elements; (b) relatively mobile elements; (c) immobile elements; (d) elements that are incorporated into waters through magmatic gases.
Mobile elements consist of Mn, Ni and Zn, and they are represented by Mn in Fig. 6a. Here, water Mn-Na+ ratios are very close to the host rock ones, although a depletion of these trace elements at the headwaters of the system can be recognized. As it was described by Varekamp et al. (2009), Mn and Ni present high concentrations after the 90´s eruptive events. Particularly, Ni has been described as an element that can be incorporated into the shallow hydrothermal system by magmatic gases (Varekamp et al. 2009). However, this phenomenon was not recognized during the last eruptive period which started in 2012. The three elements of this group are affected by dilution process along the system. In melted waters, these elements can precipitate as secondary minerals (Stumm and Morgan 1996), as it is also recognized for some near-neutral Lower Agrio River samples (Fig. 6a).
a Mn, b Sr, c Ba, d As, e RRE and f V versus Na + . The samples tendency according to the dilution process is also presented. In c, d, e and f precipitation processes are also recognized. In d the incorporation of As into waters through magmatic gases is visualized. Purple: Crater Lake; green: Springs; blue: Upper Agrio River; brown: Caviahue Lake; gray: Lower Agrio River; light blue: Melted waters; black line: NV-30 rock sample ratio (Roulleau et al. 2018)
Relatively mobile elements are Sr, Be, Co, Cr, Cs, Rb, Th, U, V and Y (not shown); the behavior of these elements is represented in Fig. 6b by Sr. All of them show ratios close to the line defined by the host rock ratio, but they are slightly more depleted than the mobile elements. Particularly, Rb and Sr can be enriched in waters after eruptive processes, as well as Co and Cr, due to olivine and sulfides dissolution. However, Co can precipitate as oxides or sulfides during non-eruptive periods (Varekamp et al. 2009). Relatively mobile elements concentrations are controlled by the dilution effect.
The group defined as immobile elements includes Ba, Bi, Cu, Hf, Mo, Sn, Ta, Ti and Zr. In Fig. 6c, Ba-Na+ ratios from the water system are represented against the Ba-Na+ ratio of NV-30 sample, showing that the former samples plot far from the latter. This decoupling respect to the host rock ratio is the consequence of Ba precipitation as baryte at the headwaters system (Gammons et al. 2005; Varekamp et al. 2009), while the rest of the elements can precipitate also at the headwaters as secondary minerals like cassiterite (SnO2) or molybdenite (MoS2), or they can be mostly retained in the host rock, as it occurs with Bi, Ti, Ta, Zr and Hf (Gammons et al. 2005; Varekamp et al. 2009).
The last group comprises the elements that are incorporated to the system through the magmatic gases, besides the water–rock interaction. It was recognized in other volcanic environments of the world that some elements (As, B, Cd, Pb, Sb and Tl) can be incorporated into the hydrological system by the gas phase or the aerosols (Aiuppa et al. 2003; Taran et al. 2008; Calabrese et al. 2011; Kaasalainen and Stefánsson 2012; Varekamp 2015). For this reason, these elements can constitute a precursor signal of volcanic activity, though more studies are needed to demonstrate this idea.
Being the As the representative of this group in Fig. 6d, it is recognized that the As-Na+ ratios of most waters are above the As-Na+ ratio of the host rock. The As enrichment is a consequence of its incorporation into waters through the magmatic gases contribution at the headwaters. The other elements that present the same behavior in the system are Tl and Pb.
It must be noted that the behavior evaluation of B, Cd and Sb could not be done due to the lack of data of these element concentrations in the rocks of this area.
In the case of REE, their behavior is similar to the relatively mobile elements, as they plot closely below the line defined by the REE-Na+ ratio of the host rock (Fig. 6e). There is a remarkable break in the slope around Na+ concentrations of 10 ppm, which can be related to hydroxysulfates precipitation. This process will be more discussed in the following section.
5.3 Hydroxysulfates Precipitation
The schwertmannite is an iron hydroxysulfate, very common in acidic waters mainly related to acid mine and rock drainage (Bigham et al. 1996; Yu et al. 1999; Bigham and Nordstrom 2000; Regenspurg et al. 2004; Jönsson et al. 2005; Regenspurg and Peiffer 2005; Sánchez-España 2007; Sánchez-España et al. 2011; Lecomte et al. 2017; Galván et al. 2018), but its occurrence is also recognized in environments related to active volcanic systems (Delmelle and Bernard 2000; Kawano and Tomita 2001; Palmer et al. 2011; Ohsawa et al. 2014). The mineral stoichiometry is Fe8O8(OH)x(SO4)y with 8 − x = 2y and 1 < y < 1.75, it has a characteristic orange color (Fig. 7a) and its structure can vary from a low internal order to amorphous (Sánchez-España et al. 2011). In the Agrio River and Caviahue Lake, schwertmannite was recognized from XRD patterns (Fig. 7b) and from chemical analysis (Alexander 2014; Rodriguez et al. 2016). Llano et al. (2020) calculated the log(KSch) in this particular system, as it was calculated for other systems in other regions of the world (Bigham et al. 1996; Yu et al. 1999; Kawano and Tomita 2001; Majzlan et al. 2004; Regenspurg and Peiffer 2005; Sánchez-España et al. 2011), obtaining two values with different methods. Using the Fe3+ activity versus pH a log(KSch) of 17.17 ± 1.29 was calculated, whereas when using the ionic activity product (IAP) versus pH an average log(IAP) of 17.64 ± 3.42 was obtained. These values are in the same range than the ones calculated by Bigham et al. (1996) of 18 ± 2.5 and by Sánchez-España et al. (2011) of 18.8 ± 1.7 and 18.8 ± 3.5 for different acid mine drainage systems.
a Schwertmannite precipitation at the Lower Agrio River; b XRD samples classified as schwertmannite; c basaluminite precipitation; d XRD sample classified as basaluminite. Sch: schwertmannite; Jar: jarosite; Bas: basalulminite (modified from Llano et al. 2020)
The Al case is different to that of Fe, as it is not well defined which precipitation is present in the Lower Agrio River. Some authors, such as Bigham and Nordstrom (2000) or Sánchez-España et al. (2011) have recognized different species of Al precipitation in other water systems, such as amorphous Al(OH)3, jurbanite (AlSO4(OH)·H2O), and hydrobasaluminite (Al4(SO4)(OH)10·12-36H2O) which recrystallizes to basaluminite (Al4(SO4)(OH)10) when the precipitate gets dehydrated, therefore forming a continuous and diffuse sequence (Sánchez-España et al. 2011). These minerals have been less studied than schwertmannite, especially in the Agrio River system. The precipitate exhibits a white color, very amorphous solid phase (Fig. 7c) with a highly noisy XRD pattern (Fig. 7d) due to its low crystallinity structure (Sánchez-España et al. 2011). Llano et al. (2020) preliminary defined the mineral as basaluminite and a log(KBas) was calculated, obtaining a value of 21.39 ± 2.05 using the Al activity and an average log(IAP) of 23.95 ± 1.26. These values are very similar to the ones obtained in acid mine drainage systems by Adams and Rawajfih (1977) of 21.7 to 24.1 and by Sánchez-España et al. (2011) of 23.9 ± 0.7 and 23.0 ± 2.7.
Analyzing the concentration distributions of the trace elements in the CCVC hydrological system, it is remarkable that most of them are only affected by dilution process. Nevertheless, in the Caviahue Lake and Lower Agrio River, it is recognized that As, Cr and V present a break in the slope in the same way as Fe and Al, being evidenced in Figs. 6d and 6f, with a high correlation between As and V (R2 = 0.99; Farnfield et al. 2012).
The poorly crystalline schwertmannite and basaluminite structures cause the high specific surface area of these precipitates, favoring the adsorption of ions and complexes onto the precipitates surface as it was described by other authors (Sánchez-España et al. 2006, 2011; Wanner et al. 2018). As, V and Cr tend to form oxoanions with high negative charges (AsO43−, VO43− and CrO42−, respectively), so they are favored to be adsorbed as they are attracted by the high density positive charges in the mineral surface. A similar process occurs in the system with P and its oxoanion PO43− (Temporetti et al. 2019). While Cr has the same structure than SO42− anion, therefore the absorption of this oxoanion into minerals internal structure is very common and has been experimentally demonstrated (e.g. Antelo et al. 2012). Many authors support the idea that As and V are adsorbed onto schwertmannite and basaluminite surface, preferentially over Cr (Bigham and Nordstrom 2000; Carlson et al. 2002; Fukushi et al. 2003; Jönsson et al. 2005; Regenspurg and Peiffer 2005; Antelo et al. 2012; Sánchez-España et al. 2016a; Wanner et al. 2018). Conversely, few authors have proposed the incorporation of these elements into the mineral structure (Fukushi et al. 2003; Regenspurg and Peiffer 2005; Antelo et al. 2012). In any case, high concentrations of As and V compared to the rest of trace elements were measured in Copahue schwertmannite (Alexander 2014; Rodríguez et al. 2016). Nevertheless, a significative abundance of Cr was not recognized in schwertmannite composition, therefore it can be predominantly taken in the basaluminite structure at the Agrio River waters (Sánchez-España 2007; Sánchez-España et al. 2016b).
As it was mentioned above, the REE show a break in the slope concentrations along the Agrio River. The adsorption of this group of elements onto colloid surfaces has been described (Lewis et al. 1997; Verplanck et al. 2004), but in acidic systems REE have a conservative behavior until pH 5.5 (Gammons et al. 2005; Wood et al. 2006). Consequently, it is here proposed that neither schwertmannite nor basaluminite precipitation affects the REE concentrations below pH 5, but at higher pH the adsorption of REE onto basaluminite occurs.
In this system, the development of REE complexes diminishes downstream, as the REE are present mostly as free ions (Gammons et al. 2005). But when REE are found in complexes, they are mostly present as LnSO4+ (being Ln any REE), favoring the adsorption of these molecules onto the hydroxo surfaces (Moller 2002; Lozano et al. 2020), and therefore their concentrations are very dependent on the pH. Moreover, heavy REE are more easily adsorbed onto minerals surface than light REE when they interact with the hydroxysulfates precipitates (Gammons et al. 2005; Wood et al. 2006; Lozano et al. 2019). As a result, REE do not coprecipitate with hydroxysulfates at the CCVC hydrological system, but they tend to be adsorbed once the system reaches pH values near 5 to 5.5. Nevertheless, it is recommended a thoroughly analysis of the REE concentrations in schwertmannite and basaluminite to complement this study.
6 Conclusions
This work has compiled most of the available analytical data from studies made at the CCVC hydrological system, describing processes that control the chemistry of the headwaters and along the Agrio River and the Caviahue Lake. Furthermore, an analysis and classification of trace elements, based on their behavior in the system, is provided.
The main characteristics of the system are originated on top of the Copahue volcano, by the interaction between the snow melted waters in the summit and the acidic volcanic gases emitted from the deep magma chamber. These gases acidify and heat the waters of the volcanic-hydrothermal system that feed the crater lake and springs, favoring a more intense water–rock interaction, therefore enriching the elements concentrations in the headwaters of the Agrio River. Some ions and elements precipitate in this part of the system as different secondary minerals, but most of them are not affected by this process. Along the main river, dilution is recognized as the main process that controls all ions and element concentrations. Dilution is caused by the income of melted waters into the main river, affecting the physico-chemical parameters of the hydrological system.
Trace elements have been clustered into four groups, according to their behavior at the headwaters: mobile elements (Mn, Ni, Zn); relatively mobile elements (e.g. Be, Cr, Rb, Sr, etc.); immobile elements (e.g. Ba, Hf, Mo, Zr, etc.); and elements that are incorporated into waters through magmatic gases (As, Tl and Pb, possibly B, Cd and Sb). Particularly, this last group of elements can provide valuable information about the volcanic activity, but further investigations must be carried out.
The other remarkable process that occurs in the hydrological system involves the precipitation of minerals of Fe and Al, which tends to precipitate as hydroxysulfates when water reaches pH values of 3 and 4–5 respectively. These processes also involve As, V and Cr, as they are adsorbed and/or absorbed by the precipitates. Something similar occurs with REE that are adsorbed onto basaluminite mineral surfaces at pH near 5.5.
This study contributes to the knowledge of the volcano-associated hydrological system, compiling most of the geochemical information of waters generated in the last 25 years and providing new interpretations about the processes controlling water characteristics of this area.
References
Adams F, Rawajfih Z (1977) Basaluminite and alunite: a possible cause of sulfate retention by acid soils. Soil Sci Soc Am J 41:686–692
Agusto M (2011) Estudio geoquímico de los fluidos volcánicos e hidrotermales del Complejo volcánico Copahue Caviahue y su aplicación para tareas de seguimiento. Ph.D. Thesis, University of Buenos Aires (in Spanish)
Agusto M, Caselli A, Tassi F, Dos Santos Afonso M, Vaselli O (2012) Seguimiento geoquímico de las aguas ácidas del sistema volcán Copahue-Río Agrio: Posible aplicación para la identificación de precursores eruptivos. RAGA 69:481–495 (in Spanish)
Agusto M, Tassi F, Caselli A, Vaselli O, Rouwet D, Capaccioni B, Caliro S, Chiodini G, Darrah T (2013) Gas geochemistry of the magmatic-hydrothermal fluid reservoir in the Copahue-Caviahue Volcanic Complex (Argentina). J Volcanol Geotherm Res 257:44–56
Agusto MR, Caselli A, Daga R, Varekamp J, Trinelli A, Dos Santos Afonso M, Guevara SR (2017) The crater lake of copahue volcano (Argentina): geochemical and thermal changes between 1995 and 2015. Geol Soc Lond Spec Publ 1:107–130
Agusto M, Varekamp J (2016) The copahue volcanic-hydrotermal system and applications for volcanic surveillance. In: Tassi F, Vaselli O, Caselli A (eds) Copahue volcano, active volcanoes of the world book series. Springer, Berlin, pp 1999–2038
Agusto M, Velez L (2017) Avances en el conocimiento del sistema volcánico-hidrotermal del Copahue: a 100 años del trabajo pionero de don Pablo Groeber. RAGA 74(1):109–124 (in Spanish)
Aiuppa A, Bellomo S, Brusca L, D'Alessandro W, Federico C (2003) Natural and anthropogenic factors affecting groundwater quality of an active volcano (Mt. Etna, Italy). Appl Geochem 18(6):863–882
Albite JM, Vigide NC, Caselli AT (2019) Caracterización de eventos glacivolcánicos en el Complejo volcánico Caviahue-Copahue y en la Formación Hualcupén, provincia del Neuquén. RAGA 76(3):183–198 (in Spanish)
Alexander E (2014) Aqueous geochemistry of an active magmato-hydrothermal system: Copahue Volcano, Río Agrio and Lake Caviahue, Neuquén, Argentina. Undergraduate Thesis, Wesleyan University
Antelo J, Fiol S, Gondar D, López R, Arce F (2012) Comparison of arsenate, chromate and molybdate binding on schwertmannite: Surface adsorption vs anion-exchange. J Colloid Interface Sci 386(1):338–343
Báez AD, Báez W, Caselli AT, Martini MA, Sommer CA (2020) The glaciovolcanic evolution of the Copahue volcano, Andean Southern Volcanic Zone, Argentina-Chile. J Volcanol Geotherm Res 396:106866
Balbis C, Petrinovic IA, Guzmán S (2016) A contribution to the hazards assessment at Copahue volcano (Argentina-Chile) by facies analysis of a recent pyroclastic density current deposit. J Volcanol Geotherm Res 327:288–298
Barcelona H, Yagupsky D, Vigide N, Senger M (2019) Structural model and slip-dilation tendency analysis at the Copahue geothermal system: inferences on the reservoir geometry. J Volcanol Geotherm Res 375:18–31
Bigham JM, Schwertmann U, Traina S, Winland R, Wolf M (1996) Schwertmannite and the chemical modeling of iron in acid sulfate waters. Geochim Cosmochim Acta 60:2111–2121
Bigham JM, Nordstrom DK (2000) Iron and aluminum hydroxusulfates from acid sulfate waters. Rev Mineral Geochem 40:351–403
Cabrera JM, Diaz MM, Schultz S, Temporetti P, Pedrozo F (2016) Iron buffer system in the water column and partitioning in the sediments of the naturally acidic Lake Caviahue, Neuquén, Argentina. J Volcanol Geotherm Res 318:19–26
Cabrera JM, Temporetti PF, Pedrozo FL (2020) Trace metal partitioning and potential mobility in the naturally acidic sediment of Lake Caviahue, Neuquén, Argentina. Andean Geol 47(1):46–60
Calabrese S, Aiuppa A, Allard P, Bagnato E, Bellomo S, Brusca L, D’Alessandro W, Parello F (2011) Atmospheric sources and sinks of volcanogenic elements in a basaltic volcano (Etna, Italy). Geochim Cosmochim Acta 75(23):7401–7425
Candela-Becerra LJ, Toyos G, Suárez-Herrera CA, Castro-Godoy S, Agusto M (2020) Thermal evolution of the Crater Lake of Copahue Volcano with ASTER during the last quiescence period between 2000 and 2012 eruptions. J Volcanol Geotherm Res 392:106752
Caraballo M, Rimstidt D, Macías F, Nieto JM, Hochella Jr M (2013) Metastability, nanocrystallinity and pseudo-solid solution effects on the undestanding of schwertmannite solubility. Chem Geol 360–361:22–31
Carlson L, Bigham JM, Schwertmann U, Kyek A, Wagner F (2002) Scavenging of as from acid mine drainage by schwertmannite and ferrihydrite: a comparison with synthetic analogues. Environ Sci Technol 36(8):1712–1719
Caselli A, Agusto M, Vélez ML, Forte P, Bengoa C, Daga R, Albite JM, Capaccioni B (2016) The 2012 eruption. In: Tassi F, Vaselli O, Caselli A (eds) Copahue volcano, active volcanoes of the world book series. Springer, Berlin, pp 61–77
Chiacchiarini P, Lavalle L, Giaveno A, Donati E (2010) First assessment of acidophilic microorganisms from geothermal Copahue-Caviahue system. Hydrometallurgy 104(3–4):334–341
Chiodini G, Cardellini C, Lamberti MC, Agusto M, Caselli A, Liccioli C, Tamburello G, Tassi F, Vaselli O, Caliro S (2015) Carbon dioxide diffuse emission and thermal energy release from hydrothermal systems at Copahue-Caviahue Volcanic Complex (Argentina). J Volcanol Geotherm Res 304:294–303
D’Alessandro W, Brusca L, Kyriakopoulos K, Michas G, Papadakis G (2008) Methana, the westernmost active volcanic system of the south Aegean arc (Greece): insight from fluids geochemistry. J Volcanol Geotherm Res 178(4):818–828
Daga R, Caselli A, Ribeiro Guevara S, Agusto M (2016) Tefras emitidas durante la fase inicial hidromagmática (julio de 2012) del ciclo eruptivo 2012-actual (2016) del volcán Copahue (Andes del sur). RAGA 74(2):191–206 (in Spanish)
Deely JM, Sheppard DS (1996) Whangaehu river, new Zealand: geochemistry of a river discharging from an active crater lake. Appl Geochem 11(3):447–460
Delmelle P, Bernard A (2000) Volcanic Lakes. In: Sigurdsson H (ed) Encyclopedia of volcanoes. Academic Press, California, pp 877–896
Delpino D, Bermúdez A (1993) La actividad del volcán Copahue durante 1992. Erupción con emisión de azufre piroclástico. Provincia de Neuquén. Dissertation, 12° Congreso Geológico Argentino (in Spanish)
Delpino D, Bermúdez A (2002) La erupción del volcán Copahue del año 2000. Impacto social y al medio natural. Provincia del Neuquén, Argentina. Dissertation, 15° Congreso Geológico Argentino (in Spanish)
Farnfield HR, Marcilla AL, Ward NI (2012) Arsenic speciation and trace element analysis of the volcanic río Agrio and the geothermal waters of Copahue, Argentina. Sci Total Environ 433:371–378
Folguera A, Ramos V (2000) Control estructural del volcán Copahue (38°S–71°O): implicancias tectónicas para el arco volcánico cuaternario (36°S–39°S). RAGA 55:229–244
Folguera A, Ramos VA, Melnick D (2002) Partición de la deformación en la zona del arco volcánico de los Andes neuquinos (36–39 S) en los últimos 30 millones de años. Rev Geol Chile 29(2):227–240
Fukushi K, Sasaki M, Sato T, Yanase N, Amano H, Ikeda H (2003) A natural attenuation of arsenic in drainage from an abandoned arsenic mine dump. Appl Geochem 18(8):1267–1278
Galván F, Murray J, Chiodi A, Pereyra R, Kirschbaum A (2018) Drenaje ácido natural en la caldera Negra Muerta y su influencia en las nacientes del río Calchaquí, provincia de Salta, NO Argentina. RAGA 75:80–94 (in Spanish)
Gammons CH, Wood SA, Pedrozo F, Varekamp J, Nelson BJ, Shope C, Baffico G (2005) Hydrogeochemistry and rare earth element behavior in a volcanically acidified watershed in Patagonia, Argentina. Chem Geol 222:249–267
Gaviria Reyes MA, Agusto M, Trinelli MA, Caselli A, Dos Santos Afonso M, Calabrese S (2016) Estudio hidrogeoquímico de las áreas termales del complejo volcánico Copahue-Caviahue. RAGA 73:256–269 (in Spanish)
Giggenbach WF (1992) Isotopic shifts in waters from geothermal and volcanic systems along convergent plate boundaries and their origin. Earth Planet Sci Lett 113:495–510
Ibáñez JM, Del Pezzo E, Bengoa C, Caselli A, Badi G, Almendros J (2008) Volcanic tremor and local earthquakes at Copahue volcanic complex, Southern Andes, Argentina. J Volcanol Geotherm Res 174(4):284–294
Jönsson J, Persson P, Sjöberg S, Lövgren L (2005) Schwertmannite precipitated from acid mine drainage: phase transformation, sulfate release and surface properties. Appl Geochem 20:179–191
Kaasalainen H, Stefánsson A (2012) The chemistry of trace elements in surface geothermal waters and steam, Iceland. Chem Geol 330:60–85
Kawano M, Tomita K (2001) Geochemical modeling of bacterially induced mineralization of schwertmannite and jarosite in sulfuric acid spring water. Am Min 86:1156–1165
Kusakabe M, Komoda Y, Takano B, Abiko T (2000) Sulfur isotopic effects in the disproportionation reaction of sulfur dioxide in hydrothermal fluids: implications for the δ34S variations of dissolved bisulfate and elemental sulfur from active crater lakes. J Volcanol Geotherm Res 97(1–4):287–307
Lamberti MC, Vigide N, Venturi S, Agusto M, Yagupsky D, Winocur D, Barcelona H, Velez ML, Cardellini C, Tassi F (2019) Structural architecture releasing deep-sourced carbon dioxide diffuse degassing at the Caviahue-Copahue volcanic complex. J Volcanol Geotherm Res 374:131–141
Lecomte K, Maza S, Sarmiento A, Depetris P (2017) Geochemical behavior of an acid drainage system: the case of the Amarillo River, Famatina (La Rioja, Argentina). Environ Sci Pollut Res 24:1630–1647
Lewis AJ, Palmer MR, Sturchio NC, Kemp AJ (1997) The rare earth element geochemistry of acid-sulphate and acid-sulphate-chloride geothermal systems from Yellowstone National Park, Wyoming, USA. Geochim Cosmochim Acta 61(4):695–706
Linares E, Ostera H, Mas L (1999) Cronología K-Ar del complejo efusivo copahue-caviahue, provincia del neuquén. RAGA 54:240–247 (in Spanish)
Llano J (2016) Hidrogeoquímica de las aguas ácidas el río Agrio inferior, provincia de Neuquén. Undergraduate Thesis, University of Buenos Aires (in Spanish)
Llano J, Agusto M, Trinelli MA, Tufo A, García S, Velásquez G, Bucarey-Parra C, Delgado Huertas A, Litvak V (2020) Procesos hidrogeoquímicos vinculados a un ambiente volcánico activo: el caso del sistema río Agrio-Volcán Copahue. RAGA 77(4):490–504
Lozano A, Ayora C, Fernandez-Martinez A (2019) Sorption of rare earth elements onto basaluminite: the role of sulfate and pH. Geochim Cosmochim Acta 258:50–62
Lozano A, Ayora C, Fernández-Martínez A (2020) Sorption of rare earth elements on schwertmannite and their mobility in acid mine drainage treatments. Appl Geochem 113:104499
Majzlan J, Navrotsky A, Schwertmann U (2004) Thermodynamics of iron oxides: Part III. Enthalpies of formation and stability of ferrihydrite (∼Fe(OH)3), schwertmannite (∼FeO(OH)3/4 (SO4)1/8), and ε-Fe2O3. Geochim Cosmochim Acta 68(5):1049–1059
Mas GR, Mas LC, Bengochea L (1996) Alteración ácido-sulfática en el campo geotérmico copahue, provincia del neuquén. RAGA 51:78–86 (in Spanish)
Melnick D, Folguera A, Ramos V (2006) Structural control on arc volcanism: the caviahue-copahue complex, central to patagonian andes transition (38°S). J S Am Earth Sci 22:66–88
Möller P (2002) Rare earth elements and yttrium in geothermal fluids. Water Sci Technol 40:97–125
Naranjo J, Polanco E (2004) The 2000 AD eruption of Copahue volcano, southern Andes. Rev Geol Chile 31:279–292
OAVV, Observatorio Argentino de Vigilancia Volcánica (2021) Reporte de actividad volcánica: Volcán Copahue del 1 al 28 de febrero de 2021. In: https://mailchi.mp/c7eee1b239cd/reporte-de-actividad-volcanica-volcan-copahue-febrero-2021. Accessed 15 Feb 2021 (in Spanish)
Ohsawa S, Sugimori K, Yamauchi H, Koeda T, Inaba H, Kataoka Y, Kagiyama T (2014) Bownish discoloration of the summit crater lake of Mt. Shinmoe-dake, Kirichima Volcano, Japan: volcanic-microbial coupled origin. Bull Volcanol 76:809–819
Palmer S, Van Hinsberg V, McKenzie J, Yee S (2011) Characterization of acid river dilution and associated trace element behavior through hydrogeochemical modeling: a case study of the Banyu Pahit River in East Java, Indonesia. Appl Geochem 26:1802–1810
Panarello H (2002) Características isotópicas y termodinámicas de reservorio del campo geotérmico Copahue-Caviahue, provincia de Neuquén. RAGA 57:182–194 (in Spanish)
Parker S, Gammons C, Pedrozo F, Wood S (2008) Diel changes in metal concentrations in a geogenically acidic river: Río Agrio, Argentina. J Volcanol Geotherm Res 178:213–223
Pedrozo FL, Díaz MM, Temporetti PF, Baffico GD, Beamud SG (2010) Características limnológicas de un sistema ácido: río Agrio-Lago Caviahue, Provincia del Neuquén, Argentina. Ecol Austral 20(2):173–184 (in Spanish)
Petrinovic I, Villarosa G, D’Elia L, Guzman S, Paez G, Outes V, Manzoni C, Delmenico A, Balbis C, Carniel R, Hernando I (2014) La erupción del 22 de diciembre de 2012 del volcán Copahue, Neuquén, Argentina: Caracterización del ciclo eruptivo y sus productos. RAGA 71:161–173 (in Spanish)
Regenspurg S, Brand A, Peiffer S (2004) Formation and stability of schwertmannite in acidic pit lakes. Geochim Cosmochim Acta 68:1185–1197
Regenspurg S, Peiffer S (2005) Arsenate and chromate incorporation in schwertmannite. Appl Geochem 20:1226–1239
Rodriguez A, Varekamp J, Van Bergen M, Kading T, Oonk P, Gammons C, Gilmore M (2016) Acid rivers and lakes at caviahue-copahue volcano as potential terrestial analogues for aqueous paleo-envionments on mars. In: Tassi F, Vaselli O, Caselli A (eds) Copahue volcano, active volcanoes of the world book series. Springer, Berlin, pp 141–172
Roulleau E, Tardani D, Sano Y, Takahata N, Vinet N, Bravo F, Muñoz C, Sanchez J (2016) New insight from noble gas and stable isotopes of geothermal/hydrothermal fluids at Caviahue-Copahue Volcanic Complex: boiling steam separation and water-rock interaction at shallow depth. J Volcanol Geotherm Res 328:70–83
Roulleau E, Bravo F, Pinti DL, Barde-Cabusson S, Pizarro M, Tardani D, Muñoz C, Sanchez J, Sano Y, Takahata N, De la Cal F, Esteban C, Morata D (2017) Structural controls on fluid circulation at the Caviahue-Copahue Volcanic Complex (CCVC) geothermal area (Chile-Argentina), revealed by soil CO2 and temperature, self-potential, and helium isotopes. J Volcanol Geotherm Res 341:104–118
Roulleau E, Tardani D, Vlastelic I, Vinet N, Sanchez J, Sano Y, Takahata N (2018) Multi-element isotopic evolution of magmatic rocks from Caviahue-Copahue Volcanic Complex (Chile-Argentina): involvement of mature slab recycled materials. Chem Geol 476:370–388
Rowe Jr GL (1994) Oxygen, hydrogen, and sulfur isotope systematics of the crater lake system of Poas volcano, Costa Rica. Geochem J 28(3) 263–287
Sánchez-España J (2007) The behavior of iron and aluminum in acid mine drainage: speciation, mineralogy, and environmental significance. In: Lectcher TM (ed) Thermodynamics, solubility and environmental issues. Elsevier, Netherlands, pp 137–150
Sánchez-España JS, Pamo EL, Pastor ES, Andrés JR, Rubí JM (2006) The removal of dissolved metals by hydroxysulphate precipitates during oxidation and neutralization of acid mine waters, Iberian Pyrite Belt. Aquat Geochem 12(3):269–298
Sánchez-España J, Yusta I, Diez-Ercilla M (2011) Schwermannite and hydrobasaluminite: a re-evaluation of their solubility and control on the iron and aluminium concentration in acidic pit lakes. Appl Geochem 26:1752–1774
Sánchez-España J, Yusta I, Burgos WD (2016a) Geochemistry of dissolved aluminum at low pH: Hydrobasaluminite formation and interaction with trace metals, silica and microbial cells under anoxic conditions. Chem Geol 441:124–137
Sánchez-España J, Yusta I, Gray J, Burgos WD (2016b) Geochemistry of dissolved aluminum at low pH: Extent and significance of Al–Fe (III) coprecipitation below pH 4.0. Geochim Cosmochim Acta 175:128–149
Stumm W, Morgan JJ (1996) Aquatic chemistry: chemical equilibria and rates in natural waters. John Wiley and Sons Inc., New York
Szentiványi J (2018) Geología del sector NE del volcán Copahue y geoquímica de los fluidos volcánicos asociados. Undergraduate Thesis, University of Buenos Aires (in Spanish)
Tamburello G, Agusto M, Caselli A, Tassi F, Vaselli O, Calabrese S, Rouwet D, Capaccioni B, Di Napoli R, Cardellini C, Chiodini G, Bitetto M, Brusca L, Bellomo S, Aiuppa A (2015) Intense magmatic degassing through the lake of Copahue volcano, 2013–2014. J Geophys Res Solid Earth 120(9):6071–6084
Taran Y, Rouwet D, Inguaggiato S, Aiuppa A (2008) Major and trace element geochemistry of neutral and acidic thermal springs at El Chichón volcano, Mexico: implications for monitoring of the volcanic activity. J Volcanol Geotherm Res 178(2):224–236
Tassi F, Agusto M, Lamberti C, Caselli A, Pecoraino G, Caponi C, Szentiványi J, Venturi S, Vaselli O (2017) The 2012–2016 eruptive cycle at Copahue volcano (Argentina) versus the peripheral gas manifestations: hints from the chemical and isotopic features of fumarolic fluids. Bull Volcanol 79(10):1–14
Tassi F, Agusto M, Vaselli O, Chiodini G (2016) Geochemistry of the magmatic-hydrothermal fluid reservoir of Copahue volcano (Argentina): insights from the chemical and isotopic features of fumarolic discharges. In: Tassi F, Vaselli O, Caselli A (eds) Copahue volcano, active volcanoes of the world book series. Springer, Berlin, pp 119–139
Temporetti P, Beamud G, Nichela D, Baffico G, Pedrozo F (2019) The effect of pH on phosphorus sorbed from sediments in a river with a natural pH gradient. Chemosphere 228:287–299
Varekamp JC (2008) The volcanic acidification of glacial Lake Caviahue, province of Neuquén, Argentina. J Volcanol Geotherm Res 178(2):184–196
Varekamp JC (2015) The chemical composition and evolution of volcanic lakes. In: Rouwet D, Christenson B, Tassi F, Vandemeulebrouck J (eds) Volcanic lakes. Advances in volcanology. Springer, Berlin, pp 93–123
Varekamp JC, De Moor JM, Merrill MD, Colvin AS, Goss AR, Vroon PZ, Hilton DR (2006) Geochemistry and isotopic characteristics of the Caviahue-Copahue volcanic complex, Province of Neuquén, Argentina. Geol Soc Am Spec Pap 407:317–342
Varekamp JC, Ouimette AP, Herman SW, Flynn KS, Bermudez, A, Delpino D (2009) Naturally acid waters from Copahue volcano, Argentina. Appl Geochem 24(2):208–220
Velez ML, Euillades P, Caselli A, Blanco M, Díaz JM (2011) Deformation of copahue volcano: inversion of InSAR data using a genetic algorithm. J Volcanol Geotherm Res 202(1–2):117–126
Verplanck PL, Nordstrom DK, Taylor HE, Kimball BA (2004) Rare earth element partitioning between hydrous ferric oxides and acid mine water during iron oxidation. Appl Geochem 19(8):1339–1354
Wanner C, Pöthig R, Carrero S, Fernandez-Martinez A, Jäger C, Furrer G (2018) Natural occurrence of nanocrystalline Al-hydroxysulfates: insights on formation, Al solubility control and as retention. Geochim Cosmochim Acta 238:252–269
Wood SA, Gammons CH, Parker SR (2006) The behavior of rare earth elements in naturally and anthropogenically acidified waters. J Alloys Compd 418(1–2):161–165
Yu J, Heo B, Choi I, Chang H (1999) Apparent solubilities of schwertmannite and ferrihydrite in natural stream waters polluted by mine drainage. Geochim Cosmochim Acta 63:3407–3416
Acknowledgements
The research leading to these results has received funding from the projects UBACyT 20020150200230BA, UBACyT 20020170200221BA, PICT-2015-3110, PICT-2016-2624 and Proyecto de Unidad Ejecutora (IDEAN) 22920160100051.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Llano, J., Lamberti, M.C., Sierra, D., Agusto, M. (2021). Hydrogeochemistry of an Acid River and Lake Related to an Active Volcano. The Case of Study: Agrio River—Copahue Volcano in Patagonia, Argentina. In: Torres, A.I., Campodonico, V.A. (eds) Environmental Assessment of Patagonia's Water Resources. Environmental Earth Sciences. Springer, Cham. https://doi.org/10.1007/978-3-030-89676-8_4
Download citation
DOI: https://doi.org/10.1007/978-3-030-89676-8_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-89675-1
Online ISBN: 978-3-030-89676-8
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)