Abstract
In a textbook on urban soils, there is a need to present and explain some of the fundamental concepts in soil science. This chapter is framed around the ecosystem functions performed by soils and covers physical, chemical, and biological functions. The soil physics topics covered include volume relationships of solids and fluids, soil water retention, soil texture and structure, soil water potentials, water flow through soils, soil water balance, and soil temperature and the soil energy balance. The soil biological topics covered include discussions of how biological processes affect soil physical properties, nutrient cycling, and soil food webs. An explanation of bioavailability leads into examination of the chemical reactions and properties in soils which underpin ecosystem functions. The chapter concludes with a brief discussion of soil functions affecting human health and well-being.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Soil functions
- Urban soils
- Ecosystem services
- Physical properties
- Soil biology
- Nutrient cycling
- Chemical properties
- Soil physics
- Soil chemistry

What you could learn from this chapter:
-
What the key physical properties of soils are and how they allow soils to function, especially considering the behaviour of water in soil
-
How the biological components (organisms) in soils control important soil functions and gain an understanding of nutrient cycling and bioavailability
-
The chemical composition of soils: minerals, compounds, and ions – and what the important types of chemical reaction are
-
Why and how soils are important for humans – in addition to the ecosystem services provided by soils.
4.1 Roles and Ecosystem Services of Soils in Urban Systems
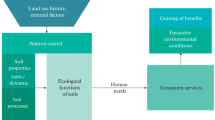
In many urban environments, the roles and function of soils are no different from non-urban soils. Morel et al. (2015) recognise four general categories of ecosystem services provided by soils in any environment: supporting plant growth, maintaining biodiversity, removal and cycling of materials, and storage of ecosystem resources (see Table 4.1, which also summarises the mechanisms behind each function, i.e. how each function works). In addition to the four types of ecosystem services, Morel et al. (2015) identify three further ecosystem services that emphasise human uses of soils: a source of raw materials, physical and cultural support of human activities, and preserving a geological and archaeological record. This chapter will address primarily the functions and ecosystem services provided by soils in general, without necessarily being restricted to urban environments. The constraints imposed by the properties, features, and composition of urban soils will be addressed in following chapters.
4.2 Soil Functions Related to Soil Physical Properties and Processes
The physical properties of soil are essential to the functioning of most terrestrial ecosystems, and some of the key phenomena are illustrated in Fig. 4.1. In this textbook we provide a summary of our view of the important concepts in soil physics, with particular reference where necessary to urban soils. In doing so we have relied on general soil science textbooks such as McKenzie et al. (2004), White (2006), and Schaetzl and Anderson (2005). For more detail about soil physical functions and concepts, readers should refer to a specialised soil physics textbook such as Hillel (2014).
Physical processes involved in maintaining ecosystem services in soils and some of the controls and interactions between them. (Graphic by Andrew W. Rate):
1. The mass loss and formation of secondary minerals allows formation of soil porosity and aggregation
2. Soil porosity allows recharge of, and leaching to, groundwater (especially with macropores from soil structure )
3. Soil porosity allows water storage, with some water available for biota, such as photosynthetic plants
4. Both soil structure and plant cover protect the soil surface against erosion
5. The biological community returns organic matter to the soil, further favouring aggregation and porosity
6. The soil pore network acts to filter particulates, including contaminants such as pathogenic bacteria
The most basic of a soil’s physical properties, treated first in many soil science textbooks, are the volume relationships . The soil volume is commonly subdivided into phases: solids, liquids, and gases. To complicate soil volume relationships a little further, soil scientists may separate the solid phase on the basis of size: the fractional volume in each of the three phases is measured (or estimated) for the soil’s fine earth fraction, having grain sizes less than 2 mm, with the coarse material (≥ 2 mm) comprising a separate volume element. This grain size distinction is particularly relevant in urban soils, which often contain large proportions of rubble or artefacts (see the definition of Technosols in Chap. 2). The obvious question at this stage is whether these volume relationships are important aspects of soil functioning. The answer lies in the porosity ; the volume of soil which is occupied by fluids, the liquids (mostly water containing dissolved material) and the gases (mostly air, but compositionally different from the above-ground atmosphere ). The pore space in soil is extremely, and surprisingly, important. The proportion of pores relative to the total soil volume affects the ability of the soil to store and conduct water, the ease with which plant roots can penetrate, and the soil’s suitability as a habitat for microorganisms and larger creatures from nematodes to earthworms, in terms of both living space safe from predation and allowing the diffusion of life-giving oxygen and carbon substrates.
Since the total soil volume is the sum of the three dominant phases, solids, liquids, and gases, then an increase in the volume fraction of any of these phases must be at the expense of the others; soil volume is a fixed-sum closed set. The presence of large, abundant solids having low porosity (such as natural or anthropogenic stone) in urban or other soils therefore results in lower pore volume, as porosity only exists between the finer grains. By a similar argument, the density of a soil, which is controlled by the packing of solid materials, also controls porosity. High density results from close packing, that is, more solids per volume than for a lower density soil. The higher volumetric solid content of dense soils leaves less space for pores and the vital functions that such pores allow.
The total volume of pores in a soil is not yet sufficient information to understand soil functioning completely. The size and connectivity of pores are critical; pores which are large (and by large, we mean ≳ 0.03 mm in diameter) hold water only weakly by capillary forces and are unable to hold water against gravitational drainage. Conversely, pores which are very small (≲ 0.2 μm or 0.0002 mm in diameter) retain water very tightly, such that plant roots cannot exert sufficient osmotic pressure to extract it. To maximise the ecosystem service provided by soil porosity, a soil needs to have a range of pore sizes: some larger, to drain excess water, and some smaller, to store water in a state in which it can be gradually released to plants and other soil organisms. An ideal range of pore sizes is not observed in all soils, even natural ones!
There are two main controls on soil pore size distribution. First, it makes sense that the spaces or pores between larger grains (sand, between 0.02 mm and 2 mm in diameter) will be larger than the pores between small grains (silt, between 0.002 mm and 0.02 mm in diameter, or clay ≤ 2 μm). We now need to introduce another soil science concept that of soil texture . The texture of a soil is a category, defined using a texture triangle , based on the relative proportions of mineral grains of different sizes in a soil (i.e. after organic matter is removed). In the international (IUSS) classification (Fig. 4.2), three ranges of grain sizes are used; sand, silt, and clay as defined above. The texture triangle defines thresholds between the texture categories; for example, a soil with 50% sand, 30% silt, and 20% clay by weight in the fine earth fraction would be classified as a clay loam in the IUSS system. There are several grain size and texture classifications in use in different countries (Minasny and McBratney 2001), which can confuse the issue.
Soil texture triangle based on mineral grain size fractions and categories from the International Union of Soil Sciences (IUSS) (drawn using the soiltexture R package by Moeys 2018)
The second control on the pore size distribution in soils is also related to grain size – the aggregation of individual grains into aggregates , or peds . Soil aggregates are arrangements of many individual soil grains held together by a range of forces and separated by pores and planes of weakness. The existence of such aggregates, and the size and geometry of the aggregates and their surrounding and internal pore spaces, is called the soil structure . Soil aggregates can range in size from dimensions similar to individual sand grains, up to several centimetres. The pore dimensions can therefore span a similar range; in addition, the aggregates themselves contain smaller ‘micropores’.
Soil structure forms when the attractive forces between soil particles exceeds the tendency to exist as single grains. Although it is beyond the scope of this book to explain the mechanisms of soil aggregation in detail, we can identify a few qualitative soil properties that favour the development of structure: moderate to high clay content; low proportions (e.g. ≤6%) of exchangeable sodium (Na+) as a fraction of total exchangeable cations (see Section 4.4.2 below); conditions favouring precipitation of cementing agents (e.g. iron oxides, carbonate minerals); specific types of organic matter, such as polysaccharides and other plant root or microbial exudates that possess adhesive qualities; plant roots themselves; and fungal hyphae. External forces such as shrink-swell cycles caused by wetting and drying, or freeze-thaw cycles, also facilitate formation of soil structure if favourable soil properties exist. The microscopic basis of soil structure is the ‘clay domain’ (Fig. 4.3).
Complexity of soil aggregation which results in soil structure . The structure-forming agents, clays, organic materials such as humus and polysaccharides, and enmeshing components such as fungal hyphae and plant roots, allow the formation of ‘clay domains’ which are the nuclei of soil structure that allow the larger grains (silt, sand) to be incorporated into aggregates
So, with the pore network in soils controlled by soil texture and structure, we are better informed to understand how soil physical properties affect the storage and movement of water. Due to its adhesive and cohesive properties (e.g. surface tension), water in soils will tend to minimise its potential energy (symbolised as the matric potential, ψM) when present in the smallest possible pores. This tendency is summarised in the relationship between volumetric water content, θV, and soil matric potential, ψM (Fig. 4.4b). Simplistically, ψM is the pressure required to remove water from the largest water-filled pore (explaining why it’s hard to get water from a dry soil!).
(a) Comparison of available and unavailable soil water for a range of textures; (b) soil water retention curves for different textures using data from Carsel and Parrish (1988) and for compacted clays adapted from Tinjum et al. (1997). The vertical lines are labelled at the potentials representing field capacity (ψFC) and permanent wilting point (ψPWP, −1500 kPa) .Water fractions were calculated, and θ-ψ relationships were plotted, from the van Genuchten equation implemented using the ‘soilphysics’ R package (de Lima et al. 2016)
Similarly, pore size distribution also affects the ability of a soil to conduct water, an important property in urban soils since the area of exposed soil into which water can infiltrate is diminished by impervious cover . The rate with which water can travel through a soil is expressed in simple terms by Darcy’s law (Eq. 4.1):
where qw is the water flux (length/time), Ks is the saturated hydraulic conductivity, ∆h is the hydraulic head driving water flow, and ∆z is the length of soil.
Darcy’s law is valid for saturated flow through relatively homogeneous soils, so it often does not apply in field situations, where most soils are seldom saturated; nevertheless, it does summarise the principles involved. The key parameter is the saturated hydraulic conductivity, which can be used to compare water flow through different soils. Values of Ks vary across several orders of magnitude, reflecting the theoretical inverse square dependence of flow rate on pore radius summarised in Poiseuille’s law (McLaren and Cameron 1990). For example, while a sand may have Ks = 1000 mm/h, Ks for a clay-textured soil may be <0.1–5 mm/h (White 2006).
The pore structure of soils is also important in other contexts that we will not cover in this chapter: transport of gases though soils and the ability of the soil pores to provide habitats for soil (micro)organisms, as described in Chap. 8.
4.2.1 Soil Energy
A further important physical role of soils in supporting ecosystems is the ability of soil to act as a heat sink or source. The net solar radiation reaching the soil surface (affected by planetary albedo, slope, slope aspect, shading, etc.) is balanced by a number of heat fluxes from and within the soil. This is expressed in a soil surface energy balance equation (Eq. 4.2):
where Jn is the net solar radiation flux, S is heat transferred within the soil, A is the ‘sensible’ (radiative) heat flux from the soil, and LE is the latent heat flux from evaporation, and transpiration by plants, of soil water.
Soil can therefore have a cooling effect on its local atmosphere , as it exports energy as water vapour to the atmosphere via the evapotranspiration (latent) heat flux (Hillel 2014). This cooling effect has also been recognised to be important for global climate regulation (Ban-Weiss et al. 2011). Soil also has a dampening effect, within the soil environment, on the extremes of atmospheric temperature , an effect which is more pronounced in subsoils compared with surface soils. The rate of heat conductance in soils also means that subsoil temperature changes lag behind those at the surface (see White 2006; Hillel 2008 for details). Both the dampening effect of depth and the lag caused by heat conductance are illustrated in Fig. 4.5. At the surface (5 cm in Fig. 4.5), soil temperatures are similar to the air temperature – the ‘weather’ temperature . At greater soil depth, soil temperatures do not increase as much during the day as at the surface, nor do temperatures decrease by as much as the surface at night. Not only is the soil temperature range less at depth, though, but the maxima and minima occur later than at the surface. The final feature shown by Fig. 4.5 is seasonal; in summer, soil temperature is cooler at depth than at the surface, but in winter the deeper soil is warmer, on average, than the surface soil.
Trends in soil temperature showing diurnal fluctuations at different depths over 10-day periods in summer and winter (note different temperature scales!) for an urban site near Canberra Airport, ACT, Australia. Data are from the OzNet data archive at www.oznet.org.au (Smith et al. 2012); curves are slightly smoothed to reduce discretisation effects. (Graphic by Andrew W. Rate)
In urban soil environments such as raised garden beds or green roofs, there is greater contact between soil and atmosphere . This means that even deeper soils can gain and lose heat more quickly, so that subsoil temperatures show a greater range which is more similar to surface soils.
4.2.2 Soil Functions Related to Hydrological Properties and Processes
The pore network of soils means that soils have a role in controlling the balance between run-off (overland flow) and infiltration (water penetrating into the soil) in the hydrological cycle (Fig. 4.6). Run-off contributes directly to stream flow and is possible when the maximum infiltration rate of the soils (essentially the hydraulic conductivity at the atmosphere-soil interface) is exceeded by the flux of liquid water added by precipitation (rainfall or melting snow or hail). Run-off contributes water to natural (streams, rivers) and anthropogenic drainage networks but, in excess, results in soil erosion (Hillel 2008).
The hydrological cycle including urban contributions. (Graphic by Andrew W. Rate inspired by a diagram by the Cary Institute of Ecosystem Studies (2020))
Infiltration of water into soil has multiple consequences. Most directly, infiltration of precipitation water increases soil water content, where it is stored in the soil pore system. Water held at matric potentials less negative than about −10 kPa will drain under the influence of Earth’s gravity; strictly vertical drainage will replenish groundwater, and drainage with a significant lateral movement contributes to surface water bodies (drainage such as streams, or static bodies such as lakes). On hillslopes, the mass of soil with high water content can result in mass movement phenomena such as soil slippage or landslides, depending on the slope steepness and soil composition (Schaetzl and Anderson 2005).
The conservative nature of water cycle is expressed by a soil water balance (Eq. 4.3). The closed nature of this balance means that if one component changes, at least one other component will also change so that the balance is maintained. In this regard, run-off from, and infiltration into, soils are commonly a complementary pair; if run-off increases, infiltration decreases, and vice versa (White 2006).
where P is precipitation, I is irrigation, U is upward capillary flow from below the root zone, ΔS is the change in soil water content, ΔV is the water content change in plants, R is run-off, D is (vertical) drainage, E is direct evaporation from soil, and T is transpiration by plants (Hillel 2008).
Both directly and indirectly, soil porosity also contributes to atmospheric compartments of the hydrological cycle. Water can evaporate from moist soil and be transpired by plants; both processes add water vapour to the air and (as observed above) exert a cooling effect on local and global atmospheres.
4.3 Soil Functions Related to Soil Biological Properties and Processes
Soils are distinguished from other solid Earth materials by the presence of an active biological community; soils are astonishingly rich in life (Fortuna 2012). After plants, the largest mass of living organisms on Earth is in soil fungi and bacteria! (Bar-On et al. 2018). In Sect. 4.2, we started to discuss how the soil’s physical properties affected some biological processes, by providing a growth medium and habitat for plants and microorganisms and allowing storage and release of the water required for their survival. Soil systems contain complex networks of effects and feedbacks, in that plants and microorganisms can mutually promote one another’s survival through food webs, trophic transfers, and nutrient cycling (Coleman et al. 2017). The biological components of soils in the form of plants and microorganisms can also affect soil physical and chemical properties, in many cases accelerating soil processes and promoting the establishment of soil conditions that are beneficial for their own survival.
4.3.1 Biological Effects on Soil Physical Properties
Soil Aggregation
Soil aggregates which are the building blocks for soil structure may be created by biological activity by enmeshment by fungal hyphae or plant roots, exudation of organic binding agents by plant roots and microbiota, and faecal pellets, or casts, of larger soil animals such as earthworms or mites (Coleman et al. 2017).
Soil Macroporosity
The penetration of plant roots and the burrowing of soil fauna create large continuous soil pores or macropores (McLaren and Cameron 1990; Schaetzl and Anderson 2005). Soil macropores are important, especially in soils with otherwise low hydraulic conductivity, for increasing overall infiltration rates.
Soil Mixing
The mixing of soils by organisms, termed bioturbation , is very important in some soil environments, increasing porosity and reducing soil density (Schaetzl and Anderson 2005). For example, the burrowing activity of earthworms and ants may be able to modify the entire upper metre of soil on timescales of centuries (de Bruyn and Conacher 1990; Feller et al. 2003).
4.3.2 Nutrient Cycling
Soils are, for many elements, the engines of terrestrial biogeochemical cycling (Chorover et al. 2007). The elements of greatest relevance are those required to sustain life: the elements C, H, and O primarily captured by photosynthesis; the macronutrients N, P, K, Ca, Mg, and S; and a range of micronutrients , of which Fe, Mn, Zn, Cu, B, Mo, Cl, and Ni are essential for plants. Additional trace elements (Co, Cr, I, Se) are required for organisms other than plants, including humans. Except for carbon, hydrogen, and oxygen which plant photosynthesis captures from the Earth’s atmosphere , all of the other elements are supplied by the soil or are reliant on soil-based processes. For any one element, the biogeochemical cycle is a conceptual model which represents the various transformations and fluxes by which an element moves within and between environmental compartments. For example, Fig. 4.7 represents a combined carbon-nitrogen cycle for soils (Yang et al. 2009). The nitrogen and carbon in soils are derived from the atmosphere by primary productivity – the growth, life, and death of photosynthetic plants. Plant litter becomes soil organic matter or ‘humus’, which in turn provides a substrate for the growth of microorganisms (mainly fungi and bacteria). Respiration by soil organisms, either directly from soil organic matter or through food webs (Sect. 4.3.3), completes the cycle by returning carbon and nitrogen to the atmosphere . Other soil biological processes contribute carbon and nitrogen to other environmental compartments such as water or retain carbon and nitrogen in the soil. We can use the same general concepts for cycling of any element like the essential elements listed above or even pollutant elements (or stable materials such as water); the cycling of many elements in soils is important, and we discuss some aspects of nutrient cycling below. It should become apparent that, to fully understand a biogeochemical cycle, we also need to know how the separate biological, chemical, and physical processes work.
Combined conceptual carbon and nitrogen cycle for soils. (From Yang et al. 2009 and used with permission from John Wiley and Sons). The boxes represent different compartments (forms) of C and/or N, and the arrows (solid lines for the C cycle, dashed lines for the N cycle) represent fluxes (transfers, transformations) of C and N. LCLUC is land cover and land use change
Nutrient Cycling Involving Plants
Terrestrial plants and other photosynthesising organisms such as algae capture atmospheric carbon to synthesise a vast array of organic compounds, from nucleic acids to lignin. A significant proportion of these carbon compounds, also containing elements derived from soils (mainly, but not limited to, N, P, and S; see Table 4.2), enter the soil environment as the organisms, or parts of them, die and begin to decompose. In addition some organic molecules are released from living organisms into the soil environment as exudates from plant roots (and microbial exudates). The transfer of organic material derived from plants into the soil environments provides the substrate for microorganisms that utilise it as a source of energy, carbon, and nutrients (Chorover et al. 2007). Plants also redistribute nutrients (and other elements, since non-essential elements are not excluded from uptake) from deeper soil or regolith into the surface soil via uptake by deep root translocation within the plant and redeposition as dead material (litter fall) at or near the soil surface (Brantley et al. 2007). Finally, certain types of plants (most notably the legumes) have the ability, in symbiosis with specific groups of bacteria, to capture nitrogen from the atmosphere without relying on soil sources of N (Haynes 1986).
Microbial Nutrient Cycling
Much nutrient cycling is associated with the heterotrophic metabolism of organic compounds in soils by soil microorganisms (bacteria, fungi, and archaea). This is important in two contexts. First, elements contained in organic molecules (including carbon (C) and also N, P, and S) are released as simple inorganic forms during soil organic matter decomposition, or mineralisation, as the soil microbiota utilise carbon compounds to produce energy and metabolites in the process of microbial respiration . The simple inorganic ions of molecules can also be reincorporated into microbial organic matter in the process of microbial immobilisation. Second, respiration is an oxidative process, and, in the absence of sufficient oxygen in the soil atmosphere (or dissolved in soil pore water), other chemical substances can act as terminal electron acceptors, or oxidants, during oxidation of carbon compounds. These include compounds or ions of several essential elements, such as NO3−, FeIII [hydr]oxides, MnIV [hydr]oxides, and SO42− (Chorover et al. 2007; Coleman et al. 2017). The metabolism of organic carbon under anoxic (oxygen-limited) conditions in soils also affects some elements indirectly. For example, if an iron(III) oxide mineral acts as the terminal electron acceptor in the anoxic oxidation of organic carbon, phosphate or trace metal ions adsorbed to the surface of that iron oxide particle, or incorporated as impurities into its structure, will be released into solution (see Table 4.2).
Nutrient cycles in soils are very complex, and in this textbook, we can only discuss a few of the principal issues involved. Some of the more important nutrient cycling processes are presented in Table 4.2, which also contains information on chemical forms of elements, since the topics of element cycling and bioavailability are where soil biology and soil chemistry overlap extensively.
4.3.3 Soil Food Webs
Stored energy, in the form of metabolisable carbon compounds, is transferred from organisms upwards through trophic levels to provide them with the energy and substrate (food) for survival. The photosynthetic organisms or primary producers capture solar energy; the herbivores and decomposers that feed on plant material pass energy, carbon, and nutrients to the next level, and so on (see Fig. 4.8). The incomplete efficiency of these transfers means that the highest proportions of stored energy, carbon, and nutrients exist in the organisms at lower trophic levels. By far the greatest proportion of terrestrial biomass on Earth exists in plants, which contain more than 35 times more biomass than the next most abundant group, soil fungi, followed by soil bacteria (Bar-On et al. 2018). The most abundant animals (by number of individuals, not mass) on Earth’s terrestrial near-surface environments are nematodes, which exist at multiple trophic levels (van den Hoogen et al. 2019). In terms of biomass, the most abundant soil animals are probably arthropods or annelids (Bar-On et al. 2018).
Simplified soil food web showing trophic transfers (energy, carbon, and nutrients) and some of the diversity of soil organisms (based on Ingham n.d.). The arrows show the direction of flow of energy, carbon, and nutrients
4.4 Soil Functions Related to Soil Chemical Properties and Processes
As with the soil physics section earlier in this chapter, we present here a summary of our view of the important concepts in soil chemistry, again making particular reference where necessary to urban soils. The presentation of this material also relies on the general soil science textbooks by McKenzie et al. (2004), White (2006), and Schaetzl and Anderson (2005). To study soil chemical functions and concepts in more depth, readers should refer to a specialised soil or environmental chemistry textbook such as Sposito (2008) or Ryan (2014).
Numerous soil chemical properties and processes are important for the delivery of ecosystem processes, and these are summarised in Fig. 4.9. This section is intended to give an overview of most of the more important chemical soil functions. We will go into some more detail about soil chemical processes in Chaps. 6 and 7. A central concept is that of chemical speciation – the existence of multiple chemical forms of an element or compound, the identity of which affects not only its chemical reactions but also biological and physical processes in the soil environment as well. Table 4.3 explains and summarises chemical speciation as it applies to soils. In addition, soil chemists recognise two ‘master variables’ – soil pH and soil redox potential. The soil pH has a profound effect on nearly every chemical process, from mineral dissolution or precipitation to the bioavailability of nutrient elements or the degradation of organic contaminants (Sposito 2008). Likewise, the soil redox potential, or Eh, can significantly affect soil chemical reactions; Eh is nearly constant in aerobic soils, but once available oxygen in a soil decreases, usually due to saturation with water and microbial respiration, a cascade of chemical reactions is initiated (McBride 1994), some of which are outlined in Table 4.2.
Chemical processes involved in maintaining ecosystem services in soils, with controls/interactions (graphic by Andrew W. Rate):
1. Weathering of soil parent materials to give secondary minerals with small grain size, plus dissolved ions
2. The ions can interact with the secondary minerals by cation exchange or chemisorption mechanisms
3. The ions released by weathering, and held in potentially bioavailable reserve by sorption, comprise essential elements for plant growth (K+, Ca2+, HPO42−, etc.)
4. Primary production by photosynthesis (and for some producers, nitrogen fixation) is facilitated by the uptake of nutrient ions from soil
5. Eventually, some of the carbon fixed by photosynthesis is added to the soil via litter fall, to form organic matter
6. The soil organic matter provides additional substrate for the adsorption of ions (including those essential for biota); some organic matter degrades to give soluble organic matter.
7. The utilisation of organic matter by heterotrophic soil organisms and the metabolic processes in plant roots result in respiration, releasing CO2 which further enhances weathering of soil parent materials
8. Soil organic matter decomposition and exudation of organic compounds by roots and microbiota provide organic acids/anions which act as metal complexing ligands to further enhance dissolution of minerals
9. Contaminants such as metals, nutrients, and organic molecules are also chemically immobilised by secondary minerals and soil organic matter
4.4.1 Formation of Secondary Minerals and Organic Matter
A fundamental process in the conversion of soil parent materials (mainly rocks and the minerals contained in them) is chemical weathering (Brantley et al. 2007). In general, the solid minerals in rocks weather by reactions such as dissolution or oxidation to produce two main products: dissolved ions and molecules in the aqueous (liquid water) phase and secondary minerals in the solid phase. For example, the weathering of the common silicate mineral orthoclase feldspar can be expressed by:
where the subscripts (s) indicate a solid phase, and (aq) indicate an aqueous phase (dissolved in water).
The chemical reaction symbolised in Eq. 4.4 emphasises the role of atmospheric CO2 dissolved in water to give carbonic acid, providing a weathering fluid which dissolves silicate minerals by acid hydrolysis. The consumption of CO2 by weathering represents a long-term control on Earth’s atmospheric composition and therefore the greenhouse effect (Kump et al. 2000). From a soil ecosystem service perspective, however, the products of this type of reaction are more immediately important: the secondary solid(s) (the phyllosilicate clay mineral kaolinite in this example) and the dissolved material (Fig. 4.9). The phyllosilicate clay minerals are important in a range of chemical reactions, as we will see below. In addition, the clay content of soils exerts significant control on pore size distribution and soil structure (see Sect. 4.2 above). The release of dissolved components is also extremely important. Loss of rock mass as dissolved ions is a key contributor to the development of porosity (Brimhall and Dietrich, 1987), and, crucially, the ions themselves (e.g. the K+ in Eq. 4.4) provide a source of available nutrients for plants and other soil biota.
Secondary minerals also form by direct precipitation from solution, given favourable conditions. For example, if a weathering reaction generates dissolved Fe3+ ions, the following reaction (Eq. 4.5) can occur.
The new secondary mineral is the ferric hydroxide Fe(OH)3 (similar to the documented oxyhydroxide mineral ferrihydrite). The existence of H+ as a product of Eq. 4.5 means that this reaction will be favoured at higher pH, since at high pH the concentration of H+ ions is low, and part of chemical equilibrium theory (Le Chatelier's principle, or the ‘equilibrium law’) means that if a product of any reaction decreases in concentration, the reaction will tend to progress further towards those products.
Finally, the stable organic matter in soils, although not a mineral phase, can be considered to be generated from ‘weathering’ (i.e. fragmentation and chemical alteration) of its parent materials – the organic detritus that enters soils during natural cycles of life and death of organisms.
4.4.2 Cation and Anion Exchange Reactions
The phyllosilicate clay minerals form from a solution generated during chemical weathering, and since parent rocks usually contain many different types of minerals, this solution contains a diverse range of dissolved ions. As a result, the clay minerals which form are seldom ‘pure’ aluminosilicates; their structures contain other cations in the structural positions of Al3+ or Si4+. For example, the structure of the common 2:1 clay mineral montmorillonite (having the nominal chemical formula (Na,Ca)0.3(Al,Mg)2Si4O10(OH)2·n(H2O)) is shown in Fig. 4.10, showing the positions of the Al3+ or Si4+ ions which, at mineral formation, are nearly always partially occupied by ‘impurity’ ions such as Mg2+ or Fe2+. Since the substituting ions (Mg2+ or Fe2+) have a lower positive charge than the dominant Al3+ or Si4+, the entire crystal structure forms with a deficit of positive charge – the clay crystals are negatively charged! An electrostatically charged particle cannot exist independently in the soil environment, and the negative charge is always balanced by exchangeable cations which are electrostatically attracted to the external surfaces and interlayer spaces of the clay crystal. In addition, since clay minerals mainly have grain sizes in the clay-size range (see Sect. 4.2), their surface area to mass ratio is high, another factor which favours chemical reactions. The exchangeable cations, most often Na+, K+, Mg2+, Ca2+, or NH4+, are of great importance for ecosystem functioning in soils (Fig. 4.9). First, the fact that they are held on a solid phase means not all the cations released during chemical weathering will be lost. Second, electrostatic interactions are relatively weak, so the cations are held in rapid and dynamic equilibrium between the mineral surface(s) and the pore water and therefore represent an important reserve of elements for plants and other soil biota which are potentially bioavailable – both essential elements and potentially toxic elements (see Chap. 6).
Crystal (chemical) structure of montmorillonite, a 2:1 phyllosilicate clay mineral in the smectite group. Image from Poppe (2004)
Other negatively charged materials also exist in soils. Soil organic matter forms from the detritus of living organisms by a wide combination of reactions including hydrolysis, oxidation, and condensation, to create high molecular weight, highly variable structures. Soil organic matter consistently contains structural elements (functional groups) such as carboxylates as part of its structure. Carboxylates are anions of weak acids, so if the soil pH is high enough (≳ pH 3.5, which is most often is), then the carboxylates are in dissociated (anionic) form. Most soil organic matter exists in the solid phase, so organic particles also carry a negative electrostatic charge which is balanced in soils by the same exchangeable cations as clays.
Other minerals can also manifest a negative electrostatic charge: the hydroxide (OH−) ions at the surface of the structure of hydroxides of aluminium, iron, and manganese are amphoteric ; that is, they can behave as weak acids or bases. Under alkaline conditions, the collective surface OH on hydroxide minerals behave as a base, losing hydrogen ions to generate negative charge which is again balanced by cations like Na+, K+, Mg2+, Ca2+, or NH4+. Iron(III) hydroxides, in particular, can also gain hydrogen ions within common soil pH ranges (≲ pH 8) to generate positive charge. In a few instances (e.g. the subsoils of highly weathered and acidic soils with low concentrations of charged clays or organic matter), then, exchange of anions such as Cl−, SO42−, or NO3− is possible.
4.4.3 Chemical Adsorption Reactions
Some ions or molecules in soils are held on solid phases much more strongly than the relatively weak associations of ion exchange. This type of reaction is called adsorption or in the usual case, where chemical bonding by electron-sharing between atoms is involved, chemisorption . Adsorption reactions still hold adsorbed ions in equilibrium with dissolved ions in the soil solution, but the equilibrium position is much further towards the surface species, the reactions are much slower to reach equilibrium, and generally rapid exchange is not observed.
Cations such as those of the trace metals Cu2+, Ni2+, and Zn2+ form strong chemical bonding with oxygen-containing structural components on soil solid phases. For example, the carboxylate groups on soil organic matter (and in some cases N-containing amino- functional groups) contain non-bonded electron pairs which can be shared to form covalent bonds with trace metal cations. Similarly, the surface hydroxides on oxide minerals and clays also contain non-bonded electron pairs allowing bonding with trace metal cations. Cation adsorption is more favourable at higher pH; this pH dependence is because bonding of a metal ion displaces hydrogen ions from the mineral or organic matter surface (Fig. 4.12). Since the O (or organic N) atoms contribute all of the electrons to the chemical bonds, the bonding is analogous to coordination complexes of metals, and the cation adsorption mechanism is sometimes called surface complexation .
Anion adsorption is somewhat different from cation adsorption. It generally only occurs significantly on inorganic materials: the phyllosilicate clays and hydroxide minerals. Again, the reaction sites are the surface hydroxides on mineral surfaces; however, bonding of an oxyanion like phosphate (H2PO4- or HPO42-) occurs between the phosphate ion and the structural cations (e.g. Fe3+ or Al3+) in the clay or hydroxide, and the previously bonded OH− anions are displaced into solution. This is an example of ligand exchange , where both the incoming phosphate ions, and the outgoing hydroxide ions or water molecules, are ligands which exist in a coordination complex with metal ions near the surface of the adsorbing mineral structure. Anion adsorption is therefore more favourable at lower pH, since under acidic conditions the low concentration of OH− anions tends to push the anion adsorption equilibrium towards reaction products (Fig. 4.12).
Adsorption of organic compounds is dependent on whether the molecule can be ionised in water and, especially for non-polar molecules, soil organic matter content. The behaviour of organic molecules in soils will be covered in more detail in Chap. 7.
4.4.4 Precipitation and Co-precipitation
Precipitation is the formation of new solid phases, by reaction of individual chemical species in solution. The reacting species are removed from the solution and included in the chemical/mineralogical structure of the new solid, or ‘precipitate’. A very large range of minerals can precipitate in soils, including phyllosilicate clays, oxides, hydroxides, carbonates, sulphides, sulphates, and phosphates (Lindsay 1979), and a few examples are listed in Box 4.1.
Box 4.1 Examples of Precipitation Reactions in Soils
-
1.
\( {\displaystyle \begin{array}{ccccccc}C{a^{2+}}_{(aq)}& +& HC{{O_3}^{-}}_{(aq)}& \rightleftharpoons & {\mathrm{CaCO}}_{3\left(\mathrm{s}\right)}& +& {\mathrm{H}}^{+}\\ {}\mathrm{calcium}& +& \mathrm{bicarbonate}& \rightleftharpoons & \mathrm{calcite}& +& \mathrm{hydrogenion}\end{array}} \)
-
2.
\( {\displaystyle \begin{array}{l}F{e^{3+}}_{(aq)}+3{\mathrm{H}}_2{\mathrm{O}}_{\left(\mathrm{l}\right)}\kern0.5em \rightleftharpoons \kern1.75em Fe{(OH)}_{3\left(\mathrm{s}\right)}+3{\mathrm{H}}^{+}\\ {}\mathrm{ferricion}+\kern2.375em \mathrm{water}\kern1.5em \rightleftharpoons \kern2.5em \mathrm{ferrihydrite}+\mathrm{hydrogenion}\end{array}} \)
-
3.
\( {\displaystyle \begin{array}{cccccc}F{e^{2+}}_{(aq)}+& H{S^{-}}_{(aq)}& \rightleftharpoons & Fe{S}_{\left(\mathrm{s}\right)}& +& {\mathrm{H}}^{+}\\ {}\mathrm{ferrousion}+& \mathrm{hydrogensulfide}& \rightleftharpoons & \mathrm{mackinawite}& +& \mathrm{hydrogenion}\end{array}} \)
-
4.
\( {\displaystyle \begin{array}{l}{{\mathrm{K}}^{+}}_{(aq)}+3F{e^{3+}}_{(aq)}+6O{H^{-}}_{(aq)}+2{{{\mathrm{SO}}_4}^{2-}}_{(aq)}\rightleftharpoons \kern1.75em KF{e}_3{(OH)}_6{\left({\mathrm{SO}}_4\right)}_{6\left(\mathrm{s}\right)}\\ {}\mathrm{potassium},\mathrm{ferric},\mathrm{hydroxide},\mathrm{and}\ \mathrm{sulfate}\ \mathrm{ions}\rightleftharpoons \kern1.5em \mathrm{jarosite}\end{array}} \)
Precipitation requires oversaturation of the solution with the reacting ions, defined by the solubility product Ksp. The solubility product is a simplified equilibrium constant for a solid substance dissolving in an aqueous solution: the product of the component ion concentrations each raised to the power of their stoichiometric coefficient (Eqs. 4.6 and 4.7).
For the reaction of a moles of ion A and b moles of ion B to give the solid AaBb(s):
Ionic charges have been omitted for clarity (subscripts (aq) show aqueous-phase (dissolved) species and (s) solid-phase species, and square brackets with subscript ‘eq’ ([ ]eq) means concentration of the enclosed species in equilibrium with the solid).
In any solution, not necessarily at equilibrium with any solid, we can calculate the ion product Qsp = [A]a × [B]b and its relationship to the equilibrium constant Ksp. If Qsp = Ksp for any solid phase, the solution is in equilibrium with that solid phase, and so the solution is said to be saturated. If Qsp < Ksp for any solid phase, the solution is undersaturated and that solid phase cannot form. If Qsp > Ksp for any solid phase, the solution is oversaturated with respect to that solid, and that solid phase will (usually) form; sometimes the solution needs to reach a certain value of Qsp/Ksp for the solid to start forming. In fact, some solids will almost never form immediately; the Ostwald step rule (Threlfall 2003) proposes that the least stable mineral variant will form first, followed by progressive recrystallisation into more stable (and less soluble) mineral phases, a process known as Ostwald ripening.
4.4.4.1 Dependence of Precipitation on pH and Redox
Many precipitation reactions are dependent on soil pH, as implied by some of the reactions in Box 4.1 which include H+ or OH− as reactants or products. For example, a reaction producing H+ (on the right side of the chemical equation) will be more favourable at high soil pH; soils with greater pH will react to consume the H+ produced, forcing the equilibrium further towards products. Similarly, redox potential will control the precipitation of some minerals; for example, sulphide minerals will form much more favourable under reducing conditions. This is because under chemically reduced conditions, the reduced forms of both iron (Fe2+) and sulphur (HS−/S2−) will be present in greater concentration, and these are both required reactants for sulphide mineral formation.
4.4.4.2 Co-precipitation
Unless a solid precipitates from a solution containing only its component ions, other ions of similar charge and ionic radius may be included in the structure of the precipitating mineral. This process is known as co-precipitation and involves inclusion of small amounts of ‘foreign’ ions in the structure of a precipitating solid, normally limited to a few mole percent of the foreign ion(s). We already know that the phyllosilicate clay minerals co-precipitate with cations in their structure other than Al3+ and Si4+ (Sect. 4.4.2), and this is a very important phenomenon in soils and sediments. Other types of mineral are also known to form co-precipitates; for example, iron oxides may include trace or greater amounts of Al, As, Cd, Co, Cr, Cu, Mn, Ni, Pb, Sn, V, and Zn (e.g. see Singh and Gilkes 1992).
4.5 Soil Functions Related to Human Concerns
Direct use of soil by human communities affects issues related to physical and emotional health and well-being, food production, community cohesion, and construction. Soil may also have indirect effects on health through differences in land use or moderation of climate or may represent an archaeological or historical archive. We have addressed soil functions related to human concerns in the other chapters in this book.
In our discussion of soil formation in urban environments (Chap. 2), we examined the archaeological function of urban soils, that is, their ability to store and preserve different types of record of human habitation and activities. Chapters 2 and 3 also discuss the geological information stored in some urban soils in terms of their original parent material. The manufactured items that we find in soil help us learn about human history, and urban soils also offer us many opportunities to learn about the natural world through urban agriculture and environmental education (Gregory et al. 2016), a topic we address in Chap. 10.
The main ‘use’ of soil in urban environments is to provide living space; we construct our dwellings on (and in some cases, from) soil. We cover some of the engineering properties of soil as a building material and foundation in Chaps. 5 and 10. Construction of buildings, however, almost always insulates us from the soil beneath, a constraint which makes access to gardens and public open spaces crucial. In contrast, the use of soil for construction or as an artisanal raw material puts humans in very direct contact with soil. Some of the most obvious activities where humans use soil as a raw material are in pottery fabrication (Oladimeji et al. 2015) and where bricks or other items for building construction are made from soil, such as rammed earth which has been used since ancient times (Spence 1975; Ghavami et al. 1999; Liu et al. 2010). The soils in cities can provide a basis for addressing a range of concerns related to individual and community health and well-being; the health risks are discussed in Chaps. 6, 7, 8, and 9, and health benefits are addressed for multiple contexts in Chap. 10.
4.6 Summary
-
The functions of soils can be expressed in terms of the services that soil provide to ecosystems, in general including support of plant growth, maintenance of biodiversity, removal and cycling of materials, and storage of ecosystem resources.
-
The sizes and arrangements of solid materials in soils, known as soil texture and structure, control soil porosity and pore size distribution. Understanding soil pores allows us to understand the storage and movement of water in soils.
-
We also need to account for soil water in the context of other Earth subsystems, and this is expressed in the soil water balance. Similarly, we can construct an energy balance for soils and use this to understand trends in soil temperature or even soil’s effect on climates.
-
The organisms that live in or on soils perform a vast range of ecosystem services, ultimately related to the ability of soils to capture and store carbon fixed by photosynthetic organisms – mainly, but not restricted to, plants. The transfers of carbon, nutrients, and energy through biological food webs in soils encapsulate some of the most important aspects of biogeochemical cycles of carbon and macro- and micronutrient elements.
-
The interactions of soil biological and physical processes control crucial soil properties such as aggregation and porosity. Soil biology and soil chemistry interact in their effects on bioavailability of the chemical elements.
-
Humans depend on all these natural soil functions and have also developed other uses for soils: we construct things from soil; we use soil to store, deliberately or otherwise, our diverse array of waste materials; the waste materials themselves preserve a record of human existence.
4.7 Further Reading
-
Hazelton P, Murphy B (2011) Understanding soils in urban environments. CSIRO Publishing, Collingwood, 160 pp.
-
Hillel D (2008) Soil in the environment: crucible of terrestrial life. Elsevier/Academic Press, Amsterdam/Boston, 307 pp.
-
McBride MB (1994) Environmental chemistry of soils. Oxford University Press, Oxford
-
Paul EA (ed) (2015) Soil microbiology, ecology and biochemistry. Academic Press, San Diego
4.8 Questions
4.8.1 Checking Your Understanding
-
1.
Explain how soil porosity and pore size distribution affects the behaviour of water in soil.
-
2.
If finer-textured soils hold on to water more tightly, how is it that loamy soils can have more plant-available water than sandy soils?
-
3.
What are the soil microbial processes involved in biogeochemical cycling of carbon and nutrients?
-
4.
What are the forms of macro- and micronutrients in soils which are available to plants? What do these have in common, and how do they differ?
-
5.
What are ‘secondary minerals’ in soils, and why are they important?
-
6.
List multiple ways by which soil can immobilise various contaminants.
4.8.2 Thinking About the Issues
-
7.
What do humans do in urban environments that damages soil structure ? Conversely, what can we do to improve urban soil structure?
-
8.
Do you think it would be possible for plants or soil organisms to degrade or remove contaminant substances? If so, which ones, and how does the degradation of removal occur? If not, why not?
-
9.
Humans have added many substances to urban soils which degrade soil quality, so is there anything we can add to soils to improve their ability to supply health ecosystem functions?
4.8.3 Contemplating Soils Creatively
-
10.
Would people place more value on soil in general if they ‘got their hands dirty’, that is, came into more frequent direct contact with soil?
References
Ban-Weiss GA, Bala G, Cao L, Pongratz J, Caldeira K (2011) Climate forcing and response to idealized changes in surface latent and sensible heat. Environ Res Lett:6. https://doi.org/10.1088/1748-9326/6/3/034032
Bar-On YM, Phillips R, Milo R (2018) The biomass distribution on Earth. Proc Natl Acad Sci 115:6506–6511. https://doi.org/10.1073/pnas.1711842115
Brantley SL, Goldhaber MB, Ragnarsdottir KV (2007) Crossing disciplines and scales to understand the critical zone. Elements 3:307–314. https://doi.org/10.2113/gselements.3.5.307
Brimhall GH, Dietrich WE (1987) Constitutive mass balance relations between chemical composition, volume, density, porosity, and strain in metasomatic hydrochemical systems: results on weathering and pedogenesis. Geochim Cosmochim Acta 51:567–587. https://doi.org/10.1016/0016-7037(87)90070-6
Carsel RF, Parrish RS (1988) Developing joint probability distributions of soil water retention characteristics. Water Resour Res 24:755–769. https://doi.org/10.1029/WR024i005p00755
Cary Institute of Ecosystem Studies (2020) Water & watersheds, Millbrook, New York. https://www.caryinstitute.org/eco-inquiry/teaching-materials/water-watersheds (accessed 20210615)
Chorover J, Kretzcschmar R, Garcia-Pichel F, Sparks DL (2007) Soil biogeochemical processes within the critical zone. Elements 3:321–326. https://doi.org/10.2113/gselements.3.5.321
Coleman DC, Crossley DA Jr, Callaham MA (2017) Fundamentals of soil ecology. Elsevier Science & Technology, Saint Louis
de Bruyn LAL, Conacher AJ (1990) The role of termites and ants in soil modification: a review. Aust J Soil Res 28:55–93. https://doi.org/10.1071/SR9900055
de Lima RP, da Silva AR, da Silva AP, Leao TP, Mosaddeghi MR (2016) Soilphysics: an R package for calculating soil water availability to plants by different soil physical indices. Comput Electron Agric 120:63–71. https://doi.org/10.1016/j.compag.2015.11.003
Feller C, Brown GG, Blanchart E, Deleporte P, Chernyanskii SS (2003) Charles Darwin, earthworms and the natural sciences: various lessons from past to future. Agric Ecosyst Environ 99:29–49. https://doi.org/10.1016/S0167-8809(03)00143-9
Fortuna A (2012) The soil biota. Nat Educ Knowledge 3:1. https://www.nature.com/scitable/knowledge/library/the-soil-biota-84078125
Ghavami K, Toledo Filho RD, Barbosa NP (1999) Behaviour of composite soil reinforced with natural fibres. Cem Concr Compos 21:39–48. https://doi.org/10.1016/S0958-9465(98)00033-X
Gregory MM, Leslie TW, Drinkwater LE (2016) Agroecological and social characteristics of New York city community gardens: contributions to urban food security, ecosystem services, and environmental education. Urban Ecosyst 19:763–794. https://doi.org/10.1007/s11252-015-0505-1
Haynes RJ (1986) Mineral nitrogen in the plant-soil system. Academic Press, Orlando
Hillel D (2008) Soil in the environment: crucible of terrestrial life. Elsevier/Academic Press, Amsterdam/Boston, 307 pp
Hillel D (2014) Environmental soil physics fundamentals, applications, and environmental considerations. Academic Press, San Diego
Ingham ER (n.d.) Soil food web. Natural Resources Conservation Service (Soils), United States Department of Agriculture. https://www.nrcs.usda.gov/wps/portal/nrcs/detailfull/soils/health/biology/?cid=nrcs142p2_053868 (accessed 20190802)
Kump LR, Brantley SL, Arthur MA (2000) Chemical weathering, atmospheric CO2, and climate. Annu Rev Earth Planet Sci 28:611–667
Lindsay WL (1979) Chemical equilibria in soils. Wiley, New York
Liu J, Hu R, Wang R, Yang L (2010) Regeneration of vernacular architecture: new rammed earth houses on the upper reaches of the Yangtze River. Front Energy Power Eng China 4:93–99. https://doi.org/10.1007/s11708-010-0002-4
McBride MB (1994) Environmental chemistry of soils. Oxford University Press, New York
McKenzie NJ, Jacquier D, Isbell R, Brown K (2004) Australian soils and landscapes: an illustrated compendium. CSIRO Publishing, Collingwood
McLaren RG, Cameron KC (1990) Soil science - an introduction to the properties and Management of new Zealand Soils. Oxford University Press, Auckland
Minasny B, McBratney AB (2001) The Australian soil texture boomerang: a comparison of the Australian and USDA/FAO soil particle-size classification systems. Aust J Soil Res 39. https://doi.org/10.1071/SR00065
Moeys J (2018) soiltexture: functions for soil texture plot, classification and transformation. R package version 1.5.1. The Comprehensive R Archive Network. https://CRAN.R-project.org/package=soiltexture (accessed 20210116)
Morel JL, Chenu C, Lorenz K (2015) Ecosystem services provided by soils of urban, industrial, traffic, mining, and military areas (SUITMAs). J Soils Sediments 15:1659–1666. https://doi.org/10.1007/s11368-014-0926-0
Oladimeji Y, Adepoju SA, Abdulsalam Z (2015) Reviving pottery enterprise: an impetus to poverty alleviation and self-reliance among women folks in Ilorin, Kwara state, Nigeria. Int J Dev Sustain 4:145–160
Poppe LJ, Paskevich VF, Hathaway JC, Blackwood DS (2004) A laboratory manual for X-Ray powder diffraction. U. S. Geological Survey Open-File Report, U.S. Department of the Interior, U.S. Geological Survey, Woods Hole, MA, USA. http://pubs.usgs.gov/openfile/of01-041/index.htm
Ryan P (2014) Environmental and low temperature geochemistry. Wiley, Chichester
Schaetzl RJ, Anderson S (2005) Soils: genesis and geomorphology. Cambridge University Press, New York
Singh B, Gilkes RJ (1992) Properties and distribution of iron oxides and their association with minor elements in the soils of South-Western Australia. J Soil Sci 43:77–98. https://doi.org/10.1111/j.1365-2389.1992.tb00121.x
Smith AB, Walker JP, Western AW, Young RI, Ellett KM, Pipunic RC et al (2012) The Murrumbidgee soil moisture monitoring network data set. Water Resour Res 48:W07701. https://doi.org/10.1029/2012WR011976
Spence RJS (1975) Predicting the performance of soil-cement as a building material in tropical countries. Build Sci 10:155–159. https://doi.org/10.1016/0007-3628(75)90031-6
Sposito G (2008) The chemistry of soils, 2nd edn. Oxford University Press, New York, 329 pp
Thompson A, Goyne KW (2012) Introduction to the sorption of chemical constituents in soils. Nat Educ Knowledge 4:7. https://www.nature.com/scitable/knowledge/library/introduction-to-the-sorption-of-chemical-constituents-94841002/
Threlfall T (2003) Structural and thermodynamic explanations of Ostwald’s rule. Org Process Res Dev 7:1017–1027. https://doi.org/10.1021/op030026l
Tinjum JM, Benson CH, Blotz LR (1997) Soil-water characteristic curves for compacted clays. J Geotech Geoenviron 123:1060–1069. https://doi.org/10.1061/(ASCE)1090-0241(1997)123:11(1060)
van den Hoogen J, Geisen S, Routh D, Ferris H, Traunspurger W, Wardle DA et al (2019) Soil nematode abundance and functional group composition at a global scale. Nature 572:194–198. https://doi.org/10.1038/s41586-019-1418-6
White RE (2006) Principles and practice of soil science: the soil as a natural resource. Blackwell Science, Malden
Yang H, Lu R, Downs RT, Costin G (2006) Goethite, α-FeO(OH), from single-crystal data. Acta Crystallogr E Struct Rep Online E62:i250–i252. https://doi.org/10.1107/S1600536806047258/wm2061Isup2.hkl
Yang X, Wittig VE, Jain AK, Post WM (2009) Integration of nitrogen cycle dynamics into the integrated science assessment model for the study of terrestrial ecosystem responses to global change. Glob Biogeochem Cycles 23:GB4029. https://doi.org/10.1029/2009GB003474
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Rate, A.W. (2022). Urban Soil Functions. In: Rate, A.W. (eds) Urban Soils. Progress in Soil Science. Springer, Cham. https://doi.org/10.1007/978-3-030-87316-5_4
Download citation
DOI: https://doi.org/10.1007/978-3-030-87316-5_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-87315-8
Online ISBN: 978-3-030-87316-5
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)