Abstract
Among various abiotic factors influencing the biology of rhizosphere, soil organic matter (SOM) and humus formation play a major role in regulating the nutrient acquisition capability of roots. Nitric oxide present in the rhizosphere is a widely distributed gaseous biomolecule which plays a pivotal role in regulating plant growth and metabolism. There exists a possible functional link associated with the soil organic matter and NO generation in the rhizosphere. It is important to understand the various biotic and abiotic sources of rhizospheric NO being accumulated by the activity of microbes and in-vivo production of NO by plant roots. Rhizosphere microclimate affects NO generation both from soil and plant roots, however, excessive accumulation of NO may turn toxic for microbial and plant growth. Plants synthesize NO both in the apoplast and symplast region of root tissues. Thus, plant derived-NO contributes to the total available NO in the rhizosphere which in turn affects root functioning. Soils harbour different types of microbes which involve nitrifying-denitryifying bacteria, photoautotrophs, chemotrophs or facultative/obligate symbionts. Nitric oxide levels in the rhizosphere largely depends upon the nature of biotic community present in the soil which in turn affects soil C:N ratio resulting from root exchange. Abiotic stress factors like heavy metal stress, drought stress or hypoxia stress are alleviated by rhizospheric NO. Furthermore, rhizospheric NO has been associated with management of mineral deficiency in plants. NO stands to be an important molecule in nitrate-sensing process of roots. NO acts differently at low and high levels of N present in the soil. Humus mediated-NO formation also results in NO-IAA crosstalk which acts upstream to PM-H+ATPase expression. Among various physiological effects exhibited by NO, protein modification at cysteine residues, tyrosine nitration and mobilization of secondary messengers have been reported to be active in response to abiotic stress. The rhizosphere-plant-atmosphere continuum of NO functioning is therefore associated with plant-environment interactions.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Rhizosphere is a unique facet of plant-environment interaction affected by various biotic and abiotic factors. In this context, it is important to assess the microbial flora of rhizosphere which largely regulates plant growth and nutrient exchange across the roots. Among various abiotic factors influencing the biology of rhizosphere, soil organic matter (SOM) and humus formation play a major role in regulating the nutrient acquisition capability of roots. A diverse group of biomolecules like sugars, amino acids, peptides, organic acids, minerals and other secondary metabolites are exchanged in the soil-root interface. Nitric oxide present in the rhizosphere is a widely distributed gaseous biomolecule which plays a pivotal role in regulating plant growth and metabolism. It is important to understand the various biotic and abiotic sources of rhizospheric NO being accumulated by the activity of microbes and in-vivo production of NO by plant roots. Nitric oxide is chemically amphipathic in nature and capable of free diffusion across plasma membranes. Plants synthesize NO both in the apoplast and symplast of root tissues. Thus, plant derived-NO contributes to the total available nitric oxide in the rhizosphere which in turn affects root functioning. Soils harbour different types of microbes which involve nitrifying-denitryifying bacteria, photoautotrophs, chemotrophs or facultative/obligate symbionts. Therefore, nitrification and denitrification activities play a major role in the formation of NH3, NO2, NO3 and NO in rhizosphere (Ġodde and Conrad 2000). Mineralization of soil components by heterotrophic microbes yield NH4 compounds which are further converted to NO2, NO3 and NO. Furthermore, bio-fertilization results in agricultural soils being rich in nitrification activities which may also result in higher accumulation of NO. Nitric oxide levels in the rhizosphere largely depend upon the nature of biotic community present in the soil which in turn affects soil C:N ratio resulting from root exchange. Thus, NO levels in the rhizosphere affect the rate of N cycling between the plant and soil. A part of the NO produced also reacts with other organic components and is responsible for ozone formation in the troposphere. Soil-generated NO in the rhizosphere has chemical abilities to form various nitrogenous forms like N2O, N2O3 or peroxynitrites (Stöhr and Ullrich 2002). This in turn affects various metabolic pathways within the plant roots. Additionally, different plant species have been reported to exhibit varied levels of NO emission resulting due to nitrification reactions (Wildt et al. 1997). Rhizospheric soils have been reported to exhibit higher nitrification abilities in comparison with non-rhizospheric soils (Chowdhury et al. 2016). Thus, microbial activity is likely to decrease with increase in soil distance from plant roots. Root exudations and mycorrhizal associations contribute to the levels of humus formation in the rhizosphere. This in turn affects the microbial flora present in the rhizosphere. Rhizospheric NO has been reported to regulate various morphological and physiological responses in plants. Regulation of root architecture and morphology is partially controlled by available free NO present in the rhizosphere. Abiotic stress factors like heavy metal stress, drought stress or hypoxia stress are alleviated by rhizospheric NO (Arasimowicz-Jelonek et al. 2011b; Molina‐Favero et al. 2007). Furthermore, rhizospheric NO has been associated with management of mineral deficiency in plants (Zhang et al. 2012). NO uptake has also been reported to be regulated by NO concentrations in the rhizosphere (Simon et al. 2013). NO plays a major role in communication between rhizospheric microbes and roots (Pande et al. 2021). The current chapter thus summarizes the aspects of plant growth and stress tolerance mechanisms mediated by rhizospheric nitric oxide.
2 Sources of NO Generation and Its Distribution in the Rhizosphere
Nitric oxide formation in the rhizosphere results from both microbial activity and plant based NO biosynthesis in the roots. Bacterial nitrification and denitrification are major microbial pathways responsible for NO accumulation in the rhizosphere. NO in soil is likely to be produced by autotrophic or heterotrophic nitrification or denitrification reactions (Robertson and Groffman 2005). Soil based variation in the levels of NO largely depend upon the types of ecosystem. Tropical moist forest lands have been reported to exhibit high turnover of NO prevalent in the rhizosphere (Butterbach-Bahl et al. 2001). NO and N2O are the major nitrogen oxides produced in the rhizosphere among which N2O flux usually appears higher than NO. Various parameters of soil texture, temperature, fertilization and microbial activity affect NO levels in the rhizosphere (Stange et al. 2000; Parton et al. 2001; Butterbach-Bahl et al. 2001). Rhizosphere microclimate affects NO generation both from soil and plant roots, however, excessive accumulation of NO may turn toxic for microbial and plant growth (Zumft 1997). Simon et al. (2009) has reported the evidences of rhizospehric NO being absorbed by plant roots. This has been attributed to NO-induced regulation of pedospheric nitrogen allocation among various components of the rhizosphere. NO level in the rhizosphere is indicative of the relative levels of microbial and plant metabolism prevalent in the zone. Microbial metabolism involves requirement of various amino acids and ammonium compounds in the soil. Thus, low levels of NO in the rhizosphere signify poor N-turnover generated by microbial metabolism (Simon et al. 2013). Various nitrifying bacteria like Rhizobium, Azotobacter or Azospirullum influence the rate of NO flux from the rhizosphere. Chemolithotrophic bacteria present in the rhizosphere may also alter nitrate use efficiency and influence the rate of NO generation (Laanbroek and Woldendorp 1995). Presence of arbuscular-vescicular mycorhizal fungi (Glomus sp.) associated with the rhizospher regulate NO generation through nitrification and denitrification activities (Zhang et al. 2013). Evidences suggest the crucial role of soil fungi in regulating N2O and NO emission from the rhizosphere (Ma et al. 2008). Fungal respiration pathway thus involves the conversion of N2O to NO. However, unlike bacterial denitrification process fungal metabolism involves aerobic conditions in the rhizosphere. Thus, oxygen levels in the rhizosphere may regulate the rate of fungal and bacterial metabolism contributing to NO generation (Ma et al. 2008). Hypoxic condition in the rhizosphere alters the rate of heterotrophic nitrification thus causing changes in the NO flux. The conversion of rhizospheric NO to N2O catalyzed by the activity of nitric oxide reductase has been reported in some fungal members (Zhang et al. 2001; Zhou et al. 2002; Watsuji et al. 2003).
Nitric oxide produced in the rhizosphere is transient and freely diffusible. Plant-derived nitric oxide also contributes to rhizospheric NO. NO by the virtue of its unpaired electron has been suggested to possess high reactivity with O2 or O2−. In this regard it is worth mentioning that certain amount of NO formed in the rhizosphere gets converted to nitrite by oxidation (Stohr and Ulrich 2002). Additionally autooxidation of NO in the rhizosphere also yields peroxynitrite species (ONOO−) (Huie and Padmaja 1993). Subsequently NO toxicity affects plant metabolism which is attributable to the formation of oxidizing species of peroxynitrite. Furthermore during hypoxic conditions NO tends to react with thiols and secondary amines. Alkaline soil in the rhizosphere supports the formation of N2O. Thus formation and distribution of NO in the rhizosphere is precisely regulated by the edaphic factors associated with the nature of microflora.
3 Rhizosphere Composition Regulates Apoplastic and Symplastic NO Production in Roots
Nitric oxide in plants is biosynthesized both by enzymatic and non-enzymatic pathways. In animal systems NO is mainly synthesized by the enzyme nitric oxide synthase (NOS). Although putative NOS like activity has also been detected in plants (Durner et al. 1998; Foissner et al. 2000) major part of NO in cytosol is produced by the enzyme nitrate reductase (cNR). Nitrite has been reported to be an important precursor of NO in plant cells (Delledonne et al. 1998). Non-enzymatic pathway of NO biosynthesis involves protonation of nitrite to form nitrous acid which subsequently yields NO and NO2−. Bethke et al. (2004) suggested that such mechanism of NO generation is likely to be prevalent in the apoplast of plant roots. Various factors like low pH, nitrite permeable transporters and nitrite present in the apoplast support apoplastic pathway of NO production in plants. However, root tissues may vary in their apoplastic nitrite content which partially depends upon the N turnover rate of rhizosphere. Interestingly Bethke et al. (2004) have reported the presence of phenolics to promote NO formation in the apoplastic regions. Rhizospheric region has been reported to contain higher amount of NO2− compare to the soil solutions away from the vicinity of plan roots (Binnerup and Sorensen 1992). Intriguing facts remain to be deciphered as to whether plant roots involve more of enzymatic or non-enzymatic pathway leading to NO generation. There are possibilities of rapid changes in root apoplastic pH mediated by various signaling events like auxin efflux, gravitropic response or changes in ion flux (Fasano et al. 2001; Pagnussat et al. 2002). Furthermore, root plasma membranes are known to possess NR activity which subsequently draws the possibility of apoplastic NO generation both through enzymatic and non-enzymatic pathways (Stohr and Ullrich 2002). According to Wildt et al. (1997) different plant species have been reported to possess variable NO emission limits in their rhizosphere. Thus, both apoplastic and symplastic NO produced by plant roots is likely to diffuse into the rhizosphere. Tobacco root cell plasma membrane has been reported to possess a nitrite: NO reductase enzyme capable of NO generation from nitrite (Stöhr et al. 2001). Earlier investigations suggested underground NO formation only under the control of microbial sources (Stöhr and Ullrich 2002). However, investigation across the last decade has put forward some intriguing facts about the rhizospheric regulation of NO generation in plant roots. The absorptive zone of roots contains abundant root hairs. This zone is active for nutrient exchange from soil solution. Furthermore, the morphology and architecture of root is under the precise control of rhizosphere nutrient levels (Forde and Lorenzo 2001; Forde 2002). Nitrate-induced lateral root formation thus coincides with cellular NO generation manifested as an effect of soil nitrate levels (Zhang and Forde 2000). Cytoslic nitrate reductase activity is induced by soil and apoplastic nitrite levels. Anoxic condition in the rhizosphere is likely to exert compartmentalisation of nitrite in the apoplast thus leading to NO generation in the apoplast (Botrel et al. 1996; Stoimenova et al. 2003). Nitrite mediated NO formation in the apoplast is upregulated by ascorbic acid and phenolic substances (Bethke et al. 2004). According to Stöhr and Stremlau (2006) it is difficult to quantify plant liberated NO present in the rhizosphere as bacterial nitrification–denitrification process remains active in the vicinity of roots. Soil liberated NO lies in the range of 1 mg N m−2 h−1 which, however, is a function of rhizosphere pH, soil temperature, moisture and fertilization (Stöhr and Ulrich 2002). Anoxic conditions result in high amount of apoplastic NO generation in plant roots. This amount of NO generation inadvertently protects cellular biomolecules from NO toxicity.
4 Rhizospheric Organic Matter Elevates NO Biosynthesis and Subsequent Upregulation of Plant Growth Hormones
Rhizospheric nitric oxide can possibly modulate the activity of various plant growth regulators like auxin (IAA), cytokinin (Cyt), abscisic acid (ABA) and ethylene (Et). Crop productivity is largely regulated by the content of soil organic matter in the field (MacCarthy et al. 1990; Magdoff and Weil 2004). Root-generated NO and humus (SOM) affect microbial metabolism in the rhizosphere. Humus originates primarily from fresh organic matter of plant and animal debris. Microbial and fungal activity in the rhizosphere causes degradation of complex organic substances. This regulates the nutrient availability of the soil viz. rhizosphere (Tipping et al. 2002; Chen et al. 2004). Investigations have suggested that a certain amount of humus with low molecular weight can penetrate root apoplast thus affecting nutrient exchange capability of the roots (Vaughan et al. 1985; Vaughan 1986; Nardi et al. 2002, 2009). Humification has been reported to directly regulate NO biosynthesis in roots which is also associated with increase in plasma membrane bound H+ ATPase activity and hormone biosynthesis (IAA, ABA, Et) (Mora et al. 2014). Furthermore, humus mediated effects can be manifested through variable concentration of growth regulators prevalent in root and shoot of plants (Mora et al. 2014). Since NO is a biologically active signaling molecule, therefore it is worthwhile to state that humus mediated regulation of PGRs is likely mediated by root NO levels. Rhizospheric humus has been suggested to elevate auxin activity (Mora et al. 2014). Interestingly, humus mediated upregulation of root PM-H+ ATPase activity enhances nitrate uptake by plants (Mora et al. 2014). In this context, Jannin et al. (2012) reported that rhizospheric humus preferably up regulates the genes associated with nitrate transport in roots. Humus-induced nitric oxide surge acts as a rapid response which triggers the expression of PM H+ATPase, IAA and Et in the roots (Zandonadi et al. 2010; Mora et al.2014). These physiological changes later manifest in the form of better root growth, root hair proliferation and increased root dry weight. Humus mediated-NO formation also results in NO-IAA crosstalk which acts upstream to PM-H+ATPase expression (Xu et al. 2010; Terrile et al. 2012; Freschi 2013). According to Terrile et al. (2012) NO can preferably S-nitrosylate the TIR-1 region of auxin receptor and down regulate IAA-oxidase activity. Humus mediated enhancement in nitrate uptake therefore promotes cytosolic NO biosynthesis in plant roots. Thus, in the context of rhizopsheric NO humus acts as a positive modulator of plant growth. However, further investigations are required to decipher the analysis of NO contributed by the plant root and microflora in the rhizosphere separately. Humus associated rhizosphere acidification is likely to regulate the microbial metabolism thus affecting NO flux from rhizosphere.
5 Rhizospheric NO Regulates Nitrate Assimilation and Root Architecture in Plants
Various investigations have deciphered the role of endogenous nitric oxide in growth promoting effects on various plant organs. However, rhizospheric NO also exerts unique regulation on root architecture and its proliferation. Autotrophic or heterotrophic nitrification promotes rhizospheric NO in soils (Fig. 1). Azospirillum brasilense is a soil dwelling plant—growth-promoting bacteria which liberates NO through its metabolic pathways. Aerobic denitrification process mediated by Azospirillum brasilense has been considered as a major source of NO flux from rhizosphere. Tomato (Solanum lycopersicum Mill.) plants investigated for Azospirillum mediated growth promoting effects were observed to exhibit better root proliferation (lateral and adventitious root) in presence of bacterial inoculation. The malleability of NO-induced root growth was confirmed by treatments of NO scavengers. Azospirillum brasilense is unique among various plant-growth-promoting bacteria (PGPB) and regulates root architecture through root hair proliferation and LR formation in wheat and tomato (Kolb and Martin 1985; Okon and Kapulnik 1986; Fallik et al. 1994; Dobbelaere et al. 1999; Creus et al. 2005). According to Hartmann and Zimmer (1994) dissimilatory nitrate reduction pathway of Azospirillum produces nitrite in addition to nitric oxide and nitrous acid. NO prodiction by Azospirillum has been suggested to be accomplished by various pathways during aerobic conditions (Molina-Favero et al. 2007). Aerobic denitrification has been suggested to be accomplished by periplasmic nitrate reductase activity (Jetten et al. 1997). Other metabolic pathways likely to be prevalent are heterotrophic denitrification of ammonium compounds which liberate hydroxylamine and NO as intermediates (Wrage et al. 2001). Different strains of Azospirillum have been used for inoculation with plants. Nutrient status of the rhizosphere regulates the nature of metabolism exhibited by Azospirillum and energised by NH4+, NO3–, or arginine availability. NO production, however, can also result from stressful situations. Rhizospheric NO is a volatile membrane permeable gaseous growth modulator likely to diffuse into plant roots. Nitrite uptake by roots can promote apoplastic NO formation in plants. NO-induced root proliferation is mediated by downstream auxin response (Pagnussat et al. 2002). Molina-Favero et al. (2008) suggest the possibility of NO-induced activation of cyclin D3 proteins necessary for cell cycle regulation, which in turn regulates cell division leading to root proliferation. Exogenous humus application has also been reported to increase PM H+-ATPase activity in maize seedlings (Zandonadi et al. 2010). Interesting observations have been obtained for the rate of reductive denitrification activity prevalent in the rhizosphere of barley crops. NO has been reported to be associated with nitrate assimilation at varying levels of nitrogen in the rhizosphere. The process of nitrate uptake through roots is followed by long distance from roots to shoots. NO stands to be an important molecule in nitrate-sensing process of roots (Sanz-luque et al. 2013, Sun et al. 2015a). NO has been reported to regulate the expression of transcripts associated with nitrate assimilation pathway. However, NO-induced modulation of NR activity showed varietal differences in its expression levels. Interestingly, NO liberated by NR activity counter-regulates the activity of nitrate transporters thus increasing N uptake. This is further manifested by increased lateral root growth (Zhang et al. 2007; Ruffel et al. 2011; Mounier et al. 2013; Sun et al. 2015b). NO acts differently at low and high levels of N present in the soil. Low N content leads to NO-induced activation of nitrate transporters to increase internal N levels in the plant roots. However, during high N-levels in soil NO inhibits the phosphorylation of 14–3–3 and causes S-nitrosylation of the protein (Frungillo et al. 2014). Non-mycorhizal beech roots (Fagus sylvatica) were reported to be associated with increased uptake of ammonium and glutamate sources induced by NO treatment (Simon et al. 2009). These observations thus imply that Rhizospheric NO-mediated N partitioning possibly occurs between plant roots and soil microbes in natural soil. Variable concentrations of NO in presence of different N levels in soil rhizosphere exhibit differences in the intensity of N-uptake in Scots pine (Pinus sylvestris L.) seedlings (Simon et al. 2013). Higher concentrations of NO supported preferential uptake of nitrate and arginine. Amonium uptake was, however, independent of NO concentration. This substantiates the fact that high concentration of NO preferentially increases nitrate and arginine uptake through roots. Thus, rate of N assimilation by plants mostly appears to be a function of rhizospheric NO concentrations. To summarize, N-sources potentially available to pine seedlings were mostly ammonium, nitrates, glutamine and arginine. The authors also stated that NO-mediated response of N-uptake varies in different plant species. Dong et al. (2015) suggests the synergistic effects of rhizospheric NO and CO2 to affect N-uptake in Fagus sylvatica seedlings. Rhizospheric CO2 is another important regulatory molecule released by microbial or plant respiration. CO2 functioning associated with such soil-root interface also regulates N-uptake (Cramer et al. 1996; Van der Merwe and Cramer 2000; Viktor and Cramer 2005). The effect of NO on N-uptake was more pronounced at ambient CO2 concentrations. High and low CO2 concentrations prioritised the uptake of organic or inorganic N-uptake in presence of NO.

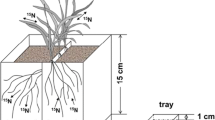
source of NO subsequently into nitrate. Furthermore, ammonia itself in solution gets translocated to rhizospheric region in the form of NO. Inside the rhizospheric region, a number of symbiotic nitrogen fixing bacteria and arbuscular mycorrhizal fungi (AMF) are found associated with plant roots that also acts NO source in plant roots
Sources of rhizospheric NO. In the aboveground parts of plants NO are being synthesized inside plant cell and is translocated to below ground. In the belowground rhizospheric region has substantial NO that is uptaken up by plant roots. Region outside rhizosphere acts as supplier of NO through soil organic matter (humus) that transport NO through diffusion into rhizosphere followed by roots. A number of free living nitrogen fixing bacteria converts NH4+ into NO which is then transported to rhizosphere where it gets converted into NO3− (nitrate) that is readily taken up by plant roots. Additionally, number of nitate fertlizers also acts as a
6 Nitric Oxide Mediated Abiotic Stress Tolerance in Plants is Partially Regulated by Rhizospheric Interactions
Unfavourable growth environment for plants are mostly associated with high nutrient depletion, heavy metal infusion, poor cation exchange capability of soils, sodicity or temperature adversities. Furthermore, anoxic and hypoxic conditions also result in physiological imbalances occurring in various plant organs. Changes in the pH, temperature and oxygen content of the rhizosphere primarily affect root metabolism and membrane functioning. Nitric oxide synthesis and its distribution in plants is regulated at the root-soil interface and accompanied by various factors like composition of microflora, available soil NO and N availability. Natural soils mostly involve NO flux obtained due to microbial metabolism. Agricultural fields, however, are subject to variable NO flux regulated by fertilization, irrigation and type of cultivation practiced. Alkaline and acidic soils show differences in microbial communities and available sources of NO. NO mediated imbalance in the reactive nitrogen species (RNS) levels triggers various nitrosative responses in plants (Corpas et al. 2011). Environmental adversities led to formation of reactive radical species thus causing harm to various cellular and metabolic processes. In this context, roots are likely to produce high amounts of apoplastic NO which prevents cytoplasmic NO toxicity. Thus beneficial effect of NO is largely mediated by signaling response associated with stress stimuli. Roots are subjected to higher rates of environmental variations in comparison with shoot or aerial organs. Thus root-mediated abiotic stress signals are transduced to aerial organs through long distance signal transduction. Among various physiological effects exhibited by NO, protein modification at cysteine residues, tyrosine nitration and mobilization of secondary messengers have been reported to be active in response to cadmium toxicity in soils (Gill et al. 2013). Persistence of cadmium in soils is mostly due to its prolonged biological half-life and common in areas contaminated with industrial effluents or phosphate fertilization (Gill et al. 2013). This heavy metal has been reported to be readily uptaken by roots. Conversely, NO has been attributed to facilitate cadmium uptake from rhizosphere which contradicts to its role in alleviating cadmium toxicity (Arasimowicz-Jelonek et al. 2011a). However, NO mediated amelioration depends upon rhizosphere composition, N turnover and NO flux from root-soil interface. Upregulation of endogenous NO biosynthesis has been reported to be regulated by cadmium stress. Hypoxia is a major soil-mediated abiotic stress signal inducing morpho-anatomical and physiological changes in plants. Water logging in soils cause oxygen deficiency which affects plants at the early vegetative stages. Liu et al. (2015) have reported that hypoxia-induced NO formation in Populus is primarily regulated by nitrate levels available in the nutrient solution. Thus nitric oxide production in roots induced by oxygen deficiency is likely to be a function of rhizospheric nitrate concentration and subsequent nitrite formation catalyzed by cNR activity in roots. Similar investigations by Wany et al. (2017) revealed that ethylene-induced aerenchyma formation in wheat roots was possibly regulated by NO activity during hypoxic conditions. Hypoxia-induced NO formation was catalyzed by NR activity prevalent in roots. The authors also reported that NO-signal during hypoxia was possibly transduced by xylem mediated long distance transport of NO-precursor or NO derivative from root to aerial shoots of Populus. Soil-dwelling chemolithotrophs are responsive to nitrification process induced by stressful conditions (Laanbroek and Woldendorp 1995). Thus, abiotic stress-induced microbial nitrification in the rhizosphere is one of the major regulators of NO production and its downstream action in plants. Mora et al. (2014) have reviewed the beneficial role of rhizospheric humus in promoting abiotic stress tolerance mediated by NO. Sodic stress-induced NO signaling primarily operates through NO-IAA crosstalk in cucumber plant roots (Gong et al. 2015).
Interestingly, plant based non-symbiotic haemoglobins have been reported to act as important endogenous regulator of NO in roots (Dordas et al. 2003). Plants subjected to hypoxia often exhibit a surge in cytosolic NO content. Hypoxia-induced haemoglobin synthesis has been reported in maize roots (Taylor et al. 1994). Rhizospheric changes associated with O2 defeciency and water logged conditions promote haemoglobin accumulation in roots. This family of protein in its oxyhaemoglobin form ligates with NO to form nitrosylhaemoglobin. Thus, haemoglobin-NO interaction triggered by hypoxia stress is involved with plant tolerance to O2 defeciency, adventitious rooting and prevention of nitrosative stress (Dordas et al. 2003). In this context, it is important to understand that exogenous nitrate levels also regulate haemoglobin mediated NO interaction in roots (Dordas et al. 2003). NO has been reported to increase Fe availability in Arachis hypogaea Linn. hsuji grown in iron deficient calcareous soils (Zhang et al. 2012). NO-induced increased Fe uptake has been suggested to be accomplished by increased FeIII reductase activity in the roots followed by increased levels of available Fe form in the rhizosphere (Zhang et al. 2012). Thus rhizospheric NO level is important in regulating the pH, available Fe content and Fe reducing ability in the soil-root interface. NO-mediated alleviation of Fe deficiency thus manifests in better plant growth and increased biomass. Zn-stress tolerance in Solanum nigrum plants have been reported to be operative through NO production and subsequent modulation of antioxidative homoeostasis. Zn stress-induced NO formation has been suggested to cause programmed cell death at the root apex followed by regulation of root architecture. Salinity stress and plant community assemblage have been reported to regulate microbial denitrification in natural wet land vegetations of coastal regions (Bañeras et al. 2012). Al-induced NO production, however, exerts negative impact on Al-tolerance of plants (Sun et al. 2015a, 2015b). NO produced by Al toxicity further decreases pectin methylation in root cell wall. This results in increased Al uptake from rhizosphere. Thus rhizosphere composition affected by nutrient availability and concentration of essential and non-essential minerals regulate NO-mediated responses during abiotic stress.
7 Rhizobacteria Mediated NO Formation in the Rhizosphere Regulates Abiotic Stress Tolerance in Plants
Root-colonizing rhizobacteria are important regulators of abiotic stress tolerance in plants. Plant-bacteria interaction in the rhizosphere triggers a series of physiological events associated with stress tolerance, free radical detoxification and amelioration of the effects of soil toxicity. The rhizosphere is suitable for root colonizing bacteria due to the fact that around 85% of soil organic carbon is obtained from roots and underground plant tissues (Barber and Martin 1976). Rhizobacteria respond to the root exudates and colonize by the mechanism of chemotaxis. A complex networking has been suggested to develop between the bacterial proteins and plant root exudates obtained in the rhizosphere (Gomez-Gomez and Boller 2002; Navarro et al. 2006). Among various biomolecules produced by rhizobacteria, NO is liberated in the rhizosphere. NO further induces resistance to soil pathogens and increases bioavailability of soil associated essential minerals (Dimkpa et al. 2009). Rhizobacteria mediated NO liberation regulates hormone biosynthesis pathway in the roots and aerial organ of the plants. Auxin, gibberllin and ethylene are the major target biomolecules modulated by NO. However, stress response induces elevation of ABA in the roots exposed to stress factors in the rhizosphere. Rhizobacteria can colonize both in the external and internal region of root cortex and hypodermis. The various soil dwelling rhizobacteria include Bacillus, Pseudomonas, Klebsiella, and Streptomyces which can also grow as endophytes in the roots (Hallmann et al. 1997; Long et al. 2008). NO mediated regulation of root architecture has been reported in Azospirullum-plant interaction (Creus et al. 2005; Molina-Favero et al. 2008). NO-mediated ethylene biosynthesis can, however, be reduced by the action of bacterial ACC deaminase activity which reduced ACC and ethylene levels in the roots. Thus, rhizobacteria colonization imparts abiotic stress tolerance mediated by reduced ethylene levels in plants. NO and IAA produced by the bacterial metabolism promotes lateral root development in plants. Furthermore, the beneficial effect of NO is manifested by increased accumulation of compatible solutes and antioxidants. Belimov et al. (2009) has suggested growth improvements and increased water use efficiency in pea plants supplemented with ACC deaminase producing bacterial strains. Plant growth promoting bacteria can possibly facilitate NO-induced salt stress amelioration. The process of NO-mediated modulation of enzyme activity (lipoxygenase, peroxidase, phenylalanine ammonialyase, catalase, superoxide dismutase) and subsequent proline accumulation has been reported to be enhanced by bacterial inoculation in salt-stressed soybean plants (Vaishnav et al. 2013). Furthermore, bacterial growth in association to exogenous nitric oxide effectively modulates ion transport mechanisms manifested by altered sodium and potassium levels. Evidences therefore imply that microbial composition at the rhizosphere region possibly regulate NO-induced processes of ion transport and metabolism in roots. Rhizobacteria-mediated NO production has been reported in response to drought, salinity and heavy metal stress (Dimkpa et al. 2009). Rhizobium sp. has been reported to trigger IAA induced-NO production in Pisum, Medicago and sugar beet plants (Ramachandran et al. 2011; Molina-Favero et al. 2007). Thus application of rhizobacteria in the form of biofertilizer promotes nutrient availability and NO production in the rhizosphere.
8 Future Perspectives: Rhizospheric NO Regulates Symbiotic Associations with Plant Roots
Nitric oxide has been reported to play a pivotal role in the aspects of plant-fungi and plant-bacterial symbiosis. Soil nitrate levels accompanied by N-uptake and rhizospheric NO production are some of the primary factors associated with the establishment of symbiotic associations. NO primarily regulates process of nodule formation and its senescence during legume-rhizobium symbiosis (Puppo et al. 2013). In this context both plant and bacterial metabolism associated with NO liberation provides important insights to the signaling process. Nitrate reductase, putative NO synthase and nitrate levels play a major role in the intensity of NO liberation (Meilhoc et al. 2011). However, excess NO causes inactivation of nitrogenase in the rhizobium colonies of root nodules. Thus bacterial enzymatic systems include haemoglobin, nitric oxide reductase and flavoredoxins which convert NO into nitrates, nitrous oxide or amonia (Cabrera et al. 2011). Bethke et al. (2004) considers both plant and bacteria as potential sources contributing to rhizospheric NO involved in symbiotic process. Different metabolic pathways are likely to be trigger NO generation in aerobic and anaerobic conditions. (Gupta et al. 2011; Mur et al. 2013). Thus O2 environment is an important determinant of NO-mediated root-bacterial signaling. According to Leach et al. (2010) NO production during soybean-Bradyrhizobium japonicum interaction is likely to be NOS dependent. Contradictory observations by Boscari et al. (2013), however, do not state the possibilities of NOS mediated NO generation during the early phase of NO-mediated symbiosis. Rhizospheric NO levels can effectively upregulate leg haemoglobin genes (LjHB1) in plants (Shimoda et al. 2005; Nagata et al. 2008). Interestingly NO burst occurring at the early stage of root-bacterial symbiosis induces haemoglobin synthesis which down regulates further NO production thus facilitating nodulation process in the roots. NO in general has been reported to exert both positive and negative regulation in the nodulation process in various plant systems (Pii et al. 2007; Leach et al. 2010; Shimoda et al. 2009). Hypoxic condition in the rhizosphere regulates NO-mediated symbiotic interaction associated with nitrate levels and subsequent NR activity (Meakin et al. 2007; Sanchez et al. 2010; Horchani et al. 2011). Nitrate mediated NO generation is likely to be accomplished by mitochondrial and bacterial electron transfer chain (Horchani et al. 2011). NO mediated regulation of nitrogenase levels has been reported to be regulated by S-nitrosylation activity (Xue et al. 2010; Puppo et al. 2013). Mycorhizal inoculation by Gigaspora margarita has also been reported to induce NO formation in Medicago trunculata (Calcagno et al. 2012). NO mediated mycorhizal symbiosis has been reported to be assisted by downregulation of defence resposne thus facilitating mycorhizal associations in the root (Boscari et al. 2013; Espinosa et al. 2014). Plasma membrane associated NR activity, NO reducatse and rhizospheric nitrate levels are important determinants of mycorhizal symbiosis (Moche et al. 2010). NO, ROS and phytohormone crosstalk has been reported to be crucial in establishing both mycorhizal and lichen symbiosis (Hichri et al. 2015). Rhizospheric NO has been reported to exhibit differential effects on N-uptake rates in mycorhizal and non-mycorhizal roots (Simon et al. 2009). Thus, further investigations are necessary to decipher complex reguations of rhizosphere NO in regulation of mycorhizal and lichen associations. The rhizosphere-plant-atmosphere continuum of NO functioning is therefore associated with plant-environment interactions.
References
Arasimowicz-Jelonek M, Floryszak-Wieczorek J, Gwóźdź A (2011a) The message of nitric oxide in cadmium challenged plants. Plant Sci 181:612–620
Arasimowicz-Jelonek M, Floryszak-Wieczorek J (2011b) Understanding the fate of peroxynitrite in plant cells—from physiology to pathophysiology. Phytochem 72:681–688
Bañeras L, Ruiz-Rueda O, López-Flores R, Quintana XD, Hallin S (2012) The role of plant type and salinity in the selection for the denitrifying community structure in the rhizosphere of wetland vegetation. Int Microbiol 15:89–99
Barber DA, Martin JK (1976) The release of organic substances by cereal roots into soil. New Phytol 76:69–80
Belimov AA, Dodd IC, Hontzeas N, Theobald JC, Safronova VI et al (2009) Rhizosphere bacteria containing 1-aminocyclopropane-1-carboxylate deaminase increase yield of plants grown in drying soil via both local and systemic hormone signalling. New Phytol 181:413–423
Bethke PC, Badger MR, Jones RL (2004) Apoplastic synthesis of nitric oxide by plant tissues. Plant Cell 16:332–341
Binnerup SJ, Sorensen J (1992) Nitrate and nitrite microgradients in barley rhizosphere as detected by a highly sensitive denitrification bioassay. Appl Environ Microbiol 58:2375–2380
Boscari A, Del Giudice J, Ferrarini A, Venturini L, Zaffini AL et al (2013) Expression dynamics of the Medicago truncatula transcriptome during the symbiotic interaction with Sinorhizobium meliloti: which role for nitric oxide? Plant Physiol 161:425–439
Botrel A, Magne C, Kaiser WM, (1996) Nitrate reduction, nitrite reduction and ammonium assimilation in barley roots in response to anoxia. Plant Physiol Biochem 34:645–652
Butterbach-Bahl K, Stange F, Papen H (2001) Regional inventory of nitric oxide and nitrous oxide emissions for forest soils of south–east Germany using the biogeochemical model PnET-NDNDC. J Geophys Res 106D:34155–34166
Cabrera JJ, Sanchez C, Gates AJ, Bedmar EJ, Mesa S et al (2011) The nitric oxide response in plant-associated endosymbiotic bacteria. Biochem Soc Trans 39:1880–1885
Calcagno C, Novero M, Genre A, Bonfante P, Lanfranco L (2012) The exudate from an arbuscular mycorrhizal fungus induces nitric oxide accumulation in Medicago truncatula roots. Mycorrhiza 22:259–269
Celeste-Molina F, CeciliaMónica C, Luciana-Lanteri M, Natalia C, Aragunde, María CL, Cell Physiol 46:99–107
Chen Y, Clapp CE, Magen H (2004) Mechanisms of plant growth stimulation by humic substances: the role of organo-iron complexes. Soil Sci Plant Nutr 50:1089–1095
Chowdhury S, Thangarajan R, Bolan N, Reilly-Wapstra J, Kunhikrishnan A et al (2016) Nitrification potential in the rhizosphere of Australian native vegetation. Soil Res 55:58–69
Corpas FJ, Leterrier M, Valderrama R, Airaki M, Chaki M et al (2011) Nitric oxide imbalance provokes a nitrosative response in plants under abiotic stress. Plant Sci 181:604–611
Cramer MD, Savidov NA, Lips SH (1996) The influence of enriched rhizosphere CO2 on N uptake and metabolism in wild-type and NR-deficient barley plants. Physiol Plant 97:47–54
Creus CM, Graziano M, Casanovas EM, Pereyra MA, Simontacchi M et al (2005) Nitric oxide is involved in the Azospirillum brasilense-induced lateral root formation in tomato. Planta 221:297–303
Delledonne M, Xia Y, Dixon RA, Lamb C (1998) Nitric oxide functions as a signal in plant disease resistance. Nature 394:585–588
Dimkpa C, Weinand T, Asch F (2009) Plant–rhizobacteria interactions alleviate abiotic stress conditions. Plant Cell Enviro 32:1682–1694
Dobbelaere S, Croonenborghs A, Thys A, Vande Brooke A, Vanderleyden J (1999) Phytostimulatory effect of Azospirillum brasilense wild type and mutant strains altered in IAA production on wheat. Plant Soil 212:155–164
Dong F, Simon J, Rienks M, Lindermayr C, Rennenberg H (2015) Effects of rhizopheric nitric oxide (NO) on N uptake in Fagus sylvatica seedings depend on soil CO2 concentration, soil N availability, and N source. Tree Physiol 35. https://doi.org/10.1093/treephys/tpv051
Dordas C, Rivoal J, Hill RD (2003) Plant haemoglobins, nitric oxide and hypoxic stress. Ann Bot 91:173–178
Durner J, Wendehenne D, Klessig DF (1998) Defense gene induction in tobacco by nitric oxide, cyclic GMP, and cyclic ADPribose. Proc Natl Acad Sci USA 95:10328–10333
Espinosa F, Garrido I, Ortega A, Casimiro I, Alvarez-Tinaut MC (2014) Redox activities and ROS, NO and phenylpropanoids production by axenically cultured intact olive seedling roots after interaction with a mycorrhizal or a pathogenic fungus. PLoS ONE 9:e100132
Fallik E, Sarig S, Okon Y (1994). Morphology and physiology of plant roots associated with Azospirillum. In: Okon Y (ed) Azospirillum plant associations, pp 77–86. CRC Press, Boca Raton, FL, U.S.A.
Fasano JM, Swanson SJ, Blancaflor EB, Dowd PE, Kao TH, Gilroy S (2001) Changes in root cap pH are required for the gravity response of the Arabidopsis root. Plant Cell 13:907–921
Foissner I, Wendehenne D, Langebartels C, Durner J (2000) In vivo imaging of an elicitor-induced nitric oxide burst in tobacco. Plant J 23:817–824
Forde B, Lorenzo H (2001) The nutritional control of plant development. Plant Soil 232:51–68
Forde BG (2002) Local and long-range signaling pathways regulating plant responses to nitrate. Ann Rev Plant Biol 53:203–224
Freschi L (2013) Nitric oxide and phytohormone interactions: current status and perspectives. Front Plant Sci 4:398
Frungillo L, Skelly MJ, Loake GJ, Spoel SH, Salgado I (2014) S-nitrosothiols regulate nitric oxide production and storage in plants through the nitrogen assimilation pathway. Nature Commun 5:5401
Ġodde M, Conrad R (2000) Influence of soil properties on the turnover of nitric oxide and nitrous oxide by nitrification and denitrification at constant temperature and moisture. Biol Fertil Soils 32:120–128
Gill SS, Hasanuzzaman M, Nahar K, Macovei A, Tuteja N (2013) Importance of nitric oxide in cadmium stress tolerance in crop plants. Plant Physiol Biochem 63:254–261
Gomez-Gomez L, Boller T (2002) Flagellin perception: a paradigm for innate immunity. Trends Plant Sci 7:251–256
Gong B, Wen D, Wang X, Wei M, Yang F et al (2015) S-Nitrosoglutathione reductase-modulated redox signaling controls sodic alkaline stress responses in Solanum lycopersicum L. Plant Cell Physiol 56:790–802
Gupta KJ, Fernie AR, Kaiser WM, van Dongen JT (2011) On the origins of nitric oxide. Trends Plant Sci 16:160–168
Hartmann A, Zimmer W (1994) Physiology of Azospirillum. In: Association AP (ed) Okon Y. CRC Press, Boca Raton, FL, U.S.A, pp 15–39
Hallmann J, Quadt Hallmann A, Mahaffee WF, Kloepper JW (1997) Bacterial endophytes in agric ultural crops. Canad J Microbiol 43:895–914
Hichri I, Boscari A, Castella C, Rovere M, Puppo A, Brouquisse R (2015) Nitric oxide: a multifaceted regulator of the nitrogen-fixing symbiosis. J Exp Bot 66:2877–2887
Horchani F, Prevot M, Boscari A et al (2011) Both plant and bacterial nitrate reductases contribute to nitric oxide production in Medicago truncatula nitrogen-fixing nodules. Plant Physiol 155:1023–1036
Huie RE, Padmaja S (1993) The reaction of NO with superoxide. Free Radical Res Commun 18:195–199
Jannin L, Arkoun M, Ourry A et al (2012) Microarray analysis of humic acid effects on Brassica napus growth: Involvement of N, C and S metabolisms. Plant Soil 359:297–319
Jetten MSM, Logemann S, Muyzer G, Robertson LA, de Vries S et al (1997) Novel principles in the microbial conversion of nitrogen compounds. Antonie Leeuwenhoek 71:75–93
Kolb W, Martin P (1985) Response of plant roots to inoculation with Azospirillum brasilense and to application of indoleacetic acid. In: . Klingmuller W (ed) Azospirillum III: genetics, physiology and ecology, pp 215–221. Springer, Berlin
Laanbroek HJ, Woldendorp JW (1995) Activity of chemolithotrophic nitrifying bacteria under stress in natural soils. In: Jones JG (ed) Advances in microbial ecology, vol 14. Springer, Boston, MA
Leach J, Keyster M, Du Plessis M, Ludidi N (2010) Nitric oxide synthase activity is required for development of functional nodules in soybean. J Plant Physiol 167:1584–1591
Liu B, Rennenberg H, Kreuzwieser J (2015) Hypoxia induces stem and leaf nitric oxide (NO) emission from poplar seedlings. Planta 241:579–589
Long HH, Schmidt DD, Baldwin IT (2008) Native bacterial endophytes promote host growth in a species-specific manner; phytohormone manipulations do not result in common growth responses. PLoS ONE 3:e2702
Ma WK, Farrell RE, Siciliano SD (2008) Soil formate regulates the fungal nitrous oxide emission pathway. Appl Environ Microbiol 74:6690–6696
MacCarthy P, Clapp CE, Malcom RE, Bloom PR (1990) Humic substances in soil and crop sciences: selected readings. American Society of Agronomy and Soil Science Society of America, Madison
Magdoff F, Weil R (2004) Soil organic matter in sustainable agriculture. CRC Press LLC, USA Martinus Nijhoff/Junk W Dordrecht, The Netherlands, pp 37–76
Meakin GE, Bueno E, Jepson B, Bedmar EJ, Richardson DJ (2007) The contribution of bacteroidal nitrate and nitrite reduction to the formation of nitrosylleghaemoglobin complexes in soybean root nodules. Microbiol 153:411–419
Meilhoc E, Boscari A, Bruand C, Puppo A, Brouquisse R (2011) Nitric oxide in legume–rhizobium symbiosis. Plant Sci 181:573–581
Moche M, Stremlau S, Hecht L, Gobel C, Feussner I et al (2010) Effect of nitrate supply and mycorrhizal inoculation on characteristics of tobacco root plasma membrane vesicles. Planta 231:425–436
Molina-Favero C, Creus CM, Simontacchi M, Puntarulo S, Lamattina L (2008) Aerobic nitric oxide production by Azospirillum brasilense Sp245 and its influence on root architecture in tomato. Mol Plant Microb Interac 21:1001–1009
Molina-Favero C, Mónica Creus C, Luciana Lanteri M, Correa-Aragunde N, Lombardo MC et al (2007) Nitric Oxide and plant growth promoting rhizobacteria: common features influencing root growth and development. Adv Bot Res 46:1–33
Mora V et al (2014) Abiotic stress tolerance in plants: Exploring the role of nitric oxide and humic substances. In: Khan M, Mobin M, Mohammad F, Corpas F (eds) Nitric oxide in plants: metabolism and role in stress physiology. Springer, Cham
Mounier E, Pervent M, Ljung K, Gojon A, Nacry P (2013) Auxin mediated nitrate signalling by NRT1.1 participates in the adaptive response of Arabidopsis root architecture to the spatial heterogeneity of nitrate availability. Plant Cell Environ 37:162–174
Mur LA, Mandon J, Persijn S, Cristescu SM, Moshkov IE et al (2013) Nitric oxide in plants: an assessment of the current state of knowledge. AoB Plants 5:pls052
Nagata M, Murakami E, Shimoda Y, Shimoda-Sasakura F, Kucho K et al (2008) Expression of a class 1 hemoglobin gene and production of nitric oxide in response to symbiotic and pathogenic bacteria in Lotus japonicus. Mol Plant Microb Int 21:1175–1183
Nardi S, Carletti P, Pizzeghello D, Muscolo A (2009) Biological activities of humic substances. In: Senesi N, Xing B, Huang PM (eds) Biophysico-chemical processes involving natural nonliving organic matter in environmental systems. Wiley, New Jersey
Nardi S, Pizzeghello D, Muscolo A, Vianello A (2002) Physiological effects of humic substances on higher plants. Soil Biol Biochem 34:1527–1536
Navarro L, Dunoyer P, Jay F, Arnold B, Dharmasiri N et al (2006) A plant miRNA contributes to antibacterial resistance by repressing auxin signaling. Science 312:436–439
Okon Y, Kapulnik Y (1986) Development and function of Azospirillum-inoculated roots. Plant Soil 90:3–16
Pagnussat GC, Simontacchi M, Puntarulo S, Lamattina L (2002) Nitric oxide is required for root organogenesis. Plant Physiol 129:954–956
Pande A, Mun BG, Lee DS, Khan M, Lee GM, Hussain A, Yun BW (2021) NO network for plant-microbe communication underground: a review. Front Plant Sci 12. https://doi.org/10.3389/fpls.2021.658679
Parton WJ, Holland EA, Del Grosso SJ, Hartman MD, Martin RE et al (2001) Generalized model for NOx and N2O emissions from soils. J Geophys Res 106D:17403–17419
Pii Y, Crimi M, Cremonese G, Spena A, Pandolfini T (2007) Auxin and nitric oxide control indeterminate nodule formation. BMC Plant Biol 7:21
Puppo A, Pauly N, Boscari A, Mandon K, Brouquisse R (2013) Hydrogen peroxide and nitric oxide: key regulators of the legume-Rhizobium and mycorrhizal symbioses. Antioxid Redox Signal 18:2202–2219
Quadt HJ, Hallmann A, Mahaffee WF, Kloepper JW (1997) Bacterial endophytes in agric ultural crops. Canad J Microbiol 43:895–914
Ramachandran VK, East AK, Karunakaran R, Downie JA, Poole SP (2011) Adaptation of Rhizobium leguminosarum to pea, alfalfa and sugar beet rhizosphere investigated by comparative transcriptomics. Genome Biol 12:106–109
Robertson GP, Groffman PM (2005) Nitrogen transformations. In: Paul EA (ed) Soil microbiology, ecology and biochemistry. Academic Press, Oxford, UK
Ruffel S, Krouk G, Ristova D, Shasha D, Birnbaum KD et al (2011) Nitrogen economics of root foraging: transitive closure of the nitrate-cytokinin relay and distinct systemic signaling for N supply vs. demand. Proc Natl Acad Sci USA 108:18524–18529
Sanchez C, Gates AJ, Meakin GE, Uchiumi T, Girard L et al (2010) Production of nitric oxide and nitrosylleghemoglobin complexes in soybean nodules in response to flooding. Mol Plant Microb Interac 23:702–711
Sanz-Luque E, Ocana-Calahorro F, Llamas A, Galvan A, Fernandez E (2013) Nitric oxide controls nitrate and ammonium assimilation in Chlamydomonas reinhardtii. J Exp Bot 64:3373–3383
Shimoda Y, Nagata M, Suzuki A, Abe M, Sato S et al (2005) Symbiotic rhizobium and nitric oxide induce gene expression of non-symbiotic hemoglobin in Lotus japonicus. Plant Cell Physiol 46:99–107
Shimoda Y, Shimoda-Sasakura F, Kucho K, Kanamori N, Nagata M et al (2009) Overexpression of class 1 plant hemoglobin genes enhances symbiotic nitrogen fixation activity between Mesorhizobium loti and Lotus japonicus. The Plant J 57:254–263
Simon J, Dong F, Buegger F, Rennenberg H (2013) Rhizospheric NO affects N uptake and metabolism in Scots pine (Pinus sylvestris L.) seedlings depending on soil N availability and N source. Plant Cell Environ 36:1019–1026
Simon J, Stoelken G, Rienks M, Rennenberg H (2009) Rhizospheric NO affects N uptake and gene expression patterns in Fagus sylvatica. FEBS Lett 583:2907–2910
Stange F, Butterbach-Bahl K, Papen H (2000) A process-oriented model of N2O and NO emissions from forest soils. 2. Sensitivity analysis and validation. J Geophys Res 105D:4385–4398
Stöhr C, Ullrich WR (2002) Generation and possible role of NO in plant roots and their apoplastic space. J Exp Bot 53:2203–2303
Stohr C, Strube F, Marx G, Ullrich WR, Rockel P (2001) A plasma membrane-bound enzyme of tobacco roots catalyses the formation of nitric oxide from nitrite. Planta 212:835–841
Stöhr C, Stremlau S (2006) Formation and possible roles of nitric oxide in plant roots. J Exp Bot 57:463–470
Stoimenova M, Libourel IGL, Ratcliff RG, Kaiser WM (2003) The role of root nitrate reduction in the anoxic metabolism of roots. II. Anoxic metabolism of tobacco roots with or without nitrate reductase activity. Plant Soil 253:155–167
Sun C et al (2015a) Decreasing methylation of pectin caused by nitric oxide leads to higher aluminium binding in cell walls and greater aluminium sensitivity of wheat roots. J Exp Bot 67:979–989
Sun H, Li J, Song W, Tao J, Huang S et al (2015b) Nitric oxide generated by nitrate reductase increases nitrogen uptake capacity by inducing lateral root formation and inorganic nitrogen uptake under partial nitrate nutrition in rice. J Exp Bot 66:2449–2459
Taylor ER, Nie XZ, Macgregor AW, Hill RD (1994) A cereal haemoglobin gene is expressed in seed and root tissues under anaerobic conditions. Plant Mol Biol 24:853–862
Terrile MC, Paris R, Calderon-Villalobos LI et al (2012) Nitric oxide influences auxin signalling through S-nitrosylation of the Arabidopsis TRANSPORT INHIBITOR RESPONSE 1 auxin receptor. Plant J 70:492–500
Tipping E, Rey-castro C, Brayan SE, Hamilton-tayl (2002) Al(III) and Fe(III) binding by humic substances in freshwaters, and implications for trace metal speciation. Geochimica et Cosmochimica Acta 663211–3224
Vaishnav J, Shekhar K, Amrita K, Sarita G, Rajarshi C et al (2013) Effect of nitric oxide signaling in bacterial-treated soybean plant under salt stress. Archv Microbiol 195
Van der Merwe CA, Cramer MD (2000) Effect of enriched rhizosphere carbon dioxide on nitrate and ammonium uptake in hydroponically grown tomato plants. Plant Soil 221:5–11
Vaughan D, Malcolm RE, Ord BG (1985) Influence of humic substances on biochemical processes in plants. In: Vaughan D, Malcolm RE (eds) Soil organic matter and biological activity. Developments in Plant and Soil Sciences, vol 16. Springer, Dordrecht. https://doi.org/10.1007/978-94-009-5105-1_3l
Vaughan D (1986) Effetto delle sostanze umiche sui processi metabolici delle piante. In: Burns RG, Dell’Agnola G, Miele S et al (eds) Sostanze Umiche effetti sul terreno e sulle piante, pp 59–81 Ramo Editoriale degli Agricoltori, Roma
Viktor A, Cramer MD (2005) The influence of root assimilated inorganic carbon on nitrogen acquisition/assimilation and carbon partitioning. New Phytol 165:157–169
Wany AK, Aprajita J, Kapuganti G (2017) Nitric oxide is essential for the development of aerenchyma in wheat roots under hypoxic stress. Plant Cell Environ 40:3002–3017
Watsuji T, Takaya N, Nakamura A, Shoun H (2003) Denitrification of nitrate by the fungus Cylindrocarpon tonkinense. Biosci Biotechnol Biochem 67:1115–1120
Wildt J, Kley D, Rockel A, Segschneider HJ (1997) Emission of NO from higher plant species. J Geophys Res 102:5919–5927
Wrage N, Velthof GL, van Beusichem ML, Oenema O (2001) Role of nitrifier denitrification in the production of nitrous oxide. Soil Biol Biochem 33:1723–1732
Xu J, Yin H, Li Y, Liu X (2010) Nitric oxide is associated with long-term zinc tolerance in Solanum nigrum. Plant Physiol 154:1319–1334
Xue Y, Liu Z, Gao X, Jin C, Wen L et al (2010) GPS-SNO: computational prediction of protein S-nitrosylation sites with a modified GPS algorithm. PloS ONE 5:e11290
Zandonadi DB, Santos MP, Dobbss LB et al (2010) Nitric oxide mediates humic acids-induced root development and plasma membrane H+-ATPase activation. Planta 231:1025–1036
Zhang H, Forde BG (2000) Regulation of Arabidopsis root development by nitrate availability. J Exp Bot 51:51–59
Zhang RQ, Zhu HH, Zhao HQ, Yao Q (2013) Arbuscular mycorrhizal fungal inoculation increases phenolic synthesis in clover roots via hydrogen peroxide, salicylic acid and nitric oxide signaling pathways. J Plant Physiol 74–79
Zhang XW, Dong YJ, Qiu XK, Hu GQ, Wang YH, Wang QH (2012) Exogenous nitric oxide alleviates iron-deficiency chlorosis in peanut growing on calcareous soil. Plant Soil Environ 58:111–120
Zhang YH, Fan JB, Zhang YL, Wang DS, Huang QW et al (2007) Accumulation and translocation in four japonica rice cultivars at different N rates. Pedosphere 17:792–800
Zhang L, Takaya N, Kitazume T, Kondo T, Shoun H (2001) Purification and cDNA cloning of nitric oxide reductase cytochrome P450nor (CYP55A4) from Trichosporon cutaneum. Eur J Biochem 268:3198–3204
Zhou ZM, Takaya N, Nakamura A, Yamaguchi M, Takeo K et al (2002) Ammonia fermentation, a novel anoxic metabolism of nitrate by fungi. J Biol Chem 277:1892–1896
Zumft WG (1997) Cell biology and molecular basis of denitrification. Microbiol Mol Biol Rev 61:533–616
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Mathur, P., Mukherjee, S. (2021). Dynamic Pool of Nitric Oxide (NO) in Rhizosphere Modulates Root Architecture, Nutrient Acquisition and Stress Tolerance in Plants. In: Mukherjee, S., Baluška, F. (eds) Rhizobiology: Molecular Physiology of Plant Roots. Signaling and Communication in Plants. Springer, Cham. https://doi.org/10.1007/978-3-030-84985-6_10
Download citation
DOI: https://doi.org/10.1007/978-3-030-84985-6_10
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-84984-9
Online ISBN: 978-3-030-84985-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)