Abstract
The biogeochemical process on a spatial and temporal scale can have a significant influence on the regulation of the stoichiometry of nutrients in the waters of coastal and nearshore ecosystems. Such changes may result in alteration of the plankton population and diversity and ultimately the entire food chain. Chilika, the first Ramsar site of India and largest brackish water lagoon of Asia, was investigated for 7 years (2013–2020) to understand the nutrient variability and their stoichiometry. During the study period, crucial parameters showed a significant variation spatially as well as seasonally (p < 0.05, n = 2520). Nutrient concentrations in Chilika were found to be 0.4 ± 0.3, 5 ± 4, 7 ± 4, 0.5 ± 0.6, and 71 ± 41 μM for nitrite (NO2), nitrate (NO3), ammonia (NH3), phosphate (P), and silicate (Si). The lagoon maintained mesotrophic condition irrespective of seasons. Shifts in the stoichiometry of dissolved inorganic nitrogen (N) to dissolved inorganic phosphate (P) and Si (N/P/Si) were investigated and found N/P and Si/P were maintained between 0.1 and 2700 with an avg. of 61 ± 125 and 0.1 and 15,439 with an avg. of 514 ± 1049, respectively, whereas N/Si varied between 0.01 and 4 with an avg. of 0.3 ± 0.3. A significant positive correlation (p < 0.01) of N/Si (r = 0.79), N/P (r = 0.79), and Si/P (r = 0.67) with chlorophyll a (Chl-a) indicated nutrient stoichiometry is the major factor that controls the productivity of the Chilika lagoon. OC (Outer Channel) recorded the lowest N/P as compared to other sectors indicating nitrogen limitation due to the mixing of seawater with poor nitrogen level. In the present study, N and P were limiting with respect to Si, and P was limiting with respect to N as evidenced from N/Si < 1; Si/P > 16 and N/P > 16, respectively. This study suggested that the NH3 has a major role in Chilika (along with NO3) for the calculation of N/P and deciding the limiting factors.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
A lagoon is recognized as a water body separated from a larger body of water like the ocean by barriers of marine origin and mostly parallel to the coastline. The Indian lagoon ecosystem is facing challenges from natural and anthropogenic aggravation which can significantly affect biodiversity and nutrient dynamics. Coastal lagoons are generally influenced by riverine and seawater influx simultaneously making brackish water ecosystems. Coastal lagoons cover about 13% of the total world’s coastline (Kjerfve 1994). The lagoons are highly useful and productive with a wide array of biodiversity and an abundance of flora, fauna, and avifauna. This also acts as the hub of the blue economy by supporting large biodiversity of fisheries, wintering ground of migratory birds, maintenance of hydrology, climate regulation, food protection coastal tourism, and a large amount of fishing harbor (Newton et al. 2018). However, anthropogenic activity is gradually increasing and degrading the ecosystems in terms of loss of ecological characters and biodiversity.
There are 17 coastal lagoons, present on the Indian coast (8 on the east coast and 9 on the west coast; Mahapatro et al. 2013), which are shallow and well mixed by waves and currents, and an average depth of the lagoon is 2 m with a photic depth of the coastal lagoons extending up to 2 m. Lagoons not only have importance for biodiversity conservation but also support the livelihood of the local community as well as the economy on an international scale. For instance, the Chilika lagoon supports more than 0.2 million fishermen. This warrants the study of such ecosystems with respect to the environmental characteristics and factors responsible for the adverse changes. The water quality indicators and its dynamics in the systems have been proved to be a critical indicator of the anthropogenic nutrient fluxes and overall ecosystem health (Muduli et al. 2021; Mishra et al. 2020). Such studies are also helpful for policymakers for the management action plan formulation (Barik et al. 2017; Robin et al. 2016; Muduli et al. 2017; Patra et al. 2016). The nutrient concentration that is maintained in the lagoon, its variability with respect to seasons, and its stoichiometry maintained with changing environmental conditions could impact the plankton biodiversity and higher food chain. In order to predict phytoplankton species composition, the stoichiometry of ambient available nutrients, such as nitrogen in terms of nitrate, nitrite, and ammonia and phosphorus, has been used by several researchers (Tilman 1982; Sommer et al. 2007). Models are also developed and validated for species composition prediction based on the stoichiometry of nutrients and phytoplankton elemental stoichiometries. The same also has been extended recently for zooplankton (Sterner 1990; Sterner and Hessen 1994). According to Siddiqui et al. (2019), eutrophication prediction can be done considering the nutrient concentration and stoichiometry. These approaches unite predictive models of populations with environmental processes and help to determine patterns of nitrogen and phosphorus limitation.
Water bodies containing low N and P naturally are very much sensitive to the nutrient fluxes as they influence the nutrient balance in lagoon ecosystems. The Redfield ratio is an important indicator of nutrient limitation for the phytoplankton growth, and its predictive power prompts for searching similar patterns and relationships in other ecosystems. Ecological stoichiometry helps to understand the balance between different chemical elements responsible for ecological functions. For instance, P is considered to be the limiting nutrient when the N/P ratio is >16, and N is considered to be a limitation for the phytoplankton growth if N/P ratios are <16. Similarly, when the stoichiometric ratio of N/P and Si maintains as 16:1:16, it is considered as a limitation of nutrients for diatoms. Lack of management policy formulation and actions has resulted in disproportionate nutrient loads in many aquatic systems around the globe. Such nutrients whether it may be N or P have the potential to alter the nutrient stoichiometry which may lead to either P limitation or N limitation (Jabir et al. 2020). Silicon loading is mostly controlled by natural factors and found to maintain consistency unlike N and P (Pandey et al. 2016).
Coastal lagoons are usually very unstable as the variations that occur in these water bodies are comparatively higher than in saline environments (Panigrahi et al. 2009). Study on nutrient dynamics in these ecosystems is crucial to demonstrate the health of a lagoon with many factors like an ocean tide, river and rivulet water mixing, and anthropogenic interferences. Ecological stoichiometry helps in the understanding of relationships between nutrient cycling and trophic status (Zhang et al. 2013). For the trophic chain, dissolved nutrients are considered as raw materials, and the lagoons act as the entry gate for the nutrients which come from continental drainage to the marine ecosystems. The supply of nutrients is higher in the lagoon ecosystem which is close to highly populated regions, because of input of industrial and domestic waste, agricultural effluents, and urban drainage. The nutrient budget on a global scale is not the same as it was during the preindustrial times as it has been changed from an almost balanced state to a nutrient enrichment state. Such changes in the lagoon and coastal waters are responsible for modifications in the environment such as increases in productivity, fishing yields, etc. When the circulation is restricted, the anthropogenic inputs can lead to excessive eutrophication in the ecosystem which consequently gets changed with varying water flows leading to alterations in water chemistry. Such changes result in different ecological consequences including species composition, phytoplankton blooms, and decline in DO (Martin et al. 2008) and eutrophication (Sonal and Kataria 2012). These forcing factors drive the quality of the habitat and also the change in biodiversity in different lagoon ecosystems.
Spatiotemporal variations in the nutrients in the lagoon could be attributed to the freshwater discharge through rivers and seawater intrusion from two mouths, Arakhkuda and Sanapatna. Apart from anthropogenic sources, atmospheric deposition and surface runoff also contribute nutrients to the Chilika lagoon (Muduli et al. 2013). Natural biogeochemical process such as nitrification and denitrification processes also attributes to a significant hike in N and P load into the lagoon (CPCB 1986). In Chilika the litter of birds (especially from Nalabana birds’ sanctuary within the lagoon) also could be a significant contributor for nutrients and holds the capability to alter the nutrient stoichiometry in the lagoon. Surface runoff along with these robust factors altogether can be a more comprehensive predictor of the eutrophic status of the Chilika lagoon which can alter the pattern of ecological nutrient limitation. Depending on the nutrient availability (either allochthonous or autochthonous sources) in the water column and other physicochemical factors, the strophic status of the ecosystem such as eutrophic, oligotrophic, or mesotrophic is defined in terms of trophic state index (TSI). Carlson (1977) initially derived TSI by considering three parameters, i.e., Chl-a, total phosphorus (TP), and transparency, which was further modified by Burns (2005). In that, total nitrogen (TN) was added along with the three parameters used for TSI calculation, and it was renamed as Trophic Level Index (TLI).
Many aquatic systems were found to be switched over from nutrient-limiting to nutrient surplus over the past few decades due to an increase in anthropogenic waste addition to the ecosystem which leads to eutrophication. Like other aquatic ecosystems, nutrient and other physiochemical parameters are the major components of the Chilika lagoon that proved to be crucial for the sustenance of the good health of the ecosystem. There are several studies on Chilika that have focused on the water quality of the lagoon (Panigrahi et al. 2009; Ganguly et al. 2015). However, the studies specifically on nutrient stoichiometry are scanty. Hence the present assessment was taken up to (1) study the spatiotemporal variability of nutrients and their stoichiometry and (2) reveal the factors controlling the nutrient stoichiometry and its influence on the Chilika ecosystem.
2 Material and Methods
2.1 Study Area
The brackish water lagoon, Chilika (19°42′N–85°21′E), spreads over three districts of Odisha state (Puri, Khurda, and Ganjam) on the east coast of India, which flows into the Bay of Bengal. It has a watershed area of over 116,500 ha. After the New Caledonian barrier reef, Australia is the largest brackish water lagoon in the world. It is the largest coastal lagoon in India and has been listed as a tentative UNESCO World Heritage site. Chilika lagoon is designated as Ramsar Convention site no. 229 Ramsar, 1981 which is the first Indian wetland that got international importance by Ramsar Convention. Chilika lagoon is about 65 km in length and 20.1 km in breadth (northeast to southwest) and maintains an average depth of 2 m. The lagoon maintains an area of 950 and 1165 km2 during summer and monsoon, respectively (Gupta et al. 2008). The lagoon is connected with the sea (Bay of Bengal) through an opening that was dredged in September 2000. To distribute the saline water throughout the lagoon, channels were dredged by the Chilika Development Authority (CDA) which enables saline water flow into the lagoon during summer and also helps to flush out the suspended matters into the sea which are received from the riverine discharge during the monsoon. The geological factors that impacted the coastal lagoon copiously are littoral drift, marine water intrusion, catchment influx, groundwater discharge, the coastal geomorphological process, inlet configuration and dimension, lagoon size, orientation with respect to prevailing winds, and water depth. Some studies explained as follows to support this; Chilika lagoon is lying parallel to the coast and maintaining the biogeochemistry of the lagoon by mixing the saline water from the sea near Satapada and freshwater flow from rivers draining into the lagoon (Cohen et al. 1999; Muduli et al. 2013; Barik et al. 2017). Daya, Makara, Bhargavi, and Luna are the major rivers which drain the copious amount of freshwater containing SPM (suspended particulate matter) and nutrients into the lagoon and attribute to significant annual and seasonal changes in hydrological conditions of the lagoon.
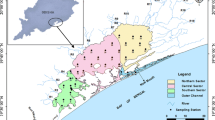
3 Methodology
Sampling from 30 prefixed locations was done (Fig. 7.1) during September 2013 and June 2020 on a monthly basis. All samples were analyzed for water quality parameters within 12 h at the shoreline laboratory facility of the Wetland Research and Training Centre (WRTC), CDA, Odisha. March to June, July to October, and November to February were considered as summer, monsoon (MON), and winter. The lagoon was considered as having four sectors named outer channel (OC), central sector (CS), southern sector (SS), and northern sector (NS) (Muduli et al. 2017; Muduli and Pattnaik 2020). 5 L Niskin sampler was used for the collection of sub-surface water samples from 0.3 m depth from the surface. Photic depth or transparency of the lagoon was measured as the Secchi disk depth. A Thermos meter with an accuracy of ±0.01 °C was used to measure the air temperature (AT) and water temperature (WT). Calibrated water quality Sonde (YSI, USA, V2, Model No. 6600) was used to measure pH and salinity. Water samples for NH3, NO2, NO3, P, and Si were collected in HDPE bottle and filtered using membrane filter paper of 0.45 μ of size 47 mm, and finally the filtered samples were analyzed using a nutrient autoanalyzer (Make: SKALAR SANplus) with precisions of ±0.01, ±0.02, ±0.01, ±0.01, and ±0.02 μM, respectively (Grasshoff et al. 1999). NO2 + NO3 + NH3 were represented as N. Modified Winkler’s method as reported in Carrit and Carpenter (1966) was followed for dissolved oxygen (DO) analysis, and biochemical oxygen demand (BOD) was estimated by 5-day incubation of samples at 20 °C. Chlorophyll a (Chl-a) sample of 1 L was filtered (GF/F filter paper, 47 mm 0.7 μm) and extracted in the dark with 90% acetone for 24 h at 4 °C. UV-VIS spectrophotometer (Make: Thermo Scientific Evolution TM 201) was used to record the absorbance following methods described in Strickland and Parsons (1972). SPSS-18 was used for multivariate regression analyses and for deriving the Pearson correlation coefficient (r).
According to the availability of data, TSI and TLI were calculated for 2019 (January to December) using Chl-a, TN and TP concentration (in μg L−1), and SD (in meters) as derived by Carlson (1977) and Burns (2005), respectively (El-Serehy et al. 2018), as mentioned below:
4 Results and Discussion
4.1 Variability of Physicochemical Parameters
4.1.1 Climatic Condition and Bathymetry
Mixing of freshwater and seawater, flushing rate, many biophysical processes, and biotic-abiotic factors with space and time influence the physicochemical variables in the Chilika lagoon. Chilika experiences monsoon, winter, and summer from July to October, November to February, and March to June, respectively. The average rainfall in the catchment is 1238.8 mm and generally decreases from northeast to southwest. Eighty percent of the annual rainfall occurs during monsoon months varying from tentatively 39 to 200 cm. Significant annual variations in rainfall and sediment flow into the rivers are observed depending on the variations in precipitation with time.
AT recorded during the study period was ranged between 12.50 and 36.00 °C with an average of 27.42 ± 3.73 °C. The WT varied proportionately with air temperature. WT is an important component of the water chemistry in the lagoon. In the lagoon, WT is mostly influenced by solar radiation, heat transfer from the atmosphere, and turbidity. It is a very important factor that controls the pH, nutrient uptake, primary productivity, plankton diversity, rate of photosynthesis, microbial activity, degradation of organic matter, oxygen solubility, etc. It was ranged between 11.84 and 35.50 °C with an average of 27.72 ± 3.43 °C. WT showed significant variation with respect to season, the lowest recorded in winter (24.56 ± 2.49 °C) as compared to summer and monsoon (average 29.6 °C) when the WT difference was insignificant.
Depth of the Chilika lagoon in association with other environmental factors could affect the water quality. For instance, turbidity (sediment churning from benthic compartment due to wind action), water column productivity and nutrient uptake, and new production in the pelagic compartment are largely dependent on the depth of the ecosystem. In this study, the depth of the lagoon was varied from 0.06 to 6 m with an average of 1.71 ± 0.75 m. NS was the shallowest with 1.15 ± 0.38 m depth on average followed by 1.46 ± 0.43, 2.10 ± 0.66, and 2.38 ± 0.93 m in CS, SS, and OC, respectively (Table 7.1).
4.1.2 Factors Responsible for SD Variability
The photic depth usually measured as Secchi disk depth (SD) is the uppermost layer of the water body that receives the sunlight, allowing flora and fauna for photosynthesis. SD depends on physical parameters like turbidity, total suspended matter, and phytoplankton pigments (Srichandan et al. 2015b). The productivity of the lagoon mostly depends on the nutrient concentration and availability of PAR (photosynthetic active radiation) at the sub-surface water level which directly depends on the SD. It could be a critical factor for phytoplankton diversity, abundance, and spatial variation. Chilika lagoon maintained the SD of 0.68 ± 0.40 m (0–2.7 m) having the highest in SS (0.93 ± 0.37 m) followed by CS (0.72 ± 0.35 m), OC (0.68 ± 0.41 m), and NS (0.37 ± 0.25 m) (Table 7.1; Fig. 7.2a). In Chilika, the regions covered with seagrass bed and submerged macrophytes recorded SD almost the same to the depth as it contained the least turbidity due to the fact that the suspended particulate matter sticks to the surface making the water more transparent (Kim et al. 2015; Patra et al. 2016). Turbidity which is responsible for lowering the SD and light penetration to the water column ranged between 0 and 636 with an avg. of 61.08 NTU. A high amount of suspended matter occurs due to flooding and intense rainstorms mostly in the monsoon seasons that leads to decline in SD. Cyclonic events such as Phailin, Titli, and Fani also had a significant impact on the flash change in SD level along with other water quality parameters (Barik et al. 2017; Muduli et al. 2017; Mishra et al. 2021) as a result of flash flood induced by severe cyclones that had landfall proximate to Chilika lagoon. SD was significantly correlated with alkalinity (r = 0.21, p < 0.01) which could be due to the fact that lower SD supports high photosynthesis which utilizes CO2 and increases the alkalinity level in the pelagic compartment (Muduli et al. 2012) (Table 7.2). The earlier reported values for SD and other physicochemical parameters are listed in Table 7.3.
(a) Sectoral variation of physicochemical parameters in monsoon, winter, and summer (CS, SS, NS, and OC represent central, southern, northern sector, and outer channel, respectively). (b) Sectoral variation of nutrients and their stoichiometry in monsoon, winter, and summer (CS, SS, NS, and OC represent central, southern, northern sector, and outer channel, respectively)
4.1.3 pH, DO, and Salinity Variability Factors
pH is the measurement of the hydrogen and hydroxyl ion concentration in the water. It is the important component of the water that determines whether it is acidic or basic. The present study observed a pH of 8.06 ± 0.6 on average and ranged between 5.99 and 10.35 which indicates the lagoon water maintained alkaline condition. pH varied significantly with space and time. pH in the lagoons is mostly affected by the physical processes such as mixing of fresh and saline water with different pH conditions and biological processes such as respiration, photosynthetic activity of the phytoplankton, etc. (Ganguly et al. 2015; Muduli et al. 2013). According to Srichandan et al. (2015a, b), the variability of pH with respect to season can impact the assimilation of phytoplankton and macrophytes in the lagoon. The lowest pH recorded in NS could be attributed to the decomposition of freshwater vegetation observed dominantly in the NS. Usually, lagoon turns alkaline during the winter due to the seawater influence and biological activity. During summer and monsoon, it gradually decreases because of the decomposition of organic matters and freshwater influx (Upadhyay et al. 2015). pH was positively correlated with transparency and DO (overall as well as for all seasons) which reveals that the pH in Chilika is predominantly controlled by photosynthetic activity which is most favored in the higher SD region of Chilika (Tables 7.2, 7.4, 7.5, and 7.6).
Dissolved oxygen (DO) indicates the oxygen quantity in dissolved form and for which regulating authorities have fixed thresholds depending on the purpose of uses. The coastal lagoon system is receiving organic load and nutrients throughout the world due to urbanization and industrialization leading to the formation of the algal bloom causing hypoxic (low oxygen concentration) conditions in the lagoon which is a matter of concern and needs its monitoring. It is the important element of an aquatic organism for the process of respiration and is produced in the process of photosynthesis by the phytoplankton, macrophytes, and submerged vegetation. Chilika lagoon maintains itself well oxygenated throughout the year (Sundaray et al. 2006; Barik et al. 2017). The DO concentrations were within the threshold range of 4 mg L−1 which is suitable for the healthy aquatic life, wildlife propagation, and fisheries (CPCB 1986). The present study also recorded a fair level of DO with respect to the threshold (7.74 ± 1.72 mg L−1 in average and ranged between 1.42 and 21.4 mg L−1). All the studies to date reported the overall DO of >5 mg L−1 whichvindicates Chilika is well oxygenated and maintains a healthy state irrespective of seasons.
DO recorded the lowest during the monsoon period and highest in winter followed by summer (Fig. 7.2a). Similar observations were also made by several researchers (Panda 2020; Upadhyay et al. 2015). During the winter higher DO was attributed to abundant phytoplankton growth leading to high primary productivity, whereas high turbid water hindering light penetration and productivity could be responsible for low DO during monsoon. Lower DO in summer as compared to winter could be due to utilization of DO for degradation of organic matter which is accelerated due to water volume reduction and growth of microbes in higher temperature by respiration process by the phytoplankton, microbes, macrophytes, and other living organisms in the lagoon (Robin et al. 2016). DO is influenced by salinity and temperature (Vijayakumar et al. 2000) as higher salinity tends to decrease in DO solubility (Mishra and Shaw 2003). In the present study, it was observed that DO did not correlate to WT significantly but negatively and significantly correlated with salinity (Table 7.2). However, the higher DO observed in low saline regions also could be due to the submerged macrophytes from which DO is sourced through the photosynthesis process (Muduli and Pattnaik 2020).
Salinity is an important factor for determining natural and biological processes in the lagoon. It is a strong determinant of the community composition of phytoplankton and their distribution (Huang 2004; Lueangthuwapranit et al. 2011). Salinity is a crucial parameter which determines the species distribution as the more tolerant species selects regions with brackish and higher saline waters, whereas the less tolerant species confine themselves in the freshwater area. Overall salinity of the lagoon was recorded as 9.87 ± 8.49 (mesohaline condition) and ranged between 0 and 36.1 (Table 7.1). The average salinity showed a trend of summer > winter > monsoon. During summer, the lagoon was completely saline water-dominated, whereas in monsoon it was freshwater-dominated. The salinity decreases to its minimum level during the southwest monsoon when the heavy rainfall increases the freshwater flux in the rivers (western catchment and Mahanadi). During peak monsoon, the water near the sea mouth is also found fresh because of the unidirectional flow of water which drains to the Bay of Bengal coming from the riverine system through the lead channel. This study showed a salinity gradient of OC > SS > CS > NS depending on the quantity of saline and freshwater mixing. NS during monsoon recorded the lowest due to mixing of freshwater from northeast rivers, whereas the OC in summer recorded highest which was attributed to minimum freshwater addition, high evaporation, and low precipitation (Mohanty and Mohanty 2002). The SS recorded higher salinity than the CS during monsoon and winter because of saline water intrusion from Rushikulya estuary through the Palur canal (Fig. 7.1). However, during summer, the CS recorded high salinity than SS which could be attributed to high salinity maintained in the OC and nearby regions (CS) due to the least freshwater flow and increased tidal saltwater mixing.
4.2 Nutrient Dynamics
Nutrients are the primary component of the aquatic food chain, and the key source into the lagoon environment is continental drainage from estuaries. The source of the nutrient can be both autochthonous and allochthonous. Inorganic nutrients such as N, P, and Si are very much crucial for the growth of the phytoplankton community in aquatic ecosystems. The freshwater influx and tidal condition in association with season greatly impact the nutrient distribution in the Chilika lagoon (Patra et al. 2016). The decline in nutrient level reflects along with increasing salinity; however, such phenomena interestingly not observed in Chilika could be due to the different point sources which increase the effluent load in the lagoon significantly (Sundaray et al. 2006). As per, the nutrient levels usually decrease during high tide and vice versa, as the high tide water is dominated by seawater with lower nutrient concentration than the riverine or estuarine ecosystem. Rainfall on the lagoon, on the Chilika watershed area, riverine freshwater discharge, seawater exchange, and in situ biogeochemical processes could change the nutrient stoichiometry and the concentration in water. Individual nutrient variability and the influencing factors have been discussed in the following sections.
4.2.1 Variability of Dissolved Inorganic Nitrogen Species
Nitrate in the aquatic environments is influenced by microbial oxidation of ammonia, advective transport into euphotic surface waters, and uptake by primary producers or denitrification in anoxic conditions (Grasshoff et al. 1999). Low summer NO3-concentrations occur either due to low discharge or high biological uptake. Atmospheric input of nitrogen, in the form of N2 gas, into aquatic systems, or associated with catchment rain events, has been recognized in the past decade as a significant allochthonous nitrogen source (Peierls et al. 2003). Loss of nitrate occurs through denitrification by microbial activity, which is the cause of the successive decrease of fixed NO2 and NO3 which gets converted to gaseous N2 and N2O. Denitrification processes within the lagoon are influenced by nitrate availability, oxygen, organic matter, temperature, and benthic in faunal activity (Nowicki et al. 1997). The major sink of nitrogen occurs through the denitrification processes in the sediments of aquatic environments, converting useable inorganic nitrogen to non-useable gaseous form, and, in the process, it alters the stoichiometric ratios of nutrients available to primary producers. NO3 in the lagoon ranged between 0.02 and 20.79 μM with an average of 5.05 ± 4.3 μM. The highest NO3 of 20.79 μM was observed in NS during the peak discharge period, in monsoon (Srichandan et al. 2019; Pattanaik et al. 2020). Irrespective of all the seasons, NS recorded the highest NO3 (Fig. 7.2b; Table 7.7) which could be due to the release of NO3 by microbial respiration of organic matter sourced from dominated vegetation in the NS. NO3 constituted ~45% of N having the highest % in monsoon (56%) followed by winter (50%) and summer (47%).
During the present study, the intermediate species NO2 (between NH3 and NO3) ranged between 0 and 3.16 μM (avg. 0.36 ± 0.32 μM). As reported by Chandran and Ramamoorthi (1984), NO2 is sourced from planktons through metabolic activity and gets released into the water. Seasonally it followed the pattern: monsoon > winter > summer, as earlier observed by Srichandan et al. (2015b). The present study also found a significant variability of NO2 with respect to seasons as confirmed by ANOVA (p = 0.01; n = 2097). A similar pattern was also recorded for other ecosystems (Pandey et al. 2015). Comparatively higher nitrite values have also been reported for the summer season which could be attributed to denitrification processes that occur in the sediment-water interface (Muduli and Pattnaik 2020). NO2 constituted ~5% of total N having the highest % in summer (5.42%) followed by winter (4.85%) and monsoon (4.37%); this could be the indication of in situ biogeochemical process through which NO2 is formed in the system (Barik et al. 2017).
Ammonium (NH4) is generated in the pelagic or benthic compartment through the degradation of organic matter by bacterially mediated deamination (Seitzinger 1988) and animal excretion (McCarthy 1981). This has been shown to be a rapid and irreversible loss process for NH3 (Lipschultz et al. 1986). The concentration of NH4 relative to other nutrients may be low, and regeneration rates are variable and may be high relative to ambient concentrations (i.e., Gilbert et al. 1982), providing a source of available nutrients. NH4 concentration can be altered through the nitrification process, i.e., ammonia oxidation to NO3. When there enough concentration of NH4 is available, NO3 remains unutilized and subsequently lost through advective processes. The main input of NH3 into the lagoon is through freshwater influx associated with local anthropogenic pollutants during monsoon which is high as compared to summer and winter (Muduli and Pattnaik 2020). In Chilika, it ranged between 0.55 and 28.99 μM with an average of 6.73 μM. NH3 recorded highest in the NS and also during the summer period (Table 7.7, Fig. 7.2a) which could be due to release of NH3 from macrophyte decomposition triggered by increasing salinity stroke as the freshwater weeds (such as Potamogeton and Ichornia, which are dominantly found in NS and CS) during monsoon keep on decomposing as the salinity keeps on increasing having peaked in summer. Apart from these few dominant macrophytes such as Phragmites karka, Schoenoplectus and Salicornia could also contribute for nutrients on decomposition according to the changing environmental characteristics. The present study showed the NH3 had a significant negative correlation with DO (r = −0.095, p < 0.05) (Table 7.2) which indicated that NH3 is formed by in situ process by decomposition of organic matter by utilizing DO (Robin et al. 2016). The phenomena occurred in all the seasons as supported by significant correlations (Tables 7.4, 7.5, and 7.6). As compared to NO3 and NO2, NH3 species constituted the highest %, i.e., more than 50% of total N. In Chilika lagoon it is very crucial to consider NH3 while calculating the N/P. Several studies have reported an N/P ratio considering NO3 as N. This may not add much error for the ecosystems with a very low % of NH3. However, in Chilika it could lead to misinterpretation as the addition of NH3 concentration to NO3 for N/P ratio calculation may change the ratio which decides the nutrient limitation. During the study period, the NH3% varied significantly with respect to season, having the highest in monsoon (56.47%) followed by winter (50.19%) and summer (46.94%) which could be the indication of the source of NH3 from freshwater discharge from rivers which diminishes as from monsoon to summer (Ganguly et al. 2015).
4.2.2 Variability of Dissolved Inorganic Phosphate
Dissolved inorganic phosphate is one of the micronutrients which controls the trophic status depending on the availability of N. As per De Busk (1999), the P in the water body could be organic or inorganic compounds either in the form of dissolved or particulate matter. In the surface water, the P is sourced from rock weathering and organic matter decomposition. Under favorable environmental conditions such as light and temperature, the P gets assimilated by phytoplankton and bacteria. As per Sobehrad 1997), the organic P available in SPM and on the surface of organic detritus is consumed by filter feeders and released as inorganic P. Lagoon water with low P declines the productivity of water as the phytoplankton growth gets hindered, whereas excess P can be the cause of the eutrophic condition which may lead to a bloom of some dominating species. To date, no studies have reported bloom caused by high P content in Chilika which could be due to the fact that the water column transparency gets declined by turbid water input from rivers which hinders photosynthesis leading to lower primary productivity (Srichandan et al. 2015a). This study showed Chilika maintains a very low concentration of P, varying from 0 to 10.19 μM with an average of 0.47 ± 0.64 μM. In Chilika lagoon, P from a point source has not been reported as there is no such industry situated in proximity to the lagoon, and whatever input that comes through the river has a minimal impact on the variability of P concentration in Chilika lagoon. As reported in DWAF (1995), high levels of P are originated from industrial effluents, domestic discharge, drainage from agricultural land, urban runoff, and atmospheric precipitation. Chilika lagoon is safe from such effluents which could influence the P level in monsoon and subsequent seasons.
The sector-wise variation in summer and winter showed a similar trend; however, its difference from monsoon might be due to the impact of abundant riverine discharge from northeast rivers. Lower P level recorded during the monsoon could be attributed to adsorption to SPM (Sobehrad 1997) and dilution effect whose factor is also earlier reported by Muduli et al. (2017). However, a couple of studies also recorded comparatively higher P level in monsoon attributing to freshwater discharge with fertilizer content and weathering to the spike (Srichandan et al. 2019). In the present study, during none of the seasons, P showed a significant correlation with salinity (Tables 7.2, 7.4, 7.5, and 7.6) which indicated it is not controlled by either freshwater input during monsoon or seawater exchange in summer. Rather, the in situ biogeochemical processes controlled the P level in Chilika. Supporting the same, P showed a significant positive correlation with turbidity indicating the P release from sediment by the churning effect.
4.2.3 Variability of Silicate
Silicate (Si) is a bio-limiting nutrient and a major constituent of diatoms. As per DWAF (1995), diatoms use Si to encase their cells. Along with N and P, this is also required for primary production. This is sourced from the terrestrial system where erosion of adjacent land takes place and also from the anthropogenic activity-influenced areas. In the Chilika lagoon, this process was evidenced by higher Si values during the monsoon. In the present study, the Si concentration in Chilika ranged from 5 to 214 μM. A gradual reduction in Si level recorded from winter season to summer (Table 7.7) could be attributed to the removal of dissolved silicate by two processes: (1) uptake of Si by diatoms for shell formation and (2) absorption on SPM which gets triggered in summer due to increase in salinity. As per Borole (1993), Si behaves conservatively, and during summer periods, silicate behaves non-conservatively. The Si observed in the OC during different seasons showed a trend of monsoon > winter > summer which indicated that the Si transfer to the Bay of Bengal through OC faces a decline from monsoon to the summer season (Table 7.7). Si is a critical factor that decides the plankton biodiversity especially the diatoms in the Chilika lagoon (Srichandan et al. 2015b). In the Veli lagoon, also a clear inverse relationship of silicate with Bacillariophyta showed the dependency of the phytoplanktons on respective nutrients (Mathew and Nair 1981). Overall as well as in respect to seasons, Si was negatively correlated with salinity (r = −0.499, p < 0.01) and SD (r = −0.320, p < 0.01) which indicated the source of Si was from riverine freshwater input and high silicate maintained in the low transparent water (Tables 7.2, 7.4, 7.5 and 7.6).
4.3 Spatiotemporal Variability in Trophic Index
During 2019, the Chilika lagoon maintained mesotrophic status as evidenced by a TSI value of 45.92. As recommended by Carlson (1977), the ecosystem is considered as eutrophic, mesotrophic, and oligotrophic if the calculated TSI value is >50, 40–50, and <40, respectively. There was no difference in trophic status with respect to the season as the TSI recorded for the individual season was as follows: summer (46.8), winter (46.58), and monsoon (44.35). TSI values indicated irrespective of seasons the lagoon maintained mesotrophic nature. Similar to seasons, all the sectors were also found to maintain mesotrophic status (TSI of 46.12, 45.23, and 42.57 for CS, NS, and SS, respectively) with the exception of the OC. The TSI of OC was calculated to be 52.75 which is very close to the eutrophic boundary. The higher TSI of OC was due to the lower transparency recorded in the OC which was attributed to surfing of water by frequent movement of motorized boats operated in the OC for tourism activities (Mohanty et al. 2016). Apart from this, the tidal fluctuations in the OC could be another factor for lower SD (Muduli and Pattnaik 2020). The present study indicates SD is the most contributing factor for TSI status, and it was ranged between 69.71 and 80.61. Such lower values of TSIChl as compared to TSISD showed, along with algae there are some other factors such as sediment particle or color which could be responsible for the light attenuation.
TLI calculated during 2019 also indicated the same trophic status (showed by TSI) of Chilika, i.e., mesotrophic. As suggested by Burns (2005), the trophic status is considered as eutrophic, mesotrophic, and oligotrophic for the TLI of >4, 3–4, and <3, respectively. This study recorded TLI values varying from 2.76 to 4.48 with an average of 3.62. Similar to TSI, the TLI also indicated mesotrophic status for individual seasons as well as sectors except the OC (TLI of 4.04, 3.55, and 3.25 in winter, summer, and monsoon, respectively; 4.17, 3.65, 3.49, and 3.30 for OC, CS, NS, and SS, respectively). The TLI recorded for OC was 4.17 which is beyond the boundary of the mesotrophic status (Burns 2005) as also revealed from TSI. The exceptional trophic status in the OC could be attributed to the factors as explained for TSI. Since there is no significant difference in the trophic status of the lagoon in different seasons and sectors explained through TSI and TLI, either of these indexes could be used for deriving the trophic status of the Chilika lagoon.
4.4 Nutrient Stoichiometry and Influencing Factors
4.4.1 N/P
The concentration of nutrients in lagoon water with specific stoichiometry plays a critical role in phytoplankton growth rate, and the ecosystems are considered to be nutrient-limited if the balance of carbon, N, and P in the environment varies from the Redfield ratio for DIC:DIN:DIP of 106:16:1 (Redfield 1958). As reported by Correll (1998), freshwater ecosystems are typically P-limited as the incorporation rate of nitrogen into plant tissue is usually controlled by P availability. NS of Chilika lagoon maintains fresh to brackish nature throughout the year, and it also showed P limiting with N/P (71.52 ± 140.02). Most of the studies also reported P limiting except few studies which reported N limiting (Table 7.8). These discrepancies could be attributed to change in sample numbers, sampling period, and sampling locations. P limitation is also reported for other ecosystems such as Apalachicola Bay, Chesapeake Bay, Hudson River, and Peel Harvey estuaries (Boynton et al. 1982; McComb et al. 1981; Myers and Iverson 1981). All the individual sectors recorded the highest N/P during the monsoon period followed by winter and summer (Table 7.7). This declining trend from monsoon to summer could be attributed to the addition of N load in monsoon from riverine freshwater discharge which gradually decreases from monsoon to summer. In the present study, N/P varied between 0.1 and 2700 μM with an average of 61.15 ± 125.16. The N/P ratio was found to be negatively correlated with salinity (r = −0.159, p < 0.01) (Table 7.2). Such an inverse relationship is also recorded for all the seasons (Tables 7.4, 7.5, and 7.6). This relationship is justified with higher N content as compared to P in the lower saline region, whereas higher saline water mostly in the OC and SS contained less concentration of N (Barik et al. 2017). The change in ratio and limitation of nutrients in relation to salinity also has been reported in several ecosystems overseas. For instance, Sakshaug and Olsen (1986) recorded P limitation in fresh and brackish waters during phytoplankton blooms in Norwegian waters, and Paasche and Erga (1988) found N limitation in marine waters. Estuaries are typically nitrogen-limited, with some variation in nutrient limitation in brackish waters observed like in Chilika ecosystems (Correll 1998). P limitation in Chilika has been reported by earlier studies (Panigrahi et al. 2009; Sarma et al. 2010; Barik et al. 2017) supporting the observations in the present study.
Nitrogen limitation is recorded for several ecosystems due to anthropogenic material influx from rivers which shifts the nutrient stoichiometry leading to N limitation (Siddiqui et al. 2019). Studies also reported N/P very close to 16, for instance, Martin et al. (2008) reported N limitation over P due to hike in P influx in the southwest coast of India. As per Klug (2006), a decline in the N/P also has the potential to alter the phytoplankton species composition. In coastal ecosystems, the phytoplankton productivity under favorable environmental conditions including nutrient stoichiometry is the cause of increasing toxic blooms. The stoichiometry study for ten large world rivers and two river-dominated coastal ecosystems similar to the Chilika lagoon ecosystem was found to be in a eutrophic state (Justic et al. 1995), and the study revealed that the nutrient stoichiometry of the river waters strongly altered the stoichiometry in the coastal waters.
Almost all studies where the nutrient stoichiometry has been reported used dissolved inorganic nitrogen (N) as NO3 or NO3 + NO2 excluding NH3. In the present study, NH3 was considered to calculate N/P (61.1) and it was found that ~50% of the decrease in N/P (30.4) was without consideration of NH3. It is noteworthy to mention that there was no change in “nutrient limitation” and it remained as P limiting in both cases. In the case of the Chilika lagoon, the inclusion of NH3 to calculate N/P did not make any difference in deciding the nutrient-limiting factor. However, in case of change in the environmental condition of Chilika in the future and any other ecosystem, maintaining relatively higher NH3 could decrease the N/P to <16 and alter the nutrient-limiting factor to N limiting. Hence is it recommended to include NH3 along with NO2 + NO3 for the N/P interpretation in further studies on the Chilika lagoon.
4.4.2 Si/P
Si/P could be used as an indicator to understand the nutrient dynamics and impact of riverine discharge on ecosystems like Chilika (Paul et al. 2008). The Si/P status maintained in the lagoon indicates the weathering forms around the lagoon which is mostly dependent on climate conditions (Turner and Rabalais 2003). This study showed a Si/P varying between 0.1 and 15,439 μM with an average of 513.66 ± 1048 μM. Similar to previous studies (Table 7.8), the present study also recorded Si/P > 16 which indicated P was limiting, making the Chilika water favorable for diatom growth (Panigrahi et al. 2009; Srichandan et al. 2015b). The abundance of diatoms due to such factors is also registered by Domingues (2007). There are examples of an ecosystem where Si limitation is encountered which leads to the dominance of phytoplankton which is not siliceous. Pereira et al. (2009) recorded such observation in Obidos lagoon, Portugal. In all the seasons (except little deviation in summer), Chilika Si/P followed a sectoral trend NS > CS > SS > OC which was in parallel with reverse salinity gradient that indicated the freshwater in the lagoon with high Si controls the Si/P (Table 7.7, Fig. 7.2b). This fact is also supported by the seasonal trend observed for Si/P as monsoon > winter > summer (lagoon dominates with freshwater and Si in monsoon which keeps on decreasing till the end of summer). Similar to N/P, Si/P correlated significantly with salinity (r = −0.242, p < 0.01) in all the seasons which also revealed the abovementioned facts (Tables 7.2, 7.4, 7.5, and 7.6).
4.4.3 N/Si
N to silicate ratio (N/Si) is a major indicator to quantify the health of a lagoon ecosystem which gives information of acute phosphorus depletion (Lucea et al. 2005). As per the Redfield ratio, the stoichiometry should be maintained as 1 (N/P/Si = 16:1:16). In the present investigation, the ratio ranged between 0 and 8.9 (avg. 0.26 ± 0.43) which is <1 indicating the N limitation over Si (Table 7.7; Srichandan et al. 2015a) similar to observations made earlier (Table 7.8). Sector-wise the trend followed as SS < CS < NS < OC, and the highest N/Si observed in the OC was attributed to the least Si recorded in the OC as compared to other sectors. On a temporal scale, the variability was also significant as the maximum ratio found in summer (0.54 ± 3.87) followed by winter (0.24 ± 0.42) and monsoon (0.21 ± 0.29) (Table 7.7). N/Si nearing 1 (Redfield ratio) in OC, i.e., 0.91, makes a most favorable condition for primary productivity as suggested by Redfield (Brzezinski 1985). Pearson correlation showed that the salinity maintained a significant correlation with N/Si (r = 0.127, p < 0.01) (Table 7.2) in all the seasons (Tables 7.4, 7.5, and 7.6) as the high saline waters contained low silicate and supported by a significant negative correlation of Si to salinity (r = −0.509, p < 0.01; Table 7.2). Hence the occurrence of silica-enriched conditions could ensure abundant Si availability to phytoplankton. Chlorophyll-a positively correlated with N/Si (r = 0.079, p < 0.01; Table 7.2) indicated the uptake of Si by phytoplankton. Low N/Si ratio in monsoon as compared to other seasons is also observed by other ecosystems when the productivity is fuelled by the supply of nutrients (Yadav and Pandey 2018). Only few studies have reported Si limitation over N (N/Si > 1) (Siddiqui et al. 2019).
5 Conclusion
This study highlighted the influencing factors of nutrient variability and the role of nutrient stoichiometry on lagoon productivity. The water quality changes due to seasons were found to be crucial for the nutrient biogeochemistry and other physicochemical parameters of the lagoon. N and P recorded in the study period along with other physicochemical parameters indicated good health of Chilika lagoon. However, on seasonal scale winter scores the best and monsoon least owing to the least transparency nutrient load from northeast rivers. The study indicated the NH3 species constituted more than 50% of total N, and it is a vital parameter to consider while calculating the N/P, and the N must include NH3. It could be misleading consideration of the only NO3 as N for the N/P stoichiometry as the addition of NH3 concentration to NO3 may shift the stoichiometry either close to Redfield ratio or far from it and may lead to misinterpretation on the nutrient-limiting factors. TSI and TLI index indicated the lagoon maintains mesotrophic condition. However, long-term monitoring of TN along with TP and other physicochemical parameters is needed for a more appropriate representation of the trophic status of the Chilika lagoon. Including the present study, Chilika has been studied in major aspects of nutrient variability such as nutrient uptake by the plankton community of Chilika, nutrient flux from riverine input, spatiotemporal variation of nutrients, etc. However, there is still some critically important figure related to nutrient dynamics yet to be studied for which it is recommended to (1) quantify the N exchange through nitrification and denitrification process, (2) estimate the nutrient exchange from sea, (3) quantify the nutrient exchange from benthic compartment, (4) estimate the nutrient uptake by macrophytes of Chilika especially the Phragmites karka spread over the NS of the lagoon, and (5) long-term monitoring of TN and TP.
References
Banarjee AC, Roy Choudhury NC (1971) Observation on some physicochemical features of Chilika Lake. Indian J Fish 13(1–2):395–429
Barik SK, Muduli PR, Mohanty B, Behera AT, Mallick S, Das A, Samal RN, Rastogi G, Pattnaik AK (2017) Spatio-temporal variability and the impact of Phailin on water quality of Chilika lagoon. Cont Shelf Res 136:39–56
Borole DV (1993) Late Pleistocene sedimentation: a case study of the Central Indian Ocean Basin. Deep-Sea Res I Oceanogr Res Pap 40(4):761–775
Boynton WR, Kemp WM, Keefe CW (1982) A comparative analysis of nutrients and other factors influencing estuarine phytoplankton production. In: Estuarine comparisons. Academic Press, New York, pp 69–90
Bita MPR, Muduli AT, Behera D, Mahapatro SK, Barik SK, Nag RN, Samal AK, Pattnaik (2016) (2016) Assessment of petroleum hydrocarbon in a tropical brackish water lagoon: Chilika India. Chemistry and Ecology 32(7):653–668. https://doi.org/10.1080/02757540.2016.1177521
Brzezinski MA (1985) The si:c:n ratio of marine diatoms: interspecific variability and the effect of some environmental variables1. Journal of Phycology 21(3):347–357. https://doi.org/10.1111/j.0022-3646.1985.00347.x
Burns N, McIntosh J, Scholes P (2005) Strategies for managing the lakes of the rotorua district new zealand. Lake and Reservoir Management 21(1):61–72. https://doi.org/10.1080/07438140509354413
Carlson RE (1977) A trophic state index for lakes 1. Limnol Oceanogr 22(2):361–369
Carrit DE, Carpenter JH (1966) Recommendation procedure for Winkler analyses of sea water for dissolved oxygen. J Mar Res 24:313–318
Chandran R, Ramamoorthi K (1984) Hydrobiological studies in the gradient zone of the vellar estuary: III. Heterotrophic bacteria. Mahasagar Bull Natl Inst Oceanogr 17(4):197–201
Cohen MCL, Lara RJ, Ramos JDF, Dittmar T (1999) Factors influencing the variability of Mg, Ca and K in waters of a mangrove creek in Bragança, North Brazil. Mangrove Salt Marshes 3(1):9–15
Correll DL (1998) The role of phosphorus in the eutrophication of receiving waters: a review. J Environ Qual 27(2):261–266
CPCB, New Delhi (1986). https://www.cpcb.nic.in/Waterquality_Criteria.php. Accessed 5.1.16
De Busk WF (1999) Document SL170. Soil and Water Science Department, Florida Cooperative Extension Service, Institute of Food and Agricultural Sciences, University of Florida
Ganguly D, Singh G, Purvaja R, Bhatta R, Paneer Selvam A, Banerjee K, Ramesh R (2018) Valuing the carbon sequestration regulation service by seagrass ecosystems of Palk Bay and Chilika India. Ocean & Coastal Management 15926–33. https://doi.org/10.1016/j.ocecoaman.2017.11.009
Domingues RB (2007) Phytoplankton succession in the Guadiana upper estuary: before and after the Alqueva dam phytoplankton succession in the Guadiana upper estuary: before and after the Alqueva dam
DWAF (1995) South African water quality guidelines for coastal marine waters, Natural environment, vol 1. DWAF, Pretoria
El-Serehy HA, Abdallah HS, Al-Misned FA, Irshad R, Al-Farraj SA, Almalki ES (2018) Aquatic ecosystem health and trophic status classification of the Bitter Lakes along the main connecting link between the Red Sea and the Mediterranean. Saudi J Biol Sci 25(2):204–212
Ganguly D, Patra S, Muduli PR, Vardhan KV, Abhilash KR, Robin RS, Subramanian BR (2015) Influence of nutrient input on the trophic state of a tropical brackish water lagoon. J Earth Syst Sci 124(5):1005–1017
Gilbert PM, Lipschultz F, McCarthy JJ, Altabet MA (1982) Isotope dilution models of uptake and remineralization of ammonium by marine plankton 1. Limnol Oceanogr 27(4):639–650
Grasshoff K, Kremling K, Ehrhardt M (1999) Methods of seawater analysis, third completely revised and extended edn. Verlag Chemie, Weinheim
Gupta GVM, Sarma VVSS, Robin RS, Raman AV, Kumar MJ, Rakesh M, Subramanian BR (2008) Influence of net ecosystem metabolism in transferring riverine organic carbon to atmospheric CO2 in a tropical coastal lagoon (Chilika Lake, India). Biogeochemistry 87(3):265–285
Huang L (2004) Species diversity and distribution for phytoplankton of the Pearl River estuary during rainy and dry seasons. Mar Pollut Bull 49(7–8):588–596. https://doi.org/10.1016/j.marpolbul.2004.03.015
Jabir T, Vipindas PV, Jesmi Y, Valliyodan S, Parambath PM, Singh A, Abdulla MH (2020) Nutrient stoichiometry (N: P) controls nitrogen fixation and distribution of diazotrophs in a tropical eutrophic estuary. Mar Pollut Bull 151:110799
Jeong KS, Kim DK, Pattnaik A, Bhatta K, Bhandari B, Joo GJ (2008) Patterning limnological characteristics of the Chilika lagoon (India) using a self-organizing map. Limnology 9(3):231–242
Justic D, Rabalais NN, Turner RE (1995) Stoichiometric nutrient balance and origin of coastal eutrophication. Mar Pollut Bull 30(1):41–46. https://doi.org/10.1016/S0272-7714(05)80014-9
Kim JY, Rastogi G, Do Y, Kim DK, Muduli PR, Samal RN, Pattnaik AK, Joo GJ (2015) Trends in a satellite-derived vegetation index and environmental variables in a restored brackish lagoon. Glob Ecol Conserv 4:614–624
Kjerfve B (1994) Coastal lagoons. Coast Lagoon Proc 1952:1–8. https://doi.org/10.2307/634562
Klug JL (2006) Nutrient limitation in the lower Housatonic River estuary. Estuar Coasts 29(5):831–840
Lucea A, Duarte CM, Agustí S, Kennedy H (2005) Nutrient dynamics and ecosystem metabolism in the Bay of Blanes (NW Mediterranean). Biogeochemistry 73(2):303–323
Lueangthuwapranit C, Sampantarak U, Wongsai S (2011) Distribution and abundance of phytoplankton: influence of salinity and turbidity gradients in the Na Thap River, Songkhla Province, Thailand. J Coast Res 27(3):585–594
Lipschultz F, Wofsy SC, Fox LE (1986) Nitrogen metabolism of the eutrophic Delaware River ecosystem 1. Limnology and Oceanography 31(4):701–716
Mahapatro D, Panigrahy RC, Panda S (2013) Coastal lagoon: present status and future challenges. Int J Mar Sci 3:178
Martin GD, Vijay JG, Laluraj CM, Madhu NV, Joseph T, Nair M, Gupta GVM, Balachandran KK (2008) Fresh water influence on nutrient stoichiometry in a tropical estuary, southwest coast of India. Appl Ecol Environ Res 6:57–64
Mathew T, Nair NB (1981) Phytoplankton of the Veli Lake, a lagoon on the south-west coast of India. Mahasagar Bull Natl Inst Oceanogr 14(1):45–54
McCarthy JJ (1981) Uptake of major nutrients by estuarine plants. In: Estuaries and nutrients. Humana Press, Totowa, pp 139–163
McComb AJ, Atkins RP, Birch PB, Gordon DM, Lukatelich RJ (1981) Eutrophication in the Peel-Harvey estuarine system, western Australia. In: Estuaries and nutrients. Humana Press, Totowa, pp 323–342
Mishra RK, Shaw BP (2003) Spatio-temporal variation of optically active substances in the coastal waters off Orissa from Rushikulya to Dhamra (east coast of India). Indian J Mar Sci 32:133–140
Mishra DR, Kumar A, Muduli PR, Equeenuddin SKM, Rastogi G, Acharyya T, Swain D (2020) Decline in phytoplankton biomass along Indian coastal waters due to COVID-19 lockdown. Remote Sens 12(16):2584. https://doi.org/10.3390/rs12162584
Mishra DR, Kumar A, Muduli P, Acharyya T, Acharya P, Singh S, Rastogi G (2021) Landfall season is critical to the impact of a cyclone on a monsoon-regulated tropical coastal lagoon. Sci Total Environ 770:145235
Mohanty RK, Mohanty SK (2002) Factors affecting finfish and shellfish biodiversity in Chilika lagoon. Fish Chimes 22(5):42–43
Mohanty RK, Mohapatra A, Mohanty SK (2009) Assessment of the impacts of a new artificial lake mouth on the hydrobiology and fisheries of Chilika Lake, India. Lakes Reserv Res Manag 14(3):231–245
Mohapatra M, Behera P, Kim JY, Rastogi G (2020) Seasonal and spatial dynamics of bacterioplankton communities in a brackish water coastal lagoon. Sci Total Environ 705:134729
Muduli PR, Pattnaik AK (2020) Spatio-temporal variation in physicochemical parameters of water in the Chilika Lagoon. In: Ecology, conservation, and restoration of Chilika Lagoon, India. Springer, New York, pp 203–229
Muduli PR, Kanuri VV, Robin RS, Kumar BC, Patra S, Raman AV, Rao GN, Subramanian BR (2012) Spatio-temporal variation of CO2 emission from Chilika Lake, a tropical coastal lagoon, on the east coast of India. Estuar Coast Shelf Sci 113:305–313
Muduli PR, Kanuri VV, Robin RS, Kumar BC, Patra S, Raman AV, Rao GN, Subramanian BR (2013) Distribution of dissolved inorganic carbon and net ecosystem production in a tropical brackish water lagoon, India. Cont Shelf Res 64:75–87
Muduli PR, Barik SK, Mohapatra D, Samal RN, Rastogi G (2017) The impact of tropical cyclone ‘Phailin’ on the hydrology of Chilika lagoon, India. Int J Environ Sci Nat Res 4(2):555632
Muduli PR, Kumar A, Kanuri VV et al (2021) Water quality assessment of the Ganges River during COVID-19 lockdown. Int J Environ Sci Technol. https://doi.org/10.1007/s13762-021-03245-x
Myers VB, Iverson RI (1981) Phosphorus and nitrogen limited phytoplankton productivity in northeastern Gulf of Mexico coastal estuaries. In: Estuaries and nutrients. Humana Press, Totowa, pp 569–582
Nayak BK, Acharya BC, Panda UC, Nayak BB, Acharya SK (2004) Variation of water quality in Chilika lake, Orissa. Indian J Mar Sci 33:164–169
Nazneen S, Raju NJ, Madhav S, Ahamad A (2019) Spatial and temporal dynamics of dissolved nutrients and factors affecting water quality of Chilika lagoon. Arab J Geosci 12(7):1–23
Newton A, Brito AC, Icely JD, Derolez V, Clara I, Angus S, Schernewski G, Inácio M, Lillebø AI, Sousa AI, Béjaoui B (2018) Assessing, quantifying and valuing the ecosystem services of coastal lagoons. J Nat Conserv 44:50–65
Nowicki BL, Kelly JR, Requintina E, Van Keuren D (1997) Nitrogen losses through sediment denitrification in Boston Harbor and Massachusetts Bay. Estuaries 20(3):626–639
Paasche E, Erga SR (1988) Phosphorus and nitrogen limitation of phytoplankton in the inner Oslofjord (Norway). Sarsia 73(3):229–243
Panda BP (2020) Relationship among the physico-chemical parameters of soil and water in different wetland ecosystems. Asian J Chem 32(7):1681–1690. https://doi.org/10.14233/ajchem.2020.22616
Pandey J, Yadav A, Singh R (2015) The N: P: Si stoichiometry and relative abundance of diatoms in Ganga River. Curr J Appl Sci Technol 9:137–147
Pandey J, Tripathi S, Pandey U (2016) Anthropogenically induced shifts in N: P: Si stoichiometry and implications in Ganga River. Air Soil Water Res 9:35–43
Panigrahi S, Acharya BC, Panigrahy RC, Nayak BK, Banarjee K, Sarkar SK (2007) Anthropogenic impact on water quality of Chilika lagoon RAMSAR site: a statistical approach. Wetl Ecol Manag 15(2):113–126
Panigrahi S, Wikner J, Panigrahy RC, Satapathy KK, Acharya BC (2009) Variability of nutrients and phytoplankton biomass in a shallow brackish water ecosystem (Chilika lagoon, India). Limnology 10(2):73–85
Patra S, Raman AV, Ganguly D, Robin RS, Muduli PR, Kanuri V, Abhilash KR, Kumar BC, Subramanian BR (2016) Influence of suspended particulate matter on nutrient biogeochemistry of a tropical shallow lagoon, Chilika, India. Limnology 17(3):223–238
Patra AP, Patra JK, Mahapatra NK, Das S, Swain GC (2010) Seasonal Variation in Physicochemical Parameters of Chilika Lake after Opening of New Mouth near Gabakunda, Orissa, India. World Journal of Fish and Marine Sciences 2:109–117.
Pattanaik S, Chanda A, Sahoo RK, Swain S, Satapathy DR, Panda CR, Choudhury SB, Mohapatra PK (2020) Contrasting intra-annl inorganic carbon dynamics and air–water CO2 exchange in Dhamra and Mahanadi Estuaries of northern Bay of Bengal, India. Limnology 21(1):129–138
Paul JT, Ramaiah N, Sardessai S (2008) Nutrient regimes and their effect on distribution of phytoplankton in the Bay of Bengal. Mar Environ Res 66(3):337–344
Pereira P, de Pablo H, Vale C, Franco V, Nogueira M (2009) Spatial and seasonal variation of water quality in an impacted coastal lagoon (Óbidos Lagoon Portugal). Environmental Monitoring and Assessment 153(1-4):281–292. https://doi.org/10.1007/s10661-008-0355-x
Peierls BL, Christian RR, Paerl HW (2003) Water quality and phytoplankton as indicators of hurricane impacts on a large estuarine ecosystem. Estuaries 26(5):1329–1343
Raman A, Satyanarayana C, Adiseshasai K, Prakash K (1990) Phytoplankton characteristics of Chilka lake, a brackish water lagoon along east coast of India. Indian Journal of Marine Sciences 19:274–277
Ramanadham R, Reddy MPM, Murty AVS (1964) Physico-chemical characteristics of the Vellar estuary in relation to shrimp farming. J Mar Biol Assoc India 6(2):183–201
Ramsar (1981). https://rsis.ramsar.org/ris/229 accessed on 18th September 2021
Redfield AM (1958) The biological control of chemical factors in the environment. Am Sci 46:205–222
Robin RS, Kanuri VV, Muduli PR, Ganguly D, Patra S, Hariharan G, Subramanian BR (2016) CO2 saturation and trophic shift induced by microbial metabolic processes in a river-dominated ocean margin (tropical shallow lagoon, Chilika, India). Geomicrobiol J 33(6):513–529
Roy JC (1954) Periodicity of the plankton diatoms of the Chilika lake for the year 1950. J Bombay Nat Hist Soc 52:112–123
Sterner RW, Hessen DO (1994) Algal nutrient limitation and the nutrition of aquatic herbivores.Annual review of ecology and systematics 25(1):1–29
Sakshaug E, Olsen Y (1986) Nutrient status of phytoplankton blooms in Norwegian waters and algal strategies for nutrient competition. Can J Fish Aquat Sci 43(2):389–396
Sarma VVSS, Prasad VR, Kumar BSK, Rajeev K, Devi BMM, Reddy NPC, Sarma VV, Kumar MD (2010) Intra-annual variability in nutrients in the Godavari estuary, India. Cont Shelf Res 30(19):2005–2014
Seitzinger SP (1988) Denitrification in freshwater and coastal marine ecosystems: ecological and geochemical significance. Limnol Oceanogr 33(4 part 2):702–724
Siddiqui SZ, Rao KVR (1995) Limnology of Chilika Lake. In: Director of Zoological survey of India (Calcutta), Fauna of Chilika Lake (wetland ecosystem series I). Zoological Survey of India, Calcutta
Siddiqui E, Pandey J, Pandey U (2019) The N:P:Si stoichiometry as a predictor of ecosystem health: a watershed scale study with Ganga River, India. Int J River Basin Manag 17(2):199–207. https://doi.org/10.1080/15715124.2018.1476370
Sobehrad S (1997) Phosphorus cycling in the estuarine environment. http://www.bellnetweb.brc.tamus.edu/phosphor.htm
Sommer T, Armor C, Baxter R, Breuer R, Brown L, Chotkowski M, Culberson S, Feyrer F, Gingras M, Herbold B, Kimmerer W (2007) The collapse of pelagic fishes in the upper San Francisco Estuary: El colapso de los peces pelagicos en la cabecera del Estuario San Francisco. Fisheries 32(6):270–277
Sonal T, Kataria HC (2012) Physico-chemical studies of water quality of Shahpura lake, Bhopal (MP) with special reference to pollution effects on ground water of its fringe areas. Curr World Environ 7(1):139–144
Srichandan S, Kim JY, Kumar A, Mishra DR, Bhadury P, Muduli PR, Pattnaik AK, Rastogi G (2015a) Interannual and cyclone-driven variability in phytoplankton communities of a tropical coastal lagoon. Mar Pollut Bull 101(1):39–52
Srichandan S, Kim JY, Bhadury P, Barik SK, Muduli PR, Samal RN, Pattnaik AK, Rastogi G (2015b) Spatiotemporal distribution and composition of phytoplankton assemblages in a coastal tropical lagoon: Chilika, India. Environ Monit Assess 187(2):1–17
Srichandan S, Baliarsingh SK, Prakash S, Lotliker AA, Parida C, Sahu KC (2019) Seasonal dynamics of phytoplankton in response to environmental variables in contrasting coastal ecosystems. Environ Sci Pollut Res 26(12):12025–12041
Strickland JDH, Parsons TR (1972) A practical handbook of seawater analysis. Fisheries Research Board of Canada, Ottawa. https://doi.org/10.1007/978-1-4615-5439-4_19
Sterner, R. W. (1990). The ratio of nitrogen to phosphorus resupplied by herbivores: zooplankton and the algal competitive arena. The American Naturalist, 136(2), 209–229v
Sundaray SK, Panda UC, Nayak BB, Bhatta D (2006) Multivariate statistical techniques for the evaluation of spatial and temporal variations in water quality of the Mahanadi river–estuarine system (India)—a case study. Environ Geochem Health 28(4):317–330
Tarafdar L, Kim JY, Srichandan S, Mohapatra M, Muduli PR, Kumar A, Mishra DR, Rastogi G (2021) Responses of phytoplankton community structure and association to variability in environmental drivers in a tropical coastal lagoon. Sci Total Environ 783:146873
Tilman D (1982) Review: structure and community competition. J Ecol 71(2):663–664
Tripathy SK (1995) Studies on the chemical properties of the Chilika lagoon, east coast of India. Doctoral dissertation, PhD thesis, Berhampur University, Odisha, India, pp 1–193
Turner RE, Rabalais NN (2003) Linking landscape and water quality in the Mississippi River Basin for 200 years. Bioscience 53(6):563–572. https://doi.org/10.1641/0006-3568(2003)053[0563:LLAWQI]2.0.CO;2
Upadhyay RK, Tiwari SK, Roy P (2015) Complex dynamics of wetland ecosystem with nonlinear harvesting: application to Chilika Lake in Odisha, India. Int J Bifurc Chaos 25(7):1540016
Vijayakumar S, Rajesh KM, Mendon MR, Hariharan V (2000) Seasonal distribution and behaviour of nutrients with reference to tidal rhythm in the Mulki estuary, southwest coast of India. J Mar Biol Assoc India 42(182):21–31
Yadav A, Pandey J (2018) The pattern of N/P/Si stoichiometry and ecological nutrient limitation in Ganga River: up-and downstream urban influences. Appl Water Sci 8(3):1–12
Zhang Z, Song X, Lu X, Xue Z (2013) Ecological stoichiometry of carbon, nitrogen, and phosphorus in estuarine wetland soils: influences of vegetation coverage, plant communities, geomorphology, and seawalls. J Soils Sediments 13(6):1043–1051
Acknowledgments
The authors are thankful to the Chief executive, CDA, for providing the data and encouragement to carry out the research. Funding from the World Bank (Credit no. 4765-IN) is acknowledged for its support to develop laboratory facilities at WRTC which was utilized to carry out the research. Thanks are also due to the research staffs of CDA who helped during the sampling and laboratory works to carry out the study.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Muduli, P.R., Barik, M., Acharya, P., Behera, A.T., Sahoo, I.B. (2022). Variability of Nutrients and Their Stoichiometry in Chilika Lagoon, India. In: Madhav, S., Nazneen, S., Singh, P. (eds) Coastal Ecosystems. Coastal Research Library, vol 38. Springer, Cham. https://doi.org/10.1007/978-3-030-84255-0_7
Download citation
DOI: https://doi.org/10.1007/978-3-030-84255-0_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-84254-3
Online ISBN: 978-3-030-84255-0
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)