Abstract
Relatively few Baccharis species have been associated with livestock poisoning. Baccharis coridifolia, B. megapotamica, and B. weirii are the most important causes of livestock loss in southern Brazil, Uruguay, Argentina, and Paraguay. B. artemisioides causes disease in cattle in a restricted zone of Argentina and the poison by B. vulneraria was reported once in dairy cattle from southern Brazil. The toxicosis has acute onset of a usually fatal clinical course consisting of muscular tremors, tachypnea, tachycardia, recumbency, and death. Lesions include reddening, edema, and necrosis of gastrointestinal mucosa and widespread lymphoid necrosis affecting lymphocytes from B-cell zones. B. halimifolia, B. glomerulifolia, and B. pteronioides are suspected of inducing toxicosis livestock in North America. B. halimifolia was also introduced from the USA into Australia and reportedly causes cattle poisoning there. The toxicosis caused by these three species was experimentally induced in chicks and laboratory rodents by feeding the plant, but the characterization of the spontaneous disease and toxic principles are less clearly established. The chemicals responsible for the phytotoxicities of B. coridifolia and B. megapotamica belong to the trichothecene class of well-studied fungal toxins (mycotoxins), specifically the macrocyclic trichothecenes. The levels of these toxins vary according to the time of year and the sex of these dioecious plants. The highest concentration (thousands of ppm) occurs in female plants at the time of flowering. Although there is good reason to think that these toxins are produced by fungal endophytes, the behavior of B. coridifolia and B. megapotamica with respect to these compounds is quite surprising. In addition, a survey of over 20 other Brazilian Baccharis plants found no further examples of plants with these toxins. In addition, feeding studies with B. halimifolia showed that this species is exquisitely sensitive to the toxic effects of these mycotoxins, a characteristic shared by virtually all other plants.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Relatively few Baccharis species have been associated with livestock poisoning. Among them, Baccharis coridifolia DC. is the most important (Barros 1993, 1998). Less frequently B. megapotamica Spreng. and B. weirii Baker, and B. vulneraria are involved in livestock deaths in southern Brazil (Driemeier et al. 2000; Pedroso et al. 2010; Oliveira Filho et al. 2011; Panziera et al. 2015), and B. artemisioides Hook. & Arn. (aka white romerillo) causes disease in cattle in a restricted zone of Argentina (Rizzo et al. 1997). B. halimifolia L. (Everist 1974), B. glomerulifolia L. (Duncan et al. 1957), and B. pteronioides Gray (Marsh et al. 1920; Stegelmeier et al. 2009) are mentioned as toxic to livestock in the USA and Australia.
2 Baccharis coridifolia
Baccharis coridifolia (Fig. 15.1) is one of the most recognized toxic plants in southern Brazil, where it grows in the states of Rio Grande do Sul, Santa Catarina, Paraná. The plant is also found in the Brazilian southeastern state of São Paulo, in large areas of Uruguay, and in northern Argentina and Paraguay (Barros 1993). The habitat of B. coridifolia is native grasslands in nonhumid areas (Tokarnia et al. 2012).
In Brazil, the plant is commonly known as “mio-mio” (Tokarnia et al. 2012) and, in the Spanish-speaking countries, as “romerillo” (Schang 1929). It is a dioecious plant that sprouts in the spring (October through November), and blooms in late summer and early fall (end of February to April). Although the plant is more toxic when flowering, the toxicosis can occur at any time of the year, and heavy cattle losses have occurred in the spring (Barros 1993).
The spontaneous poisoning occurs mainly in cattle (Barros et al. 1986; Barros 1993, 1998; Rissi et al. 2005), and less frequently in sheep (Rozza et al. 2006; Hammerschmitt et al. 2018), goats (Panziera et al. 2015), buffalo (Lértora and Negrette 2015), and horses (Alda et al. 2009). The poisoning has been experimentally reproduced in various animal species including cattle (Tokarnia and Döbereiner 1975; Varaschin et al. 1998), sheep (Tokarnia and Döbereiner 1976), horses (Costa et al. 1995), rabbits (Döbereiner et al. 1976; Rodrigues and Tokarnia 1995), and pigeons (Flores and Houssay 1917).
The toxic principles of B. coridifolia and B. megapotamica belong to the trichothecene class of well-studied fungal toxins (mycotoxins), specifically the macrocyclic trichothecenes (see ahead). The toxin levels in the plant vary according to the time of year and the sex of the dioecious plants. The highest concentration (thousands of ppm) occurs in female specimens at blooming (Jarvis et al. 1991; Varaschin et al. 1998). Accordingly doses as low as 0.25–0.5 g/kg/bodyweight of the green flowering plant may cause deaths in cattle. In the sprouting period, 2 g/kg/body weight is required for the same effect (Tokarnia and Döbereiner 1975). Sheep are comparatively resistant, and the toxic dose for this species is about twice as great as that for cattle (Tokarnia and Döbereiner 1976). Horses are particularly susceptible. The administration of a single dose of 0.06 g/kg causes severe disease, and doses in the range 0.125–0.5 g/kg are lethal (Costa et al. 1995). When dried, B. coridifolia retains about 50% of its potency for at least 17 months (Tokarnia and Döbereiner 1975).
3 The Chemical Biology of Baccharis
In the fall of 1975, a 5 kg shipment of B. megapotamica arrived in the laboratory of Prof. Morris Kupchan at the University of Virginia. Kupchan had a contract with the US National Institutes of Health (NIH) to evaluate plants collected from around the world for the presence of anticancer compounds and had evaluated several Baccharis species in the past, none of which showed any promise. However, the extract of B. megapotamica proved to be quite interesting; it exhibited very high in vitro cytotoxicity, and even better had extraordinarily high in vivo activity against mouse leukemia, a property rarely seen with crude extracts (Kupchan et al. 1976).
Subsequently, extraction of a 54 kg sample of B. megapotamica yielded compounds that belonged to a class of natural products called trichothecenes, specifically macrocyclic trichothecenes, which were responsible for the anticancer properties (Kupchan et al. 1977). The NIH was keen to obtain larger quantities of the original member of this group, baccharinoid B5 (Fig. 15.2), and so an 1800 kg collection of the plant was extracted and processed to yield a large number (>30) of related macrocyclic trichothecenes (Jarvis et al. 1987a). Morris Kupchan passed away in the fall of 1976, and the work on Baccharis was transferred to the laboratory of BBJ.
With gram quantities of B5 in hand, the NIH carried out a number of cancer preclinical studies on B5. However, an earlier trichothecene, anguidine, which was in human cancer clinical trials, proved too toxic for further development. In light of the negative results with this trichothecene, the B5 trichothecene was dropped from further consideration as an anticancer drug. Although disappointing, from this latter 1800 kg extraction, many interesting compounds were isolated and their chemistry studied (Jarvis 1992).
Later, in the 1980s, Habermehl and coworkers reported that B. coridifolia, a plant held responsible for livestock poisoning in Brazil, also contained a series of macrocyclic trichothecenes, including roridins A and E (Habermehl et al. 1985, Fig. 15.3).
Trichothecenes are a very broad group (>200 compounds) of terpenes produced by fungi. They are well-recognized mycotoxins known to be associated with both human and livestock health issues; several of them are highly toxic, especially the macrocyclic compounds (McCormick et al. 2011). Relevant to Baccharis intoxications in South American livestock are similar intoxications of Eastern European livestock caused by their eating moldy hay and straw. The fungus responsible is Stachybotrys chartarum, which produces a series of macrocyclic trichothecenes closely related in structure to compounds found in some Baccharis species (Jarvis 2003). This fungus has also been found in mold-damaged buildings and held responsible, in part, for the health problems of the building occupants (Jarvis and Miller 2005).
Up until the report of their presence in B. megapotamica, trichothecenes had never been detected in plants except in plants with obvious fungal contamination. Neither B. megapotamica nor B. coridifolia had any obvious fungal infections even though these plants were shown to contain from several hundred to thousands of ppm of macrocyclic trichothecenes (Jarvis 1991). Also later, several African plants were shown to contain trichothecenes, and these plants also showed no obvious signs of fungal activity (Loukaci et al. 2000; Zhang et al. 2002). Furthermore, trichothecenes are highly phytotoxic. When tested in plant cell cultures, macrocyclic trichothecenes were found to be among the most phytotoxic compounds known (Cutler and Jarvis 1985; Jarvis et al. 1988). All this raises the question of the sources of the plant-derived trichothecenes. Of immediate relevance is the question of whether trichothecenes are present in other species of Baccharis.
No further examples of macrocyclic trichothecene-containing Baccharis species have been found by chemical analysis of the following species: B. anomala, B. articulata, B. cultrata, B. cylindrica (a synonym of B. trimera), B. dracunculifolia, B. microptera, B. myriocephala, B. ochracea, B. pseudotenuifolia (a synonym of B. linearifolia), B. spicata, B. tridentata, B. trimera, B. usterii (a synonym of B. junciformis), (all from Brazil), B. articulata var. gaudichaudiana (a synonym of B. articulata) (Paraguay), B. magellanica, B. patagonica (both from Chile), B. glutinosa, B. halimifolia, B. neglecta, and B. sarothroides (from the United States) (Jarvis et al. 1991). This same paper reported that herbarium samples of B. coridifolia and B. megapotamica that dated back to the nineteenth century contained significant levels of the macrocyclic trichothecenes.
When B. megapotamica was grown in the greenhouse of the University of Maryland, no macrocyclic trichothecenes could be detected in the plant material. However, when fed roridin A, the plant absorbed roridin A through its roots, and the roridin A was readily transferred to the leaves where it was transformed into baccharinoid B7 (Fig. 15.4). This conversion occurs in two steps: first a rapid introduction of the OH group into 8-position followed by a slow epimerization of the 2’-OH group at the 2′-position. The plant was uninjured by this treatment; however, when the North American species, B. halimifolia, was subjected to the same feeding, it died within a day (Jarvis et al. 1981).
Trichothecenes bind to most eukaryotic ribosomes where they shut down protein biosynthesis (Dejardins et al. 1993). However, unpublished results from our laboratory showed that roridin A did not bind to the ribosomes of B. megapotamica (as is also the case with trichothecene-producing fungi) but did so with the ribosomes of B. halimifolia. In fungi, this resistance has been found to be due to methylation of ribosomes (Iglesias and Ballesta 1994), but nothing is yet known about the mechanism of resistance in the Brazilian plants.
The genus Baccharis is dioecious, and with respect to trichothecene content, there is no difference between the male and female plants of B. megapotamica; however, this is not the case with B. coridifolia. Until these plants go into flower, the levels of trichothecenes (<1000 ppm, mainly oridin A) are the same in the male and female plants of B. coridifolia, but as they go into flower, the levels of roridin A in the female plants increase dramatically to greater than 4000 ppm (Kuti et al. 1990). Furthermore, roridin A facilitates the germination of B. coridifolia seeds. When the seed coats of this plant are removed, the resulting decoated embryos do not germinate easily. Normally, removing the seed coats actually helps with germination since the seed coats contain germination-inhibiting compounds. When the decoated seeds of B. coridifolia are treated with a solution of 10−6 M roridin A, the seeds readily germinate. Decoated seeds of B. halimifolia and B. glutinosa (that readily germinate when their seed coats are removed) are killed by this solution of roridin A. This rather bizarre behavior seems to be related to the pollination of B. coridifolia. The seeds of B. coridifolia whose flowers were hand-pollinated with pollen from the male plant developed viable seeds with a high level (1300 ppm) of trichothecenes. Surprisingly, female flowers prevented from being pollinated produced seeds that would not germinate. When female B. coridifolia flowers were cross-pollinated with pollen from B. halimifolia and B. megapotamica, the resulting seeds exhibited significantly lower viability and decreased germination. Most interestingly, no trichothecenes were detected in the seeds from both these cross-pollinated experiments (Kuti et al. 1990).
There have been two quite different suggestions as to the source of the trichothecenes in the Baccharis plants. Because of the unusually high levels of these toxins in the plants as well as their bizarre effects (e.g., on germination and dependence of levels on the sex of B. coridifolia and its pollination of this plant), it has been suggested that the Baccharis plants themselves are biosynthesizing the trichothecenes (Kuti et al. 1990). However, this would require that the plants somehow acquire the trichothecene biosynthetic genes, presumably through horizontal gene transfer (HGT). Although HGT is common among microorganisms (Andam and Gogarten 2011), it is much less so between eukaryotic species such as plants and fungi (Richardson and Palmer 2007). Furthermore, the trichothecene biosynthetic pathway is complex and involves numerous genes (Desjardins et al. 1993; Trapp et al. 1998); it is difficult to see how the entire set of trichothecene biosynthetic genes could be transferred from a fungus (e.g., Myrothecium) to Baccharis plants, presumably on more than one occasion. Furthermore, these plants are found in two evolutionarily distinct lineages; B. coridifolia is placed in the clade of Baccharis subgen. Coridifoliae, while B. megapotamica and B. weirii are sister species in the clade of Baccharis subgen. Baccharis (Heiden et al. 2019).
The other possibility is that B. megapotamica, B. weirii, and B. coridifolia have an associated endophyte(s) that is actually the source of the plant-derived trichothecenes. In fact, such an endophyte was isolated from B. coridifolia and B. artemisioides and studied by Argentinean biologists (Rizzo et al. 1997). B. artemisioides joins the other three Baccharis species as a plant source for trichothecenes. These workers showed that the fungus they isolated from plant tissue produced macrocyclic trichothecenes in culture. However, in our hands, we found their fungus grew poorly in culture (not uncommon for endophytes), and we could detect no trichothecenes (unpublished results).
There are reports of two African plants found to contain appreciable levels of, in one case simple trichothecenes (Loukaci et al. 2000) and in the other case, macrocyclic trichothecenes (Zhang et al. 2002). Neither found any appreciable fungal activity to account for the presence of trichothecenes.
On the other hand, we (Jarvis et al. 1987b) and others (Habermehl et al. 1985; Habermehl 1989) have found M. verrucaria in the rhizosphere of the roots of B. coridifolia. In addition, roots of B. coridifolia, which have been surfaced sterilized and placed on agar plates, produce colonies of M. verrucaria, and only M. verrucaria, after a two-week period (Aboul-Nasr 1989).
Most if not all vascular plants have associated endophytes (Strobel and Daisy 2003) that play important roles in the lives of these plants. These endophytes produce a multitude of chemicals that have been isolated from plant extracts and were initially attributed to the biosynthetic machinery of the plants. However, a number of biologically active natural products isolated from plant extracts in the past are now recognized as being derived from endophytes (Newman and Cragg 2015). The trichothecene-containing Baccharis plants appear to have established a relationship with an endophyte that results in their accumulating the highly toxic macrocyclic trichothecenes. How this was done is an interesting question that awaits further investigation.
4 Baccharis spp. Poisoning
Typically, the intoxication occurs when livestock that is raised in zones free of the plant is transported and released into pastures infested with B. coridifolia. The risk of intoxication increases considerably if, during transport, animals are still subjected to long marches, stress, hunger, and thirst (Schang 1929; Barros et al. 1986; Barros 1993).
Although the situation described above is the one found in the vast majority of cases of B. coridifolia poisoning, there are reports of other less frequent conditions in which the disease may occur. The intoxication may, exceptionally, occur in lactating animals (mainly sheep) when they begin to graze (Tokarnia and Döberener 1975; Hammerschmitt et al. 2018). Others maintain that, during periods of drought and overcrowding, hunger forces some animals to ingest the plant (Flores and Houssay 1917), especially if they are placed in pastures where the plant is sprouting after burning (Tokarnia and Döberener 1975). Most of these observations are reported by farmers who are by no means unanimous. Many believe that, even with extreme hunger, livestock raised in pastures where B. coridifolia already exists will not ingest the plant (Barros 1993).
The magnitude of economic losses from the death of livestock poisoned by B. coridifolia is difficult to assess. Many cases are probably not communicated to diagnostic laboratories probably due mainly to two factors; most farmers and field veterinary practitioners recognize the disease without the aid of the laboratory, or the incidence of the poisoning has decreased in face of awareness about the toxicosis and the prophylactic methods used. Data on morbidity and mortality ratios are scarce in the literature; in general, both are high. Mortality rates of 20% and 50% in a period of respectively 72 and 30 h after cattle were introduced into a pasture highly infested with B. coridifolia have been reported (Barros et al. 1986; Barros 1993).
The toxicosis runs an acute and usually fatal clinical course. In fatal cases in cattle and sheep, the clinical signs begin, respectively, 5–29 h and 3–23 h after the ingestion of the fresh green plant, and death occurs respectively between 3–34 h and 2–42 h after the onset of the signs (Tokarnia and Döbereiner 1975, 1976; Barros 1993). In cattle, there is loss of appetite, mild-to-moderate bloat, instability of the hind limbs, muscle tremors, dry muzzle, ocular secretion, lack of rumination, dry small amounts of feces, mild sialorrhea, thirst, rapid and laborious breathing, groans, tachycardia, and restlessness (the animal lies down and lifts repeatedly). Finally, it remains most of the time in sternal decubitus, assuming lateral decubitus 30 min to 1 h before death. In nonfatal cases, the clinical signs are similar; there is anorexia, the absence of ruminal movements, catarrhal discharge through the nasal and conjunctival fossa with mild sialorrhea, and constipation followed by diarrhea (Tokarnia and Döbereiner 1975). Some authors maintain that diarrhea appears to be a sign of a good prognosis, indicating a recovery within a few hours to up to 2 weeks (Tokarnia and Döbereiner 1975; Rissi et al. 2005). In our experiments with B. coridifolia in cattle, however, death was preceded by severe diarrhea in 4 cases with acute clinical courses (Varaschin et al. 1998).
In sheep, the clinical signs are similar. The animal stops grazing, moves away from the lot and assumes sternal decubitus for intermittent and progressively more extended periods, and shows apathy, muscle tremors, and wheezing. Just a few hours before death, there is lateral decubitus with paddling movements (Tokarnia and Döbereiner 1976).
The predominant sign in horses is colic associated with increased heart rate, anorexia, small intestinal hypermotility and distension of the colon, and cecum by gas and, in some cases, diarrhea (Alda et al. 2009).
Necropsy findings are restricted to the digestive system, mainly to the gastrointestinal tract, and are similar in cattle, sheep, and buffalo (Tokarnia and Döbereiner 1975, 1976; Rissi et al. 2005; Rozza et al. 2006; Lértora and Negrette 2015). The lesions are not very specific. The rumen is distended by liquid. There are varying degrees of reddening, edema, and erosions in the mucous membranes of the forestomachs (Fig. 15.5), redness, and petechiae in the mucosa of the abomasum and small intestine, which has a liquid content. The liver may be yellowish, lighter than usual, or can present a slight accentuation of the lobular pattern. Hemorrhages occur in the epi- and endocardium; although these are nonspecific lesions, they occur in the vast majority of cases. Additionally, in our cases of experimental intoxication by B. coridifolia in cattle, we occasionally observed marked edema and reddening of the mucosa large intestine and hemorrhagic content in the cecum and proximal colon. In another case, macroscopic lesions were not observed (Varaschin et al. 1998). Brain edema was reported in buffalo (Lértora and Negrette 2015).
Poisoning by Baccharis coridifolia . Forestomachs, lamb. The ruminal mucosa is ulcerated, and the mucosa of the reticulum is markedly reddish. (Courtesy Drs. Welden Panziera and Márcia Hammerschmitt, Setor de Patologia Veterinária, Faculdade de Veterinária, Universidade Federal do Rio Grande do Sul)
In the spontaneous poisoning in horses, gross lesions included hemorrhages, and edema in the glandular parts of the stomach and marked edema and hemorrhages in the mucosa ileum, cecum (Fig. 15.6), and large colon (Alda et al. 2009).
Poisoning by Baccharis coridifolia , cecum, horse. There is marked transmural edema and ulceration of the mucosa. (Reproduced with permission from Alda et al. (2009) Pesqu Vet Bras 29:409–414)
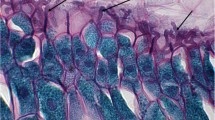
The main histological lesions consist of degeneration, necrosis, and detachment of the epithelium lining the forestomachs (Tokarnia and Döbereiner 1975, 1976; Rissi et al. 2005; Rozza et al. 2006; Lértora and Negrette 2015; Hammerschmitt et al. 2018). This lesion (Fig. 15.7) begins with acute cellular swelling, progressing to ballooning degeneration, the formation of vesicles within the epithelial lining, necrosis, and transient leukocyte infiltration. The degenerative-necrotic lesions frequently cause separation between the epithelium and the lamina propria. In experiments with cattle (Varaschin et al. 1998), and laboratory mice (Varaschin and Alessi 2003), necrotic lesions of secondary lymphoid organs (in zones of B-lymphocytes) have been consistently observed (Fig. 15.8). Occasionally, neutrophilic infiltrates and perivascular hemorrhages are observed respectively in the intestinal mucosa and in the brain (Tokarnia and Döbereiner 1975). Although perivascular hemorrhages in the brain can be potentially produced by the direct action of the toxicants, we have observed similar findings in brains of cattle and sheep that have died from different causes, and we believe that, at least in some situations, they may be mechanical artifacts produced in the brain during the opening of the calvarium with the ax or due to agonic ischemia.
Additional histological lesions have been described in B. coridifolia poisoning in buffalo (Lértora and Negrette 2015). The vascular component of the gastrointestinal mucosa had degenerative changes associated with thrombosis. The liver showed diffuse hepatocellular swelling, and there were brain edema and multifocal cerebrocortical necrosis (Lértora and Negrette 2015).
In the spontaneous poisoning in horses, histopathological observations were restricted to the gastrointestinal tract and included gastritis and enteritis with necrosis of the lining epithelium of the stomach, ileum, cecum, and colon, and marked inflammatory infiltrate, edema, and dilatation of submucosal lymphatics (Alda et al. 2009).
5 Other Species of Baccharis
B. megapotamica , B. weirii , and B. artemisioides produce the same toxic effects in livestock as B. coridifolia, and they share the same class of toxic principles (Oliveira Filho et al. 2012; Rizzo et al. 1997). Cattle from a relatively small area in Argentina, northwest of Buenos Aires and southeast of Cordoba, are spontaneously poisoned by the ingestion of B. artemisioides (Rizzo et al. 1997).
Poisoning by B. megapotamica and its sister species B. weirii is observed in livestock in southern Brazil, states of Rio Grande do Sul, and Santa Catarina. Unlike B. coridifolia, it inhabits swampy areas and is therefore known as marsh mio-mio. These two species are both toxic to livestock. Spontaneous outbreaks of intoxication have been described only with Baccharis weirii in cattle (Driemeier et al. 2000), sheep (Pedroso et al. 2010), buffalo (Oliveira-Filho et al. 2011), and goats (Panziera et al. 2015). Intoxication by B. megapotamica and B. weirii has been experimentally produced in cattle (Tokarnia et al. 1992) sheep (Armién et al. 1993), goats (Barbosa et al. 1994), and buffalo (Oliveira-Filho et al. 2012).
An outbreak of poisoning by Baccharis vulneraria (formerly Baccharidastrum triplinervium) in dairy cows was reported from Paraná, Brazil. Out of 58 cows at risk, 15 got sick, and 6 died after a clinical course of 12–60 h. The disease was clinically and pathologically similar to those caused by other species of Baccharis and was reproduced by force-feeding three calves with the aerial fresh parts of the plant (20–30 g/kg/bw), but no macrocyclic trichothecenes were detected in the chemical analysis of the plant (Langohr et al. 2005).
Baccharis pteronioides is a North American species of Baccharis that grows in clusters in gravelly soil in Texas, New Mexico, Arizona, and northern Mexico (Kingsbury 1964). It has been associated with cattle poisoning, for almost a century (Marsh et al. 1920; Manley et al. 1982). However, there are no convincing descriptions of the gross or histologic lesions of the affected cattle to support a definitive association with the ingestion of the plant. In 2004, in New, Mexico, USA, 80 from a herd of 100 cattle became sick and more than 40 died from what was presumptively diagnosed as B. pteronioides poisoning (Stegelmeier et al. 2009). Postmortem examination was delayed, and the findings described seem to indicate autolysis and putrefaction. An attempt to reproduce the intoxication in hamsters by feeding large doses of B. pteronioides resulted in a disease characterized histologically by severe necrotizing vasculitis with vascular thrombosis of hepatic and renal vessels (Stegelmeier et al. 2009). The authors concluded that, at high doses, B. pteronioides is toxic to hamsters and produces lesions that are very similar to bacterial endotoxin-produced vasculitis and infarction. However, there is yet no definite demonstration that B. pterinoides was the causative agent in spontaneous cattle outbreaks in New Mexico. No information is available concerning the toxin content in B. pteronioides.
Other North American species such as B. halimifolia and B. glomeruliflora are suspect of being toxic to livestock, but the association of these plants with livestock loss is unclear at best. B. halimifolia was introduced from the USA into Australia and Europe and reportedly causes cattle poisoning there (Everist 1974), and it was experimentally toxic to chicks (Duncan et al. 1957). There was no determination of the toxic principle either in B. halimifolia or B. glomeruliflora.
6 Treatment and Prophylaxis
Although some recommend the oral administration of activated charcoal to affected cattle in order to protect the gastrointestinal mucosa, there is no good or practical treatment available.
Livestock born and raised in pastures where the B. coridifolia exists will not ingest the plant (Barros 1993, 1998). Based on this common-sense knowledge, empirical methods to prevent B. coridifolia poisoning in ruminants were developed.
The Spaniards that colonized South America already used methods that consisted of being careful when introducing animals from B. coridifolia-free pastures to regions where the plant exists (Cobo 1653). These methods included burning dried mio-mio (to produce smoke) next to the nostrils of the animal, as well as rubbing fresh mio-mio on the muzzle and gums of the animal several times, or even satiated the animals and giving them small amounts of the plant before releasing them in the pasture infested by mio-mio.
When there is a large number of cattle to set free in a mio-mio-infested pasture, an alternative method is used by farmers, whereby the animals are gradually introduced into the pasture under a person’s supervision, allowing the animals to remain gradually more time in the field every day, always taking care in not allowing them to graze too long, and keeping them in this management until ingestion of the plant is no longer noted, which will take 5–10 days (Tokarnia et al. 2012).
Recent studies concluded that an efficient method to induce aversion to the plant is the force-feeding of sublethal doses to naïve cattle before setting them loose in a B. coridifolia-infested-pasture (Almeida et al. 2011, 2013). For better results, cattle should remain there at least 24-h after dosing.
The same authors concluded that these methods may be impractical when a large number of bovines must be moved from a B. coridifolia-free to a B. coridifolia-infested pastures due to excessive management needed. In these instances, cattle should first be held in pastures with a low density of mio-mio and remain there for approximately 7–30 days before being transferred to the dangerous pasture.
It is apparent that future studies on mio-mio toxicosis should touch two points. (1) The pathogenesis of the disease, that is, the mechanisms that lead the animals to death so quickly and (2) on the methods to control the disease.
References
Aboul-Nasr MB (1989) Studies on Baccharis coridifolia-Myrothecium species interaction. PhD thesis, University of Maryland, College Park
Alda JL, Sallis ESV, Nogueira CEW et al (2009) Intoxicação espontânea por Baccharis coridifolia (Compositae) em equinos no Rio Grande do Sul. Pesqui Vet Bras 29:409–414
Almeida MB, Assis-Brasil ND, Schild AL et al (2011) Conditioned aversion induced by Baccharis coridifolia in sheep and cattle. In: Riet-Correa F, Pfister JA, Schild AL, Wierenga T (eds) Poisoning by plants, mycotoxins and related toxins. CABI International, Wallingford, pp 613–616
Almeida MB, Schild AL, Pfister JA et al (2013) Methods of inducing conditioned food aversion to Baccharis coridifolia (mio-mio) in cattle. Ciên Rural 43:1866–1871
Andam, CP, Gogarten, JP (2011) Biased gene transfer in microbial evolution. Nat Rev Microbiol 9:543–555
Armién AG, Peixoto PV, Tokarnia CH (1993) Intoxicação experimental por Baccharis megapotamica var. megapotamica e var. weirii (Compositae) em ovinos. Pesqui Vet Bras 13:5–20
Barbosa JD, Armién AG, Tokarnia CH (1994) Intoxicação experimental por Baccharis megapotamica var. weirii (Compositae) em caprinos. Pesqui Vet Bras 13:5–20
Barros CSL (1993) Intoxicação por Baccharis coridifolia. In: Riet-Correa F, Méndez MC, Schild AL (eds) Intoxicações por Plantas e Micotoxicoses em Animais Domésticos. Editorial Agropecuaria del Hemisferio Sur, Uruguay, pp 159–169
Barros CSL (1998) Livestock poisoning by Baccharis coridifolia. In: Garland T, Barr AC (eds) Toxic plants and other natural toxicants. CAB International, New York, pp 569–572
Barros CSL, Weiblen R, Santurio JM et al (1986) Intoxicação por “mio-mio” (Baccharis coridifolia) em bovinos. In: Relatório do Centro de Diagnóstico Veterinário da Universidade Federal de Santa Maria. Imprensa Universitária, pp 20–2l
Cobo B (1653) Historia del nuevo mundo Biblioteca de Autores Españoles, [re-edited in 1964]. Madrid 92:226–227
Costa ER, Costa JN, Armién AG et al (1995) Intoxicação experimental por Baccharis coridifolia (Compositae) em eqüinos. Pesqui Vet Bras 15:19–26
Cutler HC, Jarvis BB (1985) Preliminary observations on the effects of macrocyclic trichothecenes on plant growth. Exp Environ Bot 25:115–128
Desjardins AE, Hohn TM, McCormick SP (1993) Trichothecene biosynthesis in Fusarium species: chemistry, genetics, and significance. Microbiol Rev 57:595–604
Driemeier D, Cruz CEF, Loretti AP (2000) Baccharis megapotamica var weirii poisoning in Brazilian cattle. Vet Hum Toxicol 42:220–221
Döbereiner ML, Rezende AML , Tokarnia CH (1976) Intoxicação experimental por Baccharis coridifolia em coelhos. Pesqui Agrop Bras, Ser Vet 11:27–35
Duncan WH, Piercy PL, Feurt SD, Starling R (1957) Toxicological aspects of southeastern plants. II. Compositae. Econ Bot 11:75–85
Everist SL (1974) Poisonous plants of Australia. Angus & Robertson, Sidney
Flores C, Houssay BA (1917) Estudios sobre mio-mio o romerillo Baccharis coridifolia D.C. Revta Hist Bacteriol Dep Nac Hygiene 1:59–100
Habermehl G (1989) Isolation, structure of new toxins from plants. Pure Appl Chem 61:377–380
Habermehl GG, Busam L, Hydel P et al (1985) Macrocyclic trichothecenes: cause of livestock poisoning by the Brazilian plant Baccharis coridifolia. Toxicon 23:731–745
Hammerschmitt ME, Panziera W, Vielmo A et al (2018) Spontaneous Baccharis coridifolia poisoning in suckling lambs. Acta Sci Vet 46(Suppl 1):1–4
Heiden G, Antonelli A, Rubens JP (2019) A novel phylogenetic infrageneric classification of Baccharis (Asteraceae: Astereae), a highly diversified American genus. Taxon 68:1048–1081
Iglesias M, Ballesta JF (1994) Mechanism of resistance to the antibiotic trichothecin in the producing fungi. Eur J Biochem 223:447–453
Jarvis BB (1991) Macrocyclic Trichothecenes. In: Sharma RP, Salunkhe DK (eds) Mycotoxins and Phytoalexins. CRC Press, Boca Raton, pp 361–421
Jarvis BB (1992) Macrocyclic trichothecenes from Brazilian Baccharis species: from microanalysis to large scale isolation. Phytochem Anal 3:241–249
Jarvis BB (2003) Stachybotrys chartarum: a fungus for our time. Phytochemistry 64:53–60
Jarvis BB, Miller JD (2005) Mycotoxins as harmful indoor contaminants. Appl Microbiol Biotechnol 66:367–372
Jarvis BB, Midiwo JO, Tuthill D et al (1981) Interaction between the antibiotic trichothecenes and the higher plant Baccharis megapotamica. Science 214:460–462
Jarvis BB, Çömezoglu SN, Rao MM et al (1987a) Isolation of macrocyclic trichothecenes from a large scale extract of Baccharis megapotamica. J Organomet Chem 52:45–56
Jarvis BB, Wells KM, Lee Y-W et al (1987b) Macrocyclic trichothecene mycotoxins from species of Brazilian Baccharis. Phytopathol 77:980–984
Jarvis BB, Kuti J, Bean GA (1988) Phytotoxicity of macrocyclic trichothecenes toward Baccharis cell lines. JSM Mycotoxins 1988:199–203
Jarvis BB, Mokhtari-Rejali N, Schenkel EP et al (1991) Trichothecene mycotoxins from Brazilian Baccharis species. Phytochemistry 30:789–797
Kingsbury JM (1964) Poisonous plants of the United States and Canada. Prentice Hall, Englewood Cliffs
Kupchan SM, Jarvis BB, Dailey RG Jr et al (1976) Baccharin, a novel potent antileukemic trichothecene triepoxide from Baccharis megapotamica. J Am Chem Soc 98:7092–7093
Kupchan SM, Streelman DR, Jarvis BB et al (1977) The isolation of potent new antileukemic trichothecenes from Baccharis megapotamica. J Organomet Chem 42:4221–4225
Kuti JO, Jarvis BB, Mokhtari-Rejali N et al (1990) Allelochemical regulation of reproduction and seed germination of two Brazilian Baccharis species by phytotoxic trichothecenes. J Chem Ecol 16:3441–3453
Langohr IM, Gava A, Barros CSL (2005) Intoxicação por Baccharidastrum triplinervium (Asteraceae) em bovinos. Pesqui Vet Bras 25:235–238
Lértora WJ, Negrette MS (2015) Baccharis coridifolia poisoning in water buffalo (Bubalus bubalis) in the north of the Province of Corrientes, Argentina. Braz J Vet Pathol 8:1–5
Loukaci A, Kayser O, Bindseil K-U, Siems K et al (2000) New trichothecenes isolated from Holarrhena floribunda. J Nat Prod 63:52–56
Manley GD, Edds GT, Sundlof SF (1982) Cattle deaths from poisonous plant. Folia Morphol 11:20
Marsh CD, Clawson AB, Eggleston WW (1920) Baccharis pteronioides as a poisonous plant of the Southwest. J Am Vet Med Assoc 57:430–434
McCormick SP, Stanley AM, Stover NA et al (2011) Trichothecenes: from simple to complex mycotoxins. Toxins 3:802–814
Newman DJ, Cragg GM (2015) Endophytic and epiphytic microbes as “sources” of bioactive agents. Front Chem 3:1–13
Oliveira-Filho JC, Carmo PMS, Lucena RB et al (2011) Baccharis megapotamica var weirii poisoning in water buffalo (Bubalus bubalis). J Vet Diagn Investig 23:610–614
Oliveira-Filho JC, Carmo PMS, Iversen A et al (2012) Experimental poisoning by Baccharis megapotamica var weirii in buffalo. Pesqui Vet Bras 32:383–390
Panziera W, Gonçalves MA, Lorenzett MP et al (2015) Intoxicação natural por Baccharis megapotamica var. weirii em caprinos. Pesqui Vet Bras 35:360–364
Pedroso PMO, Bandarra PM, Feltrin C et al (2010) Intoxicação por Baccharis megapotamica var. weirii em ovinos. Pesqui Vet Bras 30:403–405
Richardson AO, Palmer JD (2007) Horizontal gene transfer in plants. J Exp Bot 58:1–9
Rissi DR, Rech RR, Fighera RA et al (2005) Intoxicação espontânea por Baccharis coridifolia em bovinos. Pesqui Vet Bras 25:111–114
Rizzo I, Varsavky E, Haidukowski M et al (1997) Macrocyclic trichothecenes in Baccharis coridifolia plants and endophytes and Baccharis artemisioides plants. Toxicon 35:753–757
Rodrigues RL, Tokarnia CH. (1995) Fatores que influenciam a toxidez de Baccharis coridifolia (Compositae): um estudo experimental em coelhos. Pesqui Vet Bras 15:51–69
Rozza DB, Raymundo DL, Corrêa et al (2006) Intoxicação espontânea por Baccharis coridifolia (Compositae) em ovinos. Pesqui Vet Bras 26:21–25
Schang RJ (1929) Acción tóxica del romerillo o mio-mio (Baccharis coridifolia). Algunos conceptos nuevos. Rev Med Vet 11:151–181
Stegelmeier BL, Sani Y, Pfister JA (2009) Baccharis pteronioides toxicity in livestock and hamsters. J Vet Diagn Investig 21:208–213
Strobel G, Daisy B (2003) Bioprospecting for microbial endophytes and their natural products. Microbial Mol Biol Rev 67:491–502
Tokarnia CH, Döbereiner J (1975) Intoxicação experimental em bovinos por “mio-mio” Baccharis coridifolia. Pesqui Agrop Bras 10:79–97
Tokarnia CH, Dobereiner J (1976) Intoxicação experimental em ovinos por “mio-mio”, Baccharis coridifolia. Pesqui Agropec Bras 11:19–26
Tokarnia CH, Peixoto PV, Gava A et al (1992) Intoxicação experimental por Baccharis megapotamica var megapotamica e var weirii (Compositae) em bovinos. Pesqui Vet Bras 12:19–31
Tokarnia CH, Brito MF, Barbosa JD et al (2012) Plantas que afetam o trato digestório in Ibid Plantas Tóxicas do Brasil para Animais de Produção, 2nd. Helianthus, Rio de Janeiro, pp 95–146
Trapp SC, Jarvis BB, Hohn TM (1998) Characterization of the macrocyclic trichothecene pathway gene cluster in Myrothecium roridum. Mol Gen Genet 257:421–432
Varaschin MS, Alessi AC (2003) Poisoning of mice by Baccharis coridifolia: an experimental model. Vet Hum Toxicol 45:42–44
Varaschin MS, Barros CSL, Jarvis BB (1998) Intoxicação experimental por Baccharis coridifolia (Compositae) em bovinos. Pesqui Vet Bras 18:69–75
Zhang HJ, Tamez PA, Aydogmus Z et al (2002) Antimalarial agents from plants. III. Trichothecenes from Ficus fistulosa and Rhaphidophora decursiva. Planta Med 68:1088–1091
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Barros, C.S.L., Jarvis, B.B. (2021). Livestock Intoxication by Baccharis. In: Fernandes, G.W., Oki, Y., Barbosa, M. (eds) Baccharis. Springer, Cham. https://doi.org/10.1007/978-3-030-83511-8_15
Download citation
DOI: https://doi.org/10.1007/978-3-030-83511-8_15
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-83510-1
Online ISBN: 978-3-030-83511-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)