Abstract
In recent times, the development of therapeutic products by using nanoparticle technology has given rise to progressive increment in a number of investigations based on improvement of solubility, penetrability, stability, etc. Solid lipid nanoparticles (SLN) involve absorption and localization through transcellular and paracellular mechanism which is one of the advanced nanoparticle-based formulations of the low solubility of drugs. This chapter entails the outline of the vital features of solid lipid nanoparticles and describes the pharmacokinetic and distribution outcomes of the SLN formulation designed for various routes. The key benefits of using such a nanocarrier in specific therapeutic circumstances and to resolve production and delivery issues are discussed. The major portion covers the explanation on pharmacokinetic studies undertaken in some of the recent researches categorized for oral delivery, injectable administration, topical delivery, biologic and diagnostic products, and ocular delivery. The aim was to present a fresh perspective over the pharmacokinetic and tissue distribution characteristics by means of current state-of-the-art of SLN research.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Solid lipid nanoparticles
- Pharmacokinetics
- Tissue distribution
- Oral delivery
- Bioavailability
- Targeted delivery
- Nanocarrier
- Topical delivery
1 Introduction
Lipid components are used from so many years in pharmaceutical fields for the development of various forms such as lotion, suppository, and ointments, among others. The lipid components receive higher approvals for parenteral and oral delivery formulation due to their high binding ability with the stratum corneum and inertness [1].
The first choice for lipid vehicles is the phospholipid which have different properties such as multifunctionality, amphiphilic nature, and biocompatibility [2]. Different lipid-based delivery systems include microemulsion, microspheres, vesicle based, and SLNs.
The origination of SLN system has been attributed to resolve the issues associated with the preparation, drug entrapment, and scalability of delivery systems such as liposomes, nanoemulsions, and polymeric nanoparticles. SLNs are expected to be more stable than vessicular systems, and their fabrication requires application high-pressure homogenization. In addition to these, SLNs allow surface modifications to produce smart delivery system for targeted delivery of active molecules [2].
In the 1990s, Muller et al. (2002) [3] formulated two novel carriers, i.e., solid lipid nanoparticles (SLNs) and nanostructured lipid nanoparticles (NLC) using lipid material. The aim of the development of SLN and NLC is to utilize the advantages of nanoparticles and also lipid components [4]. Solid lipid nanoparticles (SLNs) are colloidal carriers with a size range of 1–1000 nm where lipid cores are stability using emulsifiers [5]. It has emerged as a novel carrier for the delivery of different classes of therapeutics moiety and also in other fields such as imaging agent, cosmetics, agriculture, and also in nanoreactors. Recently, novel cationic SLNs have attracted attention due to their application for the delivery of genes and also cationic nature which helps bind DNA and also protects against enzymatic degradation [6,7,8,9]. One of the promising benefits of SLN is overcoming the reticuloendothelial system and prolong the duration of active components in the body [5, 10]. Other numerous benefits of SLN include the following: encapsulation of hydrophilic and lipophilic drugs, provide physical stability, low cost in comparison with liposomes, and easy to manufacture [11, 12]. In addition, SLNs are a good choice for brain targeting [13, 14], epidermis targeting [1], and also as controlled-release vehicles [15].
SLNs are a safe and flexible carrier for the delivery of drug, gene, and nucleic acid and safe for particular administration routes [16]. New technologies have been developed for SLN production and are currently under investigation to obtain the optimum encapsulation of different drug categories and to deliver the bioactive compounds at the desired site. Details of different materials, methods, and characterizations are already covered by Geszke-Moritz and Moritz (2016) [17] and Mehnert (2001) [11]. But, SLNs have a strong lipophilic nature which hurdles dispersion in aqueous media, so a sufficient lower size is needed to spontaneously disperse them in water. For that reason, it required higher energy input to reduce size. One of the common steps in all SLN methods is applying the energy in different forms such as high pressure [18, 19], probe sonication [5], and microwaves [20]. High energy decreases the lipid components’ size and increases surface area. These higher surface area-containing particles need to be dispersed in aqueous media and for that needs stearic or electrostatics stabilizers [11]. Surfactants play two crucial roles in the stabilization of SLNs: (a) dispersion of the lipid melt in aqueous phase and (b) providing the stability of lipid nanoparticles after cooling [21]. Different types of surfactants are used for the stability of SLN. Broadly, surfactants are classified into amphoteric (such as phosphatidylcholines families), non-ionics (such as polysorbate 20, polysorbate 80), and ionic (such as sodium oleate, sodium taurodeoxycholate, sodium cholate). In comparison to the ionic lipid, non-ionic lipids have low toxicity and also irritation and that is why they are the first choice for oral and parenteral routes [21]. Major concern related to clinical safety is probability of toxicity due to surfactants for parenteral dosage forms while for oral and transdermal not problematic [22]. Lower size and higher surface increase the absorption of drugs which increase the bioavailability of drugs [23].
In spite of numerous merits, SLNs also have demerits such as physical instability which is characterized by the particle growth and burst release of drug. Particle aggregation and growth are major drawbacks for the parenteral route which can lead to emboli formation and also many more complications [24].
Different types of lipids, viz., cationic lipids, anionic lipids, and non-ionic lipids, are used for the formulation of SLN. Again, these lipids are different based on different fatty acids with chain lengths of hydrocarbon (stearic acid, palmitic acid, dodecanoic acid, and myristic acid), glycerides (tripalmitin, caprylate triglycerides, tribehenin), fatty ester (cetyl palmitate), fatty alcohols (oleyl alcohol, stearyl alcohol), and mixture of glyceryl esters (glyceryl behenate, glyceryl monostearate, glyceryl hydroxystearate). Different cationic lipids such as chloroquine phosphate, benzalkonium chloride, octadecylamine (stearylamine), dimethyldioctadecylammonium bromide, and cetylpyridinium chloride are used for the preparation of cationic SLN [21]. Different advantages and their challenges are shown in Fig. 13.1.
Different benefits, challenges , and limitations of solid lipid nanoparticles (reproduced from Scioli Montoto (2020) [25], an open-access article distributed under the Creative Commons Attribution License that permits unrestricted use, distribution, and reproduction in any medium)
2 Composition of SLN
SLNs are generally spherical with smooth exterior which also governs in vitro and in vivo performance. They are mostly comprised of solid lipid (at room temperature), emulsifiers, therapeutic moiety, and suitable solvents. Based on the loading of therapeutics moiety, SLNs are classified with different models, such as (a) shell-loaded therapeutics moiety, (b) core-loaded therapeutics moiety, and (c) matrix-type model.
Shell-loaded therapeutics moiety is developed when the solid lipid is melted into the hot liquid droplets which are further subjected to separation during cooling phase [26]. Precipitation is one of the common mechanisms for the formulation of nanoparticles. In the case of SLNs, rapid cooling leads to solidification of melted liquid lipid droplets and completion of cooling leads to the formation of SLNs. The lipid is solidified initially before the therapeutics moiety, so that therapeutics moiety gets encapsulated in the shell. On the other hand, the core-loaded therapeutic moiety is based on the fact that therapeutic moiety is crystallized first and crystallized drug gets encapsulated by lipid undergoing crystallization. This model is the best for the sustained release of therapeutics moiety. Matrix-type models are commonly applied for the highly hydrophobic therapeutics moiety where therapeutics moieties are uniformly mixed inside the lipid matrix [2].
3 Pharmacokinetics and Biodistribution of SLNs Administered via Different Routes
SLNs have shown their potential in enhancing the therapeutic activity of active ingredients against various diseases, primarily owing to the alteration of physicochemical and biopharmaceutical characteristics. These lipid-based nanoparticles may be developed for targeted distribution and enhanced pharmacokinetic profile [27]. The disease target of SLNs has been a number of fatal and epidemic diseases, wherein advanced therapeutic products are desired. A great deal of efforts have been made for cancer treatment to encapsulate and deliver lipophilic as well as hydrophilic molecules in a targeted manner [28]. The following explains about the various therapeutic strategies of SLNs employed for using specific route of administration to deliver drug and/or biologicals. The main focus is on the discussion about pharmacokinetics and tissue distribution of the nanoparticles to indicate their therapeutic potential.
4 SLNs for Oral Administration
In recent times, the use of oral nanoparticles for improving solubility, permeability, and stability for various drugs has been tried out extensively. The absorption of SLNs via paracellular and transcellular routes has shown a lot of promise to deal with issues faced due to low water-soluble drugs [29]. Enteric SLNs for targeting the duodenum were formulated incorporating tilmicosin with regard to specific region of absorption and mechanism of transport. Tilmicosin is a veterinary antibiotic which faces problems of low solubility, low penetrability, presystemic metabolism, lack of release at desired sites, etc. The outcomes of T1/2, mean residence time (MRT) , and oral absorption for enteric SLNs of tilmicosin increased several folds as compared to the commercial preparation tilmicosin. The low Cmax (755 ng/ml) and high AUC (11.31 μg·h·mL−1) values of enteric SLN formulation was due to rapid release in gastric region and slow release in intestine. These SLNs went on to achieve sustained release along with better oral absorption owing to reduced metabolism and duodenal-specific localization of drug [30]. The enteric SLNs containing enrofloxacin was prepared to enhance the bioavailability and reduce unwanted gastric mucosa response and stability issues. The formulation was developed by employing hot homogenization and ultrasonic emulsification method. The SLNs were injected through intragastric route which initially led to rapid increase in plasma concentration of enrofloxacin up to 0.52 μg/mL upon completion of 3.33 h, followed by slow reduction in concentration up to 0.03 μg/mL at 72 h. The area under curve and mean residence time was 4.26 μg h/mL and 6.80 h, respectively, for powder drug which increased up to 11.24 μg h/mL and 17.97 h for enteric SLNs of drug. In comparison with the powder drug, the bioavailability, T1/2, and MRT raised to about 2.64-, 2.67-, and 2.64-fold, respectively, after being formulated as enteric SLNs. The outcomes have shown that the SLNs can prove to be a useful technique for overcoming other similar formula-related issues as well [31].
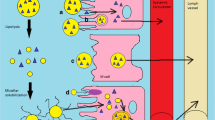
Dronedarone HCl is a potent antiarrhythmic drug which faces bioavailability issues due to low water solubility. A recent literature has explained about the development of SLNs of dronedarone HCl by using glyceryl monostearate. The pharmacokinetic studies of the prepared SLNs showed improvement in bioavailability by 2.68-fold as compared to the pure drug suspension. The outcomes were favorable for using the oral SLNs of dronedarone HCl to counter problems like low bioavailability and first pass effect. However, the bioavailability of the drug reduced from SLNs when given along with chlorpromazine which showed the uptake of SLN takes place via endocytosis [32]. Figure 13.2 shows the diagrammatic representation of different pathways of absorption of oral SLNs. To improve oral bioavailability of felodipine, SLNs were developed by using effervescent dispersion technique in order to have some edge over tradition preparation techniques. Pharmacokinetic studies performed on beagle dogs showed rise in area under curve up to 3.17-fold after oral administration. Furthermore, the Cmax increased from 34.9 μg/L for free felodipine to 329.42 μg/L for the SLN formulation. The observations were impressive and suggested that bioavailability of the drug increased considerably due to increase in solubility. The higher absorption could also be possibly due to absorption of drug lymphatic route which led to diminishing the presystemic metabolism [33].
Diagrammatic illustration of different pathways of SLN absorption via oral route (reproduced from Lin CH 2017 [38], an open-access article distributed under the Creative Commons Attribution License that permits unrestricted use, distribution, and reproduction in any medium)
The curcumin-loaded SLNs were prepared using surfactants such as tristearin and polyethylene glycol. These emulsified SLNs with varied concentration of surfactants were tested for their pharmacokinetic profile by using male Sprague–Dawley rats. The high and comparable Cmax, AUC, Tmax, and bioavailability values were observed for SLNs prepared with both 17.1 mM and 46.9 mM PEG100SE concentration. The PEGylated SLNs possess no charge on the micelle which allowed rapid permeation of the SLNs through mucus layer of epithelium [34]. A study was published by Kumar et al. where they prepared SLNs by incorporating all-trans retinoic acid (ATRA) by employing novel microemuslification method. However, ATRA is known for its effectivity against several inflammatory disorders, but low solubility and instability hinder the therapeutic efficiency. SLNs of ATRA improved their solubility and stability to a great extent and thereby can be looked upon as potential carrier. Nevertheless, pharmacokinetics and biodistribution studies may be performed to further confirm the therapeutic potential of the product [35].
Camptothecin (CPT) is a potent anticancer agent whose activity is hindered due to low bioavailability and undesirable side effects. CPT was linked with palmitic acid by using disulfide linker and loaded into SLNs to generate a redox sensitive formulation (CPT-SS-PA) for successful oral delivery. The CPT-SS-PA SLN showed sustained release as compared to CPT SLN and plain CPT suspension with the peak concentration at 4 h of 2.31 μg/mL and area of curve of 8.66 μg/L.h. Although the pharmacokinetic profiles of CPT-SS-PA SLN and CPT SLNs are comparable, CPT-SS-PA SLN showed significantly higher bioavailability. The study claimed that such modified strategies can be useful in improving oral bioavailability as well as decreasing chances of side effects [36]. The macrophage internalization of camptothecin-containing SLNs studied by using fluorescent marking is included from the research published by Martins et al. (Fig. 13.3). A group of scientists prepared SLN of lurasidone hydrochloride by using high-pressure homogenization method to improve its absorption and bioavailability when administered through oral route. The Cmax from SLNs was about 578.23 ng/ml, which was 2.76-fold higher than the plain drug suspension. The area under curve and Tmax for SLN was reported to be 5871.84 ng.h/ml and 6 h, respectively, and prolonged t1/2 and MRT showed slow elimination of drug from SLNs. The bioavailability of drug from SLN was 5.16-fold higher than drug suspension which is probably due to smaller particle and large surface area of the nanoparticles. High bioavailability may also be resulted because of lymphatic uptake of SLNs, thereby bypassing the presystemic metabolism. Furthermore, the study also established that intestinal lymphatic transport is an important mechanism for the absorption of the drug. The results of Cmax and AUC reduced in the presence of cycloheximide because of inhibition of generation of chylomicrons from the enterocytes and lymphatic transport of drug also decreased [37].
Illustration of macrophage internalization of SLNs containing camptothecin with the help of fluorescent marking. The literature indicated that different lipids incorporated within the formulations were indicated with blue color in the images (a–c). FluoSpheres® and Alexa Fluor® 594 internalization was represented around macrophage layer by green and red color (d), respectively [39]
5 SLNs for Injectable Administration
The nanoparticles administered through intravenous route are swiftly taken up and cleared from the circulation by reticuloendothelial system which are primarily accumulated in the spleen and liver. The development of SLNs or even modifying them with the help of emulsifiers may result in prolonged retention in the circulation. Loureiro et al. developed the SLNs containing resveratrol and grape skin and seed extracts for the treatment of Alzheimer’s disease. Resveratrol is quickly metabolized into glucuronic acid and sulfate conjugates in the liver and epithelial cells of the intestine, followed by elimination from the body. To deal with these issues, SLNs were associated with anti-transferrin receptor monoclonal antibody (OX26) to facilitate the transportation of the active product to the brain. The OX26 SLNs demonstrated considerable cellular uptake as compared to the normal SLNs indicating the better transcytosis of the functionalized SLNs [40]. A group of scientists developed cationic SLNs for the delivery poorly water-soluble drugs and biotechnology products. They investigated the toxicological profile of cationic SLNs by evaluating the distribution of drug in different organs at 24 and 72 h post intravenous injection. It was reported that migration of macrophages in the liver, lungs and spleen took place after administration of cationic SLNs. The permeability across blood-brain barrier was indicated as cationic SLNs moved to brain parenchyma with no damaging effects to the barrier [41].
An attempt was made to develop SLNs of buparvaquone by using modified nanoprecipitation method to improve splenic uptake of the drug. SLNs were radiolabeled with 99mTc to provide adequate stability and targeting characteristic. The biodistribution studies indicated maximum localization of SLNs in the organs of reticuloendothelial system, with considerably high accumulation in the spleen as compared to the liver. A remarkable high spleen to liver concentration ratio (11.94 at 3 h) affirmed high uptake by spleen which is probably due to circumventing the Kupffer cells and low molecular weight of the SLNs. The study was admirable in targeting the spleen for drug delivery, especially in the cases of theileriosis and other spleen-specific infections [42]. Agomelatine, a novel antidepressant, undergoes substantial presystemic metabolism which leads to very low absolute bioavailability. An attempt was made to develop SLN of agomelatine for improving bioavailability and penetration across blood-brain barrier, and pharmacokinetic studies were carried out for comparison of intravenous and intranasal delivery routes. The Cmax, area under curve, and absolute bioavailability of SLNs were 759 ng/mL, 7805.69 ng.min/mL, and 44.44%, respectively, which are significantly higher as compared to oral suspension of the drug. The optimized formulation showed better targetability from intranasal route as compared to intravenous route. The formulation also displayed direct transport % of 47.37 which showed drug delivery in the brain predominantly takes place directly from the nose to brain pathway [43].
6 SLNs for Targeted Delivery
Some of the active pharmaceutical ingredients and biotechnology products require targeting a specific organ or tissue to achieve maximum therapeutic efficiency with minimum adverse effects. SLNs and their modified derivatives may be utilized for subjugating the intracellular and extracellular barriers that have significant impact over delivery. A number of literatures pondering over detailed description of the specific type of solid lipid nanoparticles to incite targeted delivery and their pharmacokinetics study are included in this portion of the chapter [44]. The targeting of alveolar macrophages was carried out by the researcher who prepared SLN assemblies modified on the surface with mannosylated surfactant (hexadecanoic acid (aminoethyl α-D-mannopyranoside) amide) containing rifampicin as model drug for tuberculosis. The biodistribution studies were carried out over the SLNs functionalized with mannosylated surfactant, and outcomes were compared with plain drug and non-functionalized SLNs post intratracheal administration in mice. The functionalized SLNs displayed the maximum retention in pulmonary region while low distribution in extra-pulmonary regions. This outcome could be possible because of significant phagocytosis by alveolar macrophages as compared to non-functionalized SLNs. Therefore, the surface modification of lipid nanoparticles may allow better treatment focusing over the specific organ which is majorly affected by the disease [45].
A targeted oral SLN-based product for veterinary use has already been discussed previously, wherein enteric granules of tilmicosin were formulated SLNs. The product is designed for targeted delivery to the duodenum region for maximizing the oral absorption [30]. The use of glucocorticoid therapy for prolonged duration in high concentration for the treatment of rheumatoid arthritis may give rise to unwanted adverse effects. Prednisolone was incorporated in to SLNs and encapsulated with hyaluronic acid to prepare a targeted formulation against rheumatoid arthritis. The main reason behind coating the SLNs with hyaluronic acid (HA) is that it leads to prolonged circulation and localization at the inflammation site for targeted delivery. The biodistribution study showed considerable reduction in plasma drug concentration in animals administered with free drug in comparison with animals administered with HA-coated SLNs upon completion of 4 h after injection. The retention of HA-coated SLNs also translated into greater localization at the joints which is desirable in the case of rheumatoid arthritis [46].
One approach to improve bioavailability is to target the lymphatic route, especially for those drugs which are hampered by presystemic metabolism. Quetiapine fumarate was formulated into SLNs to be administered through intraduodenal injection targeting lymphatic system. SLNs were prepared using various lipids by employing microemulsion technique and subjected to in vivo pharmacokinetic studies. The optimized SLN was compared with plain drug suspension to assess the pharmacokinetic parameters. The results suggested that area under curve of the optimized SLN was considerably higher than the drug suspension. The bioavailability was also obtained similar to area under curve results, as SLNs showed 2.76 times higher bioavailability in comparison with plain drug suspension [47]. Pinheiro et al. developed quercetin lipid with surface functionalized with RVG29 peptide for targeting the brain better and improve neuronal uptake for the treatment of Alzheimer’s disease. The permeability studies using in vitro blood-brain barrier model showed 1.5 times improvement in penetrability when functionalized nanoparticles were compared with non-functionalized ones. Although the results were impressive, pharmacokinetic and biodistribution studies should be performed to assess the in vivo behavior and targetability of the formulation [48].
7 SLNs in Biologic and Diagnostic Products
Solid lipid nanoparticles are considered to be one of the promising carriers for the biological products, such as vaccines, serum, protein, and peptide drugs, as an efficient and safe substitute for the treatment of various diseases. The main attributes of SLNs are its ability to deal with challenges with regard to stability against degradation, better cell uptake, intracellular accumulation, targetability potential, etc. Furthermore, they offer several benefits with regard to their safety as SLNs are fabricated with tolerated excipients and ease of scalability [49,50,51]. The oral delivery of insulin has been mostly challenging because of degradation by gastrointestinal enzymes and inadequate absorption through intestine. An attempt was made to develop insulin-loaded cetyl palmitate-based SLNs and characterizing their potential as carrier system. SLNs were fabricated using modified solvent emulsification and evaporation method, and in vivo behavior of the product was estimated. The study displayed that after administration of SLNs into the male Wistar rats, the minimum plasma glucose concentration reached to about 73.2% of the initial concentration within 14 h of period. Apart from that, relative bioavailability of insulin-loaded in SLNs (5%) was higher than that from the oral insulin solution (1.6%). The outcomes suggested that SLNs avoid degradation of insulin and improve intestinal absorption. It was also seen that plasma glucose levels reduced significantly after oral administration of SLNs as compared to the oral insulin solution after 24 h [52]. Muntoni et al. developed the SLNs of insulin and glargine-insulin by utilizing the fatty acid coacervation technique to protect the insulin from enzymatic degradation. The pharmacokinetic studies revealed that differential gastric emptying on the uptake of drug and absorption maxima is achieved upon completion of 30 min post administration. The bioavailability obtained after duodenal and gavage administrations was observed to be 6.10% and 4.5%, respectively. The concentration estimated in the lymph was about 2.0 μg/mL mg−1 at 1.5 h post duodenal administration, which showed that intestinal uptake of drug is primarily dependent upon lymph. The outcomes of the glucose responsivity were estimated in healthy rats which showed considerable reduction in glucose at 2.5 h and even slow reduction was observed upon completion of 6 h [53].
The modification of SLNs with the help of ligand attachment may serve to achieve better bioavailability, in the case of protein drugs and peptides. Fan et al. reported the fabrication of salmon calcitonin-containing SLNs which were attached with two different peptides to form two types of SLNs. One of the products was sCT CSK-SLNs, which was presumed to have more affinity for goblet cells and sCT IRQ-SLNs for imparting cell penetrating power. The investigation conformed about superior protection to active pharmaceutical ingredient and internalization of drug through the mucosal cells of the duodenum. The mucus layer was a significant barrier to the movement of the drug across membrane, but modified SLNs showed enhanced drug absorption. The absolute bioavailability of the sCT CSK-SLNs and sCT IRQ-SLNs was found out to be 12.41% and 10.05%, respectively. There was close to twofold rise for the modified SLNs in comparison with unmodified SLNs [54]. The strategy of modification by using peptides was taken even further by Juang et al. who prepared pH-sensitive SLNs modified using peptides for effective delivery of irinotecan. Irinotecan is a potent anticancer agent for colorectal cancer, which was incorporated into a specialized carrier to possess targeted pH-dependent release and better cell internalization. The outcomes of the in vivo studies showed that targeting by pH-responsive carriers was successful as they suppressed the colorectal tumor growth and decreased associated toxicity [55].
The nanotechnology-based diagnostic agents furnish more efficient along with negligible issues with regards to safety. Correspondingly, the diagnosis through imaging techniques is considered to have potential for future prospects by using novel nano-based imaging contrasting agents. A SLN-based diagnostic product was prepared to improve contrasting characteristics while performing magnetic resonance imaging for the diagnosis of colorectal cancer. The two types of SLNs were fabricated by incorporating the gadolinium diethylenetriaminepentaacetic acid and fluorescein isothiocyanate. The prepared SLNs were intravenously administered into mice, and magnetic resonance colonography was performed for the examination of colon. The signal to noise ration raised from 1.54- to 1.74-fold in colorectal tumors after administration of gadolinium fluorescein isothiocyanate-containing SLNs. In case of SLNs containing gadolinium diethylenetriaminepentaacetic acid, signal to noise ratio raised from 1.39- to 1.57-fold [56].
8 SLNs for Topical Delivery
SLNs have also displayed promise as an advanced drug carrier for the topical delivery of a number of active pharmaceutical ingredients. The conventional drug delivery systems of some topical agents are associated with moderate to severe side effects which hamper their therapeutic potential. Presumably, the reduction of side effects and enhancement of therapeutic activity are attainable by formulating these topical agents as SLN carriers. Despite showing a lot promise, there is still obscurity about the absorption mechanisms and cellular uptake of the topical lipid nanoparticles. This portion of the chapter entails the discussion about some of the important researches on topical SLN with their pharmacokinetic considerations [57,58,59]. The SLNs of penciclovir, a potent and highly selective inhibitor of herpes viruses, was formulated and proved that penciclovir-loaded SLNs are a promising carrier for topical delivery. The in vitro percutaneous permeation and skin uptake behaviors in the rat skin penetration indicated twofold increase compared to commercial cream as a control at 12 h. The microscopic examination of the skin surface showed the apparent morphology of stratum corneum and broke the close conjugation of corneocyte layers. The amount of penciclovir penetrated into dermis from SLNs increased by 130% [60]. Meloxicam-loaded hydrogel of SLNs was studied by Kahlil et al. The 48 h in vitro study showed sustained release of drug. The formulation further studied for suppression of UV-induced erythema compare to commercial gel (0.5%) as a reference product in adult male Wistar rats. The results of the degree of erythema upon exposure to UVB radiation was monitored over 72 h and concluded that 67% of rats receiving SLN gel showed complete suppression of erythema evidenced by high mean erythemal score value. Similarly, histopathological analysis of excised skin sections also proved superiority of formulation and showed normal histology with SLN-loaded hydrogel [61].
Bhalekar et al. studied piperine (a natural alkaloid) SLN dispersion for the treatment of rheumatoid arthritis. CFA-induced arthritis model was used for in vivo pharmacodynamic study in rat. Photomicrographs of joint section obtained during histopathology study indicated that piperine SLN gel showed minimal infiltration of inflammatory cells and connective tissue proliferation as compared to test group. TNFα assay by ELISA study showed that activated macrophages was seen to have considerably decreased as related to arthritic control group it may be attributed to the selective accumulation of piperine SLNs in inflamed site, thus reducing the secretion to TNF α from the activated macrophages [62]. SLNs were used as a carrier for topical ocular delivery of tobramycin by a group of scientists and compared with marketed formulation Tobral®. Dispersion of SLNs containing 0.3% w/v was administered to eye of rabbits to evaluate the ocular tolerance and potential irritation. The pre-ocular retention study by fluorescence in conjunctival sac and on corneal surface suggested four times longer retention in eye. The aqueous humor concentration of the drug was measured for 6 h indicating that the Cmax (36.30 μg/mL−1), Tmax (4.0 h), and high AUC (155.08 μg·h·mL−1) of SLNs dispersion were significantly higher compared to solution [63].
Nair et al. investigated clarithromycin-loaded SLNs to increase the ocular permeation and improve the therapeutic potential of the drug in topical ocular drug delivery. The permeation study of SLNs showed significantly higher permeation (30.45 g/cm2/h; p < 0.0001) as compared to control (solution). Pharmacokinetics data demonstrated significant improvement of clarithromycin bioavailability (p < 0.0001) from SLN formulation, as evidenced by a 150% increase in Cmax (~1066 ng/mL) and a 2.8-fold improvement in AUC (5736 ng h/mL) (p < 0.0001) as compared to control drug solution (Cmax; 655 ng/mL and AUC; 2067 ng h/mL) [62]. Minoxidil is currently known for the treatment of androgenic alopecia, but it needs to be formulated into carrier with good penetration and non-corrosive characteristics. A 5% minoxidil was incorporated with the help of combination of polysorbates and sorbitan oleate. The skin penetration studies revealed that minoxidil is primarily distributed within the outermost layer of the skin, and only little amount was able to get to the dermis. However, the distribution of minoxidil was similar in the epidermis and dermis layer upon completion of 24 h after administration [65]. The SLNs for the topical delivery of tretinoin have also been reported as this potent anti-psoriatic agent unleashes irritating side effects, such as erythema and peeling. A biocompatible SLN of tretinoin was prepared to enhance its cutaneous delivery, stability, and pharmacodynamic features. The confocal laser screening microscopy showed that after topical application of the SLN formulation, it has penetrated into the different layers of the skin. The investigation involved preparation and examination of several nano-carriers for the delivery of tretinoin, and SLNs came out to be one of the promising systems from the study [66].
9 SLNs for Ocular Delivery
The conventional oral products face bioavailability issues because of brief contact time with ocular structures and quick washout due to tear production. Furthermore, the efficient delivery to the posterior region of the eye is difficult, and opting for alternate route may be required [67]. To resolve these issues, SLNs emerged as suitable carriers for delivery of drug as well as biotechnological agents. Lipid nanoparticles integrate the benefits of other systems like polymeric nanoparticles, nanoemulsions, and liposomes, while evading their shortcomings [68]. The ocular SLN carrier was prepared for topical administration of tobramycin. The formulation composed of ion-pair complex of tobramycin and hexadecyl phosphate was fabricated by employing warm microemulsion method. The pharmacokinetic study of SLN was performed in aqueous humor for estimating the AUC, Cmax, Tmax, and bioavailability. The outcomes suggested that bioavailability of tobramycin produced by SLN was much higher as compared to the reference eyedrops. The SLN produced increment in Cmax, Tmax, and AUC up to 1.5-, 8-, and 4-fold as compared to the reference solution. The greater bioavailability resulted from the SLN was probably due to prolonged retention which was estimated from the retention study. It was also suggested in the literature that the use of permeation enhancer like Epikuron 200 may have boosted up the corneal passage of the drug [69].
The tetrandrine-containing SLNs were developed by melt emulsification and ultra-sonication method. The observations of AUC (6581.50 μgh/L) amd Cmax (1103.43 μg/L) were all determined to be considerably higher in comparison with tetrandrine solution. In addition to these, the prolonged t1/2 (13.86 h) and MRT (19.37 h) were estimated for SLNs. The biodistribution study showed that the highest concentration showed up in lungs post intravenous administration. The SLN uptake was also significantly higher in reticuloendothelial system organs than normal drug solution. It was also ascertained that SLNs were taken to the liver and spleen through the phagocytosis or endocytosis mechanism. The localization of drug from SLN was lower than drug solution in the heart and kidney [70].
Ahmad et al. developed the SLN containing etoposide for the effective ocular delivery, specifically to the posterior region. The preparation of SLN was carried out by using melt emulsification and ultra-sonication method. The etoposide-containing SLNs displayed prolonged release of etoposide for 1 week in vitreous humor with Cmax of 46.75 μg/mL, and concentration of etoposide was maintained constant throughout the week. Contrarily, the animals administered with etoposide solution showed Cmax of 73.18 μg/mL, but very little amount of observed at day 2 after administration. AUC (657.9 μg.h/mL) and t1/2 (7.75 h) were also significantly higher in case of SLN as compared to etoposide solution. The scintigraphic study was performed by labelling the SLNs with Tc-99m to understand their distribution. It was observed that radiolabelled nanoparticle showed relatively homogeneous distribution throughout the vitreous. The nanoparticle formulation was retained at the retinal region for long period with no or little concentration observed in systemic circulation until 24-h post administration [71]. Table 13.1 summarises outcomes of pharmacokinetic studies performed on solid lipid nanoparticles administered through various routes.
Change history
11 August 2022
The below chapter was mistakenly published with an incorrect author name it should be read as below. The chapter and book have been updated with the change.
References
Rodrigues LBO, Lima FA, Alves CPB, et al. Ion pair strategy in solid lipid nanoparticles: a targeted approach to improve epidermal targeting with controlled adapalene release, resulting reduced skin irritation. Pharm Res. 2020;37:1–14.
Tekade RK, Maheshwari R, Tekade M, Chougule MB. Solid lipid nanoparticles for targeting and delivery of drugs and genes. Elsevier Inc.; 2017.
Muller R, Radtke M, Wissing SA. Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) in cosmetic and dermatological preparations. Adv Drug Deliv Rev. 2002;54:S131.
Naseri N, Valizadeh H, Zakeri-Milani P. Solid lipid nanoparticles and nanostructured lipid carriers: structure, preparation and application. Adv Pharm Bull. 2015;5(3):305–13.
Shah B, Khunt D, Bhatt H, Misra M, Padh H. Application of quality by design approach for intranasal delivery of rivastigmine loaded solid lipid nanoparticles: effect on formulation and characterization parameters. Eur J Pharm Sci. 2015;78:54–66.
Bondi ML, Azzolina A, Craparo EF, et al. Novel cationic solid-lipid nanoparticles as non-viral vectors for gene delivery. J Drug Target. 2007;15:295–301.
Doroud D, Vatanara A, Zahedifard F, et al. Cationic solid lipid nanoparticles loaded by cystein proteinase genes as a novel anti-leishmaniasis DNA vaccine delivery system: characterization and in vitro evaluations. J Pharm Pharm Sci. 2010;13:320–35.
Tenchov R, Bird R, Curtze AE, Zhou Q. Lipid nanoparticles—from liposomes to mRNA vaccine delivery, a landscape of research diversity and advancement. ACS Nano. 2021;15(11):16982–7015.
Almeida AJ, Runge S, Müller RH. Peptide-loaded solid lipid nanoparticles (SLN): influence of production parameters. Int J Pharm. 1997;149:255–65.
Brioschi AM, Calderoni S, Zara GP, Priano L, Gasco MR, Mauro A. Solid lipid nanoparticles for brain tumors therapy: state of the art and novel challenges. Prog Brain Res. 2009;180:193–223.
Mehnert W, Mäder M. Solid lipid nanoparticles: production, characterization and applications. Adv Drug Deliv Rev. 2001;47:165–96.
Ghadiri M, Fatemi S, Vatanara A, et al. Loading hydrophilic drug in solid lipid media as nanoparticles: statistical modeling of entrapment efficiency and particle size. Int J Pharm. 2012;424:128–37.
Topal GR, Mészáros M, Porkoláb G, et al. ApoE-targeting increases the transfer of solid lipid nanoparticles with donepezil cargo across a culture model of the blood–brain barrier. Pharmaceutics. 2021;13:38.
Mostafa DAE, Khalifa MKA, Gad SS. Zolmitriptan brain targeting via intranasal route using solid lipid nanoparticles for migraine therapy: formulation, characterization, in-vitro and in-vivo assessment. 2020.
Pole S, Williams AC, Barry BW, et al. Docosahexaenoic acid–mediated, targeted and sustained brain delivery of curcumin microemulsion. Drug Deliv. 2016;10:1–14.
H Muller R, Shegokar R, M Keck C. 20 years of lipid nanoparticles (SLN & NLC): present state of development & industrial applications. Curr Drug Discov Technol. 2011;8:207–27.
Geszke-Moritz M, Moritz M. Solid lipid nanoparticles as attractive drug vehicles: composition, properties and therapeutic strategies. Mater Sci Eng C. 2016;68:982–94.
Ramzan M, Kaur G, Trehan S, et al. Mechanistic evaluations of ketoconazole lipidic nanoparticles for improved efficacy, enhanced topical penetration, cellular uptake (L929 and J774A. 1), and safety assessment: in vitro and in vivo studies. J Drug Delivery Sci Technol. 2021;65:102743.
Vinchhi P, Patel JK, Patel MM. High-pressure homogenization techniques for nanoparticles. In: Emerging technologies for nanoparticle manufacturing. Cham: Springer; 2021. p. 263–85.
Shah RM, Rajasekaran D, Ludford-Menting M, Eldridge DS, Palombo EA, Harding IH. Transport of stearic acid-based solid lipid nanoparticles (SLNs) into human epithelial cells. Colloids Surf., B. 2016;140:204–12.
Rajabi M, A. Mousa S. Lipid nanoparticles and their application in nanomedicine. Curr Pharm Biotechnol. 2016;17:662–72.
Souto EB, Doktorovová S. Solid lipid nanoparticle formulations. Pharmacokinetic and biopharmaceutical aspects in drug delivery. Methods Enzymol. 2009;464:105–29.
Harde H, Das M, Jain S. Solid lipid nanoparticles: an oral bioavailability enhancer vehicle. Expert Opin Drug Deliv. 2011;8(11):1407–24.
Wissing SA, Kayser O, Müller RH. Solid lipid nanoparticles for parenteral drug delivery. Adv Drug Deliv Rev. 2004;56:1257–72.
Scioli Montoto S, Muraca G, Ruiz ME. Solid lipid nanoparticles for drug delivery: pharmacological and biopharmaceutical aspects. Front Mol Biosci. 2020;7:1–24.
Jenning V, Thünemann AF, Gohla SH. Characterisation of a novel solid lipid nanoparticle carrier system based on binary mixtures of liquid and solid lipids. Int J Pharm. 2000;199:167–77.
Paliwal R, Paliwal SR, Kenwat R, et al. Solid lipid nanoparticles: a review on recent perspectives and patents. Expert Opin Ther Pat. 2020;30(3):179–94.
Abdel Hady M, Sayed OM, Akl MA. Brain uptake and accumulation of new levofloxacin-doxycycline combination through the use of solid lipid nanoparticles: formulation; optimization and in-vivo evaluation. Colloids Surf B Biointerfaces. 2020;193:111076.
Salah E, Abouelfetouh MM, Pan Y, et al. Solid lipid nanoparticles for enhanced oral absorption: A review. Colloids Surf B Biointerfaces. 2020;196:111305.
Zhou K, Yan Y, Chen D, et al. Solid lipid nanoparticles for duodenum targeted oral delivery of tilmicosin. Pharmaceutics. 2020;12(8):731.
Li C, Zhou K, Chen D, et al. Solid lipid nanoparticles with enteric coating for improving stability, palatability, and oral bioavailability of enrofloxacin. Int J Nanomedicine. 2019;14:1619–31.
Gambhire VM, Gambhire MS, Ranpise NS. Solid lipid nanoparticles of dronedarone hydrochloride for oral delivery: optimization, in vivo pharmacokinetics and uptake studies. Pharm Nanotechnol. 2019;7(5):375–88.
He Y, Zhan C, Pi C, et al. Enhanced oral bioavailability of felodipine from solid lipid nanoparticles prepared through effervescent dispersion technique. AAPS PharmSciTech. 2020;21(5):170.
Ban C, Jo M, Park YH, et al. Enhancing the oral bioavailability of curcumin using solid lipid nanoparticles. Food Chem. 2020;302:125328.
Kumar M, Sharma G, Singla D, et al. Enhanced oral absorption of all-trans retinoic acid upon encapsulation in solid lipid nanoparticles. Pharm Nanotechnol. 2020;8(6):495–510.
Du Y, Ling L, Ismail M, et al. Redox sensitive lipid-camptothecin conjugate encapsulated solid lipid nanoparticles for oral delivery. Int J Pharm. 2018;549(1–2):352–62.
Patel MH, Mundada VP, Sawant KK. Fabrication of solid lipid nanoparticles of lurasidone HCl for oral delivery: optimization, in vitro characterization, cell line studies and in vivo efficacy in schizophrenia. Drug Dev Ind Pharm. 2019;45(8):1242–57.
Lin CH, Chen CH, Lin ZC, Fang JY. Recent advances in oral delivery of drugs and bioactive natural products using solid lipid nanoparticles as the carriers. J Food Drug Anal. 2017;25(2):219–34.
Martins S, Tho I, Reimold I, et al. Brain delivery of camptothecin by means of solid lipid nanoparticles: formulation design, in vitro and in vivo studies. Int J Pharm. 2012;439(1–2):49–62.
Loureiro JA, Andrade S, Duarte A, et al. Resveratrol and grape extract-loaded solid lipid nanoparticles for the treatment of Alzheimer’s disease. Molecules. 2017;22(2):277.
Mendonça MCP, Radaic A, Garcia-Fossa F, da Cruz-Höfling MA, Vinolo MAR, de Jesus MB. The in vivo toxicological profile of cationic solid lipid nanoparticles. Drug Deliv Transl Res. 2020;10(1):34–42.
Maithania HV, Mohanty BS, Chaudhari PR, Samad A, Devarajan PV. Shape mediated splenotropic delivery of buparvaquone loaded solid lipid nanoparticles. Drug Deliv Transl Res. 2020;10(1):159–67.
Fatouh AM, Elshafeey AH, Abdelbary A. Intranasal agomelatine solid lipid nanoparticles to enhance brain delivery: formulation, optimization and in vivo pharmacokinetics. Drug Des Devel Ther. 2017;11:1815–25.
Jorge A, Pais A, Vitorino C. Targeted siRNA delivery using lipid nanoparticles. Methods Mol Biol. 2020;2059:259–83.
Truzzi E, Nascimento TL, Iannuccelli V, et al. In vivo biodistribution of respirable solid lipid nanoparticles surface-decorated with a mannose-based surfactant: a promising tool for pulmonary tuberculosis treatment? Nanomaterials (Basel). 2020;10(3):568.
Zhou M, Hou J, Zhong Z, Hao N, Lin Y, Li C. Targeted delivery of hyaluronic acid-coated solid lipid nanoparticles for rheumatoid arthritis therapy. Drug Deliv. 2018;25(1):716–22.
Yasir M, Gaur PK, Puri D, Shehkar P, Kumar SS. Solid lipid nanoparticles approach for lymphatic targeting through intraduodenal delivery of quetiapine fumarate. Curr Drug Deliv. 2018;15(6):818–28.
Pinheiro RGR, Granja A, Loureiro JA, et al. RVG29-functionalized lipid nanoparticles for quercetin brain delivery and Alzheimer’s disease. Pharm Res. 2020;37(7):139.
Del Pozo-Rodríguez A, Solinís MÁ, Rodríguez-Gascón A. Applications of lipid nanoparticles in gene therapy. Eur J Pharm Biopharm. 2016;109:184–93.
Rajpoot K. Solid lipid nanoparticles: a promising nanomaterial in drug delivery. Curr Pharm Des. 2019;25(37):3943–59.
Hsu CY, Wang PW, Alalaiwe A, Lin ZC, Fang JY. Use of lipid nanocarriers to improve oral delivery of vitamins. Nutrients. 2019;11(1):68.
Sarmento B, Martins S, Ferreira D, Souto EB. Oral insulin delivery by means of solid lipid nanoparticles. Int J Nanomedicine. 2007;2(4):743–9.
Muntoni E, Marini E, Ahmadi N, et al. Lipid nanoparticles as vehicles for oral delivery of insulin and insulin analogs: preliminary ex vivo and in vivo studies. Acta Diabetol. 2019;56(12):1283–92.
Fan T, Chen C, Guo H, et al. Design and evaluation of solid lipid nanoparticles modified with peptide ligand for oral delivery of protein drugs. Eur J Pharm Biopharm. 2014;88(2):518–28.
Juang V, Chang CH, Wang CS, et al. pH-responsive PEG-shedding and targeting peptide-modified nanoparticles for dual-delivery of irinotecan and microRNA to enhance tumor-specific therapy. Small. 2019;15(49):e1903296.
Wang H, Ding W, Peng L, et al. Gadolinium-loaded solid lipid nanoparticles for colorectal tumor in MR colonography. J Biomed Nanotechnol. 2020;16(5):594–602.
Trombino S, Mellace S, Cassano R. Solid lipid nanoparticles for antifungal drugs delivery for topical applications. Ther Deliv. 2016;7(9):639–47.
Mu H, Holm R. Solid lipid nanocarriers in drug delivery: characterization and design. Expert Opin Drug Deliv. 2018;15(8):771–85.
Liu M, Wen J, Sharma M. Solid lipid nanoparticles for topical drug delivery: mechanisms, dosage form perspectives, and translational status. Curr Pharm Des. 2020;26(27):3203–17.
Lv Q, Yu A, Xi Y, et al. Development and evaluation of penciclovir-loaded solid lipid nanoparticles for topical delivery. Int J Pharm. 2009;372(1–2):191–8.
Khalil RM, Abd-Elbary A, Kassem MA, et al. Nanostructured lipid carriers (NLCs) versus solid lipid nanoparticles (SLNs) for topical delivery of meloxicam. Pharm Dev Technol. 2014;19(3):304–14.
Bhalekar MR, Madgulkar AR, Desale PS, Marium G. Formulation of piperine solid lipid nanoparticles (SLN) for treatment of rheumatoid arthritis. Drug Dev Ind Pharm. 2017;43(6):1003–10.
Cavalli R, Gasco MR, Chetoni P, et al. Solid lipid nanoparticles (SLN) as ocular delivery system for tobramycin. Int J Pharm. 2002;238(1–2):241–5.
Nair AB, Shah J, Al-Dhubiab BE, et al. Clarithromycin solid lipid nanoparticles for topical ocular therapy: optimization, evaluation and in vivo studies. Pharmaceutics. 2021;13(4):523.
Padois K, Cantiéni C, Bertholle V, et al. Solid lipid nanoparticles suspension versus commercial solutions for dermal delivery of minoxidil. Int J Pharm. 2011;416(1):300–4.
Raza K, Singh B, Lohan S, et al. Nano-lipoidal carriers of tretinoin with enhanced percutaneous absorption, photostability, biocompatibility and anti-psoriatic activity. Int J Pharm. 2013;456(1):65–72.
Battaglia L, Serpe L, Foglietta F, et al. Application of lipid nanoparticles to ocular drug delivery. Expert Opin Drug Deliv. 2016;13(12):1743–57.
Uner M, Yener G. Importance of solid lipid nanoparticles (SLN) in various administration routes and future perspectives. Int J Nanomedicine. 2007;2(3):289–300.
Sánchez-López E, Espina M, Doktorovova S, Souto EB, García ML. Lipid nanoparticles (SLN, NLC): overcoming the anatomical and physiological barriers of the eye - part II - ocular drug-loaded lipid nanoparticles. Eur J Pharm Biopharm. 2017;110:58–69.
Li S, Ji Z, Zou M, et al. Preparation, characterization, pharmacokinetics and tissue distribution of solid lipid nanoparticles loaded with tetrandrine. AAPS PharmSciTech. 2011;12(3):1011–8.
Ahmad I, Pandit J, Sultana Y, et al. Optimization by design of etoposide loaded solid lipid nanoparticles for ocular delivery: characterization, pharmacokinetic and deposition study. Mater Sci Eng C Mater Biol Appl. 2019;100:959–70.
Kaufman RC. U.S. Patent No. 10,028,919. Washington, DC: U.S. Patent and Trademark Office. 2018.
Kumar VV, Chandrasekar D, Ramakrishna S, et al. Development and evaluation of nitrendipine loaded solid lipid nanoparticles: influence of wax and glyceride lipids on plasma pharmacokinetics. Int J Pharm. 2007;335(1–2):167–75.
Ren J, Zou M, Gao P, et al. Tissue distribution of borneol-modified ganciclovir-loaded solid lipid nanoparticles in mice after intravenous administration. Eur J Pharm Biopharm. 2013;83(2):141–8.
Alukda D, Sturgis T, Youan BC. Formulation of tenofovir-loaded functionalized solid lipid nanoparticles intended for HIV prevention. J Pharm Sci. 2011;100(8):3345–56.
Xie S, Zhu L, Dong Z, et al. Preparation, characterization and pharmacokinetics of enrofloxacin-loaded solid lipid nanoparticles: influences of fatty acids. Colloids Surf B Biointerfaces. 2011;83(2):382–7.
Bhandari R, Kaur IP. Pharmacokinetics, tissue distribution and relative bioavailability of isoniazid-solid lipid nanoparticles. Int J Pharm. 2013;441(1–2):202–12.
Fundarò A, Cavalli R, Bargoni A, et al. Non-stealth and stealth solid lipid nanoparticles (SLN) carrying doxorubicin: pharmacokinetics and tissue distribution after i.v. administration to rats. Pharmacol Res. 2000;42(4):337–43.
Zara GP, Bargoni A, Cavalli R, et al. Pharmacokinetics and tissue distribution of idarubicin-loaded solid lipid nanoparticles after duodenal administration to rats. J Pharm Sci. 2002;91(5):1324–33.
Manjunath K, Venkateswarlu V. Pharmacokinetics, tissue distribution and bioavailability of clozapine solid lipid nanoparticles after intravenous and intraduodenal administration. J Control Release. 2005;107(2):215–28.
Jose S, Anju SS, Cinu TA, et al. In vivo pharmacokinetics and biodistribution of resveratrol-loaded solid lipid nanoparticles for brain delivery. Int J Pharm. 2014;474(1–2):6–13.
Dwivedi P, Khatik R, Khandelwal K, et al. Pharmacokinetics study of arteether loaded solid lipid nanoparticles: an improved oral bioavailability in rats. Int J Pharm. 2014;466(1–2):321–7.
Yasir M, Sara UVS. Solid lipid nanoparticles for nose to brain delivery of haloperidol: in vitro drug release and pharmacokinetics evaluation. Acta Pharm Sin B. 2014;4:454–63.
Xue M, Yang MX, Zhang W, et al. Characterization, pharmacokinetics, and hypoglycemic effect of berberine loaded solid lipid nanoparticles. Int J Nanomedicine. 2013;8:4677–87.
Luo CF, Yuan M, Chen MS, et al. Pharmacokinetics, tissue distribution and relative bioavailability of puerarin solid lipid nanoparticles following oral administration. Int J Pharm. 2011;410(1–2):138–44.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Paliwal, H., Prajapati, B.G., Khunt, D., Shirisha, C., Patel, J.K., Pathak, Y.V. (2022). Pharmacokinetic and Tissue Distribution Study of Solid Lipid Nanoparticles. In: Patel, J.K., Pathak, Y.V. (eds) Pharmacokinetics and Pharmacodynamics of Nanoparticulate Drug Delivery Systems . Springer, Cham. https://doi.org/10.1007/978-3-030-83395-4_13
Download citation
DOI: https://doi.org/10.1007/978-3-030-83395-4_13
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-83394-7
Online ISBN: 978-3-030-83395-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)