Abstract
In this work, based on numerical modeling, we carried out a comparative analysis of the flow of chemically reactive gas flow through a catalyst using the example of the selective hydrogenation of acetylene. The catalyst models are built based on an open foam cell material and a traditional granular catalyst. We obtained the results of numerical studies in the form of fields of concentration of components of the gas mixture, vector fields of gas motion, and values of conversion and selectivity.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Most chemical industry reactions are exothermic; therefore, it is necessary to prevent overheating in the gas’s contact zones and the surface of the catalyst granules [1–3]. A solution to this problem can be using an open cell foam material as a catalyst carrier. The authors of [4] investigated the flow in a layer consisting of cubes of an open cell foam material. Global and local porosity in this geometry increase convection by decreasing the superheat in the reactor. The use of such structures in a reactor is promising. Open cell foam materials are the subject of numerous studies due to their unique properties: large contact surface area and high porosity, which creates a low pressure drop and provides high energy efficiency [5–7]. Currently, metallic porous materials are widely used as multifunctional heat exchangers [8] and compact radiators for microelectronic devices [9] due to their heat transfer properties [10, 11]. Also, thermally conductive porous materials have been proposed as an effective solution for intensifying non-adiabatic catalytic processes in tubular reactors.
In chemical engineering, porous materials are widely used as catalyst carriers. During chemical reactions, the process parameters and geometric structure of fixed bed catalytic reactors, including porous materials, play a decisive role. For stable and reliable reactors in industrial conditions, it is necessary to correctly set the operating parameters to control the reactor’s coolant dynamics. The mode of operation of catalytic reactions associated with a chemical reaction or their field of application determines the characteristic requirements and limitations of reactors, including size, productivity, uniformity of heat transfer, pressure drop, and selectivity. Numerical simulations can characterize or optimize the performance of fixed bed catalytic reactors for industrial applications. For exothermic reactions, solid porous structures show good results. In [12], ceramic porous materials, honeycombs, and volumetric spheres were compared. The authors found that porous materials’ main advantage is their radial mixing characteristics of gases and heat transfer from the reactor’s pipe wall. In [13], porous metal granules with solid porous structures were compared in terms of pressure drop and heat transfer characteristics.
In this work, we performed a numerical simulation of acetylene’s selective hydrogenation to ethylene when passing through an open cell foam catalyst. Ethylene is an essential component of the petrochemical industry. The main task in the production of ethylene is its purification from by-components, for example, acetylene. In this case, purification can be carried out by hydrogenation, when acetylene is converted to ethylene. However, the acetylene and the ethylene, which is hydrogenated to ethane, can be hydrogenated. Thus, the process should take place in the presence of a catalyst with selectivity parameters that allow acetylene’s hydrogenation, but do not promote ethylene’s hydrogenation.
2 Problem Formulation and Solution Method
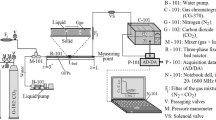
Let us carry out a comparative analysis of the reactors’ operation for acetylene’s selective hydrogenation [14]. Experimental data are needed to build a model of the movement of a chemically reacting flow. Experimental studies were carried out for the reaction of selective hydrogenation of acetylene to ethylene. The hydrogenation of acetylene to ethylene occurs in a reactor with a granular bed of a palladium catalyst or a foam catalyst with palladium deposition (see Fig. 1).
The catalyst is tested in an isothermal cylindrical reactor. The reactor is a hollow steel tube 700 mm long and 20 mm in inner diameter. There is a 45 mm bed of granular catalyst mixed with quartz in the middle of the reactor or a sample of a foam catalyst 55 mm high. In front of the catalyst and on the catalyst, quartz is poured, a shapeless granule with a 3–5 mm diameter. Gas temperature 25–75 °C, pressure 1 atm. The gas used is a fraction of argon (24%), ethylene (75%), and acetylene (1%). Passing through the catalyst bed, acetylene is hydrogenated to ethylene
In addition to the primary reaction, undesirable side reactions can occur, for example, hydrogenation of ethylene to ethane’s
As the criteria for the catalyst operation, let us estimate the acetylene conversion and selectivity as
We will carry out a detailed numerical simulation of reactors from experimental studies to analyze the effects that affect the reaction’s activity and selectivity.
The solution to the problem of the movement of a chemically reacting gas flow is carried out by the finite volume method with the division of the considered region of the reactor into triangular elements. The considered flow is stationary. For a multicomponent gas phase, the laws of conservation of mass, momentum, and energy are fulfilled.
Mass conservation equation
where \(\rho\) is the density, \(\vec{v}\) is the velocity.
The momentum conservation equation
where \(p\) is the pressure, \(\bar{\bar{\tau }}\) is the stress tensor. In Eq. (6), the stress tensor is
where \(\mu\) is the viscosity, \(\bar{\bar{I}}\) is unit tensor.
Mass conservation equation for the i-th component of the gas mixture
where \(Y_{i}\) is the mass fraction of the i-th component of the gas mixture, \(R_{i}\) is the consumption of the i-th component of the chemical reaction, \(J_{i}\) is the diffusion flux of the i-th component arising from the concentration and temperature gradients.
where \(T\) is the temperature, \(D_{{m,i}}\) is the mass diffusion coefficient, \(D_{{T,i}}\) is the temperature diffusion coefficient.
Energy conservation equation
where \(h = \sum\nolimits_{{i = 1}}^{N} {Y_{i} h_{i} }\) is the enthalpy, and
where \(\lambda\) is the thermal conductivity of the gas mixture, \(N\) is the number of components in the mixture.
For chemical reactions, the parameter \(R_{i}\) in Eq. (8) can be written as
where \(M_{{w,i}}\) is the molecular weight of the i-th component of the mixture, \(N_{R}\) is the number of reactions involving the i-th component of the mixture.
where \(C_{{j,r}}\) is the concentration of the j-th component of the mixture, \(\eta _{{i,r}}^{'}\) is the exponent for the reactant j-th component in the reaction, \(k_{{f,r}}\) is the reaction rate constant.
where \(A_{r}\) is the pre-exponential factor, \(E_{r}\) is the activation energy.
We use ANSYS Fluent 19.2 software to solve the written system of equations. Many of the component properties parameters are taken from the database of the software used. We take the physicochemical properties of the components according to the polynomial dependence on temperature.
For a detailed simulation of the reactor, it is necessary to create a geometry consisting of randomly poured granules or representing a foam material with a random arrangement of connected cells. The primary methods for creating geometry with random elements in space are the Discrete Element Method (DEM). A dynamic process is considered, in which the speed of movement depends on the physical properties of a discrete system.
Then we will consider the constructed layer of granules cut from the reactor volume and the movement of the gas mixture in space. Next, the built area for gas movement will be subdivided into a grid. In this case, it is worth paying attention to the points of contact between the individual pellets and the reactor’s walls. The constructed grid cells at such points have sharp bends, which leads to problems of solution convergence. There are four approaches to solving the convergence of the numerical solution at the points of contact: a decrease in the diameter of granules with the formation of gaps [15–17], an increase in the diameter of granules with the formation of intersections [18], a partial cutting of the surface of the granules at the points of contact [19], bringing bridges between granules at points of contact [20]. The authors of [21] analyzed the use of these approaches for fixed bed reactors, with particular attention to the parameters of pressure drop, porosity, and heat transfer. The authors showed that an adequate description of the parameters under study could be obtained only with the help of local modification of particles, for example, by cutting off part of the granules at the points of contact or by the bridge method. In [22], these two modifications of granules were studied with the numerical simulation of a chemical reaction in a fixed layer of spheres. The results obtained indicate similar values of the investigated parameters when leveling a part of the granules at the contact points and building bridges.
The acetylene hydrogenation reaction is very fast and is used in industry, for example, to purify the ethylene fraction from acetylene impurities. The advantage of using an open cell foam material as a catalyst carrier is a decrease in the resistance to gas flow as it passes through the catalyst bed and a developed catalyst surface area.
Let us carry out a comparative analysis of two types of catalysts: traditional granular and built based on a foam material. Let us create models that correspond to the experimental studies carried out. The constructed models are shown in Fig. 2. In the experiments, catalyst granules were mixed with quartz particles. When making a computational model, cylindrical catalyst granules and shapeless quartz particles were also selected.
To solve the problem numerically, we divide the entire area under consideration into finite elements of a triangular shape, the dimensions of which are sufficient to determine the specific factors of the phenomenon under study. We are using a uniform mesh with 10–4 m selected as the primary cell size. Condensation with a cell size of 2.5×10–5 m and 5×10–5 m was carried out near the granules’ surface and at the contact points. This separation of the grid allows avoiding a sharp bending of the cells.
3 Results and Discussions
Experimental studies were carried out for a feed gas temperature range of 25 to 75 °C. Numerical calculations were also performed in this range of gas flow temperatures. The results of the calculated fields of the mass content of acetylene in the cross-section of the reactor are presented in Fig. 3 for a granular catalyst and in Fig. 4 for a catalyst based on an open cell foam material.
It can be seen from the pictures of the calculation results that the mass content of acetylene is significantly lower at the outlet of the reactor for a catalyst based on an open cell foam material than at the outlet of a reactor with a traditional granular catalyst. In this case, large values in acetylene conversion are observed in the calculations over the entire temperature range of the gas flow.
Next, we carry out a quantitative analysis of the conversion of acetylene when passing through the catalyst bed. Figure 5 shows the results of experimental studies and numerical calculations.
It can be seen from the figure that the results of numerical calculations show good agreement with the results of the experiments performed. In this case, the best agreement of the results is observed for an open cell foam catalyst. The most considerable deviations are observed for a granular catalyst at the smallest and largest gas temperatures in the selected range. In both experimental and computational experiments, catalysts based on an open cell foam catalyst show the best results in comparison with a traditional granular catalyst.
Figure 6 shows the vector fields of gas movement through the catalyst bed for two studied samples. It can be seen that when passing through an open cell foam catalyst, the gas velocity field is more uniform. And the structural elements of the catalyst do not much disturb the flow. In this case, the picture for a granular catalyst shows both the formation of regions with a high velocity and stagnant circulation regions near the catalyst granules. The gas contact time with the catalyst decreases when driving at a high rate. And in stagnant circulation areas, the likelihood of side reactions increases. Both factors negatively affect the studied parameters of conversion and selectivity.
4 Conclusion
We made models of an open cell foam catalyst and a traditional granular catalyst. A mathematical model of the process of selective catalytic hydrogenation of acetylene has been constructed. The calculations of the models of laboratory reactors of the catalyst are carried out, and the parameters of conversion and selectivity are compared with laboratory data. As a result of a comparative analysis of the gas movement fields directly near the catalyst surface, factors influencing the studied reaction were determined.
References
Soloveva OV, Solovyev SA (2016) Investigation of the influence of heated catalyst feeding system on the intensity of temperature-dependent chemical reaction in the fluidized bed apparatus. IOP Confers Ser: Mater Sci Eng 158(1):012086. https://doi.org/10.1088/1757-899x/158/1/012086
Solovev SA, Soloveva OV, Antipin AV, Shamsutdinov EV (2018) Investigation of internal elements impaction on particles circulation in a fluidized bed reactor. J Phys: Conf Ser 944:012114. https://doi.org/10.1088/1742-6596/944/1/012114
Solovev SA, Khusainov RR, Nasretdinova DR (2019) Numerical investigation of the granule size effect on the reaction product yield in a catalyst fixed bed. IOP Conf Ser: Mater Sci Eng 618(1):012096. https://doi.org/10.1088/1757-899x/618/1/012096
Das S, Deen NG, Kuipers JAM (2018) Multiscale modeling of fixed-bed reactors with porous (open-cell foam) non-spherical particles: hydrodynamics. Chem Eng J 334:741–759. https://doi.org/10.1016/j.cej.2017.10.047
Lu TJ, Stone HA, Ashby MF (1998) Heat transfer in open-cell metal foams. Acta Mater 46(10):3619–3635. https://doi.org/10.1016/S1359-6454(98)00031-7
Richardson JT, Peng Y, Remue D (2000) Properties of ceramic foam catalyst supports: pressure drop. Appl Cat A: Gen 204(1):19–32. https://doi.org/10.1016/S0926-860X(00)00508-1
Giani L, Groppi G, Tronconi E (2005) Mass-transfer characterization of metallic foams as supports for structured catalysts. Ind Eng Chem Res 44(14):4993–5002. https://doi.org/10.1021/ie0490886
Haack DP, Butcher KR, Kim T, Lu TJ (2001) Novel lightweight metal foam heat exchangers. ASME Congr Proc 1–7
Banhart J (2001) Manufacture, characterisation and application of cellular metals and metal foams. Progr Mater Sci 46(6):559–632. https://doi.org/10.1016/S0079-6425(00)00002-5
Coquard R, Loretz M, Baillis D (2008) Conductive heat transfer in metallic/ceramic open-cell foams. Adv Eng Mater 10(4):323–337. https://doi.org/10.1002/adem.200700331
Ghosh I (2009) Heat transfer correlation for high-porosity open-cell foam. Int J Heat Mass Transf 52(5–6):1488–1494. https://doi.org/10.1016/j.ijheatmasstransfer.2008.07.047
Patcas FC, Garrido GI, Kraushaar-Czarnetzki B (2007) CO oxidation over structured carriers: a comparison of ceramic foams, honeycombs and beads. Chem Eng Sci 62(15):3984–3990. https://doi.org/10.1016/j.ces.2007.04.039
Kolaczkowski ST, Awdry S, Smith T, Thomas D, Torkuhl L, Kolvenbach R (2016) Potential for metal foams to act as structured catalyst supports in fixed-bed reactors. Cat Today 273:221–233. https://doi.org/10.1016/j.cattod.2016.03.047
Khusainov RR, Solovev SA, Soloveva OV, Ilyasov IR (2020) Numerical simulation and experimental study of the acetylene hydrogenation reaction. IOP Conf Ser: Mater Sci Eng 734(1):012205. https://doi.org/10.1088/1757-899X/734/1/012205
Calis HPA, Nijenhuis J, Paikert BC, Dautzenberg FM, van den Bleek CM (2001) CFD modelling and experimental validation of pressure drop and flow profile in a novel structured catalytic reactor packing. Chem Eng Sci 56:1713–1720. https://doi.org/10.1016/s0009-2509(00)00400-0
Dixon AG, Nijemeisland M (2001) CFD as a design tool for fixed-bed reactors. Ind Eng Chem Res 40:5246–5254 (2001). https://doi.org/10.1021/ie001035a
Romkes SJP, Dautzenberg FM, van den Bleek CM, Calis HPA (2003) CFD modelling and experimental validation of particle-to-fluid mass and heat transfer in a packed bed at very low channel to particle diameter ratio. Chem Eng J 96:3–13. https://doi.org/10.1016/j.cej.2003.08.026
Guardo A, Coussirat M, Recasens F, Larrayoz MA, Escaler X (2006) CFD study on particle-to-fluid heat transfer in fixed bed reactors: convective heat transfer at low and high pressure. Chem Eng Sci 61:4341–4353. https://doi.org/10.1016/j.ces.2006.02.011
Eppinger T, Seidler K, Kraume M (2011) DEM–CFD simulations of fixed bed reactors with small tube to particle diameter ratios. Chem Eng J 166:324–331. https://doi.org/10.1016/j.cej.2010.10.053
Kuroki M, Ookawara S, Ogawa KA (2009) High-fidelity CFD model of methane steam reforming in a packed bed reactor. J Chem Eng Jpn 42(1):73–78. https://doi.org/10.1252/jcej.08we256
Dixon AG, Nijemeisland M, Stitt EH (2013) Systematic mesh development for 3D CFD simulation of fixed beds: contact points study. Com Chem Eng 48:135–153. https://doi.org/10.1016/j.compchemeng.2012.08.011
Dixon AG (2017) Local transport and reaction rates in a fixed bed reactor tube: endothermic steam methane reforming. Chem Eng Sci 168:156–177. https://doi.org/10.1016/j.ces.2017.04.039
Acknowledgements
The reported research was funded by Russian Foundation for Basic Research, grant №. 19-07-01188.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this paper
Cite this paper
Solovev, S., Soloveva, O. (2022). Numerical Simulation of the Operation of a Chemical Reactor with an Open Cell Foam Catalyst. In: Beskopylny, A., Shamtsyan, M. (eds) XIV International Scientific Conference “INTERAGROMASH 2021”. Lecture Notes in Networks and Systems, vol 247. Springer, Cham. https://doi.org/10.1007/978-3-030-80946-1_3
Download citation
DOI: https://doi.org/10.1007/978-3-030-80946-1_3
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-80945-4
Online ISBN: 978-3-030-80946-1
eBook Packages: EngineeringEngineering (R0)