Abstract
Despite incredible innovation in the current medical world, cancer remains a lethal disease struggling to cure and becomes a prime reason for deaths worldwide. In recent years, surgery, chemotherapy, and radiation therapy are the major treatment modalities targeted to eradicate the various types of cancers. Conventional cancer treatments effectively destroy cancer cells, but they are also harmful to the normal healthy cells and tissues. A variety of nanomaterials recently appear as promising tools for cancer treatment due to the unique mechanism of passive and active tumor-targeted drug delivery. Various types of nanomaterials-based drug delivery platforms such as organic and inorganic nanomaterials have been significantly investigated for cancer treatment because they can load and carry anticancer drugs and accumulate drugs in the tumor site via passive or active targeting, thereby specifically carrying chemotherapeutic drugs to the preferred tumor sites. These functionalized nanomaterials will prominently enhance the chemotherapeutic drugs’ therapeutic efficacy while decreasing nonspecific adverse effects in cancer treatment. Additionally, remarkable efforts have been recently committed to developing targeted and controlled drug delivery systems from a variety of nanomaterials for cancer treatment, which can deliver drugs with a controlled manner to target tumor site and concurrently monitor therapeutic response by visualizing cancer cells. This chapter aims to emphasize the distinguished advantage of nanomaterials-based drug delivery systems and the mechanism of action underlying their selective targeted drug delivery effects and to introduce successful recent nanomaterials and their drug delivery systems for cancer treatment.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
3.1 Introduction
Cancer is the second most severe lethal disease in the current world and spreading further with continuance and growing incidence in the twenty-first century. According to the estimates from the GLOBOCAN cancer statistics 2018 (International Agency for Research on Cancer, WHO), there are 9.6 million cancer cases deaths in 2018. More than 18.1 million cancer cases are diagnosed, and this rate has been estimated to rise to 29.5 million by the year 2040 (Faisca Phillips 2019; Bray et al. 2018). The condition is so alarming that every fourth person is having a lifetime cancer risk. Is cancer treatable? The short reply to this question is “yes.” Cancer mortality rates can be decreased if cancer cases are detected and treated early with better treatment strategies (Siegel et al. 2019; Wild 2019). Cancer begins from transforming healthy normal tissues into tumor tissues in a multistage development that usually progresses from a precancerous to a malignant tumor. Many types of cancers affect the people, and the cancer cells show no symptoms at an initial stage of development (Papaccio et al. 2017; Kulikov et al. 2017). Cancer cells proliferate and continue to increase unless one of three things occur: (i) The tumor tissues are removed surgically, (ii) using radiation therapy, or (iii) using chemotherapy.
There are different methods of cancer treatment. Current cancer treatment options can be surgical intervention, radiation therapy, chemotherapy, immunotherapy and hormone therapy, or a combination of these options (Miller et al. 2019; Chowdhury et al. 2016). The types of cancer treatment that patients receive depend on the type of cancer patients have and what stage advanced it is. The treatment of cancer by surgery works best for small size solid tumors that are localized in one area (Tyson II et al. 2018; Derks et al. 2017). The surgery to remove the entire tumorous mass should not harm the surrounding normal healthy cells or tissues. Nonsurgical cancer treatment commonly followed is radiation therapy or chemotherapy medication. Radiation therapy practices with high ionizing radiation dose to eradicate cancer cells and slow tumor growth by damaging the DNA (Liu et al. 2016a; Baskar and Itahana 2017). The radiation therapy is commonly used in combination with the surgery to reduce the tumor size, so the tumor can be easily removed by surgical treatment (Bishop et al. 2018a, b). The body can safely receive a limited amount of radiation over the course of the treatment. The radiation dose to be delivered to the cancer site depends upon various factors such as the cancer type, tumor size and location in the body, age of the person, general health and medical history, and possible side effects on the nearby normal tissues (Ghahremani et al. 2018; Cabrera et al. 2016). Immunotherapy is a biological cancer therapy that supports the immune system battle against cancer, and it is not yet as extensively used as surgery, radiation therapy, and chemotherapy (Zaidi and Jaffee 2019; Ishihara et al. 2017). Hormone therapy uses hormones to stop the growth of cancers (Axelrad et al. 2020; Eeles et al. 2016).
Chemotherapy or combined chemotherapy , a very common cancer treatment, uses anticancer drugs to kill or destroy the uncontrolled proliferation of cancerous cells. Conventional chemotherapy works principally by interfering with the synthesis of DNA and mitosis, leading to the death of rapidly proliferating and dividing cancer cells (Senapati et al. 2018; Wang et al. 2016). Unfortunately, due to nonspecific drug targeting by anticancer medicines, conventional chemotherapy fails to target the tumor specifically without interacting with the normal healthy cells (Kumari et al. 2016; Wakaskar 2017; Raza et al. 2019).
This chapter aims to present the limitations of conventional cancer treatment and principal concepts of nanomaterials for cancer treatment, to emphasize the distinguished advantage of nanomaterials-based drug delivery systems (nano DDS) and the mechanism of action underlying their selective targeted drug delivery effects, and to introduce successful recent nano drug delivery system for cancer treatment and diagnosis.
3.2 Limitations of Conventional Cancer Treatment
The conventional cancer treatments effectively destroy the cancer cells, but they are also harmful to the normal healthy cells and tissues (Johnson et al. 2018; Kalyanaraman 2017). Cancer cells cannot be entirely removed by the surgery, and even the existence of a single cancer cell that is unseen can redevelop into a new tumor and metastasize to other parts of the body. The cancer treatment by the surgical procedure is not used for hematological cancers or cancers that have metastasized to other tissues or parts of the body. The radiation therapy administered both internally or externally can also destroy the normal healthy cells and induce the side effects due to the ionizing radiation. The radiation therapy is not used if the tumor is located at extremely vulnerable locations or if the cancer is at the advanced stages. Immunotherapy and hormone therapy cause side effects in the body, and hormone therapy blocks the ability to produce hormones in the body system.
Chemotherapy is considered as an effective type of cancer treatment for all types of cancers, but it damages either normal healthy tissues or cells that divide rapidly, such as cells in the macrophages, digestive tract, bone marrow, and hair follicles. The notable drawback of conventional chemotherapy is that it cannot provide specific target action only to the cancer cells. The nonspecific delivery of chemotherapeutic drugs causes severe side effects such as mucositis, myelosuppression, organ dysfunction, alopecia, and thrombocytopenia, and these side effects impose treatment delay, dose reduction, and therapy discontinuation. Furthermore, most of the available chemotherapeutic drugs often cannot penetrate the outer membranes of solid tumors and reach the inside core of solid tumors, failing to destroy the cancer cells. Also, the repeated administration of nonselective chemotherapeutic drugs can influence drug resistance.
Chemotherapeutic drugs are often eliminated from the plasma circulation engulfed by macrophages and P-glycoprotein, acting as the efflux pump, which is overexpressed on the cancer cells surface and prevents the accumulation of drugs inside the tumor. Thus, chemotherapeutic drugs stay in the plasma circulation for a very short and limited time and cannot interact with the cancer cells resulting in the chemotherapy entirely unsuccessful. The low drug solubility, large particle size, low specificity, and high toxicity of chemotherapeutic drugs are also important issues in conventional chemotherapy, making them unable to improve the bioavailability and reach the chemotherapeutic drugs at the tumor sites.
To circumvent the pitfalls as mentioned above and the limitations of conventional cancer treatments, chemotherapeutic drugs need to reformulate with various types of nanomaterials and drug delivery systems.
3.3 Nanomaterials as Drug Delivery System for Cancer Treatment
Since innovative researches and understanding of biological mechanisms of cancer tissues are emerging regularly, novel cancer treatment procedures are being developed to have improved effectiveness of the treatment, thereby enabling the patient’s survivability and improving their quality of life. With the recent technological advances in medical sciences, different types of cancer treatment have been practiced in the past, and many new therapies, such as targeted therapy, are currently being practiced. There have been significant successes in the nanotechnology medical applications (nanomedicine) in recent years, particularly in the drug delivery system (Wolfram and Ferrari 2019; Salvioni et al. 2019; van der Meel et al. 2019; Tran et al. 2017; Prasad et al. 2017).
Treating cancer cells using a nanoparticulate drug delivery system (nano DDS) approach plays a pivotal role in circumventing the limitations of conventional cancer treatment methods by providing simultaneous diagnosis and treatment. The application of nano DDS to cancer treatment could extend beyond the drug delivery system into the making of new therapeutics capable of killing the cancer cells with negligible damage to normal healthy cells and tissues. Various types of organic and inorganic nanomaterials are used to formulate chemotherapeutic drug-loaded nano DDS for cancer diagnosis and treatment. Most of the organic nanomaterials (liposomes, solid lipid nanoparticles, dendrimers, polymeric micelles, polymeric (natural or synthetic) nanoparticles, and polymer-drug conjugates) and inorganic nanomaterials (mesoporous silica nanoparticles, gold nanoparticles, magnetic nanoparticles, carbon nanotubes, and quantum dots) were developed as a vehicle in nano DDS for cancer treatment.
3.4 Unique Advantages of Nano DDS
3.4.1 Particle Size (Kumar et al. 2017; Arms et al. 2018; Ghasemiyeh and Mohammadi-Samani 2018; Tiruwa 2016; Ghasemiyeh and Mohammadi-Samani 2020; Sarcan et al. 2018)
Particle size distribution and small size with high surface area characteristics of nanoparticles are the most important key factors for drug delivery applications. The great advantage of nano DDS is that the particle size and size distributions are tunable. Several types of research have reported that nanoparticulate systems have plenty of advantages over other microparticulate systems. Nanoparticles can improve drug loading, stability, controlled drug release, high cellular uptake, in vivo pharmacokinetics, plasma circulation half-life, biodistribution, targeted drug delivery, tumor accumulation, and ability to cross the blood-brain barrier and transport the drugs to the brain due to their smaller size and flexibility (Prasad et al. 2019). Nanoparticles can also be coated with different types of polymers or surface-functionalized with targeting moieties, peptides, and nucleic acids that bind to specific cancer target sites. The nanoparticles used in a nano DDS should be small size enough to escape or avoid capture by macrophages in the circulation system. Systemically administered nano DDS should have a particle size ranging from 10 to 200 nm, particle size less than 200 nm to avoid sequestration by the liver and spleen, and particle size larger than 10 nm to avoid first-pass metabolism or elimination through the kidneys, benefiting accumulation/clearance and biodistribution behavior. The particle size of nano DDS has been shown to influence the surface functionalization and targeted drug delivery applications for cancer treatment.
3.4.2 High Drug Payload (Ghasemiyeh and Mohammadi-Samani 2018; Meunier et al. 2017; Liu et al. 2020; Qu et al. 2016; Huang et al. 2016)
An effective nanoparticulate system should load and hold a higher amount of drugs, thereby decreasing the frequent dose of uptake and increasing drug plasma concentration after administration in the body. Drug loading in the nano DDS can be done by adsorption/absorption and incorporation techniques. A high drug loading capacity and encapsulation efficiency mainly depend on the classification of drugs (e.g., biopharmaceutical classification systems (BCS) Class I–IV) and drug solubility in the nano DDS, which is related to the drug-polymer interactions, compositions of excipients, and the presence of active functional groups from drug and excipients. For instance, the solid lipid core of solid lipid nanoparticles can accommodate a higher amount of hydrophobic chemotherapeutic drugs, and liposomes can load and hold both hydrophobic and hydrophilic chemotherapeutic drugs due to their unique characteristics.
3.4.3 Controlled Drug Release (Li et al. 2016a; Kamaly et al. 2016; Deodhar et al. 2017; Liu et al. 2019a; Paris et al. 2018)
It is crucial to take consideration of both polymer biodegradation and drug release kinetics in simulated body conditions when formulating a nano DDS. The drug release behavior from nano DDS mainly depends on (i) solubility of active pharmaceutical ingredient, (ii) nano DDS degradation or erosion, (iii) desorption from the surface-attached drug or incorporated drug from the inside polymer core, (iv) drug diffusion through the nano DDS, and (v) the combination of diffusion and erosion processes. For example, the drug release of uniformly drug distributed nanospheres occurs by diffusion or matric erosion. If the active drug diffusion is more rapid than matrix erosion, then the drug release mechanism is mostly maintained by diffusion. The burst drug release from nanoparticles at the early stage is primarily attributed to surface-attached drug molecules to the large surface of nano DDS. It is indicated that the method of drug loading has a pivotal role in the drug release profile from nanoparticles. If the active pharmaceutical ingredient is entrapped in the nano DDS by the incorporation technique, then the nano DDS has a negligible amount of burst drug release and controlled drug release profile. If the nano DDS is surface-modified or coated by other synthetic or natural polymers, the drug release profile is then controlled by drug diffusion from the surface polymeric membrane.
3.4.4 Surface Modification (Ahmad et al. 2018a; Choi and Meghani 2016; Ahmad et al. 2018b; Ganesan et al. 2018; Ramalingam and Ko 2016; Ramalingam and Ko 2015; Ramalingam et al. 2016)
Surface modification or coating on the nano DDS can improve drug biodistribution, pharmacokinetics, and oral and brain drug delivery. To enhance drug targeting, it is crucial to prolong the nanoparticle circulation and minimize the opsonization in vivo, and it can be accomplished by coating or surface modification of nano DDS with biodegradable hydrophilic polymers, e.g., natural polymers such as chitosan and their derivatives, PEG, polysorbate 80, poloxamer, and polyethylene oxide. Several researches publish that PEG surface modification on nano DDS avoids opsonization and reduces phagocytosis.
3.5 Physiology of Tumor and Tumor Targeting Using Nano DDS
3.5.1 Angiogenesis and Tumor Vasculatures
A well understanding and knowledge of the angiogenesis and tumor vasculature characteristics have facilitated effective cancer treatment against various types of cancers. To develop the nano DDS, it is essential to find the biomarkers of the tumor microenvironment and the important differences in normal healthy cells (Liu et al. 2021). The process of angiogenesis in tumor sites promotes new blood vessels with discontinuous epithelium from preexisting vascular systems. The irregular blood vessels present in tumor regions have unusual morphological and physiological conditions dissimilar from normal vasculatures. The discontinuities between epithelial cells or vascular gap openings of tumors are remarkably 10 and 100 times larger in tumor models than in normal tissues. Lack of lymphatic drainage with leakiness favors the passive accumulation of long-circulating macromolecules and into the tumor (Li et al. 2016b; Park et al. 2016; Yang and Gao 2017; Wong et al. 2016). These findings suggest that the nano DDS of certain sizes can penetrate leaky tumor vasculatures and selectively carry the chemotherapeutic drugs to the tumor regions.
3.5.2 Mechanisms of Tumor Targeting by Nano DDS
Tumor-targeted drug delivery can be attained by inherent passive targeting and adopted active targeting strategies. Active drug targeting of chemotherapeutic drugs can be accomplished by conjugating the targeting moiety on the nano DDS. Passive drug targeting is achieved by loading chemotherapeutic drugs into a nano DDS that passively reaches the cancer target site or tissue through the EPR effect. For example, several studies reported that liposomes surface-modified with targeting moiety influenced the drug targeting and it can work as a drug reservoir exhibiting controlled drug release profile and drug accumulation at the tumor site (Kanamala et al. 2016; Masood 2016; Anarjan 2019; Derakhshandeh and Azandaryani 2016; Dai et al. 2016).
3.5.2.1 Passive Tumor Targeting
The EPR effect-mediated chemotherapeutic drug deliveries of nano DDS have been considered one of the strategies to accumulate the drug at the tumor sites. Compared to blood vessels in normal tissues, angiogenic blood vessels at the tumor sites have bigger size openings between nearby vascular endothelial cells. This can help the nano DDS to accumulate at the tumor tissues and then release a higher concentration of the drugs specifically into the tumor cells, thus permitting effective cancer treatment with least systemic side effects. Various studies have demonstrated that EPR plays a pivotal part in passive drug targeting. The EPR effect mainly depends on many factors, such as the nano DDS surface properties, tumor types, and immunogenicity. Passive drug targeting is due to the faulty leaky tumor vasculature with irregular epithelium, reduced level of lymphatic drainage, and lowered uptake of the interstitial fluid, supporting passive targeting of nano DDS in tumors (Kumari et al. 2016; Wakaskar 2017; Masood 2016; Mahato 2017).
3.5.2.2 Active Tumor Targeting
Passive tumor targeting can help the localization of nano DDS at the tumor sites, but it is not able to encourage cellular uptake by tumor cells. This can be accomplished by active tumor targeting. Compared to passive tumor targeting, active tumor targeting strategy relies on a biological communication between targeting ligand on the surface of nano DDS and the receptor on the target tumor cell surface. Active tumor targeting strategy can easily differentiate the normal healthy cells and tumor cells. A large number of targeting ligands and targets have been identified and evaluated for facilitating active drug targeting of nano DDS for various types of cancers (Table 3.1). Such ligands on the surface of nano DDS often actively attach to specific receptors on the tumor cell surface, increasing the drug-containing nano DDS internalization by receptor-mediated endocytosis, improving the therapeutic efficacy, controlling the delivery of chemotherapeutic drugs to healthy tissues, and also decreasing the systemic adverse effects. Hence, active tumor targeting has displayed promising outcomes in circumventing different pitfalls, such as multidrug resistance in tumors and bypassing the blood-brain barrier (Anarjan 2019; He et al. 2020; Lin et al. 2016; Nag and Delehanty 2019).
3.6 Nano DDS for Cancer Treatment
3.6.1 Organic Nanomaterials for Cancer Treatment
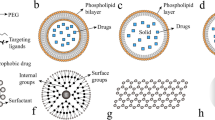
Most of the organic nanomaterials (liposomes, solid lipid nanoparticles, polymeric micelles, dendrimers, polymeric nanoparticles, and polymer-drug conjugates) are used as a carrier and targeting system for cancer treatment (Fig. 3.1).
3.6.1.1 Liposomes
Liposomes are described as phospholipid vesicles comprising of one or more concentric bilayer vesicles surrounding the discrete aqueous phase. Because liposome composition is identical to that of cellular membranes, liposomes are safer and biocompatible than other synthetic polymers. Because of the unique structure of liposomes, both hydrophobic and hydrophilic drugs can be incorporated in liposomes. Liposomes can load and hold hydrophobic drugs in the lipid bilayers and hydrophilic drugs in the aqueous core. Liposomes have several advantages than other drug delivery systems, and it is administrated as a potential nanocarrier for drug delivery of chemotherapeutic drugs (Mishra et al. 2018; Ahmed et al. 2019). Currently, there are many liposomal products in the market (Table 3.2) and clinical development (Table 3.3) for cancer treatment.
The types of phospholipids, targeting ligand, PEGylation, and stimuli-sensitive materials determined the charge of the surface of the liposomes . In addition, liposomes with surface modification protect the incorporated drug from degradation, increase the targeting, improve the pharmacokinetic and pharmacodynamics properties, and reduce the toxic side effect of the chemotherapeutic drugs (Patel 2020; Mohamed et al. 2019). PEG conjugation has been identified as a unique strategy for the evasion of RES uptake. The targeting ligands, peptides, and nucleic acid-functionalized liposomes can specifically deliver the chemotherapeutic drugs to the tumor sites. The use of liposome targeted delivery systems in combination therapies of chemotherapy and phototherapy to transport anticancer drugs and photosensitizer can reduce the side effects, significantly enhance the drug accumulation at the target site, and improve the effectiveness of chemotherapy and photodynamic therapy (Cao et al. 2018). Different types of liposomes for targeted anticancer drug delivery are summarized in Table 3.4.
3.6.1.2 Solid Lipid Nanoparticles
Solid lipid nanoparticles (SLNs) are made from biological and safe grade lipids, and it is biocompatible and less toxic compared to polymeric or inorganic nanomaterials. SLNs promote the high drug upload of multiple hydrophobic and hydrophilic drugs. SLNs are a versatile drug delivery system that has been applied to enhance the therapeutic effect of chemotherapeutic drugs. Targeted delivery of chemotherapeutic drugs from SLNs reduces the systemic side effects and improves the therapeutic action. SLNs can enhance the chemotherapeutic drug delivery applications for cancer treatment by tumor targeting mechanisms of actions such as passive, active, and codelivery mechanisms (Ganesan et al. 2018; Ramalingam and Ko 2016; Lingayat et al. 2017; Patel et al. 2018). Several studies have reported that SLNs are used as a targeted drug delivery vehicle for different types of tumors. The outcomes of SLNs as carriers of chemotherapeutic drugs are summarized in Table 3.5.
3.6.1.3 Polymeric Micelles
Polymeric micelles composed of amphiphilic block copolymers with a hydrophilic corona and hydrophobic core are colloidal nanoparticulate drug delivery systems for chemotherapeutic drugs. Polymeric micelles form a self-assembled structure spontaneously in an aqueous environment. The hydrophobic core of the polymeric micelles possesses a high drug loading of water insoluble chemotherapeutic drugs, and hydrophilic corona provides steric stability to avoid rapid uptake by the RES, resulting in extended drug circulation in the body. In addition to passive drug targeting, polymeric micelles can be surface-modified with targeting ligands for active tumor targeting to enhance the selectivity for cancer cells and improve intracellular delivery of anticancer drugs by receptor-mediated endocytosis while reducing systemic toxicity and severe side effects compared to systemic chemotherapy (Marzbali and Khosroushahi 2017; Gothwal et al. 2016; Biswas et al. 2016). Currently, many chemotherapeutic drug-loaded polymeric micelles are evaluated for effective cancer treatment (Table 3.6).
3.6.1.4 Dendrimers
Dendrimers are highly branched globular macromolecules with their 3D nonpolymeric architectures: a central core, a corona with functional groups, and a hyperbranched mantle. Dendrimers’ unique properties like polyvalency, well-defined molecular weight, nanosize, the high degree of branching, water solubility, and simple synthesis procedure make them promising drug carrier systems for anticancer drugs. The dendrimers’ biological effect is initiated by terminal moieties, and the dendrimers seem to be excellent candidates for carriers of anticancer drugs. A variety of dendrimers, including PAMAM, PEG, PPI, and PLL, have been successfully developed for drug delivery applications, and the PAMAM is most widely employed for targeted cancer therapy. Surface modification or conjugation of dendrimers with PEG and other ligands can help reduce the cytotoxicity of dendrimers and enhance plasma circulation time and accumulation of tumor through the EPR effect (Kaur et al. 2016; Augustus et al. 2017; Munir et al. 2016; Parajapati et al. 2016; Abedi-Gaballu et al. 2018; Sherje et al. 2018). Numerous researches that have been conducted to study the application of dendrimers in cancer treatment are presented in Table 3.7.
3.6.1.5 Polymeric Nanoparticles
The polymeric nanoparticulate system from natural and synthetic biodegradable polymers has earned more attention due to their biodegradability, biocompatibility, tailorability and stability, ease of coating or surface modification, and low cost. Polymeric nanoparticles, in general, can be used to improve solubility, controlled release, and bioavailability for systemic delivery of anticancer drugs. Drug-loaded polymeric nanoparticles can be developed to actively or passively accumulate in sites of the tumor by controlling their particle size or surface functionalizing with targeting moieties. Polymers like hyaluronic acid and pullulan are used to activate nanoparticles for active targeted drug delivery. These polymers degrade in physiological body conditions, and by-products of the polymers are not harmful to the body. Various natural and synthetic polymers-based nanoparticles were developed and reported for cancer treatment and diagnosis (Masood 2016; Prasad et al. 2017; Conte et al. 2016; Wong et al. 2020; Espinosa-Cano et al. 2018; Taghipour-Sabzevar et al. 2019). Natural and synthetic polymers-based nano DDS for cancer treatment are summarized in Tables 3.8 and 3.9.
3.6.1.6 Polymer-Drug Conjugates
Polymer-drug conjugates (PDCs) can be prepared as nano DDS by covalently conjugating one or more drugs to a polymer backbone before the synthesis of nanoparticles. PDCs are identified as the most examined type of nano DDS, and currently, many PDs in clinical trials and several polymer-drug conjugates are successfully transformed into clinical practice. For example, N-(2-hydroxypropyl) methacrylamide-DOX was the first chemotherapeutic PDC to reach clinical trial studies about 22 years ago. The conjugation of therapeutic drugs to polymers provides many benefits, including improved drug solubilization, stability, controlled drug delivery, enhanced efficacy and improved pharmacokinetics, biodistribution, as well as reduced toxicity and immunogenicity. The main advantage of using PDCs is that the physical and chemical characteristics of polymers can be modified to reduce the toxicity and improve the therapeutic efficacy of the loaded chemotherapeutics . In addition, PDCs have displayed increased accumulation of tumors, improved therapeutic index, prolonged circulation, controlled release of the anticancer drugs, and active tumor uptake by active targeting (Ekladious et al. 2019; Thanou and Duncan 2003; Vicent and Duncan 2006; Li and Wallace 2008) (Table 3.10).
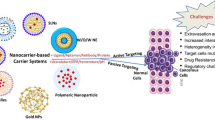
3.6.2 Inorganic Nanomaterials for Cancer Treatment
Inorganic nanomaterials have been intensively studied for cancer therapy and diagnostic imaging due to their great advantages, such as high drug loading, large surface area, improved bioavailability, reduced toxic side effects and controlled release of anticancer drugs, and their tolerance to most organic solvents. Mesoporous silica nanoparticles , gold nanoparticles, magnetic nanoparticles, carbon nanotubes, and quantum dots are commonly used in cancer treatment and diagnosis in various ways (Fig. 3.2) (Khafaji et al. 2019; Veeranarayanan and Maekawa 2019; Liu et al. 2017b).
3.6.2.1 Mesoporous Silica Nanoparticles (Senapati et al. 2018; Ahmadi Nasab et al. 2018; Moreira et al. 2016; de Oliveira Freitas et al. 2017; Yang and Yu 2016; Saini and Bandyopadhyaya 2019)
Silica nanoparticles are extensively used nanoparticle systems in cancer treatment due to its various benefits such as easy synthesis, well-controlled diameter, adjustable pore volume, and potential surface modification. There are two types of silica nanoparticles (core or shell silica nanoparticles and mesoporous silica nanoparticles (MSNs)) established for cancer treatment. Of the two types, MSNs are mostly used as a nano DDS in cancer treatment. One study demonstrated that gemcitabine-loaded MSNs are used to treat pancreatic cancer. One research group developed the rod-shaped magnetic MSNs for suicide gene therapy. The shapes of the MSNs also play a vital role in drug delivery applications. Compared to spherical MSNs, rod shape-like MSNs displayed higher drug loading, better drug release, and gene delivery.
The research carried out by Lee et al. showed how MSNs decorated with doxorubicin-loaded multiple magnetite nanocrystals promoted effective cell death in a melanoma model, confirming passive targeting and nanoparticle accumulation in the tumor site. Huan et al. used MSNs modified with polyethyleneimine/PEG to deliver doxorubicin jointly with P-glycoprotein siRNA. This research explained that nanoparticles were efficiently biodistributed, resulting in 8% of the EPR effect at the tumor site. MSNs can also be surface-functionalized with various types of ligand molecules such as aptamers, growth factors, peptides, and vitamins to actively target tumors via receptor-mediated endocytosis. In the study carried out by Kayuan et al., DOX-loaded HB5 aptamer-functionalized MSNs were used for combined chemo-photothermal therapies. This study verified that combination therapies promote cancer cell killing compared to chemo-photothermal therapy alone. MSNs achieve a satisfactory level of active targeting and reduce toxic side effects in the healthy normal cells.
3.6.2.2 Gold Nanoparticles (Sztandera et al. 2018; Peng and Liang 2019; Kumar et al. 2012; Singh et al. 2018)
Gold nanoparticles (GNPs) have been investigated for its potential application in cancer treatment, diagnostics, and targeted drug delivery. Current researches confirm numerous advantages of GNPs for cancer treatment, primarily due to enabling the control of preparation of GNPs with multiple sizes and shapes and the possibility of surface functionalization on GNPs with various functional and targeting agents. Many features of GNPs are related to their shape and size. The size of spherical GNPs influenced plasma concentration, circulation time, and cellular uptake. It was also reported that the smaller particles of GNPs permeated into the blood-brain barrier, deep layers of skin, and placental barrier. Surface functionalization of GNPs provides significant effects on plasma half-life, protection against aggregation, biocompatibility, preventing the removal by the MPS and RES, targeted transport and drug accumulation at the desired site. For the GNP-based drug delivery system, passive targeting, active targeting, or a combination of both strategies can improve tumor accumulation. A remarkable approach confirming the intracellular delivery of chemotherapeutic drugs involves their conjugation to the surface of GNPs through thiol functional groups. The examples of chemotherapeutic drugs conjugated with GNPs are listed in Table 3.11.
Due to their exceptional properties of absorption and scattering of electromagnetic radiation, GNPs are of specific interest for the PTT in cancer treatment. This PTT treatment procedure involves the utilization of electromagnetic radiation or laser radiation to generate local heating and hyperthermia for the thermal destruction of cancerous cells. The PTT efficacy may be additionally improved by the application of photothermal compounds such as transition metal oxide/sulfide nanomaterials and nanocarbons, enabling an improved transformation of light into heat.
3.6.2.3 Magnetic Nanoparticles (Zhang et al. 2018d; Kolosnjaj-Tabi and Wilhelm 2017; Fathi Karkan et al. 2017; Fathi et al. 2020; Lungu et al. 2016)
Magnetic nanoparticles (MNPs) have been discovered as a potential carrier system to modify the pharmacokinetics of loaded drugs, decrease the cytotoxicity, improve the controlled release, and increase the half-life. Due to the unique properties of higher magnetic moments and surface to volume ratios, it can be used for hyperthermia therapy of cancer treatment and targeted delivery. MNPs are in magnetic resonance imaging to enhance the image contrast of targeted tumor tissues. MNPs can be functionalized with high affinity ligands such as peptides and antibodies to enhance the selectivity further and localize MNPs at the tumor sites. Recently, the MNP application in biosensors has been extensively studied for rapid cancer diagnosis and prevention of cancer metastasis. Various types of MNPs employed in cancer treatment and diagnosis are summarized in Table 3.12.
3.6.2.4 Carbon Nanotubes (Chen et al. 2017; Son et al. 2016; Pardo et al. 2018)
Carbon nanotubes (CNTs) are very popular systems for cancer treatment and diagnosis due to their many unique properties such as structure and high specific surface area to volume. CNTs are classified into single-walled carbon nanotubes and multi-walled carbon nanotubes based on the number of graphene sheets used for the preparation. CNTs have been investigated in all the cancer treatment modalities, including thermal, photodynamic, and gene therapy, drug delivery, lymphatic targeted chemotherapy, and diagnostic techniques. Recently developed single-walled carbon nanotube-based drug delivery systems for cancer treatment are summarized in Table 3.13. CNTs may help the attached chemotherapeutic drugs to penetrate through the target cell to treat cancer.
The CNTs are used as a photosensitizer for photodynamic therapy. CNTs are used as a contrast medium for diagnostic imaging techniques, and it can be used in ultrasonography, photoacoustic imaging, PET, and MRM for cancer diagnostic applications.
3.6.2.5 Quantum Dots (Zhao et al. 2016; Fang et al. 2017; Lee et al. 2017)
Quantum dots (QDs) are nanosized crystals comprised of a semiconductor core within a shell composed of second semiconductor material. QDs have outstanding optical properties, such as high brightness, tunable emission spectra, and resistance to photo-bleaching. Quantum dots have been used in targeting and localizing tumors and sentinel lymph node mapping in vivo. New imaging techniques like quantum dots resolve the limitations of sensitivity and specificity from current imaging techniques like X-ray, ultrasound, radionuclide imaging, computed tomography, and MRI. Recent studies in surface functionalization of QDs improve their potential application in imaging of cancer. Bioconjugation of QDs with peptides and antibodies can be used for tumor-targeted drug delivery, nanodiagnostics, imaging, and photodynamic therapy. The application of quantum dot conjugates is listed in Table 3.14.
3.7 Challenges and Future Perspectives
Despite numerous advanced technologies in the production of safe biopolymers and nanomaterials, there remain controversies regarding the safety of nanoformulations. Although the benefits of some biopolymers, dendrimers, and metal-based inorganic nanomaterials are remarkable, toxicity remains a serious problem. It has been proven, for example, that PEI and excessive positive charges of dendrimers destabilize the cell membrane. Thus, advancements in biopolymer synthesis and purification techniques promise to reduce side effects and enhance treatment efficacy. The instability, immune response, potential toxicity, and chronic inflammation challenges for micelles and inorganic nanomaterials need to be focused so that more effective cancer treatment strategies can be developed. Combination therapy with nanomaterials for different types of cancers remains a challenge because of the distinct cancer development mechanisms. For targeted drug therapy, inorganic nanomaterials and micelles can be surface-functionalized with target agents such as magnetic, light, and pH imaging contrast agents; the major limitation of these clinical treatment methods is the poor tissue penetration. All the nanomaterials are not biodegradable so that it can be retained and circulated in the body system for a more extended period after administration. Various research and strategies aimed at overcoming all these challenges will facilitate nanomaterial usage as a drug delivery system and eventually enhance patient survival.
The future perspective of stimuli-responsive nanomaterials can be obtained by various strategies, including enzymatic activation, pH variants, magnetic fields, ultrasound, light, redox potential, and thermal gradients for efficient cancer treatment and diagnosis. Further advancements in the nanomaterials system can improve their application in localizing metastasis, quantitative measurement of molecular targets, and monitoring the efficacy and tracking of drug delivery.
3.8 Conclusion
This chapter has summarized a variety of nanomaterials that are either being used or have the potential to be used as nano drug delivery systems for cancer treatment. Nanomaterials-based cancer treatment has shown significant advantages and new strategies over conventional cancer treatment. Passive or active targeting can significantly remove the systemic side effects of conventional chemotherapies. Targeted drug delivery has made a considerable impact on selective recognizing of the tumor tissues, controlled drug delivery, and overcoming limitations of the conventional chemotherapies. Numerous nanomedicines have been approved by the FDA and indicated satisfactory performance in clinical practice. Although some nanomaterials have not been approved upon their clinical translation, new strategies and promising nanomaterials that are under progress show great assurance, thus providing hope for innovative cancer treatment choices in the near future.
Abbreviations
- BCS:
-
Biopharmaceutical Classification System
- CNTs :
-
Carbon nanotubes
- CS:
-
Chitosan
- DOX:
-
Doxorubicin
- DTX:
-
Docetaxel
- EPR:
-
Enhanced permeation and retention effect
- FA:
-
Folic acid
- FDA:
-
Food and Drug Administration
- GEM:
-
Gemcitabine
- GNPs:
-
Gold nanoparticles
- HA:
-
Hyaluronic acid
- MNPs:
-
Magnetic nanoparticles
- MPS:
-
Mononuclear phagocytic system
- MRI :
-
Magnetic resonance imaging
- MSNs :
-
Mesoporous silica nanoparticles
- Nano DDS:
-
Nano drug delivery system
- PAMAM:
-
Polyamidoamine
- PCL:
-
Poly (ε-caprolactone)
- PDCs:
-
Polymer-drug conjugates
- PET:
-
Positron emission tomography
- PEG :
-
Polyethylene (glycol)
- PLA:
-
Polylactic acid
- PLGA:
-
Poly (D, L-lactide-co-glycolide)
- PLL:
-
Poly-l-lysine
- PPI:
-
Poly (propylamine)
- PTT:
-
Photothermal therapy
- PTX:
-
Paclitaxel
- QDs:
-
Quantum dots
- RES:
-
Reticuloendothelial system
- SLNs :
-
Solid lipid nanoparticles
- TPGS:
-
D-Tocopherol polyethylene glycol1000 succinate
- WHO:
-
World Health Organization
References
Abedi-Gaballu F, Dehghan G, Ghaffari M, Yekta R, Abbaspour-Ravasjani S, Baradaran B et al (2018) PAMAM dendrimers as efficient drug and gene delivery nanosystems for cancer therapy. Appl Mater Today 12:177–190
Agrawal P, Singh RP, Kumari L, Sharma G, Koch B, Rajesh CV et al (2017) TPGS-chitosan cross-linked targeted nanoparticles for effective brain cancer therapy. Mater Sci Eng C 74:167–176
Ahmad N, Alam MA, Ahmad R, Naqvi AA, Ahmad FJ (2018a) Preparation and characterization of surface-modified PLGA-polymeric nanoparticles used to target treatment of intestinal cancer. Artif Cells Nanomed Biotechnol 46(2):432–446
Ahmad N, Ahmad R, Alam MA, Ahmad FJ (2018b) Enhancement of oral bioavailability of doxorubicin through surface modified biodegradable polymeric nanoparticles. Chem Cent J 12(1):65
Ahmadi Nasab N, Hassani Kumleh H, Beygzadeh M, Teimourian S, Kazemzad M (2018) Delivery of curcumin by a pH-responsive chitosan mesoporous silica nanoparticles for cancer treatment. Artif Cells Nanomed Biotechnol 46(1):75–81
Ahmed KS, Hussein SA, Ali AH, Korma SA, Lipeng Q, Jinghua C (2019) Liposome: composition, characterisation, preparation, and recent innovation in clinical applications. J Drug Target 27(7):742–761
Alconcel SN, Baas AS, Maynard HD (2011) FDA-approved poly (ethylene glycol)–protein conjugate drugs. Polym Chem 2(7):1442–1448
Alven S, Nqoro X, Buyana B, Aderibigbe BA (2020) Polymer-drug conjugate, a potential therapeutic to combat breast and lung cancer. Pharmaceutics 12(5):406
Amjad MW, Amin MCIM, Katas H, Butt AM (2012) Doxorubicin-loaded cholic acid-polyethyleneimine micelles for targeted delivery of antitumor drugs: synthesis, characterization, and evaluation of their in vitro cytotoxicity. Nanoscale Res Lett 7(1):687
Anarjan FS (2019) Active targeting drug delivery nanocarriers: ligands. Nano-Struct Nano-Objects 19:100370
Anirudhan TS, Anila MM, Franklin S (2017) Synthesis characterization and biological evaluation of alginate nanoparticle for the targeted delivery of curcumin. Mater Sci Eng C 78:1125–1134
Anselmo AC, Mitragotri S (2016) Nanoparticles in the clinic. Bioeng Transl Med 1(1):10–29
Arms L, Smith DW, Flynn J, Palmer W, Martin A, Woldu A et al (2018) Advantages and limitations of current techniques for analyzing the biodistribution of nanoparticles. Front Pharmacol 9:802
Augustus EN, Allen ET, Nimibofa A, Donbebe W (2017) A review of synthesis, characterization and applications of functionalized dendrimers. Am J Polym Sci 7(1):8–14
Axelrad JE, Bazarbashi A, Zhou J, Castañeda D, Gujral A, Sperling D et al (2020) Hormone therapy for cancer is a risk factor for relapse of inflammatory bowel diseases. Clin Gastroenterol Hepatol 18(4):872–80.e1
Bakhtiary Z, Barar J, Aghanejad A, Saei AA, Nemati E, Ezzati Nazhad Dolatabadi J et al (2017) Microparticles containing erlotinib-loaded solid lipid nanoparticles for treatment of non-small cell lung cancer. Drug Dev Ind Pharm 43(8):1244–1253
Banerjee I, De K, Mukherjee D, Dey G, Chattopadhyay S, Mukherjee M et al (2016) Paclitaxel-loaded solid lipid nanoparticles modified with Tyr-3-octreotide for enhanced anti-angiogenic and anti-glioma therapy. Acta Biomater 38:69–81
Barenholz YC (2012) Doxil®—the first FDA-approved nano-drug: lessons learned. J Control Release 160(2):117–134
Barenholz YC (2016) Doxil®–the first FDA-approved Nano-drug: from basics via CMC, cell culture and animal studies to clinical use. Nanomed Design Deliv Detect 51:315–345
Barkat MA, Beg S, Pottoo FH, Ahmad FJ (2019) Nanopaclitaxel therapy: an evidence based review on the battle for next-generation formulation challenges. Nanomedicine 14(10):1323–1341
Baskar R, Itahana K (2017) Radiation therapy and cancer control in developing countries: can we save more lives? Int J Med Sci 14(1):13
Bhattacharya D, Behera B, Sahu SK, Ananthakrishnan R, Maiti TK, Pramanik P (2016) Design of dual stimuli responsive polymer modified magnetic nanoparticles for targeted anti-cancer drug delivery and enhanced MR imaging. New J Chem 40(1):545–557
Bishop AJ, Zagars GK, Torres KE, Bird JE, Feig BW, Guadagnolo BA (2018a) Malignant peripheral nerve sheath tumors: a single institution’s experience using combined surgery and radiation therapy. Am J Clin Oncol 41(5):465
Bishop AJ, Zagars GK, Demicco EG, Wang W-L, Feig BW, Guadagnolo BA (2018b) Soft tissue solitary fibrous tumor: combined surgery and radiation therapy results in excellent local control. Am J Clin Oncol 41(1):81–85
Biswas S, Kumari P, Lakhani PM, Ghosh B (2016) Recent advances in polymeric micelles for anti-cancer drug delivery. Eur J Pharm Sci 83:184–202
Bobde Y, Biswas S, Ghosh B (2020) PEGylated N-(2 hydroxypropyl) methacrylamide-doxorubicin conjugate as pH-responsive polymeric nanoparticles for cancer therapy. React Funct Polym 151:104561
Bobo D, Robinson KJ, Islam J, Thurecht KJ, Corrie SR (2016) Nanoparticle-based medicines: a review of FDA-approved materials and clinical trials to date. Pharm Res 33(10):2373–2387
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68(6):394–424
Butt AM, Amin MCIM, Katas H (2015) Synergistic effect of pH-responsive folate-functionalized poloxamer 407-TPGS-mixed micelles on targeted delivery of anticancer drugs. Int J Nanomedicine 10:1321
Cabrera AR, Kirkpatrick JP, Fiveash JB, Shih HA, Koay EJ, Lutz S et al (2016) Radiation therapy for glioblastoma: executive summary of an American Society for Radiation Oncology evidence-based clinical practice guideline. Pract Radiat Oncol 6(4):217–225
Cao Y, Dong H, Yang Z, Zhong X, Chen Y, Dai W et al (2017) Aptamer-conjugated graphene quantum dots/porphyrin derivative theranostic agent for intracellular cancer-related microRNA detection and fluorescence-guided photothermal/photodynamic synergetic therapy. ACS Appl Mater Interfaces 9(1):159–166
Cao J, Chen Z, Chi J, Sun Y, Sun Y (2018) Recent progress in synergistic chemotherapy and phototherapy by targeted drug delivery systems for cancer treatment. Artif Cells Nanomed Biotechnol 46(sup1):817–830
Cerqueira BBS, Lasham A, Shelling AN, Al-Kassas R (2017) Development of biodegradable PLGA nanoparticles surface engineered with hyaluronic acid for targeted delivery of paclitaxel to triple negative breast cancer cells. Mater Sci Eng C 76:593–600
Chen Z, Zhang A, Wang X, Zhu J, Fan Y, Yu H et al (2017) The advances of carbon nanotubes in cancer diagnostics and therapeutics. J Nanomater 2017:1–13
Choi J-S, Meghani N (2016) Impact of surface modification in BSA nanoparticles for uptake in cancer cells. Colloids Surf B Biointerfaces 145:653–661
Chou H, Lin H, Liu JM (2015) A tale of the two PEGylated liposomal doxorubicins. Onco Targets Ther 8:1719
Chowdhury S, Yusof F, Salim WWAW, Sulaiman N, Faruck MO (2016) An overview of drug delivery vehicles for cancer treatment: nanocarriers and nanoparticles including photovoltaic nanoparticles. J Photochem Photobiol B Biol 164:151–159
Chu X, Li K, Guo H, Zheng H, Shuda S, Wang X et al (2017) Exploration of graphitic-C3N4 quantum dots for microwave-induced photodynamic therapy. ACS Biomater Sci Eng 3(8):1836–1844
Conte C, Maiolino S, Pellosi DS, Miro A, Ungaro F, Quaglia F (2016) Polymeric nanoparticles for cancer photodynamic therapy. Light-responsive nanostructured systems for applications in nanomedicine, 61–112
Corti A, Fiocchi M, Curnis F (2017) Targeting CD13 with Asn-Gly-Arg (NGR) peptide-drug conjugates. In Next-generation therapies and technologies for immune-mediated inflammatory diseases. Springer, Cham , pp 101–122
Cui Y-N, Xu Q-X, Davoodi P, Wang D-P, Wang C-H (2017) Enhanced intracellular delivery and controlled drug release of magnetic PLGA nanoparticles modified with transferrin. Acta Pharmacol Sin 38(6):943–953
Dabbagh A, Hedayatnasab Z, Karimian H, Sarraf M, Yeong CH, Madaah Hosseini HR et al (2019) Polyethylene glycol-coated porous magnetic nanoparticles for targeted delivery of chemotherapeutics under magnetic hyperthermia condition. Int J Hyperthermia 36(1):104–114
Dai L, Liu J, Luo Z, Li M, Cai K (2016) Tumor therapy: targeted drug delivery systems. J Mater Chem B 4(42):6758–6772
Dai M, Wu C, Fang H-M, Li L, Yan J-B, Zeng D-L et al (2017) Thermo-responsive magnetic liposomes for hyperthermia-triggered local drug delivery. J Microencapsul 34(4):408–415
Dawidczyk CM, Kim C, Park JH, Russell LM, Lee KH, Pomper MG et al (2014) State-of-the-art in design rules for drug delivery platforms: lessons learned from FDA-approved nanomedicines. J Control Release 187:133–144
de Oliveira Freitas LB, de Melo Corgosinho L, Faria JAQA, dos Santos VM, Resende JM, Leal AS et al (2017) Multifunctional mesoporous silica nanoparticles for cancer-targeted, controlled drug delivery and imaging. Microporous Mesoporous Mater 242:271–283
Deng Z, Xiao Y, Pan M, Li F, Duan W, Meng L et al (2016) Hyperthermia-triggered drug delivery from iRGD-modified temperature-sensitive liposomes enhances the anti-tumor efficacy using high intensity focused ultrasound. J Control Release 243:333–341
Deodhar GV, Adams ML, Trewyn BG (2017) Controlled release and intracellular protein delivery from mesoporous silica nanoparticles. Biotechnol J 12(1):1600408
Derakhshandeh K, Azandaryani AH (2016) Active-targeted nanotherapy as smart cancer treatment. Smart Drug Delivery System:91–116
Derks M, van Lonkhuijzen LR, Bakker RM, Stiggelbout AM, de Kroon CD, Westerveld H et al (2017) Long-term morbidity and quality of life in cervical cancer survivors: a multicenter comparison between surgery and radiotherapy as primary treatment. Int J Gynecol Cancer. 27(2):350–356
Eeles RA, Morden JP, Gore M, Mansi J, Glees J, Wenczl M et al (2016) Adjuvant hormone therapy may improve survival in epithelial ovarian cancer: results of the AHT randomized trial. Obstet Gynecol Surv 71(4):223–224
Ekladious I, Colson YL, Grinstaff MW (2019) Polymer–drug conjugate therapeutics: advances, insights and prospects. Nat Rev Drug Discov 18(4):273–294
Eryılmaz E, Canpolat C (2017) Novel agents for the treatment of childhood leukemia: an update. Onco Targets Ther 10:3299
Espinosa-Cano E, Palao-Suay R, Aguilar MR, Vázquez B, San Román J (2018) Polymeric nanoparticles for cancer therapy and bioimaging. In: Nanooncology. Springer, pp 137–172.
Faisca Phillips AM (2019) Recent developments in anti-cancer drug research. Curr Med Chem 26(41):7282–7284
Fan Z, Zhou S, Garcia C, Fan L, Zhou J (2017) pH-Responsive fluorescent graphene quantum dots for fluorescence-guided cancer surgery and diagnosis. Nanoscale 9(15):4928–4933
Fang M, Chen M, Liu L, Li Y (2017) Applications of quantum dots in cancer detection and diagnosis: a review. J Biomed Nanotechnol 13(1):1–16
Fathi Karkan S, Mohammadhosseini M, Panahi Y, Milani M, Zarghami N, Akbarzadeh A et al (2017) Magnetic nanoparticles in cancer diagnosis and treatment: a review. Artif Cells Nanomed Biotechnol 45(1):1–5
Fathi M, Barar J, Erfan-Niya H, Omidi Y (2020) Methotrexate-conjugated chitosan-grafted pH-and thermo-responsive magnetic nanoparticles for targeted therapy of ovarian cancer. Int J Biol Macromol 154:1175–1184
Feng R, Deng P, Song Z, Chu W, Zhu W, Teng F et al (2017) Glycyrrhetinic acid-modified PEG-PCL copolymeric micelles for the delivery of curcumin. React Funct Polym 111:30–37
Ganesan P, Ramalingam P, Karthivashan G, Ko YT, Choi D-K (2018) Recent developments in solid lipid nanoparticle and surface-modified solid lipid nanoparticle delivery systems for oral delivery of phyto-bioactive compounds in various chronic diseases. Int J Nanomed 13:1569
Garg NK, Singh B, Jain A, Nirbhavane P, Sharma R, Tyagi RK et al (2016) Fucose decorated solid-lipid nanocarriers mediate efficient delivery of methotrexate in breast cancer therapeutics. Colloids Surf B Biointerfaces 146:114–126
Ghahremani F, Shahbazi-Gahrouei D, Kefayat A, Motaghi H, Mehrgardi MA, Javanmard SH (2018) AS1411 aptamer conjugated gold nanoclusters as a targeted radiosensitizer for megavoltage radiation therapy of 4T1 breast cancer cells. RSC Adv 8(8):4249–4258
Ghasemiyeh P, Mohammadi-Samani S (2018) Solid lipid nanoparticles and nanostructured lipid carriers as novel drug delivery systems: applications, advantages and disadvantages. Res Pharm Sci 13(4):288
Ghasemiyeh P, Mohammadi-Samani S (2020) Potential of nanoparticles as permeation enhancers and targeted delivery options for skin: advantages and disadvantages. Drug Des Devel Ther. 14:3271
Gothwal A, Khan I, Gupta U (2016) Polymeric micelles: recent advancements in the delivery of anticancer drugs. Pharm Res 33(1):18–39
Gu Z, Gao D, Al-Zubaydi F, Li S, Singh Y, Rivera K et al (2018) The effect of size and polymer architecture of doxorubicin–poly (ethylene) glycol conjugate nanocarriers on breast duct retention, potency and toxicity. Eur J Pharm Sci 121:118–125
Guo F, Guo D, Zhang W, Yan Q, Yang Y, Hong W et al (2017) Preparation of curcumin-loaded PCL-PEG-PCL triblock copolymeric nanoparticles by a microchannel technology. Eur J Pharm Sci 99:328–336
Hardiansyah A, Yang M-C, Liu T-Y, Kuo C-Y, Huang L-Y, Chan T-Y (2017) Hydrophobic drug-loaded PEGylated magnetic liposomes for drug-controlled release. Nanoscale Res Lett 12(1):1–11
He R, Yin C (2017) Trimethyl chitosan based conjugates for oral and intravenous delivery of paclitaxel. Acta Biomater 53:355–366
He Z, Zhang Y, Feng N (2020) Cell membrane-coated nanosized active targeted drug delivery systems homing to tumor cells: a review. Mater Sci Eng C 106:110298
Huang J, Li Y, Orza A, Lu Q, Guo P, Wang L et al (2016) Magnetic nanoparticle facilitated drug delivery for cancer therapy with targeted and image-guided approaches. Adv Funct Mater 26(22):3818–3836
Huang L, Chaurasiya B, Wu D, Wang H, Du Y, Tu J et al (2018) Versatile redox-sensitive pullulan nanoparticles for enhanced liver targeting and efficient cancer therapy. Nanomed Nanotechnol Biol Med 14(3):1005–1017
Ishihara D, Pop L, Takeshima T, Iyengar P, Hannan R (2017) Rationale and evidence to combine radiation therapy and immunotherapy for cancer treatment. Cancer Immunol Immunother 66(3):281–298
Jain A, Jain SK (2018) Stimuli-responsive smart liposomes in cancer targeting. Curr Drug Targets 19(3):259–270
Jhaveri A, Deshpande P, Pattni B, Torchilin V (2018) Transferrin-targeted, resveratrol-loaded liposomes for the treatment of glioblastoma. J Control Release 277:89–101
Johnson SB, Park HS, Gross CP, Yu JB (2018) Use of alternative medicine for cancer and its impact on survival. JNCI 110(1):121–124
Ju R-J, Cheng L, Qiu X, Liu S, Song X-L, Peng X-M et al (2018) Hyaluronic acid modified daunorubicin plus honokiol cationic liposomes for the treatment of breast cancer along with the elimination vasculogenic mimicry channels. J Drug Target 26(9):793–805
Kalyanaraman B (2017) Teaching the basics of cancer metabolism: developing antitumor strategies by exploiting the differences between normal and cancer cell metabolism. Redox Biol 12:833–842
Kamaly N, Yameen B, Wu J, Farokhzad OC (2016) Degradable controlled-release polymers and polymeric nanoparticles: mechanisms of controlling drug release. Chem Rev 116(4):2602–2663
Kanamala M, Wilson WR, Yang M, Palmer BD, Wu Z (2016) Mechanisms and biomaterials in pH-responsive tumour targeted drug delivery: a review. Biomaterials 85:152–167
Katuwavila NP, Perera A, Samarakoon SR, Soysa P, Karunaratne V, Amaratunga GA et al (2016) Chitosan-alginate nanoparticle system efficiently delivers doxorubicin to MCF-7 cells. J Nanomater 2016:1–12
Kaur D, Jain K, Mehra NK, Kesharwani P, Jain NK (2016) A review on comparative study of PPI and PAMAM dendrimers. J Nanopart Res 18(6):146
Khafaji M, Zamani M, Golizadeh M, Bavi O (2019) Inorganic nanomaterials for chemo/photothermal therapy: a promising horizon on effective cancer treatment. Biophys Rev 11:335–352
Khodadust R, Unsoy G, Gunduz U (2014) Development of poly (I: C) modified doxorubicin loaded magnetic dendrimer nanoparticles for targeted combination therapy. Biomed Pharmacother 68(8):979–987
Kim Y-M, Park S-C, Jang M-K (2017) Targeted gene delivery of polyethyleneimine-grafted chitosan with RGD dendrimer peptide in αvβ3 integrin-overexpressing tumor cells. Carbohydr Polym 174:1059–1068
Kolosnjaj-Tabi J, Wilhelm C (2017) Magnetic nanoparticles in cancer therapy: how can thermal approaches help? Nanomedicine (Lond) (Future Medicine) 12(6):573–575
Kuang Y, Zhang K, Cao Y, Chen X, Wang K, Liu M et al (2017) Hydrophobic IR-780 dye encapsulated in cRGD-conjugated solid lipid nanoparticles for NIR imaging-guided photothermal therapy. ACS Appl Mater Interfaces 9(14):12217–12226
Kulhari H, Pooja D, Shrivastava S, Kuncha M, Naidu V, Bansal V et al (2016) Trastuzumab-grafted PAMAM dendrimers for the selective delivery of anticancer drugs to HER2-positive breast cancer. Sci Rep 6(1):1–13
Kulikov AV, Luchkina EA, Gogvadze V, Zhivotovsky B (2017) Mitophagy: link to cancer development and therapy. Biochem Biophys Res Commun 482(3):432–439
Kumar A, Ma H, Zhang X, Huang K, Jin S, Liu J et al (2012) Gold nanoparticles functionalized with therapeutic and targeted peptides for cancer treatment. Biomaterials 33(4):1180–1189
Kumar B, Jalodia K, Kumar P, Gautam HK (2017) Recent advances in nanoparticle-mediated drug delivery. J Drug Deliv Sci Technol 41:260–268
Kumar R, Singh M, Meena J, Singhvi P, Thiyagarajan D, Saneja A et al (2019) Hyaluronic acid-dihydroartemisinin conjugate: synthesis, characterization and in vitro evaluation in lung cancer cells. Int J Biol Macromol 133:495–502
Kumari P, Ghosh B, Biswas S (2016) Nanocarriers for cancer-targeted drug delivery. J Drug Target 24(3):179–191
Lee JJ, Yazan LS, Abdullah CAC (2017) A review on current nanomaterials and their drug conjugate for targeted breast cancer treatment. Int J Nanomed 12:2373
Li C, Wallace S (2008) Polymer-drug conjugates: recent development in clinical oncology. Adv Drug Deliv Rev 60(8):886–898
Li M, Song W, Tang Z, Lv S, Lin L, Sun H et al (2013) Nanoscaled poly (L-glutamic acid)/doxorubicin-amphiphile complex as pH-responsive drug delivery system for effective treatment of nonsmall cell lung cancer. ACS Appl Mater Interfaces 5(5):1781–1792
Li J, Wang H, Yang B, Xu L, Zheng N, Chen H et al (2016a) Control-release microcapsule of famotidine loaded biomimetic synthesized mesoporous silica nanoparticles: controlled release effect and enhanced stomach adhesion in vitro. Mater Sci Eng C 58:273–277
Li X, Wu M, Pan L, Shi J (2016b) Tumor vascular-targeted co-delivery of anti-angiogenesis and chemotherapeutic agents by mesoporous silica nanoparticle-based drug delivery system for synergetic therapy of tumor. Int J Nanomed 11:93
Li J, Liang H, Liu J, Wang Z (2018a) Poly (amidoamine)(PAMAM) dendrimer mediated delivery of drug and pDNA/siRNA for cancer therapy. Int J Pharm 546(1-2):215–225
Li H, Tong Y, Bai L, Ye L, Zhong L, Duan X et al (2018b) Lactoferrin functionalized PEG-PLGA nanoparticles of shikonin for brain targeting therapy of glioma. Int J Biol Macromol 107:204–211
Li Y, Chen M, Yao B, Lu X, Zhang X, He P et al (2019a) Transferrin receptor-targeted redox/pH-sensitive podophyllotoxin prodrug micelles for multidrug-resistant breast cancer therapy. J Mater Chem B 7(38):5814–5824
Li S, Li X, Ding J, Han L, Guo X (2019b) Anti-tumor efficacy of folate modified PLGA-based nanoparticles for the co-delivery of drugs in ovarian cancer. Drug Des Devel Ther. 13:1271
Li Z, Xu H, Shao J, Jiang C, Zhang F, Lin J et al (2019c) Polydopamine-functionalized black phosphorus quantum dots for cancer theranostics. Appl Mater Today 15:297–304
Lin W, Ma G, Kampf N, Yuan Z, Chen S (2016) Development of long-circulating zwitterionic cross-linked micelles for active-targeted drug delivery. Biomacromolecules 17(6):2010–2018
Lingayat VJ, Zarekar NS, Shendge RS (2017) Solid lipid nanoparticles: a review. Nanosci Nanotechnol Res 2:67–72
Liu J, Yang Y, Zhu W, Yi X, Dong Z, Xu X et al (2016a) Nanoscale metal− organic frameworks for combined photodynamic & radiation therapy in cancer treatment. Biomaterials 97:1–9
Liu Y, Zhou C, Wang W, Yang J, Wang H, Hong W et al (2016b) CD44 receptor targeting and endosomal pH-sensitive dual functional hyaluronic acid micelles for intracellular paclitaxel delivery. Mol Pharm 13(12):4209–4221
Liu B, Han L, Liu J, Han S, Chen Z, Jiang L (2017a) Co-delivery of paclitaxel and TOS-cisplatin via TAT-targeted solid lipid nanoparticles with synergistic antitumor activity against cervical cancer. Int J Nanomedicine 12:955
Liu Y, Zhang G, Guo Q, Ma L, Jia Q, Liu L et al (2017b) Artificially controlled degradable inorganic nanomaterial for cancer theranostics. Biomaterials 112:204–217
Liu Q, Jing Y, Han C, Zhang H, Tian Y (2019a) Encapsulation of curcumin in zein/caseinate/sodium alginate nanoparticles with improved physicochemical and controlled release properties. Food Hydrocoll 93:432–442
Liu P, Chen N, Yan L, Gao F, Ji D, Zhang S et al (2019b) Preparation, characterisation and in vitro and in vivo evaluation of CD44-targeted chondroitin sulphate-conjugated doxorubicin PLGA nanoparticles. Carbohydr Polym 213:17–26
Liu D, Zhang Q, Wang J, Fan L, Zhu W, Cai D (2019c) Hyaluronic acid-coated single-walled carbon nanotubes loaded with doxorubicin for the treatment of breast cancer. Die Pharmazie 74(2):83–90
Liu Y, Yang G, Jin S, Xu L, Zhao CX (2020) Development of high‐drug‐loading nanoparticles. ChemPlusChem 85(9):2143–2157.
Liu Z, Parida S, Prasad R, Pandey R, Sharma D, Barman I (2021) Vibrational spectroscopy for decoding cancer microbiome interactions: Current evidence and future Perspective. Semin Cancer Biol. https://doi.org/10.1016/j.semcancer.2021.07.004.
Lungu II, Radulescu M, Mogosanu GD, Grumezescu AM (2016) pH sensitive core-shell magnetic nanoparticles for targeted drug delivery in cancer therapy. Rom J Morphol Embryol 57(1):23–32
Luo T, Magnusson J, Préat V, Frédérick R, Alexander C, Bosquillon C et al (2016) Synthesis and in vitro evaluation of polyethylene glycol-paclitaxel conjugates for lung cancer therapy. Pharm Res 33(7):1671–1681
Lyon PC, Griffiths LF, Lee J, Chung D, Carlisle R, Wu F et al (2017) Clinical trial protocol for TARDOX: a phase I study to investigate the feasibility of targeted release of lyso-thermosensitive liposomal doxorubicin (ThermoDox®) using focused ultrasound in patients with liver tumours. J Therap Ultrasound 5(1):1–8
Ma G, Zhang C, Zhang L, Sun H, Song C, Wang C et al (2016) Doxorubicin-loaded micelles based on multiarm star-shaped PLGA–PEG block copolymers: influence of arm numbers on drug delivery. J Mater Sci Mater Med 27(1):17
Mahato R (2017) Nanoemulsion as targeted drug delivery system for cancer therapeutics. J Pharm Sci Pharm 3(2):83–97
Manjili HK, Malvandi H, Mousavi MS, Attari E, Danafar H (2018) In vitro and in vivo delivery of artemisinin loaded PCL–PEG–PCL micelles and its pharmacokinetic study. Artif Cells Nanomed Biotechnol 46(5):926–936
Marzbali MY, Khosroushahi AY (2017) Polymeric micelles as mighty nanocarriers for cancer gene therapy: a review. Cancer Chemother Pharmacol 79(4):637–649
Masood F (2016) Polymeric nanoparticles for targeted drug delivery system for cancer therapy. Mater Sci Eng C 60:569–578
Mehta D, Leong N, McLeod VM, Kelly BD, Pathak R, Owen DJ et al (2018) Reducing dendrimer generation and PEG chain length increases drug release and promotes anticancer activity of PEGylated polylysine dendrimers conjugated with doxorubicin via a cathepsin-cleavable peptide linker. Mol Pharm 15(10):4568–4576
Meunier M, Goupil A, Lienard P (2017) Predicting drug loading in PLA-PEG nanoparticles. Int J Pharm 526(1-2):157–166
Miller KD, Nogueira L, Mariotto AB, Rowland JH, Yabroff KR, Alfano CM et al (2019) Cancer treatment and survivorship statistics, 2019. CA Cancer J Clin 69(5):363–385
Mishra H, Chauhan V, Kumar K, Teotia D (2018) A comprehensive review on Liposomes: a novel drug delivery system. J Drug Deliv Therap 8(6):400–404
Mo L, Song JG, Lee H, Zhao M, Kim HY, Lee YJ et al (2018) PEGylated hyaluronic acid-coated liposome for enhanced in vivo efficacy of sorafenib via active tumor cell targeting and prolonged systemic exposure. Nanomed Nanotechnol Biol Med 14(2):557–567
Moghimipour E, Rezaei M, Kouchak M, Ramezani Z, Amini M, Ahmadi Angali K et al (2018) A mechanistic study of the effect of transferrin conjugation on cytotoxicity of targeted liposomes. J Microencapsul 35(6):548–558
Mohamed M, Abu Lila AS, Shimizu T, Alaaeldin E, Hussein A, Sarhan HA et al (2019) PEGylated liposomes: immunological responses. Sci Technol Adv Mater 20(1):710–724
Moreira AF, Dias DR, Correia IJ (2016) Stimuli-responsive mesoporous silica nanoparticles for cancer therapy: a review. Microporous Mesoporous Mater 236:141–157
Munir M, Hanif M, Ranjha NM (2016) Dendrimers and their applications: a review article. Pakistan J Pharm Res 2(1):55–66
Muntoni E, Martina K, Marini E, Giorgis M, Lazzarato L, Salaroglio IC et al (2019) Methotrexate-loaded solid lipid nanoparticles: protein functionalization to improve brain biodistribution. Pharmaceutics 11(2):65
Nag OK, Delehanty JB (2019) Active cellular and subcellular targeting of nanoparticles for drug delivery. Pharmaceutics 11(10):543
Nayak R, Meerovich I, Dash AK (2019) Translational multi-disciplinary approach for the drug and gene delivery systems for cancer treatment. AAPS PharmSciTech 20(4):160
Nguyen HT, Tran TH, Thapa RK, Dai Phung C, Shin BS, Jeong J-H et al (2017) Targeted co-delivery of polypyrrole and rapamycin by trastuzumab-conjugated liposomes for combined chemo-photothermal therapy. Int J Pharm 527(1-2):61–71
Pan M, Li W, Yang J, Li Z, Zhao J, Xiao Y et al (2017) Plumbagin-loaded aptamer-targeted poly D, L-lactic-co-glycolic acid-b-polyethylene glycol nanoparticles for prostate cancer therapy. Medicine 96(30):e7405
Pandey H, Rani R, Agarwal V (2016) Liposome and their applications in cancer therapy. Brazilian Arch Biol Technol 59
Papaccio F, Paino F, Regad T, Papaccio G, Desiderio V, Tirino V (2017) Concise review: cancer cells, cancer stem cells, and mesenchymal stem cells: influence in cancer development. Stem Cells Transl Med 6(12):2115–2125
Parajapati SK, Maurya SD, Das MK, Tilak VK, Verma KK, Dhakar RC (2016) Potential application of dendrimers in drug delivery: a concise review and update. J Drug Deliv Therap 6(2):71–88
Pardo J, Peng Z, Leblanc RM (2018) Cancer targeting and drug delivery using carbon-based quantum dots and nanotubes. Molecules 23(2):378
Paris JL, Mannaris C, Cabañas MV, Carlisle R, Manzano M, Vallet-Regí M et al (2018) Ultrasound-mediated cavitation-enhanced extravasation of mesoporous silica nanoparticles for controlled-release drug delivery. Chem Eng J 340:2–8
Park J-S, Kim I-K, Han S, Park I, Kim C, Bae J et al (2016) Normalization of tumor vessels by Tie2 activation and Ang2 inhibition enhances drug delivery and produces a favorable tumor microenvironment. Cancer Cell 30(6):953–967
Patel V (2020) Liposome: a novel carrier for targeting drug delivery system. Asian J Pharm Res Develop 8(4):67–76
Patel SG., Patel MD, Patel AJ, Chougule MB, Choudhury H (2018) Solid lipid nanoparticles for targeted brain drug delivery. In Nanotechnology-based targeted drug delivery systems for brain tumors. Academic Press, pp 191–244.
Peng J, Liang X (2019) Progress in research on gold nanoparticles in cancer management. Medicine 98(18):e15311
Phan QT, Patil MP, Tu TT, Le CM, Kim G-D, Lim KT (2020) Polyampholyte-grafted single walled carbon nanotubes prepared via a green process for anticancer drug delivery application. Polymer 193:122340
Pooja D, Reddy TS. Kulhari H, Kadari A, Adams DJ, Bansal V, Sistla R (2020). N-acetyl-d-glucosamine-conjugated PAMAM dendrimers as dual receptor-targeting nanocarriers for anticancer drug delivery. Eur J Pharm Biopharm 154:377–386.
Pranatharthiharan S, Patel MD, Malshe VC, Pujari V, Gorakshakar A, Madkaikar M et al (2017) Asialoglycoprotein receptor targeted delivery of doxorubicin nanoparticles for hepatocellular carcinoma. Drug Deliv 24(1):20–29
Prasad R, Pandey R, Varma A, Barman I (2017) Polymer based nanoparticles for drug delivery systems and cancer therapeutics. In: Natural Polymers for Drug Delivery (eds. Kharkwal H and Janaswamy S), CAB International, UK 53–70
Prasad R, Kumar V, Kumar M, Choudhary D (2019) Nanobiotechnology in Bioformulations. Springer International Publishing (ISBN 978-3-030-17061-5) https://www.springer.com/gp/book/9783030170608
Priya S, Rekha M (2017) Redox sensitive cationic pullulan for efficient gene transfection and drug retention in C6 glioma cells. Int J Pharm 530(1–2):401–414
Ptacek J, Zhang D, Qiu L, Kruspe S, Motlova L, Kolenko P et al (2020) Structural basis of prostate-specific membrane antigen recognition by the A9g RNA aptamer. Nucleic Acids Res
Qi D, Gong F, Teng X, Ma M, Wen H, Yuan W et al (2017) Design and evaluation of mPEG-PLA micelles functionalized with drug-interactive domains as improved drug carriers for docetaxel delivery. J Biomater Sci Polym Ed 28(14):1538–1555
Qu J, Zhang L, Chen Z, Mao G, Gao Z, Lai X et al (2016) Nanostructured lipid carriers, solid lipid nanoparticles, and polymeric nanoparticles: which kind of drug delivery system is better for glioblastoma chemotherapy? Drug Deliv 23(9):3408–3416
Rahman M, Beg S, Anwar F, Kumar V, Ubale R, Addo RT et al (2017) Liposome-based nanomedicine therapeutics for rheumatoid arthritis. Crit Rev Ther Drug Carrier Syst. 34(4):283–316
Raja MA, Arif M, Feng C, Zeenat S, Liu C-G (2017) Synthesis and evaluation of pH-sensitive, self-assembled chitosan-based nanoparticles as efficient doxorubicin carriers. J Biomater Appl 31(8):1182–1195
Ramalingam P, Ko YT (2015) Enhanced oral delivery of curcumin from N-trimethyl chitosan surface-modified solid lipid nanoparticles: pharmacokinetic and brain distribution evaluations. Pharm Res 32(2):389–402
Ramalingam P, Ko YT (2016) Improved oral delivery of resveratrol from N-trimethyl chitosan-g-palmitic acid surface-modified solid lipid nanoparticles. Colloids Surf B Biointerfaces 139:52–61
Ramalingam P, Yoo SW, Ko YT (2016) Nanodelivery systems based on mucoadhesive polymer coated solid lipid nanoparticles to improve the oral intake of food curcumin. Food Res Int 84:113–119
Rana S, Shetake NG, Barick K, Pandey B, Salunke H, Hassan P (2016) Folic acid conjugated Fe 3 O 4 magnetic nanoparticles for targeted delivery of doxorubicin. Dalton Trans 45(43):17401–17408
Ranjbar-Navazi Z, Eskandani M, Johari-Ahar M, Nemati A, Akbari H, Davaran S et al (2018) Doxorubicin-conjugated D-glucosamine-and folate-bi-functionalised InP/ZnS quantum dots for cancer cells imaging and therapy. J Drug Target 26(3):267–277
Raza A, Hayat U, Rasheed T, Bilal M, Iqbal HM (2019) “Smart” materials-based near-infrared light-responsive drug delivery systems for cancer treatment: a review. J Mater Res Technol 8(1):1497–1509
Rodenak-Kladniew B, Islan GA, de Bravo MG, Durán N, Castro GR (2017) Design, characterization and in vitro evaluation of linalool-loaded solid lipid nanoparticles as potent tool in cancer therapy. Colloids Surf B Biointerfaces 154:123–132
Rosiere R, Van Woensel M, Gelbcke M, Mathieu V, Hecq J, Mathivet T et al (2018) New folate-grafted chitosan derivative to improve delivery of paclitaxel-loaded solid lipid nanoparticles for lung tumor therapy by inhalation. Mol Pharm 15(3):899–910
Saberinasab A, Raissi H, Hashemzadeh H (2019) Understanding the effect of vitamin B6 and PEG functionalization on improving the performance of carbon nanotubes in temozolomide anticancer drug transportation. J Phys D Appl Phys 52(39):395402
Saif MW (2013) US Food and Drug Administration approves paclitaxel protein-bound particles (Abraxane®) in combination with gemcitabine as first-line treatment of patients with metastatic pancreatic cancer. JOP 14(6):686–688
Saini K, Bandyopadhyaya R (2019) Transferrin-conjugated polymer-coated mesoporous silica nanoparticles loaded with gemcitabine for killing pancreatic cancer cells. ACS Appl Nano Mater 3(1):229–240
Salvioni L, Rizzuto MA, Bertolini JA, Pandolfi L, Colombo M, Prosperi D (2019) Thirty years of cancer nanomedicine: success, frustration, and hope. Cancers 11(12):1855
Sarcan ET, Silindir-Gunay M, Ozer AY (2018) Theranostic polymeric nanoparticles for NIR imaging and photodynamic therapy. Int J Pharm 551(1-2):329–338
Schmidt LH, Brand C, Stucke-Ring J, Schliemann C, Kessler T, Harrach S et al (2017) Potential therapeutic impact of CD13 expression in non-small cell lung cancer. PLoS One 12(6):e0177146
Senapati S, Mahanta AK, Kumar S, Maiti P (2018) Controlled drug delivery vehicles for cancer treatment and their performance. Signal Transduct Target Ther 3(1):1–19
Serinan E, Altun Z, Aktaş S, Çeçen E, Olgun N (2018) Comparison of cisplatin with lipoplatin in terms of ototoxicity. J Int Adv Otol 14(2):211
Serini S, Cassano R, Corsetto PA, Rizzo AM, Calviello G, Trombino S (2018) Omega-3 PUFA loaded in resveratrol-based solid lipid nanoparticles: Physicochemical properties and antineoplastic activities in human colorectal cancer cells in vitro. Int J Mol Sci 19(2):586
Seyfoori A, Sarfarazijami S, Seyyed Ebrahimi S (2019) pH-responsive carbon nanotube-based hybrid nanogels as the smart anticancer drug carrier. Artif Cells Nanomed Biotechnol 47(1):1437–1443
Shao J, Zhang J, Jiang C, Lin J, Huang P (2020) Biodegradable titanium nitride MXene quantum dots for cancer phototheranostics in NIR-I/II biowindows. Chem Eng J 400:126009
Sherje AP, Jadhav M, Dravyakar BR, Kadam D (2018) Dendrimers: a versatile nanocarrier for drug delivery and targeting. Int J Pharm 548(1):707–720
Siegel RL, Miller KD, Jemal A (2019) Cancer statistics, 2019. CA Cancer J Clin 69(1):7–34
Silverman JA, Deitcher SR (2013) Marqibo®(vincristine sulfate liposome injection) improves the pharmacokinetics and pharmacodynamics of vincristine. Cancer Chemother Pharmacol 71(3):555–564
Singh S, Mehra NK, Jain N (2016a) Development and characterization of the paclitaxel loaded riboflavin and thiamine conjugated carbon nanotubes for cancer treatment. Pharm Res 33(7):1769–1781
Singh RP, Sharma G, Singh S, Kumar M, Pandey BL, Koch B et al (2016b) Vitamin E TPGS conjugated carbon nanotubes improved efficacy of docetaxel with safety for lung cancer treatment. Colloids Surf B Biointerfaces 141:429–442
Singh P, Pandit S, Mokkapati V, Garg A, Ravikumar V, Mijakovic I (2018) Gold nanoparticles in diagnostics and therapeutics for human cancer. Int J Mol Sci 19(7):1979
Sofias AM, Dunne M, Storm G, Allen C (2017) The battle of “nano” paclitaxel. Adv Drug Deliv Rev 122:20–30
Son KH, Hong JH, Lee JW (2016) Carbon nanotubes as cancer therapeutic carriers and mediators. Int J Nanomedicine 11:5163
Song L, Pan Z, Zhang H, Li Y, Zhang Y, Lin J et al (2017) Dually folate/CD44 receptor-targeted self-assembled hyaluronic acid nanoparticles for dual-drug delivery and combination cancer therapy. J Mater Chem B 5(33):6835–6846
Sztandera K, Gorzkiewicz M, Klajnert-Maculewicz B (2018) Gold nanoparticles in cancer treatment. Mol Pharm 16(1):1–23
Taghipour-Sabzevar V, Sharifi T, Moghaddam MM (2019) Polymeric nanoparticles as carrier for targeted and controlled delivery of anticancer agents. Ther Deliv 10(8):527–550
Tang Y, Li Y, Xu R, Li S, Hu H, Xiao C et al (2018a) Self-assembly of folic acid dextran conjugates for cancer chemotherapy. Nanoscale 10(36):17265–17274
Tang X, Lyu Y, Xie D, Li A, Liang Y, Zheng D (2018b) Therapeutic effect of sorafenib-loaded TPGS-b-PCL nanoparticles on liver cancer. J Biomed Nanotechnol 14(2):396–403
Thanou M, Duncan R (2003) Polymer-protein and polymer-drug conjugates in cancer therapy. Curr Opin Investig Drugs (London, England: 2000) 4(6):701–709
Tiruwa R (2016) A review on nanoparticles–preparation and evaluation parameters. Indian J Pharm Biol Res 4(2):27–31
Tran S, DeGiovanni P-J, Piel B, Rai P (2017) Cancer nanomedicine: a review of recent success in drug delivery. Clin Transl Med 6(1):44
Tsuboi S, Sasaki A, Sakata T, Yasuda H, Jin T (2017) Immunoglobulin binding (B1) domain mediated antibody conjugation to quantum dots for in vitro and in vivo molecular imaging. Chem Commun 53(68):9450–9453
Tyson MD II, Koyama T, Lee D, Hoffman KE, Resnick MJ, Wu X-C et al (2018) Effect of prostate cancer severity on functional outcomes after localized treatment: comparative effectiveness analysis of surgery and radiation study results. Eur Urol 74(1):26–33
van der Meel R, Sulheim E, Shi Y, Kiessling F, Mulder WJ, Lammers T (2019) Smart cancer nanomedicine. Nat Nanotechnol 14(11):1007–1017
Veeranarayanan S, Maekawa T (2019) External stimulus responsive inorganic nanomaterials for cancer theranostics. Adv Drug Deliv Rev 138:18–40
Vicent MJ, Duncan R (2006) Polymer conjugates: nanosized medicines for treating cancer. Trends Biotechnol 24(1):39–47
Vinothini K, Rajendran NK, Ramu A, Elumalai N, Rajan M (2019) Folate receptor targeted delivery of paclitaxel to breast cancer cells via folic acid conjugated graphene oxide grafted methyl acrylate nanocarrier. Biomed Pharmacother 110:906–917
Wakaskar RR (2017) Passive and active targeting in tumor microenvironment. Int J Drug Develop Res 9(2):37–41
Wang H, Yu J, Lu X, He X (2016) Nanoparticle systems reduce systemic toxicity in cancer treatment. Nanomedicine (Lond) (Future Medicine) 11(2):103–106
Wang W, Zhang L, Chen T, Guo W, Bao X, Wang D et al (2017a) Anticancer effects of resveratrol-loaded solid lipid nanoparticles on human breast cancer cells. Molecules 22(11):1814
Wang T, Hou J, Su C, Zhao L, Shi Y (2017b) Hyaluronic acid-coated chitosan nanoparticles induce ROS-mediated tumor cell apoptosis and enhance antitumor efficiency by targeted drug delivery via CD44. J Nanobiotechnol 15(1):7
Wang W, Li M, Zhang Z, Cui C, Zhou J, Yin L et al (2017c) Design, synthesis and evaluation of multi-functional tLyP-1-hyaluronic acid-paclitaxel conjugate endowed with broad anticancer scope. Carbohydr Polym 156:97–107
Wang D, Ren Y, Shao Y, Yu D, Meng L (2017d) Facile preparation of doxorubicin-loaded and folic acid-conjugated carbon nanotubes@ poly (N-vinyl pyrrole) for targeted synergistic chemo–Photothermal Cancer treatment. Bioconjug Chem 28(11):2815–2822
Wang D, Ren Y, Shao Y, Meng L (2017e) Multifunctional polyphosphazene-coated multi-walled carbon nanotubes for the synergistic treatment of redox-responsive chemotherapy and effective photothermal therapy. Polym Chem 8(45):6938–6942
Wang W, Chen T, Xu H, Ren B, Cheng X, Qi R et al (2018a) Curcumin-loaded solid lipid nanoparticles enhanced anticancer efficiency in breast cancer. Molecules 23(7):1578
Wang Y, Ren J, Liu Y, Liu R, Wang L, Yuan Q et al (2018b) Preparation and evaluation of folic acid modified succinylated gelatin micelles for targeted delivery of doxorubicin. J Drug Deliv Sci Technol 46:400–407
Wang X, Chang Z, Nie X, Li Y, Hu Z, Ma J et al (2019) A conveniently synthesized Pt (IV) conjugated alginate nanoparticle with ligand self-shielded property for targeting treatment of hepatic carcinoma. Nanomed Nanotechnol Biol Med 15(1):153–163
Wild CP (2019) The global cancer burden: Necessity is the mother of prevention. Nat Rev Cancer 19(3):123–124
Wolfram J, Ferrari M (2019) Clinical cancer nanomedicine. Nano Today 25:85–98
Wong P-P, Bodrug N, Hodivala-Dilke KM (2016) Exploring novel methods for modulating tumor blood vessels in cancer treatment. Curr Biol 26(21):R1161–R11R6
Wong KH, Lu A, Chen X, Yang Z (2020) Natural ingredient-based polymeric nanoparticles for cancer treatment. Molecules 25(16):3620
Wu P-H, Onodera Y, Ichikawa Y, Rankin EB, Giaccia AJ, Watanabe Y et al (2017a) Targeting integrins with RGD-conjugated gold nanoparticles in radiotherapy decreases the invasive activity of breast cancer cells. Int J Nanomedicine 12:5069
Wu J, Deng C, Meng F, Zhang J, Sun H, Zhong Z (2017b) Hyaluronic acid coated PLGA nanoparticulate docetaxel effectively targets and suppresses orthotopic human lung cancer. J Control Release 259:76–82
Xiong XY, Pan X, Tao L, Cheng F, Li ZL, Gong YC et al (2017) Enhanced effect of folated pluronic F87-PLA/TPGS mixed micelles on targeted delivery of paclitaxel. Int J Biol Macromol 103:1011–1018
Xu W, Siddiqui IA, Nihal M, Pilla S, Rosenthal K, Mukhtar H et al (2013) Aptamer-conjugated and doxorubicin-loaded unimolecular micelles for targeted therapy of prostate cancer. Biomaterials 34(21):5244–5253
Xu G, Chen Y, Shan R, Wu X, Chen L (2018) Transferrin and tocopheryl-polyethylene glycol-succinate dual ligands decorated, cisplatin loaded nano-sized system for the treatment of lung cancer. Biomed Pharmacother 99:354–362
Yang S, Gao H (2017) Nanoparticles for modulating tumor microenvironment to improve drug delivery and tumor therapy. Pharmacol Res 126:97–108
Yang Y, Yu C (2016) Advances in silica based nanoparticles for targeted cancer therapy. Nanomed Nanotechnol Biol Med 12(2):317–332
Yang Y, Yang Y, Xie X, Wang Z, Gong W, Zhang H et al (2015) Dual-modified liposomes with a two-photon-sensitive cell penetrating peptide and NGR ligand for siRNA targeting delivery. Biomaterials 48:84–96
Yang Y, Zhao Y, Lan J, Kang Y, Zhang T, Ding Y et al (2018a) Reduction-sensitive CD44 receptor-targeted hyaluronic acid derivative micelles for doxorubicin delivery. Int J Nanomedicine 13:4361
Yang C, Song W, Zhang D, Yu H, Yin L, Shen N et al (2018b) Poly (L-glutamic acid)-g-methoxy poly (ethylene glycol)-gemcitabine conjugate improves the anticancer efficacy of gemcitabine. Int J Pharm 550(1-2):79–88
Yao S, Li L, Su X-T, Wang K, Lu Z-J, Yuan C-Z et al (2018) Development and evaluation of novel tumor-targeting paclitaxel-loaded nano-carriers for ovarian cancer treatment: in vitro and in vivo. J Exp Clin Cancer Res 37(1):29
Yu C, Zhou Q, Xiao F, Li Y, Hu H, Wan Y et al (2017) Enhancing doxorubicin delivery toward tumor by hydroxyethyl starch-g-polylactide partner nanocarriers. ACS Appl Mater Interfaces 9(12):10481–10493
Zahednezhad F, Zakeri-Milani P, Shahbazi Mojarrad J, Valizadeh H (2020) The latest advances of cisplatin liposomal formulations: essentials for preparation and analysis. Expert Opin Drug Deliv 17(4):523–541
Zaidi N, Jaffee EM (2019) Immunotherapy transforms cancer treatment. J Clin Invest 129(1):46–47
Zhai J, Luwor RB, Ahmed N, Escalona R, Tan FH, Fong C et al (2018) Paclitaxel-loaded self-assembled lipid nanoparticles as targeted drug delivery systems for the treatment of aggressive ovarian cancer. ACS Appl Mater Interfaces 10(30):25174–25185
Zhang B, Zhang Y, Yu D (2017) Lung cancer gene therapy: transferrin and hyaluronic acid dual ligand-decorated novel lipid carriers for targeted gene delivery. Oncol Rep 37(2):937–944
Zhang J, Zhao X, Xian M, Dong C, Shuang S (2018a) Folic acid-conjugated green luminescent carbon dots as a nanoprobe for identifying folate receptor-positive cancer cells. Talanta 183:39–47
Zhang J, Yang C, Pan S, Shi M, Li J, Hu H et al (2018b) Eph A10-modified pH-sensitive liposomes loaded with novel triphenylphosphine–docetaxel conjugate possess hierarchical targetability and sufficient antitumor effect both in vitro and in vivo. Drug Deliv 25(1):723–737
Zhang Y, Guo Z, Cao Z, Zhou W, Zhang Y, Chen Q et al (2018c) Endogenous albumin-mediated delivery of redox-responsive paclitaxel-loaded micelles for targeted cancer therapy. Biomaterials 183:243–257
Zhang H, Liu XL, Zhang YF, Gao F, Li GL, He Y et al (2018d) Magnetic nanoparticles based cancer therapy: current status and applications. Sci China Life Sci 61(4):400–414
Zhang X, Zhang R, Huang J, Luo M, Chen X, Kang Y et al (2019) Albumin enhances PTX delivery ability of dextran NPs and therapeutic efficacy of PTX for colorectal cancer. J Mater Chem B 7(22):3537–3545
Zhao M-X, Zhu B-J, Yao W-J, Chen D-F (2016) Therapeutic effect of quantum dots for cancer treatment. RSC Adv 6(114):113791–113795
Zhao Q-S, Hu L-L, Wang Z-D, Li Z-P, Wang A-W, Liu J (2017) Resveratrol-loaded folic acid-grafted dextran stearate submicron particles exhibits enhanced antitumor efficacy in non-small cell lung cancers. Mater Sci Eng C 72:185–191
Zhao Z, Chen C, Xie C, Zhao Y (2020) Design, synthesis and evaluation of liposomes modified with dendritic aspartic acid for bone-specific targeting. Chem Phys Lipids 226:104832
Zheng YB, Gong JH, Liu XJ, Li Y, Zhen YS (2017) A CD13-targeting peptide integrated protein inhibits human liver cancer growth by killing cancer stem cells and suppressing angiogenesis. Mol Carcinog 56(5):1395–1404
Zheng G, Zheng M, Yang B, Fu H, Li Y (2019) Improving breast cancer therapy using doxorubicin loaded solid lipid nanoparticles: synthesis of a novel arginine-glycine-aspartic tripeptide conjugated, pH sensitive lipid and evaluation of the nanomedicine in vitro and in vivo. Biomed Pharmacother 116:109006
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Ramalingam, P. et al. (2022). Recent Advances in Nanomaterials-Based Drug Delivery System for Cancer Treatment. In: Krishnan, A., Ravindran, B., Balasubramanian, B., Swart, H.C., Panchu, S.J., Prasad, R. (eds) Emerging Nanomaterials for Advanced Technologies. Nanotechnology in the Life Sciences. Springer, Cham. https://doi.org/10.1007/978-3-030-80371-1_3
Download citation
DOI: https://doi.org/10.1007/978-3-030-80371-1_3
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-80370-4
Online ISBN: 978-3-030-80371-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)