Abstract
Cancer is considered the world’s deadliest disease, and its underlying pathology is intricate. Though cancer patients have had access to chemotherapeutics since aeons, the traditional treatments sometimes cause discomfort, have unwanted side effects, and are not site-specific. In this regard, several nanomaterials have lately been developed and explored specifically for use in cancer treatment ascribable to their unique optically active, magnetic, and electrical properties owing to nano-sized (1 to 100 nm) particles. Targeting, improved bioavailability, and low toxicity are vital reasons for nanomedicines becoming increasingly popular. Nanoparticles actively and passively target the cancer cells and kill them. Various ligands, including aptamers, biomolecules, peptides and/or antibodies have been in use for active targeting of cancer, while the cell characteristics, like leaky vasculature, angiogenesis etc., of cancer cells facilitate passive targeting. Focusing on nano oncology research investigations and clinical applications, this review traces the development and current state of targeted cancer therapy using nanomaterials. Also, this article furnishes account of challenges hindering the reach of these materials to clinical applications.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cancers manifest as anomalous aggregations of cellular material, frequently observed as a prevalent indication of inflammatory processes. It is a pathological condition characterised by the presence of a fluid-filled cavity, which may or may not originate from the proliferation of abnormally enlarged neoplastic cells. Cancer is a phenomenon characterised by the emergence of a novel growth that possesses the capacity to impact neighbouring tissues, propagate throughout the body and, in the absence of intervention, culminate in the demise of the afflicted individual [1, 2]. Conventional treatment options available do not address to the needs of patients. These suffer from limitations of poor bioavailability, non-site-specific delivery, toxicity and associated adverse reactions. Thus, the prime goal of delivery of drugs to cancerous tissues lies in achieving targeted drug delivery to a specific anatomical region, thereby maximising therapeutic efficacy while minimising the potential for adverse effects or tissue injury resulting from diffusion to non-targeted regions [3,4,5].
The key purpose of drug delivery research is to advance the field by formulating therapeutic interventions that possess practical clinical applications, ultimately benefiting patients. In the last one decade, significant progress has been made in the domain of drug delivery technology, leading to the emergence of nanoparticulate-based drug delivery that optimises patient compliance and convenience [6]. With the advent of modern technologies, it is now feasible to administer drugs over extended periods of time, spanning weeks to months, while precisely controlling their release kinetics, along with improved biopharmaceutical performance. Nano-guided cancer treatment is burgeoning as a revolutionary offshoot of multidisciplinary research which is likely to surpass conventional cancer diagnosis and treatment methods [7,8,9]. Nanotechnology has been a boon for the many horrendous diseases including cancer. Delivering of the administered doses of therapeutic factors (drugs/plant bioactives/RNA etc.) into tumour cells while bypassing normal cells with the use of nanosized biomaterials is known as nanoparticulate targeted cancer delivery. This strategy tends to overcome the challenges associated with traditional chemotherapies. Moreover, nanocarriers owing to their higher surface-to-volume ratio enable improved bioavailability of poorly bioavailable drugs following their administration. Despite notable advancements, there remain certain domains that necessitate further efforts prior to the attainment of clinical relevance. An area of significant interest lies within the realm of targeted delivery to cancer [10,11,12,13]. The use of nanoparticle-based drug carriers in cancer not only improves the efficacy of therapy and reduces associated toxicity, but also provides a shield to normal cells. Nanoparticles should direct anticancer drugs to tumour tissues, while circumventing the all-phagocytic barriers with minimal systemic activity. After intracellular administration, the active medicine should exclusively affects malignant cells.
Thus, through current article, the authors would furnish an account on the use of nanotechnology for targeted cancer treatment, challenges associated with nano-oncology and regulatory perspective on the use of nanotechnology.
Nanotechnology-enabled carrier systems for cancer treatment
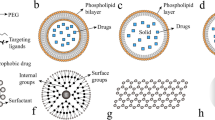
Nanotechnology brings a whole buffet of technologies to the table, from nanochips and nanoscale probes to liposomes and polymeric micelles for the diagnosis and treatment of carcinomas [12]. The marvellous nanoparticles and tiny wonders can assume an impressive array of forms and sizes, owing to the availability of numerous manufacturing technologies. Nanoparticles can be employed as drug carriers like liposomes [14, 15], polymer-drug conjugates [16, 17] and immunoconjugates [18, 19] due to their ability to change form and size during production. Lipid-based delivery systems, including solid lipid and nanostructured lipidic carriers, liposomes, self-emulsifying delivery systems [20] [21], polymer-based nanovectors [22, 23], dendrimers [24, 25] and inorganic nanoparticles [26, 27], form different classes of nano biomaterials employed for cancer theranostics. Lipid-based systems comprise the maximum share of all nanocarriers that have been explored owing to their biocompatibility and potential to scale up [28]. The nanoformulations have advantages over the traditional approaches as these make use of either passive targeting (non-ligand-mediated nanoparticles) or active targeting (ligand-coupled nanoformulations) to localise the actives to affected tissue only. Hence, normal tissues are not affected, and there are hardly any off-site-related complications. The various delivery carriers have been explained in the following section, while underscoring their role in cancer treatment and how these are helping to target the cancer cells.
Lipidic nanocarrier systems
Liposomes
Liposomal nanosystems represent vesicular delivery carriers that encapsulate the drug within the vesicles, facilitating its targeted transportation to the desired region of action. The limitations associated with conventional chemotherapy can be effectively addressed through the implementation of such strategies that enhance drug delivery specifically to the tumour tissue while minimising drug reaching the normal tissues [29]. The utilisation of liposomal drug delivery systems for chemotherapeutic agents presents notable benefits, as these carriers effectively shield the drug from degradation, prolong their presence within the bloodstream and enhance their pharmacokinetic characteristics. Upon successful permeation into the tumour, the preferential accumulation of nanocarriers within the tumour microenvironment is facilitated by the compromised lymphatic drainage in the tissue. The lymphatic function within tumours exhibits a notable deficiency, leading to a limited uptake of the interstitial fluid. Hence, the nanocarriers that have successfully traversed the perivascular region exhibit suboptimal clearance rates and tend to accumulate within the tumour interstitium. The phenomenon of spontaneous accumulation or “passive” targeting is commonly referred to as the enhanced permeability and retention (EPR) effect in the scientific community. The exploitation of the enhanced permeability and retention (EPR) effect is thus a highly efficacious approach for the precise localization of nanopreparations, specifically liposomes, to the tumour site, which has been extensively elucidated in scientific literature. Unaltered liposomes exhibit a swift elimination from the bloodstream due to the phagocytic activity of the reticuloendothelial system (RES), with the liver and spleen serving as the primary sites of clearance [30]. The surface grafting of polyethylene glycol (PEG) leads to the emergence of “stealth” or stabilised liposomes, thereby enhancing their in vivo stability and prolonging their circulation time, typically ranging from 24 to 48 h. These liposomes are commonly referred to as long-circulating liposomes. The performance of PEG as a stabiliser is contingent upon several key factors, namely the length of the polymer chain, the optimal surface density and the ideal configuration of the polymer chains [31].
Solid lipid nanoparticles (SLNs)
This is a novel colloidal drug delivery system that effectively combines the characteristics of liposomes and polymeric nanoparticles. SLNs can provide both the stability of the solid core and the biocompatibility of lipid nanocarriers, averting the limitations of liposomes and polymeric nanoparticles such as long-term stability, toxicity, sterilisation and scalability [32]. It has been discovered that SLNs improve the solubility, bioavailability and therapeutic efficacy of water-insoluble pharmaceuticals. However, these are susceptible to non-specific uptake, which limits their effectiveness in the treatment of certain cancers, like prostate cancer. In this regard, surface modification of SLNs in order to circumvent absorption via RES improves tumour selectivity [33].
Nanostructured lipidic carriers
These are the advanced SLNs, and these carrier systems have demonstrated the potential to enhance the therapeutic effectiveness of encapsulated cargo by employing either an active or passive targeting strategy against various cancer types. The utilisation of targeted nanomedicine holds great promise in facilitating the precise delivery of drug carriers to the designated tumour-targeted tissue/cells, thereby minimising any potential impact on the surrounding healthy tissue/cells. The utilisation of active targeting involves the exploitation of the binding affinity between a cancer-specific ligand and the surface of the NLCs. This strategic approach enhances the therapeutic effectiveness and safety profile of the cancer therapeutics [34].
Self-emulsifying drug delivery carriers
Nanomedicines have been proposed researched for targeted anticancer therapy. The number of nanomedicines utilised in clinical settings is less than the number of nanotechnology-based cancer therapy studies undertaken. This may be attributable to technology transfer problems from the laboratory to the bedside. In this regard, SEDDS, the isotropic mixtures of oil, surfactant and cosolvent, are an emerging system for systemic drug delivery owing to ease of formulation, thermodynamic stability and capability of encapsulating lipophilic pharmaceuticals [35].
These mixtures spontaneously form an oil-in-water nanoemulsion on encountering the physiological media. The research efforts have been undertaken to actively target such systems in different cancers. In this regard, Giarra et al. (2019) had optimised the formulation of SEDDS coated with enoxaparin (Enox), which showed enhanced cellular uptake compared to uncoated SEDDS in cancer cells. Low molecular weight heparins (LMWH), viz., enoxaparin (Enox), are natural glycosaminoglycans employed as the gold standard for the treatment of thrombosis. Owing to their negative charge, these are able to bind a large amount of intracellular and extracellular matrix components, which are also involved in tumour progression and influence their activity. It has been demonstrated that LMWH has the ability to bind to several drug transporters of the ABC and non-ABC families, directly inhibit ATPase activity and reduce the efflux of chemotherapeutic agents, thus enhancing their cytotoxicity. Furthermore, heparin and derivatives have also been reported to interact with lung resistance protein (LRP), the main non-ABC transport protein and with vascular endothelial, fibroblast and angiogenetic growth factor receptors, which are strictly involved in tumoural angiogenetic processes. Based on these data, LMWH may be useful as ligands on delivery systems for targeting chemo-resistant cells [36].
Polymeric nanoparticles
Polymeric nanomaterials are often used to carry drugs as they are biocompatible, biodegradable, and can have many different shapes. Polymers could self-assemble into polymeric nanoparticles that could hold healing drugs or imaging agents at the same time. Coated with PEG, these can stay in the blood longer, escape being quickly recognised and thrown out by the immune system, and slowly release drugs in tumours while also making it easier to target tumours. Polymers can still build up in diseased tissues where they are supposed to, either passively through the increased EPR effect or actively through cell surface ligands or receptors [37].
Inorganic nanoparticles
In recent years, the advent of nanoparticles has presented a promising platform for the advancement of this therapeutic approach. In the realm of nanoparticles, it is the inorganic nanoparticles that emerge as the most fitting materials owing to their distinctive optical, electrical, thermal and magnetic properties, as well as their remarkable proficiency in facilitating drug delivery. These have been meticulously categorised into distinct classes, namely metal, carbon, silicon, magnetic and composite inorganic nanoparticles. The utilisation of these agents in cancer therapy has been extensive as well [38]. The modification of surface with ligands, like CD47, on platelet membranes facilitates the evasion of nanoparticle clearance by the immune system. Moreover, it is noteworthy to highlight that the existence of numerous receptors on the platelet membrane surface allows for direct interactions with distinct constituents present at the tumour location, thereby facilitating precise and targeted outcomes [39].
Table 1 summarises various carrier systems and the targeting ligands employed to improve their potential to target cancer.
Targeted nano drug delivery in cancer: mechanisms
The use of nanoparticle-based drug carriers in cancer improves the efficacy of therapy, reduces associated toxicity and provides a shield to normal cells. Nanoparticles should direct anticancer drugs to tumour tissues, while circumventing the all-phagocytic barriers with minimal systemic activity, thereby reducing exposure to normal cells [4, 40, 41].
For cancer therapy to be effective, controlled drug release mechanisms for nanomaterial-based cancer-targeted delivery systems are essential [42]. The controlled release is achieved through stimuli-responsive nanomaterials. Stimulus-responsive nanocarriers that take advantage of the distinct tumour microenvironment are frequently used in these systems. Nanomaterials can experience structural changes that result in regulated drug release when they are stimulated by a variety of factors, including pH, temperature, enzymes or external fields. While thermosensitive materials react to temperature variations characteristic of malignant locations, pH-sensitive nanocarriers take advantage of the acidic environment of tumour tissues for drug release. Specific enzymes that are prevalent in the tumour microenvironment are necessary for enzyme-triggered release because they enable drug liberation upon enzymatic breakdown. Furthermore, on-demand drug release is made possible by external stimuli such as light, ultrasound or magnetic fields, which improves treatment accuracy. These mechanisms provide promising opportunities for enhanced cancer therapy by ensuring targeted medication delivery to cancer cells while limiting systemic toxicity through nanomaterial-based delivery systems [43].
Utilising the increased permeability and retention effect of tumours, passive targeting targets therapeutic drugs specifically to malignant tissues while limiting exposure to healthy cells. Active targeting increases the uptake of medications at the tumour site by using ligands or antibodies to bind selectively to receptors that are overexpressed on cancer cells. When combined, these tactics improve cancer therapy’s therapeutic outcomes by increasing treatment specificity and lowering off-target side effects. Hence, in a nutshell, passive targeting uses non-ligand-mediated nanoparticles, while active targeting uses ligand-coupled nanoformulations for targeting the cancerous cells [44, 45].
Passive targeting
It is widely recognised that under certain conditions, such as inflammation or hypoxia, frequently observed in tumours, the endothelium of blood vessels demonstrates heightened permeability in comparison to its normal state [46]. In such conditions, neoplasms that demonstrate accelerated growth exhibit angiogenesis, wherein they actively initiate developing new blood vessels or encompass pre-existing vasculature in proximity. The newly devised permeable vessels enable the selective and enhanced transportation of macromolecules with molecular size greater than 40 kD and nanovector systems into the stroma of the tumour [47].
Furthermore, the conventional lymphatic drainage is lacking within the tumour milieu and serves as a contributing element to the accumulation of nanoparticles (NPs) [48]. The aforementioned characteristics, however, are not pertinent to small molecule pharmaceuticals, as these demonstrate a significantly short duration within the systemic circulation system and quickly disperse from the tumour site. Thus, the phenomenon of encapsulating molecular pharmaceutical entities within nanoscale drug carrier systems improves their pharmacokinetic characteristics, resulting in prolonged systemic circulation duration [48, 49]. Moreover, this methodology provides a certain level of precision targeting towards neoplastic tissues while simultaneously minimising undesired side effects. The phenomenon of tumour targeting, commonly known as “passive” targeting, is contingent upon the intrinsic attributes of the carrier, encompassing its dimensions and duration of circulation, alongside the distinctive properties exhibited by the tumour, notably its vascularity and permeability [49,50,51]. Figure 1 portrays the active and passive targeting approaches of cancer targeting.
Active targeting through surface tuning
“Active targeting” describes explicit interactions amidst a drug or drug carrier and the target cells, which are generally mediated by definite ligand-receptor interactions [52, 53]. It is only possible for ligand-receptor interactions to occur when the two components are close to one another. When you hear the term “active targeting,” you might imagine employing a cruise missile to point a drug or drug carrier in a certain direction. However, present medication delivery techniques are unable to focus on a specific target. They arrive at the desired area by blood flow and extravasation, which is followed by intratumoural retention and dispersion. Simply put, “active targeting” refers to a certain “ligand-receptor type interaction” for intracellular localisation. In order to improve delivery to the tumour location, it is believed that increasing the EPR effect and/or lengthening blood circulation duration through PEGylation (i.e., changing the exterior plane of nanovectors with polyethylene glycol) will be effective [54]. Instead of enhancing tumour accumulation per se, active pharmaceutical targeting is often employed to improve target cell recognition and uptake. Antibodies, ligands, proteins/peptides and other biomolecules are employed for active targeting in cancers [54].
Immunoglobulins
Antibodies possess a recognised ability to identify cancerous cells, particularly those that exhibit a high expression of receptors or surface antigens. Since the inception of the initial monoclonal antibody (mAb) targeting tumour antigens in 1975, several mAbs have obtained clearance from the FDA for the purpose of cancer therapy [55, 56]. Nevertheless, certain mAbs are presently undergoing evaluation in clinical studies[57]. Indeed, antibody-based immunotherapy has been employed for the treatment of cancer. Additionally, the precise targeting capabilities and strong binding affinities of antibodies towards their intended targets have been harnessed to facilitate drug administration through carriers, such as lipid-based vectors [58]. The utilisation of monoclonal antibody (mAb)-conjugated drug carriers for targeted therapy is widely recognised as a significant and potentially curative approach [59]. However, it is important to note that prolonged administration of these therapies may lead to the development of immunological memory towards the antibodies. The utilisation of antibody fragments or chimeric antibodies has the potential to significantly mitigate the immunogenicity associated with full antibodies [60]. An instance of a successful use of cetuximab, a recombinant monoclonal antibody consisting of a variable (murine) and a constant region (human), involves its utilisation in cancer treatment through the specific targeting of the epidermal growth factor receptor [61]. The employment of twofold targeting antibodies is a developing approach that enhances the efficacy of tumour targeting, as antibodies possess dual epitope binding sites with ability to interact with either one or double target sites [62].
DNA/RNA aptamers
Short, single-stranded DNA or RNA aptamers possess intricate 3-D conformations. The application of Systemic Evolution of Ligands by Exponential Enrichment (SELEX) has resulted in the generation of nucleotide ligands with the ability to bind to specific sites on cancer cells [63]. The utilisation of cell-based SELEX exhibits significant promise due to its ability to selectively target cancer cells without requiring an extensive understanding of the protein expressions on their cell surfaces. This approach enables the development of distinct aptamers that can effectively target various types of cancers. Aptamers exhibit greater stability in vivo compared to antibodies and can be synthesised through chemical means using in vitro selection, whereas the production of antibodies targeting specific antigens necessitates the involvement of biological systems [64]. In vitro production of aptamers necessitates the availability of many targets. The targeting of membrane antigen specific to prostate and nucleolin is achieved by exploring aptamer-conjugated lipid-based drug delivery systems. Aptamers, although very nascent in their development and not as widely recognised as antibodies, are currently being discussed with novel cancer-targeting lipid-based drug carriers [65]. The combination of nucleotide aptamers and lipid-based vectors has been identified as a highly effective approach for antibody-guided applications in vivo, mostly attributed to their low immunogenicity [66]. The limited affinity of aptamers in comparison to antibodies poses a constraint on their utility. Aptamers possessing a single epitope binding site exhibit diminished binding affinities. The enhancement of aptamer binding affinity can be achieved through a number of approaches, while the utilisation of multivalent designs that incorporate a flexible linker is most advantageous [67, 68].
Other ligands
Transferrin, biotin, hyaluronic acid, folic acid and natural or synthetic proteins/peptides with short varied amino acid sequences may recognise target cancer cells [43, 69]. Small peptides or ligands target cancer cell receptors with remarkable specificity. Transferrin, a serum glycoprotein, interacts to cell surface transferrin receptors to internalise iron. Due to iron needs, malignant cancer cells like the bladder, brain, breast, lung and lymphoma upregulate transferrin receptors. Transferrin-conjugated drug delivery methods allow receptor-mediated endocytosis of pharmaceuticals [70]. Hyaluronic acid, another such ligand molecule, targets the CD44 receptor. This linear glycosaminoglycan binds to malignancies with CD44-overexpression, including head and neck, stomach, colon, liver and breast cancers. However, cellular absorption and drug carrier efficacy are highly dependent upon the molecular weight of HA used [71, 72]. Folic acid, vitamin B9, interacts with the folate receptor to help cells internalise. Folate receptor targeting is a beneficial strategy since it is not highly expressed in normal tissues, but it is strongly expressed in many malignancies, notably female cancers including cervical, breast and ovarian [73, 74]]. Natural ligands improve tumour access and treatment.
Cell targeting peptides (CtPs) are synthesised from libraries of peptides and employed as targeting ligands. CtPs are more stable than antibodies and have ten amino acids. CtP amino acid sequences identify targets, and appropriate sequences for interactions with specific cancer cell surface receptors are essential [75]. The arginylglycylaspartic acid (RGD) peptide, which binds to integrin receptors overexpressed on multiple cancer cell types, is the most thoroughly researched CtP [75, 76].
Site-specific delivery using extravascular vesicles
The concept of site-specific targeting refers to the ability to selectively deliver a substance or agent to a specific location within a biological system. In this approach, extracellular vesicles (EVs), nanovesicles enclosed by a lipid bilayer, are actively released by various cellular entities. This diverse population encompasses microvesicles, exosomes and apoptotic bodies. Extracellular vesicles (EVs) are lipid-based vesicles that exist within the confines of a cell, typically exhibiting a diameter ranging from 50 to 2000 nm. These vesicles have been widely explored and are recognised for their association with intercellular communication processes [77]. The utilisation of electric vehicles for drug delivery has been proposed in recent times, owing to their inherent ability to transport therapeutic agents and various advantages they possess in comparison to liposomes. Hence, it is conceivable that the incorporation of diverse therapeutic agents, such as miRNA, siRNA or chemical drugs, alongside a synergistic approach involving the administration of anticancer drugs and oncolytic adenovirus enclosed within extracellular vesicles (EVs), presents a viable strategy for the delivery of pharmaceutical compounds for cancer therapeutics [78].
Table 1 delineates various literature instances for targeted drug delivery in cancer using antibodies, aptamers, ligands and /or peptides.
Challenges in nano-targeted delivery in cancer
Currently, the volume of research and knowledge dedicated to nanotechnology has profoundly flourished. However, only a few of them have actually advanced to clinical trials. Hence, the clinical translation of each nanoformulation is characterised by unique challenges including clinical EPR effect, tumor heterogenity, drug resistance, extravasation and lack of regulations (Fig. 2).
Clinical EPR effect
The EPR effect, while present, exhibits a relatively modest level of tumour specificity, resulting in a mere 20–30% increase in delivery vis-a-vis normal tissues. The manifestation of the EPR effect is contingent upon the inherent biological characteristics of the tumour [89], specifically: (1) the extent of angio- and lymph-genesis, (2) the extent of perivascular tumour expansion and the density of the stromal reaction, and (3) the intratumoural pressure [90]. The collective influence of these various factors, in conjunction with the physicochemical attributes of nanocarriers, shall ultimately dictate their efficacy in delivering drugs.
The permeability of recently developed tumour blood vessels impacts the penetration of nanomedicine, while also leading to increased interstitial pressure. Interestingly, this elevated pressure hinders the accumulation of drug carriers within the tumour. Furthermore, as a consequence of the disparity between pro- and anti-angiogenic signalling inside distinct regions of the tumour, the vasculature exhibits anomalous characteristics such as dilation, tortuosity and the presence of saccular channels. Additionally, the interconnections and branching within the vessels appear quite disorganised [91, 92].
As a consequence of the disparate blood supply, neoplastic cells exhibit irregular growth patterns, wherein those in close proximity to vascular structures undergo more rapid proliferation compared to those situated within the central region of the tumour, which experience limited access to essential nutrients and oxygen [92]. This elucidates the presence of hypoxic/necrotic regions within the central regions of sizable tumours, specifically those measuring 1–2 cm in diameter in murine models. Furthermore, it frequently poses a challenge for nanomedicines to effectively penetrate and access the blood vessels located within the central region of the tumours exhibiting a relatively lower degree of leakage compared to what might be anticipated, primarily owing to the elevated interstitial pressure. The said phenomenon has been observed in a diverse array of murine and human tumours. The presence of elevated interstitial pressure exerts a dual effect on drug delivery, impeding the process of convection and concurrently exerting compressive forces on nascent blood capillaries. This leads to the flow of blood to get redirected from the tumour’s core to its periphery [93, 94].
Nevertheless, it is also conceivable to chemically or mechanically modulate the EPR effect in order to attain vascular normalisation, thereby resulting in an augmented accumulation of nanocarriers. Within the realm of chemical EPR enhancers, it is possible to identify various substances such as bradykinin (also known as kinin), nitric oxide, peroxynitrite, prostaglandins, vascular permeability factor (VPF)/vascular endothelial growth factor (VEGF) and a multitude of other cytokines. These particular molecules have the capacity to induce hypertension or promote vascular normalisation, thereby potentially leading to a temporary enhancement of tumour perfusion. Alternative methodologies employ ultrasound [94], radiation, hyperthermia or photo-immunotherapy techniques to manipulate the vasculature of tumours and enhance the permeation of nanosystems. However, it is important to acknowledge that all of the aforementioned methodologies possess inherent limitations and contraindications, necessitating meticulous deliberation [95]. Quantitative evaluation of the EPR effect for individual dose is essential to comprehend the effect of dose and potential effects of successive multi-doses on bioefficacy.
Tumour extravasation and infiltration
A drug carrier possesses the capability to selectively identify and locate its intended target cell within a solid tumour, even amidst the presence of various cell types. This is achieved by traversing the intricate network of blood vessels within the tumour and adopting the structure of either a soluble macromolecule or a nanoparticle. The carrier system, however, needs to transverse through blood vessel openings in order to penetrate the tumour tissue and disseminate there. The transportation of molecules from the blood compartment to the tumour tissue is controlled by the synergistic influence of convection and diffusion. The occurrence of convective motion is a direct result of the existence of a pressure gradient. Unfortunately, a significant proportion of solid neoplasms demonstrate elevated interstitial fluid pressure (IFP), with a range of values between 5 and 40 mm Hg depending on the size of the tumour. In contrast, it is generally observed that healthy tissue tends to sustain an interstitial fluid pressure (IFP) of 3 mm Hg. The extravasation of drug carriers through convective fluid flow depends on the difference in colloid osmotic pressures in the compartments, as well as the disparity between the interstitial fluid pressure (IFP) within the tumour and the hydrostatic pressure within the capillaries (ranging from 10 to 30 mm Hg). Drug carriers possess the capability to infiltrate compartments of cancerous tissue through the utilisation of stochastic Brownian motion. This motion allows them to navigate through intercellular gaps, commonly referred to as fenestrae, which possess dimensions that are adequately spacious to accommodate a wide array of nanoparticles [96, 97]. The biological characteristics of the vasculature, including its distribution, density and sizes of fenestrae, as well as nanoparticles characteristics, such as surface properties, shape, size and amount localised to the tumour blood vessel, can affect the speed of diffusional extravasation. According to a study, the amount of a test particle that can penetrate a solid tumour depends on the size of the particle, again placing severe restrictions on the test particle’s ability to travel a significant distance. Accordingly, there is little chance that any nanoparticle will actually come in contact with bulk of target cells within the tumour. The intratumoural distribution of nanoparticles that can interact with extracellular matrix (ECM) components and non-target cells in a tumour becomes much worse when the mobility of the particles is further reduced [98]. Another potential obstacle for applications employing nanoparticles is the possibility that they may be large enough to physically block tumour vascular fenestrae, suppression of self-entry and subsequent entry of chemical substances until they are expelled from the aforementioned sites. Intratumoural distribution is a crucial factor when developing clinically applicable targeted carrier systems, even if intracellular trafficking and systemic biodistribution are of considerable interest to the majority of drug delivery studies [98].
Tumour heterogeneity
Histology and genomic profiling are two clinical methods used to classify tumours into one of many potential subtypes. For example, one of the 120 subtypes of tumours of the CNS can be categorised [99]. In particular, this single classification fails to capture the variety of intratumour cancer cells, which is crucial for initiatives involving the delivery of tailored medications to treat human disease. As a result of their propensity for mutation, cancer cells are known to change with a tumour both spatially and over time. The same tumour can therefore give rise to many human cell lines. The intratumoural heterogeneity induced by genetic variation and epigenetic change is supported by both cancer stem cell and clonal evolution hypotheses [100]. Haematopoietic stem cell-derived blood cells exhibit organisational variability, as predicted by the cancer stem cell theory. Somatic stem cells are more prone to acquire oncogenic genetic alterations than less persistent non-stem cells. Furthermore, it is established that cancer stem cells are responsible for metastasis, recurrence and resistance to chemotherapy and radiation. In fact, there is a theory that says cancer is a sort of stem cell malfunction. Henceforth, the ongoing discourse persists regarding the categorisation of cancer as a malady originating from stem cells. A tumour does not exhibit characteristics of a monoculture or a concentrated population of a single cell type. Cell population heterogeneity exists in even cultivated cell lines, with cancer stem-like cells displaying a unique set of surface markers from other bulk cells that can tolerate traditional cytotoxic treatments. Surface markers that identify a population of cancer stem-like cells from that tumour may not be the same as isolated cell lines from that tumour, suggesting that cancer stem cells may have a different origin. The epithelial-mesenchymal switch is a renowned transition of differentiated cells towards a more oncogenic phenotype, which includes cancer stem-like cells in metastasis. Multiple cell subpopulations in various phases of transition between these diverse cell phenotypes may exist simultaneously in a tumour in a state of dynamic equilibrium. Together, these cells have the capacity to produce a tumour microenvironment that is incredibly complicated. Together, these cells have the capacity to produce a tumour microenvironment that is incredibly complicated. All things considered, selecting a single population amongst diverse, shifting and moving populations to target with a single surface marker is equivalent to selecting cancer cells. Due to the given surface marker’s shared features with normal cells within the tumour, recognition and classification of a specific cancer cell variety by just one surface marker can end up in an incorrect estimation of cancers. Therefore, single surface marker techniques are frequently considered to be obsolete. One approach to identifying and locating cancer cells is the multiple surface marker method [101].
Likewise, “active targeting” might not always be ensured by the simple existence of a particulate ligand on the exterior of nanoparticles. Cancer cell lines that are cultured and maintained in vitro through tissue culture techniques may not accurately reflect the inherent characteristics of the original cancer cells identified within a patient’s tumour, thereby limiting their suitability for identifying potential receptors for “active targeting”. Additionally, it is just unclear how frequently and in what amounts each cancer cell expresses a certain receptor in tumour cells. Because of this, promising findings from early in vitro studies may not be directly comparable to those from xenograft model investigations using these well-established cancerous cell lines in rats or, more critically, to those from spontaneous human tumours [102]. Due to these challenges in determining an effective ligand-receptor interaction, the efficacy of “active targeting” is questioned in this instance.
Drug resistance
Also, cancer cells can become resistant to nanoparticles either due to target’s own mutations or difficulty in building medications that target some of the identified targets with complex structure. The mutations may cause the targeted therapy to no longer interact well with the target, and the tumour may find a whole new growth path that is unrelated to the target [103].
Lack of regulatory controls
While there is much anticipation for the future of nanomedicine, the area still lacks comprehensive regulatory guidelines [104]. Regardless, many nanomaterials and nanotechnologies have been authorised by regulators and used in clinical settings for a wide variety of drugs, including the liposomal preparations, Doxil® and AmBisome®, albumin-drug nanoparticles such as Abraxane® and polymeric micelles such as Eligard® to name a few [104]. Moreover, numerous new nanomedicines perform through interaction with genetic materials or biomolecules essential for regular genome function and cellular division, thereby potentially inducing genotoxicity and mutagenicity [105]. Also, clinical trials for cancer therapies encounter many clinical and regulatory challenges, which is a matter of concern [106]. Therefore, a solid regulatory framework is required to manage these crucial features of nanotechnology-based cancer-targeting techniques.
Issues of biocompatibility, stability and clearance of nanomaterials
The critical aspects related to nanoparticles are their biocompatibility, stability and clearance from the body. Biocompatibility can be encountered using biocompatible lipids/polymers for the fabrication of nanomaterials [34]. However, stability is a vital concern, as nanomaterials are prone to agglomeration, degradation or changes in their physical and chemical properties over time. Hence, the application of apt stabilisation strategy like surface functionalization, coating with biocompatible materials or encapsulation can help enhance their stability and performance [68]. Also, clearance of nanomaterials is vital for ensuring that nanomaterials are safely removed from the body after use. Designing nanomaterials with appropriate size, shape and surface properties can influence their clearance rates and minimise potential accumulation in organs or tissues [105].
In a nutshell, addressing these issues requires a multidisciplinary approach involving materials scientists, biologists, pharmacologists and regulatory agencies to develop safe and effective nanomaterials for various biomedical applications.
Conclusions
The most effective method of cancer therapy medication administration is one that delivers the drug only to the targeted tumour. Furthermore, in order to address the potential limitation of incomplete tumour removal through single-target approaches, it may be imperative to concurrently direct efforts towards multiple targets. It may be advantageous to develop “magic shotgun” strategies to deliver the medicine to diverse targets or deliver many drugs (101, 103). Advancements in nanotechnology have the potential to revolutionise healthcare by improving diagnosis, treatment and patient care on the whole. This would allow for early detection and strategizing interventions for the delivery of precision medicine and improved treatment outcomes.
Numerous strategies and procedures have been investigated over time for medication targeting to tumours. But most of them have been unsuccessful in either early-stage or late-stage clinical testing. This may be the result of a number of factors, chief amongst them the overinterpretation and/or misinterpretation of some of the mechanistics underlying tumour-targeted drug delivery, as well as the fact that some of the formulations tested fell short of expectations in monotherapy regimens or were too complex for the market to scale up. The advancement of targeted drug delivery utilising nanomaterials in cancer treatment relies heavily on interdisciplinary collaboration amongst researchers from a spectrum of domains. In order to tackle associated challenges with cancer targeting, a diverse group of experts, including biomedical engineers, chemists, material scientists, pharmacologists, computer scientists and oncologists, need to come together. Collaboration would promote advancements in imaging technologies, which will pave the way for non-invasive real-time tracking of drug distribution and therapeutic response. Potentially improving cancer treatment efficacy while decreasing off-target effects and optimising patient outcomes, interdisciplinary collaborations would speed up the translation of tailored nanomedicines from bench to bedside by combining knowledge from different fields.
References
Greten FR, Grivennikov SI (2019) Inflammation and cancer: triggers, mechanisms, and consequences. Immunity 51(1):27–41
van Weverwijk A, de Visser KE (2023) Mechanisms driving the immunoregulatory function of cancer cells. Nat Rev Cancer 23(4):193–215
Senapati S, Mahanta AK, Kumar S, Maiti P (2018) Controlled drug delivery vehicles for cancer treatment and their performance. Signal Transduct Target Ther 3(1):7
Yao Y, Zhou Y, Liu L, Xu Y, Chen Q, Wang Y, Wu S, Deng Y, Zhang J, Shao A (2020) Nanoparticle-based drug delivery in cancer therapy and its role in overcoming drug resistance. Front Mol Biosci 7:193
Moyo AA, Jagadhane KS, Bhosale SR, Shinde SB, Marealle AI, Shimpale VB, Anbhule PV (2023) Anbhule, anticancer and apoptotic effects of Hymenodictyon floribundum (Hochst. & Steud.) BL Rob. Stem Bark Hydroethanolic Extract. J Chemistry Africa 7(3):1235–1250
Mudgil M, Gupta N, Nagpal M, Pawar P (2012) Nanotechnology: a new approach for ocular drug delivery system. Int J Pharm Pharm Sci 4(2):105–112
Thakor AS, Gambhir SS (2013) Nanooncology: the future of cancer diagnosis and therapy. Ca: Cancer J Clinicians 63(6):395–418
Bhosale SR, Bhosale RR, Patil DN, Dhavale RP, Kolekar GB, Shimpale VB, Anbhule PV (2023) Bioderived mesoporous carbon@ tungsten oxide nanocomposite as a drug carrier vehicle of doxorubicin for potent cancer therapy. J Langmuir 39(33):11910–11924
Bhattacharya T, Soares GABE, Chopra H, Rahman MM, Hasan Z, Swain SS, Cavalu S (2022) Applications of phyto-nanotechnology for the treatment of neurodegenerative disorders. J Materials 15(3):804
Jin C, Wang K, Oppong-Gyebi A, Hu J (2020) Application of nanotechnology in cancer diagnosis and therapy-a mini-review. Int J Med Sci 17(18):2964
Mosleh-Shirazi S, Abbasi M, Reza Moaddeli M, Vaez A, Shafiee M, Kasaee SR, Amani AM, Hatam S (2022) Nanotechnology advances in the detection and treatment of cancer: an overview. Nanotheranostics 6(4):400
Kemp JA, Kwon YJ (2021) Cancer nanotechnology: current status and perspectives. Nano convergence 8(1):34
Hsieh CY, Ko PW, Chang YJ, Kapoor M, Liang YC, Lin HH, Horng JC, Hsu MH (2019) Design and synthesis of benzimidazole-chalcone derivatives as potential anticancer agents. Molecules 24(18):3259
Fulton MD, Najahi-Missaoui W (2023) Liposomes in cancer therapy: how did we start and where are we now. Int J Mol Sci 24(7):6615
Allahou LW, Madani SY, Seifalian A (2021) Investigating the application of liposomes as drug delivery systems for the diagnosis and treatment of cancer. Int J Biomater 2021:3041969
S. Wadhwa,R.J. Mumper, Polymer-drug conjugates for anticancer drug delivery. Critical Reviews™ in Therapeutic Drug Carrier Systems, 2015. 32(3)
Alven S, Nqoro X, Buyana B, Aderibigbe BA (2020) Polymer-drug conjugate, a potential therapeutic to combat breast and lung cancer. Pharmaceutics 12(5):406
Petrilli R, Pinheiro DP, de Cássia Evangelista F, de Oliveira GF, Galvão LG, Marques RFV, Lopez C, Pessoa J.O. Eloy (2021) Immunoconjugates for cancer targeting: a review of antibody-drug conjugates and antibody-functionalized nanoparticles. Curr Med Chem 28(13):2485–2520
Smaglo BG, Aldeghaither D, Weiner LM (2014) The development of immunoconjugates for targeted cancer therapy. Nat Rev Clin Oncol 11(11):637–648
Bhardwaj K, Sharma A, Kumar R, Tyagi V, Kumar R (2023) Improving oral bioavailability of herbal drugs: a focused review of self-emulsifying drug delivery system for colon cancer. Current Drug Delivery 21(3):389–402
Sharma T, Jain A, Kaur R, Saini S, Katare O, Singh B (2020) Supersaturated LFCS type III self-emulsifying delivery systems of sorafenib tosylate with improved biopharmaceutical performance: QbD-enabled development and evaluation. Drug Deliv Transl Res 10:839–861
Yu J, Qiu H, Yin S, Wang H, Li Y (2021) Polymeric drug delivery system based on pluronics for cancer treatment. Molecules 26(12):3610
Sung YK, Kim SW (2020) Recent advances in polymeric drug delivery systems. Biomaterials Research 24(1):1–12
Mukherjee S, Mukherjee S, Abourehab MA, Sahebkar A, Kesharwani P (2022) Exploring dendrimer-based drug delivery systems and their potential applications in cancer immunotherapy. Eur Polym J 177:111471
Nanjwade BK, Bechra HM, Derkar GK, Manvi F, Nanjwade VK (2009) Dendrimers: emerging polymers for drug-delivery systems. Eur J Pharm Sci 38(3):185–196
Ateeq H, Zia A, Husain Q, Khan MS (2023) Role of inorganic nanocomposite materials in drug delivery systems. synthesis and applications of nanomaterials and nanocomposites. Springer, pp 171–195
Zhao C, Rehman FU, Shaikh S, Sajid Z, Mian AA, He N (2022) Metallic nanoscale-knife application in cancer theranostics. Smart Mater Med 4:313–336
Liu Q, Zou J, Chen Z, He W, Wu W (2023) Current research trends of nanomedicines. J Acta Pharmaceutica Sinica B 13(11):4391–4416
Mukherjee A, Bisht B, Dutta S, Paul MK (2022) Current advances in the use of exosomes, liposomes, and bioengineered hybrid nanovesicles in cancer detection and therapy. Acta Pharmacol Sin 43(11):2759–2776. https://doi.org/10.1038/s41401-022-00902-w
Onzi G, Guterres SS, Pohlmann AR, Frank LA (2021) Passive Targeting and the enhanced permeability and retention (EPR) effect. the ADME encyclopedia: a comprehensive guide on biopharmacy and pharmacokinetics. Springer International Publishing, Cham, pp 1–13
Nag OK, Awasthi VJP (2013) Surface engineering of liposomes for stealth behavior 5(4):542–569
Mishra V, Bansal KK, Verma A, Yadav N, Thakur S, Sudhakar K, Rosenholm JMJP (2018) Solid lipid nanoparticles: emerging colloidal nano drug delivery systems 10(4):191
Akanda M, Getti G, Nandi U, Mithu MS, Douroumis D (2021) Bioconjugated solid lipid nanoparticles (SLNs) for targeted prostate cancer therapy. Int J Pharm 599:120416
Jampílek J, Kráľová KJNIB (2019) Recent advances in lipid nanocarriers applicable in the fight against cancer. Nanoarchitectonics in Biomedicine 599:219–294
Maji I, Mahajan S, Sriram A, Medtiya P, Vasave R, Khatri DK, Kumar R, Singh SB, Madan J, Singh PK (2021) Solid self emulsifying drug delivery system: Superior mode for oral delivery of hydrophobic cargos. J Control Release 337:646–660
Giarra S, Lupo N, Campani V, Carotenuto A, Mayol L, De Rosa G, Bernkop-Schnürch A (2019) In vitro evaluation of tumor targeting ability of a parenteral enoxaparin-coated self-emulsifying drug delivery system. J. Drug Delivery Sci Technol 53:101144. https://doi.org/10.1016/j.jddst.2019.101144
Zhang D, Liu L, Wang J, Zhang H, ZhangZ, Xing G, Wang X, Liu M (2022) Drug-loaded PEG-PLGA nanoparticles for cancer treatment. Front Pharmacol 13:990505
Zhang Y, Chen Q, Zhu Y, Pei M, Wang K, Qu X, Zhang Y, Gao J, Qin H (2022) Targeting inorganic nanoparticles to tumors using biological membrane-coated technology. MedComm 3(4):2688–2663
Kunde SS, Wairkar S (2021) Platelet membrane camouflaged nanoparticles: Biomimetic architecture for targeted therapy. Int J Pharm 598:120395. https://doi.org/10.1016/j.ijpharm.2021.120395
Pattni BS, Torchilin VP (2015) Targeted drug delivery systems: strategies and challenges. In: Devarajan P, Jain S (eds) Targeted drug delivery: concepts and design. Advances in Delivery Science and Technology. Springer.
Hossen S, Hossain MK, Basher M, Mia M, Rahman M, Uddin MJ (2019) Smart nanocarrier-based drug delivery systems for cancer therapy and toxicity studies: a review. J Adv Res 15:1–18
Kumar A, Behl T, Chadha S (2020) Synthesis of physically crosslinked PVA/Chitosan loaded silver nanoparticles hydrogels with tunable mechanical properties and antibacterial effects. J Int J Biol Macromolecules 149:1262–1274
Kaur N, Popli P, Tiwary N, Swami R (2023) Small molecules as cancer targeting ligands: shifting the paradigm. J Control Release 355:417–433
Tiwari H, Rai N, Singh S, Gupta P, Verma A, Singh AK, Kajal P, Salvi SK, Singh V (2023) Gautam, Recent advances in nanomaterials-based targeted drug delivery for preclinical cancer diagnosis and therapeutics. Bioengineering 10(7):760
Yoo J, Park C, Yi G, Lee D, Koo H (2019) Active targeting strategies using biological ligands for nanoparticle drug delivery systems. Cancers 11(5):640
Baghban R, Roshangar L, Jahanban-Esfahlan R, Seidi K, Ebrahimi-Kalan A, Jaymand M, Kolahian S, Javaheri T, Zare P (2020) Tumor microenvironment complexity and therapeutic implications at a glance. Cell Communication and Signaling 18:1–19
Lugano R, Ramachandran M, Dimberg A (2020) Tumor angiogenesis: causes, consequences, challenges and opportunities. Cell Mol Life Sci 77:1745–1770
Wei Q-Y, Xu Y-M, Lau AT (2020) Recent progress of nanocarrier-based therapy for solid malignancies. Cancers 12(10):2783
Ejigah V, Owoseni O, Bataille-Backer P, Ogundipe OD, Fisusi FA, Adesina SK (2022) Approaches to improve macromolecule and nanoparticle accumulation in the tumor microenvironment by the enhanced permeability and retention effect. Polymers 14(13):2601
Hirsjarvi S, Passirani C, Benoit J-P (2011) Passive and active tumour targeting with nanocarriers. Curr Drug Discov Technol 8(3):188–196
Bhargav AG, Mondal SK, Garcia CA, Green JJ, Quiñones-Hinojosa A (2020) Nanomedicine revisited: next generation therapies for brain cancer. Advanced Therapeutics 3(10):2000118
Vyas SP, Singh A, Sihorkar V (2001) Ligand-receptor-mediated drug delivery: an emerging paradigm in cellular drug targeting. Critical Reviews™ in Therapeutic Drug Carrier Systems 18(1):1–76
Tietjen GT, Bracaglia LG, Saltzman WM, Pober JS (2018) Focus on fundamentals: achieving effective nanoparticle targeting. Trends Mol Med 24(7):598–606
Din FU, Aman W, Ullah I, Qureshi OS, Mustapha O, Shafique S, Zeb A (2017) Effective use of nanocarriers as drug delivery systems for the treatment of selected tumors. Int J Nanomedicine 12:7291–7309
Lu R-M, Hwang Y-C, Liu I-J, Lee C-C, Tsai H-Z, Li H-J, Wu H-C (2020) Development of therapeutic antibodies for the treatment of diseases. J Biomed Sci 27(1):1–30
Zahavi D, Weiner L (2020) Monoclonal antibodies in cancer therapy. Antibodies 9(3):34
Glennie MJ, Johnson PW (2000) Clinical trials of antibody therapy. Immunol Today 21(8):403–410
Chen L, Hong W, Ren W, Xu T, Qian Z, He Z (2021) Recent progress in targeted delivery vectors based on biomimetic nanoparticles. Signal Transduct Target Ther 6(1):225
Alley SC, Okeley NM, Senter PD (2010) Antibody–drug conjugates: targeted drug delivery for cancer. Curr Opin Chem Biol 14(4):529–537
Vaisman-Mentesh A, Gutierrez-Gonzalez M, DeKosky BJ, Wine Y (2020) The molecular mechanisms that underlie the immune biology of anti-drug antibody formation following treatment with monoclonal antibodies. Front Immunol 11:1951
Shepard HM, Phillips GL, Thanos CD, Feldmann M (2017) Developments in therapy with monoclonal antibodies and related proteins. Clin Med 17(3):220
Pillay V, Gan HK, Scott AM (2011) Antibodies in oncology. New Biotechnol 28(5):518–529
Ahmad NA, Zulkifli RM, Hussin H, Nadri MH (2021) In silico approach for Post-SELEX DNA aptamers: a mini-review. J Mol Graph Model 105:107872
Chen K, Liu B, Yu B, Zhong W, Lu Y, Zhang J, Liao J, Liu J, Pu Y, Qiu L (2017) Advances in the development of aptamer drug conjugates for targeted drug delivery. Wiley Interdisciplinary Reviews: Nanomedicine and Nanobiotechnology 9(3):e1438
Gao F, Yin J, Chen Y, Guo C, Hu H, Su J (2022) Recent advances in aptamer-based targeted drug delivery systems for cancer therapy. Front Bioeng Biotechnol 10:972933
Sun H, Zhu X, Lu PY, Rosato RR, Tan W, Zu Y (2014) Oligonucleotide aptamers: new tools for targeted cancer therapy. Mol Ther Nucleic Acids 3:E182
Hasegawa H, Savory N, Abe K, Ikebukuro K (2016) Methods for improving aptamer binding affinity. Molecules 21(4):421
Hu X, Tang L, Zheng M, Liu J, Zhang Z, Li Z, Yang Q, Xiang S, Fang L, Ren Q (2022) Structure-guided designing pre-organization in bivalent aptamers. J Am Chem Soc 144(10):4507–4514
Zhong Y, Meng F, Deng C, Zhong Z (2014) Ligand-directed active tumor-targeting polymeric nanoparticles for cancer chemotherapy. Biomacromol 15(6):1955–1969
Tortorella S, Karagiannis TC (2014) Transferrin receptor-mediated endocytosis: a useful target for cancer therapy. J Membr Biol 247:291–307
Dosio F, Arpicco S, Stella B, Fattal E (2016) Hyaluronic acid for anticancer drug and nucleic acid delivery. Adv Drug Deliv Rev 97:204–236
Akim A, Zafar MN, Safdar N, Sung YY, Muhammad TST (2021) Understanding hyaluronan receptor (CD44) interaction, HA-CD44 activated potential targets in cancer therapeutics. Advanced Pharmaceutical Bulletin 11(3):426
Ebrahimnejad P, Taleghani AS, Asare-Addo K, Nokhodchi A (2022) An updated review of folate-functionalized nanocarriers: a promising ligand in cancer. Drug Discovery Today 27(2):471–489
Scaranti M, Cojocaru E, Banerjee S, Banerji U (2020) Exploiting the folate receptor α in oncology. Nat Rev Clin Oncol 17(6):349–359
Mousavizadeh A, Jabbari A, Akrami M, Bardania H (2017) Cell targeting peptides as smart ligands for targeting of therapeutic or diagnostic agents: a systematic review. Colloids Surf, B 158:507–517
Bhosale RR, Gangadharappa HV, Gowda DV, Osmani RAMA, Vaghela R, Kulkarni PK, Sairam KV, Gurupadayya B (2018) Current perspectives on novel drug carrier systems and therapies for management of pancreatic cancer: an updated inclusive review. Critical Reviews™ in Therapeutic Drug Carrier Systems 35(3):195–292
Walker S, Busatto S, Pham A, Tian M, Suh A, Carson K, Quintero A, Lafrence M, Malik H, Santana MX (2019) Extracellular vesicle-based drug delivery systems for cancer treatment. Theranostics 9(26):8001
Wu M, Wang M, Jia H, Wu P (2022) Extracellular vesicles: Emerging anti-cancer drugs and advanced functionalization platforms for cancer therapy. Drug Delivery 29(1):2513–2538
Kos J, Obermajer N, Doljak B, Kocbek P, Kristl J (2009) Inactivation of harmful tumour-associated proteolysis by nanoparticulate system. Int J Pharm 381(2):106–112. https://doi.org/10.1016/j.ijpharm.2009.04.037
Vinothini K, Rajendran NK, Ramu A, Elumalai N, Rajan M (2019) Folate receptor targeted delivery of paclitaxel to breast cancer cells via folic acid conjugated graphene oxide grafted methyl acrylate nanocarrier. Biomed Pharmacother 110:906–917. https://doi.org/10.1016/j.biopha.2018.12.008
Taymouri S, Alem M, Varshosaz J, Rostami M, Akbari V, L. (2019) Firoozpour, Biotin decorated sunitinib loaded nanostructured lipid carriers for tumor targeted chemotherapy of lung cancer. J Drug Delivery Sci Technol 50:237–247. https://doi.org/10.1016/j.jddst.2019.01.024
Deng X, Cao M, Zhang J, Hu K, Yin Z, Zhou Z, Xiao X, Yang Y, Sheng W, Wu Y, Zeng Y (2014) Hyaluronic acid-chitosan nanoparticles for co-delivery of MiR-34a and doxorubicin in therapy against triple negative breast cancer. Biomaterials 35(14):4333–4344
Mashreghi M, Zamani P, Moosavian SA, Jaafari MR (2020) Anti-EpCAM aptamer (Syl3c)-functionalized liposome for targeted delivery of doxorubicin: in vitro and in vivo antitumor studies in mice bearing C26 colon carcinoma. Nanoscale Res Lett 15(1):101. https://doi.org/10.1186/s11671-020-03334-9
Bhagwat GS, Athawale RB, Gude RP, Md S, Alhakamy NA, Fahmy UA, Kesharwani P (2020) Formulation and development of transferrin targeted solid lipid nanoparticles for breast cancer therapy. Front Pharmacol 11:614290
Kazemi Y, Dehghani S, Soltani F, Abnous K, Alibolandi M, Taghdisi SM, Ramezani M (2022) PNA-ATP aptamer-capped doxorubicin-loaded silica nanoparticles for targeted cancer therapy. Nanomed: Nanotechnol Biol Med 45:102588. https://doi.org/10.1016/j.nano.2022.102588
Moon Y, Shim MK, Choi J, Yang S, Kim J, Yun WS, Cho H, Park JY, Kim Y, Seong JK, Kim K (2022) Anti-PD-L1 peptide-conjugated prodrug nanoparticles for targeted cancer immunotherapy combining PD-L1 blockade with immunogenic cell death. Theranostics 12(5):1838–7640
Liu W, Su J, Shi Q, Wang J, Chen X, Zhang S, Li M, Cui J, Fan C, Sun B, Wang G (2021) RGD Peptide-conjugated selenium nanocomposite inhibits human glioma growth by triggering mitochondrial dysfunction and ROS-dependent MAPKs activation. Front Bioeng Biotechnol 9:781608
Luo K, Yin S, Zhang R, Yu H, Wang G, Li J (2020) Multifunctional composite nanoparticles based on hyaluronic acid-paclitaxel conjugates for enhanced cancer therapy. Int J Pharm 589:119870. https://doi.org/10.1016/j.ijpharm.2020.119870
Subhan MA, Yalamarty SSK, Filipczak N, Parveen F, Torchilin VP (2021) Recent advances in tumor targeting via EPR effect for cancer treatment. Journal of personalized medicine 11(6):571
Nakamura Y, Mochida A, Choyke PL, Kobayashi H (2016) Nanodrug delivery: is the enhanced permeability and retention effect sufficient for curing cancer? Bioconjug Chem 27(10):2225–2238
Shi J, Kantoff PW, Wooster R, Farokhzad OC (2017) Cancer nanomedicine: progress, challenges and opportunities. Nat Rev Cancer 17(1):20–37
Goel S, Duda DG, Xu L, Munn LL, Boucher Y, Fukumura D, Jain RK (2011) Normalization of the vasculature for treatment of cancer and other diseases. Physiol Rev 91(3):1071–1121
Munir MU (2022) Nanomedicine penetration to tumor: challenges, and advanced strategies to tackle this issue. Cancers 14(12):2904
Souri M, Soltani M, Kashkooli FM, Shahvandi MK, Chiani M, Shariati FS, Mehrabi MR, Munn LL (2022) Towards principled design of cancer nanomedicine to accelerate clinical translation. Materials Today Bio 13:100208
Mahmood J, Alexander AA, Samanta S, Kamlapurkar S, Singh P, Saeed A, Carrier F, Cao X, Shukla HD, Vujaskovic Z (2020) A combination of radiotherapy, hyperthermia, and immunotherapy inhibits pancreatic tumor growth and prolongs the survival of mice. Cancers 12(4):1015
Bae YH, Park K (2011) Targeted drug delivery to tumors: myths, reality and possibility. J Control Release 153(3):198–205
Ferretti S, Allegrini PR, Becquet MM, McSheehy PM (2009) Tumor interstitial fluid pressure as an early-response marker for anticancer therapeutics. Neoplasia 11(9):874–881
Holback H, Yeo Y (2011) Intratumoral drug delivery with nanoparticulate carriers. Pharm Res 28:1819–1830
Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D, Hawkins C, Ng H, Pfister SM, Reifenberger G (2021) The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol 23(8):1231–1251
Reagan M (2019) Causes of cancer: Genetic, epigenetic, viral, microenvironmental, and environmental contributions to cancer. In: Cancer, prevention, early detection, treatment and recovery, pp 53–74
Prince M, Sivanandan R, Kaczorowski A, Wolf G, Kaplan M, Dalerba P, Weissman I, Clarke M, Ailles L (2007) Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proc Natl Acad Sci 104(3):973–978
Rotow J, Bivona TG (2017) Understanding and targeting resistance mechanisms in NSCLC. Nat Rev Cancer 17(11):637–658
Garcia-Mayea Y, Mir C, Masson F, Paciucci R, LLeonart M (2020) Insights into new mechanisms and models of cancer stem cell multidrug resistance. In: Seminars in cancer biology, vol 60. Academic Press, pp 166–180
Foulkes R, Man E, Thind J, Yeung S, Joy A, Hoskins C (2020) The regulation of nanomaterials and nanomedicines for clinical application: current and future perspectives. Biomaterials science 8(17):4653–4664
Leopold L, Zhao C, McConnachie L, Khurana RK, Sharma T, Singh B, Ho RJY (2018) Surface-engineered nanomaterials: environmental and safety considerations in pharmaceutical and medicinal products. In: Singh B, Kanwar JR, Garg S (eds) Nanobioengineering. CRC Press, pp 1–14
Rosenblum D, Joshi N, Tao W, Karp JM, Peer D (2018) Progress and challenges towards targeted delivery of cancer therapeutics. Nat Commun 9(1):1410
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
N/A.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sharma, T., Gorivale, S. & Bhandari, P. Targeted drug delivery in cancer using nanomaterials: advances and challenges. J Nanopart Res 26, 127 (2024). https://doi.org/10.1007/s11051-024-06023-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-024-06023-1