Abstract
The early immunohistochemical studies of carbonic anhydrases (CAs) were mainly focused on their normal tissue distribution. Only a few studies included samples from pathologic diseases, particularly cancer. This line of research remained inactive until the discovery of CA IX—the first cancer-associated isozyme. The association of CA IX with hypoxic regions of tumors became obvious, and experimental results confirmed hypoxia regulation. CA IX is now widely considered a biomarker of tumor hypoxia and prognosis. Even though it has several characteristics of a promising biomarker, the implementation of CA IX in clinical pathology has progressed slowly. CA IX research has also produced promising therapeutic molecules, some of which are already in clinical trials. CA XII is another cancer-associated isozyme; however, it is not yet used as a clinical biomarker in routine diagnostics nor is it utilized in therapeutic applications. Surprisingly, the well-known isozyme CA II has turned out to be an attractive candidate as a diagnostic marker, at least in the special case of gastrointestinal stromal tumors. As a conclusion, certain CA isozymes have definite promise as histopathological markers and therapeutic targets. Even though the implementation of new approaches is a slow process in clinical medicine, the first step has been taken to utilize the unique properties of CA isozymes in diagnostics and therapy.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
2.1 Short Introduction of Mammalian Carbonic Anhydrases
Among vertebrates, the α-carbonic anhydrases (CAs) are the dominant enzyme forms, and they have distinctive subcellular localizations, tissue distributions, activities, and biological functions. Their key roles include various biological functions, such as the regulation of pH homeostasis, ion transport, gluconeogenesis, ureagenesis, respiration, bone resorption, as well as formation of biological fluids. Thirteen active isozymes have been identified in mammals thus far: five cytoplasmic (CA I, CA II, CA III, CA VII, and CA XIII), five membrane-associated (CA IV, CA IX, CA XII, CA XIV, and CA XV), two mitochondrial (CA VA and CA VB), and one secreted form (CA VI) (Fig. 2.1) (Sly and Hu 1995; Parkkila and Parkkila 1996; Lehtonen et al. 2004; Hilvo et al. 2005). In addition, the CA gene family includes three carbonic anhydrase-related proteins (CARPs) that lack CA catalytic activity due to missing histidine residues in their active sites (Tashian et al. 2000). As with their catalytic activities, all isozymes also differ in their affinity for CA inhibitors. Some of the developed CA inhibitors are clinically used as therapeutic agents in the management of certain diseases, such as glaucoma and epilepsy (Thiry et al. 2007; Mincione et al. 2007; Supuran and Scozzafava 2007). It has become evident that CAs and their inhibitors offer interesting opportunities for both developing novel drugs and as diagnostic tools to improve the health and wellbeing of humans and other species.
A typical subcellular localization pattern of the enzymatically active mammalian carbonic anhydrases. CA IV and CA XV are anchored to the plasma membrane through a glycosyl phosphatidylinositol (GPI) linkage. CA XV is not expressed in human tissues (Hilvo et al. 2005). CA IX, XII, and XIV are transmembrane enzymes whose catalytic sites are located to the cell exterior. CA IX and XII are confined to the basolateral plasma membrane, whereas CA XIV has also shown expression at the apical membrane (Kaunisto et al. 2002). CA VA and VB are mitochondrial enzymes, and CA VI is secreted via secretory granules into milk and saliva. The remaining isozymes are expressed in the cytosol
2.2 CA II as a Biomarker
CA II, although it is the most active and widely expressed isozyme in human tissues, has attracted much less attention in cancer research compared to the classical tumor-associated isozymes, CA IX and XII. It was first demonstrated by immunohistochemistry that colorectal adenomas and adenocarcinomas do not stain for CA II (Gramlich et al. 1990). Low expression of CA II was later confirmed in a study where CA I, II, and XIII were investigated in parallel tissue sections (Kummola et al. 2005), as well as in a study where the expression of various CA isozymes was analyzed using a microarray technique (Niemela et al. 2007).
CA II is highly expressed in the epithelium of the pancreatic ducts, where it is involved in the production of bicarbonate-rich pancreatic juice (Parkkila and Parkkila 1996; Parkkila et al. 1995a). The expression rate of CA II in the ductal cells, which is sustained after malignant transformation, does not correlate with the malignancy of tumors, suggesting a limited value for CA II reactivity in histopathological diagnostics of pancreatic adenocarcinoma (Parkkila et al. 1995a).
It is well-known that CA II is prominently present in both gastric parietal cells and bicarbonate-producing surface epithelial cells of the stomach, whereas it is only weakly expressed in the esophageal epithelium (Parkkila et al. 1994). Recently, the expression and clinical significance of CA II, IX, and XII were investigated in Barrett’s esophagus and esophageal adenocarcinoma (EAC) (Nortunen et al. 2018). Even though CA II was significantly downregulated in metastatic disease, it was concluded that none of the tested isozymes could provide additional value as a biomarker for esophageal adenocarcinoma.
Two recent articles have provided congruent results showing that the reduction of CA II expression in gastric tumors is associated with tumor growth, metastasis, and poor prognosis. The first report demonstrated that the expression of CA II was significantly higher in the normal gastric mucosa than in the intraepithelial neoplasia and gastric carcinoma (Li et al. 2012). The positive signal for CA II was significantly more frequent in gastric carcinoma at early stages compared to more advanced stages. The rate of positive CA II signal was significantly lower in poorly differentiated gastric carcinoma than in moderately or well-differentiated tumors. The patients with CA II-positive tumors showed a better survival rate than those with CA II-negative tumors. The second publication confirmed that CA II expression is significantly decreased in gastric cancer compared with the normal gastric mucosa (Hu et al. 2014). Low expression was significantly associated with tumor size, depth of invasion, lymph node involvement, distant metastasis, and TNM stage, and it predicted poor survival in gastric cancer patients.
Viikilä et al. recently investigated the expression of CA II, VII, IX, and XII in a series of colorectal carcinoma samples from 593 patients (Viikila et al. 2016). Only CA II and XII showed statistically significant correlations to patient survival in that higher expression indicated poorer prognosis. CA II staining associated with the patient age group, while no other significant correlation was reported between the isozymes and various clinicopathological parameters.
The most promising results for CA II as a potential biomarker were published in 2010, when the enzyme was identified in gastrointestinal stromal tumors (GISTs) (Parkkila et al. 2010). GISTs represent the most common mesenchymal tumor category of the gastrointestinal tract and include tumors with low malignancy and those that behave as highly malignant metastasizing neoplasias. KIT expression is a nearly consistent phenotypic feature of GISTs, and oncogenic activation of KIT or PDGFRA receptor tyrosine kinase signaling is considered pathogenetically important (Miettinen and Lasota 2006). GISTs originate from Cajal cells of the gastrointestinal wall (Kindblom et al. 1998); thus, the tumors can arise in various gastrointestinal locations. The Western blotting experiments first indicated that CA II is highly expressed in GIST cell lines (Parkkila et al. 2010). Subsequently, CA II expression was analyzed in 175 GISTs, of which 95% showed positive immunostaining. The CA II expression in GISTs did not correlate with particular KIT or PDGFRA mutation types. CA II was absent or expressed at low levels in the other mesenchymal tumor categories that were analyzed in that particular study. High CA II expression was associated with a better disease-specific survival rate than low or no expression. The results indicated that CA II is quite selective to this tumor type among various mesenchymal tumors; therefore, it might be a useful biomarker in diagnostics.
Recently, CA II expression was studied in another type of gastrointestinal tumors, called pseudomyxoma peritonei (Jarvinen et al. 2017). The specimens were collected from 89 patients with this malignancy; the tissue sections were immunostained for CA II, and the expression was analyzed against the survival of the patients. Positive CA II expression was found in 65% of patients. The 5-year overall survival rates for the CA II-positive and -negative cases were 80 and 59%, respectively, reaching a clear statistical significance. It was suggested based on the results that the expression of CA II acts as an independent prognostic biomarker in pseudomyxoma peritonei.
CA II is expressed in at least many, if not in most, cases of hematological malignancies. Leppilampi et al. investigated the presence of CA isozymes in malignant hematopoietic cell lines and malignant blast cells of bone marrow samples (Leppilampi et al. 2002). Three out of six malignant hematopoietic cell lines expressed CA II, whereas no expression was detected for CA I, IX, or XII. The positive reactions were found in 62% of acute myeloid leukemia samples, 73% of acute lymphoblastic leukemias, and 50% of chronic myelomonocytic leukemias. The results indicated that CA II expression is not restricted to one cell lineage but may result from a genetic aberration that occurs in both myeloid and lymphatic lineages or in their progenitor cells.
CA II is expressed in various brain tumors, including astrocytic tumors, oligodendrogliomas, ependymal and choroid plexus tumors, and tumors of nerve sheath cell origin (Parkkila et al. 1995b). In brain tumors, a significant fraction of CA II reactivity is, in fact, located in the capillary endothelium (Haapasalo et al. 2007). A similar pattern of ectopic CA II expression has been demonstrated in the endothelium of neovessels of several other cancers, including melanoma and esophageal, renal, and lung tumors (Yoshiura et al. 2005).
2.3 CA IX as a Biomarker
Among different CA isozymes, CA IX has shown the highest promise as a potential biomarker of certain tumors. In fact, there are already over 1000 publications available on the combined topics of CA IX and cancer, although CA IX is not fully specific for cancer cells only. Among various normal tissues, CA IX is abundantly present in the basolateral plasma membranes of the gastrointestinal epithelia (Pastorekova et al. 1997). The highest levels of the enzyme are present in the epithelial cells of both gastric and gall bladder mucosa. The expression is prominent also in the proximal gut, i.e., in the duodenum and jejunum, and it is moderate in the ileum and colon, while it diminishes towards the rectum (Saarnio et al. 1998a). In contrast to the gastric epithelium, where the enzyme is highly expressed by the surface epithelial cells and parietal cells, the expression in the intestinal epithelium is confined to the deep proliferating areas, the crypts of Lieberkühn. As the cells migrate along the intestinal villus, they differentiate and gradually lose their CA IX expression, suggesting that it may have a role in the proliferation and differentiation of epithelial cells. In addition to the stomach, gallbladder, and intestine, CA IX expression has been detected in the human biliary epithelium, pancreatic ducts, male reproductive organs, and mesothelium (Pastorekova et al. 1997; Saarnio et al. 2001; Kivela et al. 2000a; Karhumaa et al. 2001; Ivanov et al. 2001).
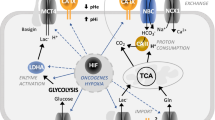
CA IX is a highly active enzyme with catalytic activity in the same range with CA II (Hilvo et al. 2008). The high enzyme activity of CA IX may be of potential significance for tumor growth and invasion. According to the current paradigm, CA IX functionally contributes to the acidification of the extracellular microenvironment surrounding cancer cells by interacting directly with ion transport proteins; accordingly, it may neutralize the intracellular space of tumor cells (Ivanov et al. 1998; Svastova et al. 2004). The protons produced by CA IX may remain outside and increase the acidity of the tumor microenvironment (Pastorekova et al. 2006). In fact, there are several pieces of evidence that CA IX physically and functionally interacts with bicarbonate transport proteins AE1, AE2, and AE3 and with sodium-dependent electroneutral bicarbonate cotransporter (NBCn1) (Morgan et al. 2007; Svastova et al. 2012; McDonald et al. 2018). CA IX also associates with proteins involved in amino acid transport, including AA transport heavy chain subunit, CD98hc (SLC3A2), L-type AA transporter, LAT1 (SLC7A5), alanine-serine-cysteine-preferring transporter 2, ASCT2 (SLC1A5), and sodium-coupled neutral amino acid transporter 2, SNAT2 (SLC38A2) (McDonald et al. 2018).
It is well-documented that CA IX is highly expressed in various tumors, including those that arise from the GI tract (Pastorekova et al. 2006, 2004). As the first example, many colorectal tumors overexpress CA IX (Niemela et al. 2007; Saarnio et al. 1998b). CA IX is the only CA isozyme that is overexpressed in hereditary nonpolyposis colorectal cancer (HNPCC) (Niemela et al. 2007). In colorectal tumors, CA IX has shown high expression in premalignant lesions and often a lower signal in poorly differentiated malignancies, suggesting that it might be a useful marker in the early diagnosis of colorectal tumorigenesis. More recent studies have indicated, however, that CA IX is not a prognostic factor in colorectal cancer (Viikila et al. 2016).
Turner et al. were the first to suggest that tumor-associated CA IX may play a role in the proliferation and regeneration of esophageal squamous epithelium, and loss of its expression may be related to cancer progression in Barrett’s-associated adenocarcinomas (Turner et al. 1997). Later, it was reported that 44.5% of esophageal carcinomas show strong CA IX expression, and the high expression is an independent prognostic factor for shorter overall and disease-free survival (Birner et al. 2011). In another study, high CA IX expression was associated with tumorigenesis markers BMI1, MCM4, and MCM7, suggesting that CA IX may play a role in early tumorigenesis (Huber et al. 2015). In contrast to the previous results, CA IX was not found to be a significant prognostic marker in patients with esophageal adenocarcinoma. In the most recent study, Nortunen et al. analyzed CA IX expression in a series of esophageal adenocarcinomas and Barrett’s esophagus (Nortunen et al. 2018). The normal squamous epithelium of the esophagus was almost completely negative for CA IX. The signal was strong in gastric metaplasia and present in all cell types across the epithelium. The expression levels decreased from gastric metaplasia to intestinal metaplasia, dysplastic lesions, and finally to adenocarcinoma. In intestinal metaplasia, the expression was still strong and showed basal dominance. The CA IX expression in carcinomas showed no significant correlation with clinicopathological variables or survival, although high CA IX expression appeared to associate weakly with nodal spread.
In the liver, CA IX is localized to the biliary epithelial cells (Pastorekova et al. 1997). Analogously, CA IX is expressed by the biliary epithelial tumors (Saarnio et al. 2001). It seems plausible that CA IX expression indicates better prognosis in intrahepatic cholangiocarcinoma (Gu 2015), even though CA IX is typically associated with poor prognosis in many other tumor categories. One of these examples is hepatocellular carcinoma, in which CA IX is expressed only in a minority of cases. In a large cohort of patients, Kang et al. showed that 15.1% of the cases expressed CA IX at low level, and 5.2% belonged to the high CA IX-positive group (Kang et al. 2015). CA IX expression was a prognostic factor for poorer survival after surgery for hepatocellular carcinoma. In addition, the high CA IX-positive group had even a poorer prognosis than the low CA IX-positive group. Recently, it was further confirmed that CA IX is, indeed, a predictive factor for poor prognosis after radical surgery for hepatocellular carcinoma (Hyuga et al. 2017). Finkelmeier et al. recently published an interesting study where they analyzed circulating CA IX enzyme levels in patients with hepatocellular carcinoma or cirrhosis (Finkelmeier et al. 2018). They included 215 patients with hepatocellular carcinoma in the study. The median serum CA IX concentration in patients with hepatocellular carcinoma was 370 pg/ml, and it was significantly higher than in controls (41 pg/ml). The patients with high serum CA IX concentrations (>400 pg/ml) had an increased mortality risk. Surprisingly, the serum CA IX concentration in cirrhotic patients did not differ significantly from the patients with hepatocellular carcinoma. Higher CA IX levels in cirrhotic patients correlated with portal hypertension and esophageal varices, and the patients with ethanol-induced cirrhosis had the highest CA IX levels.
Ectopic expression through hypoxia regulation is an important hallmark of CA IX; therefore, CA IX is most highly expressed in tumors that originate from CA IX-negative tissues. Because the normal gastric mucosa contains the highest levels of CA IX among normal tissues, it was not surprising that gastric carcinomas showed relatively low expression (Leppilampi et al. 2003). Nakamura et al. have shown that the expression of CA IX in gastric cancer is predominantly regulated by the methylation of a single CpG rather than by hypoxia (Nakamura et al. 2011). A subgroup of gastric cancers retain CA IX expression in cancer cells at the invasion front (Chen et al. 2005). It has been shown that overexpression of CA IX is important for tumor invasion and metastasis; as such, it may serve as a useful prognostic indicator of long-term survival in patients with gastric adenocarcinoma (Senol et al. 2016).
CA IX is not prominently expressed in any normal neural tissues. Interestingly, it is highly expressed in some cases of brain tumors. The first publication on this topic was published by Haapasalo et al., showing a positive signal for CA IX in 78% of astrocytomas (Haapasalo et al. 2006). The staining pattern followed the distribution of hypoxic regions within tumor specimens. The CA IX immunoreactivity showed a strong association with tumor malignancy grades. CA IX showed no association with p53 expression, nor did it correlate with epidermal growth factor receptor-amplification, apoptosis, or cell proliferation. CA IX intensity had significant prognostic value in survival analysis, which was confirmed later by other researchers (Cetin et al. 2018). In oligodendroglial tumors, CA IX was positive in 80% of the cases, and it again correlated with poorer outcome (Jarvela et al. 2008). CA IX expression has also been studied in rare pediatric brain tumors, including primitive neuroectodermal tumors and medulloblastomas (Nordfors et al. 2010). CA IX-positive staining was observed in 23% of the cases, and its expression predicted poor prognosis of patients. Since the presence of hypoxic and necrotic areas represents an important criterion in glioma diagnostics, CA IX has become a useful biomarker for this tumor category. Accordingly, the neuropathologists of Tampere University Hospital already utilize CA IX immunostaining as a routine diagnostic asset for this clinical purpose (Dr. Hannu Haapasalo, personal communication).
CA IX expression has been studied in a variety of lung cancers. Ramsey et al. analyzed CA IX immunohistochemical expression in pulmonary/pleural tumors, including metastatic clear cell renal carcinoma of the lung, mesothelioma, squamous cell carcinoma, small cell carcinoma, and adenocarcinoma (Ramsey et al. 2012). All cases of metastatic clear cell carcinomas and mesotheliomas were positive for CA IX. Most cases of lung squamous cell carcinoma and small cell carcinoma were positive, while the enzyme was less frequently present in pulmonary adenocarcinoma. The high expression of CA IX in mesotheliomas was later confirmed in a series of 27 malignant pleural mesotheliomas (Kivela et al. 2013). According to the immunohistochemical and follow-up data, CA IX expression predicts a poor survival rate in lung cancer (Kim et al. 2004, 2005). The presence of CA IX has been specifically linked to the expression of proteins that are involved in angiogenesis, apoptosis inhibition, and cell–cell adhesion disruption, which explains the strong association of the enzyme with poor clinical outcomes (Giatromanolaki et al. 2001). However, recent studies have indicated that the inclusion of CA IX as one criterion does not improve the prognostic accuracy of blood biomarkers for the diagnostics of non-small cell lung cancer (Carvalho et al. 2016).
Cervical cancer was one of the first cancer types in which CA IX expression was studied in detail (Liao et al. 1994). Loncaster et al. (2001) showed clinical evidence that CA IX expression in cervical cancer correlates with the levels of tumor hypoxia and associates with a poor prognosis of the disease (Loncaster et al. 2001). The authors suggested that the level of CA IX expression may be used to select patients who would benefit most from hypoxia-modification therapies or bioreductive drugs. Maseide et al. noticed that high CA IX expression predicts a poor prognosis for patients with soft tissue sarcoma (Maseide et al. 2004). Hynninen et al. investigated the expression of CA II, CA IX, and CA XII in a series of gynecological malignancies, including adenocarcinomas and mesenchymal tumors, such as sarcomas and leiomyomas (Hynninen et al. 2012). Positive staining of CA II, CA IX, and CA XII was detected in many cases of sarcomas. The study confirmed the earlier positive results of CA IX expression in leiomyosarcoma (Mayer et al. 2008), and it also added new data on the expression levels of CA IX in stromal sarcomas and mixed Müllerian tumors. In addition to the results for CA IX, the findings showed that CA II and CA XII are often weakly or moderately expressed in these mesenchymal tumors. Among these tumors, all isozymes showed variable staining results, suggesting that they have only limited value in sarcoma diagnostics if used alone. The specimens involved a total of 33 leiomyomas, all of which were negative for CA II and CA XII. Previously, using another series of tumors, Mayer et al. reported that all leiomyomas are negative for CA IX (Mayer et al. 2008). In contrast, Hynninen et al. reported that 33 leiomyoma specimens included five cases that were CA IX-positive. The biological role and mechanism of CA IX induction in leiomyomas remained unresolved, however. There were at least no other visible signs of hypoxia in the tissue sections.
Ovarian tumors is another category of tumors in which CA IX is highly expressed (Hynninen et al. 2006). Most cases of borderline mucinous cystadenomas, mucinous cystadenocarcinomas, and serous cystadenocarcinomas are moderately or strongly positive for CA IX. In malignant tumors, the expression patterns have shown clear correlations to hypoxic regions. The high expression levels of CA IX in mucinous and serous cystadenocarcinomas suggested that these tumors could be considered potential candidates for CA IX‐targeted therapy.
The current literature already includes a number of publications on CA IX expression in breast tumors. A study by Bartosová et al. indicated that ectopic activation of the CA9 gene may be implicated in breast carcinogenesis, and it also suggested that CA IX could be a breast cancer marker (Bartosova et al. 2002). The main conclusion from several expression studies is that CA IX indicates poor prognosis in breast cancer (Brennan et al. 2006; Hussain et al. 2007; Chia et al. 2001), even though Span et al. demonstrated that CA IX is more predictive than prognostic in this cancer type (Span et al. 2007).
The expression of CA IX has been examined in head and neck squamous cell carcinoma (HNSCC) (Beasley et al. 2001). The enzyme was related to the location of tumor microvessels, angiogenesis, necrosis, and tumor stage, and it was considered a potential target for future therapy in HNSCC. The follow-up studies have included CA IX in the panels of possible predictive markers in HNSCC (Schutter et al. 2005; Le et al. 2007). Although combinations of markers have been associated with treatment outcome, their clinical value as predictive factors must still be established (Hoogsteen et al. 2007).
Both CA IX and CA XII have been investigated in both the normal skin and skin tumors (Syrjanen et al. 2014). In the normal skin, the highest expression of CA IX was detected in hair follicles, sebaceous glands, and basal parts of the epidermis. CA XII was detected in all epithelial components of the skin. Both CA IX and CA XII expression levels were significantly different in epidermal, appendageal, and melanocytic tumor categories. Both CA IX and XII showed the most intense immunostaining in epidermal tumors, whereas virtually all melanocytic tumors were devoid of CA IX and XII immunostaining. In premalignant lesions, CA IX expression significantly increased when the tumors progressed to more severe dysplasia forms.
The story about CA IX in kidney tumors and its role as a prognostic marker is rather complicated because of an alternative mechanism of upregulation of CA IX expression due to von Hippel-Lindau gene mutations in certain tumors. Human renal cancer cell lines and renal cancers have shown high expression of CA IX mRNA and CA IX protein (McKiernan et al. 1997; Parkkila et al. 2000). CA IX is mostly, but not fully, specific to clear cell renal cell carcinoma among various kidney tumors. Notably, the most common tumor of the pediatric kidney, Wilms’ tumor, has shown 63% positivity for CA IX, although the median fraction of positive cells was only 5% (Dungwa et al. 2011). Sandlund et al. assessed CA IX expression in different subtypes of renal cell cancer (Sandlund et al. 2007). They found that the expression is higher in conventional clear cell renal cell carcinoma compared to other renal cancer types. They also reported that the patients with clear cell carcinoma have a less favorable prognosis when the CA IX expression is low. In line with those findings, CA IX has also been described as a prognostic marker in metastatic clear cell carcinoma (Bui et al. 2003). Limited CA IX expression in the area of 85% or less has been associated with poorer cancer-specific survival (Pickering and Larkin 2015). This exact criterion seems to be valid in metastatic tumors only, and based on present knowledge, CA IX expression may not represent a reliable prognostic factor in localized clear cell renal cell carcinomas (Pickering and Larkin 2015). The present literature available on CA IX in renal cancer suggests that the enzyme may represent not only a useful prognostic marker for metastatic clear cell renal cell carcinoma but also a promising therapeutic target for novel oncological applications, including immunotherapy and radioisotopic methods (Pastorekova et al. 2006; Bleumer et al. 2006).
The thyroid cancers express both CA IX and CA XII with different patterns (Takacova et al. 2014). Among various thyroid tumors, the highest expression of CA IX was reported in medullary thyroid carcinoma and anaplastic carcinoma, where positive immunostaining was reported in 91.67 and 100% of cases, respectively. Only limited expression of CA IX was detected in well-differentiated tumors (3.8% of papillary thyroid carcinoma and 12% of follicular thyroid carcinoma). The highest positivity for CA XII was found in papillary carcinoma (91.7%).
Very recent investigations have demonstrated CA IX expression in lymphoma cells (Mehes et al. 2019). More specifically, positive signal was found in Reed-Sternberg cells of Hodgkin’s lymphoma in 39/81 samples (48.1%). In contrast, CA XII expression in these cells was present in only 18/77 samples (23.4%). For the CA IX-positive group, 72 month-progression-free survival was significantly lower compared with the CA IX-negative cases, while the overall survival did not differ significantly.
As this chapter indicates, there have been many studies investigating the prognostic value of CA IX expression in patients with solid tumors. In 2016, van Kuijk et al. published the first meta-analysis covering 147 studies on CA IX expression in various tumor categories (excluding renal cancer because of different regulation) (Kuijk et al. 2016). Overall, the results showed that high CA IX expression is an adverse prognostic marker in solid tumors (excluding renal tumors). A strong association between high expression and poor prognosis was reported in the majority of different tumor sites, supporting a pivotal role of CA IX in disease progression and treatment resistance in various cancers.
2.4 CA IX Immunoassay Data
Currently, CA IX immunoassay reagents and methods are available from several commercial sources, even though it is not always possible to define the original manufacturer based on the incomplete datasheet or other public data. Some examples of the commercial assays are shown in Table 2.1. Many articles have been already published where CA IX concentrations were detected from human biological fluids, including serum, plasma, pleural effusion, or urine. In most studies, the data have been collected from patients with renal cancer (Lucarini et al. 2018; Gigante et al. 2012; Li et al. 2008; Zavada et al. 2003; Zhou et al. 2010; Papworth et al. 2010; Pena et al. 2010). Based on immunohistochemical data, it is obvious that CA IX expression is much more common in clear cell renal cell carcinoma than in other neoplasms of the kidney (Genega et al. 2010). Immunoassay results of CA IX have recently shown that the expression levels are very high in the plasma of patients with clear cell renal cell carcinoma (Lucarini et al. 2018). High serum CA IX levels are significantly associated with shorter overall survival (Gigante et al. 2012), suggesting that increased shedding of the enzyme into circulation is a hallmark for poor prognosis.
Woelber et al. analyzed the CA IX levels in both ovarian (Woelber et al. 2010) and cervical cancer patients (Woelber et al. 2011). They found that the enzyme concentration did not change significantly during the first-line therapy of ovarian cancer and were not prognostically relevant. Similarly, the serum concentrations of CA IX did not correlate with intratumoral expression of the enzyme or other clinicopathological variables in cervical cancer. Ilie et al. analyzed CA IX levels from patients with non-small cell lung cancer and found that the high plasma level of CA IX is an independent biomarker of poor prognosis (Ilie et al. 2010). Ostheimer et al. combined three markers, including osteopontin, vascular endothelial growth factor, and CA IX (Ostheimer et al. 2014). They found that high pretreatment plasma levels of these markers additively correlated with prognosis in M0-stage non-small cell lung cancer. CA IX concentrations have also been studied from pleural effusions of patients with various pathologies (Liao and Lee 2012). It was found that CA IX levels were significantly higher in the effusions collected from the patients with malignant diseases compared to those collected from patients with various benign diseases. Rosenberg et al. investigated CA IX levels by ELISA in patients with head and neck cancer (Rosenberg et al. 2016). They found that high pretreatment CA IX concentration is a negative prognostic factor in locally advanced tumors.
2.5 CA XII in Cancer
CA XII is broadly similar in overall structure to CA IX, excluding the proteoglycan-like domain of CA IX. The expression of CA XII is also induced by hypoxic conditions (Ivanov et al. 1998), but its distribution in tissues does not correlate with hypoxic regions to the same extent as CA IX. It has been demonstrated that the expression of CA XII is under estrogen receptor regulation, and the expression in breast tumors is associated with positive estrogen alpha receptor status (Watson et al. 2003; Wykoff et al. 2001; van't Veer et al. 2002).
Like CA IX, CA XII may also function in metabolons together with ion transport proteins. It has been reported that it regulates the function of the chloride–bicarbonate exchanger (AE2) (Hong et al. 2015; Waheed and Sly 2017). Mutations in the CA12 gene have been associated with an autosomal recessive form of salt wasting disease that results in hyponatremia in some Bedouin families (Feldshtein et al. 2010; Muhammad et al. 2011). The clinical symptoms of the trait include high sweat chloride concentration, dehydration, and failure to thrive in infancy.
Expression of CA XII has been quite extensively studied in both normal tissues and several types of cancer. It is expressed in the normal kidney (Parkkila et al. 2000), colon (Kivela et al. 2000b), endometrium (Karhumaa et al. 2000), and skin (Syrjanen et al. 2014), and its heterogenous expression pattern in tumors may reduce its potential as a biomarker (Pastorekova et al. 2006). Expression of CA XII transcripts in nonpigmented epithelial cells of retina has also been reported (Liao et al. 2003), and these epithelial cells from glaucoma patients showed increased CA12 gene expression. From these results, it has been concluded that CA XII is expressed in ciliary cells, and thus, may be involved in aqueous humor production.
One of the first publications on CA XII demonstrated its distribution in colorectal tumors (Kivela et al. 2000b). Most cases of adenomatous polyps were positive for CA XII, and the staining became more diffusely spread within the lesion with a more severe grade of dysplasia. Among 20 malignant colorectal tumors, 19 showed positive reactions that were typically diffuse, being present in both the superficial and deep parts of the mucosa. In contrast, the normal colorectal mucosa shows CA XII-positive signal in the superficial part of the mucosa, whereas staining is usually absent in the deep part of the mucosa. Another early publication described the expression of CA XII in various tumor categories (Ivanov et al. 2001). The highest rates of distinct positive signal were reported in cervical carcinomas and intraepithelial neoplasias, endometrial carcinoma, ovarian carcinomas and cystadenomas, breast ductal and lobular carcinomas, renal cancers excluding Wilms’ tumor, colon adenomas, and gliomas.
The original discovery of CA XII was published almost simultaneously by two independent groups (Ivanov et al. 1998; Tureci et al. 1998). The first findings already linked CA XII to kidney function and renal cancer. In the human kidney, CA XII was located to the basolateral plasma membrane of the epithelial cells in the thick ascending limb of Henle, distal convoluted tubules, and collecting ducts (Parkkila et al. 2000). A weak basolateral signal was also detected in the epithelium of the proximal convoluted tubules. In a series of 31 renal tumors, the enzyme showed moderate or strong expression in most oncocytomas and clear cell carcinomas.
CA XII seems to be expressed in the normal stratified squamous epithelium, including the esophagus and skin (Nortunen et al. 2018; Syrjanen et al. 2014). In pathological tumor stage 2–3 of esophageal squamous cell carcinoma, the 3-year survival rate of patients with the high-grade expression of CA XII (29.1%) was significantly lower than that of patients with the low-grade expression of CA XII (70.3%) (Ochi et al. 2015). A multivariate analysis showed that the expression of CA XII was one of the most important independent prognostic factors following radical esophagectomy in tumor stage 3-4 carcinomas.
An alternatively spliced form of CA XII is expressed in brain tumors (Haapasalo et al. 2008). RT-PCR revealed that the enzyme present in diffuse astrocytomas is mainly encoded by a shorter mRNA variant. Anti-CA XII antibody recognized both isoforms in the glioblastoma cell lines. Most diffusely infiltrating astrocytomas (98%) showed positive immunoreactions for CA XII protein. Importantly, the expression correlated with poorer patient prognosis in univariate and multivariate survival analyses. The absence of 11 amino acids in the short variant, which seems to be a common form in astrocytomas, may affect the normal quaternary structure and biological function of CA XII.
To date, there have been a few studies elucidating the prognostic value of CA XII. Watson et al. examined the CA XII expression in a series of 103 cases of invasive breast cancer and found a positive correlation with a lower relapse rate and a better survival (Watson et al. 2003). Kim et al. analyzed both CA IX and XII expression in cervical cancer and found CA IX and CA XII transcript expression in 62.7 and 88.1% of tumors, respectively (Kim et al. 2006). Multivariate analysis revealed that CA IX expression was the most significant factor associated with lower metastasis-free survival, whereas CA XII expression was linked to a lower risk of metastasis and better survival.
By comparing the current literature on CA IX and XII, it is obvious that CA IX has greater promise as a histological marker protein. Nevertheless, CA XII is physiologically an interesting member of the CA family, and its exact roles still deserve further investigations.
2.6 Carbonic Anhydrases as Therapeutic Targets
CA research has produced large amounts of data on the distribution, functions, and clinical relevance of different isozymes. These enzymes have been considered therapeutic targets for treatments of various diseases, such as glaucoma, mountain sickness, brain edema, cancer, and epilepsy. In most cases, CA II has been considered the main target enzyme, even though several isozymes are typically expressed in target tissues, and inhibitors can inhibit several isozymes with different affinities. Several drugs with CA inhibitory properties are clinically used, and more are under development to treat important diseases. The clinically approved drugs with CA inhibition properties include acetazolamide, methazolamide, ethoxzolamide, dichlorphenamide, dorzolamide, brinzolamide, topiramate, zonisamide, lacosamide, imatinib, and statins (Pastorekova et al. 2004; Temperini et al. 2010; Parkkila et al. 2012, 2009). Because other chapters of this book describe different clinical targets in great detail, only glaucoma and cancer are briefly mentioned here as examples.
Glaucoma has been a major disease target where CA inhibition has been proven a useful therapeutic option. In the 1950s, the effect of acetazolamide on intraocular pressure was first demonstrated (Becker 1955). The first generation CA inhibitors for treatment of glaucoma included systematically acting drugs: acetazolamide, methazolamide, and dichlorphenamide (Scozzafava and Supuran 2013). The second generation CA inhibitors were topically acting sulfonamides, dorzolamide, and brinzolamide, which possess both water solubility and liposolubility to penetrate the cornea, and they can enter the ciliary process where the CAs are present. More recently, the third generation CA sulfonamide inhibitors have been developed based on the novel “tail approach.” These topically administered compounds have shown 2–3 times higher effects in reducing intraocular pressure compared to dorzolamide (Scozzafava and Supuran 2013). Supuran’s group has suggested that dithiocarbamates could represent the fourth CA inhibitor group for antiglaucoma therapy (Scozzafava and Supuran 2013; Carta et al. 2012). Many dithiocarbamates inhibit CA II in the low nanomolar range, are easy to synthesize, and possess excellent water solubility. Therefore, some dithiocarbamates may arrive in clinical testing soon.
The connections of CAs with various forms of cancer are obvious; therefore, at least CA IX and CA XII are considered potential targets for cancer therapy. Supuran’s and DeSimone’s groups have recently published an extensive review article covering CA IX as a potential therapeutic target enzyme in primary tumors, metastases, and cancer stem cells (Supuran et al. 2018). Their review describes state-of-the-art of studies on CA IX including structural, functional, and biomedical aspects, as well as the development of molecules with diagnostic and therapeutic potential. Crystal structures of both CA IX and CA XII have been previously described (Alterio et al. 2009; Whittington et al. 2001). The knowledge of the three-dimensional structures of both enzymes has made the design of selective inhibitors more straightforward. There are also several pieces of promising results showing that inactivation of CA IX and/or CA XII indeed inhibits the growth or invasion capacity of cancer cells (Doyen et al. 2013; Lou et al. 2011; Boyd et al. 2017; Pettersen et al. 2015). Combination therapies using CA inhibitors together with other anticancer drugs may represent novel options for improved treatment efficiency, especially in hard-to-treat cancers (Amiri et al. 2016).
2.7 Concluding Remarks
During the last two decades, a great number of publications have suggested CA isozymes as potential biomarkers and therapeutic targets. Most of the studies have illustrated CA expression in various tumors, and many suggested CA isozymes—mainly CA IX and XII—as prognostic factors. In addition, immunological assays have been recently developed in order to monitor CA IX levels in biological samples. The observed association between cancers and different CA isozymes has already stimulated translational CA research, which will hopefully lead to attractive novel discoveries that will provide new hope for cancer patients.
References
Alterio V, Hilvo M, Di Fiore A, Supuran CT, Pan P, Parkkila S, Scaloni A, Pastorek J, Pastorekova S, Pedone C, Scozzafava A, Monti SM, De Simone G (2009) Crystal structure of the catalytic domain of the tumor-associated human carbonic anhydrase IX. Proc Natl Acad Sci USA 106(38):16233–16238. https://doi.org/10.1073/pnas.0908301106
Amiri A, Le PU, Moquin A, Machkalyan G, Petrecca K, Gillard JW, Yoganathan N, Maysinger D (2016) Inhibition of carbonic anhydrase IX in glioblastoma multiforme. Eur J Pharm Biopharm 109:81–92. https://doi.org/10.1016/j.ejpb.2016.09.018
Bartosova M, Parkkila S, Pohlodek K, Karttunen TJ, Galbavy S, Mucha V, Harris AL, Pastorek J, Pastorekova S (2002) Expression of carbonic anhydrase IX in breast is associated with malignant tissues and is related to overexpression of c-erbB2. J Pathol 197(3):314–321. https://doi.org/10.1002/path.1120
Beasley NJ, Wykoff CC, Watson PH, Leek R, Turley H, Gatter K, Pastorek J, Cox GJ, Ratcliffe P, Harris AL (2001) Carbonic anhydrase IX, an endogenous hypoxia marker, expression in head and neck squamous cell carcinoma and its relationship to hypoxia, necrosis, and microvessel density. Cancer Res 61(13):5262–5267
Becker B (1955) The mechanism of the fall in intraocular pressure induced by the carbonic anhydrase inhibitor, diamox. Am J Ophthalmol 39(2 Pt 2):177–184
Birner P, Jesch B, Friedrich J, Riegler M, Zacherl J, Hejna M, Wrba F, Schultheis A, Schoppmann SF (2011) Carbonic anhydrase IX overexpression is associated with diminished prognosis in esophageal cancer and correlates with Her-2 expression. Ann Surg Oncol 18(12):3330–3337. https://doi.org/10.1245/s10434-011-1730-3
Bleumer I, Oosterwijk E, Oosterwijk-Wakka JC, Voller MC, Melchior S, Warnaar SO, Mala C, Beck J, Mulders PF (2006) A clinical trial with chimeric monoclonal antibody WX-G250 and low dose interleukin-2 pulsing scheme for advanced renal cell carcinoma. J Urol 175(1):57–62
Boyd NH, Walker K, Fried J, Hackney JR, McDonald PC, Benavides GA, Spina R, Audia A, Scott SE, Libby CJ, Tran AN, Bevensee MO, Griguer C, Nozell S, Gillespie GY, Nabors B, Bhat KP, Bar EE, Darley-Usmar V, Xu B, Gordon E, Cooper SJ, Dedhar S, Hjelmeland AB (2017) Addition of carbonic anhydrase 9 inhibitor SLC-0111 to temozolomide treatment delays glioblastoma growth in vivo. JCI Insight 2(24). https://doi.org/10.1172/jci.insight.92928
Brennan DJ, Jirstrom K, Kronblad A, Millikan RC, Landberg G, Duffy MJ, Ryden L, Gallagher WM, O’Brien SL (2006) CA IX is an independent prognostic marker in premenopausal breast cancer patients with one to three positive lymph nodes and a putative marker of radiation resistance. Clin Cancer Res 12(21):6421–6431
Bui MH, Seligson D, Han KR, Pantuck AJ, Dorey FJ, Huang Y, Horvath S, Leibovich BC, Chopra S, Liao SY, Stanbridge E, Lerman MI, Palotie A, Figlin RA, Belldegrun AS (2003) Carbonic anhydrase IX is an independent predictor of survival in advanced renal clear cell carcinoma: implications for prognosis and therapy. Clin Cancer Res 9(2):802–811
Carta F, Aggarwal M, Maresca A, Scozzafava A, McKenna R, Masini E, Supuran CT (2012) Dithiocarbamates strongly inhibit carbonic anhydrases and show antiglaucoma action in vivo. J Med Chem 55(4):1721–1730. https://doi.org/10.1021/jm300031j
Carvalho S, Troost EG, Bons J, Menheere P, Lambin P, Oberije C (2016) Prognostic value of blood-biomarkers related to hypoxia, inflammation, immune response and tumour load in non-small cell lung cancer—a survival model with external validation. Radiother Oncol 119(3):487–494. https://doi.org/10.1016/j.radonc.2016.04.024
Cetin B, Gonul II, Gumusay O, Bilgetekin I, Algin E, Ozet A, Uner A (2018) Carbonic anhydrase IX is a prognostic biomarker in glioblastoma multiforme. Neuropathology 38(5):457–462. https://doi.org/10.1111/neup.12485
Chen J, Rocken C, Hoffmann J, Kruger S, Lendeckel U, Rocco A, Pastorekova S, Malfertheiner P, Ebert MP (2005) Expression of carbonic anhydrase 9 at the invasion front of gastric cancers. Gut 54(7):920–927
Chia SK, Wykoff CC, Watson PH, Han C, Leek RD, Pastorek J, Gatter KC, Ratcliffe P, Harris AL (2001) Prognostic significance of a novel hypoxia-regulated marker, carbonic anhydrase IX, in invasive breast carcinoma. J Clin Oncol 19(16):3660–3668. https://doi.org/10.1200/JCO.2001.19.16.3660
De Schutter H, Landuyt W, Verbeken E, Goethals L, Hermans R, Nuyts S (2005) The prognostic value of the hypoxia markers CA IX and GLUT 1 and the cytokines VEGF and IL 6 in head and neck squamous cell carcinoma treated by radiotherapy +/- chemotherapy. BMC Cancer 5(1):42
Doyen J, Parks SK, Marcie S, Pouyssegur J, Chiche J (2013) Knock-down of hypoxia-induced carbonic anhydrases IX and XII radiosensitizes tumor cells by increasing intracellular acidosis. Front Oncol 2:199. https://doi.org/10.3389/fonc.2012.00199
Dungwa JV, Hunt LP, Ramani P (2011) Overexpression of carbonic anhydrase and HIF-1alpha in Wilms tumours. BMC Cancer 11:390. https://doi.org/10.1186/1471-2407-11-390
Feldshtein M, Elkrinawi S, Yerushalmi B, Marcus B, Vullo D, Romi H, Ofir R, Landau D, Sivan S, Supuran CT, Birk OS (2010) Hyperchlorhidrosis caused by homozygous mutation in CA12, encoding carbonic anhydrase XII. Am J Hum Genet 87(5):713–720. https://doi.org/10.1016/j.ajhg.2010.10.008
Finkelmeier F, Canli O, Peiffer KH, Walter D, Tal A, Koch C, Pession U, Vermehren J, Trojan J, Zeuzem S, Piiper A, Greten FR, Grammatikos G, Waidmann O (2018) Circulating hypoxia marker carbonic anhydrase IX (CA9) in patients with hepatocellular carcinoma and patients with cirrhosis. PLoS One 13(7):e0200855. https://doi.org/10.1371/journal.pone.0200855
Genega EM, Ghebremichael M, Najarian R, Fu Y, Wang Y, Argani P, Grisanzio C, Signoretti S (2010) Carbonic anhydrase IX expression in renal neoplasms: correlation with tumor type and grade. Am J Clin Pathol 134(6):873–879. https://doi.org/10.1309/AJCPPPR57HNJMSLZ
Giatromanolaki A, Koukourakis MI, Sivridis E, Pastorek J, Wykoff CC, Gatter KC, Harris AL (2001) Expression of hypoxia-inducible carbonic anhydrase-9 relates to angiogenic pathways and independently to poor outcome in non-small cell lung cancer. Cancer Res 61(21):7992–7998
Gigante M, Li G, Ferlay C, Perol D, Blanc E, Paul S, Zhao A, Tostain J, Escudier B, Negrier S, Genin C (2012) Prognostic value of serum CA9 in patients with metastatic clear cell renal cell carcinoma under targeted therapy. Anticancer Res 32(12):5447–5451
Goodison S, Chang M, Dai Y, Urquidi V, Rosser CJ (2012) A multi-analyte assay for the non-invasive detection of bladder cancer. PLoS One 7(10):e47469. https://doi.org/10.1371/journal.pone.0047469
Gramlich TL, Hennigar RA, Spicer SS, Schulte BA (1990) Immunohistochemical localization of sodium-potassium-stimulated adenosine triphosphatase and carbonic anhydrase in human colon and colonic neoplasms. Arch Pathol Lab Med 114(4):415–419
Gu M (2015) CA9 overexpression is an independent favorable prognostic marker in intrahepatic cholangiocarcinoma. Int J Clin Exp Pathol 8(1):862–866
Haapasalo JA, Nordfors KM, Hilvo M, Rantala IJ, Soini Y, Parkkila AK, Pastorekova S, Pastorek J, Parkkila SM, Haapasalo HK (2006) Expression of carbonic anhydrase IX in astrocytic tumors predicts poor prognosis. Clin Cancer Res 12(2):473–477. https://doi.org/10.1158/1078-0432.CCR-05-0848
Haapasalo J, Nordfors K, Jarvela S, Bragge H, Rantala I, Parkkila AK, Haapasalo H, Parkkila S (2007) Carbonic anhydrase II in the endothelium of glial tumors: a potential target for therapy. Neuro Oncol 9(3):308–313. https://doi.org/10.1215/15228517-2007-001
Haapasalo J, Hilvo M, Nordfors K, Haapasalo H, Parkkila S, Hyrskyluoto A, Rantala I, Waheed A, Sly WS, Pastorekova S, Pastorek J, Parkkila AK (2008) Identification of an alternatively spliced isoform of carbonic anhydrase XII in diffusely infiltrating astrocytic gliomas. Neuro Oncol 10(2):131–138. https://doi.org/10.1215/15228517-2007-065
Hilvo M, Baranauskiene L, Salzano AM, Scaloni A, Matulis D, Innocenti A, Scozzafava A, Monti SM, Di Fiore A, De Simone G, Lindfors M, Janis J, Valjakka J, Pastorekova S, Pastorek J, Kulomaa MS, Nordlund HR, Supuran CT, Parkkila S (2008) Biochemical characterization of CA IX, one of the most active carbonic anhydrase isozymes. J Biol Chem 283(41):27799–27809. https://doi.org/10.1074/jbc.M800938200
Hilvo M, Tolvanen M, Clark A, Shen B, Shah GN, Waheed A, Halmi P, Hanninen M, Hamalainen JM, Vihinen M, Sly WS, Parkkila S (2005) Characterization of CA XV, a new GPI-anchored form of carbonic anhydrase. Biochem J 392(Pt 1):83–92. https://doi.org/10.1042/BJ20051102
Hong JH, Muhammad E, Zheng C, Hershkovitz E, Alkrinawi S, Loewenthal N, Parvari R, Muallem S (2015) Essential role of carbonic anhydrase XII in secretory gland fluid and HCO3 (-) secretion revealed by disease causing human mutation. J Physiol 593(24):5299–5312. https://doi.org/10.1113/JP271378
Hoogsteen IJ, Marres HA, Bussink J, van der Kogel AJ, Kaanders JH (2007) Tumor microenvironment in head and neck squamous cell carcinomas: predictive value and clinical relevance of hypoxic markers. A Review. Head Neck 29(6):591–604
Huber AR, Tan D, Sun J, Dean D, Wu T, Zhou Z (2015) High expression of carbonic anhydrase IX is significantly associated with glandular lesions in gastroesophageal junction and with tumorigenesis markers BMI1, MCM4 and MCM7. BMC Gastroenterol 15:80. https://doi.org/10.1186/s12876-015-0310-6
Hussain SA, Ganesan R, Reynolds G, Gross L, Stevens A, Pastorek J, Murray PG, Perunovic B, Anwar MS, Billingham L, James ND, Spooner D, Poole CJ, Rea DW, Palmer DH (2007) Hypoxia-regulated carbonic anhydrase IX expression is associated with poor survival in patients with invasive breast cancer. Br J Cancer 96(1):104–109
Hu X, Huang Z, Liao Z, He C, Fang X (2014) Low CA II expression is associated with tumor aggressiveness and poor prognosis in gastric cancer patients. Int J Clin Exp Pathol 7(10):6716–6724
Hynninen P, Vaskivuo L, Saarnio J, Haapasalo H, Kivela J, Pastorekova S, Pastorek J, Waheed A, Sly WS, Puistola U, Parkkila S (2006) Expression of transmembrane carbonic anhydrases IX and XII in ovarian tumours. Histopathology 49(6):594–602. https://doi.org/10.1111/j.1365-2559.2006.02523.x
Hynninen P, Parkkila S, Huhtala H, Pastorekova S, Pastorek J, Waheed A, Sly WS, Tomas E (2012) Carbonic anhydrase isozymes II, IX, and XII in uterine tumors. APMIS 120(2):117–129. https://doi.org/10.1111/j.1600-0463.2011.02820.x
Hyuga S, Wada H, Eguchi H, Otsuru T, Iwgami Y, Yamada D, Noda T, Asaoka T, Kawamoto K, Gotoh K, Takeda Y, Tanemura M, Umeshita K, Doki Y, Mori M (2017) Expression of carbonic anhydrase IX is associated with poor prognosis through regulation of the epithelialmesenchymal transition in hepatocellular carcinoma. Int J Oncol 51(4):1179–1190. https://doi.org/10.3892/ijo.2017.4098
Ilie M, Mazure NM, Hofman V, Ammadi RE, Ortholan C, Bonnetaud C, Havet K, Venissac N, Mograbi B, Mouroux J, Pouyssegur J, Hofman P (2010) High levels of carbonic anhydrase IX in tumour tissue and plasma are biomarkers of poor prognostic in patients with non-small cell lung cancer. Br J Cancer 102(11):1627–1635. https://doi.org/10.1038/sj.bjc.6605690
Ivanov SV, Kuzmin I, Wei MH, Pack S, Geil L, Johnson BE, Stanbridge EJ, Lerman MI (1998) Down-regulation of transmembrane carbonic anhydrases in renal cell carcinoma cell lines by wild-type von Hippel-Lindau transgenes. Proc Natl Acad Sci USA 95(21):12596–12601
Ivanov S, Liao SY, Ivanova A, Danilkovitch-Miagkova A, Tarasova N, Weirich G, Merrill MJ, Proescholdt MA, Oldfield EH, Lee J, Zavada J, Waheed A, Sly W, Lerman MI, Stanbridge EJ (2001) Expression of hypoxia-inducible cell-surface transmembrane carbonic anhydrases in human cancer. Am J Pathol 158(3):905–919. https://doi.org/10.1016/S0002-9440(10)64038-2
Jarvela S, Parkkila S, Bragge H, Kahkonen M, Parkkila AK, Soini Y, Pastorekova S, Pastorek J, Haapasalo H (2008) Carbonic anhydrase IX in oligodendroglial brain tumors. BMC Cancer 8:1. https://doi.org/10.1186/1471-2407-8-1
Jarvinen P, Kivela AJ, Nummela P, Lepisto A, Ristimaki A, Parkkila S (2017) Carbonic anhydrase II: a novel biomarker for pseudomyxoma peritonei. APMIS 125(3):207–212. https://doi.org/10.1111/apm.12653
Kang HJ, Kim IH, Sung CO, Shim JH, Yu E (2015) Expression of carbonic anhydrase 9 is a novel prognostic marker in resectable hepatocellular carcinoma. Virchows Arch 466(4):403–413. https://doi.org/10.1007/s00428-014-1709-0
Karhumaa P, Parkkila S, Tureci O, Waheed A, Grubb JH, Shah G, Parkkila A, Kaunisto K, Tapanainen J, Sly WS, Rajaniemi H (2000) Identification of carbonic anhydrase XII as the membrane isozyme expressed in the normal human endometrial epithelium. Mol Hum Reprod 6(1):68–74
Karhumaa P, Kaunisto K, Parkkila S, Waheed A, Pastorekova S, Pastorek J, Sly WS, Rajaniemi H (2001) Expression of the transmembrane carbonic anhydrases, CA IX and CA XII, in the human male excurrent ducts. Mol Hum Reprod 7(7):611–616
Kaunisto K, Parkkila S, Rajaniemi H, Waheed A, Grubb J, Sly WS (2002) Carbonic anhydrase XIV: luminal expression suggests key role in renal acidification. Kidney Int 61(6):2111–2118. https://doi.org/10.1046/j.1523-1755.2002.00371.x
Kim SJ, Rabbani ZN, Vollmer RT, Schreiber EG, Oosterwijk E, Dewhirst MW, Vujaskovic Z, Kelley MJ (2004) Carbonic anhydrase IX in early-stage non-small cell lung cancer. Clin Cancer Res 10(23):7925–7933
Kim SJ, Rabbani ZN, Dewhirst MW, Vujaskovic Z, Vollmer RT, Schreiber EG, Oosterwijk E, Kelley MJ (2005) Expression of HIF-1alpha, CA IX, VEGF, and MMP-9 in surgically resected non-small cell lung cancer. Lung Cancer 49(3):325–335
Kim JY, Shin HJ, Kim TH, Cho KH, Shin KH, Kim BK, Roh JW, Lee S, Park SY, Hwang YJ, Han IO (2006) Tumor-associated carbonic anhydrases are linked to metastases in primary cervical cancer. J Cancer Res Clin Oncol 132(5):302–308
Kindblom LG, Remotti HE, Aldenborg F, Meis-Kindblom JM (1998) Gastrointestinal pacemaker cell tumor (GIPACT): gastrointestinal stromal tumors show phenotypic characteristics of the interstitial cells of Cajal. Am J Pathol 152(5):1259–1269
Kivela AJ, Parkkila S, Saarnio J, Karttunen TJ, Kivela J, Parkkila AK, Pastorekova S, Pastorek J, Waheed A, Sly WS, Rajaniemi H (2000a) Expression of transmembrane carbonic anhydrase isoenzymes IX and XII in normal human pancreas and pancreatic tumours. Histochem Cell Biol 114(3):197–204
Kivela A, Parkkila S, Saarnio J, Karttunen TJ, Kivela J, Parkkila AK, Waheed A, Sly WS, Grubb JH, Shah G, Tureci O, Rajaniemi H (2000b) Expression of a novel transmembrane carbonic anhydrase isozyme XII in normal human gut and colorectal tumors. Am J Pathol 156(2):577–584. https://doi.org/10.1016/S0002-9440(10)64762-1
Kivela AJ, Knuuttila A, Rasanen J, Sihvo E, Salmenkivi K, Saarnio J, Pastorekova S, Pastorek J, Waheed A, Sly WS, Salo JA, Parkkila S (2013) Carbonic anhydrase IX in malignant pleural mesotheliomas: a potential target for anti-cancer therapy. Bioorg Med Chem 21(6):1483–1488. https://doi.org/10.1016/j.bmc.2012.09.018
Kummola L, Hamalainen JM, Kivela J, Kivela AJ, Saarnio J, Karttunen T, Parkkila S (2005) Expression of a novel carbonic anhydrase, CA XIII, in normal and neoplastic colorectal mucosa. BMC Cancer 5(1):41
Lehtonen J, Shen B, Vihinen M, Casini A, Scozzafava A, Supuran CT, Parkkila AK, Saarnio J, Kivela AJ, Waheed A, Sly WS, Parkkila S (2004) Characterization of CA XIII, a novel member of the carbonic anhydrase isozyme family. J Biol Chem 279(4):2719–2727. https://doi.org/10.1074/jbc.M308984200
Leppilampi M, Saarnio J, Karttunen TJ, Kivela J, Pastorekova S, Pastorek J, Waheed A, Sly WS, Parkkila S (2003) Carbonic anhydrase isozymes IX and XII in gastric tumors. World J Gastroenterol 9(7):1398–1403
Leppilampi M, Koistinen P, Savolainen ER, Hannuksela J, Parkkila AK, Niemela O, Pastorekova S, Pastorek J, Waheed A, Sly WS, Parkkila S, Rajaniemi H (2002) The expression of carbonic anhydrase II in hematological malignancies. Clin Cancer Res 8(7):2240–2245
Le QT, Kong C, Lavori PW, O’Byrne K, Erler JT, Huang X, Chen Y, Cao H, Tibshirani R, Denko N, Giaccia AJ, Koong AC (2007) Expression and prognostic significance of a panel of tissue hypoxia markers in head-and-neck squamous cell carcinomas. Int J Radiat Oncol Biol Phys 69(1):167–175
Liao ND, Lee WY (2012) Detection of carbonic anhydrase IX protein in the diagnosis of malignant pleural effusion by enzyme-linked immunosorbent assay and immunocytochemistry. Cancer Cytopathol 120(4):269–275. https://doi.org/10.1002/cncy.21191
Liao SY, Brewer C, Zavada J, Pastorek J, Pastorekova S, Manetta A, Berman ML, DiSaia PJ, Stanbridge EJ (1994) Identification of the MN antigen as a diagnostic biomarker of cervical intraepithelial squamous and glandular neoplasia and cervical carcinomas. Am J Pathol 145(3):598–609
Liao SY, Ivanov S, Ivanova A, Ghosh S, Cote MA, Keefe K, Coca-Prados M, Stanbridge EJ, Lerman MI (2003) Expression of cell surface transmembrane carbonic anhydrase genes CA9 and CA12 in the human eye: overexpression of CA12 (CAXII) in glaucoma. J Med Genet 40(4):257–261
Li G, Feng G, Gentil-Perret A, Genin C, Tostain J (2008) Serum carbonic anhydrase 9 level is associated with postoperative recurrence of conventional renal cell cancer. J Urol 180(2):510–513; discussion 513–514. https://doi.org/10.1016/j.juro.2008.04.024
Li XJ, Xie HL, Lei SJ, Cao HQ, Meng TY, Hu YL (2012) Reduction of CAII expression in gastric cancer: correlation with invasion and metastasis. Chin J Cancer Res 24(3):196–200. https://doi.org/10.1007/s11670-012-0196-6
Loncaster JA, Harris AL, Davidson SE, Logue JP, Hunter RD, Wycoff CC, Pastorek J, Ratcliffe PJ, Stratford IJ, West CM (2001) Carbonic anhydrase (CA IX) expression, a potential new intrinsic marker of hypoxia: correlations with tumor oxygen measurements and prognosis in locally advanced carcinoma of the cervix. Cancer Res 61(17):6394–6399
Lou Y, McDonald PC, Oloumi A, Chia S, Ostlund C, Ahmadi A, Kyle A, Auf dem Keller U, Leung S, Huntsman D, Clarke B, Sutherland BW, Waterhouse D, Bally M, Roskelley C, Overall CM, Minchinton A, Pacchiano F, Carta F, Scozzafava A, Touisni N, Winum JY, Supuran CT, Dedhar S (2011) Targeting tumor hypoxia: suppression of breast tumor growth and metastasis by novel carbonic anhydrase IX inhibitors. Cancer Res 71(9):3364–3376. https://doi.org/10.1158/0008-5472.CAN-10-4261
Lucarini L, Magnelli L, Schiavone N, Crisci A, Innocenti A, Puccetti L, Cianchi F, Peri S, Supuran CT, Papucci L, Masini E (2018) Plasmatic carbonic anhydrase IX as a diagnostic marker for clear cell renal cell carcinoma. J Enzyme Inhib Med Chem 33(1):234–240. https://doi.org/10.1080/14756366.2017.1411350
Maseide K, Kandel RA, Bell RS, Catton CN, O’Sullivan B, Wunder JS, Pintilie M, Hedley D, Hill RP (2004) Carbonic anhydrase IX as a marker for poor prognosis in soft tissue sarcoma. Clin Cancer Res 10(13):4464–4471
Mayer A, Hockel M, Wree A, Leo C, Horn LC, Vaupel P (2008) Lack of hypoxic response in uterine leiomyomas despite severe tissue hypoxia. Cancer Res 68(12):4719–4726. https://doi.org/10.1158/0008-5472.CAN-07-6339
McDonald PC, Swayampakula M, Dedhar S (2018) Coordinated regulation of metabolic transporters and migration/invasion by carbonic anhydrase IX. Metabolites 8(1). https://doi.org/10.3390/metabo8010020
McKiernan JM, Buttyan R, Bander NH, Stifelman MD, Katz AE, Chen MW, Olsson CA, Sawczuk IS (1997) Expression of the tumor-associated gene MN: a potential biomarker for human renal cell carcinoma. Cancer Res 57(12):2362–2365
Mehes G, Matolay O, Beke L, Czenke M, Jona A, Miltenyi Z, Illes A, Bedekovics J (2019) Hypoxia related carbonic anhydrase IX expression is associated with unfavourable response to first-line therapy in classical Hodgkin’s lymphoma. Histopathology. https://doi.org/10.1111/his.13808
Miettinen M, Lasota J (2006) Gastrointestinal stromal tumors: review on morphology, molecular pathology, prognosis, and differential diagnosis. Arch Pathol Lab Med 130(10):1466–1478. https://doi.org/10.1043/1543-2165(2006)130[1466:GSTROM]2.0.CO;2
Mincione F, Scozzafava A, Supuran CT (2007) The development of topically acting carbonic anhydrase inhibitors as anti-glaucoma agents. Curr Top Med Chem 7(9):849–854
Morgan PE, Pastorekova S, Stuart-Tilley AK, Alper SL, Casey JR (2007) Interactions of transmembrane carbonic anhydrase, CAIX, with bicarbonate transporters. Am J Physiol Cell Physiol 293(2):C738-748. https://doi.org/10.1152/ajpcell.00157.2007
Muhammad E, Leventhal N, Parvari G, Hanukoglu A, Hanukoglu I, Chalifa-Caspi V, Feinstein Y, Weinbrand J, Jacoby H, Manor E, Nagar T, Beck JC, Sheffield VC, Hershkovitz E, Parvari R (2011) Autosomal recessive hyponatremia due to isolated salt wasting in sweat associated with a mutation in the active site of Carbonic Anhydrase 12. Hum Genet 129(4):397–405. https://doi.org/10.1007/s00439-010-0930-4
Nakamura J, Kitajima Y, Kai K, Hashiguchi K, Hiraki M, Noshiro H, Miyazaki K (2011) Expression of hypoxic marker CA IX is regulated by site-specific DNA methylation and is associated with the histology of gastric cancer. Am J Pathol 178(2):515–524. https://doi.org/10.1016/j.ajpath.2010.10.010
Niemela AM, Hynninen P, Mecklin JP, Kuopio T, Kokko A, Aaltonen L, Parkkila AK, Pastorekova S, Pastorek J, Waheed A, Sly WS, Orntoft TF, Kruhoffer M, Haapasalo H, Parkkila S, Kivela AJ (2007) Carbonic anhydrase IX is highly expressed in hereditary nonpolyposis colorectal cancer. Cancer Epidemiol Biomarkers Prev 16(9):1760–1766. https://doi.org/10.1158/1055-9965.EPI-07-0080
Nordfors K, Haapasalo J, Korja M, Niemela A, Laine J, Parkkila AK, Pastorekova S, Pastorek J, Waheed A, Sly WS, Parkkila S, Haapasalo H (2010) The tumour-associated carbonic anhydrases CA II, CA IX and CA XII in a group of medulloblastomas and supratentorial primitive neuroectodermal tumours: an association of CA IX with poor prognosis. BMC Cancer 10:148. https://doi.org/10.1186/1471-2407-10-148
Nortunen M, Huhta H, Helminen O, Parkkila S, Kauppila JH, Karttunen TJ, Saarnio J (2018) Carbonic anhydrases II, IX, and XII in Barrett’s esophagus and adenocarcinoma. Virchows Arch 473(5):567–575. https://doi.org/10.1007/s00428-018-2424-z
Ochi F, Shiozaki A, Ichikawa D, Fujiwara H, Nakashima S, Takemoto K, Kosuga T, Konishi H, Komatsu S, Okamoto K, Kishimoto M, Marunaka Y, Otsuji E (2015) Carbonic anhydrase XII as an independent prognostic factor in advanced Esophageal squamous cell carcinoma. J Cancer 6(10):922–929. https://doi.org/10.7150/jca.11269
Ostheimer C, Bache M, Guttler A, Kotzsch M, Vordermark D (2014) A pilot study on potential plasma hypoxia markers in the radiotherapy of non-small cell lung cancer. Osteopontin, carbonic anhydrase IX and vascular endothelial growth factor. Strahlenther Onkol 190(3):276–282. https://doi.org/10.1007/s00066-013-0484-1
Papworth K, Sandlund J, Grankvist K, Ljungberg B, Rasmuson T (2010) Soluble carbonic anhydrase IX is not an independent prognostic factor in human renal cell carcinoma. Anticancer Res 30(7):2953–2957
Parkkila S, Parkkila AK, Saarnio J, Kivela J, Karttunen TJ, Kaunisto K, Waheed A, Sly WS, Tureci O, Virtanen I, Rajaniemi H (2000) Expression of the membrane-associated carbonic anhydrase isozyme XII in the human kidney and renal tumors. J Histochem Cytochem 48(12):1601–1608. https://doi.org/10.1177/002215540004801203
Parkkila S, Parkkila AK (1996) Carbonic anhydrase in the alimentary tract. Roles of the different isozymes and salivary factors in the maintenance of optimal conditions in the gastrointestinal canal. Scand J Gastroenterol 31(4):305–317
Parkkila S, Parkkila AK, Juvonen T, Rajaniemi H (1994) Distribution of the carbonic anhydrase isoenzymes I, II, and VI in the human alimentary tract. Gut 35(5):646–650
Parkkila S, Parkkila AK, Juvonen T, Lehto VP, Rajaniemi H (1995a) Immunohistochemical demonstration of the carbonic anhydrase isoenzymes I and II in pancreatic tumours. Histochem J 27(2):133–138
Parkkila AK, Herva R, Parkkila S, Rajaniemi H (1995b) Immunohistochemical demonstration of human carbonic anhydrase isoenzyme II in brain tumours. Histochem J 27(12):974–982
Parkkila S, Innocenti A, Kallio H, Hilvo M, Scozzafava A, Supuran CT (2009) The protein tyrosine kinase inhibitors imatinib and nilotinib strongly inhibit several mammalian alpha-carbonic anhydrase isoforms. Bioorg Med Chem Lett 19(15):4102–4106. https://doi.org/10.1016/j.bmcl.2009.06.002
Parkkila S, Lasota J, Fletcher JA, Ou WB, Kivela AJ, Nuorva K, Parkkila AK, Ollikainen J, Sly WS, Waheed A, Pastorekova S, Pastorek J, Isola J, Miettinen M (2010) Carbonic anhydrase II. A novel biomarker for gastrointestinal stromal tumors. Mod Pathol 23(5):743–750. https://doi.org/10.1038/modpathol.2009.189
Parkkila S, Vullo D, Maresca A, Carta F, Scozzafava A, Supuran CT (2012) Serendipitous fragment-based drug discovery: ketogenic diet metabolites and statins effectively inhibit several carbonic anhydrases. Chem Commun (camb) 48(29):3551–3553. https://doi.org/10.1039/c2cc30359k
Pastorekova S, Parkkila S, Parkkila AK, Opavsky R, Zelnik V, Saarnio J, Pastorek J (1997) Carbonic anhydrase IX, MN/CA IX: analysis of stomach complementary DNA sequence and expression in human and rat alimentary tracts. Gastroenterology 112(2):398–408
Pastorekova S, Parkkila S, Pastorek J, Supuran CT (2004) Carbonic anhydrases: current state of the art, therapeutic applications and future prospects. J Enzyme Inhib Med Chem 19(3):199–229. https://doi.org/10.1080/14756360410001689540
Pastorekova S, Parkkila S, Zavada J (2006) Tumor-associated carbonic anhydrases and their clinical significance. Adv Clin Chem 42:167–216
Pena C, Lathia C, Shan M, Escudier B, Bukowski RM (2010) Biomarkers predicting outcome in patients with advanced renal cell carcinoma: results from sorafenib phase III treatment approaches in renal cancer global evaluation trial. Clin Cancer Res 16(19):4853–4863. https://doi.org/10.1158/1078-0432.CCR-09-3343
Pettersen EO, Ebbesen P, Gieling RG, Williams KJ, Dubois L, Lambin P, Ward C, Meehan J, Kunkler IH, Langdon SP, Ree AH, Flatmark K, Lyng H, Calzada MJ, Peso LD, Landazuri MO, Gorlach A, Flamm H, Kieninger J, Urban G, Weltin A, Singleton DC, Haider S, Buffa FM, Harris AL, Scozzafava A, Supuran CT, Moser I, Jobst G, Busk M, Toustrup K, Overgaard J, Alsner J, Pouyssegur J, Chiche J, Mazure N, Marchiq I, Parks S, Ahmed A, Ashcroft M, Pastorekova S, Cao Y, Rouschop KM, Wouters BG, Koritzinsky M, Mujcic H, Cojocari D (2015) Targeting tumour hypoxia to prevent cancer metastasis. From biology, biosensing and technology to drug development: the METOXIA consortium. J Enzyme Inhib Med Chem 30(5):689–721. https://doi.org/10.3109/14756366.2014.966704
Pickering LM, Larkin J (2015) Kidney cancer: carbonic anhydrase IX in resected clear cell RCC. Nat Rev Urol 12(6):309–310. https://doi.org/10.1038/nrurol.2015.124
Ramsey ML, Yuh BJ, Johnson MT, Yeldandi AV, Zynger DL (2012) Carbonic anhydrase IX is expressed in mesothelioma and metastatic clear cell renal cell carcinoma of the lung. Virchows Arch 460(1):89–93. https://doi.org/10.1007/s00428-011-1178-7
Rosenberg V, Pastorekova S, Zatovicova M, Vidlickova I, Jelenska L, Slezak P (2016) High serum carbonic anhydrase IX predicts shorter survival in head and neck cancer. Bratisl Lek Listy 117(4):201–204
Saarnio J, Parkkila S, Parkkila AK, Waheed A, Casey MC, Zhou XY, Pastorekova S, Pastorek J, Karttunen T, Haukipuro K, Kairaluoma MI, Sly WS (1998a) Immunohistochemistry of carbonic anhydrase isozyme IX (MN/CA IX) in human gut reveals polarized expression in the epithelial cells with the highest proliferative capacity. J Histochem Cytochem 46(4):497–504. https://doi.org/10.1177/002215549804600409
Saarnio J, Parkkila S, Parkkila AK, Haukipuro K, Pastorekova S, Pastorek J, Kairaluoma MI, Karttunen TJ (1998b) Immunohistochemical study of colorectal tumors for expression of a novel transmembrane carbonic anhydrase, MN/CA IX, with potential value as a marker of cell proliferation. Am J Pathol 153(1):279–285. https://doi.org/10.1016/S0002-9440(10)65569-1
Saarnio J, Parkkila S, Parkkila AK, Pastorekova S, Haukipuro K, Pastorek J, Juvonen T, Karttunen TJ (2001) Transmembrane carbonic anhydrase, MN/CA IX, is a potential biomarker for biliary tumours. J Hepatol 35(5):643–649
Sandlund J, Oosterwijk E, Grankvist K, Oosterwijk-Wakka J, Ljungberg B, Rasmuson T (2007) Prognostic impact of carbonic anhydrase IX expression in human renal cell carcinoma. BJU Int 100(3):556–560
Scozzafava A, Supuran CT (2013) Glaucoma and the applications of carbonic anhydrase inhibitors. Subcell Biochem 75:349–359. https://doi.org/10.1007/978-94-007-7359-2_17
Senol S, Aydin A, Kosemetin D, Ece D, Akalin I, Abuoglu H, Duran EA, Aydin D, Erkol B (2016) Gastric adenocarcinoma biomarker expression profiles and their prognostic value. J Environ Pathol Toxicol Oncol 35(3):207–222. https://doi.org/10.1615/JEnvironPatholToxicolOncol.2016016099
Shimizu Y, Furuya H, Bryant Greenwood P, Chan O, Dai Y, Thornquist MD, Goodison S, Rosser CJ (2016) A multiplex immunoassay for the non-invasive detection of bladder cancer. J Transl Med 14:31. https://doi.org/10.1186/s12967-016-0783-2
Sly WS, Hu PY (1995) Human carbonic anhydrases and carbonic anhydrase deficiencies. Annu Rev Biochem 64:375–401
Span PN, Bussink J, De Mulder PH, Sweep FC (2007) Carbonic anhydrase IX expression is more predictive than prognostic in breast cancer. Br J Cancer 96(8):1309; author reply 1310
Supuran CT, Scozzafava A (2007) Carbonic anhydrases as targets for medicinal chemistry. Bioorg Med Chem 15(13):4336–4350
Supuran CT, Alterio V, Di Fiore A, DA K, Carta F, Monti SM, De Simone G (2018) Inhibition of carbonic anhydrase IX targets primary tumors, metastases, and cancer stem cells: three for the price of one. Med Res Rev 38(6):1799–1836. https://doi.org/10.1002/med.21497
Svastova E, Hulikova A, Rafajova M, Zat’ovicova M, Gibadulinova A, Casini A, Cecchi A, Scozzafava A, Supuran CT, Pastorek J, Pastorekova S (2004) Hypoxia activates the capacity of tumor-associated carbonic anhydrase IX to acidify extracellular pH. FEBS Lett 577(3):439–445
Svastova E, Witarski W, Csaderova L, Kosik I, Skvarkova L, Hulikova A, Zatovicova M, Barathova M, Kopacek J, Pastorek J, Pastorekova S (2012) Carbonic anhydrase IX interacts with bicarbonate transporters in lamellipodia and increases cell migration via its catalytic domain. J Biol Chem 287(5):3392–3402. https://doi.org/10.1074/jbc.M111.286062
Syrjanen L, Luukkaala T, Leppilampi M, Kallioinen M, Pastorekova S, Pastorek J, Waheed A, Sly WS, Parkkila S, Karttunen T (2014) Expression of cancer-related carbonic anhydrases IX and XII in normal skin and skin neoplasms. APMIS 122(9):880–889. https://doi.org/10.1111/apm.12251
Takacova M, Bullova P, Simko V, Skvarkova L, Poturnajova M, Feketeova L, Babal P, Kivela AJ, Kuopio T, Kopacek J, Pastorek J, Parkkila S, Pastorekova S (2014) Expression pattern of carbonic anhydrase IX in medullary thyroid carcinoma supports a role for RET-mediated activation of the HIF pathway. Am J Pathol 184(4):953–965. https://doi.org/10.1016/j.ajpath.2014.01.002
Tanaka N, Kato H, Inose T, Kimura H, Faried A, Sohda M, Nakajima M, Fukai Y, Miyazaki T, Masuda N, Fukuchi M, Kuwano H (2008) Expression of carbonic anhydrase 9, a potential intrinsic marker of hypoxia, is associated with poor prognosis in oesophageal squamous cell carcinoma. Br J Cancer 99(9):1468–1475. https://doi.org/10.1038/sj.bjc.6604719
Tashian RE, Hewett-Emmett D, Carter N, Bergenhem NC (2000) Carbonic anhydrase (CA)-related proteins (CA-RPs), and transmembrane proteins with CA or CA-RP domains. Exs 90:105–120
Temperini C, Innocenti A, Scozzafava A, Parkkila S, Supuran CT (2010) The coumarin-binding site in carbonic anhydrase accommodates structurally diverse inhibitors: the antiepileptic lacosamide as an example and lead molecule for novel classes of carbonic anhydrase inhibitors. J Med Chem 53(2):850–854. https://doi.org/10.1021/jm901524f
Thiry A, Dogne JM, Supuran CT, Masereel B (2007) Carbonic anhydrase inhibitors as anticonvulsant agents. Curr Top Med Chem 7(9):855–864
Tureci O, Sahin U, Vollmar E, Siemer S, Gottert E, Seitz G, Parkkila AK, Shah GN, Grubb JH, Pfreundschuh M, Sly WS (1998) Human carbonic anhydrase XII: cDNA cloning, expression, and chromosomal localization of a carbonic anhydrase gene that is overexpressed in some renal cell cancers. Proc Natl Acad Sci USA 95(13):7608–7613
Turner JR, Odze RD, Crum CP, Resnick MB (1997) MN antigen expression in normal, preneoplastic, and neoplastic esophagus: a clinicopathological study of a new cancer-associated biomarker. Hum Pathol 28(6):740–744
van Kuijk SJ, Yaromina A, Houben R, Niemans R, Lambin P, Dubois LJ (2016) Prognostic significance of carbonic anhydrase IX Expression in cancer patients: a meta-analysis. Front Oncol 6:69. https://doi.org/10.3389/fonc.2016.00069
van ‘t Veer LJ, Dai H, van de Vijver MJ, He YD, Hart AA, Mao M, Peterse HL, van der Kooy K, Marton MJ, Witteveen AT, Schreiber GJ, Kerkhoven RM, Roberts C, Linsley PS, Bernards R, Friend SH (2002) Gene expression profiling predicts clinical outcome of breast cancer. Nature 415(6871):530–536. https://doi.org/10.1038/415530a
Viikila P, Kivela AJ, Mustonen H, Koskensalo S, Waheed A, Sly WS, Pastorek J, Pastorekova S, Parkkila S, Haglund C (2016) Carbonic anhydrase enzymes II, VII, IX and XII in colorectal carcinomas. World J Gastroenterol 22(36):8168–8177. https://doi.org/10.3748/wjg.v22.i36.8168
Waheed A, Sly WS (2017) Carbonic anhydrase XII functions in health and disease. Gene 623:33–40. https://doi.org/10.1016/j.gene.2017.04.027
Watson PH, Chia SK, Wykoff CC, Han C, Leek RD, Sly WS, Gatter KC, Ratcliffe P, Harris AL (2003) Carbonic anhydrase XII is a marker of good prognosis in invasive breast carcinoma. Br J Cancer 88(7):1065–1070
Whittington DA, Waheed A, Ulmasov B, Shah GN, Grubb JH, Sly WS, Christianson DW (2001) Crystal structure of the dimeric extracellular domain of human carbonic anhydrase XII, a bitopic membrane protein overexpressed in certain cancer tumor cells. Proc Natl Acad Sci USA 98(17):9545–9550. https://doi.org/10.1073/pnas.161301298
Woelber L, Mueller V, Eulenburg C, Schwarz J, Carney W, Jaenicke F, Milde-Langosch K, Mahner S (2010) Serum carbonic anhydrase IX during first-line therapy of ovarian cancer. Gynecol Oncol 117(2):183–188. https://doi.org/10.1016/j.ygyno.2009.11.029
Woelber L, Kress K, Kersten JF, Choschzick M, Kilic E, Herwig U, Lindner C, Schwarz J, Jaenicke F, Mahner S, Milde-Langosch K, Mueller V, Ihnen M (2011) Carbonic anhydrase IX in tumor tissue and sera of patients with primary cervical cancer. BMC Cancer 11:12. https://doi.org/10.1186/1471-2407-11-12
Wykoff CC, Beasley N, Watson PH, Campo L, Chia SK, English R, Pastorek J, Sly WS, Ratcliffe P, Harris AL (2001) Expression of the hypoxia-inducible and tumor-associated carbonic anhydrases in ductal carcinoma in situ of the breast. Am J Pathol 158(3):1011–1019. https://doi.org/10.1016/S0002-9440(10)64048-5
Yoshiura K, Nakaoka T, Nishishita T, Sato K, Yamamoto A, Shimada S, Saida T, Kawakami Y, Takahashi TA, Fukuda H, Imajoh-Ohmi S, Oyaizu N, Yamashita N (2005) Carbonic anhydrase II is a tumor vessel endothelium-associated antigen targeted by dendritic cell therapy. Clin Cancer Res 11(22):8201–8207
Zavada J, Zavadova Z, Zat’ovicova M, Hyrsl L, Kawaciuk I (2003) Soluble form of carbonic anhydrase IX (CA IX) in the serum and urine of renal carcinoma patients. Br J Cancer 89(6):1067–1071. https://doi.org/10.1038/sj.bjc.6601264
Zhou GX, Ireland J, Rayman P, Finke J, Zhou M (2010) Quantification of carbonic anhydrase IX expression in serum and tissue of renal cell carcinoma patients using enzyme-linked immunosorbent assay: prognostic and diagnostic potentials. Urology 75(2):257–261. https://doi.org/10.1016/j.urology.2009.09.052
Acknowledgements
The studies of the author’s group are supported by grants from the Sigrid Juselius Foundation, Academy of Finland and the Jane & Aatos Erkko Foundation.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Parkkila, S. (2021). Carbonic Anhydrase Isozymes as Diagnostic Biomarkers and Therapeutic Targets. In: Chegwidden, W.R., Carter, N.D. (eds) The Carbonic Anhydrases: Current and Emerging Therapeutic Targets. Progress in Drug Research, vol 75. Springer, Cham. https://doi.org/10.1007/978-3-030-79511-5_2
Download citation
DOI: https://doi.org/10.1007/978-3-030-79511-5_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-79510-8
Online ISBN: 978-3-030-79511-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)