Abstract
Structure-activity relationship analysis is a powerful tool to elucidate the structural requirements for high-affinity vitamin D receptor (VDR) ligands. This chapter systematically interrogates the structural features of 1α,25(OH)2D3, the vitamin D metabolite with the highest VDR affinity. It can be concluded that the C1α and C25 hydroxyl groups of 1α,25(OH)2D3 are very important for binding. Optimal spatial arrangement of both hydroxyl groups was achieved with either a hydrophobic semi-flexible secosteroid scaffold or a simplified, flexible carbon chain. Y-shaped ligands with high affinity confirmed a highly inducible VDR ligand-binding pocket, which has been visualized by X-ray crystallography. Substitution of the secosteroid scaffold by other hydrophobic spacers such as carboranes or aromatic ring systems has led to many non-secosteroid VDR ligands. Exploration of ligand substitution has led to the development of antagonists that are accommodated by the inducible VDR ligand-binding pocket but alter the overall conformation of VDR in ways that prevent interactions with coactivator proteins from occurring and ultimately result in reduced gene transcription.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Vitamin D
- Vitamin D receptor
- 1,25-Dihydroxyvitamin D3
- Structure-activity relationship
- Agonist
- Antagonist
- Coactivator
8.1 Introduction
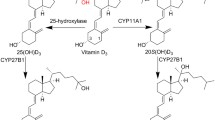
The vitamin D receptor (VDR) takes a special place among nuclear receptors because the biosynthesis of its endogenous ligand, 1α,25-dihydroxyvitamin D3 (1α,25(OH)2D3), is dependent on sun exposure. Poor living conditions during the industrial revolution, when people were destined to work and live inside with minimal light exposure, caused bone deformations and skin diseases with symptoms that were alleviated by light therapy. Once irradiated food was found to have the same medicinal effect [1], isolation of vitamin D was rapidly accomplished [2]. Identification of the corresponding receptor turned out to be difficult; however, vitamin D does not bind VDR at physiological concentrations. Once radiolabeled vitamin D was generated [3], 25(OH)D3 and 1α,25(OH)2D3 were identified [4, 5], which in turn enabled the identification and cloning of VDR [6, 7]. The genomic function of VDR regulates genes involved in calcium homeostasis, cell proliferation, and cell differentiation. The endocrine receptor is expressed in the epithelia of the endocrine organs, digestive tract, kidneys, and thymus [8] but is also found in leukocytes and bone cells. VDR can be found in the cytosol or membrane-bound [9]. In the nucleus, VDR is liganded and binds DNA and the retinoid X receptor (RXR) [10]. VDR-specific gene promoter sequences have been identified [11]. The transcriptional complex includes, among other proteins, nuclear receptor coactivators and corepressors that interact with RNA polymerase II [12], changing chromatin packing and enabling specific gene transcription [13].
This chapter is not a complete review describing more than 3000 VDR ligands that have been reported since 1970. Therefore, I refer the reader to five chapters within the recent edition of Vitamin D and cited references, as well as excellent recent reviews [14,15,16,17,18,19,20]. This chapter highlights the relationship between molecular ligand structure and VDR affinity. Other downstream biological effects can be found in the references and the recent two volumes of Vitamin D. Most VDR ligands have been characterized by their ability to compete with tritium-labeled 1α,25(OH)2D3. Transcription assays have been used to distinguish between agonists and antagonists. VDR coactivator recruitment has been studied with two-hybrid assays or biochemically using homogeneous time-resolved fluorescence (HTRF). Tertiary assays employed for the characterization of VDR ligands included, among others, cell differentiation, cell proliferation, cellular calcium uptake, intestinal calcium transport, and serum calcium changes. Herein, we predominately report VDR affinity independent of cell permeability, metabolic stability, plasma binding (vitamin D-binding protein), and other factors that change with the structure of a small molecule.
8.2 Secosteroid VDR Ligands
Calcitriol, or 1α,25(OH)2D3, has the highest affinity for VDR among all vitamin D metabolites. The competitive VDR binding of 1α,25(OH)2D3 using [3H]-1α,25(OH)2D3 as a probe has been reported in the range of 0.04–0.16 nM with protein isolated from the tissue and cells or recombinantly expressed VDR as full-length receptor or ligand-binding domain [21, 22]. Other assays such as biochemical coactivator recruitment assays reported EC50 of 1.2 nM for 1α,25(OH)2D3 [23]. For most cases, the affinities of new compounds in comparison to 1α,25(OH)2D3 are reported as percent affinity in this chapter.
1α,25(OH)2D3 has six chiral centers and two trisubstituted double bonds that can adopt an E or Z configuration. First, we will compare VDR binding of 1α,25(OH)2D3 epimers and stereoisomers depicted in Figs. 8.1, 8.2, 8.3, and 8.4.
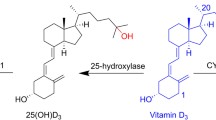
1α,25(OH)2D3 is the metabolic product of vitamin D3 , which lacks a hydroxyl group in the C1 and C25 positions (Fig. 8.1). 25-Hydroxyvitamin D-1α hydroxylase, located primarily in the kidneys but also in other tissues, stereospecifically introduces a C1α-hydroxyl group [24,25,26,27]. The 3-OH group is present in vitamin D3 and its precursor 7-dehydrocholersterol. The evaluation of A-ring diastereomers of 1α,25(OH)2D3 demonstrated that binding to VDR is more impacted by the stereochemistry of the C1-position than the C3-position [28]. VDR affinity for 2 was 24% in comparison to 1α,25(OH)2D3 but only 0.2% and 0.8% for 3 and 4, respectively. 1α,25(OH)2-3-Epi-D3 has been identified as a natural metabolite of 1α,25(OH)2D3 [29] and was intensively studied in vivo. 1β,25(OH)2D3 was first synthesized in 1977 [30] and has also recently been identified as a natural metabolite of vitamin D [31]. 1β,25(OH)2D3 has been reported as a non-genomic antagonist of 1α,25(OH)2D3 [28].
Secosteroids in contrast to steroids have a “broken” B-ring resulting in a triene system with 5(Z),7(E) configuration (Fig. 8.2). The formation of secosteroids occurs via a retro Diels-Alder reaction in the presence of light followed by a [1,7] sigmatropic rearrangement. In the skin, this conversion occurs with high stereoselectivity.
Isomer 5 retains a good affinity toward VDR, which is 13% in comparison to 1α,25(OH)2D3 [32]. The E,E stereochemistry can be generated by light in the presence of iodine [33] or by a cheletropic addition-elimination with sulfur dioxide [34]. In contrast, isomers 6 and 7 were not observed for photochemical reactions but synthesized using a chromium(0)-mediated isomerization reaction with a vinylallene precursor [34]. The affinity toward VDR was 0.82% for 6 and 1.6% for 7 in comparison to 1α,25(OH)2D3. Thus, the position of the A-ring with respect to the B- and C-ring is more important for VDR binding than the location of the terminal alkene.
The stereochemistry of the fused B,C-ring system of 1α,25(OH)2D3 originates from 7-dehydrocholesterol. Interestingly, the configuration of the fused system has a direct influence on the equilibrium of the thermal [1,7] sigmatropic rearrangement reaction (Fig. 8.3). When 1α,25(OH)2D3 was heated at 80 °C, only 12% of the pre-1α,25(OH)2D3 was detected [35]. However, when epimer 8 was heated at 80 °C, a 95:5 ratio in favor of the pre-structure was formed.
Pure 8 was synthesized by epimerization of Grundmann’s ketone and retained a VDR affinity of 15% in comparison with 1α,25(OH)2D3 [35]. Interestingly, no reports were found for compounds 9 and 10.
The synthesis of 11 has been reported [36]. Later, an improved route was developed but VDR binding was not reported [37]. However, inhibition of human breast cancer cell (MCF-7) proliferation was more pronounced in the presence of 11 than 1α,25(OH)2D3. Epimer 12 demonstrated inhibition of T-cell proliferation at picomolar concentrations [38]. The VDR binding was 88% in comparison to 1α,25(OH)2D3 [39].
Next, the importance of functional groups and substituents with respect to VDR binding is discussed. Analogs that lack certain structural elements are compared to 1α,25(OH)2D3 (Fig. 8.5).
25OHD3 is a metabolic product of vitamin D3 and was identified in 1968 [4]. It is abundant in blood and used to determine the vitamin D status in humans [40]. The binding toward VDR is 900-fold less than 1α,25(OH)2D3 [41]. In contrast to 13, the affinity of 14 was only 1/8 less effective than 1α,25(OH)2D3, making the 1α-OH group significantly more important for VDR binding than the 3-OH group [41]. Compound 15 lacking the methylene group was first synthesized in 1990 and has been shown to induce the differentiation of HL-60 cells at the same concentration as 1α,25(OH)2D3 [42]. The VDR binding was 30% in comparison to 1α,25(OH)2D3 [43]. Compound 16 was reported to be three times more potent than 1α,25(OH)2D3 with respect to porcine VDR binding [44]. Thus the presence of C18 impaired VDR binding, contrasting with compound 17, in which a lack of the C21 methyl reduced affinity toward chick VDR to 10% that of 1α,25(OH)2D3 [45]. Interestingly, substitution of C20 by oxygen reduced VDR affinity to 0.1% in comparison to 1α,25(OH)2D3 [45], emphasizing the importance of hydrophobicity for good receptor binding. Compound 18 with possible (R) and (S) configurations has not been reported. VDR binding of 19 has not been investigated; however, the ability to differentiate HL-60 cells compared to 1α,25(OH)2D3 was 1% at the same concentration [46]. For a similar molecule with a terminal alkene in the 2-position, a 1.9% VDR affinity was reported in comparison to the parent compound [47]. Compound 20, also known as alfacalcidol, was first reported in 1973 [48, 49]. Alfacalcidol is converted to 1α,25(OH)2D3 in vivo and, therefore, exhibits similar activities. The VDR affinity was 900-fold less than 1α,25(OH)2D3 [41]. Thus, it can be concluded that hydroxyl groups in the C1α and C25 positions are the most important substituents to promote VDR binding.
Further investigations into the significance of the bicyclic structure of 1α,25(OH)2D3 with respect to VDR binding is represented by compounds depicted in Fig. 8.6.
Removal of the five-membered ring of 1α,25(OH)2D3 was investigated with 21 and analogs thereof [50]. The relative stereochemistry of C17 marginally influenced VDR binding; however, large differences between these epimers were observed for anti-proliferation and calcium homeostasis. VDR binding of 21 was 60% in comparison to 1α,25(OH)2D3. The VDR affinity of the C20 epimer of 21 was 70%. The same report characterized compounds like 22 with a VDR affinity of 80% in comparison to 1α,25(OH)2D3. The synthesis of 23 was reported but VDR binding was not determined [51]. However, 24 with the terminal alkene moved from position 10 to position 2, exhibited 80 times lower affinity toward VDR than 1α,25(OH)2D3 [21].
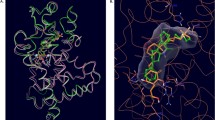
Overall, it can be concluded that structural changes to the hydrophobic core of 1α,25(OH)2D3 can still result in high-affinity ligands for VDR. The ligand-binding domain of VDR consists of 12 helices when bound to 1α,25(OH)2D3. The most essential features of 1α,25(OH)2D3 are the C1α and C25 hydroxyl groups, which have been shown to form canonical hydrogen bonds with VDR (Fig. 8.7).
Crystal structure of 1α,25(OH)2D3 bound to human VDR [PDB ID:1DB1] [52]
The interaction of 25-OH with His305 (loop H6-H7) and His 397 (H11) is vital to the conformational change VDR undergoes when interacting with coregulator proteins. 1-OH interacts with Ser237 (H3) and Arg274 (H5), anchoring the ligand in the binding pocket. 3-OH interacts with Ser278 (H5) and Tyr143 (loop H1-H2), but compounds like 2 and 14 have shown that these contacts merely provide further stabilization to the complex. 1α,25(OH)2D3 only fills 56% of the VDR-binding pocket, which helps explain the large variety of high-affinity ligands that have been developed for VDR. Important, however, is the spacing and orientation of the hydroxyl groups, which are supported by ring structures, a diene moiety, and chiral carbon centers. The majority of the central VDR ligand pocket surface is hydrophobic and assembled by leucine, isoleucine, and valine side chains.
8.3 Non-secosteroid VDR Ligands
The first non-secosteroid ligands with a diarylmethane moiety were reported by Ligand Pharmaceuticals (Fig. 8.8). The quaternary carbon center bearing two ethyl substituents was superior to other alkyl substituents, and aligned well with the fused ring system of VDR-bound 1α,25(OH)2D3 [53].
The racemic mixture of LG190178 exhibited a VDR affinity of 0.3% in comparison to 1α,25(OH)2D3 [54]. The synthesis of individual LG190178 stereoisomers identified the (2S, 2’R) isomer as the most active compound with a 28.3% VDR affinity in comparison to 1α,25(OH)2D3 [55]. The systematic development of these ligands resulted in 26, which was equally as active as 1α,25(OH)2D3 in a cell-based transcription assay [56]. An analog of 26, which replaced the ethyl groups adjacent to the tertiary alcohol with trifluoromethyl groups, showed a fivefold improvement in the transcription assay [57]. In recent years, many similar compounds were developed with thiophene, pyrrol [58], and other heterocycles, though compound 27, which contains a pyridine substituent, exhibited the highest affinity toward VDR at 37% of the VDR-1α,25(OH)2D3 interaction [59].
Another successful approach for non-secosteroidal VDR ligand design was the incorporation of a dicarba-closo-dodecaborane as a hydrophobic moiety, rather than the fused ring system of natural VDR ligands (Fig. 8.9).
The development of carborane-based VDR ligands with different side chains resulted in 28, which exhibited an affinity of 640 nM (IC50) [60]. The (R) isomer was one-fifth as active. Subsequent research identified compound 29 being twice as potent as 28 in a HL-60 differentiation assay [61].
Other approaches for VDR ligands included the incorporation of aromatic ring structures, which are absent from natural VDR ligand, 1α,25(OH)2D3. The earliest examples were identified from a library of bis-aromatic compounds (Fig. 8.10).
CD4528 was characterized with a CYP24A1 reporter assay demonstrating an EC50 of 1.7 nM [62]. For the same assay, the EC50 of 1α,25(OH)2D3 was 1.0 nM. Other related VDR ligands exhibited similar low nanomolar activities, for example, CD4849 (0.5 nM) [63]. A recent study employing the A-ring structure of 1α,25(OH)2D3 resulted in 32, which displayed a VDR affinity 24% that of 1α,25(OH)2D3 [64]. Less active was 33, exhibiting a 0.01% VDR affinity in comparison to 1α,25(OH)2D3 [65]. Other nutritional ligands with low VDR affinities were reported by Haussler et al. [66].
VDR is highly expressed in the intestine and has been described as a bile acid sensor due to its ability to bind lithocholic acid (Fig. 8.11) [67].
The affinity of VDR for lithocholic acid was less than 0.005% in comparison to 1α,25(OH)2D3. The corresponding acetate was more potent with a 0.01% VDR affinity in comparison to 1α,25(OH)2D3 [68]. A later VDR-binding study reported an IC50 of 30 μM for both 35 and 36 [69]. Recently, a methylsulfonate analog of lithocholic acid showed an IC50 of 1.2 μM [70]. For estrone analog 38, an EC50 of 850 nM was reported in a VDR transactivation assay [71].
The first Y-shaped VDR ligand called Gemini was introduced in 2000 by Norman et al. [72] (Fig. 8.12).
Gemini exhibited a VDR affinity of 38% in comparison to 1α,25(OH)2D3. Based on an available crystal structure of VDR bound to 1α,25(OH)2D3, it was hypothesized that VDR might accommodate this second side chain in a so-called A pocket (alternative pocket) [73]. The later reported crystal structure of VDR bound to Gemini confirmed the adaptability of VDR to accommodate Y-shaped ligands [74]. Further developments resulted in 40 and its C20 epimer, 41, which were 36- and 22-fold more active in a gene reporter assay than 1α,25(OH)2D3 [75].
8.4 VDR Antagonists
Throughout the last two decades, many different antagonists with strong VDR affinities have been reported [76]. The earliest disclosed antagonists were derivatives from the natural-occurring vitamin D metabolite, (23S,25R)-1α,25(OH)2D3-26,23-lactone [77], such as compound TEI-9647 and its C23 epimer TEI-9648 (Fig. 8.13).
The VDR affinity of TEI-9647 was 10% compared to 1α,25(OH)2D3. Its C23 epimer TEI-9648 bound VDR with a 8% affinity [78]. Further research confirmed that these unsaturated esters underwent a conjugate addition reaction with cysteine residues in the human VDR ligand pocket [79]. Methyl substitutions at positions 2 and 24 (43) significantly increased VDR affinity (63% in comparison to 1α,25(OH)2D3) [80]. The introduction of a cyclopropyl group on the lactone ring resulted in 44, which surpassed the VDR affinity of 1α,25(OH)2D3 (166%) [81].
Based on the structure of calcipotriol (45), an approved treatment for psoriasis and a high-affinity VDR ligand, other related ligands were able to be synthesized by Schering (Fig. 8.14).
In contrast to agonist calcipotriol, ZK159222 and ZK168281 were poor inducers of VDR transcription and reduced 1α,25(OH)2D3-meditated transcription [82]. For ZK191784, a VDR affinity of 33% in comparison to 1,25-(OH)2D3 was reported. [83] Thus, the VDR affinities of these ligands are strong but induce an antagonistic VDR conformation. Additionally, these antagonists were investigated in vivo and demonstrated promising anti-inflammatory properties [76].
Amide-based VDR antagonists that were inspired by calcitriol lactone were introduced in 2004 (Fig. 8.15).
The corresponding lactam 49 exhibited a VDR affinity of 10.2 nM in comparison to 1α,25(OH)2D3 with 0.5 nM [84]. The measured VDR binding of 50 was 1.9 nM (IC50). Importantly, 50 inhibited VDR-mediated transcription at nanomolar concentrations without showing any agonist activity in the absence of 1α,25(OH)2D3 [85]. Further improvement was achieved with 51, which exhibited 52% VDR-binding affinity compared to 1α,25(OH)2D3 and inhibited 1α,25(OH)2D3-mediated transcription with an IC50 of 90 nM [86]. For ortho-aniline compound 52, an IC50 of 107 nM was reported [87].
Introduction of a bulky adamantane group was conceived as another approach to change the conformation of VDR (Fig. 8.16).
Compound 53 exhibited 2% VDR affinity compared to 1α,25(OH)2D3 [88]. In the presence of 1α,25(OH)2D3, VDR-mediated transcription was inhibited at 100 nM. Among a series of diastereomeric analogs, 54 exhibited the highest affinity toward VDR with 17% affinity in comparison to 1α,25(OH)2D3 [89]. Further improvements resulted in ligands with an internal alkyne named ADTK1-4 [90]. The compound with the highest VDR affinity among this group was 55 reaching 90% of the VDR-1α,25(OH)2D3 interaction. This compound behaved as a partial agonist. Finally, a library of VDR ligands with a diyne system were synthesized and evaluated, achieving a 7% VDR affinity in comparison to 1α,25(OH)2D3 [91].
A series of Y-shaped VDR ligands that were inspired by Gemini were developed and, dependent upon their substitution pattern, were found to be antagonists, partial agonists, or superagonists (Fig. 8.17).
Among a series of diastereomers with a butyl substituent in the C22 position, 56 was identified as a VDR antagonist [92]. The VDR affinity was 4.1% in comparison to 1α,25-(OH)2D3 [92]. Interestingly, the C20 epimer of 56 was identified as an agonist with a 2.5% affinity toward VDR in comparison to 1α,25(OH)2D3. The influence of the alkyl chain length with respect to VDR binding was investigated for very similar compounds containing a methylene group in the C2 position (57–60) [47, 93]. The presence of a butyl substituent resulted in antagonist 59, which had a VDR affinity of 61% in comparison to 1α,25(OH)2D3. Partial agonists 57, 58, and 60 exhibited lower VDR affinity. Further structural changes to compound 59 included the introduction of two methyl substituents in the C24 position or elongation of the hydroxyl-bearing carbon chain by one carbon. Both of these structural changes also resulted in antagonistic ligands; however, implementing the carbon chain inherent to 1α,25(OH)2D3 resulted in a superagonist with higher VDR affinity than 1α,25(OH)2D3 [47]. Other superagonists were produced with the introduction of C22 substituents for 20-epi-1α,25-(OH)2D3 (12) [94]. Compound 63 exhibited the highest affinity for VDR (797%) followed by 62 and 61. Finally, antagonists were produced with an isopropyl group at C24 (64) [92]. Among different diastereomers, 64 exhibited the highest VDR affinity of 1.4% in comparison to 1α,25(OH)2D3.
A high-throughput screen of 390,000 compounds identified PPARδ agonist GW0742 as a novel VDR antagonist (Fig. 8.18) [95].
The VDR affinity of 65 was 8.7 μM. The structural change of the acid function into an hydroxyl group resulted in partial VDR agonist 66 with an EC50 of 120 nM [96]. The evaluation of a library of compounds related to 65 demonstrated that CF3 substituents in the meta- and ortho-position reduced the affinity toward PPARδ without influencing the affinity for VDR [97]. Recently, VDR antagonist 67 was reported with activity of 660 nM [98]. This compound did not bind PPARδ. Virtual screening of known nuclear receptor ligands for application in VDR binding identified several compounds as possible candidates. VDR binding was demonstrated for several compounds, including H6036 [99].
8.5 Concluding Remarks and Future Directions
Novel VDR ligand design and synthesis is still a very active research area, with many international research groups working together to develop new drug candidates for disorders caused by vitamin D deficiency, cancer, and inflammatory diseases. Recently discovered VDR ligands are being investigated in clinical trials, reflecting the need for new medications in the respective disease areas. A great number of ligands have been elucidated in complex with VDR using X-ray crystallography. The structural information has guided new ligand design while also demonstrating that the VDR ligand pocket is amendable to very different ligand shapes. However, as highlighted in this chapter, hydrogen bonding on opposite ends of the ligand pocket is essential for high VDR affinity. Another important feature is distinct spacing of these groups by a flexible hydrophobic spacer. Recently, endogenous ligands for VDR such as lithocholic acid and fatty acids have been identified, although their biological function is still unclear. Furthermore, new vitamin D metabolites have been identified in the last few decades, offering new areas of research in the field of vitamin D.
References
Steenbock H, Black A. Fat-soluble vitamins. XVII. The induction of growthpromoting and calcifying properties in a ration by exposure to ultraviolet light. J Biol Chem. 1924;61:405–22.
Askew FA, Bourdillon RB, Bruce HM, Jenkins RGC, Webster TA. The distillation of vitamin D. Proc R Soc. 1930;B107:76–90.
Chalk KJ, Kodicek E. The association of 14C-labelled vitamin D2 with rat serum proteins. Biochem J. 1961;79:1–7.
Blunt JW, DeLuca HF, Schnoes HK. 25-hydroxycholecalciferol. A biologically active metabolite of vitamin D3. Biochemistry. 1968;7(10):3317–22.
Holick MF, Schnoes HK, DeLuca HF, Suda T, Cousins RJ. Isolation and identification of 1,25-dihydroxycholecalciferol. A metabolite of vitamin D active in intestine. Biochemistry. 1971;10(14):2799–804.
Brumbaugh PF, Haussler MR. 1Alpha,25-dihydroxyvitamin D3 receptor: competitive binding of vitamin D analogs. Life Sci. 1973;13(12):1737–46.
McDonnell DP, Mangelsdorf DJ, Pike JW, Haussler MR, O’Malley BW. Molecular cloning of complementary DNA encoding the avian receptor for vitamin D. Science. 1987;235(4793):1214–7.
Wang YJ, Zhu JG, DeLuca HF. Where is the vitamin D receptor? Arch Biochem Biophys. 2012;523(1):123–33.
Huhtakangas JA, Olivera CJ, Bishop JE, Zanello LP, Norman AW. The vitamin D receptor is present in caveolae-enriched plasma membranes and binds 1 alpha,25(OH)(2)-vitamin D-3 in vivo and in vitro. Mol Endocrinol. 2004;18(11):2660–71.
Orlov I, Rochel N, Moras D, Klaholz BP. Structure of the full human RXR/VDR nuclear receptor heterodimer complex with its DR3 target DNA. EMBO J. 2012;31(2):291–300.
Haussler MR, Jurutka PW, Mizwicki M, Norman AW. Vitamin D receptor (VDR)-mediated actions of 1 alpha,25(OH)(2)vitarnin D-3: genomic and non-genomic mechanisms. Best Pract Res Clin Endocrinol Metab. 2011;25(4):543–59.
White JH, Salehi-Tabar R, Dimitrow V, Bouttier M. Diverse mechanism of transcriptional regulation by the vitamin D receptor. In: Feldman D, editor. Vitamin D, vol. 1. 4th ed. London: Elsevier; 2018. p. 175–87.
Nurminen V, Neme A, Seuter S, Carlberg C. The impact of the vitamin D-modulated epigenome on VDR target gene regulation. Biochim Biophys Acta. 2018;1861(8):697–705.
Verlinden L, Bouillon R, De Clercq P, Verstuyf A. Analogs of calcitriol. In: Feldman D, editor. Vitamin D, vol. 2. 4th ed. Academic Press, Elsevier; 2018. p. 583–611.
Stites RE, Mackrell JG, Stayrock KR. Nonsecosteroidal ligands and molulators of the vitamin D receptor. In: Feldman D, editor. Vitamin D, vol. 2. Academic Press, Elsevier; 2018. p. 615–25.
Makishima M, Yamada S. Bile acid-derived vitamin D receptor ligands. In: Feldman D, editor. Vitamin D, vol. 2. Academic Press, Elsevier; 2018. p. 629–41.
Yu OB, Arnold LA. Moldulating vitamin D receptor-coregulator binding with small molecules. In: Feldman D, editor. Vitamin D, vol. 2. Academic Press, Elsevier; 2018. p. 657–64.
Saitoh H. Vitamin D receptor antagonists. In: Feldman D, editor. Vitamin D, vol. 2. Academic Press, Elsevier; 2018. p. 679–91.
Maestro MA, Molnar F, Carlberg C. Vitamin D and its synthetic analogs. J Med Chem. 2019;62(15):6854–75.
Maestro MA, Molnar F, Mourino A, Carlberg C. Vitamin D receptor 2016: novel ligands and structural insights. Expert Opin Ther Pat. 2016;26(11):1291–306.
Plonska-Ocypa K, Grzywacz P, Sicinski RR, Plum LA, DeLuca HF. Synthesis and biological evaluation of a des-C,D-analog of 2-methylene-19-nor-1alpha,25-(OH)2D3. J Steroid Biochem Mol Biol. 2007;103(3–5):298–304.
Mottershead DG, Polly P, Lyons RJ, Sutherland RL, Watts CK. High activity, soluble, bacterially expressed human vitamin D receptor and its ligand binding domain. J Cell Biochem. 1996;61(3):325–37.
Molnar F, Sigueiro R, Sato Y, Araujo C, Schuster I, Antony P, et al. 1alpha,25(OH)2-3-epi-vitamin D3, a natural physiological metabolite of vitamin D3: its synthesis, biological activity and crystal structure with its receptor. PLoS One. 2011;6(3):e18124.
Monkawa T, Yoshida T, Wakino S, Shinki T, Anazawa H, Deluca HF, et al. Molecular cloning of cDNA and genomic DNA for human 25-hydroxyvitamin D3 1 alpha-hydroxylase. Biochem Biophys Res Commun. 1997;239(2):527–33.
Takeyama K, Kitanaka S, Sato T, Kobori M, Yanagisawa J, Kato S. 25-Hydroxyvitamin D3 1alpha-hydroxylase and vitamin D synthesis. Science. 1997;277(5333):1827–30.
St-Arnaud R, Messerlian S, Moir JM, Omdahl JL, Glorieux FH. The 25-hydroxyvitamin D 1-alpha-hydroxylase gene maps to the pseudovitamin D-deficiency rickets (PDDR) disease locus. J Bone Miner Res. 1997;12(10):1552–9.
Zehnder D, Bland R, Williams MC, McNinch RW, Howie AJ, Stewart PM, et al. Extrarenal expression of 25-hydroxyvitamin d(3)-1 alpha-hydroxylase. J Clin Endocrinol Metab. 2001;86(2):888–94.
Norman AW, Bouillon R, Farach-Carson MC, Bishop JE, Zhou LX, Nemere I, et al. Demonstration that 1 beta,25-dihydroxyvitamin D3 is an antagonist of the nongenomic but not genomic biological responses and biological profile of the three A-ring diastereomers of 1 alpha,25-dihydroxyvitamin D3. J Biol Chem. 1993;268(27):20022–30.
Bischof MG, Siu-Caldera ML, Weiskopf A, Vouros P, Cross HS, Peterlik M, et al. Differentiation-related pathways of 1 alpha,25-dihydroxycholecalciferol metabolism in human colon adenocarcinoma-derived Caco-2 cells: production of 1 alpha,25-dihydroxy-3epi-cholecalciferol. Exp Cell Res. 1998;241(1):194–201.
Paaren HE, Schoenen HK, DeLuca HF. Synthesis of 1β-hydroxyvitamin D3 and 1β,25-dihydroxyvitamin D3. Chem Commun. 1977;23:890–2.
Pauwels S, Jans I, Billen J, Heijboer A, Verstuyf A, Carmeliet G, et al. 1beta,25-Dihydroxyvitamin D3: a new vitamin D metabolite in human serum. J Steroid Biochem Mol Biol. 2017;173:341–8.
Wecksler WR, Norman AW. Studies on the mode of action of calciferol XXV. 1 alpha,25-dihydroxy-5,6-trans-vitamin D3, the 5E-isomer of 1 alpha,25-dihydroxyvitamin D3. Steroids. 1980;35(4):419–25.
Kobayashi T, Moriuchi S, Shimura F, Katsui G. Synthesis and biological activity of 5,6-trans-vitamin D3 in anephric rats. J Nutr Sci Vitaminol (Tokyo). 1976;22(4):299–306.
VanAlstyne EM, Norman AW, Okamura WH. 7,8-Cis geometric isomers of the steroid hormone 1a,25-dihydroxyvitamin D. J Am Chem Soc. 1994;116:6207–16.
Maynard DF, Trankle WG, Norman AW, Okamura WH. 14-epi stereoisomers of 25-hydroxy- and 1 alpha,25-dihydroxyvitamin D3: synthesis, isomerization to previtamins, and biological studies. J Med Chem. 1994;37(15):2387–93.
Kurek-Tyrlik A, Michalak K, Wicha J. Synthesis of 17-epi-calcitriol from a common androstane derivative, involving the ring B photochemical opening and the intermediate triene ozonolysis. J Org Chem. 2005;70(21):8513–21.
Michalak K, Wicha J. Total synthesis of a CD-ring: side-chain building block for preparing 17-epi-calcitriol derivatives from the Hajos-Parrish dione. J Org Chem. 2011;76(16):6906–11.
Binderup L, Latini S, Binderup E, Bretting C, Calverley M, Hansen K. 20-epi-vitamin D3 analogues: a novel class of potent regulators of cell growth and immune responses. Biochem Pharmacol. 1991;42(8):1569–75.
Zhou X, Zhu GD, Van Haver D, Vandewalle M, De Clercq PJ, Verstuyf A, et al. Synthesis, biological activity, and conformational analysis of four seco-D-15,19-bisnor-1alpha,25-dihydroxyvitamin D analogues, diastereomeric at C17 and C20. J Med Chem. 1999;42(18):3539–56.
Bischoff-Ferrari HA, Giovannucci E, Willett WC, Dietrich T, Dawson-Hughes B. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr. 2006;84(1):18–28.
Procsal DA, Okamura WH, Norman AW. Structural requirements for the interaction of 1 alpha, 25-(OH) 2- vitiamin D3 with its chick interestinal receptor system. J Biol Chem. 1975;250(21):8382–8.
Perlman KL, Sicinski RR, Schnoes HK, DeLuca HF. 1α,25-dihydroxy-19-nor-vitamin D3, a novel vitamin D-related compound with potential therapeutic activity. Tetrahedron Lett. 1990;31(13):1823–4.
Bouillon R, Sarandeses LA, Allewaert K, Zhao J, Mascarenas JL, Mourino A, et al. Biologic activity of dihydroxylated 19-nor-(pre)vitamin D3. J Bone Miner Res. 1993;8(8):1009–15.
Sicinski RR, Perlman KL, Prahl J, Smith C, DeLuca HF. Synthesis and biological activity of 1 alpha, 25-dihydroxy-18-norvitamin D3 and 1 alpha, 25-dihydroxy-18,19-dinorvitamin D3. J Med Chem. 1996;39(22):4497–506.
Kubodera N, Miyamoto K, Matsumoto M, Kawanishi T, Ohkawa H, Mori T. Synthetic studies of vitamin D analogues. X. Synthesis and biological activities of 1 alpha,25-dihydroxy-21-norvitamin D3. Chem Pharm Bull (Tokyo). 1992;40(3):648–51.
Ostrem VK, Lau WF, Lee SH, Perlman K, Prahl J, Schnoes HK, et al. Induction of monocytic differentiation of HL-60 cells by 1,25-dihydroxyvitamin D analogs. J Biol Chem. 1987;262(29):14164–71.
Sakamaki Y, Inaba Y, Yoshimoto N, Yamamoto K. Potent antagonist for the vitamin D receptor: vitamin D analogues with simple side chain structure. J Med Chem. 2010;53(15):5813–26.
Haussler MR, Zerwekh JE, Hesse RH, Rizzardo E, Pechet MM. Biological activity of 1alpha-hydroxycholecalciferol, a synthetic analog of the hormonal form of vitamin D3. Proc Natl Acad Sci U S A. 1973;70(8):2248–52.
Holick MF, Semmler EJ, Schnoes HK, DeLuca HF. 1 Hydroxy derivative of vitamin D3 : a highly potent analog of 1, 25-dihydroxyvitamin D3. Science. 1973;180(4082):190–1.
Verstuyf A, Verlinden L, van Etten E, Shi L, Wu Y, D'Halleweyn C, et al. Biological activity of CD-ring modified 1alpha,25-dihydroxyvitamin D analogues: C-ring and five-membered D-ring analogues. J Bone Miner Res. 2000;15(2):237–52.
Kutner A, Zhao H, Fitak H, Chodyn M, Halkes SJ, Wilson SR, et al. Inventors new pharmaceutically active compounds. 1995. Patent, WO1995019963A1.
Rochel N, Wurtz JM, Mitschler A, Klaholz B, Moras D. The crystal structure of the nuclear receptor for vitamin D bound to its natural ligand. Mol Cell. 2000;5(1):173–9.
Kakuda S, Okada K, Eguchi H, Takenouchi K, Hakamata W, Kurihara M, et al. Structure of the ligand-binding domain of rat VDR in complex with the nonsecosteroidal vitamin D3 analogue YR301. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2008;64(Pt 11):970–3.
Boehm MF, Fitzgerald P, Zou A, Elgort MG, Bischoff ED, Mere L, et al. Novel nonsecosteroidal vitamin D mimics exert VDR-modulating activities with less calcium mobilization than 1,25-dihydroxyvitamin D3. Chem Biol. 1999;6(5):265–75.
Hakamata W, Sato Y, Okuda H, Honzawa S, Saito N, Kishimoto S, et al. (2S,2'R)-analogue of LG190178 is a major active isomer. Bioorg Med Chem Lett. 2008;18(1):120–3.
Kashiwagi H, Ono Y, Ohta M, Morikami K, Takahashi T. Systematic SAR study of the side chain of nonsecosteroidal vitamin D(3) analogs. Bioorg Med Chem. 2012;20(14):4495–506.
Kashiwagi H, Ohta M, Ono Y, Morikami K, Itoh S, Sato H, et al. Effects of fluorines on nonsecosteroidal vitamin D receptor agonists. Bioorg Med Chem. 2013;21(3):712–21.
Ge Z, Hao M, Xu M, Su Z, Kang Z, Xue L, et al. Novel nonsecosteroidal VDR ligands with phenyl-pyrrolyl pentane skeleton for cancer therapy. Eur J Med Chem. 2016;107:48–62.
Taniguchi K, Katagiri K, Kashiwagi H, Harada S, Sugimoto Y, Shimizu Y, et al. A novel nonsecosteroidal VDR agonist (CH5036249) exhibits efficacy in a spontaneous benign prostatic hyperplasia beagle model. J Steroid Biochem Mol Biol. 2010;121(1–2):204–7.
Fujii S, Masuno H, Taoda Y, Kano A, Wongmayura A, Nakabayashi M, et al. Boron cluster-based development of potent nonsecosteroidal vitamin D receptor ligands: direct observation of hydrophobic interaction between protein surface and carborane. J Am Chem Soc. 2011;133(51):20933–41.
Fujii S, Kano A, Masuno H, Songkram C, Kawachi E, Hirano T, et al. Design and synthesis of tetraol derivatives of 1,12-dicarba-closo-dodecaborane as non-secosteroidal vitamin D analogs. Bioorg Med Chem Lett. 2014;24(18):4515–9.
Perakyla M, Malinen M, Herzig KH, Carlberg C. Gene regulatory potential of nonsteroidal vitamin D receptor ligands. Mol Endocrinol. 2005;19(8):2060–73.
Ciesielski F, Sato Y, Chebaro Y, Moras D, Dejaegere A, Rochel N. Structural basis for the accommodation of bis- and tris-aromatic derivatives in vitamin D nuclear receptor. J Med Chem. 2012;55(19):8440–9.
Gogoi P, Seoane S, Sigueiro R, Guiberteau T, Maestro MA, Perez-Fernandez R, et al. Aromatic-based design of highly active and noncalcemic vitamin D receptor agonists. J Med Chem. 2018;61(11):4928–37.
Chen F, Su Q, Torrent M, Wei N, Peekhaus N, Mcmasters D, et al. Identification and characterization of a novel nonsecosteroidal vitamin D receptor ligand. DrugDev Res. 2007;68(2):51–60.
Haussler MR, Haussler CA, Bartik L, Whitfield GK, Hsieh JC, Slater S, et al. Vitamin D receptor: molecular signaling and actions of nutritional ligands in disease prevention. Nutr Rev. 2008;66(10 Suppl 2):S98–112.
Makishima M, Lu TT, Xie W, Whitfield GK, Domoto H, Evans RM, et al. Vitamin D receptor as an intestinal bile acid sensor. Science. 2002;296:1313–6.
Adachi R, Honma Y, Masuno H, Kawana K, Shimomura I, Yamada S, et al. Selective activation of vitamin D receptor by lithocholic acid acetate, a bile acid derivative. J Lipid Res. 2005;46(1):46–57.
Ishizawa M, Matsunawa M, Adachi R, Uno S, Ikeda K, Masuno H, et al. Lithocholic acid derivatives act as selective vitamin D receptor modulators without inducing hypercalcemia. J Lipid Res. 2008;49(4):763–72.
Masuno H, Kazui Y, Tanatani A, Fujii S, Kawachi E, Ikura T, et al. Development of novel lithocholic acid derivatives as vitamin D receptor agonists. Bioorg Med Chem. 2019;27(16):3674–81.
Arichi N, Fujiwara S, Ishizawa M, Makishima M, Hua DH, Yamada KI, et al. Synthesis and biological evaluation of steroidal derivatives bearing a small ring as vitamin D receptor agonists. Bioorg Med Chem Lett. 2017;27(15):3408–11.
Norman AW, Manchand PS, Uskokovic MR, Okamura WH, Takeuchi JA, Bishop JE, et al. Characterization of a novel analogue of 1alpha,25(OH)(2)-vitamin D(3) with two side chains: interaction with its nuclear receptor and cellular actions. J Med Chem. 2000;43(14):2719–30.
Mizwicki MT, Keidel D, Bula CM, Bishop JE, Zanello LP, Wurtz JM, et al. Identification of an alternative ligand-binding pocket in the nuclear vitamin D receptor and its functional importance in 1alpha,25(OH)2-vitamin D3 signaling. Proc Natl Acad Sci U S A. 2004;101(35):12876–81.
Ciesielski F, Rochel N, Moras D. Adaptability of the Vitamin D nuclear receptor to the synthetic ligand Gemini: remodelling the LBP with one side chain rotation. J Steroid Biochem Mol Biol. 2007;103(3–5):235–42.
Huet T, Maehr H, Lee HJ, Uskokovic MR, Suh N, Moras D, et al. Structure-function study of gemini derivatives with two different side chains at C-20, Gemini-0072 and Gemini-0097. MedChemComm. 2011;2(5):424–9.
Teske KA, Yu O, Arnold LA. Inhibitors for the vitamin D receptor-coregulator interaction. Vitam Horm. 2016;100:45–82.
Ishizuka S, Yamaguchi H, Yamada S, Nakayama K, Takayama H. Stereochemistry of 25-hydroxyvitamin D3-26,23-lactone and 1 alpha, 25-dihydroxyvitamin D3-26,23-lactone in rat serum. FEBS Lett. 1981;134(2):207–11.
Miura D, Manabe K, Ozono K, Saito M, Gao Q, Norman AW, et al. Antagonistic action of novel 1alpha,25-dihydroxyvitamin D3-26, 23-lactone analogs on differentiation of human leukemia cells (HL-60) induced by 1alpha,25-dihydroxyvitamin D3. J Biol Chem. 1999;274(23):16392–9.
Kakuda S, Ishizuka S, Eguchi H, Mizwicki MT, Norman AW, Takimoto-Kamimura M. Structural basis of the histidine-mediated vitamin D receptor agonistic and antagonistic mechanisms of (23S)-25-dehydro-1alpha-hydroxyvitamin D3-26,23-lactone. Acta Crystallogr D Biol Crystallogr. 2010;66(Pt 8):918–26.
Saito N, Saito H, Anzai M, Yoshida A, Fujishima T, Takenouchi K, et al. Dramatic enhancement of antagonistic activity on vitamin D receptor: a double functionalization of 1alpha-hydroxyvitamin D3 26,23-lactones. Org Lett. 2003;5(25):4859–62.
Saito N, Matsunaga T, Saito H, Anzai M, Takenouchi K, Miura D, et al. Further synthetic and biological studies on vitamin D hormone antagonists based on C24-alkylation and C2alpha-functionalization of 25-dehydro-1alpha-hydroxyvitamin D(3)-26,23-lactones. J Med Chem. 2006;49(24):7063–75.
Bury Y, Steinmeyer A, Carlberg C. Structure activity relationship of carboxylic ester antagonists of the vitamin D(3) receptor. Mol Pharmacol. 2000;58(5):1067–74.
Zugel U, Steinmeyer A, Giesen C, Asadullah K. A novel immunosuppressive 1alpha,25-dihydroxyvitamin D3 analog with reduced hypercalcemic activity. J Invest Dermatol. 2002;119(6):1434–42.
Kato Y, Nakano Y, Sano H, Tanatani A, Kobayashi H, Shimazawa R, et al. Synthesis of 1alpha,25-dihydroxyvitamin D3-26,23-lactams (DLAMs), a novel series of 1 alpha,25-dihydroxyvitamin D3 antagonist. Bioorg Med Chem Lett. 2004;14(10):2579–83.
Nakano Y, Kato Y, Imai K, Ochiai E, Namekawa J, Ishizuka S, et al. Practical synthesis and evaluation of the biological activities of 1 alpha,25-dihydroxyvitamin D-3 antagonists, 1 alpha,25-dihydroxyvitamin D-3-26,23-lactams. Designed on the basis of the helix 12-folding inhibition hypothesis. J Med Chem. 2006;49(8):2398–406.
Cho K, Uneuchi F, Kato-Nakamura Y, Namekawa J, Ishizuka S, Takenouchi K, et al. Structure-activity relationship studies on vitamin D lactam derivatives as vitamin D receptor antagonist. Bioorg Med Chem Lett. 2008;18(15):4287–90.
Lamblin M, Spingarn R, Wang TT, Burger MC, Dabbas B, Moitessier N, et al. An o-aminoanilide analogue of 1alpha,25-dihydroxyvitamin D(3) functions as a strong vitamin D receptor antagonist. J Med Chem. 2010;53(20):7461–5.
Inaba Y, Yamamoto K, Yoshimoto N, Matsunawa M, Uno S, Yamada S, et al. Vitamin D3 derivatives with adamantane or lactone ring side chains are cell type-selective vitamin D receptor modulators. Mol Pharmacol. 2007;71(5):1298–311.
Igarashi M, Yoshimoto N, Yamamoto K, Shimizu M, Ishizawa M, Makishima M, et al. Identification of a highly potent vitamin D receptor antagonist: (25S)-26-adamantyl-25-hydroxy-2-methylene-22,23-didehydro-19,27-dinor-20-epi-vita min D3 (ADMI3). Arch Biochem Biophys. 2007;460(2):240–53.
Kudo T, Ishizawa M, Maekawa K, Nakabayashi M, Watarai Y, Uchida H, et al. Combination of triple bond and adamantane ring on the vitamin D side chain produced partial agonists for vitamin D receptor. J Med Chem. 2014;57(10):4073–87.
Watarai Y, Ishizawa M, Ikura T, Zacconi FC, Uno S, Ito N, et al. Synthesis, biological activities, and X-ray crystal structural analysis of 25-hydroxy-25(or 26)-adamantyl-17-[20(22),23-diynyl]-21-norvitamin D compounds. J Med Chem. 2015;58(24):9510–21.
Inaba Y, Yoshimoto N, Sakamaki Y, Nakabayashi M, Ikura T, Tamamura H, et al. A new class of vitamin D analogues that induce structural rearrangement of the ligand-binding pocket of the receptor. J Med Chem. 2009;52(5):1438–49.
Anami Y, Sakamaki Y, Itoh T, Inaba Y, Nakabayashi M, Ikura T, et al. Fine tuning of agonistic/antagonistic activity for vitamin D receptor by 22-alkyl chain length of ligands: 22S-hexyl compound unexpectedly restored agonistic activity. Bioorg Med Chem. 2015;23(22):7274–81.
Yamamoto K, Inaba Y, Yoshimoto N, Choi M, DeLuca HF, Yamada S. 22-Alkyl-20-epi-1alpha,25-dihydroxyvitamin D3 compounds of superagonistic activity: syntheses, biological activities and interaction with the receptor. J Med Chem. 2007;50(5):932–9.
Nandhikonda P, Yasgar A, Baranowski AM, Sidhu PS, McCallum MM, Pawlak AJ, et al. Peroxisome proliferation-activated receptor delta agonist GW0742 interacts weakly with multiple nuclear receptors, including the vitamin D receptor. Biochemistry. 2013;52(24):4193–203.
Teske K, Nandhikonda P, Bogart JW, Feleke B, Sidhu P, Yuan N, et al. Modulation of transcription mediated by the vitamin D receptor and the peroxisome proliferator-activated receptor delta in the presence of GW0742 analogs. J Biomol Res Ther. 2014;3(1):1000111.
Teske KA, Rai G, Nandhikonda P, Sidhu PS, Feleke B, Simeonov A, et al. Parallel chemistry approach to identify novel nuclear receptor ligands based on the GW0742 scaffold. ACS Comb Sci. 2017;19(10):646–56.
Teske KA, Bogart JW, Arnold LA. Novel VDR antagonists based on the GW0742 scaffold. Bioorg Med Chem Lett. 2018;28(3):351–4.
Teske K, Nandhikonda P, Bogart JW, Feleke B, Sidhu P, Yuan N, et al. Identification of Vdr antagonists among nuclear receptor ligands using virtual screening. Nucl Recept Res. 2014;1:1–8.
Acknowledgments
This work was supported by the University of Wisconsin-Milwaukee, the Milwaukee Institute for Drug Discovery, the UWM Research Growth Initiative, NIH R03DA031090, the UWM Research Foundation, the Lynde and Harry Bradley Foundation, and the Richard and Ethel Herzfeld Foundation.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Mutchie, T.R., Webb, D.A., Di Milo, E.S., Arnold, L.A. (2021). Strategies for the Design of Vitamin D Receptor Ligands. In: Badr, M.Z. (eds) Nuclear Receptors. Springer, Cham. https://doi.org/10.1007/978-3-030-78315-0_8
Download citation
DOI: https://doi.org/10.1007/978-3-030-78315-0_8
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-78314-3
Online ISBN: 978-3-030-78315-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)